Abstract
Exosomes are small (~100 nm) membrane-bound extracellular vesicles released by various types of cells into biological fluids. They contain proteins, mRNAs and miRNAs as cargo. Different cell types can take up exosomes by endocytosis and the cargo contained within them can be transferred horizontally to these recipient cells. Exosomal proteins and miRNAs can be functional and regulate physiological cell events modifying the microenvironment in target cells, a key event of liver pathology. Exosome-mediated cell-cell communication can alter tumor growth, cell migration, anti-viral infection and hepatocyte regeneration, indicating that exosomes have great potential development as diagnostic or therapeutic tools. Analyses of circulating total or exosomal miRNAs have identified a large number of candidate miRNAs that are regulated in liver diseases, and the diagnostic testing using single or multiple miRNAs shows good sensitivity and specificity. Some candidate miRNAs have been identified to play an important role in various liver disorders. This review summarizes recent findings on the role of extracellular vesicles in liver diseases and their diagnostic and therapeutic potential, mainly focusing on exosomes but also includes microvesicles in liver pathology.
Keywords: exosomes, microvesicles, microRNAs, liver disease, cancer
Introduction
Exosomes are one of three types of extracellular vesicles (EVs) secreted in a wide variety of cells. EVs are spherical particles enclosed by a phospholipid bilayer. There are three types of vesicles according to their size: exosomes (40~100 nm), microvesicles or microparticles (0.1–1 μm) and apoptotic bodies (1–4 μm) [1]. Exosomes are derived from multivesicular bodies (MVBs), which are the type of endosomes containing membrane-bound vesicles. These vesicles form by budding into the lumen of the MVB, and some MVBs are degraded in lysosomes. Some MVBs, however, are fused with the plasma membrane releasing internal vesicles as exosomes via exocytosis. Microvesicles are released directly from plasma membrane of cells by outward blebbing without MVBs. EVs have been the topic of great interest in recent years in medical research. In particular, EVs are of great interest in liver pathology because they regulate cell-cell communication and a number of pathophysiological events in various types of cells via horizontal transfer of their cargo [2]. EVs contain a variety of cargoes including proteins, mRNAs and microRNAs (miRNAs) and they can be transferred from donor cells to recipient cells and can activate or regulate cell activities such as protein expression, cell proliferation and differentiation or antiviral responses.
miRNAs are small non-coding RNAs which regulate gene expression by binding to target mRNAs and interfering with their translation (Figure 1). Therefore, cargoes of EVs especially miRNAs have attracted research interests in recent years. It is important to study EVs and their functions to understand mechanisms of liver disorders. More importantly, EVs can be used as specific biomarkers to diagnose liver diseases. This review summarizes latest liver cell derived-EV research mainly focusing on exosomes but also includes microvesicles in liver pathology.
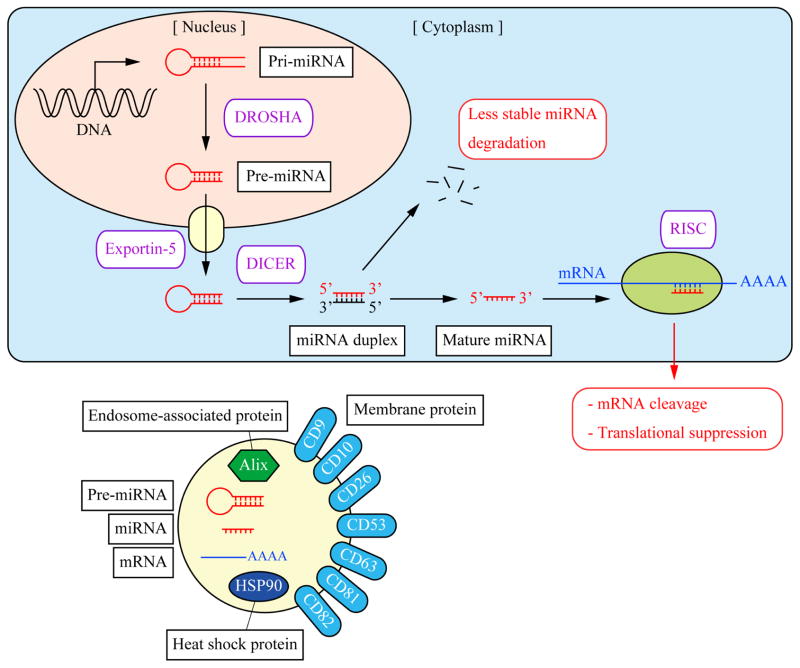
Figure 1. miRNA synthesis and contents of exosomes.
The pathway of miRNA synthesis and its function (top). In the nucleus, miRNAs are transcribed from DNA forming a primary transcript, pri-miRNA. The RNase III enzyme DROSHA cleaves pri-miRNA to produce a precursor, pre-miRNA that is transported to the cytoplasm through a nuclear export protein Exportin-5. In the cytoplasm, pre-miRNAs are cleaved by DICER to form a miRNA duplex. In general, only one strand of the miRNA duplex is used as the mature miRNA while the other strand is degraded because of less stability. Mature miRNA binds to the 3′UTR of target mRNA and is loaded into RNA-induced silencing complex (RISC). As a result, this complex inhibits the process of mRNA translation or enhances mRNA degradation leading to translational suppression.
Contents of exosomes (bottom). Typical exosomes contain various cargoes including pre-miRNAs, mature miRNAs, and mRNAs as well as membrane marker proteins, endosome-associated (MVB-derived) proteins, and heat shock proteins (chaperones).
Circulating EVs as biomarkers of liver diseases
Searching for a novel biomarker of liver diseases has been a challenge for researchers. The isolation of EVs, however, may be a potential approach due to the unique molecules such as proteins and miRNAs could be transported only in exosomes or microvesicles. Sugimachi et al has compared expression profile of miRNAs in serum exosomes between patients with and without hepatocellular carcinoma (HCC) recurrence after liver transplantation and found that miR-718 is significantly decreased in exosomes of patients with HCC recurrence [3]. A study comparing 30 patients with HCC and 30 patients with chronic hepatitis B virus (HBV) infection has demonstrated that serum exosomes from HCC patients contain a higher level of miR-21 [4]. Another study recruiting 20 HCC patients and 20 HBV patients has reported that miR-18a, -221, -222 and -224 are increased and miR-101, -106b, -122 and -195 are decreased in serum exosomes from HCC patients although no significant difference was observed for miR-21 [5]. Microvesicles may also have a potential for a novel biomarker. A study comparing patients with chronic hepatitis C virus (HCV) infection with healthy individuals has shown that patients with HCV secrete increased levels of microvesicles derived from CD4+ and CD8+ T cells [6]. Although further studies are required to identify a unique biomarker that is specific in the certain liver disorder, secreted EVs in positive patients may be a good candidate for identification or diagnosis for liver diseases.
Exosomal alteration in liver diseases
Drug-induced liver injury
As EVs can be used as a novel biomarker, several groups have performed profiling exosomal cargoes secreted in liver diseases. A recent study has analyzed exosomal protein levels in urine of rats with drug-induced liver injury (DILI) and reported that exosomes in DILI rat urine contain reduced exosomal proteins, including CD26 and CD81 and candidate marker proteins compared with exosomes in control rat urine [7]. Another study has demonstrated, however, that exosomes isolated from blood serum of rats with DILI contain higher expression levels of exosomal proteins, including HSP70 and HSP90 and other candidate marker proteins although Alix, endosome-associated protein is decreased compared with exosomes from healthy rats [8]. This study also demonstrated that protein expression profiles differ between liver extracts and circulating exosomes isolated from blood serum. These observations indicate that a biomarker for liver diseases could be identified only in exosomal cargoes isolated from specific body fluids and may not be identified in liver tissue or serum. In fact, another study has shown that total miRNA concentrations are increased in plasma of porcine models of DILI compared with control pigs but this enhancement is not observed in isolated exosomes from plasma [9].
Alcoholic and non-alcoholic fatty liver disease
Alcohol-fed mice were found to secrete significantly increased amounts of exosomes in the blood, and miRNA array has shown increased levels of candidate miRNAs in the exosomes of alcohol-fed mice [10]. Furthermore, previous studies have demonstrated that human monocytes, precursors of macrophages, release significant amounts of exosomes when exposed to ethanol, and these exosomes can stimulate naïve monocytes to polarize and differentiate into M2-macrophages. Monocytes exposed to alcohol also secrete exosomes containing increased levels of miR-27a leading to increased cytokine secretion, followed by activation and polarization of other monocytes [11, 12]. Alcohol treatment also induces EV release from hepatocytes, which is associated with the activated caspase-3. Additionally alcohol-induced EVs contain CD40 ligand that promotes macrophage activation followed by inflammation in alcoholic liver disease [13].
Patients with non-alcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH) secrete increased levels of microvesicles derived from macrophages and natural killer T cells [6]. Another EV study has shown that the expression levels of various proteins within vesicles are enhanced in a mouse model of NAFLD, and that protein expression pattern differs between exosomes and microvesicles [14]. Another study has demonstrated that lipids induce EV release from primary hepatocytes and the Huh7 cells (well differentiated hepatocyte derived cellular carcinoma cell lines) and those secreted EVs activate an inflammatory response in macrophages leading to NASH [15]. These findings suggest that cells under disease conditions may secrete elevated amounts of EVs containing unique cargoes, and this could be a trigger for various pathophysiological events.
Cell-cell communication via exosomes
There is strong evidence that EVs can play an important role in cell-cell communication by transferring proteins and miRNAs from the source to the target cells (Figure 2). Such communication allows a cell to influence neighboring cells and alter the liver’s microenvironment. A study has also demonstrated that exosomes secreted from mouse and human mast cell lines can be taken up by other mast cells, and their cargos, mRNAs and miRNAs are still functional in recipient cells in vitro, which means that transferred exosomal mRNAs can be translated and the new proteins could regulate physiological events in recipient cells [16].
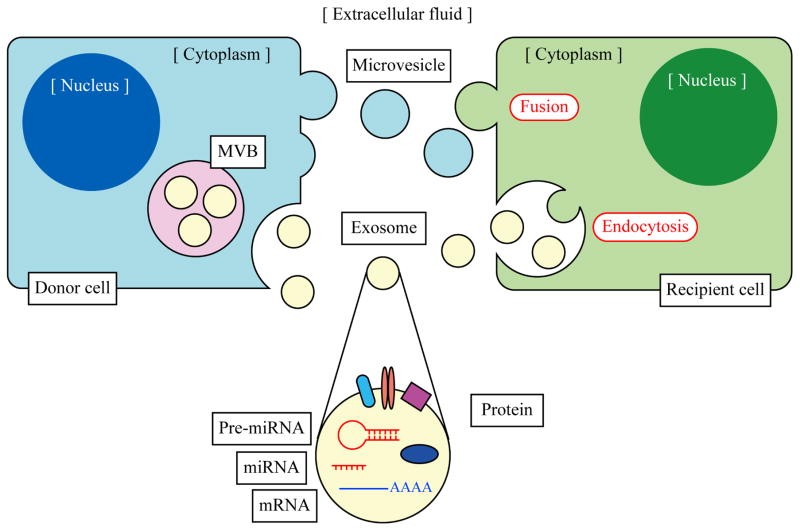
Figure 2. Cell-cell communication via exosomes and microvesicles.
Exosomes are incorporated into vesicles in MVBs by budding into their lumen. MVBs are fused with the plasma membrane releasing internal exosomes to outside the cell by exocytosis. Microvesicles are formed and released directly from the plasma membrane by outward blebbing. These EVs are taken up by the other cell by fusion with the plasma membrane or endocytosis and EV cargoes are transferred horizontally from the donor cell to the recipient cell. EVs can contain various molecules including mRNAs, miRNAs, pre-miRNAs and proteins. These EV cargoes regulate physiological cell events in the recipient cell. Some EVs are circulating through the body in extracellular fluid and their cargoes can be useful as a biomarker to diagnose liver diseases.
Liver cancer
Recent studies have revealed that cancer cells can modulate surrounding hepatic environments to aid their growth, proliferation and invasion. Exosomes containing mRNAs, miRNAs and natural antisense RNAs secreted by colorectal cancer cells can be taken up by other cancer cells such as hepatoma and lung cancer cells in vitro [17]. Human HCC cells can secrete exosomes that contain a variety of miRNAs, which attenuate protein expression of the TGF-β pathway in other HCC cells. As a result, growth of HCC cells is increased dramatically with HCC cell-derived exosomes [18]. Another study using an immortalized non-tumorigenic hepatocyte cell line, MIHA has demonstrated that non-motile MIHA cells show significantly higher cell migration and invasion after incubated with exosomes isolated from HCC cells [19]. Similar results are also observed in studies with cholangiocarcinoma (CCA). A study using CCA cells derived from human patients were shown to secrete exosomes that increased migration and invasion of human cholangiocyte cell line H69 [20]. The exposure of mesenchymal stem cells to CCA cell-derived exosomes enhance their migratory capability and the release of cytokines such as CXCL1, CCL2 and IL-6 leading to cell proliferation [21]. These results suggest that malignant cells can co-operate with other cancer cells or even “educate” non-cancer cells for their cell growth, migration and invasion by modifying microenvironment by secreting exosomes.
Viral hepatitis
Exosomes and intercellular communication may also be involved in immune regulation and antiviral response during viral infection. It is known that virus-infected cells can secrete exosomes, which contain virus-derived miRNAs leading to suppression of target genes in recipient cells [22]. Exosomes isolated from HCV-infected patients’ serum contain HCV RNA and elevated levels of proteins Ago2 and HSP90 helping viral receptor-mediated transmission to hepatocytes [23]. Virus-infected cells release type I and III interferons (IFNs) that are a cytokine to stimulate immune system and antiviral responses [24]. Liver cells stimulated by type I IFN, IFN-α secrete exosomes which contain antiviral molecules and can attenuate hepatitis B virus (HBV) replication [25]. Exosomes isolated from liver endothelial cells stimulated by either type I or III IFNs can also suppress viral replication in HCV-infected liver cells [26]. These findings suggest that both viral infection and antiviral response are mediated by cell-cell communication through exosomes.
Exosomes as therapeutic tools for liver diseases
Exosomes can modulate microenvironment not only to support cancer cell proliferation or viral transmission, but also to facilitate cell regeneration and recovery to maintain homeostasis. Mesenchymal stem cells (MSCs) provide important approach for the therapy of liver disease. For example, direct injection of exosomes isolated from human MSCs into mouse liver alleviates carbon tetrachloride-induced liver fibrosis by suppressing collagen and TGF-β1 expression in vivo [27]. Induction of cellular senescence in activated hepatic stellate cells can reduce the secretion of extracellular matrix components and subsequently inhibit liver fibrosis. We have demonstrated that human MSC derived extracellular vesicles induced cellular senescence in cultured human hepatic stellate cells (Figure 3). Intrasplenic injection of MSC-derived exosomes can enhance protein expression including PCNA and cyclin D1 leading to liver recovery, and alleviate liver damage caused by carbon tetrachloride administration in mice [28]. Primary hepatocytes as well as MSCs can also enhance liver cell proliferation. Hepatocyte-derived exosomes induce hepatocyte proliferation in vitro while exosomes isolated from Kupffer cells or endothelial cells have no effect [29]. Furthermore, the cargo of hepatocyte-derived exosomes induces generation of sphingosine-1-phosphate leading to hepatocyte regeneration and liver repair after ischemia/reperfusion injury in vivo. Endothelial cell-derived exosomes can regulate hepatic stellate cell signaling and migration via sphingosine-1-phosphate [30]. These studies indicate that exosomes and their components are involved in mechanisms of liver cell regeneration and migration, and hence they can be a potential therapeutic tool for liver disease and damage.
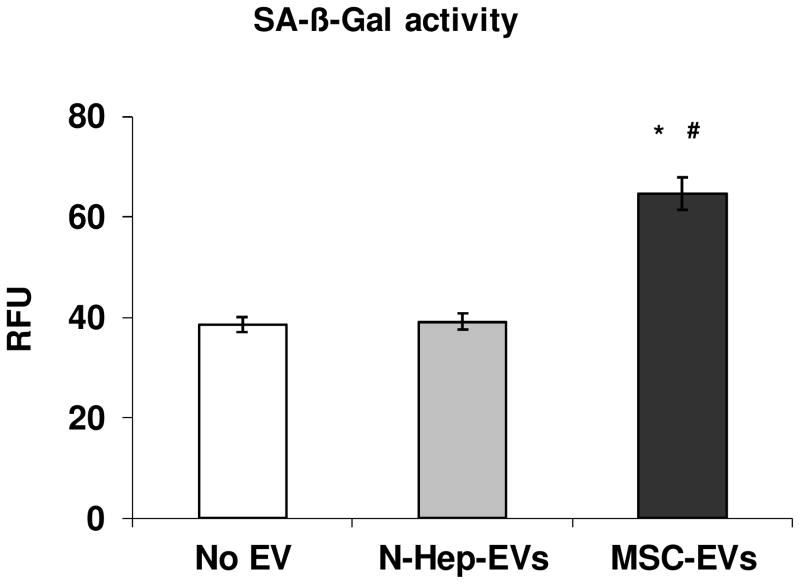
Figure 3. Enhanced Cellular Senescence in Cultured Human Hepatic Stellate Cells treated with MSC derived extracellular vesicles (EVs).
Human hepatic stellate cells (ScienCell Research Laboratories, Carlsbad, CA) were incubated with 30 μg/ml MSC derived EVs or normal human hepatocytes (N-Heps) derived EVs for 48 hours and compared to no EV treatment controls. Lysates were allowed to incubate with SA-β-Gal Substrate for 1 hr at 37°C. SA-β-Gal activities were measured using 96-Well Cellular Senescence Assay Kit from Cell Biolabs, Inc (San Diego, CA). Human MSCs and N-Heps were also purchased from ScienCell Research Laboratories. Significantly increased SA-β-Gal activities were observed in cultured human hepatic stellate cells incubated with MSC-EVs when compared to no EV or N-Heps-EVs controls. * p<0.05 relative to no EV group; # p<0.05 relative to N-Heps-EVs group.
Microvesicles may also be used as a therapeutic tool. Microvesicles derived from liver stem cells inhibit HCC cell growth and this is mediated by various miRNAs contained in those microvesicles [31]. An intravenous anesthetic propofol has antitumor effects, and it has been shown that mice with propofol administration secrete macrophage-derived microvesicles and those microvesicles and their cargo miR-142-3p significantly inhibits tumor growth in vivo [32]. In liver cancers such as HCC, malignant cells can not only modify microenvironment to help their proliferation and invasion, but also be controlled by other cells via cell-cell communication using exosomes and microvesicles (Figure 4).
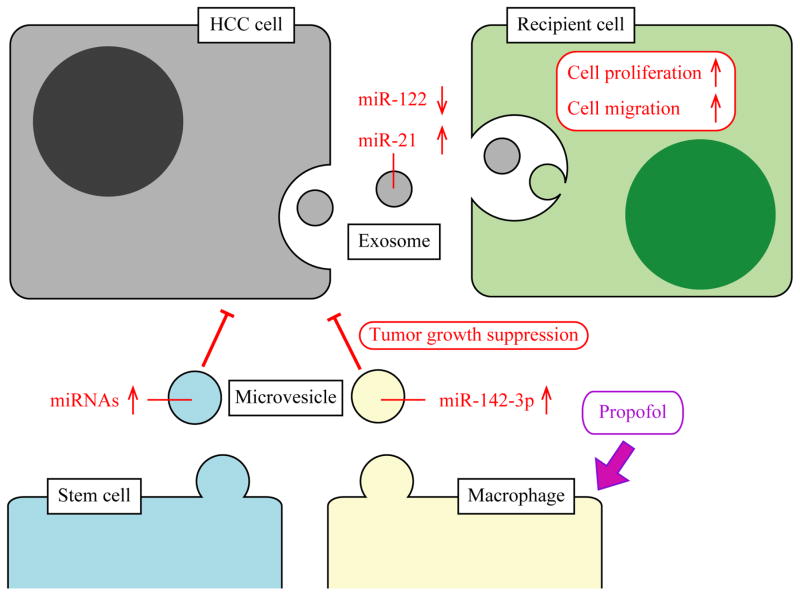
Figure 4. Exosomes and microvesicles in hepatocellular carcinoma.
HCC cells secrete exosomes that can be taken up by other HCC cells leading to enhancement of cell proliferation, migration and invasion in the recipient cell. Previous studies have shown that exosomes secreted from HCC cells contain decreased and increased levels of miR-122 and miR-21, respectively. Liver stem cells secrete microvesicles which contain elevated levels of various miRNAs. An anesthetic propofol induces macrophages to secrete microvesicles which contain elevated miR-142-3p. These microvesicles can inhibit HCC cell growth suggesting that microvesicles could be used as a therapeutic tool for liver cancer.
Diagnosis of liver disease using circulating proteins and miRNAs
Diagnosis of liver disease can be challenging, especially for chronic liver diseases, because of its progression through several stages: hepatitis → fibrosis → cirrhosis → HCC, and this can take 5–50 years to attain the advanced disease stages or the progression may be arrested in the early stage with no obvious symptoms. Diagnosis of HCC, for example, is generally based on imaging including ultrasound, MRI or CT scan, or elevated serum α-fetoprotein level although it can produce false diagnoses and it is not very feasible to discriminate the specific stages such as HCC from chronic HBV infection or cirrhosis. As early diagnosis of HCC can improve the survival rate, scientists have been making an effort to search a novel diagnostic method to discriminate liver disease stages, and circulating proteins or miRNAs have attracted great interests as a tool of diagnosis.
Table 1 shows selected diagnostic studies with circulating miRNAs performed for human patients with liver disease. These latest studies have usually focused on total circulating miRNAs including exosomes, microvesicles and free miRNAs. Some studies have isolated exosomes before miRNA analyses but only limited number of studies is available although exosomes are a main component of EVs circulating in the serum [10]. Majority of recent studies has used a miRNA panel with ~10 miRNAs for diagnostic analyses to increase sensitivity and specificity. Although single miRNA can be useful to discriminate HCC from healthy individuals with good sensitivity and specificity, miRNA panels may be more reliable to discriminate more sensitive cases such as HCC patients from non-HCC individuals who are infected by HBV or HCV and showing chronic liver disease or cirrhosis but not HCC (Table 1). A meta-analysis studying 13 publications has shown that a miRNA panel analyzing multiple miRNAs can produce better accuracy than single miRNA diagnosis for those cases [33].
Table 1.
Selected studies for diagnosis of liver disease using human circulating miRNAs
| Biomarker | Sample | Cases | Controls | Control condition | Sensitivity | Specificity | AUC | Regulation | [Ref.] |
|---|---|---|---|---|---|---|---|---|---|
| HCC | |||||||||
| miR-143 | Serum | 95 | 127 | Healthy | 73 | 83 | 0.795 | Up | [45] |
| miR-215 | Serum | 95 | 127 | Healthy | 80 | 91 | 0.816 | Up | [45] |
| miRNA panel1 | Serum | 112 | 42 | Healthy | 82 | 98 | 0.949 | Up/Down | [46] |
| miR-221 | Serum | 30 | 60 | Non-HCC | 87 | 40 | 0.655 | Up | [47] |
| miRNA panel2 | Serum | 27 | 135 | Non-HCC | 70 | 80 | 0.752 | Up | [48] |
| miR-101 | Serum | 67 | 61 | Cirrhosis | 96 | 90 | 0.976 | Down | [49] |
| miRNA panel3 | Serum | 103 | 78 | Cirrhosis | 82 | 85 | 0.892 | Up/Down | [50] |
| miRNA panel4 | Plasma | 67 | 82 | HBV | 86 | 64 | 0.802 | Up | [51] |
| CCA | |||||||||
| miRNA panel5 | Bile | 46 | 50 | Healthy | 67 | 96 | N/A | Up | [52] |
| miR-192 | Serum | 51 | 32 | Healthy | 74 | 72 | 0.803 | Up | [53] |
| miRNA panel6 | Urine | 22 | 21 | Healthy | 82 | 71 | 0.849 | Up | [54] |
| HBV | |||||||||
| miRNA panel7 | Plasma | 100 | 100 | Cirrhosis | 80 | 70 | 0.858 | Up | [55] |
| DILI | |||||||||
| miRNA panel8 | Plasma | 41 | 40 | Non-DILI | 90 | 90 | 0.96 | Up/Down | [56] |
Notes:
miR-19a, -195, -192, -146a;
miR-29a, -29c, -133a, -143, -145, -192, -505;
miR-122, -1228, -141-3p, -192, -199a, -206, -26a, -433-3p;
miR-20a, 25-3p, -30a, -92a-3p, -132-3p, -185, -320a, -324-3p;
miR-191, -486-3p, -1274b, -16, -484;
miR-192, -21;
miR-18a, -21, -29c-3p, -106b, -122, -185;
miR-382, -483-3p, -125a, -1290, -378i, -449b-3p, -151a-3p, -1915, -652, -27a-3p, -122, -151a, -151b, -885, -26a, -4783-3p, -2861
Strong candidate biomarkers for liver diseases
Exosomes contain diverse specific types of proteins/RNAs/miRNAs reflective of cell functions and conditions. The increasing number of exosomal proteins/RNAs/miRNAs have been found to be potential biomarkers for a variety of diseases including acute and chronic liver diseases. Studies for identification of candidate biomarkers in circulating total or exosomal miRNAs have reported a large number of miRNAs to date, but some miRNAs have been identified in several studies showing strong evidence that those miRNAs are involved in the specific liver disorders.
miR-122
miR-122 is the most frequently identified and well-studied miRNA in liver diseases. It is the major miRNA in liver, and decrease of exosomal miR-122 expression has been observed in HCC [5]. miR-122 can alleviate tumor growth and miR-122 knockdown upregulates HCC cell viability by inhibiting apoptosis in vitro suggesting that miR-122 may be a key for tumor proliferation and migration via exosomal intercommunication and hence could be a good therapeutic or diagnostic target for HCC. [34].
Whereas reduced levels of miR-122 are observed in HCC, they are, in contrast, elevated in alcoholic liver disease. In a rodent model of chronic alcohol feeding, elevated serum miR-122 levels were found to correlate with liver damage [35]. Alcohol drinking induces gut bacteria permeability and endotoxin (also known as lipopolysaccharide, LPS) elevation in blood. Hepatic cells exposed to alcohol secrete exosomes which contain elevated miR-122, and these exosomes are taken up by macrophages and sensitize them to LPS leading to enhanced cytokine secretion in vitro [36]. These current studies strongly support that this miRNA plays an important role in liver pathology but also suggest that even same miRNA can be expressed differently depending on liver diseases.
miR-21
miR-21 is another candidate exosomal biomarker identified in several liver disease studies. Previous studies indicate that miR-21 may have opposite functions to miR-122. In HCC, miR-122 expression is lower in serum or circulating exosomes of positive patients compared with healthy individuals, but miR-21, on the other hand, is higher in HCC patients [37]. Although miR-122 knockdown inhibits apoptosis and increases HCC cell viability in vitro [34], miR-21 knockdown enhances apoptosis and decreases cell viability [38]. A study using human CCA specimens has revealed elevated miR-21 expression in the CCA tissue compared with noncancerous biliary epithelium. This study has also demonstrated that overexpression of miR-21 promotes CCA growth by targeting the key enzyme for prostaglandin conversion, 15-hydroxyprostaglandin dehydrogenase (15-PGDH) [39]. These studies support the notion that miR-21 may be another strong candidate biomarker in liver pathology.
Other miRNAs
miR-192 is often identified from patients with liver diseases as well as miR-122 and miR-21. Although only limited number of studies is available to date and functions of miR-192 in liver pathology are largely unknown, a study has revealed that miR-192 suppresses metastasis of HCC cells in vivo and in vitro by targeting an oncogene involved in different types of cancer, SLC39A6 [40].
Also, miR-155 is one of miRNAs which are associated with regulations of inflammation and its expression levels are elevated in the serum and peripheral monocytes of patients with chronic HCV infection [41]. A study using miR-155 knockout mice has shown that administration of exosomes containing miR-155 mimic leads to miR-155 expression in isolated hepatocytes of recipient knockout mice indicating its cellular uptake [42]. Another study using miR-155 knockout mice has demonstrated that miR-155 deficiency attenuates hepatic steatosis and fibrosis, but not liver injury and inflammation induced by methionine-choline-deficient diet [43]. These earlier studies suggest that some candidate miRNAs may be useful to understand mechanisms and to seek a novel biomarker or treatment in liver pathology.
Conclusions and perspectives
Despite the progress in research for liver diseases to date, diagnosis of liver pathology remains a difficult task. Because methods such as imaging are not completely reliable, invasive liver biopsy continues to be a gold standard for diagnosis. Thus the development of novel, reliable and noninvasive diagnostic method has been of major interests. Recent research indicates that EVs and their cargoes may have a great potential as biomarkers for the diagnosis of liver pathology. Current studies suggest that circulating miRNAs and proteins may be a useful tool for diagnostic testing and in fact, especially miRNA panels show good sensitivity and specificity against various liver disorders. Figure 5 summarizes potential achievements in EV research. However, the current miRNA-based diagnoses have some limitations. First, researchers have a large number of options for EV studies: disorder, circulating miRNAs or local miRNAs in specific tissues, body fluid, and exosomes, microvesicles or total miRNAs and experimental conditions. Biomarkers could differ depending on liver diseases and even same miRNA may be expressed differently in a different disorder. miR-122 is increased in exosomes of alcohol-fed mice but decreased in exosomes of HCC patients. Chronic alcohol feeding increases miR-122 in exosomes but decreases in liver tissues. Exosomal cargoes may differ between serum exosomes and urine exosomes. Although exosomes constitute majority of extracellular vesicles, cargoes may differ between exosomes and microvesicles and specific biomarker could exist in specific EVs. Moreover, careful attention should be paid to experimental conditions involving EV research for reliable and reproducible results. The secretion levels of EVs and protein composition in their cargoes can vary depending on culture medium with or without serum in vitro [44]. Second, although numerous miRNAs have been identified in samples from liver diseases, their functions and roles in liver pathology are largely unknown. Functional analyses of exosomal cargoes may be important in understanding the mechanisms of liver pathology and/or to identify therapeutic targets. Identification of donor and recipient cells for cell-cell communication in the specific liver disease and underlying mechanisms also need to be further clarified.
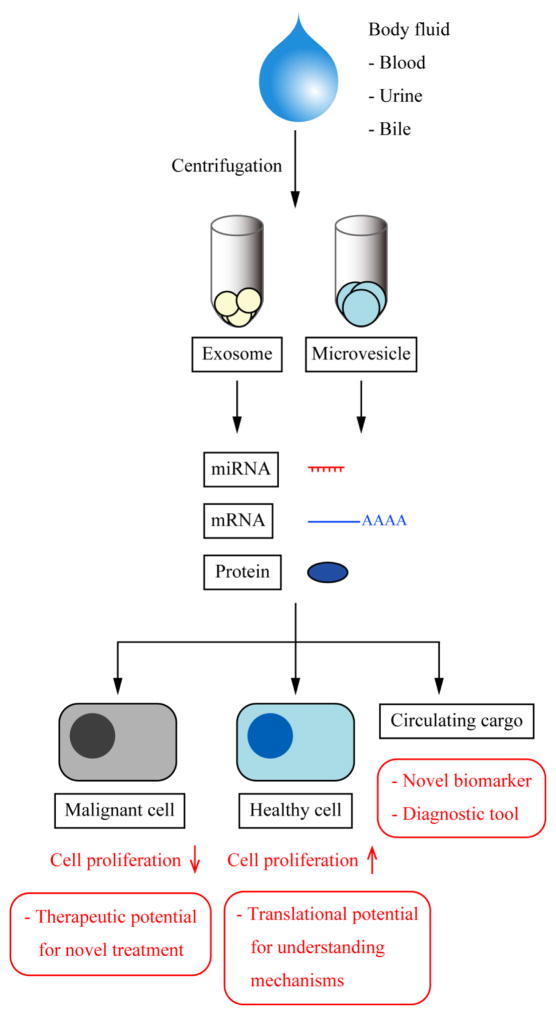
Figure 5. Pathological potentials in EV research.
EV research could contribute to liver pathology. Major EVs, exosomes and microvesicles can be isolated by centrifugation from various body fluids. Circulating EV cargoes could be analyzed as disease-specific biomarkers leading to a novel non-invasive diagnostic method. EVs can be taken up by other liver cells and modify microenvironment by enhancing cell proliferation and migration, and this could lead to better understanding of pathophysiology of liver disorders. EVs could also be used as a tumor suppressor suggesting that EVs have a therapeutic potential to treat liver cancer.
Key points.
Exosomes are small membrane-bound vesicles secreted by various types of cells
Exosomes contain proteins, mRNAs and miRNAs as their cargoes and can transfer them horizontally to the other type of cells
Exosomal cargo can be functional after being taken up by the recipient cell and regulate physiological events
Exosomal miRNAs are associated with liver pathology and have a great potential as a therapeutic target or as a molecular biomarker which may be useful for diagnostic testing
Acknowledgments
Statement of financial support: This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White, a VA Research Career Scientist Award, a VA Merit award to Drs. Alpini (5I01BX000574), Meng (1I01BX001724), Glaser (5I01BX002192) and NIH DK107310, DK076898, DK054811 and DK062975 grants to Drs. Alpini, Meng and Glaser. This manuscript is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Abbreviations
- CCA
cholangiocarcinoma
- DILI
drug-induced liver injury
- EV
extracellular vesicle
- MVBs
multivesicular bodies
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IFN
interferon
- LPS
lipopolysaccharide
- miRNA
microRNA
- MSC
mesenchymal stem cell
- N-Heps
normal human hepatocytes
- MVB
multivesicular bodies
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- PGDH
15-hydroxyprostaglandin dehydrogenase
- Pre-miRNA
miRNA precursor
- Pri-miRNA
primary transcript of miRNA
Footnotes
Conflict of interest: No conflict of interest to report.
Individual contributions: Keisaku Sato assisted in the overall design and drafted the manuscript; Fanyin Meng helped design and interpret the related microRNA study publications, provided technical support and helped to finalize the manuscript; Shannon Glaser contributed to overall design and helped with manuscript finalization and provided funding; Gianfranco Alpini developed the overall design, provided funding and helped draft the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship.
- 1.Lemoinne S, Thabut D, Housset C, Moreau R, Valla D, Boulanger CM, et al. The emerging roles of microvesicles in liver diseases. Nat Rev Gastroenterol Hepatol. 2014;11:350–361. doi: 10.1038/nrgastro.2014.7. [DOI] [PubMed] [Google Scholar]
- 2.Masyuk AI, Masyuk TV, LaRusso NF. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J Hepatol. 2013;59:621–625. doi: 10.1016/j.jhep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H, et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112:532–538. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894. doi: 10.1155/2014/864894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohn W, Kim J, Kang SH, Yang SR, Cho JY, Cho HC, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47:e184. doi: 10.1038/emm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, et al. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143:448–458. doi: 10.1053/j.gastro.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conde-Vancells J, Rodriguez-Suarez E, Gonzalez E, Berisa A, Gil D, Embade N, et al. Candidate biomarkers in exosome-like vesicles purified from rat and mouse urine samples. Proteomics Clin Appl. 2010;4:416–425. doi: 10.1002/prca.200900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Suarez E, Gonzalez E, Hughes C, Conde-Vancells J, Rudella A, Royo F, et al. Quantitative proteomic analysis of hepatocyte-secreted extracellular vesicles reveals candidate markers for liver toxicity. J Proteomics. 2014;103:227–240. doi: 10.1016/j.jprot.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker LA, Lee KC, Palacios Jimenez C, Alibhai H, Chang YM, Leckie PJ, et al. Circulating microRNAs Reveal Time Course of Organ Injury in a Porcine Model of Acetaminophen-Induced Acute Liver Failure. PLoS One. 2015;10:e0128076. doi: 10.1371/journal.pone.0128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015;13:261. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saha B, Bruneau JC, Kodys K, Szabo G. Alcohol-induced miR-27a regulates differentiation and M2 macrophage polarization of normal human monocytes. J Immunol. 2015;194:3079–3087. doi: 10.4049/jimmunol.1402190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha B, Momen-Heravi F, Kodys K, Szabo G. MicroRNA Cargo of Extracellular Vesicles from Alcohol-exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages. J Biol Chem. 2016;291:149–159. doi: 10.1074/jbc.M115.694133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, et al. Alcohol stimulates macrophage activation through caspase dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64:651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, et al. Lipid-induced Signaling Causes Release of Inflammatory Extracellular Vesicles from Hepatocytes. Gastroenterology. 2016 Jan 4; doi: 10.1053/j.gastro.2015.12.037. pii: S0016-5085(0015)01865-X. doi:01810.01053/j.gastro.02015.01812.01037. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 17.Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep. 2012;28:1551–1558. doi: 10.3892/or.2012.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, et al. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36:1008–1018. doi: 10.1093/carcin/bgv081. [DOI] [PubMed] [Google Scholar]
- 20.Dutta S, Reamtong O, Panvongsa W, Kitdumrongthum S, Janpipatkul K, Sangvanich P, et al. Proteomics profiling of cholangiocarcinoma exosomes: A potential role of oncogenic protein transferring in cancer progression. Biochim Biophys Acta. 2015;1852:1989–1999. doi: 10.1016/j.bbadis.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles. 2015;4:24900. doi: 10.3402/jev.v4.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10:e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fensterl V, Sen GC. Interferons and viral infections. Biofactors. 2009;35:14–20. doi: 10.1002/biof.6. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 26.Giugliano S, Kriss M, Golden-Mason L, Dobrinskikh E, Stone AE, Soto-Gutierrez A, et al. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. 2015;148:392–402. e313. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64:60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R, Ding Q, Yaqoob U, de Assuncao TM, Verma VK, Hirsova P, et al. Exosome Adherence and Internalization by Hepatic Stellate Cells Triggers Sphingosine 1-Phosphate-dependent Migration. J Biol Chem. 2015;290:30684–30696. doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonsato V, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, et al. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30:1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX, Jin HY, et al. Propofol exerts anti-hepatocellular carcinoma by microvesicle-mediated transfer of miR-142-3p from macrophage to cancer cells. J Transl Med. 2014;12:279. doi: 10.1186/s12967-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Zhu R. Diagnostic value of circulating microRNAs for hepatocellular carcinoma. Mol Biol Rep. 2014;41:6919–6929. doi: 10.1007/s11033-014-3578-7. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Wu S, Tong L, Luan T, Lin L, Lu S, et al. miR-122 affects the viability and apoptosis of hepatocellular carcinoma cells. Scand J Gastroenterol. 2009;44:1332–1339. doi: 10.3109/00365520903215305. [DOI] [PubMed] [Google Scholar]
- 35.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu WH, Ren LN, Wang X, Wang T, Zhang N, Gao Y, et al. Combination of exosomes and circulating microRNAs may serve as a promising tumor marker complementary to alpha-fetoprotein for early-stage hepatocellular carcinoma diagnosis in rats. J Cancer Res Clin Oncol. 2015;141:1767–1778. doi: 10.1007/s00432-015-1943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najafi Z, Sharifi M, Javadi G. Degradation of miR-21 induces apoptosis and inhibits cell proliferation in human hepatocellular carcinoma. Cancer Gene Ther. 2015;22:530–535. doi: 10.1038/cgt.2015.51. [DOI] [PubMed] [Google Scholar]
- 39.Lu L, Byrnes K, Han C, Wang Y, Wu T. miR-21 targets 15-PGDH and promotes cholangiocarcinoma growth. Mol Cancer Res. 2014;12:890–900. doi: 10.1158/1541-7786.MCR-13-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lian J, Jing Y, Dong Q, Huan L, Chen D, Bao C, et al. miR-192, a prognostic indicator, targets the SLC39A6/SNAIL pathway to reduce tumor metastasis in human hepatocellular carcinoma. Oncotarget. 2016;7:2672–2683. doi: 10.18632/oncotarget.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bala S, Tilahun Y, Taha O, Alao H, Kodys K, Catalano D, et al. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J Transl Med. 2012;10:151. doi: 10.1186/1479-5876-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bala S, Csak T, Momen-Heravi F, Lippai D, Kodys K, Catalano D, et al. Biodistribution and function of extracellular miRNA-155 in mice. Sci Rep. 2015;5:10721. doi: 10.1038/srep10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csak T, Bala S, Lippai D, Kodys K, Catalano D, Iracheta-Vellve A, et al. MicroRNA-155 Deficiency Attenuates Liver Steatosis and Fibrosis without Reducing Inflammation in a Mouse Model of Steatohepatitis. PLoS One. 2015;10:e0129251. doi: 10.1371/journal.pone.0129251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Lee Y, Johansson HJ, Mager I, Vader P, Nordin JZ, et al. Serum-free culture alters the quantity and protein composition of neuroblastoma-derived extracellular vesicles. J Extracell Vesicles. 2015;4:26883. doi: 10.3402/jev.v4.26883. [DOI] [PMC free article] [PubMed] [Google Scholar]