Abstract
The aim of the study is to determine the influence of area-level socio-economic status and healthcare access in addition to tumor hormone-receptor subtype on individual breast cancer stage, treatment, and mortality among Non-Hispanic (NH)-Black, NH-White, and Hispanic US adults. Analysis was based on 456,217 breast cancer patients in the SEER database from 2000 to 2010. Multilevel and multivariable-adjusted logistic and Cox proportional hazards regression analysis was conducted to account for clustering by SEER registry of diagnosis. NH-Black women had greater area-level access to healthcare resources compared with women of other races. For instance, the average numbers of oncology hospitals per million population in counties with NH-Black, NH-White, and Hispanic women were 8.1, 7.7, and 5.0 respectively; average numbers of medical doctors per million in counties with NH-Black, NH-White, and Hispanic women were 100.7, 854.0, and 866.3 respectively; and average number of Ob/Gyn in counties with NH-Black, NH-White, and Hispanic women was 155.6, 127.4, and 127.3, respectively (all p values <0.001). Regardless, NH-Black women (HR 1.39, 95 % CI 1.36–1.43) and Hispanic women (HR 1.05, 95 % CI 1.03–1.08) had significantly higher breast cancer mortality compared with NH-White women even after adjusting for hormone-receptor subtype, area-level socioeconomic status, and area-level healthcare access. In addition, lower county-level socio-economic status and healthcare access measures were significantly and independently associated with stage at presentation, surgery, and radiation treatment as well as mortality after adjusting for age, race/ethnicity, and HR subtype. Although breast cancer HR subtype is a strong, important, and consistent predictor of breast cancer outcomes, we still observed significant and independent influences of area-level SES and HCA on breast cancer outcomes that deserve further study and may be critical to eliminating breast cancer outcome disparities.
Keywords: Breast cancer outcomes, Hormone-receptor subtype, Racial disparities, Healthcare access, Socioeconomic status
Introduction
Racial and socio-economic disparities in breast cancer outcomes have been well established for many decades [1]; however, the survival gap between racial and socio-economic groups has remained intractable and widened over time [1–3]. To date, the causes of breast cancer outcome disparities have not been fully explained, and are likely multifactorial and pervasive across many levels including individual, community, provider, and health systems. The disparity is partly explained by differential access to high-quality screening and healthcare resources [4, 5], factors that operate at both individual and area levels and have been shown to influence stage at diagnosis and receipt of guideline-adherent treatment [6], and therefore survival [7]. Underlying biological differences in breast cancer between Black and White women may also be an underlying cause of disparities in outcomes, particularly in mortality rates [8–10], as Black women are more likely to be diagnosed with aggressive, hormone-receptor (HR)-negative subtypes [10–17].
Recent advances in molecular epidemiology provide evidence for racial differences in the aggressiveness and survival of breast cancer by HR subtype [8, 10]. When classified according to the presence or absence of tumor markers of estrogen receptor (ER), progesterone receptor (PR), and human epidermal receptor-2 (Her2), patients diagnosed with the HR-negative subtypes (ER−, PR−, and/or Her2−) experience the lowest survival rates compared with patients diagnosed with HR-positive (ER+, PR+, and/or Her2+) subtypes [10]. Furthermore, the association between SES and breast cancer incidence and survival varies by hormone-receptor subtype, with consistent associations in HR+ subtypes but not HR− subtypes [18]. Nevertheless, the results of studies examining racial and SES disparities in breast cancer mortality have been somewhat inconsistent. Some studies report that adjusting for SES eliminates racial disparities in outcomes [19, 20], while others find sustained racial differences even after accounting for SES [21, 22]. These differences may be due to inadequate consideration of HR subtypes in addition to upstream socio-economic and healthcare factors. Socioeconomic status has been shown to influence breast cancer survival outcomes when measured at both individual and area levels, with results consistently showing worse outcomes for patients with lower individual and/or area-level SES [7, 19, 23]. Fewer studies have examined other area-level factors such as access to healthcare resources [4, 24, 25], while most studies have focused on examining insurance status. However, area-level availability of healthcare resources likely plays a key role in breast cancer survival outcome through the receipt of timely and high-quality treatment, which in turn influences survival. Poor availability of healthcare resources may complicate disease outcomes especially for patients with the fast growing and aggressive HR− subtypes, who are more likely to be Black women.
To date, very few studies have simultaneously examined the influence of area-level socio-economic status (SES) and healthcare access (HCA) in addition to HR subtype in predicting breast cancer outcomes, as well as examining if racial disparities in survival remain after accounting for both biological differences of tumors and socio-economic and healthcare access differences. This study aims to investigate the independent association of HR subtypes, area-level SES and healthcare access on stage, treatment, and survival disparities among NH-Black, NH-White, and Hispanic women diagnosed with breast cancer and represented in the SEER database.
Methods
Data source
Our analyses used data obtained from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database (November 2012 submission) linked with the Area Resource File. The study sample included Non-Hispanic (NH) white, NH-Black, and Hispanic women diagnosed with breast cancer between 2000 and 2010 in the SEER database. The SEER 18 population-based dataset includes all breast cancer cases diagnosed in the following SEER cancer registries: Atlanta, Georgia; Connecticut; Detroit, Michigan; Hawaii; Iowa; New Mexico; San Francisco-Oakland, California; Seattle, Washington; Utah; Los Angeles, California; San Jose-Monterey, California; Rural Georgia; Greater California; Kentucky; and New Jersey. SEER covers about 28 % of the U.S. population, although the regions included tend to be more urban and suburban compared with the general U.S. population.
Outcome variables
There were four primary outcome variables of interest in this study: (1) stage at diagnosis based on the SEER summary stage variable (excluding in situ cases) and classified as late stage (if regional or distant) or early stage (if localized); (2) surgical treatment given or not, regardless of reason; (3) radiation treatment given or not, regardless of reason; and (4) breast cancer survival.
Area-level availability of healthcare resources
County-level availability of healthcare resources was assessed based on variables in the 2004 Area Resource File (ARF), a publication of the U.S. Health Resources and Services Administration. The ARF is a collection of data on health resources at the county level assembled from multiple sources, detailed in http://ahrf.hrsa.gov. We based our assessment of county-level availability on the following variables for the year 2000: the proportion of the population insured, number of hospitals with oncology services per million people, number of physicians per million people, and number of obstetrician-gynecologist (Ob-Gyn) per million people. We included the percent of individuals with health insurance, as this may influence the concentration of hospitals, clinics, or medical personnel in particular neighborhoods. We included the percentage of Ob-Gyn per million, as this could serve as a proxy for overall access to women’s health services. Multiple studies have discussed the geographic level at which it is best to measure area-level variables in order to reduce the likelihood of residual confounding in disparities research [26–29]. We assessed healthcare resources at the county level because the decisions regarding community resources and infrastructure such as hospitals or clinics are often made at the county or municipal level.
Area-level socio-economic status
County-level SES was assessed based on the following variables for the year 2000: percent of adult population with less than a 9th grade education, percent of families living below the federal poverty level, proportion of the population unemployed, and proportion of the population that is African-American. We also designated each county as rural, urban non-metropolitan (areas with an urban population of <2500 not adjacent to a metropolitan area), or urban metropolitan (metropolitan areas or areas with urban populations <2500 adjacent to metropolitan areas) based on the United States Department of Agriculture classification scheme.
Breast cancer hormone-receptor subtype
The SEER program captures estrogen and progesterone receptor status information for breast cancer cases. Variable coding details of HR subtypes have been previously published [30] and are available on the SEER website (http://seer.cancer.gov/seerstat/databases/ssf/). In brief, tumors are classified as positive or negative for estrogen receptor (ER±) and progesterone receptor (PR±). Information on human epidermal receptor factor 2 (Her2±) was captured starting in 2010, and was not included in the current analysis. We further classified breast cancer hormone-receptor status into two subtypes (HR+ or HR−) using the SEER dataset HR status recode variable; HR+ if ER status recode or PR status recode were both positive, and HR− if ER status recode and PR status recode were both negative. We excluded all patients with unknown and borderline breast cancer subtypes (n = 76,078), corresponding to a total of 456,217 breast cancer patients used for statistical analyses.
Ethics and consent statement
This study was considered exempt by the Institutional Review Board at the University of Alabama at Birmingham, as the SEER database is a publicly available and non-identifiable secondary data source.
Statistical analysis
We described the distribution of socio-demographic characteristics and access to healthcare resources by race/ethnicity using Chi-Square tests for categorical variables and ANOVA for continuous variables. We compared the estimated overall survival by HR status among NH-Black, NH-White, and Hispanic patients using Kaplan–Meier curves. We conducted consecutive multilevel regression modeling to examine the independent and joint associations between county-level SES, healthcare availability, and HR subtypes with each study outcome accounting for clustering by SEER registry of diagnosis. The HR subtype model included age, race/ethnicity, and HR subtype; the SES model included age, race/ethnicity, and SES; the HCA model included age, race/ethnicity, and HCA; and the fully adjusted model included age, race/ethnicity, HR subtype, SES, and HCA. To estimate the probability of breast cancer mortality by race, HR subtype, SES, and HCA, we fit Cox proportional hazards models with time-to-breast cancer-related death as the outcome and censored patients at the time of death, or end of follow-up (December 2010). Since county-level variables (SES and availability of healthcare resources) were not normally distributed, we transformed these variables by dividing each by their population standard deviation. In addition, since 1 % increase in county-level variables may not be clinically meaningful, we presented odds and hazard ratios in statistical models associated with standard deviation increases in county-level variables. That is, instead of presenting odds ratios associated with each 1 % increase in % families living below poverty, we presented the odds ratios associated with 1 SD increase in % families living below poverty. We used SAS version 9.4 for all statistical analyses. We considered p values ≤0.05 and confidence intervals excluding the null value (odds ratio or hazard ratio = 1.00) as statistically significant.
Results
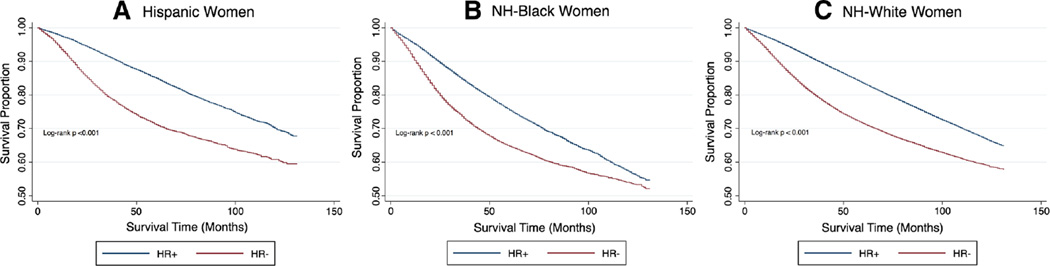
We identified 456,217 female breast cancer cases over the 10-year observation period; most patients were NH-White (81.2 %), while 10.1 % were NH-Black, and 8.7 % were Hispanic women (Table 1). NH-Black women had significantly lower breast cancer survival over the observation period compared with NH-White and Hispanic women, corresponding with the shortest length of follow-up time (46.1 months vs. 53.6 and 48.2 months, respectively; p value <0.001). NH-Black women had lower 5-year survival compared with women of other racial groups across all hormone-receptor (HR)-subtypes, including HR-positive subtypes (Fig. 1). Compared with 17.1 % of NH-Whites, 33.0 % of NH-Blacks and 23.1 % of Hispanics were diagnosed with HR− breast cancer subtypes (p < 0.001). NH-White women (34.8 %) were less likely to have a late-stage breast cancer diagnosis when compared with NH-Black (45.5 %) and Hispanic (42.5 %) women (p value <0.001). In addition, NH-Black women were less likely to receive surgery and radiation treatment (9.7 % did not receive surgery, and 53.2 % did not receive radiation) for their disease, compared with NH-White (4.9 % did not receive surgery, and 49.2 % did not receive radiation) and Hispanic (6.0 % did not receive surgery, and 51.7 % did not receive radiation) women (p value <0.001).
Table 1.
Socio-demographic and healthcare characteristics of breast cancer cases by race/ethnicity, SEER 2000–2010
| Race/ethnicity N (%) | ||||
|---|---|---|---|---|
| NH-Blacks N = 46,069 (10.1) |
NH-Whites N = 370,275 (81.2) |
Hispanic N = 39,891 (8.7) |
p value* | |
| Length of follow-up, months‡ | 46.1 (35.0) | 53.6 (36.8) | 48.2 (35.4) | <0.001 |
| Age at diagnosis (%) | ||||
| 40–44 | 4635 (10.1) | 23,129 (6.3) | 4896 (12.3) | <0.001 |
| 45–54 | 13,101 (28.4) | 81,726 (22.1) | 12,483 (31.3) | |
| 55–64 | 12,328 (26.8) | 95,631 (25.8) | 10,079 (25.3) | |
| 65–74 | 8947 (19.4) | 82,971 (22.4) | 7365 (18.5) | |
| 75+ | 7058 (15.3) | 86,800 (23.4) | 5068 (12.7) | |
| Late stage (%) | ||||
| No | 25,102 (54.5) | 241,430 (65.2) | 22,923 (57.5) | <0.001 |
| Yes/unknown | 20,967 (45.5) | 128,827 (34.8) | 16,968 (42.5) | |
| Surgical treatment (%) | ||||
| Yes | 41,592 (90.3) | 352,178 (95.1) | 37,494 (94.0) | <0.001 |
| No/unknown | 4477 (9.7) | 18,079 (4.9) | 2397 (6.0) | |
| Radiation treatment (%) | ||||
| Yes | 21,566 (46.8) | 188,150 (50.8) | 19,285 (48.3) | <0.001 |
| No/unknown | 24,503 (53.2) | 182,107 (49.2) | 20,606 (51.7) | |
| County-level healthcare access‡ | ||||
| % No health insurance | 16.0 (4.0) | 14.7 (4.5) | 18.6 (4.9) | <0.001 |
| # Oncology hospitals per million | 8.1 (7.1) | 7.7 (10.9) | 5.0 (4.0) | <0.001 |
| # Medical doctors per million | 1000.7 (533.9) | 854.0 (529.5) | 866.3 (407.0) | <0.001 |
| # OB GYN per million | 155.6 (81.8) | 127.4 (70.1) | 127.3 (48.5) | <0.001 |
| County-level socio-economic status‡ | ||||
| % Black population | 26.8 (18.1) | 10.5 (11.7) | 9.0 (7.7) | <0.001 |
| % Unemployed | 7.0 (2.0) | 6.0 (2.1) | 7.2 (2.3) | <0.001 |
| % Less than 9th grade education | 8.4 (4.2) | 7.9 (4.6) | 11.2 (4.9) | <0.001 |
| % Families below poverty level | 11.3 (5.0) | 8.7 (4.6) | 10.8 (4.4) | <0.001 |
| Residence | ||||
| Rural | 285 (0.6) | 5,764 (1.6) | 76 (0.2) | <0.001 |
| Urban | 45,784 (99.4) | 364,493 (98.4) | 39,815 (99.8) | |
| Breast cancer hormone-receptor subtype | ||||
| HR+ | 30,872 (67.0) | 306,993 (82.9) | 30,673 (76.9) | <0.001 |
| HR− | 15,197 (33.0) | 63,264 (17.1) | 9,218 (23.1) | |
Among 456,217 cases
Mean (SD)
Estimated using ANOVA or Chi-Square test
Fig. 1.
Kaplan–Meier plots for time-to-breast cancer-related death among races, stratified by breast cancer subtype. SEER 2000—2010. a Hispanic women, b NH-Black women, and c NH-White women
NH-Black women (mean # hospitals: 8.1) were more likely to reside in counties with a higher average number of oncology hospitals per million people compared with NH-Whites (mean # hospitals: 7.7) and Hispanics (mean # hospitals: 5.0). NH-Black women (mean # doctors: 1000.7) were also more likely to live in counties with a higher average number of medical doctors per million people compared with NH-Whites (mean # doctors: 854.0) and Hispanics (mean # doctors: 886.3), and higher average number of obstetricians-gynecologists per million (mean # ob-gyn: 155.6) compared with NH-Whites (mean # ob-gyn: 127.4) and Hispanics (mean # ob-gyn: 127.3) (all p values <0.001). In addition, Hispanic women resided in counties with a higher proportion of individuals with no health insurance compared with NH-Whites or NH-Blacks (p value <0.001). However, NH-Black and Hispanic women resided in communities with a greater proportion of unemployed and families living below poverty level compared with NH-White women. NH-White women were more likely to reside in rural areas (1.6 %) when compared with NH-Black (0.6 %) and Hispanic (0.2 %) women (p value <0.001).
Late-stage breast cancer diagnosis
In the model accounting for HR subtype (Table 2), after adjusting for age, NH-Black women (OR 1.42, 95 % CI 1.39–1.45) and Hispanic women (OR 1.29, 95 % CI 1.26–1.31) were more likely to be diagnosed at late stage compared with NH-White women. In addition, compared with HR+ subtypes, diagnosis with HR− subtypes was associated with a 29 % increased odds of late-stage diagnosis (OR 1.29, 95 % CI 1.27–1.31). In the area-level SES model, breast cancer patients residing in a county with a higher proportion of Black residents (OR per SD increase: 0.98, 95 % CI 0.97–0.99) and patients living in counties with higher unemployment rates (OR per SD increase: 0.98 95 % CI 0.97–0.99) experienced a 2 % reduced odds of late-stage diagnosis. However, patients residing in counties with a higher proportion of families below poverty level were at increased odds of late-stage diagnosis by 6 % (OR per SD increase: 1.06, 95 % CI 1.04–1.09). In the area-level HCA model, breast cancer patients residing in counties with a higher proportion of individuals without health insurance (OR per SD increase: 1.04, 95 % CI 1.03–1.05) were at increased odds of late-stage diagnosis, while patients residing in counties with more medical doctors were at reduced odds of late-stage diagnosis (OR per SD increase: 0.98, 95 % CI 0.96–0.99). In the fully adjusted model, NH-Blacks and Hispanics, patients with HR− subtypes, and adults residing in counties with a higher proportion of families below poverty level remained at significantly higher odds of late-stage diagnosis. In all models, older age was associated with decreased odds of late-stage breast cancer diagnosis.
Table 2.
Multivariate adjusted odds ratios for late-stage diagnosis, SEER 2000–2010
| HR subtype AOR (95 % CI)a |
SES AOR (95 % CI)b |
HCA AOR (95 % CI)c |
Fully adjusted AOR (95 % CI)d |
|
|---|---|---|---|---|
| Race/ethnicity | ||||
| NH-White (referent) | – | – | – | – |
| NH-Black | 1.42 (1.39–1.45) | 1.47 (1.44–1.51) | 1.47 (1.44–1.50) | 1.42 (1.39–1.45) |
| Hispanic | 1.29 (1.26–1.31) | 1.29 (1.26–1.32) | 1.29 (1.26–1.32) | 1.28 (1.25–1.31) |
| Age at diagnosis | ||||
| 40–44 (referent) | – | – | – | – |
| 45–54 | 0.87 (0.85–0.89) | 0.86 (0.84–0.89) | 0.86 (0.84–0.89) | 0.87 (0.85–0.89) |
| 55–64 | 0.72 (0.71–0.74) | 0.72 (0.70–0.73) | 0.72 (0.70–0.73) | 0.72 (0.71–0.74) |
| 65–74 | 0.59 (0.58–0.61) | 0.58 (0.57–0.60) | 0.58 (0.57–0.60) | 0.59 (0.58–0.61) |
| 75+ | 0.60 (0.59–0.62) | 0.59 (0.57–0.60) | 0.59 (0.57–0.60) | 0.60 (0.59–0.62) |
| Breast cancer hormone-receptor subtype | ||||
| HR+ (referent) | – | – | ||
| HR− | 1.29 (1.27–1.31) | 1.29 (1.27–1.31) | ||
| County-level socio-economic status† | ||||
| % Black population (SD = 13.24) | 0.98 (0.97–0.99) | 0.99 (0.98–1.00) | ||
| % Unemployed (SD = 2.20) | 0.98 (0.97–0.99) | 0.98 (0.96–0.99) | ||
| % Less than 9th grade education (SD = 4.74) |
1.01 (1.00–1.03) | 1.01 (1.00–1.03) | ||
| % Families below poverty level (SD = 4.75) |
1.06 (1.04–1.09) | 1.06 (1.04–1.08) | ||
| County-level healthcare access† | ||||
| % No health insurance (SD = 4.68) | 1.04 (1.03–1.05) | 1.00 (0.98–1.02) | ||
| # Oncology hospitals per million (SD = 10.08) |
1.00 (0.99–1.01) | 1.00 (0.99–1.01) | ||
| # Medical doctors per million (SD = 520.55) |
0.98 (0.96–0.99) | 0.98 (0.96–0.99) | ||
| # OB GYN per million (SD = 70.47) | 1.00 (0.98–1.01) | 1.00 (0.99–1.01) |
AOR adjusted odds ratios
Bold indicates significance p value ≤0.05
Adjusted for race, age, SEER registry, and HR subtype
Adjusted for race, age, SEER registry, and county-level SES
Adjusted for race, age, SEER registry, and county-level HCA
Adjusted for race, age, SEER registry, HR subtype, and county-level SES and HCA
ORs associated with standard deviation increase
Surgical treatment for breast cancer
In the model accounting for HR subtype (Table 3), after adjusting for age and stage at diagnosis, NH-Black women (OR 0.55, 95 % CI 0.53–0.57) and Hispanic women (OR 0.81, 95 % CI 0.77–0.85) were less likely to receive surgical treatment compared with NH-White women. In addition, diagnosis with HR− subtype (OR 0.82, 95 % CI 0.80–0.85) was associated with decreased odds of surgical treatment. In the area-level SES model, patients residing in counties with higher proportion of unemployed (OR per SD increase: 1.07, 95 % CI 1.04–1.10) were at increased odds of surgical treatment. In the area-level HCA model, patients residing in a county with a higher proportion of individuals without health insurance (OR per SD increase: 0.96, 95 % CI 0.94–0.99) and larger number of medical doctors per population (OR per SD increase: 0.93, 95 % CI 0.90–0.96) were less likely to receive surgical treatment. In the fully adjusted model, NH-Black (OR 0.55, 95 % CI 0.53–0.58) and Hispanic race (OR 0.82, 95 % CI 0.78–0.86), HR− subtype (OR 0.82, 95 % CI 0.79–0.85), patients residing in counties with larger proportion of individuals without health insurance (OR per SD increase: 0.94, 95 % CI 0.89–0.98), and patients residing in counties with higher number of medical doctors per million population (OR per SD increase: 0.94, 95 % CI 0.91–0.97) remained at lower odds of receiving surgery. Patients residing in counties with higher proportion of unemployed (OR per SD increase: 1.07, 95 % CI 1.03–1.10) were more likely to receive surgical treatment. In all models, older age was associated with decreased odds of surgical treatment following cancer diagnosis.
Table 3.
Multivariate adjusted odds ratios for surgical treatment, SEER 2000–2010
| HR subtype AOR (95 % CI)a |
SES AOR (95 % CI)b |
HCA AOR (95 % CI)c |
Fully adjusted AOR (95 % CI)d |
|
|---|---|---|---|---|
| Race/ethnicity | ||||
| NH-White (referent) | – | – | – | – |
| NH-Black | 0.55 (0.53–0.57) | 0.54 (0.52–0.56) | 0.54 (0.52–0.57) | 0.55 (0.53–0.58) |
| Hispanic | 0.81 (0.77–0.85) | 0.80 (0.77–0.84) | 0.81 (0.77–0.85) | 0.82 (0.78–0.86) |
| Age at diagnosis | ||||
| 40–44 (referent) | – | – | – | – |
| 45–54 | 0.78 (0.73–0.83) | 0.78 (0.73–0.83) | 0.78 (0.73–0.83) | 0.78 (0.73–0.83) |
| 55–64 | 0.61 (0.57–0.65) | 0.61 (0.58–0.65) | 0.61 (0.58–0.65) | 0.61 (0.57–0.65) |
| 65–74 | 0.55 (0.51–0.58) | 0.55 (0.52–0.59) | 0.55 (0.52–0.59) | 0.55 (0.51–0.58) |
| 75+ | 0.30 (0.28–0.32) | 0.31 (0.29–0.32) | 0.31 (0.29–0.33) | 0.30 (0.28–0.32) |
| Breast cancer hormone-receptor subtype | ||||
| HR+ (referent) | – | – | ||
| HR− | 0.82 (0.80–0.85) | 0.82 (0.79–0.85) | ||
| County-level socio-economic status† | ||||
| % Black population (SD = 13.24) | 0.97 (0.95–0.99) | 1.00 (0.97–1.03) | ||
| % Unemployed (SD = 2.20) | 1.07 (1.04–1.10) | 1.07 (1.03–1.10) | ||
| % Less than 9th grade education (SD = 4.74) |
0.97 (0.94–1.00) | 0.99 (0.95–1.02) | ||
| % Families below poverty level (SD = 4.75) |
0.96 (0.92–1.01) | 0.97 (0.93–1.02) | ||
| County-level healthcare access† | ||||
| % No health insurance (SD = 4.68) | 0.96 (0.94–0.99) | 0.94 (0.89–0.98) | ||
| # Oncology hospitals per million (SD = 10.08) | 1.01 (0.99–1.03) | 1.01 (0.99–1.02) | ||
| # Medical doctors per million (SD = 520.55) |
0.93 (0.90–0.96) | 0.94 (0.91–0.97) | ||
| # OB GYN per million (SD = 70.47) | 1.01 (0.98–1.04) | 1.00 (0.97–1.03) |
AOR adjusted odds ratios
Bold indicates significance p value ≤0.05
Adjusted for race, age, SEER registry, late-stage diagnosis, and HR subtype
Adjusted for race, age, SEER registry, late-stage diagnosis, and county-level SES
Adjusted for race, age, SEER registry, late-stage diagnosis, and county-level HCA
Adjusted for race, age, SEER registry, late-stage diagnosis, HR subtype, and county-level SES and HCA
ORs associated with standard deviation increase
Radiation treatment for breast cancer
In the model accounting for HR subtype (Table 4), after adjusting for age and stage at diagnosis, NH-Black women (OR 0.84, 95 % CI 0.82–0.85) and Hispanic women (OR 0.89, 95 % CI 0.87–0.91) were less likely to receive radiation therapy compared with NH-White women. Furthermore, diagnosis with HR− (OR 0.84, 95 % CI 0.83–0.85) was associated with lower odds of radiation therapy. Women aged 65 years and older were less likely to receive radiation therapy, while women between ages 45 and 64 years were more likely to receive radiation therapy compared with those aged 40–44 years. In the area-level SES model, patients residing in counties with higher proportion of Blacks (OR per SD increase: 1.02, 95 % CI 1.01–1.03) and unemployed (OR per SD increase: 1.06, 95 % CI 1.05–1.08) experienced higher odds of radiation therapy, while patients residing in counties with a higher proportion of families living below the poverty level (OR per SD increase: 0.87, 95 % CI 0.85–0.89) were 13 % less likely to receive radiation therapy. In the area-level HCA model, patients residing in counties with a higher proportion of adults with no health insurance (OR per SD increase: 0.92, 95 % CI 0.91–0.93) were less likely to receive radiation treatment. In the fully adjusted models, Black and Hispanic women as well as women with HR− subtypes were less likely to receive radiation therapy. In addition, patients residing in counties with higher proportion of families below poverty line (OR per SD increase: 0.86, 95 % CI 0.84–0.87) were 14 % less likely to receive radiation therapy. However, patients residing in counties with higher proportion of unemployed (OR per SD increase: 1.06, 95 % CI 1.05–1.08) experienced increased odds of radiation therapy.
Table 4.
Multivariate adjusted odds ratios for radiation therapy, SEER 2000–2010
| HR Subtype AOR (95 % CI)a |
SES AOR (95 % CI)b |
HCA AOR (95 % CI)c |
Fully adjusted AOR (95 % CI)d |
|
|---|---|---|---|---|
| Race/ethnicity | ||||
| NH-White (referent) | – | – | – | – |
| NH-Black | 0.84 (0.82–0.85) | 0.83 (0.81–0.85) | 0.83 (0.81–0.84) | 0.85 (0.83–0.87) |
| Hispanic | 0.89 (0.87–0.91) | 0.90 (0.88–0.92) | 0.90 (0.88–0.92) | 0.90 (0.90–0.92) |
| Age at diagnosis | ||||
| 40–44 (referent) | – | – | – | – |
| 45–54 | 1.04 (1.02–1.07) | 1.05 (1.02–1.07) | 1.05 (1.02–1.07) | 1.04 (1.02–1.07) |
| 55–64 | 1.07 (1.05–1.10) | 1.08 (1.06–1.11) | 1.08 (1.05–1.11) | 1.07 (1.05–1.10) |
| 65–74 | 0.91 (0.89–0.94) | 0.93 (0.91–0.95) | 0.93 (0.90–0.95) | 0.92 (0.89–0.94) |
| 75+ | 0.47 (0.46–0.48) | 0.48 (0.47–0.49) | 0.48 (0.47–0.49) | 0.47 (0.46–0.49) |
| Breast cancer hormone-receptor subtype | ||||
| HR+ (referent) | – | – | ||
| HR− | 0.84 (0.83–0.85) | 0.84 (0.83–0.86) | ||
| County-level socio-economic status† | ||||
| % Black population (SD = 13.24) | 1.02 (1.01–1.03) | 1.01 (1.00–1.02) | ||
| % Unemployed (SD = 2.20) | 1.06 (1.05–1.08) | 1.06 (1.05–1.08) | ||
| % Less than 9th grade education (SD = 4.74) |
0.98 (0.97–1.00) | 0.99 (0.96–0.99) | ||
| % Families below poverty level (SD = 4.75) |
0.87 (0.85–0.89) | 0.86 (0.85–0.88) | ||
| County-level healthcare access† | ||||
| % No health insurance (SD = 4.68) | 0.92 (0.91–0.93) | 1.03 (1.01–1.05) | ||
| # Oncology hospitals per million (SD = 10.08) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | ||
| # Medical doctors per million (SD = 520.55) | 1.00 (0.99–1.02) | 1.00 (0.99–1.02) | ||
| # OB GYN per million (SD = 70.47) | 1.01 (1.00–1.03) | 1.01 (1.00–1.03) |
AOR adjusted odds ratios
Bold indicates significance p value ≤0.05
Adjusted for race, age, SEER registry, late-stage diagnosis, and HR subtype
Adjusted for race, age, SEER registry, late-stage diagnosis and county-level SES
Adjusted for race, age, SEER registry, late-stage diagnosis, and county-level HCA
Adjusted for race, age, SEER registry, late-stage diagnosis, HR subtype, and county-level SES and HCA
ORs associated with standard deviation increase
Breast cancer survival
In the Cox model accounting for HR subtype, stage, and treatment (Table 5), NH-Black women had 42 % higher hazards of breast cancer mortality compared with NH-White women (HR 1.42, 95 % CI 1.39–1.44), while Hispanic women had 4 % higher hazards (HR 1.04, 95 % CI 1.01–1.07). Furthermore, breast cancer mortality was about 4.7 times higher among women aged 75 years and older compared with those less than 44 years (HR 4.69, 95 % CI 4.54–4.85). There was about a twofold increased risk of breast cancer mortality among women diagnosed with HR− subtypes compared with HR+ subtype (HR 1.91, 95 % CI 1.88–1.94). In the area-level SES model, patients residing in counties with a higher proportion of families below the poverty level were at 9 % increased hazards of breast cancer mortality (HR 1.09, 95 % CI 1.07–1.10). In the area-level HCA model, patients residing in counties with a higher proportion of adults without health insurance experienced marginally higher breast cancer mortality (HR per SD increase: 1.01, 95 % CI 1.01–1.02), while patients residing in counties with higher number of medical doctors were at 6 % reduced hazards of breast cancer mortality (HR per SD increase: 0.94, 95 % CI 0.92–0.95). In the fully adjusted model, the racial and subtype differences in hazards of breast cancer mortality remained, and residing in counties with a higher proportion of families below poverty level (HR per SD increase: 1.07, 95 % CI 1.05–1.09) remained associated with increased hazards of breast cancer mortality. In addition, residing in counties with a higher number of medical doctors (HR per SD increase: 0.96, 95 % CI 0.95–0.98) remained associated with reduced hazards of breast cancer mortality.
Table 5.
Multivariate Hazard Ratios (AHR) for breast cancer mortalityb, SEER 2000–2010
| HR Subtype AHR (95 % CI)a |
SES AHR (95 % CI)b |
HCA AHR (95 % CI)c |
Fully adjusted AHR (95 % CI)d |
|
|---|---|---|---|---|
| Race/ethnicity | ||||
| NH-White (referent) | – | – | – | – |
| NH-Black | 1.42 (1.39–1.44) | 1.50 (1.47–1.54) | 1.56 (1.53–1.59) | 1.39 (1.36–1.43) |
| Hispanic | 1.04 (1.01–1.07) | 1.05 (1.02–1.08) | 1.07 (1.04–1.10) | 1.05 (1.03–1.08) |
| Age at diagnosis | ||||
| 40–44 (referent) | – | – | – | – |
| 45–54 | 1.01 (0.98–1.05) | 1.00 (0.96–1.04) | 1.00 (0.97–1.04) | 1.01 (0.98–1.05) |
| 55–64 | 1.29 (1.25–1.34) | 1.25 (1.21–1.30) | 1.25 (1.21–1.30) | 1.29 (1.24–1.33) |
| 65–74 | 2.03 (1.96–2.10) | 1.90 (1.84–1.97) | 1.91 (1.84–1.98) | 2.02 (1.95–2.09) |
| 75+ | 4.69 (4.54–4.85) | 4.30 (4.16–4.44) | 4.32 (4.17–4.46) | 4.69 (4.53–4.85) |
| Breast cancer hormone-receptor subtype | ||||
| HR+ (referent) | – | – | ||
| HR− | 1.91 (1.88–1.94) | 1.90 (1.87–1.93) | ||
| County-level socio-economic status† | ||||
| % Black population (SD = 13.24) | 0.99 (0.98–0.99) | 1.00 (0.99–1.01) | ||
| % Unemployed (SD = 2.20) | 0.99 (0.98–1.00) | 1.01 (1.00–1.02) | ||
| % Less than 9th grade education (SD = 4.74) |
0.98 (0.97–0.99) | 1.00 (0.99–1.02) | ||
| % Families below poverty level (SD = 4.75) |
1.09 (1.07–1.10) | 1.07 (1.05–1.09) | ||
| County-level healthcare access† | ||||
| % No health insurance (SD = 4.68) | 1.01 (1.01–1.02) | 0.94 (0.93–0.96) | ||
| # Oncology hospitals per million (SD = 10.08) | 1.01 (1.01–1.02) | 1.00 (1.00–1.01) | ||
| # Medical doctors per million (SD = 520.55) | 0.94 (0.92–0.95) | 0.95 (0.94–0.97) | ||
| # OB GYN per million (SD = 70.47) | 1.01 (0.99–1.02) | 0.99 (0.98–1.01) |
AHR adjusted hazard ratios
Bold indicates significance p value ≤0.05
Adjusted for race, age, SEER registry, surgical treatment, radiation therapy, and late-stage diagnosis
Adjusted for race, age, SEER registry, surgical treatment, radiation therapy, late-stage diagnosis, and SES
Adjusted for race, age, SEER registry, surgical treatment, radiation therapy, late-stage diagnosis, and HCA
Adjusted for race, age, SEER registry, surgical treatment, radiation therapy, late-stage diagnosis, SES and, HCA
HRs associated with standard deviation increase
Discussion
In a large sample of racially diverse breast cancer patients in the SEER database, we examined the influence of area-level SES, healthcare access, and HR subtypes on breast cancer stage at presentation, receipt of treatment, and survival and determined if racial disparities in breast cancer outcomes persisted after adjustment for these variables. Regardless of SES and healthcare access, diagnosis with HR− subtypes was associated with increased odds of late-stage diagnosis, decreased odds of surgery and radiation therapy, and lower survival in all racial groups. However, our results demonstrate that although HR subtype is an important predictor of breast cancer outcome, there remains a small but important independent influence of area-level SES and healthcare access on outcomes that may be critical to eliminating breast cancer outcome disparities. We further observed that regardless of HR subtype, SES, or healthcare access, racial disparities remained in stage at diagnosis, surgery and radiation treatment, and survival, with larger differences among NH-Blacks compared with NH-Whites.
The importance of HR subtype on stage at diagnosis, treatment, and survival has been well-documented [10–17]. HR-negative tumors are known to be fast growing and highly aggressive, likely contributing to our finding of later stage at diagnosis for HR-negative compared with HR-positive subtypes in all racial groups. This association remained after adjusting for area-level factors, specifically SES and healthcare access, suggesting that the effect of the biologically aggressive HR-negative tumors subtype is a stronger predictor of later stage at diagnosis in patients than screening and timely diagnosis—factors closely related to SES and healthcare access. Previous work by our group has shown significant associations between area-level SES and HR+ but not HR− breast cancers [18], suggesting that SES measures may be more important as prognostic, rather than etiologic factors. Furthermore, we observed that patients of all races diagnosed with HR-negative subtypes were less likely to receive surgery and radiation compared with patients with HR-positive subtypes, even after adjusting for stage at presentation. It is well known that HR-positive breast tumors are more likely to respond to adjuvant hormonal therapy and aromatase inhibitors than HR-negative breast tumors. Considering that surgery, radiation, and chemotherapy are still the mainstay of treatment for most HR-negative breast tumors (apart from the HER2-positive subset), it appears that there may be serious gaps in receipt of appropriate treatment for HR-negative breast cancer. This is consistent with prior studies showing that a high proportion of breast cancer patients received non-guideline concordant treatment for breast cancer, likely explaining poorer survival outcomes especially among racial minorities [31–33]. Future studies will be needed to identify factors associated with receipt of adequate treatment among patients with HR-negative breast cancer subtypes. Additionally, diagnosis with HR-negative subtypes increased breast cancer mortality, independent of SES and healthcare access. The fatality of HR-negative breast cancer subtypes has been well-documented [10, 14, 15], and our study adds to this line of research by providing evidence that accounting for SES and healthcare access does not attenuate the association.
A large number of studies have examined the influence of SES, at both the individual and area level, on breast cancer outcomes [4, 5, 7, 9, 19, 21], while a smaller number of studies have examined the influence of HCA on outcomes [4, 5]. However, very few studies have simultaneously accounted for HR subtypes, SES, and healthcare access at the area level. Since availability of and access to high-quality healthcare is a prerequisite for the receipt of routine screening, timely diagnostic work-up, and guideline-adherent treatment, the lack of consideration for healthcare access in many studies is a major limitation. Further, since access to high-quality healthcare is strongly linked to SES, there are likely racial barriers to healthcare access that mirrors the racial distribution of wealth and income in the US. In addition, beyond individual variables such as education, income, and health insurance, the geographic distribution of healthcare facilities and resources in the US likely contributes to disparities through other dimensions of healthcare access such as the logistics of transportation and travel time to specialty physicians, in addition to other barriers such as cost sharing and out-of-pocket costs [34]. However, inclusion of healthcare access in cancer outcomes research is complicated by the lack of consistent and systematic inclusion of the necessary variables in most cancer databases. Here, we defined healthcare access using a set of four variables that capture the availability of healthcare personnel and facilities at the county level. We observed that the indices for healthcare access (i.e., oncology hospitals, medical doctors, health insurance, and OB/GYNs) were highest for NH-Blacks compared with the other racial groups, although this may possibly be due to higher proportion of these facilities in urban centers. However, further research is needed to better understand healthcare availability and utilization patterns among racial minority groups, as this may have important implications for the interventions to improve the receipt of timely and adequate cancer treatment, as well as patient adherence to treatment.
Consistent with previous studies [11, 13], we observed a higher prevalence of HR-negative subtypes of breast cancer among NH-Black women compared with NH-White and Hispanic women. Genetics and lifestyle risk factors likely account for this distribution, however, the specific mechanism leading to a higher prevalence of HR-negative subtypes among Black women remains unclear. Nevertheless, late-stage diagnosis, receipt of adequate treatment, and survival are downstream events that are likely influenced by both SES and healthcare access. For instance, previous studies have reported that non-White patients experience significant delays in diagnosis and treatment initiation, and are less likely to receive guideline-adherent treatment [35–37], contributing to observed disparities in survival. However, these disparities are reduced among higher SES individuals [20], suggesting that SES may act as a mediator of the race–breast cancer outcome relationship. Given that breast cancer diagnosis and treatment must be preceded by an interaction with the healthcare system facilities and personnel, we expected to find similar associations to SES with breast cancer stage, treatment, and survival. However, the lack of strong and consistent associations suggests that area-level availability of healthcare resources may be a poor proxy for utilization of healthcare resources. There is likely low utilization of existing healthcare resources among racial minorities due to individual (e.g., lack of health insurance, mistrust of healthcare professionals, fear of disease), structural (e.g., distance to facility and public safety), or cultural (belief in home remedies or misconceptions regarding surgery or chemotherapy) reasons. Improved understanding of health utilization patterns among racial minority groups may be critical to eliminating breast cancer outcome disparities, ensuring that all patients, especially those with HR-negative subtypes, receive timely and guideline-adherent diagnosis and treatment necessary for survival.
There are several strengths and limitations of the current study. Our analysis is strengthened through the use of the large SEER population-based database, limiting the likelihood of selection bias and increasing the likelihood of detecting statistically significant differences given the large sample size. Further, the assessment of healthcare access and SES using county-level information provided a unique source of data usually lacking in other large cancer databases. However, as has been reported previously, county-level variables may not fully capture individual level variation in exposures, such as SES and HCA [38]. Nonetheless, since healthcare resources such as facilities and personnel are often allocated based on population size and geography, inclusion of county-level measures may provide a valid measure of healthcare resources available to individuals. Future studies including individual measures of SES and HCA will be highly valuable. Another limitation of this study is the lack of data on other individual level variables such as Her2 status, which was not publicly available at the time of this analysis, as well as other potential confounders such as chronic medical conditions or BMI, variables that may also influence breast cancer outcomes.
In summary, we found that breast cancer HR subtype remained an important predictor of stage at diagnosis, treatment, and survival among all racial groups after adjusting for area-level SES and healthcare access. However, the association between HCA and breast cancer outcomes after adjusting for HR subtype was inconsistent. Racial disparities remained after accounting for HR subtype, SES, and HCA. Further studies are needed to better understand the relationship between the availability and utilization of healthcare resources among cancer patients, as this is likely a highly modifiable factor that may be enhanced to improve breast cancer outcomes, especially among racial minorities.
Acknowledgments
Dr. Akinyemiju was supported by Grant U54 CA118948 from the NIH. Mr. Moore was supported by Grant R25 CA47888 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Compliance with ethical standards
Conflicts of interest None.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER cancer statistics review, 1975–2012. Vol based on Nov 2014 SEER data submission. Bethesda: National Cancer Institute; 2015. [Google Scholar]
- 2.McCarthy AM, Yang J, Armstrong K. Increasing disparities in breast cancer mortality from 1979 to 2010 for US black women aged 20 to 49 years. Am J Public Health. 2015;105(Suppl 3):S446–S448. doi: 10.2105/AJPH.2014.302297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aizer AA, Wilhite TJ, Chen MH, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Trinh QD, Hu JC, Nguyen PL. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532–1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 4.Akinyemiju TF, Soliman AS, Johnson NJ, Altekruse SF, Welch K, Banerjee M, Schwartz K, Merajver S. Individual and neighborhood socioeconomic status and healthcare resources in relation to black–white breast cancer survival disparities. J Cancer Epidemiol. 2013;2013:490472. doi: 10.1155/2013/490472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jadav S, Rajan SS, Abughosh S, Sansgiry SS. The role of socioeconomic status and health care access in breast cancer screening compliance among hispanics. J Public Health Manag Pract. 2015;21(5):467–476. doi: 10.1097/PHH.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 6.Sheppard VB, Oppong BA, Hampton R, Snead F, Horton S, Hirpa F, Brathwaite EJ, Makambi K, Onyewu S, Boisvert M, Willey S. Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol. 2015;22(9):2902–2911. doi: 10.1245/s10434-015-4397-3. [DOI] [PubMed] [Google Scholar]
- 7.Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, Zhao X, Budhwani H. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiol. 2015;39(5):745–751. doi: 10.1016/j.canep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Tao L, Gomez SL, Keegan TH, Kurian AW, Clarke CA. Breast cancer mortality in African-American and non-hispanic white women by molecular subtype and stage at diagnosis: a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1039–1045. doi: 10.1158/1055-9965.EPI-15-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parise CA, Caggiano V. The influence of socioeconomic status on racial/ethnic disparities among the ER/PR/HER2 breast cancer subtypes. J Cancer Epidemiol. 2015;2015:813456. doi: 10.1155/2015/813456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akinyemiju T, Moore JX, Altekruse SF. Breast cancer survival in African-American women by hormone receptor subtypes. Breast Cancer Res Treat. 2015 doi: 10.1007/s10549-015-3528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012 doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 12.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th. Chicago, IL: American Joint Committee on Cancer; 2011. [Google Scholar]
- 13.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG, Cronin KA. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):1–8. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihemelandu CU, Leffall LD, Dewitty RL, Naab TJ, Mezghebe HM, Makambi KH, Adams-Campbell L, Frederick WA. Molecular breast cancer subtypes in premenopausal and post-menopausal African-American women: age-specific prevalence and survival. J Surg Res. 2007;143:109–118. doi: 10.1016/j.jss.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 15.Ihemelandu CU, Naab TJ, Mezghebe HM, Makambi KH, Siram SM, Leffall LD, DeWitty RL, Frederick WA. Basal cell-like (triple-negative) breast cancer, a predictor of distant metastasis in African American women. Am J Surg. 2008;195:153–158. doi: 10.1016/j.amjsurg.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Newman L. Breast cancer disparities: high-risk breast cancer and African ancestry. Surg Oncol Clin N Am. 2014;23:579–592. doi: 10.1016/j.soc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Parise CA, Caggiano V. Breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J Cancer Epidemiol. 2014;2014:11. doi: 10.1155/2014/469251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akinyemiju TF, Pisu M, Waterbor JW, Altekruse SF. Socioeconomic status and incidence of breast cancer by hormone receptor subtype. Springer. 2015;4:508. doi: 10.1186/s40064-015-1282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinglass J, Rydzewski N, Yang A. The socioeconomic gradient in all-cause mortality for women with breast cancer: findings from the 1998 to 2006 national cancer data base with follow-up through 2011. Ann Epidemiol. 2015;25(8):549–555. doi: 10.1016/j.annepidem.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) registries. J Natl Cancer Inst Monogr. 2014;49:236–243. doi: 10.1093/jncimonographs/lgu020. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parise CA, Caggiano V. Disparities in race/ethnicity and socioeconomic status: risk of mortality of breast cancer patients in the California Cancer Registry, 2000–2010. BMC Cancer. 2013;13:449. doi: 10.1186/1471-2407-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, Wang M, Niknam BA, Ludwig JM, Wang W, Even-Shoshan O, Fox KR. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310(4):389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 23.Shariff-Marco S, Yang J, John EM, Kurian AW, Cheng I, Leung R, Koo J, Monroe KR, Henderson BE, Bernstein L, Lu Y, Kwan ML, Sposto R, Vigen CL, Wu AH, Keegan TH, Gomez SL. Intersection of race/ethnicity and socioeconomic status in mortality after breast cancer. J Community Health. 2015;40(6):1287–1299. doi: 10.1007/s10900-015-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roseland ME, Pressler ME, Lamerato LE, Krajenta R, Ruterbusch JJ, Booza JC, Schwartz K, Simon MS. Racial differences in breast cancer survival in a large urban integrated health system. Cancer. 2015;121(20):3668–3675. doi: 10.1002/cncr.29523. [DOI] [PubMed] [Google Scholar]
- 25.Akinyemiju TF, Soliman AS, Yassine M, Banerjee M, Schwartz K, Merajver S. Healthcare access and mammography screening in Michigan: a multilevel cross-sectional study. Int J Equity Health. 2012;11:16. doi: 10.1186/1475-9276-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan JX, Hanson HA, Zick CD, Brown BB, Kowaleski-Jones L, Smith KR. Geographic scale matters in detecting the relationship between neighbourhood food environments and obesity risk: an analysis of driver license records in Salt Lake County, Utah. BMJ Open. 2014;4(8):e005458. doi: 10.1136/bmjopen-2014-005458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Comparing individual- and area-based socioeconomic measures for the surveillance of health disparities: a multilevel analysis of Massachusetts births, 1989–1991. Am J Epidemiol. 2006;164(9):823–834. doi: 10.1093/aje/kwj313. [DOI] [PubMed] [Google Scholar]
- 28.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diez Roux AV. A glossary for multilevel analysis. J Epidemiol Community Health. 2002;56(8):588–594. doi: 10.1136/jech.56.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, Cronin KA. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):1–8. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joslin CE, Brewer KC, Davis FG, Hoskins K, Peterson CE, Pauls HA. The effect of neighborhood-level socioeconomic status on racial differences in ovarian cancer treatment in a population-based analysis in Chicago. Gynecol Oncol. 2014;135(2):285–291. doi: 10.1016/j.ygyno.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bristow RE, Powell MA, Al-Hammadi N, Chen L, Miller JP, Roland PY, Mutch DG, Cliby WA. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105(11):823–832. doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez DR, Liao KP, Giordano S, Nguyen H, Smith BD, Elting LS. Adherence to national guidelines for antiemesis prophylaxis in patients undergoing chemotherapy for lung cancer: a population-based study. Cancer. 2013;119(7):1428–1436. doi: 10.1002/cncr.27899. [DOI] [PubMed] [Google Scholar]
- 34.Devoe JE, Baez A, Angier H, Krois L, Edlund C, Carney PA. Insurance + access not equal to health care: typology of barriers to health care access for low-income families. Ann Fam Med. 2007;5(6):511–518. doi: 10.1370/afm.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Sociodemographic disparities in advanced ovarian cancer survival and adherence to treatment guidelines. Obstet Gynecol. 2015;125(4):833–842. doi: 10.1097/AOG.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hines RB, Barrett A, Twumasi-Ankrah P, Broccoli D, Engelman KK, Baranda J, Ablah EA, Jacobson L, Redmond M, Tu W, Collins TC. Predictors of guideline treatment nonadherence and the impact on survival in patients with colorectal cancer. J Natl Compr Cancer Netw. 2015;13(1):51–60. doi: 10.6004/jnccn.2015.0008. [DOI] [PubMed] [Google Scholar]
- 37.Bristow RE, Chang J, Ziogas A, Anton-Culver H, Vieira VM. Spatial analysis of adherence to treatment guidelines for advanced-stage ovarian cancer and the impact of race and socioeconomic status. Gynecol Oncol. 2014;134(1):60–67. doi: 10.1016/j.ygyno.2014.03.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The public health disparities geocoding project. Am J Epidemiol. 2002;156(5):471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]