Abstract
Cardiomyopathy is a frequent cause of death in patients with Friedreich ataxia (FA), and a characteristic pathological feature is the focal accumulation of iron (Fe) in cardiomyocytes. This restricted localization of the metal contrasts with the diffuse cardiac Fe overload in hemochromatosis and transfusion siderosis. Nevertheless, heart Fe in FA contributes to cardiomyocyte necrosis, inflammation, and scarring as the disease progresses. A putative mechanism of cardiomyopathy in FA is Fe-mediated oxidative damage. Two other transition metals zinc (Zn) and copper (Cu), are diffusely distributed throughout normal hearts and the hearts of patients with FA. The myocardium in FA is also prone to deposits of calcium in the form of scattered concretions. In this study, heart tissues (left and right ventricular walls and ventricular septum) of 23 patients with genetically confirmed FA and 8 normal controls were obtained at autopsy and analyzed for Fe, Zn, Cu, and calcium. The principal assay methods were inductively coupled plasma optical emission spectrometry and plasma mass spectrometry. Total levels of Fe in bulk extracts were not significantly higher than normal, and the concentrations of Zn also remained in the normal range. Cu levels, however, were significantly lower in FA. In conclusion, the decrease of Cu may be important in consideration of the potential benefit of Cu supplements in FA cardiomyopathy.
The mutation in Friedreich ataxia (FA), a pathogenic homozygous guanine-adenine-adenine trinucleotide repeat expansion, leads to a systemic deficiency of frataxin. This small mitochondrial protein is essential for the biogenesis and delivery of iron (Fe)-sulfur clusters, whereas other roles, such as Fe storage and detoxification, are less well defined. FA affects central and peripheral nervous systems, dorsal root ganglia, heart, skeleton, and the endocrine pancreas of children, adolescents, and adults. The most common cause of death in FA is cardiomyopathy, and in some patients, heart disease precedes the neurological manifestations.1,2 Frataxin levels in the left ventricular wall (LVW) of FA hearts are at or below the detection limit of an enzyme-linked immunosorbent assay (<15 ng/g wet weight), whereas those in control LVW samples have an average of 235.4 ± 75.1 ng/g wet weight (mean ± SD).3 The description of Fe excess in cardiomyocytes of FA hearts4 antedates the discovery of the mutation.5 Chemical assays of total Fe in bulk extracts of FA hearts do not reveal a net increase,1,3 but in situ quantification by X-ray fluorescence shows elevated levels in highly localized, randomly distributed, regions of the heart. In contrast, zinc (Zn) remains normal.3,6 The piecemeal Fe excess in the myocardium of patients with FA is unlike the diffuse distribution in Fe overload diseases, such as primary hereditary hemochromatosis7,8 and transfusion siderosis.9 In the present study, Fe, Zn, copper (Cu), and calcium (Ca) were measured in an autopsy series of FA hearts to identify a potential impact of these metals on the pathogenesis of FA cardiomyopathy. Surprisingly, this analysis discovered cardiac Cu deficiency.
Methods
The Institutional Review Board of Veterans Affairs Medical Center (Albany, New York) approved this study. Frozen heart specimens were collected from 23 deceased patients (11 men and 12 women) with FA over a period of 10 years through a tissue donation program supported by Friedreich’s Ataxia Research Alliance (Downingtown, Pennsylvania). Ages of death were 10 to 69 (34.7 ± 15.4 [mean ± SD]). All patients had homozygous guanine-adenine-adenine trinucleotide repeat expansions. Twenty-two had heart failure or arrhythmia during life. Microscopic examination showed cardiomyocyte hypertrophy, endomysial fibrosis, and Fe-positive inclusions in all patients. Eight frozen heart samples from subjects without heart disease (3 men, 5 women; age of death 49.9 ± 11.8 years [mean ± SD]) were obtained from National Disease Research Interchange (Philadelphia, Pennsylvania) for use as a control group. Hearts were thawed under clean room conditions, and samples were dissected from LVW, right ventricular wall (RVW), and ventricular septum (VS) by stainless steel instruments. For one FA heart, only the LVW was available for analysis. Formalin-fixed, paraffin-embedded, tissue samples of the 23 FA hearts were also available. Sections were stained by hematoxylin and eosin, and by Perls’s Fe stain.
All elemental determinations in heart tissue were performed in the Laboratory of Inorganic and Nuclear Chemistry at the New York State Department of Health (Wadsworth Center), Albany, New York. Frozen tissue samples were thawed, weighed, and freeze-dried to constant weight before being digested in concentrated nitric acid with microwave-assisted heating (MARS5; CEM Corporation, Matthews, North Carolina). Elemental measurements were obtained using inductively coupled plasma optical emission spectrometry (ICP-OES; Perkin Elmer Optima 5300 DV; Shelton, Connecticut). The samples were analyzed a second time using single quadrupole inductively coupled plasma mass spectrometry (ICP-MS; Perkin Elmer DRC II; and NexION 300D, Shelton, Connecticut). The detection limit for Cu by ICP-OES (4 ng/g) was insufficient for this determination. Therefore, all Cu data were based on ICP-MS measurements. All metal levels were expressed as μg/g wet weight to compare with other data in the studies.
Tissue concentrations of each metal were analyzed separately by repeated measures analysis of variance with a between-group effect of disease (2 levels, FA and normal), a within-patient myocardial location effect (3 levels, LVW, RVW, VS), and their interaction. Multiple comparisons were by Tukey’s test. There was no evidence that Fe, Zn, or Cu distributions were other than normal (Anderson–Darling test) nor was there any indication of heteroscedasticity (all SD were well within an order of magnitude). For Ca, the patients with FA demonstrated non-normal distributions in all 3 myocardial locations (p <0.01; Anderson–Darling test) due to pronounced upward skew. The skew could not be corrected by transformation, and a Mann–Whitney test was therefore used for each of the 3 myocardial locations separately. Statistical software included JMP (version 10.0 General Linear Model; SAS Statistical Software, Cary, North Carolina) and Minitab (version 17, Mann–Whitney tests; Minitab, Inc., State College, Pennsylvania).
Results
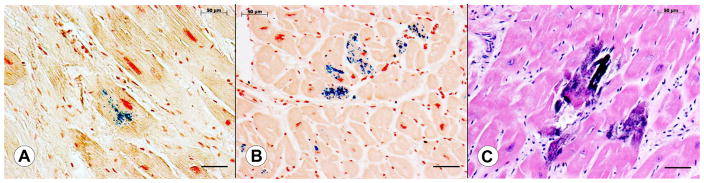
Figure 1 shows the appearance of Fe in sections of LVW from 2 different patients with FA and Ca in a third patient with FA. Accumulation of Fe ranges from small granules in a single cardiomyocyte (Figure 1) to involvement of several adjacent fibers (Figure 1). In addition to abnormal Fe, Figure 1 also displays the characteristic fibrosis of the heart in FA cardiomyopathy. The section stained by hematoxylin and eosin (Figure 1) reveals intensely basophilic material that may be interpreted as calcification. These concretions are also Fe-positive.
Figure 1.
Fe and Ca accumulation in cardiomyocytes in FA. (A to B) Perls’s Fe stain generates intense blue reaction product. The sections were counter-stained by Brazilin. The Fe reaction ranges from a few minute granules in a single fiber (A) to granules in many adjacent cardiomyocytes (B). (C) Hematoxylin and eosin stain. Several fibers in the LVW of a 10-year-old boy with severe FA cardiomyopathy show intensely basophilic Ca deposits. Bars, 50 μm.
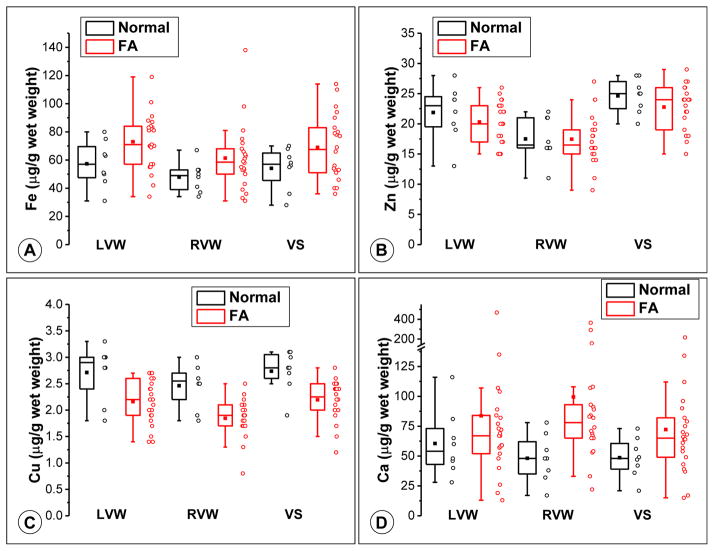
Results for Fe, Cu, Zn, and Ca in FA and control hearts are displayed in Tukey box-and-whisker plots (Figure 2). Pooled Fe levels in normal controls were 53.1 ± 14.6 μg/g (mean ± SD) compared with 71.3 ± 20.6 μg/g (mean ± SD) in FA. The higher levels in patients with FA did not achieve statistical significance (main effect of group; p = 0.064). There was a significant effect, however, of location in FA (main effect of location; p = 0.001) with RVW Fe levels significantly lower than LVW and VS, which did not differ from each other (Figure 2). There was no indication that disease had differing effects in the 3 locations (interaction effect; p = 0.94; Figure 2).
Figure 2.
Tukey box plots of Fe (A), Zn (B), Cu (C), and Ca (D) concentrations are shown together with the raw data points to the right of each box. In the box plots, the central rectangles span the first quartile to the third quartile (the IQR), and the lines within the rectangles mark the medians. The square symbols mark the means. The “whiskers” above and below the boxes are drawn to the furthest points within 1.5 × IQR from the boxes (the nonoutlier ranges). See text for the statistical analysis of the observations.
Pooled Zn levels were not significantly different, averaging 21.5 ± 2.9 μg/g (mean ± SD) in FA and 23.1 ± 3.4 μg/g (mean ± SD) in normal hearts (Figure 2), indicating that the disease had no effect on the levels of this metal (group effect; p = 0.36). There was, however, a significant effect of location (p <0.001; Figure 2): Zn was highest in VS, lowest in RVW, and all locations were significantly different from one another. Considering patients with FA only, RVW Zn was significantly lower than LVW Zn (p = 0.03) and VS (p <0.001), but LVW and VS were not different from one another (p = 0.06). There was no indication that disease had differing effects in the 3 locations (interaction effect, p = 0.52).
For Cu, there was a statistically significant effect of disease (p <0.001) and location (p <0.001) without an interaction effect (p = 0.90). Cu levels across the 3 locations in normal subjects were 2.72 ± 0.42 μg/g (mean ± SD) compared with 2.18 ± 0.40 μg/g (mean ± SD) in patients with FA. In FA and controls, overall LVW and VS Cu levels were not different, but RVW Cu was significantly lower than both LVW Cu and VS Cu (Figure 2). Considering patients with FA only, RVW Cu was significantly lower than LVW Cu (p = 0.01) and VS (p = 0.003), but LVW and VS were not different from one another (p = 0.99). There was no significant interaction effect of disease and location on Cu, indicating that the effect of disease was not different in the 3 myocardial locations.
Ca demonstrated upwardly skewed levels in patients with FA because of several very high outliers (Figure 2). Mann–Whitney tests applied to the samples from the 3 regions separately showed no difference in LVW (p = 0.38; median: 54.0 μg/g, interquartile range [IQR]: 41.5 to 77.0 μg/g in normal subjects; and median: 67 μg/g, IQR: 52.0 to 84.0 μg/g in FA) or VS (p = 0.08; 48 μg/g, IQR: 37.5 to 62.8 μg/g in normal subjects; and median: 65 μg/g, IQR: 47.5 to 84 in FA). RVW Ca was significantly higher in FA (p = 0.008; median: 48 μg/g, IQR: 33.5 to 65.5 μg/g in normal subjects; and 78 μg/g, IQR: 65 to 96.5 μg/g in FA).
Discussion
Levels of Fe, Zn, Cu, and Ca in normal myocardium determined here are similar to previously reported results (in μg/g wet weight)10–17 although the cited investigators did not separately assay metal concentrations in LVW, RVW, and VS. Ca in normal heart is known to be highly variable,10,13 ranging from 24 to 96 μg/g10 or 24 to 265 μg/g,13 which is similar to our normal control specimens (Figure 2).
The present study confirms previous conclusions that total heart Fe and Zn levels in FA do not increase significantly compared with control hearts.3 The loss of Cu from all 3 anatomical sites in FA, however, is a new observation and is further analyzed in the following section. A plausible explanation for the wide range of Ca in FA hearts (Figure 2) is random dystrophic calcification of heart fibers in cases of necrotizing cardiomyopathy (Figure 1). The elevated Ca concentration in RVW remains unexplained.
As illustrated in Figure 1, Fe accumulation in FA hearts affects a few fibers at a time. Koeppen et al3 presented evidence that Fe in FA cardiomyopathy is part of an inflammatory process and diffuse Fe toxicity in the pathogenesis of FA-related heart disease may be excluded. Although RVW does not fully escape damage in FA, the significantly lower Fe levels (Figure 2) may be related to the more limited damage in comparison with LVW and VS, or, as an alternate explanation, differential vulnerability.
The more limited lesion of RVW resembles that in the right and left atria of patients with FA. The atria show only a minor excess of connective tissue between working fibers, and Fe-positive inclusions are few. It may be suggested that greater wall stress in the left ventricle and incorrectly assembled intercalated discs18 combine to elicit a more destructive process in LVW and VS.
It is unknown whether low Cu levels contribute to the pathogenesis of FA cardiomyopathy or are simply due to depletion as heart disease evolves. Cu deficiency has been connected with experimental heart disease in animals and hereditary human cardiomyopathy for many years,19–23 but it is unlikely that the significant Cu deficiency in all heart regions in patients with FA causes a deficit in mitochondrial energy production. Cu is still present in vast excess over what is required for the metalation of mitochondrial Cu,Zn-superoxide dismutase and cytochrome c oxidase.24 In heart biopsies of patients with FA, the activity of the Cu-dependent mitochondrial complex IV (cytochrome C oxidase) was normal.25
Restoration of frataxin in heart and other tissues of patients with FA is an obvious goal, and recent successful gene therapy of a mouse model of FA is promising.26,27 Fe chelation therapy must consider that FA cardiomyopathy is not due to diffuse Fe overload, and the benefit of Fe removal from a few fibers (Figure 1) may not be clinically apparent over relatively short trial periods. In a 6-month-long tightly controlled trial with deferiprone in 72 patients with FA, the oral Fe chelator had no convincing beneficial effect on the neurological disease.28 Among cardiac markers, however, the left ventricular mass index declined sharply at 2 dose levels (20 and 40 mg/kg/day, respectively). Therapy with Fe chelators must also consider the affinity of the drugs for other metals and potential loss of Cu from the heart in FA.
Liebes and Medeiros15 compared Cu levels in non-cardiomyopathic and cardiomyopathic human hearts and concluded that mean levels of 2.21 ± 0.42 μg/g wet weight (mean ± SD, n = 14) in the former and 2.09 ± 0.53 μg/g wet weight (mean ± SD, n = 19) in the latter were not significantly different. Although Cu values for control and FA hearts reported here show a comparable difference of their means (2.73 μg/g and 2.18 μg/g, respectively), our statistical analysis yielded significance at p <0.001. In addition, in the compilation of 57 elements in 59 normal human hearts, Iyengar et al11 reported a mean Cu level of 4 μg/g wet weight and a range of 1.9 to 4.4 μg/g. These levels lend further support to the conclusion that heart Cu in FA is low. Although it remains unclear whether the heart is damaged by lack of Cu, the evidence gained from animal models suggests that Cu supplementation may be beneficial.19,20
Cu homeostasis in mammals is as complex as Fe homeostasis, and many proteins rely on Cu for proper function. Cu deficiency in humans is generally nutritional, and therapeutic oral Zn intake, such as for the treatment of Wilson’s disease, effectively reduces Cu uptake from the diet. The reports contain no information on suboptimal intestinal Cu absorption in FA, but lack of dietary Cu appears less likely than failure of the FA heart to retain or import the metal. Nevertheless, clinicians may want to determine serum Cu levels, and if low in FA, prescribe Cu supplements. They should also advise their patients not to take Zn-containing nutritional preparations.
Acknowledgments
Dr. Koeppen received grants from Friedreich’s Ataxia Research Alliance, R01NS069454 from National Institutes of Health, and Neurochemical Research, Inc.
National Disease Research Interchange receives financial support from National Institutes of Health (2 U42 OD011158).
The authors gratefully acknowledge the families who permitted the use of autopsy tissues for research.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Michael S, Petrocine SV, Qian J, Lamarche JB, Knutson MD, Garrick MD, Koeppen AH. Iron and iron-responsive proteins in the cardiomyopathy of Friedreich’s ataxia. Cerebellum. 2006;5:257–267. doi: 10.1080/14734220600913246. [DOI] [PubMed] [Google Scholar]
- 2.Koeppen AH. Friedreich’s ataxia: pathology, pathogenesis, and molecular genetics. J Neurol Sci. 2011;303:1–12. doi: 10.1016/j.jns.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koeppen AH, Ramirez RL, Becker AB, Bjork ST, Levi S, Santambrogio P, Parsons PJ, Kruger PC, Yang KX, Feustel PJ, Mazurkiewicz JE. The pathogenesis of cardiomyopathy in Friedreich ataxia. PLoS One. 2015;10:e0116396. doi: 10.1371/journal.pone.0116396. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamarche JB, Côté M, Lemieux B. The cardiomyopathy of Friedreich’s ataxia morphological observations in 3 cases. Can J Neurol Sci. 1980;7:389–396. doi: 10.1017/s0317167100022927. [DOI] [PubMed] [Google Scholar]
- 5.Campuzano V, Montermini L, Moltò MD, Pianese L, Cossée M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Cañizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez RL, Qian J, Santambrogio P, Levi S, Koeppen AH. Relation of cytosolic iron excess to cardiomyopathy of Friedreich’s ataxia. Am J Cardiol. 2012;110:1820–1827. doi: 10.1016/j.amjcard.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keschner HW. The heart in hemochromatosis. South Med J. 1951;44:927–931. doi: 10.1097/00007611-195110000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Olson LJ, Edwards WD, McCall JT, Ilstrup DM, Gersh BJ. Cardiac iron deposition in idiopathic hemochromatosis: histologic and analytic assessment of 14 hearts from autopsy. J Am Coll Cardiol. 1987;10:1239–1243. doi: 10.1016/s0735-1097(87)80124-9. [DOI] [PubMed] [Google Scholar]
- 9.Buja LM, Roberts WC. Iron in the heart: etiology and clinical significance. Am J Med. 1971;51:209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 10.Wester PO. Concentration of 24 trace elements in human heart tissue determined by neutron activation analysis. Scand J Clin Lab Invest. 1965;17:357–370. doi: 10.3109/00365516509077063. [DOI] [PubMed] [Google Scholar]
- 11.Iyengar GV, Kollmer WE, Bowen HJM. The Elemental Composition of Human Tissues and Body Fluids: A Compilation of Values for Adults. Weinheim and New York: Verlag Chemie; 1978. pp. 55–58. [Google Scholar]
- 12.Chipperfield B, Chipperfield JR. Differences in metal content of the heart muscle in death from ischemic heart disease. Am Heart J. 1978;95:732–737. doi: 10.1016/0002-8703(78)90503-3. [DOI] [PubMed] [Google Scholar]
- 13.Julshamn K, Andersen KJ, Svendsen E, Ringdal O, Egholm M. Trace elements intake in the Faroe Islands III. Element concentrations in human organs in populations from Bergen (Norway) and the Faroe Islands. Sci Total Environ. 1989;84:25–33. doi: 10.1016/0048-9697(89)90367-7. [DOI] [PubMed] [Google Scholar]
- 14.Saltzman BE, Gross SB, Yeager DW, Meiners BG, Gartside PS. Total body burdens and tissue concentrations of lead, cadmium, copper, zinc, and ash in 55 human cadavers. Environ Res. 1990;52:126–145. doi: 10.1016/s0013-9351(05)80248-8. [DOI] [PubMed] [Google Scholar]
- 15.Liebes R, Medeiros DM. Decreased nuclear encoded subunits of cytochrome c oxidase and increased copper, zinc-superoxide dismutase activity are found in cardiomyopathic human hearts. Int J Cardiol. 1997;62:259–267. doi: 10.1016/s0167-5273(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 16.Frustaci A, Magnavita N, Chimenti C, Caldarulo M, Sabbioni E, Pietra R, Cellini C, Possati GF, Maseri A. Marked elevation of myocardial trace elements in idiopathic dilated cardiomyopathy compared with secondary cardiac dysfunction. J Am Coll Cardiol. 1999;33:1578–1583. doi: 10.1016/s0735-1097(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 17.Rahil-Khazen R, Bolann BJ, Myking A, Ulvik RJ. Multi-element analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES) J Trace Elem Med Biol. 2002;16:15–25. doi: 10.1016/S0946-672X(02)80004-9. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez RL, Becker AB, Mazurkiewicz JE, Feustel PJ, Gelman BB, Koeppen AH. Pathology of intercalated discs in Friedreich cardiomyopathy. J Am Coll Cardiol. 2015;66:1739–1740. doi: 10.1016/j.jacc.2015.06.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klevay LM. Cardiovascular disease from copper deficiency-a history. J Nutr. 2000;130:489S–492S. doi: 10.1093/jn/130.2.489S. [DOI] [PubMed] [Google Scholar]
- 20.Zheng L, Han P, Liu J, Li R, Yin W, Wang T, Zhang W, Kang YJ. Role of copper in regression of cardiac hypertrophy. Pharmacol Ther. 2015;148:66–84. doi: 10.1016/j.pharmthera.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Jaksch M, Ogilvie I, Yao J, Kortenhaus G, Bresser H-G, Gerbitz K-D, Shoubridge EA. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum Mol Genet. 2000;9:795–801. doi: 10.1093/hmg/9.5.795. [DOI] [PubMed] [Google Scholar]
- 22.Kim B-E, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab. 2010;11:353–363. doi: 10.1016/j.cmet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papadopoulou LC, Sue CM, Davidson MM, Tanji K, Nishino I, Sadlock JE, Krishna S, Walker W, Selby J, Glerum DM, Van Coster R, Lyon G, Scalais E, Lebel R, Kaplan P, Shanske S, De Vivo DC, Bonilla E, Hirano M, DiMauro S, Schon EA. Fatal infantile cardioencephalopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999;23:333–337. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- 24.Leary SC, Winge DR, Cobine PA. “Pulling the plug” on cellular copper: the role of mitochondria in copper export. Biochim Biophys Acta. 2009;1793:146–153. doi: 10.1016/j.bbamcr.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rötig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Gen. 1997;17:215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- 26.Perdomini M, Belbellaa B, Monassier L, Reutenauer L, Messaddeq N, Cartier N, Crystal RG, Aubourg P, Puccio H. Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich’s ataxia. Nat Med. 2014;20:542–547. doi: 10.1038/nm.3510. [DOI] [PubMed] [Google Scholar]
- 27.Gérard C, Xiao X, Filali M, Coulombe Z, Arsenault M, Couet J, Li J, Drolet M-C, Chapdelaine P, Chikh A, Tremblay JP. An AAV9 coding for frataxin clearly improved the symptoms and prolonged the life of Friedreich ataxia mouse models. Mol Ther Meth Clin Dev. 2014;1:14044. doi: 10.1038/mtm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandolfo M, Arpa J, Delatycki MB, Le Quan Sang KH, Mariotti C, Munnich A, Sanz-Gallego I, Tai G, Tarnopolsky MA, Taroni F, Spino M, Tricta F. Deferiprone in Friedreich ataxia: a 6-month randomized controlled trial. Ann Neurol. 2014;76:509–521. doi: 10.1002/ana.24248. [DOI] [PubMed] [Google Scholar]