Abstract
The episodic memory system can differentiate similar events based on the temporal information associated with the events. Temporal context, which is at least partially determined by the events that precede or follow the critical event, may be a cue to differentiate events. The purpose of the present study is to investigate whether the hippocampal dentate gyrus (DG)/CA3 and CA1 subfields are sensitive to changes in temporal context and, if so, whether the subregions show a linear or threshold-like response to similar temporal contexts. Participants incidentally encoded a series of object picture triplets and 20 of them were included in final analyses. The third picture in each triplet was operationally defined as the target and the first two pictures served as temporal context for the target picture. Each target picture was presented twice with temporal context manipulated to be either repeated, high similarity, low similarity, or new on the second presentation. We extracted beta parameters for the repeated target as a function of the type of temporal context. We expected to see repetition suppression, a reduction in the beta values, in response to repetition of the target. If temporal context information is included in the representation of the target within a given region, this repetition suppression should be greater for target images that were preceded by their original context than for target images preceded by a new context. Neuroimaging results showed that CA1, but not DG/CA3, modifies the target’s representation based on its temporal context. Right CA1 did not distinguish high similarity temporal context from repeated context but did distinguish low similarity temporal context from repeated context. These results indicate that CA1 is sensitive to temporal context and suggest that it does not differentiate between a substantially similar temporal context and an identical temporal context. In contrast, DG/CA3 does not appear to process temporal context as defined in the current experiment.
Keywords: temporal context, CA1, dentate gyrus/CA3
Introduction
The hippocampus as a whole is known to be involved in processing temporal context (Agster et al., 2002; Ezzyat and Davachi, 2014; Hsieh et al., 2014; Kesner et al., 2002;). In the current study we investigate how specific hippocampal subfields process temporal context and how these regions distinguish similar temporal context input. Temporal context is defined as the information and events surrounding a target event in time. Some studies have suggested that differing temporal context changes the way a target event is processed. If so, temporal context may provide a cue to separate the representations of similar events in the hippocampus (Turk-Browne et al., 2012).
For example, imagine that you teach three sections of the same course. Prior to teaching the first section, you enjoy a buffet breakfast. Prior to teaching the second and third sections, you grade quizzes from previous classes. In this example, “eating breakfast” or “grading quizzes” provides temporal context for each of the three critical events “teaching class”. The “teaching class” event is similar in all instances (the time of day, material, and classroom are constant) and therefore it is the differing temporal context that makes the critical events distinguishable. We might expect the experience of, and therefore the representation of, the critical event to differ somewhat due to the preceding event. If so, the second and third teaching events might be more similar to one another than they would be to the first teaching event. The current study aims to investigate how encoding of an event differs due to changes in temporal context and how those changes in encoding are modulated by the similarity of temporal context.
The hippocampus includes the CA subfields (CA1, CA2, and CA3), dentate gyrus (DG), and subiculum (Amaral and Witter, 1989). Animal studies have shown that “time cells” in CA1 encode temporal information, meaning that the firing pattern of CA1 neurons changes as a function of time (Kraus et al., 2013; MacDonald et al., 2013; MacDonald et al., 2011; Mankin et al., 2012;). The firing pattern of CA1 neurons is also affected by the preceding events, suggesting that CA1 encodes preceding events, perhaps as temporal context for an event (MacDonald et al., 2011). CA2, in addition to CA1, has also been identified as a region that processes temporal information (Mankin et al., 2015). In contrast, CA3 neurons showed highly reproducible firing patterns over time, regardless of temporal context changes, which suggests that CA3 neurons are not involved in temporal coding (Mankin et al., 2012).
Although evidence from animal studies strongly suggests that CA1 processes temporal information, there is little evidence from human studies investigating the role of CA1 in temporal information processing. For example, studies have shown that CA1, CA2/CA3/DG, and subiculum represent temporal regularities (Schapiro et al., 2012) and differentiate spatial and temporal context (Copara et al., 2014). A study by Schlichting and colleagues (2014) also showed that right CA1 activation was associated with generalization across related events that occurred at different points in time and, moreover, reinstatement of a CA1 activation pattern predicted successful inference across those events. The accumuluated evidence thus far in human studies is therefore partially consistent with animal studies in finding that CA1 and CA2, but not CA3, encode temporal information. The first aim of the current study is to further investigate the neural correlates of temporal context processing in hippocampal subfields in human participants.
An additional question about hippocampal subfield function is how CA1 and DG/CA3 respond to similar event information. DG/CA3 is thought to be involved in pattern separation which is a process of orthogonalizing similar inputs into distinct outputs such that any minor change creates a distinct event (Bakker et al., 2008; Duncan et al., 2012; Guzowski et al., 2004; Lacy et al., 2011; Yassa and Stark, 2011). In contrast, CA1 representations appear to differ linearly with respect to the degree of similarity of information (Guzowski et al., 2004; Lacy et al., 2011; Leutgeb et al., 2007; Vazdarjanova and Guzowski, 2004; Yassa and Stark, 2011). That is, minor changes result in minor differences in the representation.
The evidence for these conclusions comes from studies investigating the encoding of similar events. Gilbert et al. (2001) studied the function of CA1 and DG in temporal and spatial pattern separation. Temporal pattern separation was tested by training rats to select the first of two open arms after sequential exposure to each of the eight arms in a maze. The two open arms were separated in time by exposure to 0, 2, 4, or 6 arms. The results showed that lesions to CA1 resulted in a deficit in distinguishing the sequence of the arms such that rats’ performance decreased as a function of decreased temporal distance. This result suggests that CA1 is involved in temporal pattern separation. Lesions to DG resulted in a deficit in distinguishing spatial differences such that rats’ performance decreased as a function of decreased spatial distance. This result suggests that DG is involved in spatial pattern separation.
Farovik et al. (2010) studied temporal pattern separation by training rats to distinguish a learned temporal sequence of odors from the reversed sequence. Similarity was manipulated by varying the temporal interval between the odors in the study session with a smaller temporal interval making it more difficult to distinguish the learned from the reversed sequence. Rats with CA3 lesions failed to distinguish the studied sequence from the reversed sequence when the inter-odor interval was 3 seconds, whereas CA1 lesions did not affect rats’ performance. When the interval was extended to 10 seconds, both CA3 and CA1 lesions impaired memory performance. The authors concluded that CA3 was critical for separating two highly similar inputs, which is consistent with the role of CA3 in pattern separation. In contrast, CA1 became involved only when the interval was extended and then inputs were more clearly differentiated.
At least one human study has investigated how hippocampal subfields respond to the degree of similarity in spatial and temporal input (Azab et al., 2014). Azab et al. (2014) presented four objects sequentially in different positions on a 3×3 grid. Each trial was presented twice, in an incidental memory task, either as an identical repetition, spatial position change, temporal sequence change, or both spatial and temporal changes (spatiotemporal change condition) and repetition suppression was measured in hippocampal subfields. DG/CA3 showed more adaptation to identical repetitions than to spatial, temporal, or spatiotemporal change trials with no significant difference between any of the change conditions. These results suggest that DG/CA3 is a general pattern separator that is sensitive to changes in both temporal and spatial information. Left and right CA1 produced different patterns. In left CA1, identical repetitions produced more adaptation than either temporal or spatiotemporal change trials, but did not distinguish between identical repetitions and spatial change trials, suggesting that left CA1 is specialized for detecting temporal changes. In right CA1, identical repetitions produced more adaptation than either spatial or spatiotemporal change trials, but did not distinguish between identical repetitions and temporal change trials, suggesting that right CA1 is specialized for detecting spatial changes.
In summary, both animal and human studies have shown that CA1 contributes to temporal pattern separation (Azab et al., 2014; Farovik et al., 2010; Gilbert et al., 2001). However, there is no direct evidence to indicate whether CA1 separates similar temporal context in the same way it has been shown to separate similar item information. Thus, the current study will test whether CA1 responds to similar temporal context by producing representations whose similarity is proportional to the similarity of the input.
In contrast to CA1, the studies reviewed above provide inconsistent conclusions regarding the involvement of DG and CA3 in temporal pattern separation. Gilbert et al.’s (2001) study showed that CA1, rather than DG, contributed to temporal pattern separation, whereas Farovik et al. (2010) and Azab et al.’s (2014) studies showed that both CA1 and DG/CA3 contributed to temporal pattern separation. In addition, the temporal pattern separation function of DG/CA3 reported in the latter two studies is in conflict with the finding that DG/CA3 does not process temporal information (Mankin et al., 2012). One possibility is that DG/CA3 was responsive to an idiosyncrasy of the paradigm used in Farovik et al. (2010) and Azab et al.’s (2014) experiments rather than temporal pattern separation in general. Both of these experiments involved distinguishing a learned sequence from a changed sequence created from the same set of items [except that Farovik et al. (2010) trained rats on an explicit version of the task whereas Azab et al. (2014) measured human brain activation on an implicit version of the task]. In an effort to reconcile the mixed evidence about DG/CA3 in temporal context separation, the current study will test how DG and CA3 respond to similar temporal input.
In the current experiment, we manipulated the similarity of temporal context information that preceded a critical event. We used object triplets as stimuli, of which the first two objects were temporal context for the third “target” object. Each triplet was presented twice. During the second presentation of the target object, the preceding temporal context was an identical repetition (repeated condition), extremely similar to the original temporal context (high similarity), somewhat similar to the original temporal context (low similarity), or entirely different from the original temporal context (new condition). In these “repeated target” conditions the target objects were always identical across the two presentations. We measured the activation within hippocampal subfields associated with the second presentation of the target object. Since the target was identical across the two presentations, any difference in hippocampal subfield activation associated with the repeated target should be due to manipulation of the temporal context. We propose that if a hippocampal subfield processes temporal context there will be repetition suppression (less activation) when the temporal contexts are the same across the two presentations than when the temporal contexts are entirely different. If a hippocampal subfield separates similar temporal input in a thresholded/nonlinear manner, the activation of the subfield should be reduced when the target object is preceded by repeated temporal context as compared to either high or low similarity temporal context. In this case, activation should not differ based on whether the target object follows high similarity, low similarity, or new temporal context. In contrast, if a hippocampal subfield responds to similar temporal input in a linear way, it should produce more activation (i.e. less repetition suppression) when the preceded temporal context is less similar. Therefore the identical repeated context should lead to less activation than a highly similar context, a high similarity context should lead to less activation than a low similarity temporal context, and a low similarity temporal context should lead to less activation than a new temporal context. Based on previous studies, we predicted that CA1 would process temporal context and separate similar temporal context linearly. In contrast, we predicted that DG/CA3 would not process temporal context.
Material and methods
2.1 Participants
Twenty-five Virginia Tech community members (18 Female, age: mean 24 years, standard deviation 3.12, range 20–30 years) participated in the experiment for monetary compensation. All participants had normal or corrected-to-normal visual acuity. Three participants were excluded from analysis due to poor resolution in the structural scans (caused by excessive motion). These participants were excluded prior to mask creation and data analysis. Two participants were excluded due to excessive movement during the functional scans. These participants were excluded following inspection with the Artifact Detection Tools as described below (Mazaika et al., 2005). The final sample size included in the analyses was 20.
2.2 Procedure
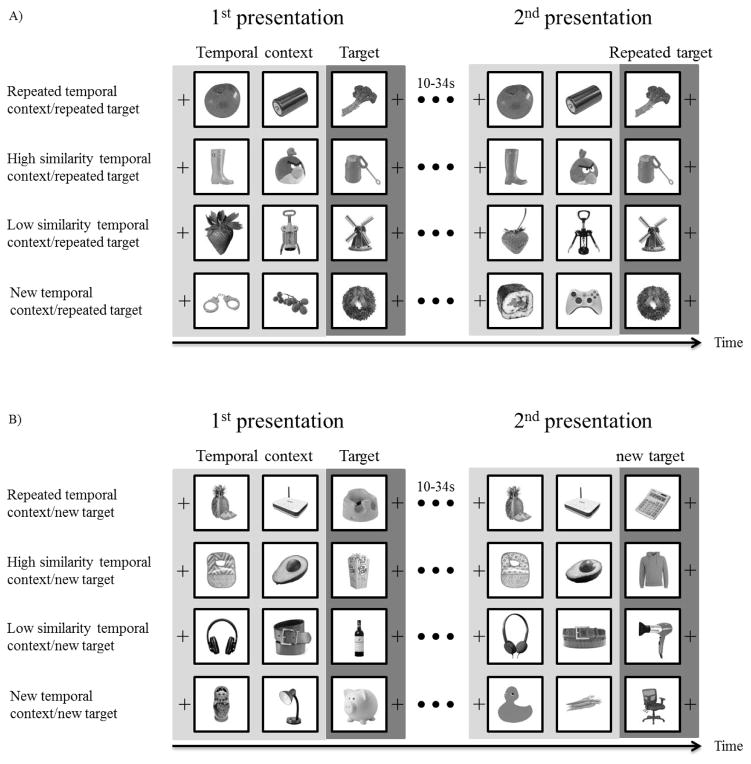
Stimuli consisted of 768 full-color images of easily recognizable objects on a white background (see Figure 1). 208 pictures were selected from the Bank of Standardized stimuli (Brodeur et al., 2010) with the rest found online. All pictures were edited to be the same size (500×500 pixels). Three types of pictures were collected to be used as stimuli: 96 high similarity pairs of pictures, 96 low similarity pairs of pictures, and 576 single pictures. Similar picture pairs did not include pictures of the same object taken from different viewpoints but rather were all images of distinct objects. The categorization of high and low similarity pairs was based on ratings from a separate group of participants in a pilot study. In the pilot study, 12 participants rated the similarity of 247 pairs of pictures on a scale from 1, “not very similar”, to 7, “almost identical”. The 96 picture pairs with the highest similarity ratings were selected as high similarity pairs (Mstandardized=0.56 SD=0.31) and the 96 picture pairs with the lowest similarity ratings were selected as low similarity pairs (Mstandardized=−0.53 SD=0.33). The 576 single pictures were not similar to any of the other pictures. Pictures were randomly selected from these overall image pools for to form the experimental trials for each participant.
Figure 1.
Experimental procedure. Each triplet was presented twice, with a 10 to 34 second interval, filled by presentation of other triplets, between the two presentations. The temporal contexts were either repeated in identical form to the first presentation, high similarity, low similarity, or unrelated across the two presentations of a triplet. (a) Repeated target conditions in which target pictures were identical in both presentations of the triplet. (b) New target conditions in which target pictures were unrelated in the two presentations of the triplet. These conditions served as a control for BOLD signal carryover from the preceding context pictures. Images were presented in full color in the experiment.
Pictures were grouped as triplets. In each triplet the third picture served as the target item and the first two pictures served as temporal context for the target item. The triplets were presented twice with the interval between the first and second presentation of each triplet being 10s to 34s (separated by 1, 2, or 3 triplets). The target (third) item in each triplet was a repeated item in half of the trials and a new, unrelated item, in the remaining trials. This manipulation was critical to determining whether any effects of temporal context were specific to the representation of the target item or whether the effects are due to carryover of the activation from the first and second items in the triplet. That is, since the first and second items in the triplet necessarily precede the third item with the same timing on each trial we could not modify the item order and timing as necessary for a typical fast-event-related fMRI design. The alternate approach we developed was to compare old targets directly to new targets with both sets of targts having the same preceding context manipulations. Any effect of temporal context on representation of the previously encoded item should occur only for the old targets and not the new targets.
Temporal context was manipulated in one of four ways during the second presentation of the triplet: repeated temporal context, high similarity temporal context, low similarity temporal context, and new temporal context. The triplet types (repeated, high similarity, low similarity, and new) were counterbalanced between the repetition delays within participants. Repeated temporal context indicates that the first two pictures were identical in both presentations of the triplet. High similarity temporal context indicates that the first and second pictures of the triplet during the second presentation of the triplet were highly similar to the first and second pictures, respectively, during the first presentation. Low similarity temporal context indicates that the first and second pictures during the second presentation of the triplet were somewhat similar to the first and second pictures, respectively, in the first presentation. New temporal context indicates that there was no relationship between the temporal context for the first and second presentations of the triplets. The same set of temporal context manipulations also preceded previously unstudied items. These “new target” trials served as a control for the trials on which the target was repeated (see Figure 1B). The combination of 2 target item manipulations and 4 temporal context manipulations formed 8 conditions: repeated temporal context/repeated item, high similarity temporal context/repeated item, low similarity temporal context/repeated item, new temporal context/repeated item, repeated temporal context/new item, high similarity temporal context/new item, low similarity temporal context/new item, and new temporal context/new item condition.
Each of the 6 blocks of the experiment was 9.7 minutes long and included 32 triplets, 4 from each of the 8 conditions, which were presented twice. Triplets were separated by a fixation sign presented for a jittered interval pseudorandomly selected to be either 2 (50% chance) or 4 (50% chance) seconds. Pictures were presented one after another in the center of the screen for 2 seconds. There was no delay between the pictures within a triplet. The jittered inter-triplet interval and the presentation order of triplets in different conditions were predetermined in order to improve design efficiency. We chose those designs, from a randomly generated set, with minimum collinearity between the time series for each condition (r values <.03).
Participants were instructed to give a “pleasant” or “unpleasant” binary judgment to each picture during the two seconds that the picture was visible. We used an this incidental encoding task in order to exclude the use of non-temporal strategies to encode the temporal information and thereby decrease contamination from non-temporal memory processes to our analyses. Participants were told that they would see new pictures, repeated pictures, and similar pictures in the experiment but were not informed about the specific manipulation of repeated pictures. They were told that the fixation sign between each triplet was used to give them a short break between pleasantness ratings. Post-experiment debriefings were used to determine whether participants noticed the presentation patterns of the triplets. None of the participants reported awareness of the experimental manipulations.
MRI data were acquired at the Virginia Tech Carilion Research Institute Human Neuroimaging Lab using a 3T Siemens Tim Trio scanner equipped with a 12-channel head coil. Prescreening interviews ensured safety in the scanner. Headphones and earplugs were provided to attenuate scanner noise. Padding and adjustable head restraints minimized head movement. High resolution functional MRI scanning took place during all six blocks of the experiment. Functional images were acquired with a T2*-weighted EPI sequence (repetition time/TR, 2000 ms; echo time/TE, 30 ms; field of view, 220 mm). Each volume included partial coverage of the head, parallel to the hippocampus, across 28 slices with 1.77×1.77×2.25 mm voxels. Six anatomical images were collected for each participant: three using an MPRAGE T1-weighted sequence (voxel size=0.599×0.599×0.6 mm) and another three using a T2-weighted scan sequence (voxel size=0.599×0.599×0.6 mm).
2.3 fMRI data analysis
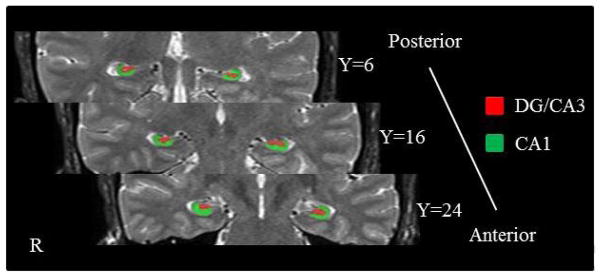
All anatomical images were co-registered to the first T2-weighted image. To improve the resolution of the anatomical image, averaged T2-weighted anatomical images were obtained by averaging the three T2-weighted images and averaged T1-weighted anatomical images were obtained by averaging the three T1-weighted anatomical images. CA1 and DG/CA3 were manually segmented on the averaged high-resolution structural image for each participant according to the Duvernoy (2005) hippocampus atlas and Yushkevich et al.’ (2009) segmentation guidelines (Figure 2). The volume of each hippocampal subfield was calculated in MarsBaR (Matthew et al., 2002) and the means across participants are reported in Table 1.
Figure 2.
Hippocampal segementation. Coronal slices along the anterior-posterior axis.
Table 1.
Mean Volume and Standard Deviation in Left and Right CA1 and DG/CA3
| Mean (mm3) | SD | |
|---|---|---|
| Left CA1 | 310.21 | 76.20 |
| Right CA1 | 327.70 | 94.21 |
| Left DG/CA3 | 169.03 | 61.75 |
| Right DG/CA3 | 178.78 | 51.02 |
Minimal preprocessing of the functional data was performed using Statistical Parametric Mapping (SPM8) software. T2*-weighted EPI data were slice-time corrected with sinc interpolation to account for differences in the timing of slice acquisition. The functional images for a single participant were brought into spatial alignment by using a six-parameter, rigid-body transformation (realignment). Following realignment, the EPI images for each participant were co-registered to the averaged T2-weighted structural image for each participant. First-level general linear model (GLM) analyses of the fMRI functional data were conducted using SPM8. Outliers were identified at the individual-subject level using the Artifact Detection Tools (http://gablab.mit.edu/index.php/software) in SPM8 with thresholds for global signal intensity (z=5), translational movement (0.5 mm), and rotational movement (0.005 rad). TRs identified as outliers were modeled as covariates of no interest.
The GLM included fourteen covariates of interest that were convolved with the hemodynamic response function. Eight of the covariates were used for the analyses reported below: (1) repeated target pictures preceded by the same temporal context, (2) repeated target pictures preceded by a high similarity temporal context, (3) repeated target pictures preceded by a low similarity temporal context, (4) repeated target pictures preceded by a new temporal context, (5) new target pictures preceded by the repeated temporal context, (6) new target pictures preceded by a high similarity temporal context, (7) new target pictures preceded by a low similarity temporal context, (8) new target pictures preceded by a new temporal context. The rest of vectors represented new pictures (images being presented for the first time not otherwise captured in the covariates of interest listed above), repeated temporal context pictures (the first two images presented in the triplet), high similarity temporal context pictures, low similarity temporal context pictures, new temporal context pictures in the second presentation, and fixation were not included in subsequent analyses. Beta values were extracted from CA1 and DG/CA3 for each of the 8 covariates of interest, all of which include only the presentation of the target (third) picture in each triplet, and assigned an alpha level of 0.05. A 2×2×4 repeated-measures ANOVA was applied to the beta values produced by presentation of the repeated target to test for effects of hemisphere, hippocampal subfield, and temporal context conditions. Planned comparisons with paired samples t-test were then conducted to evaluate differences between the four temporal context conditions.
Results
3.1 Behavioral results
The pleasantness rating task was used to encourage participants to attend to each picture and responses were subjective. Results showed that 98% of the pictures were given pleasantness ratings. An average of 63% (SD=.02) of the target pictures were rated as pleasant. There was no pleasantness rating difference between any of the eight conditions of interest (F(7, 312)=1.04, p=.41), thus any brain activation difference associated with the target pictures in different conditions should not be due to the pleasantness responses.
3.2 fMRI results
A repeated measures ANOVA with a Greenhouse-Geisser correction examining the beta values associated with presentation of repeated targets showed that there was no main effect for hemisphere (F(1,19)=0.83, p=.38), hippocampal subfield (F(1,19)=1.64, p=.22), or temporal context condition (F(3,57)=0.54, p=.66). However, there was a significant interaction of hemisphere (left, right), hippocampal subfield (CA1, DG/CA3), and temporal context condition (repeated, high similarity, low similarity, and new temporal context) (F(2.036, 38.685)=4.04, p=.03). This indicates that CA1 and DG/CA3 responded differently to the manipulation of temporal context similarity and there was a hemispheric difference. Therefore, we analyzed left and right CA1 and DG/CA3 separately.
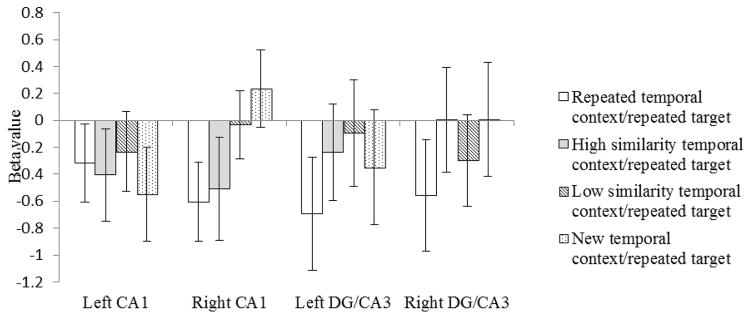
In left CA1 (Figure 3), none of the four conditions were significantly different from one another (ps>.05). There was an overall adaption effect such that the repeated targets (collapsed across all four temporal context conditions) produced significantly lower beta values than did the new targets (Meanrepeated target=−0.38, Meannew target=−0.09, t(19)=1.73, p=.04).
Figure 3.
Beta values to repeated target images following temporal context that was an identical repeat (white bars), high in similarity to the original temporal context (grey bars), low in similarity to the original temporal context (striped bars), or unrelated to the original temporal context (dotted bars). Error bars indicate the standard error of the means.
In right CA1 (Figure 3), the beta values for the repeated targets were significantly lower, and therefore indicated greater adaptation, when the target was presented after a repeated temporal context than when it was presented after a new temporal context (t(19)=−2.41, p=.01). The beta values for the repeated targets presented after a high similarity temporal context were not significantly different from the beta values for the repeated temporal context/repeated target condition (t(19)=−0.24, p=.41), but were significantly lower than the beta value for the new temporal context/repeated target condition (t(19)=−1.79, p=.04). These findings suggest that right CA1 did not separate the representation of an object that followed a highly similar temporal context from an object that followed an identical temporal context. The beta values for the repeated targets were significantly higher, and therefore indicated less adaptation, when the target followed a low similarity temporal context than when it followed a repeated temporal context (t(19)=−1.89, p=.04), and did not differ significantly from the beta values for the new temporal context/repeated target condition (t(19)=−0.95, p=1.73). These findings suggest that right CA1 did separate the representation of an object that followed a similar but easily distinguishable temporal context from an object that followed the original temporal context.
In left DG/CA3 (Figure 3), the difference in the beta values for repeated targets following a repeated temporal context and those following a low similarity temporal context approached statistical significance (t(19)=−1.5, p=.08)-. In right DG/CA3 (Figure 3), the difference between the repeated temporal context/repeated target condition and both the new temporal context/repeated target conditions (t(19)=−1.4, p=.09) and the high similarity temporal context/repeated target conditions (t(19)=−1.36, p=.09) approached significance. There was no statistically significant difference in any of the other comparisons within left and/or right DG/CA3: repeated temporal context/repeated target vs new temporal context/repeated target (tLeft(19)=−.79, p=.22), repeated temporal context/repeated target vs high similarity temporal context/repeated target (tLeft(19)=−1.17, p=.13), repeated temporal context/repeated target vs low similarity temporal context/repeated target (tRight(19)=−.57, p=.29), high similarity temporal context/repeated target vs low similarity temporal context/repeated target (tLeft(19)=−.37, p=.36, tRight(19)=.64, p=.26), high similarity temporal context/repeated target vs new temporal context/repeated target (tLeft(19)=.28, p=.39, tRight(19)=−.003, p=.50), low similarity temporal context/repeated target vs new temporal context/repeated target(tLeft(19)=.80, p=.22, tRight(19)=−.92, p=.18). These findings suggest that left DG/CA3 may not have separated the representations for the first and second presentations of an object when it follows an identical temporal context but did separate the representations of objects that followed any change in temporal context. Right DG/CA3 was able to distinguish repeated temporal context from both new and highly similar temporal context. However, these findings cannot be confidently interpreted without considering whether a carryover in adaptation from the repeated temporal context pictures to the repeated target picture was responsible for the differences in beta values.
In order to control for any BOLD signal carryover, we included four control conditions in the experiment with the same temporal context manipulations as those described above but a new target picture as the third item during the second presentation of each object triplet. Any differences in adaptation for these new target items must be based on carryover from the preceding temporal context items rather than adaptation of the ROI to the object itself. We subtracted the beta values for new targets with repeated, high similarity, low similarity, and new temporal context from the corresponding beta values for repeated targets. This subtraction (repeated minus new) should reflect temporal context processing without the contamination of BOLD signal carryover from previous repeated pictures.
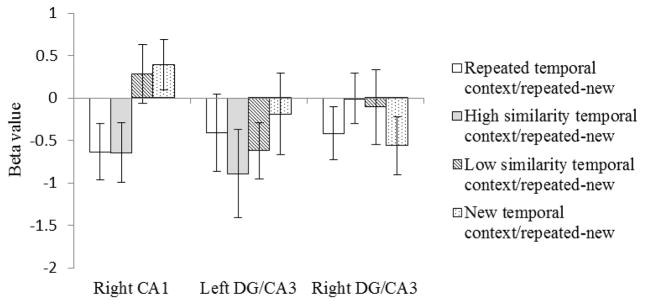
In right CA1 (Figure 4), the subtraction of beta values for the new targets from repeated targets produced the same pattern as the initial analyses. The subtracted beta values for the repeated temporal context condition were significantly lower, indicating greater adaptation, than the subtracted beta values for the new temporal context condition (t(19)=−2.23, p=.02). There was no significant difference between the subtracted beta values for the repeated temporal context and high similarity temporal context conditions (t(19)=0.02, p=.49), but the subtracted beta values for the high similarity temporal context condition were significantly lower than those for the new temporal context condition (t(19)=2.92, p=.004). There was no significant difference between the subtracted beta values for the low similarity temporal context condition and the new temporal context condition (t(19)=−0.26, p=.39). The difference between the subtracted beta values for the low similarity temporal context condition and the repeated temporal context condition approached significance (t(19)=−1.54, p=.07). Finally, the subtracted beta values for the high similarity temporal context condition were significantly lower than those for the low similarity temporal context condition (t(19)=−1.89, p=.04). These results suggest that right CA1 processes temporal context. When there is similar temporal context input, right CA1 does not distinguish it from repeated temporal context as long as the similarity level is high. When the similarity decreases, right CA1 distinguishes similar temporal context from repeated temporal context and separates the representation of the object following the low similarity temporal from the previous presentation.
Figure 4.
Subtracted beta values. The new target betas in each condition are subtracted from the repeated target betas shown in Figure 3. Error bars indicate the standard error of the means.
In contrast to right CA1, in left and right DG/CA3 the subtraction of beta values for the new target from the beta values for the repeated target did not produce the same pattern as the original analyses (Figure 4). In the subtraction analysis, there was no significant difference between any of four conditions for left and right DG/CA3 (ps>.05). These null results observed in DG/CA3 were not due to manipulation failure because we observed an adaption effect in left DG/CA3 when comparing the repeated target to the new target collapsed across the four temporal context conditions (MeanLeft/repeated target=−0.34, MeanLeft/new target=0.19, t(19)=−1.88, p=.037, MeanRight/repeated target=−0.21, MeanRight/new target=0.06, t(19)=−1.32, p=.10). That is, repeating an object image produced repetition suppression in left DG/CA3 but that repetition suppression did not vary according to the nature of the stimuli preceding the target. Therefore, these results indicate that DG/CA3 does not rely on temporal context to differentiate object representations. Rather, repeated items are recognized as such, regardless of the preceding items, and therefore show reduced activation compared to new items. Both old and new targets show decreased activation in DG/CA3 when presented following a series of repeated items, due to carryover of the BOLD signal from the preceding images.
A post-hoc analysis was done to investigate the involvement of parahippocampal cortex (PHc) in temporal context processing. The beta values in an anatomically-defined PHc ROI were significantly higher in the repeated temporal context/repeated target condition than in the new temporal context/repeated target condition (t(19)left=2.72, p=.007; t(19)right=3.04, p=.003). The effect when comparing the high smiliarity temporal context/repeated target condition to the new temporal context/repeated target condition was also statistically significant (t(19)left=3.25, p=.002; t(19)right=2.69, p=.007). The effects for repeated targets did not appear to be driven by carryover effects based on the finding that new target items following repeated context or high similarity items produced less activation than did old target items (t(19)left/repeated temporal context=1.38, p=.09; t(19)right/repeated temporal context=0.77, p=.22; t(19)left/high similarity temporal context=1.36, p=.09; t(19)right/high similarity temporal context=0.92, p=.18). Note that the effects found in PHc are in the opposite direction to the effects in right CA1 such that PHc did not show adaptation but rather increased activation for repeated or highly similar temporal context. An old target that was preceded by either repeated or high similarity temporal context items led to greater PHc activation than did an old target that was preceded by either low similarity or new temporal context items.
Discussion
In the current study, we investigated the involvement of hippocampal subfields CA1 and DG/CA3 in distinguishing the representations of items with varying temporal context. We compared the beta values associated with repeated target pictures when those targets were preceded by repeated, high similarity, low similarity, and new temporal context. Hippocampal subfields that use temporal context as a cue when representing events should show increased repetition suppression (and therefore reduced activation) when target pictures are preceded by repeated temporal context as compared to new temporal context. In addition, differences in repetition suppression based on the similarity of new temporal context to the original temporal context should provide information about the role of hippocampal subfields in separating similar events. As the beta values associated with target pictures might be contaminated by fMRI signal carryover from preceding temporal context pictures, we included a set of control conditions in which the manipulation of temporal context was the same as the experimental conditions except that the target pictures were new. Any beta value differences among new target pictures preceded by repeated, high similarity, low similarity, and new temporal context can only reflect signal carryover from preceding pictures. After removing any carryover signal, the beta values associated with repeated targets should reflect the influence of temporal context only.
Our results indicated that right CA1 was sensitive to temporal context when representing an object image. Right CA1 appears to be involved in temporal context processing because target pictures preceded by repeated temporal context produced significantly lower beta values in this ROI than did target pictures preceded by new temporal context. In contrast, DG/CA3 was not sensitive to repeated temporal context when representing an object image since there was no difference between the beta values for target pictures following repeated or new temporal contexts. (Note that our conclusions with respect to DG/CA3 are based on a null effect and therefore should not be considered definitive.) DG/CA3 was sensitive to repetition of items themselves as seen in carryover effects from repeated temporal context items to both old and new target items. In these item-level adaptation effects, DG/CA3 distinguished between repeated images and high similarity replacements of those images. This finding replicates previous studies which have demonstrated that DG is involved in pattern separation of similar information.
We did not find any differences between conditions in left CA1 and thus our conclusions are specific to right CA1. Interestingly, some prior work on human hippocampal subfields suggests that right CA1 and not left CA1 is critical for generalizing across events from different time points, which is consistent with our results (Schlicting et al., 2014). In contrast, Azab and colleagues (2014) found that right CA1 was specifically sensitive to spatial changes whereas left CA1 was sensitive to temporal changes. Although this is now the third study with human participants that differentiates right and left CA1 function, the inconsistencies in identifying those functions make it difficult to conclude that the roles of left and right CA1 in temporal context processing are qualitatively different. Further study is required.
The finding that CA1, but not DG/CA3, processes temporal information and bridges events across time is somewhat suprising. DG is often described as the area responsible for pattern separation, which would suggest that DG distinguishes between similar events regardless of the dimension upon which those events differ. However, our finding that DG does not separate similar temporal information is consistent with findings from the animal literature, which has primarily identified pattern separation in DG for similar spatial information (Leutgeb et al, 2007). In addition, Gilbert and colleagues (2001) specifically compared the responses of CA1 and DG to similar spatial and temporal information and found that DG distinguishes similar spatial representations but only CA1 distinguished similar temporal representations.
Another important point of consideration is the combination of CA3 and DG into a single mask in this experiment. The current technology in human fMRI makes it difficult to reliably separate CA3 and DG. Therefore, our findings in this ROI are likely to represent an amalgamation of the functions of CA3 and DG. Past findings have consistently shown that neurons in CA3 are not responsive to temporal context changes (Mankin et al., 2012) whereas the firing patterns of CA1 neurons appear to record elapsed time (Kraus et al., 2013; MacDonald et al., 2013; MacDonald et al., 2011; Mankin et al., 2012). Therefore, the results from the current study are largely consistent with previous studies of pattern separation in animal populations (Eichenbaum et al., 1999; Lee et al., 2004; Moser et al., 2008) and with the limited available evidence from human populations. CA1 provides binding of item information with its context in both the temporal and spatial dimensions (Mankin et al., 2015). DG and CA3 appear to bind item information with context information that occurs during the same temporal window but not outside of that temporal window.
The involvement of CA1 but not DG/CA3 in temporal context processing sheds light on how temporal information is transmitted within hippocampal subfields. There are two well identified circuits in the hippocampus (Witter, 1993; Lavenex and Amaral, 2000). One projection originates from cells in layer II in entorhinal cortex and sends information to CA3, then CA1, and back to the deep layer of entorhinal cortex. A second projection arises from cells in layer III in entorhinal cortex, sends information to CA1 directly, and then back to the deep layer of entorhinal cortex. Since CA3 does not appear to be involved in processing temporal context, temporal information is likely conveyed to CA1 by the second pathway, via direct projections from layer III of the entorhinal cortex. At least one prior study provides evidence to support this conclusion by showing that inhibition of the direct pathway from entorhinal layer III to CA1 impairs animals’ ability to associate events across time (Suh et al., 2011).
The current study also investigated the nature of hippocampal subfield responses to similar temporal input. This question builds upon the first set of findings about whether CA1 and DG/CA3 are sensitive to temporal context at all. Because DG/CA3 were not responsive to temporal context we would not expect to find modulations in DG/CA3 activation based on varying similarity of that temporal context. Because right CA1 was sensitive to temporal context in general, we can further explore the nature of its response to temporal context of varying similarity. It should be noted that fMRI data cannot reveal whether the hippocampal subfields we investigated are critical for temporal context processing and episodic separation but only whether they are sensitive to the nature of temporal context.
We found that right CA1 failed to distinguish high similarity temporal context from repeated temporal context when representing an object. In this experiment, high similarity temporal context took the form of objects whose differences were so minor, in comparison to the objects used during the first presentation of the triplet, that it was unlikely participants were consciously aware that the images were new. On the other hand, right CA1 was able to distinguish low similarity temporal context from repeated temporal context when representing an object. Low similarity temporal context took the form of objects that would receive the same verbal label as the objects presented previously but that could be easily identified as different objects. Although the beta values in right CA1 for the low similarity temporal context condition were lower than those for the new temporal context condition this difference was not statistically significant. These results show that right CA1 does not produce a linear response to temporal context based on its similarity with previous events. There was almost no numerical difference between the target items following repeated and high similarity temporal context and the difference between the target items following low similarity and new temporal context was not significant. The relationship between changes in temporal input and output in CA1 resembles a sigmoid function: when there were small changes in temporal input, CA1 processed the highly similar temporal context as though it were a repeated temporal context. When the changes in temporal input increased, CA1 was able to identify that the low similarity temporal context was not repeated and process it as new temporal context. This finding is consistent with previous evidence that CA1 was not involved in distinguishing a learned versus reversed sequence of events when the inter-event interval was short. CA1 became involved only when the interval was extended, which indicates that CA1 primarily distinguishes temporal information when it differs substantially. (Farovik et al., 2012).
In contrast to its role as a pattern separator in item and spatial context processing, DG/CA3 did not show a pattern separation effect intemporal context processing for repeated targets as opposed to new targets. Rather, DG/CA3 appeared to distinguish identical from high similarity items preceding a target regardless of the status of the target itself. Since neither DG/CA3 nor CA1 appeared to distinguish highly similar temporal input, a remaining question is whether any hippocampal subfield or brain region outside of the hippocampus distinguishes highly similar temporal input. CA2 is a one candidate for a region that provides this function. Although CA2 was included in the CA1 mask in the current study, and therefore the effects reported in CA1 also reflect the influence of CA2, it’s possible that effects in CA2 were overshadowed by the pattern in CA1. Previous research has found that the firing pattern of CA2 neurons becomes progressively dissimilar over time and that of CA2 neurons are more involved in temporal information processing than either CA1 or DG/CA3 neurons (Mankin et al., 2015). However, the anatomical and physiological features of CA2 are less well understood (Jones and McHugh, 2011). We could not specifically test the involvement of CA2 in the current study due to limitations in the resolution of structural image that we collected.
The relationship between conscious detection of differences in stimuli and representation of those stimuli in hippocampal subregions cannot be determined definitively from our data but we speculate that our high similarity images were unlikely to be consciously identified as different from one another whereas our low similarity images are likely to be identified as different. If this speculation is correct, we propose that stimuli that are not distinguished consciously are likely to be represented as the same items in CA1. We found that right CA1 did not distinguish between repeated context and high similarity context, which indicates that the representation of high similiarity context images were comparable to the representation of the original context images. In contrast, the implications of our findings in DG/CA3 with respect to this question are less clear. We found that left and right DG/CA3 produce less activation to both old and new targets that followed repeated temporal context items than those targets that followed high similarity, low similarity, or new temporal context items. This finding suggests that DG/CA3 may in fact distinguish between similar items that are not consciously identified as new (such as our high similiarity items) but only at the item level and not at the level of temporal context. Finally, the current study suggests that different neural mechanisms underlie the processing of temporal and non-temporal (i.e. spatial) context within hippocampal subfields. Temporal context is different from other types of episodic context (i.e. spatial context) in that temporal context occurs at a different time point (before or after) than the critical event (Kahana, 2002; Polyn and Kahana, 2008). Non-temporal context happens during the same time window as the critical event. Both temporal and non-temporal context processing involve the hippocampus (Agster et al., 2002; Davachi, 2006; Davachi et al., 2003; Diana et al., 2007; Eichenbaum et al., 2007; Eichenbaum et al, 1994; Ezzyat and Davachi, 2014; Hsieh et al., 2014; Kesner et al., 2002), but it seems that subfields within the hippocampus play different roles in associating item information with these categories of context. It has been well established in the animal and human literatures that CA1 and DG/CA3 bind an item with its spatial context. The current study provides some of the first evidence in humans that only CA1 associates events with their temporal context. Even though CA1 participates in both temporal and non-temporal context processing, it appears to respond in a different manner to similar temporal and non-temporal context inputs. CA1 transforms similar spatial context input into dissimilar output linearly (Guzowski et al., 2004; Lacy et al., 2011; Leutgeb et al., 2007; Vazdarjanova and Guzowski, 2004; Yassa and Stark, 2011). However, the current study found that CA1 does not transform similar temporal input into dissimilar output linearly. The nonlinear relationship seen in the current experiment could be driven by the nature of our context similarity manipulation and therefore more evidence is needed in order to definitively conclude how CA1 processes similar temporal contexts.
The findings for the PHc ROI may seem contradictory to the hippocampal findings at first glance. however we think they provide a clear replication and extension of a previous study (Diana et al., 2013). In Diana and colleagues’ 2013 study, context features were reinstated prior to presentation of an item memory probe. This paradigm was similar in many respects to the organization of the trials in the current experiment. The 2013 study found that increased PHc activation during reinstatement of context features predicted successful recollection of the item probe. The current study can be viewed in a similar light: PHc activation during the second presentation of temporal context items serves as a measure of successful context reinstatement. The current study also extends previous PHc findings by suggesting that PHc representations of context do not differentiate between repeated information and highly similar information.
Although we have also previously found adaptation in PHc in relation to repetition of context information (Diana et al., 2012), the adaptation paradigm required immediate repetition of stimuli such that no intervening items interrupted encoding of the first and second study events. In a sense, the 2012 findings can be viewed as habituation whereas the current findings can be viewed as context reinstatement and retrieval/expectation of the target picture induced by that context reinstatement. With respect to the wider literature on temporal context, our PHc findings do not replicate Turk-Browne et al. (2012), which may be related to the use of scene images in that study, but are consistent with Hsieh et al.’s findings (2014) that PHc is sensitive to temporal position but not the specific surrounding events.
Conclusions
The current study investigated the role of hippocampal subfields CA1 and DG/CA3 in processing temporal context as well as the responses of these subfields to similar temporal context inputs. We found that right CA1, but not DG/CA3, processed temporal context and was sensitive to temporal context when representing events. CA1 was not able to distinguish highly similar temporal contexts from repeated temporal contexts. However, right CA1 did distinguish low similarity temporal contexts from repeated temporal contexts and recognized these low similarity temporal contexts as new contexts.
Highlights.
Right CA1 representation of an event was affected by the event’s temporal context.
Right CA1 did not distinguish high similarity temporal context from repeated context.
Right CA1 did distinguish low similarity temporal context from repeated context.
DG/CA3 was not sensitive to temporal context when representing an event.
Acknowledgments
Funding for this project was provided by National Institutes of Health grant MH083945
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. The Journal of neuroscience. 2002;22(13):5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Azab M, Stark SM, Stark CE. Contributions of human hippocampal subfields to spatial and temporal pattern separation. Hippocampus. 2014;24(3):293–302. doi: 10.1002/hipo.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur MB, Dionne-Dostie E, Montreuil T, Lepage M. The Bank of Standardized Stimuli (BOSS), a new set of 480 normative photos of objects to be used as visual stimuli in cognitive research. PloS one. 2010;5(5):e10773. doi: 10.1371/journal.pone.0010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copara MS, Hassan AS, Kyle CT, Libby LA, Ranganath C, Ekstrom AD. Complementary roles of human hippocampal subregions during retrieval of spatiotemporal context. The Journal of Neuroscience. 2014;34(20):6834–6842. doi: 10.1523/JNEUROSCI.5341-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current opinion in neurobiology. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in cognitive sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Adaptation to cognitive context and item information in the medial temporal lobes. Neuropsychologia. 2012;50(13):3062–3069. doi: 10.1016/j.neuropsychologia.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Parahippocampal cortex activation during context reinstatement predicts item recollection. Journal of Experimental Psychology: General. 2013;142(4):1287. doi: 10.1037/a0034029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Springer Science & Business Media; 2005. [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behavioral and Brain Sciences. 1994;17(03):449–472. [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23(2):209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annual review of neuroscience. 2007;30:123. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L. Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 2014;81(5):1179–1189. doi: 10.1016/j.neuron.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Eichenbaum H. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learning & Memory. 2010;17(1):12–17. doi: 10.1101/lm.1616209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: a double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11(6):626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44(4):581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hsieh LT, Gruber MJ, Jenkins LJ, Ranganath C. Hippocampal Activity Patterns Carry Information about Objects in Temporal Context. Neuron. 2014;81(5):1165–1178. doi: 10.1016/j.neuron.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, McHugh TJ. Updating hippocampal representations: CA2 joins the circuit. Trends in neurosciences. 2011;34(10):526–535. doi: 10.1016/j.tins.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Kahana MJ. Associative symmetry and memory theory. Memory & cognition. 2002;30(6):823–40. doi: 10.3758/bf03195769. [DOI] [PubMed] [Google Scholar]
- Duncan K, Sadanand A, Davachi L. Memory’s penumbra: episodic memory decisions induce lingering mnemonic biases. Science. 2012;337(6093):485–487. doi: 10.1126/science.1221936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua La. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience. 2002;116(2):286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “time cells”: time versus path integration. Neuron. 2013;78(6):1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learning & Memory. 2011;18(1):15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10(4):420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430(6998):456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Carrow S, Place R, Eichenbaum H. Distinct hippocampal time cell sequences represent odor memories in immobilized rats. The Journal of Neuroscience. 2013;33(36):14607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Diehl GW, Sparks FT, Leutgeb S, Leutgeb JK. Hippocampal CA2 activity patterns change over time to a larger extent than between spatial contexts. Neuron. 2015;85(1):190–201. doi: 10.1016/j.neuron.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK. Neuronal code for extended time in the hippocampus. Proceedings of the National Academy of Sciences. 2012;109(47):19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett Matthew, Anton Jean-Luc, Valabregue Romain, Poline Jean-Baptiste. Region of interest analysis using an SPM toolbox. Abstract presented at the Eighth International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. Jun, [Google Scholar]
- Mazaika PK, Whitfield S, Cooper JC. Detection and repair of transient artifacts in fMRI data. Neuroimage. 2005;26(Suppl 1):S36. [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Kahana MJ. Memory search and the neural representation of context. Trends in cognitive sciences. 2008;12(1):24–30. doi: 10.1016/j.tics.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Kustner LV, Turk-Browne NB. Shaping of object representations in the human medial temporal lobe based on temporal regularities. Current Biology. 2012;22(17):1622–1627. doi: 10.1016/j.cub.2012.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Zeithamova D, Preston AR. CA1 subfield contributions to memory integration and inference. Hippocampus. 2014;24(10):1248–1260. doi: 10.1002/hipo.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334(6061):1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. The Journal of neuroscience. 2004;24(29):6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal—hippocampal system: A review of current anatomical data. Hippocampus. 1993;3(S1):33–44. [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends in neurosciences. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Avants BB, Pluta J, Das S, Minkoff D, Mechanic-Hamilton D, … Grossman M. A high-resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4 T. Neuroimage. 2009;44(2):385–398. doi: 10.1016/j.neuroimage.2008.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]