Abstract
There has been increasing interest in the rostromedial tegmental nucleus (RMTg), given its potential regulatory role in many aversion-related behaviors. The RMTg contains mostly GABAergic neurons, sends a dense inhibitory projection to dopamine neurons in the midbrain, and is rich with μ-opioid receptors (MOR). Like most addictive drugs, ethanol has both aversive and rewarding properties. However, the cellular mechanisms underlying the effects of ethanol, particularly the aversive effect that limits its intake are not well understood. Recent studies have linked aversion with synaptic inhibition of dopamine neurons in the ventral tegmental area. To determine a potential role that the RMTg plays in the effect of ethanol, in this study, we employed a neurotoxin, dermorphin-saporin (DS), to lesion RMTg neurons prior to assessing ethanol-related behaviors. Rats were infused with DS bilaterally into the RMTg. This manipulation substantially increased the intake and preference for ethanol but not sucrose. It also reduced the number of neurons with MOR and glutamic acid decarboxylase 67 immunoreactivity within the RMTg. These changes did not occur after intra-RMTg infusion of blank saporin or vehicle. Importantly, intra-RMTg DS infusion significantly enhanced expression of conditioned place preference induced by ethanol (2g/kg, i.p.), and slowed the extinction process. These results suggest that MOR-expressing GABAergic neurons in the RMTg contribute significantly to the regulation of ethanol consumption and related behaviors.
Keywords: conditioning place preference, dermorphin-saporin, intracranial infusion, rat, ethanol
1. Introduction
Alcohol use disorders are a serious economic and health problem in our society, but the underlying neurobiological mechanisms are not completely understood. Ethanol has both rewarding and aversive properties, and a balance between them may influence ethanol consumption. Ethanol’s rewarding property has been linked to its ability to increase the activity of dopamine neurons in the ventral tegmental area (VTA) and the release of dopamine in the target projection regions (Di Chiara, 1998; Nicola and Malenka, 1997; Thomas et al., 2001), however, significantly less is known about its aversive property.
There has been increasing interest in the rostromedial tegmental nucleus (RMTg), because it encodes aversive signals, is excited by various aversive stimuli (Jhou et al., 2009a; Lecca et al., 2012), and its activation produces conditioned aversion (Jhou et al., 2013). The RMTg exhibits distinct neuroanatomical, physiological, and behavioral properties. It consists of GABAergic neurons that project intensively to midbrain dopamine neurons, and exerts a major inhibitory drive on the dopamine system (Lecca et al., 2011). The RMTg could act as a hub that converges and integrates widespread multimodal signals towards the dopamine system (Lecca et al., 2011). Several lines of evidence have demonstrated that mu opioid receptors (MOR), but not delta opioid receptors (DOR), are the main opioid receptors in the RMTg. Histologically, high levels of MORs are one of the important markers that distinguish the RMTg from surrounding regions (Jhou et al., 2009a). Electrophysiological data demonstrated that the MOR agonist DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin) hyperpolarizes RMTg neurons that project to the VTA; but the delta-opioid agonist DPDPE (D-Ala2, D-Leu5 enkephalin) did not have a significant effect. Furthermore, only DAMGO, but not DPDPE, decreased the amplitude of the GABAA IPSCs evoked by RMTg stimulation in dopamine neurons (Matsui and Williams, 2011), suggesting that GABAergic transmission from the RMTg to VTA dopamine neurons is activated by mu, but not delta opioid receptors.
The RMTg receives strikingly focused afferents from the lateral habenula (LHb), a key structure in the brain that regulates aversion-related behavior. LHb neurons are activated by a variety of aversive stimuli, such as stress, fear, disappointment, or negative prediction errors (Hikosaka, 2010; Lecourtier and Kelly, 2007; Matsumoto and Hikosaka, 2009; Stamatakis and Stuber, 2012). Stimulation of the LHb in vivo inhibits dopamine neurons, an action mediated by GABAA receptors (Ji and Shepard, 2007), probably via the activation of RMTg neurons. In the current study, we measured changes in MOR expression and drinking-related behaviors induced by intra-RMTg infusion of dermorphin-saporin (DS). Dermorphin has a much higher affinity for MORs than DORs, as reflected by the very different KD values: 1.24 vs 78 nM (Krumins, 1987). DS could induce lesions in MOR-expressing neurons, including the GABA neurons in the VTA of rats (Reynolds et al., 2011; Shank et al., 2007). Here, we provide the evidence that ablation of RMTg neurons robustly increases ethanol intake and preference, as well as enhances ethanol-induced conditioned place preference. Our findings suggest that the RMTg plays a significant role in ethanol-related behaviors.
2. Materials and Methods
2.1. Animals and housing
All procedures were approved by the Animal Care and Utilization Committee of Rutgers, the State University of New Jersey, in accordance with National Institutes of Health guidelines, minimizing the number of animals used and their suffering. All experiments were conducted on adult female Sprague-Dawley rats (250–350 g at the start of the experiments). The rats were individually housed. Food and water were available ad libitum unless indicated otherwise. We conducted the current study in female rats to take advantage of their stable head size, their higher alcohol drinking levels, and the request for more studies in females by the NIH (see Discussion for more).
2.2. Intermittent-access to 20% ethanol two-bottle free choice drinking procedure
We used the intermittent-access to 20% ethanol two-bottle free choice drinking procedure (I2BC) described previously (Li et al., 2011). Briefly, after acclimating to the homecage environment, all animals had 24-hour concurrent access to two bottles, one with 20% ethanol (v/v) and another with water only, starting on Monday afternoon. After 24 hours, the ethanol bottle was replaced with a second water bottle that was available for the next 24 hours. This pattern was repeated on Wednesdays and Fridays. On all other days the rats had unlimited access to two bottles of water. In each ethanol drinking session, the placement of the ethanol bottle was alternated to control for side preferences.
The amount of fluid intake was determined by weighing the bottles before and after 24 hours of access. Ethanol consumption was determined by calculating grams of ethanol consumed per kilogram of body weight. Two bottles, one containing water and one containing 20% ethanol, were placed in a cage without rats to evaluate the spillage due to the experimental manipulations during the test sessions. The spillage was always < 1.0 ml (< 2.5% of the total fluid intake) during 24 hours. Animals in the control group were allowed unlimited access to water and food. Body weights of all rats were recorded weekly, and no significant difference between the control and the ethanol-drinking rats was found at the end of the experiments. Rats under the I2BC paradigm escalated their ethanol intake and preference, in keeping with previous report (Li et al., 2011). Animals in the control group were allowed access to water and food without limitation. There was no significant difference in body weight between the control and the ethanol-drinking rats at the end of the experiments.
2.3. Intra-RMTg infusion
After a stable baseline drinking level had been reached (about 6–8 weeks in the I2BC drinking paradigm), rats received bilateral intra-RMTg infusion (1.5 pmol/100 nl/side, 10 nl/min) of dermorphin-saporin (DS, Advanced Targeting Systems, Inc. San Diego, CA, USA), or the equivalent amount of blank saporin (BS, Advanced Targeting Systems, Inc.) or artificial cerebrospinal fluid (aCSF) under isoflurane anesthesia at the following stereotaxic coordinates (in mm: AP: −6.8; ML:± 0.8; DV: −7.9 from the skull’s surface) according to the rat brain atlas (Paxinos and Watson, 2007). Following the infusion, the pipette remained in place for 10 min before being withdrawn. Thereafter the burr hole was sealed with sterile bone wax; and the scalp was sutured. Before recovery from anesthesia, rats were given the pain-killer meloxicam (1.0 mg/kg, s.c.), and returned to their home cage. Rats were allowed to recover for ≥7 days before resuming drinking, during which time they were handled daily. The same investigator executed all procedures.
2.4. Sucrose self-administration
A separate group (n = 6) of rats was trained to drink 2% sucrose (wt/vol) solution under an I2BC procedure, similar to that for ethanol described above. We selected 2% sucrose according to previous studies (Li et al., 2012; Nie et al., 2011). These rats had reached a stable baseline drinking level after 6 drinking sessions, and then received intra-RMTg infusion of DS, BS, or vehicle. Seven days after infusion, these rats resumed sucrose drinking for an additional 12 sessions.
2.5. Ambulation measurement
We measured the non-habituated locomotor activity, as described (Li et al., 2012), under low-light conditions utilizing standard locomotor testing chambers (TruScan Photobeam Activity Monitors, 40L × 40W × 40H cm; Coulbourn Instruments, Whitehall, PA, USA). We quantified ambulation as the total distance (cm) traveled in the horizontal plane. The interruption of two consecutive photobeams resulted in a count of “ambulation” by the control software. On each testing day, the rats rested for 10 min after being transported into the testing room and then were placed in the assessment chamber where locomotion in the X-Y plane was assessed during a 60-minute session.
2.6. Quantization of MOR and glutamic acid decarboxylase 67 immunoreactivity
The RMTg expresses high levels of the GABA synthesizing enzyme glutamic acid decarboxylase 67 (GAD67) and the MOR immunoreactivity that distinguish it from surrounding regions (Barrot et al., 2012). We infused the RMTg with DS (a total of 3 pmol (100 ng) for each rat), a dose able to effectively eliminate the MOR-expressing neurons within 7 to 28 days (Shank et al., 2007). To determine whether DS infusion could have reduced the number of MOR-expressing GABAergic neurons by day 14 and 34 after DS infusion, after obtaining behavior data, we sacrificed the animals and quantified MOR and GAD67 immunoreactivity as described (Unterwald et al., 1998). To further validate that DS induced a loss of neurons by day 34 after DS infusion, we quantified NeuN immunoreactivity as described (Jhou et al., 2013). Briefly, the rats were euthanized and transcardially perfused with saline followed by 4% paraformaldehyde (PFA). Brains were removed, post-fixed (overnight, at 4 °C) in 4% PFA, and cryoprotected. Serial 30-μm coronal sections of the midbrain were cut on a freezing microtome (Microm HM550, Walldorf, German), and a 1-in-4 series of brain sections was processed for immunohistochemistry using primary antibodies against GAD67 (1:400; MAB5406, Millipore Bioscience Research Reagents, Billerica, MA, USA), MOR (1:400; ab10275, Abcam, Cambridge, MA, USA), and NeuN (1:1000; MAB377, Millipore) at 4 °C overnight. After rinsing in 0.01 M PBS, the sections were incubated with biotinylated anti-mouse or anti-rabbit IgG (1:500; Vector Lab, Burlingame, CA, USA) at room temperature for 2 hours. After rinsing, sections were incubated in a solution containing avidin-biotin-horseradish peroxidase complex for 45 min (Vector Elite Kit, Vector Lab). Horseradish peroxidase activity was visualized with diaminobenzidine staining (Vector Lab). Sections from each experimental group were processed simultaneously. The sections stained without primary antibody served as negative controls. Sections were mounted onto chrome-alum slides, dehydrated, and cover slipped.
Immunopositive cells were visualized with a CCD camera connected to a microscope (Nikon Eclipse 80i, Japan). The images were obtained at 10× and 20× magnifications. The RMTg region was identified and cells were counted manually as previously described (Jhou et al., 2009a). Two investigators who were blinded to the treatment history counted the number of immunopositive cells of each image [100 × images of RMTg region]. For each animal, RMTg cells were counted in 10 consecutive coronal sections (bregma −6.0 to −7.0 mm). Counts were determined for each hemisphere individually, and an average value for both hemispheres of each section was calculated. The average value across all sections for each animal was then determined. Since we counted only the neuronal cell bodies with obvious light emission and the light of some positive neurons might be too weak to detect, the numbers of MOR-IR neurons and GAD67-IR neurons should be regarded as the minimum number of positive neurons in the sections.
2.7. Conditioned place preference
Two weeks after intra-RMTg infusion, we started examining the expression and extinction of ethanol-induced Conditioned Place Preference (CPP) in 24 ethanol-naïve rats.
Apparatus
We conducted the CPP test in a standard rat place preference apparatus (MED-CPP2-013C, Med Associates INC. Georgia, VT). Its two chambers (30 L×20W×20H, in cm) differed by floor tactile cues (mesh vs. rod) and wall patterns (black vs. white), and are separated by a guillotine door. We used Photobeam detectors to detect the locomotion of a rat, defined by the position of its two front paws within the chamber.
CPP test
We used an unbiased procedure (Cunningham et al., 2003), which consists of the following three phases on 6 consecutive days.
Pre-conditioning (Day 1)
A rat was placed in the apparatus and allowed access to the entire apparatus for 15 min. Most rats spent a similar time in the two chambers. A few rats (<15%) that had a strong initial preference (>75%) for one chamber were excluded from the analysis to reduce the impact of unconditioned preferences and “ceiling” or “floor” effects. We completed the preconditioning phase before, as well as two weeks after, intra-RMTg infusion; we did not find a significant difference. Therefore, the analysis was based on data obtained after intra-RMTg infusion.
Conditioning (Days 2–5)
Starting the day after pre-conditioning, experiments consisted of eight 15-min sessions (4 saline and 4 ethanol pairings). On each day, one ethanol session and one saline session were performed; they were separated by at least 6 hours. Animals were confined in one chamber immediately after intra-RMTg infusion. Ethanol pairing with net vs grid chamber were conducted in a counterbalanced manner across rats. The order of ethanol and saline injection was counterbalanced across all groups. The doses of ethanol (2g/kg) for CPP were based on our prior study (Zuo et al., 2015).
Post-conditioning (Day 6)
On day 6, the guillotine door was opened, and the time spent in each chamber was recorded during 15 minutes. The preference score was defined as the difference in time spent in the ethanol-paired chamber on the post-conditioning day vs the pre-conditioning day. Positive or negative scores indicated preference or aversion, respectively.
Extinction
After being tested for CPP expression, rats underwent 7 extinction trials at 48-hour intervals within 14 days using the indicated schedule; during this time no drug was administered. In each session, rats were allowed to investigate both chambers for 15 min and the time spent in each chamber was recorded. The time spent in ethanol-paired chamber during extinction period was standardized into the percentage of the time spent on post-test Day 6.
2.8. Data analysis
All data are expressed as mean ± S.E.M. (standard error of the mean). The ethanol or sucrose drinking data were measured during 24 hours and averaged over 5 continuous drinking sessions before intra-RMTg infusion, serving as the baseline level. The baseline locomotor activity was recorded for 60 minutes at one week before intra-RMTg infusion. These data were analyzed to extract the significant main effects and interactions; a two-way repeated measure ANOVA (RM ANOVA) and a Bonferroni or Tukey post hoc comparison were utilized. Comparisons of the number of NeuN-, MOR- and GAD67-IR cells among different groups were performed using t-test or one-way ANOVA followed by a post hoc Bonferroni test.
CPP expression data of each rat were expressed as the percentage of time spent in the ethanol-paired chamber over total time spent in the apparatus. The data from the three treatment groups were analyzed using one-way ANOVA followed by Tukey post hoc comparison test. CPP extinction data were analyzed using two-way RM ANOVA for the treatment and extinction time, followed by Tukey post hoc comparison test. Significance was set at p < 0.05.
3. Results
3.1. Intra-RMTg infusion of dermorphin-saporin increases self-administration of ethanol but not sucrose
Thirty-three of the 36 rats (91.6%) under the I2BC paradigm significantly escalated their ethanol consumption (data not shown) and maintained it at a stable level 6–8 weeks into the drinking program, in agreement with our previous report (Li et al., 2010). Three rats drank less than 3 g/kg of ethanol in 24 hours and were excluded from further study. The remaining rats that consumed ethanol at the level of 5.50 ± 0.35 g/kg (n=33) in 24 hours were randomly assigned to one of three groups. Each group received a single intra-RMTg infusion of DS (n=12), BS (n=12), or vehicle (aCSF, n=9), respectively. These rats resumed ethanol drinking seven days after intra-RMTg infusion.
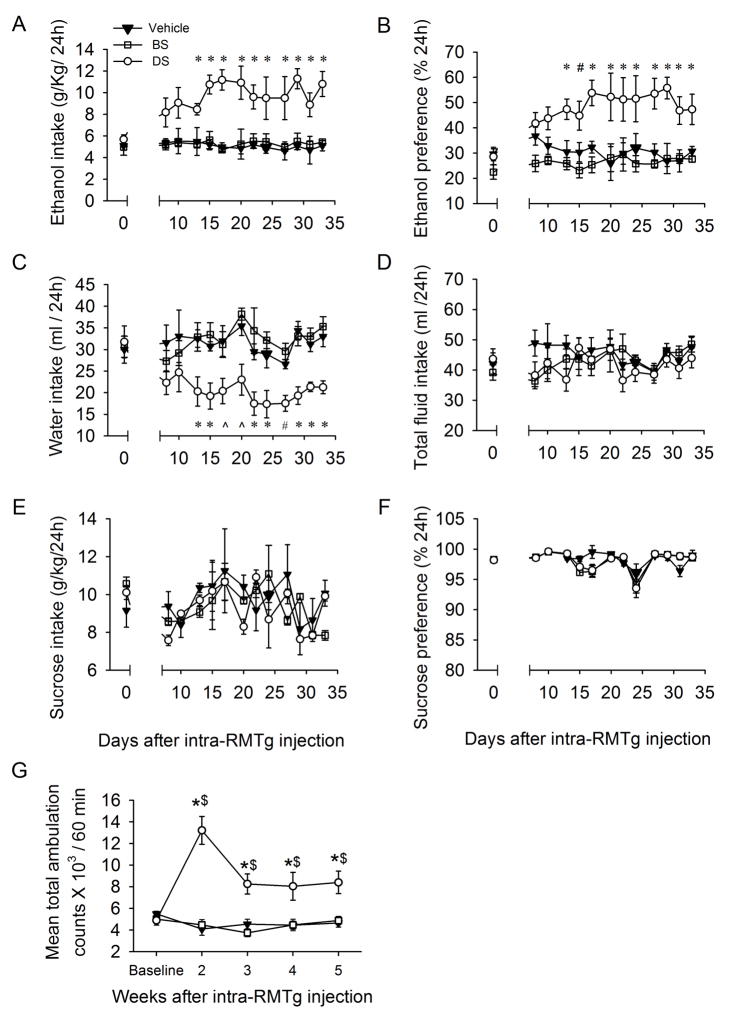
Ethanol intake in 24 hours of the DS group was significantly higher than those in BS or vehicle groups from day 14 to day 34 after RMTg infusion (Fig. 1A; F (2, 288) = 22.367, p< 0.001). Ethanol preference was also significantly higher in the DS group from day 11 to 34 (Fig. 1B, F (2, 288) = 36.284, p < 0.001). Conversely, the 24 hour water consumption of the DS group was significantly less than either BS or vehicle groups from day 11 to day 34 after RMTg infusion (Fig. 1C; F(2, 288) = 17.273, p < 0.001). There was no significant difference regarding total fluid intake among DS, BS, and vehicle groups during all sessions (Fig. 1D).
Fig. 1.
Intra-RMTg dermorphin–saporin substantially increases the intake of ethanol and the basal locomotor activity. The rats in DS-injected group, but not in BS- or vehicle-groups, robustly escalated the intake (A) and preference (B) for ethanol during 24 hours after the onset of drinking sections. Moreover, DS infusion reduced water intake (C), but did not affect the total fluid intake (D). However, intra-RMTg DS infusion did not alter the intake (E) and preference (F) for sucrose. (G), the mean total ambulation for rats injected with DS were substantially elevated compared with vehicle (100 nl/side, n = 9), BS (1.5 pmol/100nl/side, n = 9) at 2, 3, 4 and 5 weeks after intra-RMTg infusion. * p < 0.05, vs. both BS and vehicle; # p < 0.05, vs. BS group; ^ p < 0.05, vs. vehicle group, *$ p < 0.05, vs. baseline (two-way RM ANOVA followed by Tukey post hoc analysis), n = 9 rats in each group.
We also measured the intake of sucrose, using intermittent access to 2% sucrose in a two-bottle choice procedure (Li et al., 2011). Intra-RMTg DS infusion altered neither sucrose intake (Fig. 1E, F(2, 180) = 0.449; p = 0.654) nor preference (Fig. 1F; F(2, 180) = 0.808; p = 0.479), compared to BS- or vehicle-groups (all p > 0.05). These results indicate that intra-RMTg DS infusion selectively affects ethanol-drinking behavior.
3.2. Intra-RMTg DS infusion increases locomotion
To determine whether the change in ethanol intake resulted from the change in locomotion, we evaluated the effect of intra-RMTg DS infusion on basal locomotor activity. The mean total ambulation distance within 60 minutes was assessed once each week (before infusion, and 2–5 weeks after infusion) in DS, BS, and vehicle groups (Fig. 1G). While the basal locomotor activity showed no difference among the three groups, the mean total ambulation counts were significantly higher in DS than those in either the vehicle or BS groups on day 14 (all p < 0.001) and on days 21–35 after infusion. However, the levels of ambulation of the DS group were lower during days 21–35 than that on day 14 (all p < 0.001). A two-way RM ANOVA indicated a main effect of treatment (F(2, 96) = 52.103, p < 0.001), a main effect of time (F(4, 96) = 3.433, p = 0.019), and a significant treatment × time interaction for mean total ambulation during the entire experimental period (F(8, 96) = 8.922, p < 0.001). Conversely, locomotor activity in the BS group was not different from that in the vehicle group at any time point. Together, these data indicate that intra-RMTg infusion of DS specifically induces hypermobility.
3.3. Histological identification of infusion sites in the RMTg
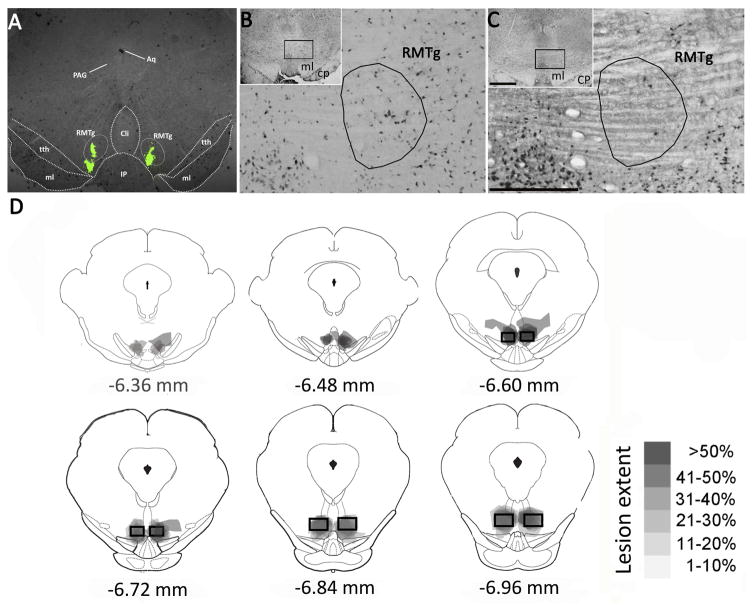
In order to assess the efficacy of DS on neuron loss, we examined the distribution of the neuronal marker NeuN in RMTg-containing brain sections of rats on day 34 after intra-RMTg infusion of DS or BS after obtaining behavioral data. As shown in Figure 2, in 9 of 12 rats in the DS group, lesions were concentrated in the RMTg, identified according to previous reports (Jhou et al., 2009a; Kaufling et al., 2009). The number of NeuN immunoreactive neurons within the RMTg was significantly smaller in DS-injected rats than that in BS-injected rats, indicating that DS had effectively ablated neurons in the RMTg (see Table 1). Nevertheless, as illustrated in Figure 2D, DS-induced lesions extended to some regions outside the RMTg, including the adjacent caudal linear nucleus of the raphe (CLi), the dorsal interpeduncular nucleus (IPN), the trigeminothalamic tract (tth) and the posterior VTA (pVTA). The remaining 3 rats in the DS group showed no lesions in the RMTg and adjacent areas, no significant reduction of NeuN positive cells, no significant change on consumption of and preference for ethanol, compared to the BS-injected rats, before infusion and during the 34 day observation period.
Fig. 2.
Intra-RMTg DS infusion reduces NeuN, glutamic acid decarboxylase (GAD-67) and μ opioid receptor (MOR) immunoreactiviy (IR) in the RMTg. Panel (A) is a representative photograph showing the accurate location in the rostral RMTg using bilateral stereotaxic microinjection of diluted green retrobead IX (100 nl/side). Panel (B) illustrates a transverse section containing RMTg area in BS-injected rat (case 010206) stained by NeuN immunostaining (AP: −6.72 mm from bregma). A representative photomicrograph at corresponding levels shows the histological confirmation of lesions of the RMTg area in DS-injected rat (case 010910) in panel (C), as well as relatively sparing neurons in the adjacent caudal linear nucleus of the raphe (CLi), dorsal interpeduncular nucleus (IPN) and trigeminothalamic tract (tth). Panel (D) depicts the outlines of RMTg area lesions in DS-injected rats. Semi-transparent shading shows lesion sites overlaid for each rat, so that darkest areas indicate areas of greatest damage. The grey scale is provided to demonstrate the lesion extent in different brain regions. Square boxes bracket the location where most GABAergic VTA-projecting neurons are found (Jhou et al., 2009). Aq, aqueduct; PAG, periaqueductal gray; CP, cerebral peduncle; CLi, caudal linear nucleus of the raphe; tth, trigeminothalamic tract; IP, interpeduncular nucleus. Scale bar=400 μm in C and 1 mm in inset.
Table 1.
Quantitation of NeuN immunoreactive (IR) cells in the RMTg and its surrounding areas from blank-saporin (BS) and dermorphin-saporin (DS) treated rats. Values are expressed as mean ± SEM.
| NeuN IR cell counting (number/mm2) | |||
|---|---|---|---|
|
| |||
| Brain regions | BS group n=9 | DS group n=9 | p-Value |
| RMTg | 292±36.7 | 28.5 ± 3.4### | P<0.001 |
| CLi | 468.4±54 | 350.8 ± 33.7 | P=0.08 |
| IPN | 1441.7±164.2 | 1090.7±143 | P=0.126 |
| tth | 323.2±29.6 | 280.8±37.7 | P=0.389 |
| pVTA | 336±25.1 | 296.6±16.1 | P=0.236 |
p <0.001 vs BS by t-test. CLi, caudal linear nucleus of the raphe; tth, trigeminothalamic tract; IPN, interpeduncular nucleus. pVTA, posterior ventral tegmental area.
3.4. Intra-RMTg DS infusion decreases MOR and GAD67 immunoreactivity
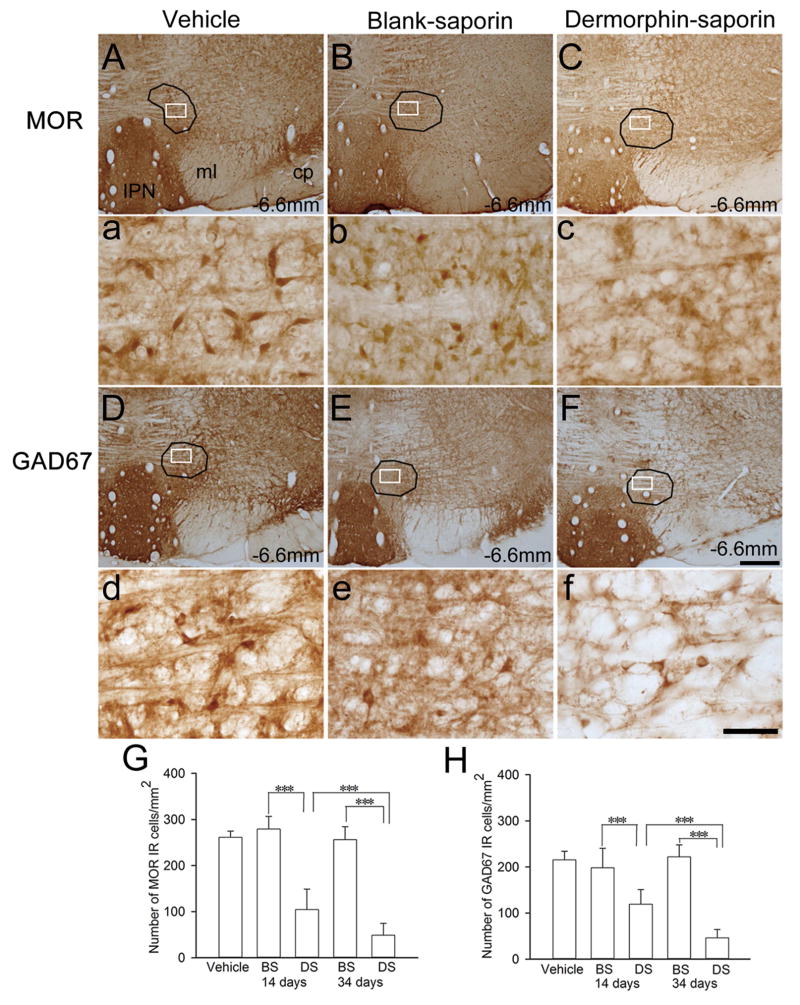
As mentioned, the RMTg expresses high levels of the GABA synthesizing enzyme GAD67 and the MOR immunoreactivity that distinguish it from surrounding regions (Jhou et al., 2009a; Kaufling et al., 2009, 2010; Olson and Nestler, 2007; Perrotti et al., 2005). To further assess the effect of DS, we examined GAD67 and MOR expression by immunohistochemistry. As illustrated in the vehicle-injected rats in Figure 3A and D, MOR and GAD67 immunoreactive (IR) cells in the RMTg were mainly distributed in an area that was lateral to caudal linear nucleus of the raphe, the median raphe nucleus (MnR), and partially embedded within the decussation of the superior cerebellar peduncle, which was consistent with previous reports (Jhou et al., 2009a; Jhou et al., 2009b; Jhou et al., 2012). On day 34 after DS infusion, but not BS or vehicle infusion, we observed an extensive loss of cell bodies with MOR immunoreactivity within the RMTg area (Fig. 3C). Conversely, there was no significant difference in the numbers of MOR IR cells between BS and vehicle-treated groups (Fig. 3A and B). Similarly, on day 34 after RMTg infusion, the number of GAD67 IR cells within the RMTg in the DS-treated rats was markedly lower than that in the BS and vehicle-treated rats (Fig. 3F). There was no difference in the numbers of GAD67 IR cells between BS and vehicle-treated groups on day 14 or day 34 (Fig. 3D and E). Figure 3G and H illustrated the statistical result of MOR and GAD67 IR cell quantification on days 14 and 34 after intra-RMTg injection.
Fig. 3.
Intra-RMTg DS injection reduces glutamic acid decarboxylase (GAD-67) and μ opioid receptor (MOR) immunoreactivity (IR) in the RMTg. Figure (A–C) and (D–F) shows representative photomicrographs of MOR- and GAD67-IR in coronal sections of the RMTg at the level of −6.72 mm from bregma on 34th day after intra-RMTg injection with vehicle, BS or DS. The statistical result of MOR and GAD67 IR cell quantification on 14th and 34th day of injection has been summarized in (G) and (H). *** p < 0.001, vs. either vehicle- or BS-treated rats (n = 5 rats in each group). cp, cerebral peduncle; IPN, interpeduncular nucleus; ml, medial lemniscus. Scale bar = 100 mm in F and 100 μm in f.
3.5. Intra-RMTg DS infusion enhances the expression of ethanol induced conditioned place preference and slows the extinction
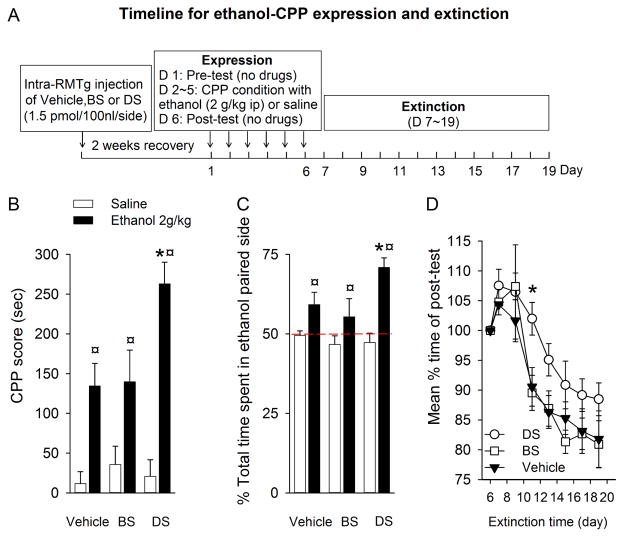
As shown in Figure 4B and C, the rats in all three groups (DS, BS, and vehicle) that were conditioned with ethanol (2g/kg, i.p. for 4 days) spent more time and a greater percentage of time in the ethanol-paired chamber than the rats that were only conditioned with saline (F(1, 37) = 22.787, p < 0.001). Importantly, the rats that received a DS infusion spent a significantly longer time in the ethanol-paired chamber than the BS or vehicle groups (p =0.001 vs. vehicle; p = 0.007 vs. BS). There was no significant difference between BS and vehicle groups, and among vehicle-, BS- and DS-treated rats that were only conditioned with saline.
Fig. 4.
Intra-RMTg DS injection significantly enhances the expression of ethanol-induced CPP, slows the extinction, and attenuates the expression of ethanol CPA. Panel (A) depicts the timeline for the expression of ethanol induced CPP and extinction. During the post-test session, all rats conditioned with ethanol (2 g/kg) spent a longer time (B) and a greater percentage of time (C) in the ethanol-paired chamber than the rats conditioned with saline only. Furthermore, RMTg area lesions by DS markedly enhanced ethanol-induced CPP expression compared to BS and vehicle. (D) During the extinction phase, rats injected with DS extinguished slower than those in the BS- or vehicle-injected rats. ¤ p < 0.001, compared respectively to Vehicle-, BS- and DS-treated rats that conditioned with saline only; * p < 0.05, compared to BS and vehicle treated rats that conditioned with ethanol. (n=8 in each group).
We next determined if intra-RMTg DS infusion might affect the extinction from ethanol-induced CPP. As described in the Methods section, after ethanol-induced CPP expression, rats underwent 7 extinction trials at 48-hour intervals within 14 days. As shown in Figure 4D, an extinguished CPP was observed in all groups (F(2,126) = 8.854, p < 0.001). However, CPP extinguished much slower in the DS group than BS- or vehicle groups (F(2, 21) = 4.586, p = 0.03). Bonferroni post hoc comparison found a significant difference among the DS, BS, and vehicle groups (p < 0.05).
4. Discussion
We show here a robust increase in the intake of ethanol, preference for ethanol, and locomotor activity in rats that received an intra-RMTg infusion of DS, which destroyed MOR-expressing neurons in the RMTg. Conversely; no such change was found in rats that received BS or vehicle infusion. Moreover, DS infusion significantly enhanced ethanol-induced CPP expression and slowed down extinction. These results suggest that RMTg MOR-expressing neurons play a significant role in ethanol-related behavior.
Using MOR and GAD67 antibodies, we identified a group of MOR-expressing GABAergic neurons within the RMTg, extending previous reports of high levels of GAD67 and MOR-expressing neurons (Barrot et al., 2012) and the co-localization of GAD67 and MOR immunostaining in the RMTg (Jalabert et al., 2011) to the females. To investigate the role of these neurons in ethanol-related behaviors, we used a specific pharmacological tool, dermorphin-saporin (DS). As a potent MOR agonist, dermorphin induces internalization of MORs upon binding (Giagnoni et al., 1984), while saporin is a ribosome-inactivating cytotoxin (Wiley and Kline, 2000). As expected, DS effectively destroyed a substantial portion of MOR-expressing cells. The parallel reduction in MOR- and GAD67-labeling neurons suggests that most, if not all of the MORs are localized on GABAergic neurons (Jalabert et al., 2011). Intra-RMTg DS infusion greatly increased ethanol intake and preference. These results suggest that RMTg MOR-expressing GABAergic neurons may normally be responsible for ethanol aversion and act as a brake for drinking. Conversely, these neurons may normally inhibit the euphoric effect of ethanol, and their removal makes drinking more rewarding. We tested these ideas by CPP experiments. As expected, ethanol (2g/kg, i.p.) elicited CPP. Importantly, RMTg lesion increased CPP expression, suggesting that ethanol may become more rewarding, and/or less aversive.
Significant evidence implicates the endogenous opioid system in the mechanisms underlying the effects of ethanol (Mendez and Morales-Mulia, 2008). Ethanol modulates opioidergic signaling and function at different levels, including biosynthesis, release, and degradation of opioid peptides, as well as binding of endogenous ligands to opioid receptors. Ethanol increases the release of β-endorphin from the hypothalamic arcuate nucleus, which can modulate activity of other neurotransmitter systems such as mesolimbic dopamine. β-endorphin and MORs play importance roles in some of the ethanol-related behaviors, including drinking and CPP (Kuzmin et al., 2003). Blocking MORs with an opioid antagonist such as naltrexone significantly reduces ethanol consumption in animal models (see for example (Kamdar et al., 2007)) and in humans with variable efficacy (Anton et al., 2004; Anton et al., 2006; O’Malley et al., 1992; Volpicelli et al., 1992). Importantly, as mentioned, the RMTg is rich in MORs.
The current study has some limitations. Specifically, given that moderate levels of MORs are expressed in areas surrounding the RMTg (Ding et al., 1996; Mansour et al., 1994), MOR-expressing neurons in these areas may also be lesioned by intra-RMTg DS injection. To minimize this possibility, we injected a small volume and a low dose of DS. Despite these precautions, there were lesions outside the RMTg (Fig. 2). Therefore, we cannot conclude that all changes in ethanol-related behaviors induced by intra-RMTg DS injection were exclusively due to the loss of cells within the RMTg.
Our results from the combination of ethanol drinking and place conditioning behavior studies strongly suggest that the RMTg MOR-expressing neurons may impact the rewarding/aversion balance of ethanol. Our results are in line with previous reports that showed that a lesion of the RMTg inhibits fear-conditioned freezing (Jhou et al., 2009a) and that cocaine-induced avoidance behaviors were abolished by lesions of the RMTg, or by optogenetic inactivation of the RMTg (Jhou et al., 2013). The consistent trend of our results from two independent ethanol-related behavioral studies (voluntary drinking and CPP) suggest that ethanol is more reinforcing or less aversive to the rats with RMTg destruction. Our results are also in general agreement with the evidence that inhibiting the activity of VTA-GABA neurons increases the activity of VTA-dopamine neurons and is behaviorally rewarding (Creed et al., 2014). Conversely, direct excitation of VTA-GABA neurons disrupts reward-related behaviors (van Zessen et al., 2012), and stimulation of VTA-GABA neurons or inhibition of VTA-dopamine neurons promotes aversion (Tan et al., 2012). Interestingly, Taha and colleagues showed that lesions of the LHb increases ethanol consumption (Haack et al., 2014); which may be due to the reduction of RMTg activity, since RMTg receives a major excitatory input from LHb.
Equally interesting is our finding regarding the hyperactivity in rats that received RMTg DS infusion. This finding indicates that RMTg GABA neurons may normally play an inhibitory role over locomotion (Huff and LaLumiere, 2015). Dopamine cells are implicated in many aspects of motivated behavior (Barrot et al., 2012) and locomotor activity (Beninger, 1983). Since a previous study showed that GABA projections from the RMTg are inhibited by opioids, which consequently disinhibits VTA-dopamine neurons (Matsui and Williams, 2011), MORs in the RMTg could act as a regulator of VTA-dopamine neuron function. Therefore, we infer that destruction of RMTg neurons would reduce GABA inputs to VTA, disinhibiting dopamine neurons, elevating mesolimbic dopamine levels, and increasing the basal locomotor activity. Thus, the increases in ethanol consumption, preference, and reward could be explained by ethanol modulating VTA neurons in this lesion-induced, disinhibited state. It is unlikely that the rewarding properties of abused drugs are secondary to their ability to induce behavioral activation since drugs such as opiates, ethanol, and barbiturates retain their rewarding properties at doses eliciting depression of motor behavior (Di Chiara and Imperato, 1988). The rewarding and motor-stimulating properties often coincide because they are both dependent, at least in part, on the activation of the mesolimbic dopamine system. Thus, activation of the mesolimbic dopamine system would result in or contribute to an interceptive pleasurable effect (reward) and a motor-activating effect. Motor activation; however, does not seem to be essential for experiencing pleasure (Di Chiara and Imperato, 1988).
We conducted the current study in female rats to take advantage of their stable head size (Rodd-Henricks et al., 2000), which helps to increase the accuracy of microinjection to a small brain region such as the RMTg. Moreover, female rodents drink more alcohol than their male counterparts (Hwa et al., 2011). Although we did not monitor the estrous cycle, which may have an effect on our results, we believe this is less of a concern since we established control groups (BS and vehicle groups) so that any effect of a given phase of the estrous cycle will be distributed across experimental conditions. Other studies have found that total daily ethanol intake was unaffected by the stage of estrous cycle when females were allowed to cycle freely, although the microstructure of ethanol drinking was reported to vary across the estrous phase (Ford et al., 2002; Torres et al., 2014). Our current study on female rats has additional significance, as recently emphasized by the NIH that more studies in females are needed (Clayton and Collins, 2014).
In summary, we demonstrated that destruction of RMTg MOR-expressing GABAergic neurons by DS elevated the intake and preference for ethanol, enhanced the expression and slowed the extinction of ethanol CPP. These results were paralleled with increased locomotion and a massive loss of MOR-expressing GABAergic neurons. These were not seen in rats injected with BS or vehicle. These findings indicate that RMTg MOR-expressing GABAergic neurons play a significant role in the regulation of ethanol consumption and CPP.
Highlights.
RMTg infusion of dermorphin-saporin (DS) increased ethanol intake and locomotion
DS infusion reduced protein expression of glutamic acid decarboxylase 67 and MORs
DS infusion decreased neurons with glutamic acid decarboxylase 67 and MOR in RMTg
RMTg DS infusion enhanced ethanol-induced CPP acquisition and slowed CPP extinction
Acknowledgments
This work was supported by grants (AA022292 and AA021657) from the National Institute of Health and the New Jersey Health foundation (PC109-13).
List of abbreviations
- aCSF
artificial cerebrospinal fluid
- AUDs
alcohol use disorders
- BS
blank saporin
- CLi
caudal linear nucleus of the raphe
- CPP
conditioned place preference
- DOR
delta opioid receptor
- DS
dermorphin saporin
- GAD67
glutamic acid decarboxylase 67
- i.p
intraperitoneal
- IPN
interpeduncular nucleus
- IR
immunoreactive
- LHb
lateral habenula
- MnR
median raphe nucleus
- MOR
mu opioid receptor
- pVTA
posterior VTA
- RMTg
rostromedial tegmental nucleus
- s.c
subcutaneously
- SEM
standard error of the mean
- tth
trigeminothalamic tract
- VTA
ventral tegmental area
Footnotes
The authors declare no conflict of interest.
Authors Contribution
RF and JHY were responsible for the study concept and design. RF performed the stereotaxic surgery. RF and XC performed behavioral, histological and molecular biological experiments. RF and JHY analyzed the data and drafted the manuscript. WHZ, JL and SK helped on the data analysis and provided critical comments on the design. LHZ, AS, AB provided critical comments on the manuscript. JHY supervised the study and provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci. 2012;32:14094–14101. doi: 10.1523/JNEUROSCI.3370-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed MC, Ntamati NR, Tan KR. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front Behav Neurosci. 2014;8:8. doi: 10.3389/fnbeh.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res. 2002;26:635–643. [PubMed] [Google Scholar]
- Giagnoni G, Parolaro D, Casiraghi L, Crema G, Sala M, Andreis C, Gori E. Dermorphin interaction with peripheral opioid receptors. Neuropeptides. 1984;5:157–160. doi: 10.1016/0143-4179(84)90051-9. [DOI] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One. 2014;9:e92701. doi: 10.1371/journal.pone.0092701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, LaLumiere RT. The rostromedial tegmental nucleus modulates behavioral inhibition following cocaine self-administration in rats. Neuropsychopharmacology. 2015;40:861–873. doi: 10.1038/npp.2014.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A. 2011;108:16446–16450. doi: 10.1073/pnas.1105418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Xu SP, Lee MR, Gallen CL, Ikemoto S. Mapping of reinforcing and analgesic effects of the mu opioid agonist endomorphin-1 in the ventral midbrain of the rat. Psychopharmacology (Berl) 2012;224:303–312. doi: 10.1007/s00213-012-2753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. gamma-Aminobutyric acid cells with cocaine-induced DeltaFosB in the ventral tegmental area innervate mesolimbic neurons. Biol Psychiatry. 2010;67:88–92. doi: 10.1016/j.biopsych.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Krumins SA. Characterization of dermorphin binding to membranes of rat brain and heart. Neuropeptides. 1987;9:93–102. doi: 10.1016/0143-4179(87)90048-5. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology. 2011;36:589–602. doi: 10.1038/npp.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2012;37:1164–1176. doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH. Region-specific induction of FosB/DeltaFosB by voluntary alcohol intake: effects of naltrexone. Alcohol Clin Exp Res. 2010;34:1742–1750. doi: 10.1111/j.1530-0277.2010.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nie H, Bian W, Dave V, Janak PH, Ye JH. Microinjection of glycine into the ventral tegmental area selectively decreases ethanol consumption. J Pharmacol Exp Ther. 2012;341:196–204. doi: 10.1124/jpet.111.190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci. 2011;31:17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M, Morales-Mulia M. Role of mu and delta opioid receptors in alcohol drinking behaviour. Curr Drug Abuse Rev. 2008;1:239–252. doi: 10.2174/1874473710801020239. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci. 1997;17:5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci U S A. 2011;108:4459–4464. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 7. 2007. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Bilsky EJ, Meng ID. Selective ablation of mu-opioid receptor expressing neurons in the rostral ventromedial medulla attenuates stress-induced mechanical hypersensitivity. Life Sci. 2011;89:313–319. doi: 10.1016/j.lfs.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Shank EJ, Seitz PK, Bubar MJ, Stutz SJ, Cunningham KA. Selective ablation of GABA neurons in the ventral tegmental area increases spontaneous locomotor activity. Behav Neurosci. 2007;121:1224–1233. doi: 10.1037/0735-7044.121.6.1224. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O’Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcohol Clin Exp Res. 2014;38:108–115. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterwald EM, Anton B, To T, Lam H, Evans CJ. Quantitative immunolocalization of mu opioid receptors: regulation by naltrexone. Neuroscience. 1998;85:897–905. doi: 10.1016/s0306-4522(97)00659-3. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wiley RG, Kline IR. Neuronal lesioning with axonally transported toxins. J Neurosci Methods. 2000;103:73–82. doi: 10.1016/s0165-0270(00)00297-1. [DOI] [PubMed] [Google Scholar]
- Zuo W, Fu R, Hopf FW, Xie G, Krnjevic K, Li J, Ye JH. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol. 2015 doi: 10.1111/adb.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]