Abstract
Background/aims
To clarify the pathogenesis of fibrosis in inflammatory orbital diseases, we analyzed the gene expression in orbital biopsies and compared our results to those reported for idiopathic pulmonary fibrosis.
Methods
We collected 140 biopsies from 138 patients (58 lacrimal gland; 82 orbital fat). Diagnoses included healthy controls (n=27), nonspecific orbital inflammation (NSOI) (n=61), thyroid eye disease (TED) (n=29), sarcoidosis (n=14), and granulomatosis with polyangiitis (GPA) (n=7). Fibrosis was scored on a zero to three scale by two expert, ophthalmic pathologists. Gene expression was quantified using Affymetrix U133 plus 2.0 microarray.
Results
Within orbital fat, fibrosis was greatest among subjects with GPA (2.75±0.46) and significantly increased in tissue from subjects with GPA, NSOI, or sarcoidosis (p<0.01), but not for TED, compared to healthy controls (1.13±0.69). For lacrimal gland, the average score among controls (1.36±0.48) did not differ statistically from any of the 4 disease groups. Seventy-three probe sets identified transcripts correlating with fibrosis in orbital fat (false discovery rate < 0.05) after accounting for batch effects, disease type, age and sex. Transcripts with increased expression included fibronectin, lumican, thrombospondin, and collagen types I and VIII, each of which has been reported upregulated in pulmonary fibrosis.
Conclusion
A pathologist's recognition of fibrosis in orbital tissue correlates well with increased expression of transcripts considered essential in fibrosis. Many of the transcripts implicated in orbital fibrosis have been previously implicated in pulmonary fibrosis. TED differs from other causes of orbital fat inflammation in that fibrosis is not a major component. Marked fibrosis is less common in the lacrimal gland compared to orbital adipose tissue.
Keywords: Orbital inflammation, fibrosis, gene expression, lacrimal gland
INTRODUCTION
Fibrosis is an important component of the inflammatory response. In many diseases including proliferative vitreoretinopathy, mucous membrane pemphigoid, cirrhosis, scleroderma, idiopathic pulmonary fibrosis, and retroperitoneal fibrosis, the fibrotic component of the disease is a dominant clinical feature. The ability to prevent or reverse fibrosis requires an understanding of its pathogenesis.
Exophthalmos can be due to infections, malignancies, or inflammation. The inflammatory processes include Graves disease (also known as thyroid eye disease or TED), sarcoidosis, granulomatosis with polyangiitis (GPA) (previously known as Wegener's granulomatosis), IgG4 disease, and Erdheim-Chester disease [1,2]. Fibrosis can be a prominent component in orbital inflammation. It is considered to be an ominous prognostic finding [3].
In order to clarify the pathogenesis of orbital inflammatory diseases, we have assembled an international consortium of orbital surgeons and ophthalmic pathologists. We have collected a library of formalin-fixed biopsies of either the lacrimal gland or orbital fat. We have scored these biopsies for fibrosis and then correlated the fibrosis score with gene expression. Finally we compared genes with increased expression in orbital fibrosis to transcripts reportedly increased in idiopathic pulmonary fibrosis.
METHODS
Tissue and pathology review
The orbital biopsies were performed by surgeons at ten different centers from 3 continents. All biopsies were fixed in formalin. The diagnosis was made on the basis of clinical information and pathological review at the center where the biopsy was obtained. Normal, uninflamed control orbital tissue was obtained from subjects with no history of orbital disease at the time of surgery such as fat typically excised and discarded as part of routine blepharoplasty or retrobulbar fat that is snared with the eye during enucleation. The pathology was further reviewed by two of the authors (DJW and HEG). Most pathological diagnoses were accepted but in a small number of cases, an alternative diagnosis was suggested and the tissue was not included in further analysis. DJW and HEG independently scored the fibrosis as absent (0), mild (1), moderate (2), or severe (3). One pathologist based the grading scheme on the percent of tissue that was fibrotic: no fibrosis was graded as 0; up to 1/3 of the specimen containing fibrosis was graded as mild (1); 1/3 to 2/3 of the specimen containing fibrosis was graded as moderate (2); and greater than 2/3 of the specimen containing fibrosis was graded as severe (3). The other pathologist graded the tissue more qualitatively overall as displaying no fibrosis versus mild, moderate or severe fibrosis.
Microarray
All tissue was sent to Oregon Health & Science University, Portland, Oregon, where RNA was extracted. cDNA was synthesized from the RNA and hybridized in two batches to Affymetrix U 133 plus 2.0 arrays, which include about 54,000 probe sets. The methodology for the RNA extraction and microarray have been previously described [4]. Further, we have reported on the RNA quality (Rosenbaum et al., manuscript submitted) and the correlation between our array data and quantitative PCR [5].
Statistical methods
Each batch of Affymetrix cel files of a tissue type was preprocessed by Robust Multiarray Analysis separately [6]. Then, a linear regression model was fitted to the preprocessed data to estimate trend in intensity with respect fibrosis score while accounting for batch effects, disease type, age and sex to all data of a tissue type independently. To fit the model, we used RUVinv in conjunction with empirical Bayes and false discovery rate adjustment for multiple test corrections [7]. These methods are available in ruv and limma packages of R Statistical Computing Environment (http://www.r-project.org). For negative controls, we used the human housekeeping genes reported in Eisenberg and Levanon (2003) [8].
RESULTS
We analyzed 150 biopsies including 85 from orbital fat and 65 from the lacrimal gland. The 85 orbital biopsies were obtained from 82 subjects with two biopsies read from 3 subjects. The 65 lacrimal biopsies were obtained from 56 subjects. The lacrimal biopsies included from two subjects who provided two separate tissue blocks and 7 subjects for whom two tissues from the same block were independently scored. Table 1 shows the age, gender, and number of biopsies for each diagnosis for the orbit and lacrimal gland respectively. Each pathologist scored the tissue for fibrosis independently using a 0 to 3 scale. All except nine normal control tissues were scored by both pathologists. The scores were averaged. For 64 lacrimal gland biopsies scored by both pathologists, 20 received an identical score (31.3%). In 35 instances (54.7%) the scores differed by one. In 9 instances, the scores differed by two (14.1%). For the orbital biopsies, 77 biopsies were scored by both pathologists. In 38 instances (49.3%), the scores were identical. Thirty-one times the scores differed by one (40.3%) and 8 times the scores differed by two (10.4%). The average fibrosis scores for diseases affecting orbital fat are shown in Figure 1A. The greatest degree of fibrosis was noted among subjects with GPA (p<0.0001 relative to healthy controls). Subjects with NSOI (p<0.002) or sarcoidosis (p=0.005) also demonstrated significantly more fibrosis than what was detected in control tissue, although each value was significantly less than for GPA (p<0.01). In contrast, patients with TED had an average fibrosis score that did not differ from controls.
Table 1.
Subject demographics
| Orbital Adipose | Lacrimal Gland | |||
|---|---|---|---|---|
| Disease | Female:Male | Age (mean ± SD) | Female:Male | Age (mean ± SD) |
| GPA | 4:2 | 41.7 ± 10.8 | 1:0 | 12.9 |
| NSOI | 16:8 | 50.8 ± 23.9 | 27:10 | 46.8 ± 17.4 |
| Sarcoidosis | 5:2 | 47.7 ± 12.3 | 6:2 | 35.4 ± 11.5 |
| TED | 19:6 | 51.6 ± 14.0 | 4:1 | 54.6 ± 6.2 |
| Normal | 14:6 | 63.6 ± 14.5 | 6:1 | 68.4 ± 8.9 |
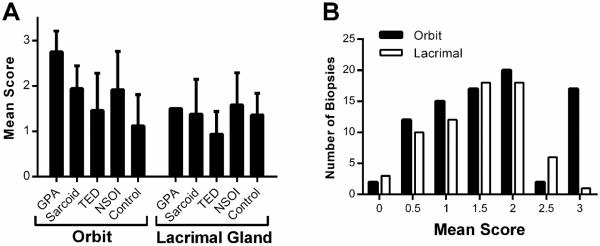
Figure 1.
Orbital adipose tissue from subjects with GPA had the highest fibrosis scores. A: Orbital adipose and lacrimal gland fibrosis scores for each disease group (mean ± standard deviation). B: The distribution of fibrosis scores relative to the site of biopsy.
Fibrosis scores for lacrimal gland diseases are also shown in Figure 1A. In contrast to inflammation affecting orbital fat, none of the disease processes resulted in fibrosis that differed statistically from the healthy controls. However, we only had a single lacrimal gland with the diagnosis of GPA. Figure 1B shows the frequency scores for fibrosis for each tissue site. The values should not be compared directly because the frequency for specific diagnoses differed between the tissues. However, it is apparent that a fibrosis score of 3 was far more likely to be scored in disease affecting orbital adipose tissue.
We then generated a list of transcripts that correlated with the scores of fibrosis in the orbit (FDR <0.05) while accounting for batch effects, disease type, age and sex. This is shown in Tables 2 and 3 with probe sets indicating increased and decreased expression, respectively. It includes 73 probe sets. Several of these code for proteins that are strongly implicated in fibrosis [9] including several types of collagen, thrombospondin, lumican, and fibronectin. With one exception, we were not able to correlate increased transcript expression with fibrosis within the lacrimal gland, a result that we attribute to the relative lack of fibrosis in that tissue. The only exception was an increase in nuclear-pore complex interacting protein-like 2.
Table 2.
Probe sets that indicate increased gene expression in orbital adipose with fibrosis.
| Probe Set | Gene Title | Fold Change* | FDR p-value |
|---|---|---|---|
| 243085_at | --- | 1.2181 | 0.0374 |
|
| |||
| 224694_at | anthrax toxin receptor 1 | 1.3928 | 0.0016 |
| 220092_s_at | 1.2915 | 0.0310 | |
|
| |||
| 202965_s_at | calpain 6 | 1.4451 | 0.0406 |
|
| |||
| 224619_at | cancer susceptibility candidate 4 | 1.1945 | 0.0449 |
|
| |||
| 207173_x_at | cadherin 11, type 2, OB-cadherin (osteoblast) | 1.4538 | 0.0014 |
| 207172_s_at | 1.3217 | 0.0100 | |
|
| |||
| 202404_s_at | collagen, type I, alpha 2 | 1.5119 | 0.0016 |
|
| |||
| 221900_at | collagen, type VIII, alpha 2 | 1.3230 | 0.0098 |
|
| |||
| 225681_at | collagen triple helix repeat containing 1 | 1.5247 | 0.0265 |
|
| |||
| 232343_at | dynactin 5 (p25) | 1.1912 | 0.0252 |
|
| |||
| 213853_at | DnaJ (Hsp40) homolog, subfamily C, member 24 | 1.2404 | 0.0450 |
|
| |||
| 201430_s_at | dihydropyrimidinase-like 3 | 1.2101 | 0.0406 |
|
| |||
| 212231_at | F-box protein 21 | 1.1767 | 0.0494 |
|
| |||
| 212464_s_at | fibronectin 1 | 1.5643 | 0.0015 |
| 211719_x_at | 1.5108 | 0.0016 | |
| 216442_x_at | 1.5068 | 0.0016 | |
| 210495_x_at | 1.4716 | 0.0036 | |
| 214701_s_at | 1.4095 | 0.0077 | |
|
| |||
| 226930_at | fibronectin type III domain containing 1 | 1.6789 | 0.0406 |
|
| |||
| 209524_at | hepatoma-derived growth factor, related protein 3 | 1.2324 | 0.0406 |
|
| |||
| 211868_x_at | immunoglobulin (IgG) heavy locus; IgG heavy constant alpha 1; IgG heavy constant alpha 2 (A2m marker); IgG heavy constant delta; IgG heavy constant gamma 1 (G1m marker); IgG heavy constant gamma 2 (G2m marker); IgG heavy constant gamma 3 (G3m marker); IgG heavy constant mu; IgG heavy variable 4–31 | 1.2907 | 0.0406 |
|
| |||
| 211635_x_at | IgG heavy constant alpha 1; IgG heavy constant alpha 2 (A2m marker); IgG heavy constant delta; IgG heavy constant gamma 1 (G1m marker); IgG heavy constant gamma 3 (G3m marker); IgG heavy constant gamma 4 (G4m marker); IgG heavy constant mu; IgG heavy variable 4–31 | 1.4915 | 0.0252 |
|
| |||
| 211640_x_at | IgG heavy constant gamma 1 (G1m marker); IgG heavy constant mu | 1.5036 | 0.0415 |
| 216541_x_at | 1.4027 | 0.0406 | |
| 211647_x_at | 1.3756 | 0.0382 | |
|
| |||
| 216853_x_at | IgG lambda variable 3–19; NULL | 1.4989 | 0.0265 |
|
| |||
| 201744_s_at | lumican | 1.6664 | 0.0006 |
|
| |||
| 218729_at | latexin | 1.3505 | 0.0489 |
|
| |||
| 37005_at | MINOS1-NBL1 readthrough; neuroblastoma 1, DAN family BMP antagonist | 1.2373 | 0.0016 |
|
| |||
| 201621_at | neuroblastoma 1, DAN family BMP antagonist | 1.2149 | 0.0098 |
|
| |||
| 1554591_at | prostate cancer associated transcript 4 (non-protein coding) | 1.2300 | 0.0277 |
|
| |||
| 227276_at | plexin domain containing 2 | 1.2265 | 0.0406 |
|
| |||
| 204517_at | peptidylprolyl isomerase C (cyclophilin C) | 1.2210 | 0.0449 |
|
| |||
| 211737_x_at | pleiotrophin | 1.4403 | 0.0098 |
| 209466_x_at | 1.3701 | 0.0449 | |
|
| |||
| 224901_at | stearoyl-CoA desaturase 5 | 1.2773 | 0.0277 |
|
| |||
| 228844_at | solute carrier family 13 (sodium-dependent citrate transporter), member 5 | 1.2062 | 0.0449 |
|
| |||
| 212354_at | sulfatase 1 | 1.3868 | 0.0449 |
|
| |||
| 201107_s_at | thrombospondin 1 | 1.4172 | 0.0252 |
| 201108_s_at | 0.0406 | ||
| 201109_s_at | 0.0449 | ||
|
| |||
| 201666_at | TIMP metallopeptidase inhibitor 1 | 1.2909 | 0.0406 |
|
| |||
| 210986_s_at | tropomyosin 1 (alpha) | 1.2643 | 0.0491 |
|
| |||
| 1553718_at | zinc finger protein 548 | 1.2220 | 0.0449 |
Based on a linear trend
Table 3.
Probe sets that indicate decreased gene expression in orbital adipose with fibrosis.
| Probe Set | Gene Title | Fold Change* | FDR p-value |
|---|---|---|---|
| 206548_at | --- | −1.2552 | 0.0075 |
|
| |||
| 49452_at | acetyl-CoA carboxylase beta | −1.2267 | 0.0449 |
|
| |||
| 207275_s_at | acyl-CoA synthetase long-chain family member 1 | −1.3043 | 0.0449 |
|
| |||
| 209612_s_at | alcohol dehydrogenase 1B (class I), beta polypeptide | −1.7190 | 0.0014 |
| 209613_s_at | −1.5021 | 0.0397 | |
|
| |||
| 207175_at | adiponectin, C1Q and collagen domain containing | −1.8639 | 0.0101 |
|
| |||
| 204151_x_at | aldo-keto reductase family 1, member C1 | −1.4219 | 0.0006 |
| 216594_x_at | −1.2731 | 0.0449 | |
|
| |||
| 209699_x_at | aldo-keto reductase family 1, member C2; aldo-keto reductase family 1 member C2-like | −1.4008 | 0.0014 |
|
| |||
| 218803_at | checkpoint with forkhead and ring finger domains, E3 ubiquitin protein ligase | −1.2139 | 0.0305 |
|
| |||
| 209683_at | family with sequence similarity 49, member A | −1.2642 | 0.0305 |
|
| |||
| 215602_at | FYVE, RhoGEF and PH domain containing 2 | −1.2499 | 0.0075 |
|
| |||
| 201540 at | four and a half LIM domains 1 | −1.4247 | 0.0274 |
| 214505_s_at | −1.4073 | 0.0397 | |
| 210299_s_at | −1.3667 | 0.0480 | |
|
| |||
| 226648_at | hypoxia inducible factor 1, alpha subunit inhibitor | −1.1994 | 0.0458 |
|
| |||
| 214767_s_at | heat shock protein, alpha-crystallin-related, B6 | −1.4170 | 0.0406 |
| 226304_at | −1.3860 | 0.0449 | |
|
| |||
| 1566472_s_at | all-trans-retinol 13,14-reductase-like; retinol saturase (all-trans-retinol 13,14-reductase) | −1.2564 | 0.0406 |
|
| |||
| 202016_at | mesoderm specific transcript | −1.3110 | 0.0449 |
|
| |||
| 231736_x_at | microsomal glutathione S-transferase 1 | −1.3182 | 0.0077 |
| 224918_x_at | −1.2982 | 0.0139 | |
|
| |||
| 1555740_a_at | melanocortin 2 receptor accessory protein | −1.3791 | 0.0305 |
|
| |||
| 205913_at | perilipin 1 | −1.7415 | 0.0077 |
|
| |||
| 205478_at | protein phosphatase 1, regulatory (inhibitor) subunit 1A | −1.5391 | 0.0077 |
|
| |||
| 1563542_a_at | sex comb on midleg-like 4 (Drosophila) | −1.2117 | 0.0372 |
|
| |||
| 215505_s_at | striatin, calmodulin binding protein 3 | −1.1932 | 0.0449 |
|
| |||
| 229477_at | thyroid hormone responsive | −1.4388 | 0.0449 |
Based on a linear trend
Fibrosis in specific tissues might be regulated by unique mediators, or it could be that there is substantial overlap between fibrosis within the orbit and fibrosis in other tissues. In order to test this latter hypothesis, we compared the list of transcripts increased in orbital disease with a list previously reported for idiopathic pulmonary fibrosis [9]. As shown in Table 4, many transcripts up regulated in the orbit affected by fibrosis have also been detected as up regulated in idiopathic pulmonary fibrosis.
Table 4.
Genes with increased expression in orbital fibrotic disease (Table 2) in common with genes with increased expression in idiopathic pulmonary fibrosis [9].
| Orbital Adipose | Lung | |||
|---|---|---|---|---|
|
|
||||
| Gene Title | Fold Change* | FDR p-value | Fold Change | Q value |
| anthrax toxin receptor 1 | 1.3928 | 0.0016 | 0.81 | 2.10E-04 |
| 1.2915 | 0.031 | |||
|
| ||||
| cadherin 11, type 2, OB-cadherin (osteoblast) | 1.4538 | 0.0014 | 0.64 | 8.40E-03 |
| 1.3217 | 0.01 | |||
|
| ||||
| calpain 6 | 1.4451 | 0.0406 | 1.96 | 6.70E-04 |
|
| ||||
| collagen triple helix repeat containing 1 | 1.5247 | 0.0265 | 8.41 | 2.20E-07 |
|
| ||||
| collagen, type I, alpha 2 | 1.5119 | 0.0016 | 1.21 | 4.90E-03 |
|
| ||||
| collagen, type VIII, alpha 2 | 1.323 | 0.0098 | 1.44 | 2.60E-07 |
|
| ||||
| dihydropyrimidinase-like 3 | 1.2101 | 0.0406 | 1 | 1.30E-04 |
|
| ||||
| fibronectin 1 | 1.5643 | 0.0015 | 2.25 | 2.10E-04 |
| 1.5108 | 0.0016 | |||
| 1.5068 | 0.0016 | |||
| 1.4716 | 0.0036 | |||
| 1.4095 | 0.0077 | |||
|
| ||||
| fibronectin type III domain containing 1 | 1.6789 | 0.0406 | 14.44 | 2.80E-08 |
|
| ||||
| immunoglobulin heavy constant alpha 1; immunoglobulin heavy constant alpha 2 (A2m marker); immunoglobulin heavy constant delta; immunoglobulin heavy constant gamma 1 (G1m marker); immunoglobulin heavy constant gamma 3 (G3m marker); immunoglobulin heavy constant gamma 4 (G4m marker); immunoglobulin heavy constant mu; immunoglobulin heavy variable 4–31 | 1.4915 | 0.0252 | 3.61 | 9.30E-03 |
|
| ||||
| immunoglobulin heavy locus; immunoglobulin heavy constant alpha 1; immunoglobulin heavy constant alpha 2 (A2m marker); immunoglobulin heavy constant delta; immunoglobulin heavy constant gamma 1 (G1m marker); immunoglobulin heavy constant gamma 2 (G2m marker); immunoglobulin heavy constant gamma 3 (G3m marker); immunoglobulin heavy constant mu; immunoglobulin heavy variable 4–31 | 1.2907 | 0.0406 | 10.89 | 5.40E-08 |
|
| ||||
| immunoglobulin heavy constant gamma 1 (G1m marker); immunoglobulin heavy constant mu | 1.5036 | 0.0415 | 4.84 | 6.60E-03 |
| 1.4027 | 0.0406 | |||
| 1.3756 | 0.0382 | |||
|
| ||||
| latexin | 1.3505 | 0.0489 | 2.25 | 4.90E-06 |
|
| ||||
| lumican | 1.6664 | 6.00E-04 | 2.25 | 6.50E-04 |
|
| ||||
| sulfatase 1 | 1.3868 | 0.0449 | 7.29 | 3.60E-10 |
|
| ||||
| thrombospondin 1 | 1.4609 | 0.0252 | 0.81 | 4.00E-02 |
| 1.4199 | 0.0406 | |||
| 1.4172 | 0.0449 | |||
|
| ||||
| TIMP metallopeptidase inhibitor 1 | 1.2909 | 0.0406 | 0.81 | 7.60E-03 |
Based on a linear trend
DISCUSSION
Fibrosis is a characteristic feature of many inflammatory diseases. We believe that our study is the first to compare the likelihood of fibrosis among various forms of orbital disease. Our results indicate that GPA is especially likely to be associated with fibrosis. We have recently noted that many patients with NSOI might have a limited form of GPA (Rosenbaum, JT, Choi, D, Wilson, DJ, et al., Orbital Pseudotumor Can Be a Localized Form of Granulomatosis with Polyangiitis Based on Gene Expression Profiling, manuscript submitted), so it should not be surprising that this entity is also associated with fibrosis. On the other hand, fibrosis was minimal among patients with TED. We anticipated that fibrosis would also accompany disease in the lacrimal gland, but our data indicate that fibrosis is generally mild in lacrimal specimens and statistically the fibrosis from diseased, lacrimal tissue did not differ from fibrosis in healthy controls.
IgG4 related disease has recently been defined as a multisystem disease associated with storiform fibrosis [10]. IgG4 related disease is well described within the orbit [11–14]. We recently reported on the prevalence of IgG4 staining in tissue from patients with orbital inflammatory disease [4]. We found only a slight correlation between the degree of fibrosis and IgG4 staining. Further, we found that many patients with GPA, NSOI, or sarcoidosis had IgG4 staining in their orbital tissue [4]. We did not specifically investigate the role of IgG4 in the present study, but the majority of the tissues described in this present report were included in our previous report on IgG4 [4], and none of subjects in the prior report had a multisystem disease that could be classified as IgG4 related disease.
The two pathologists scoring fibrosis in tissue for this project agreed in only a minority of instances, 31% for lacrimal gland and 49% for orbital adipose tissue. In 14% and 10% for lacrimal gland and orbital fat respectively, the pathologists differed by a score of two. In fact, one of the pathologists who scored 9 lacrimal gland biopsies twice (because two separate blocks were evaluated) provided discordant scores 8 of the 9 instances. The intra-observer and inter-observer discrepancies could result from the subjective nature of the scoring system as well as from sampling error. The pathologists accurately recognized fibrosis since tissue with greater fibrosis expressed transcripts associated with fibrosis in another tissue, the lung. We speculate that a fibrosis score based on transcript expression might eventually be standard in biopsied tissue because it can be quantified and would minimize the sampling errors that confront a histopathologist.
We noted more fibrosis in orbital fat than in lacrimal specimens. A possible explanation for this is that the adipocytes themselves contribute to fibrosis. For example, the adipokines, leptin and adiponectin, have been implicated in hepatic fibrosis [15]. In skin affected by scleroderma, adipocytes reportedly demonstrate the potential to transform into myofibroblasts that are a critical part of the fibrosing process [16]. Future studies should compare the levels of adipokines in the lacrimal gland versus the anterior orbit as these cytokines might represent a potential therapeutic target.
The transcripts which we found to be up regulated in association with fibrosis include many that code for proteins generally implicated in fibrosis including collagens, fibronectin, and thrombospondin. This suggests that pathological grading of fibrosis has an expected molecular correlate with gene expression and it validates the histopathological interpretation. The finding of overlapping, up regulated genes in fibrosis affecting the lung and orbit also supports the validity of the technique and indicates that fibrosis in diverse tissues must share common elements in pathogenesis. An analysis of gene expression in fibrosing diseases holds the promise to discover novel targets for pharmacotherapy.
ACKNOWLEDGEMENTS
RNA extraction and microarray assays were performed in the OHSU Gene Profiling Shared Resource. We are grateful to Kristina Vartanian for her excellent technical support for the microarray work.
Financial support: NIH Grants EY020249, EY010572 and RR024140, Research to Prevent Blindness, the William and Mary Bauman Foundation, the Mas Family Foundation, and the Stan and Madelle Rosenfeld Family Trust. None of the funding organizations had a role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Competing Interest Disclosures: Dr. Rosenbaum has in the past consulted for Genentech and was a co-investigator on a studied funded by Genentech to evaluate the use of rituximab for orbital inflammatory diseases. The other authors report no potential competing interest.
REFERENCES
- 1.Rootman J. Orbital Inflammations. In: BenEzra D, editor. Ocular Inflammation: Basic and Clinical Concepts. Jerusalem: 1999. [Google Scholar]
- 2.Lutt J, Lim L, Phal P, Rosenbaum J. Orbital inflammatory disease. Semin Arthritis Rheum. 2008;37(4):207–22. doi: 10.1016/j.semarthrit.2007.06.003. doi. [DOI] [PubMed] [Google Scholar]
- 3.Hsuan JD, Selva D, McNab AA, Sullivan TJ, Saeed P, O'Donnell BA. Idiopathic sclerosing orbital inflammation. Arch. Ophthalmol. 2006;124(9):1244–50. doi: 10.1001/archopht.124.9.1244. doi: 124/9/1244 [pii] 10.1001/archopht.124.9.1244. [DOI] [PubMed] [Google Scholar]
- 4.Wong AJ, Planck SR, Choi D, et al. IgG4 immunostaining and its implications in orbital inflammatory disease. PLoS One. 2014;9(10):e109847. doi: 10.1371/journal.pone.0109847. doi: 10.1371/journal.pone.0109847 PONE-D-14-25948 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vartanian K, Slottke R, Johnstone T, et al. Gene expression profiling of whole blood: comparison of target preparation methods for accurate and reproducible microarray analysis. BMC Genomics. 2009;10(1):2. doi: 10.1186/1471-2164-10-2. doi: 10.1186/1471-2164-10-2 1471-2164-10-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31(4):e15. doi: 10.1093/nar/gng015. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagnon-Bartsch JA, Jacob L, Speed TP. Removing Unwanted Variation from High Dimensional Data with Negative Controls. 2013 http://statistics.berkeley.edu/sites/default/files/tech-reports/ruv.pdf.
- 8.Eisenberg E, Levanon EY. Human housekeeping genes are compact. Trends Genet. 2003;19(7):362–5. doi: 10.1016/S0168-9525(03)00140-9. doi: S0168-9525(03)00140-9 [pii] 10.1016/S0168-9525(03)00140-9 [doi] [DOI] [PubMed] [Google Scholar]
- 9.DePianto DJ, Chandriani S, Abbas AR, et al. Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis. Thorax. 2014 doi: 10.1136/thoraxjnl-2013-204596. doi: thoraxjnl-2013-204596 [pii] 10.1136/thoraxjnl-2013-204596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–92. doi: 10.1038/modpathol.2012.72. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 11.Mehta M, Jakobiec F, Fay A. Idiopathic fibroinflammatory disease of the face, eyelids, and periorbital membrane with immunoglobulin G4-positive plasma cells. Arch Pathol Lab Med. 2009;133(8):1251–5. doi: 10.5858/133.8.1251. doi: 10.1043/1543-2165-133.8.1251. [DOI] [PubMed] [Google Scholar]
- 12.Plaza JA, Garrity JA, Dogan A, Ananthamurthy A, Witzig TE, Salomão DR. Orbital inflammation with IgG4-positive plasma cells: manifestation of IgG4 systemic disease. Arch. Ophthalmol. 2011;129(4):421–8. doi: 10.1001/archophthalmol.2011.16. doi: 10.1001/archophthalmol.2011.16. [DOI] [PubMed] [Google Scholar]
- 13.Wallace ZS, Khosroshahi A, Jakobiec FA, et al. IgG4-related systemic disease as a cause of “idiopathic” orbital inflammation, including orbital myositis, and trigeminal nerve involvement. Surv Ophthalmol. 2012;57(1):26–33. doi: 10.1016/j.survophthal.2011.07.004. doi: 10.1016/j.survophthal.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Mulay K, Aggarwal E, Jariwala M, Honavar SG. Orbital immunoglobulin-G4-related disease: case series and literature review. Clin Experiment Ophthalmol. 2013 doi: 10.1111/ceo.12284. doi: 10.1111/ceo.12284. [DOI] [PubMed] [Google Scholar]
- 15.Saxena NK, Anania FA. Adipocytokines and hepatic fibrosis. Trends Endocrinol Metab. 2015;26(3):153–61. doi: 10.1016/j.tem.2015.01.002. doi: S1043-2760(15)00003-X [pii] 10.1016/j.tem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marangoni RG, Korman BD, Wei J, et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67(4):1062–73. doi: 10.1002/art.38990. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]