Abstract
Background and Aims
Rates of progression to esophageal adenocarcinoma in subjects with Barrett's esophagus (BE) are lower than previously estimated. Identification of predictors of progression will enable risk stratification of BE subjects, potentially making current surveillance programs more efficient. We aimed to assess the potential of demographic and lifestyle factors, obesity and medications in predicting progression in BE.
Methods
BE subjects were identified from the General Practice Research Database using validated diagnostic codes. BE subjects developing esophageal cancer (EC) 12 months after their index BE diagnosis were defined as progressors. Time to event analysis was used to assess the overall risk of progression to EC. Cox proportional hazards models and time varying marginal structural models were used to assess predictors of progression.
Results
9,660 BE patients were included in the analysis. The mean age (SD) of the study subjects was 63 (13.5) years. 62.6 % were males. 103 subjects (1.1%) progressed to EC. The mean (SD) follow-up since initial diagnosis was 4.8 (3.3) years. The incidence of EC was 2.23 per 1000 person-years of follow-up. Increasing age, male gender, and being overweight (BMI 25- 29.9) were found to be independent predictors of progression. When time varying models were used, PPI and statin use were protective against progression.
Conclusions
In this large population-based cohort of BE subjects, increasing age, male gender, and being overweight predicted progression to EC whereas PPI and statin use were protective against EC development. These factors may aid in developing a risk score to predict the risk of progression and develop chemopreventive strategies in BE subjects.
Keywords: Esophageal Carcinoma, Barrett's esophagus, Chemoprevention, Risk-stratification
INTRODUCTION
Barrett's esophagus (BE) is a premalignant condition in which the normal squamous epithelium lining the lower esophagus is replaced by metaplastic columnar epithelium.1, 2 BE is a strong risk factor for esophageal adenocarcinoma (EAC), a malignancy associated with dismal 5-year survival rate of less than 20%.3 The incidence of EAC in the western world has increased by 6-fold over the past 4 decades.4
EAC is diagnosed at an earlier stage in BE patients enrolled in a surveillance program and has a better prognosis than EAC diagnosed outside surveillance.5–7 Despite the evidence from retrospective studies that support endoscopic surveillance,5, 8 current practice patterns may not be effective in reducing EAC-related mortality.9 Though patients with BE have 30 to 40 times higher risk of EAC compared with the general population, the absolute risk of progression to adenocarcinoma is low and is estimated to be 0.5% or less annually.10 This could explain the lack of consensus on cost effectiveness of current surveillance programs.11, 12
Predictors of progression to EAC in BE subjects are currently not well understood. This has led to uniform recommendations on surveillance intervals for all BE subjects, with dysplasia grade being the primary risk stratification tool.13 Identification of additional risk factors would enable the stratification of BE subjects into high-risk and low-risk groups, potentially enabling surveillance and/or therapy to be focused on high-risk groups, rendering this approach more effective and efficient.
Studies have suggested male sex,11, 14 increasing age,11 central obesity,15 hiatal hernia size,16 BE segment length,17 duration of BE,18 presence of specialized intestinal metaplasia,14 and BE-associated dysplasia grade14, 17 as potential predictors of malignant transformation in BE. Additionally, medications such as aspirin / NSAIDs;19–21 statins,22–26 and proton pump inhibitors (PPI)27–31 have been reported to protect against progression.
Several of these studies were done on small single-center cohorts with contradictory results. Moreover, adjustment for confounding variables was not performed due to small sample sizes. Several studies assessing the impact of medications on BE progression also used only baseline data on drug exposure to assess the impact on progression, rendering the results to be biased given the lack of availability of data on consumption during the study interval. We hence aimed to identify factors predictive of progression, particularly obesity and protective against progression to esophageal carcinoma (EC) in a large population-based cohort of BE subjects from the General Practice Research Database (GPRD). GPRD is a claims based database and hence provides additional data on drug exposure during patient follow-up.
METHODS
The study was approved by the University Hospitals Case Medical Center Institutional Review Board and the Independent Scientific Advisory Committee at the GPRD.
GPRD is a large primary care database from the United Kingdom (U.K.). It was established in 1987 and currently has data on over 8 million people. GPRD is representative of the U.K. population and is now part of the Clinical Practice Research Datalink. Previous studies on GPRD have shown excellent agreement between the diagnoses recorded in the database and that on paper-based records.32 GPRD data have been used to perform successful epidemiologic studies investigating associations between risk factors and malignancies.33 We have previously validated the diagnosis of BE from the GPRD database using specific diagnostic codes in a study assessing the association between BE and diabetes mellitus-type2 (DM2).34
All patients with a diagnosis of BE in the GPRD database between May 1991 and April 2010 were identified using GPRD specific diagnostic codes (Appendix 1). The first date of BE diagnosis in the GPRD database was defined as the index date. The collected data were analyzed to identify BE patients who progressed to EC. EC was identified using GPRD-specific diagnostic codes (Appendix 1). Because pathology reports were not available for review in the GPRD database, all esophageal cancers identified in BE subjects were assumed to be histologically adenocarcinoma given that the occurrence of squamous cell esophageal carcinoma (SCC) in BE subjects is rare. Additional sensitivity analyses were conducted varying the estimates of this proportion to 93% given previous studies.35
“Progressors” were defined as BE subjects who developed EC 12 months after the index date. Patients who developed EC within 12 months of index date were excluded from the study because these could potentially be prevalent cancers. “Non-progressors” were defined as BE subjects who did not have a diagnosis of EC in the entire GPRD follow-up.
Predictors of interest investigated in the current study were age, gender, smoking, hiatal hernia (presence or absence), obesity, DM2, and medications (which were previously reported to be associated with BE progression). Other potential predictors of progression such as length of BE segment and BE-associated dysplasia grade were not included in the study because those variables were not available for review in the GPRD database.
Age was modeled as a continuous variable. History of smoking was abstracted as to whether the patient “ever smoked” (consisting of current and ex-smokers) or “never smoked.” Information on presence of hiatal hernia was abstracted using specific diagnostic codes (Appendix 1).
Obesity was defined using body mass index (BMI). Study subjects were classified into 3 categories - overweight (BMI 25- 29.9), obese-I (BMI 30- 34.9), and obese-II (>34.9). We analyzed the variation in weight of study subjects throughout the entire follow-up period in GPRD. A mean change of only 0.0122 kg/m2 with standard deviation of 2.54 was observed during a median follow-up duration of 4.04 years (interquartile range, 2.05–6.92 years). To enable maximal sample size and power, we used the weight over the entire follow-up period available in the GPRD. The most recent weight and height values were used to construct the BMI variable. When multiple weight values were available, the average of weights was used. DM2 was defined as a diagnosis of DM2 at baseline (by standard diagnosis codes for DM2 in GPRD) and a medication code indicating either an oral hypoglycemic or insulin prescription being filled at least once before the index date. This definition was designed to increase the specificity of DM2 diagnosis. The definition for DM2 used in the current study was identical to the definition used in our previous publication that analyzed GPRD database.34
Medications evaluated in the current study included PPI, NSAIDs, statins, insulin, metformin and other anti-diabetic medications (OAD). This information was obtained using the British National Formulary (BNF) mapping codes and product codes (Appendix 1).
Additional analyses were conducted varying the definition of incident cancer to 6 months and 18 months from date of diagnosis, randomly excluding 36% of the cases (proportion of patients who may not have intestinal metaplasia on histology despite having columnar metaplasia of the esophagus14) and assessing influence of PPI dosage (once versus twice a day) on progression risk. Given the absence of histology information in GPRD, we performed analysis on a subset of patients who had not had endoscopy within the last 3 years (as a surrogate marker for BE without dysplasia).
Statistical Analysis
Time to event analysis was used to assess the overall risk of progression to EC. Cox proportional hazards models were used to assess predictors of progression. The Cox model of time to cancer diagnosis included all the potential confounders described above and medication exposure described below. In addition, analyses of medications of interest were conducted using a time varying model. The number of days of medication availability was calculated using proportion days covered (PDC) available in the claims data, where PDC is defined as the number of covered days on a prescription divided by the total days of follow-up. This model calculates the percent time BE subjects were on the medications of interest, which was hypothesized to afford a more precise estimate of exposure to the medication compared with a conventional model looking at exposure at baseline or during follow-up (yes vs no). In a time-varying model, the proportional effect of treatment may vary depending on the percent of medication coverage at a particular instance of time. The analytical data set was created in SAS (versions 9.2 and 9.3; SAS, Cary, NC) and the statistical analyses were conducted in Stata SE software (version 11.2; Stata, College Station, Tex).
RESULTS
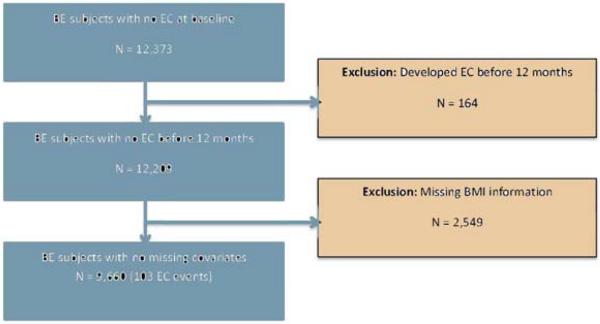
Twelve thousand three hundred seventy-three BE subjects were identified in the GPRD database during the study period. 164 (1.3%) of these subjects developed EC within 12 months of the index date and hence were excluded as potentially prevalent cancers. Among the 12,209 subjects left, 2549 subjects had missing BMI values and were excluded from further analysis. Figure 1 summarizes identification, inclusion, and exclusion of BE subjects. The final study population consisted of 9660 patients with mean (SD) age of 63 (13.5) years. 62.6% of the study subjects were males. Baseline characteristics of the study population are presented in Table 1. Comparison of baseline characteristics of the complete cohort (N = 12,209) and the cohort with BMI data (N = 9660) are detailed in the appendix (Supplementary table 1).
Figure 1.
Flowchart showing identification, inclusion and exclusion of BE subjects from the GPRD database.
Table 1.
Baseline characteristics of the final study population
| Variables | Total N = 9660 | Males N = 6042 | Females N = 3618 |
|---|---|---|---|
| Age (years) - mean (SD) | 63 (13.5) | 61 (13.3) | 66 (13.3) |
| Median | 64 | 62 | 67 |
| 10th – 90thpercentile | 45 – 80 | 43 – 79 | 48 – 83 |
| Follow-up (years) – mean (SD) | 4.8 (3.3) | 4.7 (3.3) | 4.9 (3.4) |
| N (%) | N (%) | N (%) | |
| Ever smoker* | 4,940 (51.91%) | 3,522 (59.24%) | 1,418 (39.7%) |
| BMI (<25) | 3,345 (34.63%) | 2,070 (34.26%) | 1,275 (35.24%) |
| Overweight (BMI 25- 29.9) | 4,256 (44.06%) | 2,896 (47.93%) | 1,360 (37.59%) |
| Obese I (BMI 30-34.9) | 1,955 (20.24%) | 1,039 (17.20%) | 916 (25.32%) |
| Obese II (BMI > 34.9) | 104 (1.08%) | 37 (0.61%) | 67 (1.85%) |
| Hiatal hernia | 2,058 (21.30%) | 1,181 (19.55%) | 877 (24.24%) |
| Diabetes | 651 (6.74%) | 418 (6.92%) | 233 (6.44%) |
| PPIs | 8,177 (84.65%) | 5,115 (84.66%) | 3,062 (84.63%) |
| NSAIDs | 6,015 (62.27%) | 3,549 (58.74%) | 2,466 (68.16%) |
| Statins | 2,668 (27.62%) | 1,751 (28.98%) | 917 (25.35%) |
| Metformin | 630 (6.52%) | 385 (6.37%) | 245 (6.77%) |
| Insulin | 226 (2.34%) | 151 (2.50%) | 75 (2.07%) |
| OAD | 767 (7.94%) | 483 (7.99%) | 284 (7.85%) |
Smoking percentages were taken among patients without missing information, so percentages are not reflective of the total.
Of the 9660 BE subjects, 103 (1.1%) subjects developed EC 12 months after from the index date and were classified as incident cancers. The mean (SD) follow-up period of study subjects was 4.8 (3.3) years. The overall incidence rate of EC in the cohort was 2.23 per 1000 person years of follow-up.
Results of univariate and multivariate analyses are presented in Table 2. Univariate analysis showed significant association between increasing age, male gender, overweight (BMI 25- 29.9), and progression to EC. On multivariate analysis (adjusting for age, gender, smoking, BMI, hiatal hernia, DM2, PPI, NSAIDs, Statin, Metformin, Insulin, and OAD), increasing age, male gender, and being overweight continued to be independent risk factors predictive of progression to EC. Obese-I (BMI 30-34.9) patients showed a trend toward significance as a risk factor for predicting progression (p = 0.08). Increasing hazard ratios for the 3 BMI groups - overweight, Obese-I and Obese-II (1.63, 1.72 and 2.24) demonstrated a statistically significant trend across the 3 groups (p= 0.034), suggesting increased risk of progression with higher BMI.
Table 2.
Predictors of progression to esophageal carcinoma: demographic and lifestyle factors
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CL) | P Value | HR (95% CL) | P Value | |
| Age (per yr) | 1.03 (1.01, 1.04) | 0.001 | 1.04 (1.02, 1.05) | <0.0001 |
| Male sex | 2.39 (1.48, 3.87) | <0.0001 | 2.79 (1.69, 4.62) | <0.0001 |
| Ever smoker | 1.22 (0.82, 1.82) | 0.317 | 1.08 (0.71, 1.63) | 0.722 |
| BMI (normal) | Reference | |||
| Overweight (BMI 25-29.9) | 1.64 (1.03, 2.60) | 0.037 | 1.63 (1.02, 2.61) | 0.040 |
| Obese I (BMI 30-34.9) | 1.30 (0.72, 2.35) | 0.383 | 1.72 (0.93, 3.17) | 0.084 |
| Obese II (BMI > 34.9) | 1.28 (0.17, 9.34) | 0.810 | 2.24 (0.30, 16.81) | 0.436 |
| Hiatal hernia | 1.00 (0.62, 1.63) | 0.987 | 0.99 (0.61, 1.60) | 0.958 |
| Diabetes | 0.61 (0.19, 1.94) | 0.404 | 0.33 (0.06, 1.85) | 0.207 |
On conventional analysis (using the strategy of baseline exposure as yes/no), none of the medications were protective against progression to EC (Table 3). However, in the time-varying model for medications, using PDC to determine exposure to medications during the follow-up intervals (Table 4), PPI use (HR = 0.43, p <0.0001) and statin use (HR = 0.61, p = 0.002) were protective against progression to EC. Once a day versus twice a day PPI use did not appear to influence the protective effect of PPIs (Supplementary table 2). NSAIDs and metformin use showed a trend toward protection against progression to EC but were not statistically significant predictors.
Table 3.
Conventional model: predictors of progression to esophageal carcinoma – medications
| Drugs | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| PPIs | 0.98 (0.57, 1.69) | 0.938 | 1.05 (0.59, 1.86) | 0.86 |
| NSAIDs | 0.94 (0.63, 1.40) | 0.746 | 0.94 (0.63, 1.41) | 0.77 |
| Statins | 0.75 (0.44, 1.30) | 0.309 | 0.61 (0.34, 1.10) | 0.102 |
| Metformin | 0.64 (0.20, 2.02) | 0.449 | 0.51 (0.09, 2.81) | 0.437 |
| Insulin | 0.65 (0.09, 4.68) | 0.673 | 0.57 (0.10, 3.35) | 0.537 |
| OAD | 0.85 (0.35, 2.10) | 0.732 | 3.38 (0.82, 13.95) | 0.092 |
Table 4.
Time varying model: predictors of progression to esophageal carcinoma - medications
| Drugs | HR (95% CI) | P value |
|---|---|---|
| PPIs | 0.43 (0.36, 0.52) | <0.0001 |
| NSAIDs | 0.15 (0.01, 1.86) | 0.14 |
| Statins | 0.61 (0.45, 0.83) | 0.002 |
| Metformin | 0.57 (0.27, 1.21) | 0.142 |
| OAD | 1.26 (0.89, 1.78) | 0.193 |
Varying the definition of incident cancers (from 6 to 18 months) also did not substantially affect the incidence rate of EC or the associations delineated in the original model (Supplementary tables 3a,b,c). Similar results were seen with random exclusion of 36% of cases (as presumably not showing intestinal metaplasia14 (Supplementary tables 4a,b,c). Selective exclusion of 36% of “non-progressors” presuming that all EC occurred in subjects with IM, resulted a slightly higher incidence rate of EC [3.49 (95% CI, 2.87 – 4.23) vs 2.23 (95% CI, 1.84 – 2.71) per 1000 person-years of follow-up].
DISCUSSION
In this large population-based cohort study with data from United Kingdom primary care practices, incidence of EC in BE subjects was 2.23 per 1000 person-years of follow-up. Increasing age, male sex, and overweight (BMI: 25-29.9) were identified as independent risk factors that predict progression of BE to EC. Increasing BMI was associated with an increasing risk of progression to EC. In addition, using time-varying marginal structural models using the proportion of days covered from this claims-based database, we identified PPI and statin use to be protective against progression to EC. NSAIDs and metformin use showed a trend toward protection although they did not meet statistical significance.
The current study has 3 unique strengths. To our knowledge, this is the largest cohort of BE patients in whom the role of clinical factors and medications in predicting progression has been assessed allowing us to adjust estimates for several clinically relevant covariates. Secondly, a novel “time varying” statistical model was used instead of the conventional model, to accurately assess the association between medication use and BE progression. In the conventional model, exposure to medication is determined at baseline or at follow-up as a dichotomous variable. It does not account for time of follow-up before exposure to medication, which leads to immortal time bias.36 The immortal time bias, when present, can generate an illusion of treatment effectiveness in observational studies.37 By using the PDC method, we were able to more accurately determine the association between exposure to these medications and risk of progression in BE, as shown by the difference in results between the conventional and time varying models. Finally, the GPRD database has comprehensive prescription refill information for all medications irrespective of which pharmacy a patient went to, thereby allowing a more reliable estimation of medication use by subjects. Moreover, our results also stayed consistent despite varying definitions of incident cancers from 6 months to 18 months.
There are currently limited data on the influence of obesity on risk of progression in BE, with studies being limited by the small number of subjects who progressed during follow-up.18, 38 Hardikar et al38 reported no association between BMI and neoplastic progression based on a cohort consisting of 411 BE patients with 45 EC cases. Sikkema et al18 also did not find an association between BMI and progression in a cohort of 713 BE patients with 26 progressors. However, we found that being overweight (BMI: 25-29.9) was an independent predictor for BE progression. In addition, we observed a statistically significant increasing trend (p=0.034) in the hazards ratios for the 3 obesity groups (HR: 1.63, 1.72, and 2.24 for overweight, Obese-I and Obese-II, respectively), suggesting increased risk of malignant progression with higher BMI. Lack of statistical significance in the individual groups with higher BMI is likely due to the smaller number of subjects with EC in these subgroups. The large number of progressors in our study likely provided us with increased power to demonstrate this relationship. BMI of ≥30 correlates strongly with central obesity39, which has also been shown to increase progression risk.15 Central obesity has been hypothesized to increase progression risk in BE through mechanical and non-mechanical systemic mechanisms.15
Recent systematic reviews and meta-analyses have shown protective effects of statins 40 and PPIs 31 on BE progression. Measurement of drug exposure in most of the studies included was performed by assessment at baseline, by patient interviews or records from VA pharmacies. Near universal exposure to PPIs in some cohorts precluded assessment of the protective effects of PPIs25. The present study does not have these limitations given more precise and accurate assessment of drug exposure from the GPRD claims-based database. Our assessment of the influence of NSAIDs and aspirin on BE progression was however limited by the lack of over-the-counter medication use captured in the GPRD database, leading to potential under-reporting of NSAID and ASA use. Recent studies have shown a potential for metformin to be a chemopreventive agent in obesity-associated cancers.41, 42 Given that central obesity is believed to play an important role in neoplastic progression of BE, the association between metformin use and progression in BE was examined. Although a protective trend for metformin was observed, this was without statistical significance. A recent randomized controlled trial also did not support the use of metformin as a chemopreventive agent in BE.43
There were some limitations in the current study that warrant consideration. Despite the large sample size of both BE subjects (9660) and those who progressed to EC (103), subjects had to be excluded from the initially identified population due to missing data. This is was largely on account of missing data on BMI. We systematically compared the excluded and included subjects to detect any differences (Supplementary Table 1). Although the 2 groups revealed differences in several baseline characteristics, multivariable models in both groups showed similar predictor variables (Supplementary Tables 5a and 5b). This gives us confidence that the associations that we identified are valid. The diagnostic criteria for BE in the United Kingdom include only demonstration of columnar metaplasia without the requirement for intestinal metaplasia (IM). Given the absence of histopathology data in the GPRD database, it is difficult to estimate the proportion of BE subjects who had IM. Recent studies have however reported that esophageal columnar metaplasia without IM also has neoplastic potential44 and is associated with biomarkers that predict progression in IM.45, 46 Moreover, the diagnosis of BE remained valid with multiple sensitivity analyses (including the requirement for endoscopy) performed in a prior study assessing the association of BE with DM2.34 Exclusion of 36% 14of subjects presumably without intestinal metaplasia did not materially change results (Supplementary tables 4a,b,c).
Another limitation was the unavailability of histopathology data in the GPRD database, which limited our ability to assess dysplasia grade, which is the most widely accepted and used marker for risk stratification in BE subjects.17 Given the population-based nature of the data in GPRD, we speculate that the large majority of BE subjects in these data had no dysplasia at baseline as shown in similar population-based studies.47 A subset analysis of subjects without endoscopy in the last 3 years (presumably without dysplasia) did not reveal any substantial change in associations (Supplementary tables 6a,b,c). In the current study, EC in a BE subject was assumed to be EAC as the occurrence of SCC in BE is uncommon. A previous paper from the GPRD database had estimated that up to 7% of ECs in BE could potentially be SCC.35 We performed a sensitivity analysis randomly excluding 7% of malignant progressors and found that the associations identified in the current study remained valid (Supplementary tables 7a,b,c). Length of BE segment is another risk factor,17 which could not be assessed as endoscopic data are not part of GPRD.
In summary, in this large population-based observational study on BE subjects, increasing age, male sex and increasing BMI were found to be risk factors that predicted progression to EC. PPI and statin use were identified as independent factors that protect against progression to EC. These results remained valid with a number of sensitivity analyses. NSAIDs and metformin use showed a trend toward protection against malignant progression. Subjects with high BMI may constitute a group of subjects who could be targeted by suitable chemopreventive agents. Prospective studies are needed to confirm these associations.
Supplementary Material
Acknowledgments
Grant Support: Supported by an investigator-initiated grant from Takeda Pharmaceuticals, Inc. Prasad Iyer and Amitabh Chak are members of the National Cancer Institute–supported Barrett's Esophagus Translational Research Network (U54 CA163060).
Abbreviations
- BE
Barrett's esophagus
- BNF
British National Formulary
- BMI
Body mass index
- DM2
Diabetes mellitus-type 2
- EAC
Esophageal adenocarcinoma
- EC
Esophageal cancer
- GPRD
General Practice Research Database
- IM
Intestinal metaplasia
- OAD
Other anti-diabetic medications
- OTC
Over the counter
- PDC
Proportion days covered
- PPI
Proton Pump Inhibitor
- UK
United Kingdom
- VA
Veterans Affairs
Appendix- I
Diagnostic Codes for Barrett's esophagus
Barrett's esophagus: Medcode, 4614; read code, J101611
Barrett's ulcer of the esophagus: Medcode, 5596; read code, J102500
Diagnostic Codes for esophageal cancer
Medcodes: 4865, 56077, 64274, 8244, 44228, 99155, 63470, 61695, 42416, 54171, 1062, 30700, 53591, 41362, 50789, 67497, and 4865
Diagnostic Codes for hiatal hernia
Medcodes: 256, 48153, 10104, 33779, and 12086
British National Formulary (BNF) mapping codes for medications
PPI: BNF 010305*
NSAIDSs: BNF 10010100*
Statins: BNF 02120400*
Product codes of medications
Insulin: 12300, 26795, 21459, 27151, 19029, 31466, 16142, 19877, 6447, 29567, 5892, 11337, 5021, 6209, 14345, 29953, 21583, 36920, 19491, 14299, 21590, 28442, 28101, 31465, 31467, 36355, 36356, 7318, 10264, 322, 38986, 18224, 14313, 14362, 5214, 26060, 23231, 12297, 14339, 14938, 10572, 18592, 24593, 1588, 1594, 1592, 7349, 9565, 14944, 1840, 21235, 16129, 26621, 36430, 15710, 12654, 12638, 27402, 22983, 22945, 23993, 17336, 41959, 24846, 4706, 30209, 14930, 13622, 26098, 25479, 4129, 27396, 9521, 1842, 36513, 9503, 14505, 12299, 24795, 23099, 10067, 7228, 6061, 7267, 13416, 4163, 14301, 14330, 10184, 6958, 35260, 6965, 7393, 7400, 5953, 10259, 7266, 6057, 10225, 7237, 36853, 7402, 28588, 13516, 14340, 36066, 15484, 18590, 38422, 30236, 8118, 7772, 7771, 14357, 10229, 4760, 43950, 14918, 5891, 1886, 9737, 10208, 1595, 1593, 14290, 14928, 10207, 15961, 25812, 11080, 10175, 14925, 27461, 5501, 23992, 46001, 35468, 33966, 13729, 14933, 13819, 28183, 30686, 4247, 7350, 1843, 10243, 14270, 4715, 39006, 42395, 18593, 10001, 39086, 28185, 36146, 31258, 5701, 5250, 27177, 43953, 34097, 10915, 10910, 7793, 17809, 22155, 1649, 4199, 4093, 10277, 16160, 4198, 43991, 19513, 11107, 8841, 25736, 25735, 36194, 16152, 21422, 28096, 33167, 33232, 44378, 19878, 41120, 13837, 14644, 29837, 9341, 21374, 21110, 14649, 11055, 11056, 21395, 22697, 42954, 21232, 45158, 20422, 30819, 24993, 15199, 25133, 44480, 24002, 35253, 21554, 31205, 2456, 5255, 10245, 2455, 3551, 7319, 2929, 5845, 2221, 2454, 7231, 7300, 1805, 2812, 3550, 10244, 12818, 5933, 4790, 13277, 3439, 3396, 2220, 10484, 27614, 1806, 21347, 10887, 17731, 8203, 22058, 26403, 20995, 9618, 24800, 8895, 36031, 14619, 27280, 2459, 10547, 18461, 7537, 18931, 9376, 1844, 8322, 1587, 17712, 12035, 16700, 41834, 16682, 26498, 4784, 44251, 34031, 6554, 6470, 11408, 6781, 5649, 13096, 15951, 14191, 13108, 17643, 11346, 11245, 11086, 12840, 11271, 14642, 6991, 9702, 6228, 5267, 7127, 9619, 11345, 43833, 43568, 12892, 13274, 37055, 38236, 6138, 6831, 5962, 37427, 10145, 40555, 42305, 44601, 42797, 26338, 15294, 15895, 10014, 10010, 10011, 10012, 10013, 10015, 10008, 10009, 10016, 10017, 18149, 5345, 4896, 6009, 7062, 16389, 5059, 5164, 5557, 6091, 38774, 38808, 17405, 29090, 17377, 6753, 6981, 22946, 17076, 33914, 19977, 28666, 35057, 35081, 35078, 7075, 35017, 35218, 20634, 28851, 31438, 31439, 20635, 20636, 39150, 6724, 1589, 1591, 13969, 5769, 11521, 21223, 13009, 5634, 14646, 5967, 6730, 30305, 16959, 5620, 7412, 10133, 6378, 5789, 11878, 16866, 13036, 18446, 24554, 22328, 30918, 31699, 25422, 9363, 1751, 13474, 22060, 34713, 40085, 2321, 23636, 38093.
Metformin: 39560, 25678, 26258, 40007, 40110, 39729, 16044, 38400, 7166, 7610, 45581, 39598, 7048, 38355, 40233, 39988, 11990, 44250, 735, 43270, 23, 33087, 34598, 34323, 34004, 34135, 34917, 34504, 93, 34836, 33674, 34020, 34742, 34697, 31146, 27501, 42161, 17580, 14164, 6855, 7325, 11760, 11737, 11609, 11610, 11604, 11717, 11601, 7375, 43684, 43619, 38551, 39203, 37902, 37874, 31077, 30316, 18220.
OAD: 23, 93, 735, 7048, 7166, 7610, 11990, 16044, 25678, 26258, 27501, 31146, 33087, 33674, 34004, 34020, 34135, 34323, 34504, 34598, 34697, 34742, 3483 6, 34917, 38355,38400, 39560, 39598, 39729, 39988, 40007, 40110, 40233, 42161, 43270, 44250, 45581, 6855, 7325, 7375, 11601, 11604, 11609, 11610, 11717, 11737, 11760,14164, 17580, 18220, 30316, 31077, 37874, 37902, 38551, 39203, 43619, 43684, 469, 479, 548, 3740, 4307, 5174, 5227, 5621, 5678, 5989, 7695, 9105, 9662, 9699, 9707, 9748, 9865, 10051, 11316, 11321, 11366, 11483, 12897, 13628, 15232,15955, 19472, 20287, 20889, 23945, 27125, 35022, 35144, 35149, 35150, 35251, 35462, 35561, 36774, 36948, 37617, 37875, 39149, 40642, 40693, 41204, 41431, 45775, 45821, 322,5021, 5214, 5892, 6209, 6447, 7318, 10264, 11337, 14299, 14313, 14345, 14362, 16142, 18224, 19491, 19877, 21583, 21590, 26060, 28101, 28442, 29567, 29953, 31465, 31467,36355, 36356, 36920, 38986, 1588, 1592, 1594, 1840, 1842, 4129, 4706, 7349, 9521, 9565, 10572, 12297, 12638, 12654, 13622, 14339, 14930, 14938, 14944, 15710, 16129, 17336, 18592, 21235,22945, 22983, 23231, 23993, 24593, 24846, 25479, 26098, 26621, 27396, 27402, 30209, 36430, 36513, 41959, 32, 547, 1253, 1254, 1847, 1964, 1965, 2219, 4862, 5276,5316, 5353, 5627, 5636, 6337, 7284, 7332, 7409, 7744, 7912, 8034, 8168, 8390, 8976, 10427, 11284, 11695, 11946, 12245, 12259, 12455, 12513, 13331, 15374, 16602,17343, 17698, 17706, 19336, 19658, 21424, 21489, 21564, 21832, 21892, 22145, 22858, 24848, 25636, 26118, 26218, 27969, 28708, 29326, 29939, 30460, 31212, 31474, 33562, 33673,34399, 34507, 34563, 34676, 34706, 34802, 34932, 34957, 36856, 40365, 40425, 41558, 41559, 41593, 42790, 43065, 43465, 44304, 44473, 44738, 45215, 45831.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Author Contributions: Rajesh Krishnamoorthi: Initial manuscript draft, interpretation of analysis, critical review of manuscript.
Bijan Borah: Statistical analysis, interpretation of analysis, critical review of manuscript.
Herbert Heien: Statistical analysis, interpretation of analysis
Ananya Das: Secured funding, critical review of manuscript
Amitabh Chak: Secured funding, interpretation of analysis, critical review of manuscript
Prasad Iyer: Secured funding, interpretation of analysis, critical review of manuscript, overall study supervision
References
- 1.Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. Jama. 2002;287:1972–1981. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ, Souza RF. Barrett's esophagus. The New England journal of medicine. 2014;371:836–45. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. Journal of the National Cancer Institute. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 4.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiology Biomarkers & Prevention. 2010;19:1468–1470. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 5.Corley DA, Levin TR, Habel LA, Weiss NS, Buffler PA. Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 6.Kastelein F, van Olphen SH, Steyerberg EW, Spaander MC, Bruno MJ. Impact of surveillance for Barrett's oesophagus on tumour stage and survival of patients with neoplastic progression. Gut. 2015 doi: 10.1136/gutjnl-2014-308802. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Naik AD, Duan Z, Shakhatreh M, Helm A, Pathak A, Hinojosa-Lindsey M, Hou J, Nguyen T, Chen J, Kramer JR. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett's oesophagus. Gut. 2015 doi: 10.1136/gutjnl-2014-308865. [DOI] [PubMed] [Google Scholar]
- 8.Verbeek RE, Leenders M, ten Kate FJ, van Hillegersberg R, Vleggaar FP, van Baal JW, van Oijen MG, Siersema PD. Surveillance of Barrett's esophagus and mortality from esophageal adenocarcinoma: A population-based cohort study. The American journal of gastroenterology. 2014;109:1215–1222. doi: 10.1038/ajg.2014.156. [DOI] [PubMed] [Google Scholar]
- 9.Corley DA, Mehtani K, Quesenberry C, Zhao W, de Boer J, Weiss NS. Impact of endoscopic surveillance on mortality from Barrett's esophagus–associated esophageal adenocarcinomas. Gastroenterology. 2013;145:312–319. e1. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma P. Barrett's esophagus. New England journal of medicine. 2009;361:2548–2556. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- 11.de Jonge PJ, Van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett's oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030–1036. doi: 10.1136/gut.2009.176701. [DOI] [PubMed] [Google Scholar]
- 12.Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N. Surveillance of Barrett's oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling. Health Technol Assess. 2006;10:1–142. iii–iv. doi: 10.3310/hta10080. [DOI] [PubMed] [Google Scholar]
- 13.Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV, Decker GA, Fanelli RD, Fisher DA, Foley KQ. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointestinal Endoscopy. 2012;76:1087–1094. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. Journal of the National Cancer Institute. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Sharma AN, Murad MH, Buttar NS, El–Serag HB, Katzka DA, Iyer PG. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology. 2013;11:1399–1412. e7. doi: 10.1016/j.cgh.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weston AP, Sharma P, Mathur S, Banerjee S, Jafri AK, Cherian R, McGregor D, Hassanein RS, Hall M. Risk stratification of Barrett's esophagus: updated prospective multivariate analysis. The American journal of gastroenterology. 2004;99:1657–1666. doi: 10.1111/j.1572-0241.2004.30426.x. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge PJ, van Blankenstein M, Grady WM, Kuipers EJ. Barrett's oesophagus: epidemiology, cancer risk and implications for management. Gut. 2014;63:191–202. doi: 10.1136/gutjnl-2013-305490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sikkema M, Looman C, Steyerberg E, Kerkhof M, Kastelein F, Van Dekken H, van Vuuren A, Bode W, van der Valk H, Ouwendijk R. Predictors for neoplastic progression in patients with Barrett's Esophagus: a prospective cohort study. The American journal of gastroenterology. 2011;106:1231–1238. doi: 10.1038/ajg.2011.153. [DOI] [PubMed] [Google Scholar]
- 19.Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, Sanchez CA, Rabinovitch PS, Reid BJ. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett's oesophagus: a prospective study. The lancet oncology. 2005;6:945–952. doi: 10.1016/S1470-2045(05)70431-9. [DOI] [PubMed] [Google Scholar]
- 20.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Annals of oncology. 2012;23:1403–1415. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Zhang XQ, Ding XW, Yang RK, Huang SL, Kastelein F, Bruno M, Yu XJ, Zhou D, Zou XP. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett's esophagus: a meta-analysis. Br J Cancer. 2014;110:2378–88. doi: 10.1038/bjc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantor ED, Onstad L, Blount PL, Reid BJ, Vaughan TL. Use of statin medications and risk of esophageal adenocarcinoma in persons with Barrett's esophagus. Cancer Epidemiology Biomarkers & Prevention. 2012;21:456–461. doi: 10.1158/1055-9965.EPI-11-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastelein F, Spaander MC, Biermann K, Steyerberg EW, Kuipers EJ, Bruno MJ. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett's esophagus. Gastroenterology. 2011;141:2000–2008. doi: 10.1053/j.gastro.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 24.Beales IL, Vardi I, Dearman L. Regular statin and aspirin use in patients with Barrett's oesophagus is associated with a reduced incidence of oesophageal adenocarcinoma. European journal of gastroenterology & hepatology. 2012;24:917–923. doi: 10.1097/MEG.0b013e3283543f01. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen DM, Richardson P, El–Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett's esophagus. Gastroenterology. 2010;138:2260–2266. doi: 10.1053/j.gastro.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett's esophagus: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology. 2013;11:620–629. doi: 10.1016/j.cgh.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Serag HB, Aguirre TV, Davis S, Kuebeler M, Bhattacharyya A, Sampliner RE. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett's esophagus. The American journal of gastroenterology. 2004;99:1877–1883. doi: 10.1111/j.1572-0241.2004.30228.x. [DOI] [PubMed] [Google Scholar]
- 28.Hillman LC, Chiragakis L, Shadbolt B, Kaye GL, Clarke AC. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett's oesophagus. Medical journal of Australia. 2004;180:387–391. doi: 10.5694/j.1326-5377.2004.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald R. Barrett's oesophagus and oesophageal adenocarcinoma: how does acid interfere with cell proliferation and differentiation? Gut. 2005;54:i21–i26. doi: 10.1136/gut.2004.041558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastelein F, Spaander MC, Steyerberg EW, Biermann K, Valkhoff VE, Kuipers EJ, Bruno MJ. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett's esophagus. Clinical Gastroenterology and Hepatology. 2013;11:382–388. doi: 10.1016/j.cgh.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229–37. doi: 10.1136/gutjnl-2013-305997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ: British Medical Journal. 1991;302:766. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y-X, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clinical Gastroenterology and Hepatology. 2005;3:587–594. doi: 10.1016/s1542-3565(05)00152-7. [DOI] [PubMed] [Google Scholar]
- 34.Iyer PG, Borah BJ, Heien HC, Das A, Cooper GS, Chak A. Association of Barrett's esophagus with type II diabetes mellitus: results from a large population-based case-control study. Clinical Gastroenterology and Hepatology. 2013;11:1108–1114. e5. doi: 10.1016/j.cgh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solaymani-Dodaran M, Logan R, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett's oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070–1074. doi: 10.1136/gut.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suissa S. Immortal time bias in pharmacoepidemiology. American journal of epidemiology. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 37.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiology and drug safety. 2007;16:241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 38.Hardikar S, Onstad L, Blount PL, Odze RD, Reid BJ, Vaughan TL. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett's esophagus. PloS one. 2013;8:e52192. doi: 10.1371/journal.pone.0052192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. The American journal of clinical nutrition. 2002;76:743–743. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut. 2013 doi: 10.1136/gutjnl-2013-305997. gutjnl-2013-305997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. Bmj. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes ZODIAC-16. Diabetes care. 2010;33:322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chak A, Buttar NS, Foster NR, Seisler DK, Marcon NE, Schoen R, Cruz-Correa MR, Falk GW, Sharma P, Hur C, Katzka DA, Rodriguez LM, Richmond E, Sharma AN, Smyrk TC, Mandrekar SJ, Limburg PJ. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2015;13:665–72. e1–4. doi: 10.1016/j.cgh.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelty CJ, Gough MD, Van Wyk Q, Stephenson TJ, Ackroyd R. Barrett's oesophagus: intestinal metaplasia is not essential for cancer risk. Scandinavian journal of gastroenterology. 2007;42:1271–1274. doi: 10.1080/00365520701420735. [DOI] [PubMed] [Google Scholar]
- 45.Hahn HP, Blount PL, Ayub K, Das KM, Souza R, Spechler S, Odze RD. Intestinal differentiation in metaplastic, non-goblet columnar epithelium in the esophagus. The American journal of surgical pathology. 2009;33:1006. doi: 10.1097/PAS.0b013e31819f57e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu W, Hahn H, Odze RD, Goyal RK. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell–containing epithelium. The American journal of gastroenterology. 2009;104:816–824. doi: 10.1038/ajg.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR, Dunagan KT, Lutzke LS, Wu T-T, Wang KK. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population-based study. The American journal of gastroenterology. 2011;106:1447–1455. doi: 10.1038/ajg.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.