Abstract
Aims
Diabetic bladder dysfunction (DBD) has been extensively studied in animal models of type 1 diabetes. We aimed to examine the functional and morphological alterations of the urinary bladder in a type 2 diabetes model, FVBdb/db mice.
Methods
FVBdb/db mice and age-matched FVB/NJ control mice were tested at either 12, 24 or 52 weeks of age. Body weight, blood glucose and glycated hemoglobin (HbA1c) levels were measured. Bladder function was assessed by measurement of 24-hour urination behavior and conscious cystometry. Bladder was harvested for Masson's Trichrome staining and morphometric analysis.
Results
The body weights of FVBdb/db mice were twice as those of FVB/NJ control mice. The blood glucose and HbA1c levels were higher in FVBdb/db mice at 12 and 24 weeks, but not at 52 weeks. A significant increase in the mean volume per void, but decrease in the voiding frequency, in FVBdb/db mice was observed. Cystometry evaluation showed increased bladder capacity, voided volume, and peak micturition pressure in FVBdb/db mice compared with FVB/NJ mice. Morphometric analysis revealed a significant increase in the areas of detrusor muscle and urothelium in FVBdb/db mice. In addition, some FVBdb/db mice, especially males at 12 and 24 weeks, showed small-volume voiding during 24-hour urination behavior measurement, and detrusor overactivity in the cystometry measurement.
Conclusions
The FVBdb/db mouse, displaying DBD characterized by not only increased bladder capacity, void volume, and micturition pressure, but also bladder overactivity, is a useful model to further investigate the mechanisms of type 2 diabetes-related bladder dysfunction.
Keywords: Bladder, Type 2 diabetes, FVBdb/db mice, Obesity
1. Introduction
The prevalence of diabetes mellitus (DM) is continuously increasing, with estimates to reach 366 million cases in 2030 according to the World Health Organization (Wild et al., 2004). DM seriously affects multiple organ systems including the urinary bladder, predisposing to diabetic bladder dysfunction (DBD), one of the most common and costly complications that affects over 50% of patients (Brown et al., 2005). In 1976, Frimodt-Moller first described the classic symptoms of diabetic cystopathy, including increased bladder capacity and post voiding residual volumes, accompanied by decreased bladder sensation and contraction (Moller, 1976). Currently, DBD refers to an umbrella description for a group of clinical symptoms that encompass not only the above classic voiding problems, which are generally symptoms of later stage bladder dysfunction in DM, but also storage problems such as overactive bladder (OAB) and urge incontinence (Brown et al., 2005;Liu and Daneshgari, 2014). OAB is defined by the International Continence Society as symptoms of urgency, usually with frequency and nocturia, in the absence of causative infection or pathological conditions (Abrams et al., 2003). Detrusor overactivity (DO) is a urodynamic diagnosis frequently associated with OAB symptoms and is characterized by involuntary detrusor contractions during bladder filling (Abrams et al., 2003).
Even though type 1 diabetes mellitus (T1DM) accounts only for 5-10% of all diagnosed cases of diabetes (American Diabetes Association, 2010), most studies have focused on DBD in T1DM due to the low cost and reproducibility of streptozotocin (STZ)-induced T1DM animal models (Daneshgari et al., 2009). In such rodent models, we have characterized the full spectrum of temporal effects of T1DM on the bladder including function, morphometry, contractility, nerve innervation and vasculature (Daneshgari et al., 2006b;Xiao et al., 2013;Liu and Daneshgari, 2014). We demonstrated that the bladder undergoes temporal progression from an initial compensated phase to a later decompensated phase (>20 weeks of DM) (Daneshgari et al., 2006b;Xiao et al., 2013;Liu and Daneshgari, 2014). Polyuria causes bladder hypertrophy in the early stage of DM, whereas oxidative stress in the bladder caused by chronic hyperglycemia may play an important role in the late stage failure of bladder function (Xiao et al., 2013). The pathophysiology of DBD is multifactorial, including disturbances of the detrusor, urothelium, and nerve innervations (Liu and Daneshgari, 2014).
On the other hand, type 2 diabetes mellitus (T2DM) accounts for 90–95% of all cases of diabetes, but few studies have been conducted on DBD associated with T2DM. DBD in T2DM is undoubtedly more complex than in T1DM since a large proportion of T2DM patients (30-80%) have metabolic syndrome (MetS) (Kumar et al., 2013), including obesity, elevated triglyceride levels, low high-density lipoprotein cholesterol levels, and hypertension, some or all of which may affect bladder function independently (Tai et al., 2010). The higher incidence and the distinctive complex pathophysiology of T2DM, compared with T1DM, should encourage more research to elucidate structural and functional changes of urinary bladder in T2DM, as well as the underlying molecular mechanisms, which may suggest different therapeutic targets from those for T1DM.
The db/db mouse model on the FVB/NJ background (FVBdb/db) is a well-established obese T2DM mouse model, originating from autosomal recessive mutation in the leptin receptor, resulting in impaired signaling of leptin (Chua S Jr et al., 2002;Chen and Wang, 2005). Defective leptin signaling in the hypothalamus causes persistent hyperphagia and obesity, along with severe insulin resistance and hyperglycemia (Chua S Jr et al., 2002). Chua et al. found the blood glucose levels in FVBdb/db mice increased at 1 month of age and sustained at high levels during their investigated period of 7 months (Chua S Jr et al., 2002). The circulating insulin in the FVBdb/db mice increased at 3 months of age, and continued to increase at 5 and 7 months of age. In this study, we determined the temporal functional and morphological alterations of the bladder of FVBdb/db mice up to 52 weeks of age. We chose three time points, 12, 24 and 52 weeks of age, based on the previous observation on the temporal progression of bladder dysfunction in STZ-induced T1DM rodents (Daneshgari et al., 2006a; Daneshgari et al., 2006b; Liu and Daneshgari, 2014).
2. Materials and Methods
2.1. Experimental animals and design
Parent strains of FVBdb/db mice and background strain FVB/NJ mice were bought from The Jackson Laboratory (Bar Harbor, Maine). The mice for the experiments were obtained through colony breeding by laboratory personnel in the Health Sciences Animal Facility at Case Western Reserve University. Both male and female mice were tested at either 12, 24 or 52 weeks of age. Mice in each time point were divided into two sets. One set was used for bladder function assessment by 24-hour urination behavior and conscious cystometry. The other set had no surgical manipulation performed so intact bladders could be harvested for measurement of bladder weight and histological staining. Prior to euthanasia, mice were tested for non-fasting blood glucose levels using a glucometer (OneTouch SureStep, Johnson & Johnson, Milpitas, CA), and glycated hemoglobin (HbA1c) levels were measured by a commercial analyzer (in2itTM HbA1c analyzer, Bio-Rad, Hercules, CA). All protocols were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. The number of mice used for experiments and analysis of different parameters is shown in Supplementary Table 1.
2.2. Twenty-four hour fluid consumption and urination behavior measurement
Twenty-four hour urination behavior measurement was performed using mouse micturition chambers with a wire mesh bottom designed by Med Associates Inc. (St. Albans, VT) (Liu et al., 2015). The mice were placed in the micturition chambers. An electric balance with a data port connected to a data acquisition system was present below the bottom opening. Changes in the weight of the collection were recorded (MED-CMG, Catamount Research and Development, Saint Albans, VT). Twenty-four hours prior to micturition analysis, solid food was removed from the cages and replaced with lactose-free milk, to reduce the frequency and weight of feces generated during testing, thereby preventing feces droppings from interfering with measurement of urine output (Liu et al., 2006). The volume of milk consumed was recorded.
2.3. Suprapubic bladder catheter implantation and conscious cystometry
A suprapubic catheter was implanted 2 days prior to cystometry (Liu et al., 2015). Under anesthesia, the bladder was exposed and a circular purse string suture of 7-0 silk was used on the dome of bladder. A small incision was made and the catheter (PE-10 tubing with a flared tip) was inserted into the bladder and tightened. The catheter was tunneled subcutaneously and externalized at the back of the neck. The distal end of the tubing was sealed. Abdominal and skin incisions were closed separately.
For cystometry measurement, mice were placed in individual metabolic cages. A pressure transducer (BP-100, CB Sciences, Dover, NH) and a flow pump (Kent Scientific Corporation, Torrington, CT) were connected to the implanted bladder catheter. The bladder was filled with room temperature 0.9% saline (1ml/hr for FVB/NJ mice and 2 ml/hr for FVBdb/db mice) while bladder pressure was recorded. Different infusion rates were used based on the following considerations (Liu et al., 2008): (1) the bladder capacity of mice with diabetes is much larger than that of control mice - mice with diabetes actually produce urine faster than control mice, and therefore the higher infusion rate is closer to their real situation; (2) it would take much longer to induce voiding in FVBdb/db mice if we used the same infusion rate as in control mice, and a long non-physiological infusion time might affect bladder function. Urine was collected in a beaker on a force transducer (FT-03 D, Grass Instrument Co, Quincy, MA) placed beneath each cage. The pressure and force transducers were connected to an amplifier (ETH-400, CB Sciences), and multiport controller software (MED-CMG, Catamount Research and Development. Saint Albans, VT) was used for data recording (20 samples/second). After an initial 0.5-1 hour stabilization period, the data on 4-6 representative micturition cycles were collected and the mean values were calculated. The bladder capacity was calculated by multiplying intercontraction interval by the infusion rate. Voided volume was the volume expelled at micturition. Peak micturition pressure was measured at the peak of the bladder contraction. From the cystometry pressure and void volume tracings, DO was defined as obvious increase in voiding and/or non-voiding contraction, with the latter defined as intravesical pressure rises greater than 5 cmH2O from baseline pressure without a release of fluid from the urethra (Schnegelsberg et al., 2010).
2.4. Bladder fixation and staining
After mice were euthanized, their bladders were harvested and subsequently equilibrated in 37 °C Krebs' solution for 15 minutes, sectioned at the equatorial midline, and fixed in 10% neutral buffered formalin (pH 7.0) (Liu and Daneshgari, 2006). After fixation, tissues were dehydrated and embedded in paraffin. Serial 5 μm tissue sections were placed on microscope slides, and dewaxed for Masson's Trichrome staining.
2.5. Morphometric analysis
Stained slides were scanned (Leica SCN400 Slide Scanner). The images were analyzed with Image Pro 6.0 image analysis software (Media Cybernetics, Silver Springs, MD) (Xiao et al., 2015). The thickness of bladder wall was measured. The whole cross-sectional tissue area and the areas occupied by smooth muscle (red), collagen (blue) and urothelium (pink) were measured.
2.6. Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). All data were expressed as mean ± SEM. For two group comparisons at 12, 24 or 52 weeks old, unpaired two-tail t-test was used. For parameters in Table 1, Fisher's exact test was used to compare the percentage of FVBdb/db group to FVB/NJ group. P<0.05 was considered statistically significant.
Table 1.
Number of FVB/NJ and FVBdb/db mice with or without DO at 12, 24, and 52 weeks of age in observed mice
| Time points (wks) | Group | # with DO in total | % | # with DO in male mice | % | # with DO in female mice | % |
|---|---|---|---|---|---|---|---|
| 12 | FVB/NJ | 0/12 | 0 | 0/6 | 0 | 0/6 | 0 |
| FVBdb/db | 6/10 | 60.00 | 5/7 | 71.43 | 1/3 | 33.33 | |
| P value | 0.0028 | 0.0210 | 0.3333 | ||||
|
| |||||||
| 24 | FVB/NJ | 1/16 | 6.25 | 0/9 | 0 | 1/7 | 14.29 |
| FVBdb/db | 4/13 | 30.77 | 4/8 | 50.00 | 0/5 | 0 | |
| P value | 0.1440 | 0.0294 | 1.0000 | ||||
|
| |||||||
| 52 | FVB/NJ | 0/4 | 0 | 0/1 | 0 | 0/3 | 0 |
| FVBdb/db | 0/6 | 0 | 0/2 | 0 | 0/4 | 0 | |
| P value | 1.0000 | 1.0000 | 1.0000 | ||||
3. Results
3.1. Body weight and metabolic parameters of FVBdb/db mice
FVBdb/db mice showed a significant weight gain throughout the study period. Body weight of FVBdb/db mice was around two-fold of that in FVB/NJ mice (Supplementary Fig. 1A). A significant increase in bladder weight was also observed in FVBdb/db mice compared with FVB/NJ mice at all time points (Supplementary Fig. 1B). Blood glucose (Supplementary Fig. 1C) and HbA1c levels (Supplementary Fig. 1D) were significantly increased in FVBdb/db mice compared with FVB/NJ mice at 12 and 24 weeks, but returned to almost normal levels by 52 weeks of age.
3.2. Twenty-four hour drinking, urination behavior and cystometry in FVBdb/db mice
Figs. 1A and 1B are representative tracings of 24-hour urination behavior measurement. FVBdb/db mice consumed more fluid than FVB/NJ mice at 12 and 24 weeks, but not 52 weeks (Fig. 1C). Twenty four-hour total void volume in FVBdb/db mice was significantly increased at 12 weeks, higher but not significant at 24 weeks, and similar at 52 weeks, compared with FVB/NJ mice (Fig. 1D). Mean volume per void was increased (Fig. 1E), but voiding frequency was decreased (Fig. 1F) in FVBdb/db mice compared with FVB/NJ mice at all time points. These data suggest that diabetes causes increased urine output and bladder capacity, along with fewer voiding episodes.
Figure 1.
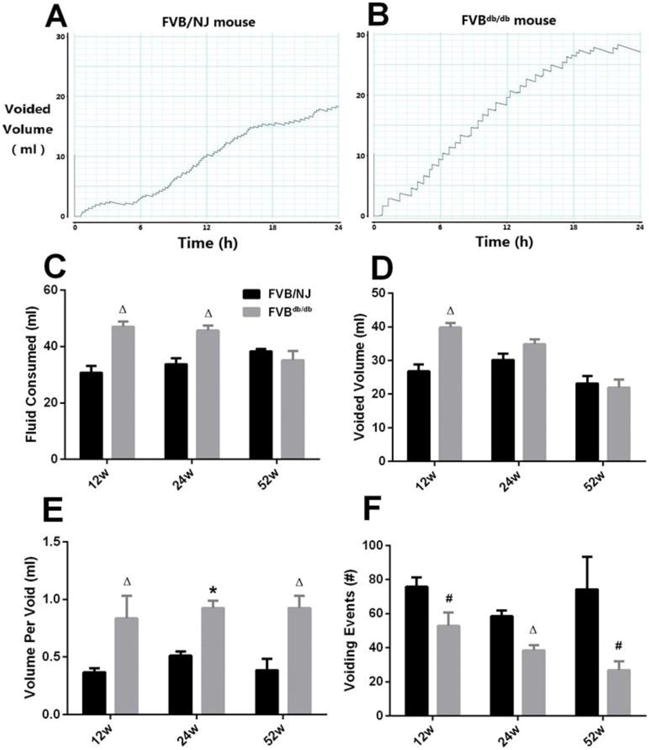
Representative tracings of 24-hour urination behavior measurement from a 12-week-old male FVB/NJ (A) and a FVBdb/db (B) mouse. 24-hour fluid consumed (C) and voided volume (D), mean volume per void (E), and 24-hour urination frequency (F) were quantified and compared. Results are expressed as mean + SE. #P<0.05 vs. FVB/NJ mice at the same time point; ΔP<0.01 vs. FVB/NJ mice at the same time point; *P<0.0001 vs. FVB/NJ mice at the same time point.
Cystometry measurement showed a regular and periodic emptying of the bladder in FVB/NJ mice (Fig. 2A) and some FVBdb/db mice (Fig. 2B). There were significant increases in bladder capacity (Fig. 2C), mean volume per void (Fig. 2D) in FVBdb/db mice compared with FVB/NJ mice. FVBdb/db mice also showed higher peak micturition pressure than that in FVB/NJ mice throughout the investigated period (Fig. 2E).
Figure 2.
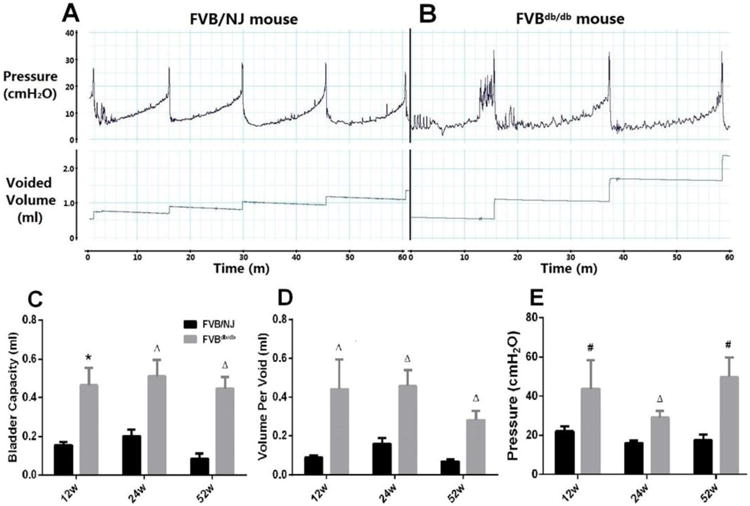
Representative cystometry tracings from a 12-week-old male FVB/NJ (A) and a FVBdb/db (B) mouse. Cystometric parameters including bladder capacity (C), mean volume per void (D), and the peak micturition pressure (E) were quantified and compared. The infusion rate is 1ml/hr for FVB/NJ mice; 2 ml/hr for FVBdb/db mice. Results are expressed as mean + SE. #P<0.05 vs. FVB/NJ mice at the same time point; ΔP<0.01 vs. FVB/NJ mice at the same time point; *P<0.0001 vs. FVB/NJ mice at the same time point.
In addition, we found some FVBdb/db mice, especially male FVBdb/db mice at 12 and 24 weeks, showed obvious small-volume voiding. This is illustrated by blunt steps (Fig. 3A) instead of sharp steps which indicate large-volume voiding (Fig. 1B), in 24-hour urination behavior measurement. The cystometry from these mice demonstrated significant amounts of small-volume voiding and non-voiding contractions between regular voiding (Fig. 3B, 3C). The above observations suggested these mice had DO, and the data from these mice were not included in the Figure 1 and 2. The number of the mice with the characteristics of DO was counted and summarized in Table 1. The percentage of male mice with DO was significantly higher than that of controls at 12 and 24 weeks (Table 1).
Figure 3.
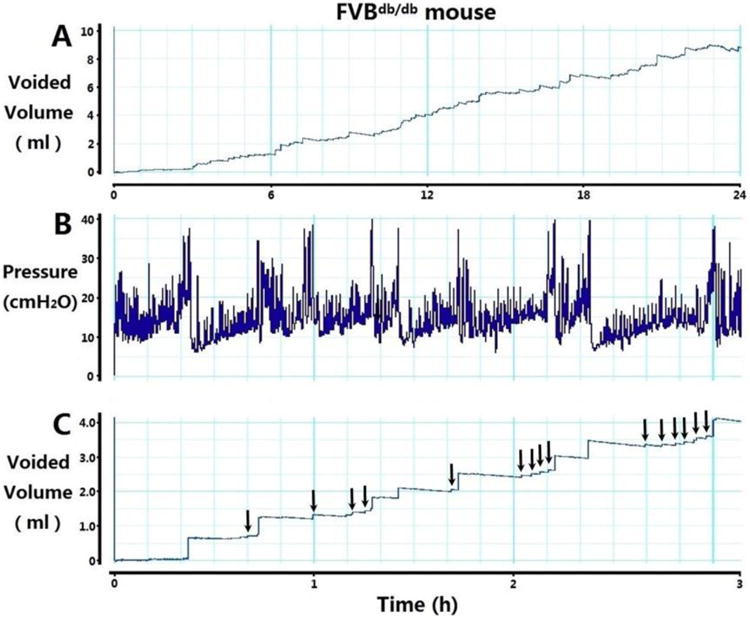
Representative tracings of 24-hour urination behavior measurement (A) and intravesical pressure during conscious cystometry (B) with corresponding voided volume (C) from a 12-week-old male FVBdb/db mouse, showing detrusor overactivity. Arrows in C indicate small-volume voids.
3.3. Morphometric analysis
Morphometric analysis of stained bladder sections (Fig. 4A, 4B) revealed a significant increase in the thickness of the bladder wall of FVBdb/db mice compared with FVB/NJ mice (Fig. 4C). Moreover, FVBdb/db mice exhibited significant increases in the whole tissue area (Fig. 4D), areas of urothelium (Fig. 4E) and smooth muscle (Fig. 4F) at all time points, and area of collagen at 12 and 52 weeks (Fig. 4G), compared with FVB/NJ mice.
Figure 4.
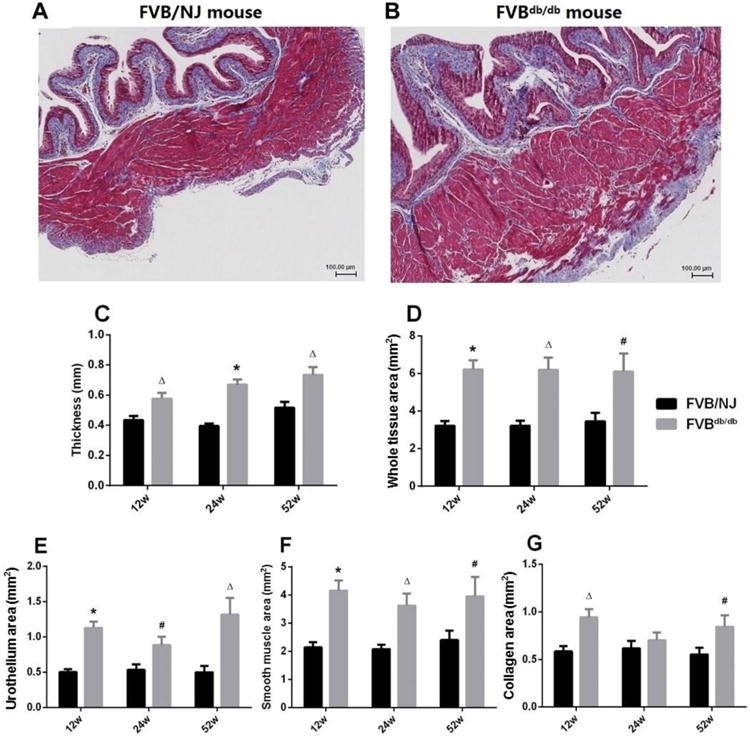
Representative images of Masson's trichrome staining sections at equatorial midline of urinary bladders from a12-week-old male FVB/NJ (A) and a FVBdb/db (B) mouse. The thickness of bladder wall (C), the whole cross-sectional tissue area (D), and the areas occupied by urothelium (inner light magenta, E), smooth muscle (outer magenta, F), and collagen (blue, G) were measured and compared. Scale bar, 100 μm.
4. Discussion
The classic symptoms of diabetic cystopathy are decreased bladder sensation, increased bladder capacity, and impaired bladder emptying with resultant elevated post-void residual urine (Moller, 1976). Long-time high glucose levels in urine and increased post-void residual urine put the patients at an increased risk for pyelonephritis and subsequent impairment of renal function (Geerlings et al., 2001;Hora et al., 2006;Geerlings, 2008). However, inconsistencies with those “classic” findings have been found in recent clinical studies (Kaplan et al., 1995;Ueda et al., 2000), in which the incidence of detrusor overactivity was reported around 25%-55%. It is now clear that DBD manifestations are a combination of storage and voiding bladder problems. DBD in animal models of T1DM has been investigated widely (Liu and Daneshgari, 2014). We have reported that DBD in T1DM follows a characteristic temporal course of progression, from compensated to decompensated bladder function (Daneshgari et al., 2006b;Liu and Daneshgari, 2014). DBD in T2DM has become more interesting since T2DM is the most common form, and is usually associated with MetS (Tai et al., 2010). Therefore, animal models of T2DM could encompass features such as obesity, insulin resistance, glucose intolerance and dyslipidemia to reflect the complexity and heterogeneous nature of the human disease. Current available rodent models of T2DM can be classified as chemically-, diet- or genetically-induced models (Chen and Wang, 2005). FVBdb/db mouse is a monogenic model, and is induced by homozygous deficiency of leptin receptor (Chen and Wang, 2005). Due to the lack of the normal leptin receptor, FVBdb/db mice cannot respond to leptin, therefore consume more food and become obese. FVBdb/db mice then develop insulin resistance and hyperglycemia, providing the opportunity to study the combined effects of obesity and hyperglycemia on bladder function.
In the present study, the body weights of FVBdb/db mice were around double those of control mice at 12, 24 and 52 weeks. Interestingly, we found blood glucose and HbA1C levels were increased significantly at 12 and 24 weeks but were back to normal levels between 40 to 52 weeks. This is probably due to an increase in insulin arising from an expansion of β-cell number in response to excessive food intake (Chua S Jr et al., 2002). The increased secretion of insulin from the expanded beta cells overwhelms the insulin resistance, finally reducing the level of blood glucose.
Bladder hypertrophy is a noticeable characteristic in FVBdb/db mice. Quantification of the bladder wall components of FVBdb/db mice revealed the primary source of the increased bladder weight was detrusor muscle, with contributions from urothelium and collagen too. An earlier study has shown that diabetes-induced polyuria can stimulate DNA synthesis, which in turn results in increased protein synthesis, causing increased cell mass and hyperplasia in smooth muscle and urothelium layers (Folkman and Moscona, 1978;Eika et al., 1993). The change of morphology might be a compensatory response to the increased urine output in FVBdb/db mice.
The results of 24-hour urination behavior measurement showed that fluid input and total urine volume in FVBdb/db mice were higher than those of the control mice at 12 and 24 weeks, but not at 52 weeks. As we know, it is hyperglycemia that causes increased urine output and fluid consumption. Since the blood glucose levels returned to normal in FVBdb/db mice at 52 weeks it is reasonable to see that the urine output and fluid consumption were similar with those in the control mice. Mean volume per void in FVBdb/db mice increased at 12 and 24 weeks, suggesting diabetes induces an increased bladder capacity. At 52 weeks, although the blood glucose levels were normal, mean volume per void was still high, indicating the effects of diabetes on bladder were not reversed. Although a previous study showed short-term (3-week induction) diabetes-and polyuria-induced functional and morphological alterations of the bladder can mostly be reversed in STZ-induced T1DM rats (Xiao et al., 2015), long-term diabetes duration may need a longer time to reverse the alterations and even make it irreversible (Cellek et al., 2003). Chua et al. reported that 4 weeks old FVBdb/db mice present hyperglycemia (Chua S Jr et al., 2002). For mice at 52 weeks of age in the present study, hyperglycemia was maintained for at least 36 weeks. This long-time diabetes duration may make the reversal difficult.
Cystometry evaluation showed increased bladder capacity, voiding volume, and peak voiding pressure in FVBdb/db mice. Similarly, Oger-Roussel et al., showed that 18 weeks old Goto-Kakizaki rat, another T2DM animal model characterized by modest hyperglycemia and mild insulin resistance, exhibited increased bladder capacity, longer intercontraction interval, and higher voiding pressure (Oger-Roussel et al., 2014). It has been postulated that hypertrophy of the smooth muscle and urothelium may be implicated in the development of increased micturition pressure, as a compensatory response to the polyuria associated with diabetes (Liu and Daneshgari, 2014). The increased bladder capacity may be due to the increased urine output (Liu and Daneshgari, 2006) and diabetes-related autonomic neuropathy. Altered metabolism of glucose, MetS-related ischemia, accumulated free radical formation, and metabolic derangement of the Schwann cell in diabetes can cause impairment of nerve conduction (Lee et al., 2009), consequently resulting in the increased bladder capacity.
Most interestingly, in addition to the above typical observations commonly seen in T1DM models (Liu and Daneshgari, 2005; Xiao et al., 2013; Liu and Daneshgari, 2014), we found some FVBdb/db mice, especially in male FVBdb/db mice at 12 and 24 weeks, voided frequently in small volumes. Cystometry from those mice showed a significant amount of unstable contractions with or without voiding between regular large-volume voids, suggesting the mice had detrusor overactivity.
The pathogenesis of OAB in T2DM could be associated with autonomic neuropathy, detrusor muscle overactivity, impaired urethra relaxation, and/or detrusor-sphincter dyssynergia. Ho et al. reviewed urodynamic findings from 94 female T2DM patients, among whom 34 had been diagnosed as having OAB (Ho et al., 2010). Patients in the OAB group had significantly higher percentages of increased bladder sensation and detrusor overactivity, and the most frequent urodynamic finding is increased bladder sensation, followed by detrusor overactivity (Ho et al., 2010). Studies demonstrated that diabetes can cause the alterations of nerve growth factor, urothelial chemicals, enzyme, and receptors in bladder (Yoshimura et al., 2005;Lee et al., 2009;Gillespie et al., 2009). These changes could irritate the sensory nerve and/or increase the excitability of the detrusor muscle, and cause OAB (Yoshimura et al., 2005;Lee et al., 2009;Gillespie et al., 2009). On the other hand, Wang et al. showed that mice with conditional hepatic double-knockout of Irs1 and Irs2 genes mice, a T2DM mice model, present OAB (Wang et al., 2012). The TNF-α levels increased in serum and bladder in those mice. Systemic inhibition of TNF-α–mediated signaling in mice reverses OAB without affecting hyperglycemia in these animals. They demonstrated that TNF-α can stimulate bladder smooth muscle cell directly, which may account for the bladder hyperactivity. In addition, diabetes can cause alterations in sympathetic and parasympathetic control of urethral function, leading to impaired urethra relaxation (Torimoto et al., 2004; Torimoto et al., 2005). Detrusor-sphincter dyssynergia was found in approximately 30% of diabetic rats, but never in controls (Yang et al., 2007). Those changes can increase outlet resistance, leading to bladder remodeling and OAB.
The extent and duration of hyperglycemia and/or MetS may relate to DO in FVBdb/db mice, since DO was seen most at 12 and 24 weeks, but none at 52 weeks. Chiu et al. reported that higher HbA1c levels increased the risk of OAB in patients with T2DM (Chiu et al., 2012). In hepatic-specific insulin receptor substrate 1 and 2 deletions-induced T2DM model, Wang et al. found that DO only presented in the mice at the early stage of diabetes (Wang et al., 2012). In addition, obesity and other components in MetS may also contribute to DO in FVBdb/db mice. Recently, both basic (Rahman et al., 2007;Lee et al., 2008) and clinical (Tai et al., 2010;Uzun and Zorba, 2012) investigations have suggested a link between MetS and OAB/DO. Rahman et al. found that rats fed a high fat diet (HFD) developed hyperlipidemia and DO (Rahman et al., 2007). Male rats fed a high fructose diet demonstrated unstable bladder contractions (Lee et al., 2008). In a clinical investigation, Uzun et al. found the MetS correlates with OAB in female patients. MetS was diagnosed in 201 (64%) of 313 patients with OAB and 73 (35%) of 208 patients without OAB (Uzun and Zorba, 2012). The pathogenesis of OAB/DO in T2DM/MetS could be associated with oxidative stress (Masuda et al., 2008). As we now know, increased oxidative stress, systemically and in tissues, is a hallmark of T2DM/MetS (Matsuda and Shimomura, 2013). Oxidative stress has been implicated in DO in animal models (Masuda et al., 2008). Induction of oxidative stress in the bladder with intravesical H2O2 can cause DO by stimulation of bladder C-fibers (Masuda et al., 2008).
The present study also showed that male FVBdb/db mice are more likely to have DO. A recent clinical study reported that male gender was one of the independent risk factors for OAB in T2DM patients (Liu et al., 2011). The prevalence of OAB was greater in the men than in the women with T2DM (Liu et al., 2011). The gender difference in susceptibility is not clear, probably related to the effects of different hormone levels on bladder and prostate. In addition, the effects of MetS/T2DM on prostate may increase the susceptibility in male. Gharaee-Kermani et al. found obesity/T2DM-related chronic inflammation causes prostate fibrosis and voiding dysfunction in mice (Gharaee-Kermani et al., 2013).
In conclusion, the FVBdb/db mouse displayed DBD characterized not only by increased bladder capacity, void volume, and micturition pressure, but also bladder overactivity. Similarly, recent clinical studies also showed the mixed picture of DBD in T2DM patients (Lee et al., 2009; Karoil et al., 2014). Therefore, FVBdb/db mouse model is a useful model for further understanding the pathophysiology of T2DM-related bladder dysfunction.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants U01DK076162, P20-DK090871, and R01-DK083733. Nan Xiao was partially supported by The Postgraduate Study Abroad Scholarship Program from the China Scholarship Council. We thank Amad Awadallah for his assistance in tissue sectioning and preparation of histological samples. We would also like to acknowledge the use of the Leica SCN400 Slide Scanner in the Genetics Department Imaging Facility at Case Western Reserve University made available through a National Center for Research Resource (NIH-NCRR) Shared Instrumentation Grant (1S10RR031845). Finally we would like to thank the individuals who assisted with data collection: Amin Rabie, Manisha Bhatia, Tamara Nsouli, Marissa Mendel, Edward Kim, and Hannah Daneshvar.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van KP, Victor A, Wein A. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JS, Wessells H, Chancellor MB, Howards SS, Stamm WE, Stapleton AE, Steers WD, van den Eeden SK, McVary KT. Urologic complications of diabetes. Diabetes Care. 2005;28(1):177–185. doi: 10.2337/diacare.28.1.177. [DOI] [PubMed] [Google Scholar]
- Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52(9):2353–2362. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- Chen D, Wang MW. Development and application of rodent models for type 2 diabetes. Diabetes Obes Metab. 2005;7(4):307–317. doi: 10.1111/j.1463-1326.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- Chiu AF, Huang MH, Wang CC, Kuo HC. Higher glycosylated hemoglobin levels increase the risk of overactive bladder syndrome in patients with type 2 diabetes mellitus. Int J Urol. 2012;19(11):995–1001. doi: 10.1111/j.1442-2042.2012.03095.x. [DOI] [PubMed] [Google Scholar]
- Chua S, Jr, Liu SM, Li Q, Yang L, Thassanapaff VT, Fisher P. Differential beta cell responses to hyperglycaemia and insulin resistance in two novel congenic strains of diabetes (FVB- Lepr (db)) and obese (DBA- Lep (ob)) mice. Diabetologia. 2002;45(7):976–990. doi: 10.1007/s00125-002-0880-z. [DOI] [PubMed] [Google Scholar]
- Daneshgari F, Huang X, Liu G, Bena J, Saffore L, Powell CT. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am J Physiol Regul Integr Comp Physiol. 2006a;290(6):R1728–R1735. doi: 10.1152/ajpregu.00654.2005. [DOI] [PubMed] [Google Scholar]
- Daneshgari F, Leiter EH, Liu G, Reeder J. Animal models of diabetic uropathy. J Urol. 2009;182(6 Suppl):S8–13. doi: 10.1016/j.juro.2009.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006b;176(1):380–386. doi: 10.1016/S0022-5347(06)00582-9. [DOI] [PubMed] [Google Scholar]
- Eika B, Levin RM, Monson FC, Murphy M, Longhurst PA. 3H-thymidine uptake by the rat urinary bladder after induction of diabetes mellitus. J Urol. 1993;150(4):1316–1320. doi: 10.1016/s0022-5347(17)35768-3. [DOI] [PubMed] [Google Scholar]
- Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Geerlings SE. Urinary tract infections in patients with diabetes mellitus: epidemiology, pathogenesis and treatment. Int J Antimicrob Agents. 2008;31(Suppl 1):S54–S57. doi: 10.1016/j.ijantimicag.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Geerlings SE, Stolk RP, Camps MJ, Netten PM, Collet JT, Schneeberger PM, Hoepelman AI. Consequences of asymptomatic bacteriuria in women with diabetes mellitus. Arch Intern Med. 2001;161(11):1421–1427. doi: 10.1001/archinte.161.11.1421. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R, Vezina CA, Sarma AV, Macoska JA. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate. 2013;73(10):1123–1133. doi: 10.1002/pros.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JI, van Koeveringe GA, de wachter SG, de Vente J. On the origins of the sensory output from the bladder: the concept of afferent noise. BJU Int. 2009;103(10):1324–1333. doi: 10.1111/j.1464-410X.2009.08377.x. [DOI] [PubMed] [Google Scholar]
- Ho CH, Tai HC, Yu HJ. Urodynamic findings in female diabetic patients with and without overactive bladder symptoms. Neurourol Urodyn. 2010;29(3):424–427. doi: 10.1002/nau.20727. [DOI] [PubMed] [Google Scholar]
- Hora M, Reischig T, Hes O, Ferda J, Klecka J. Urological complications of congenital nephrogenic diabetes insipidus--long-term follow-up of one patient. Int Urol Nephrol. 2006;38(3-4):531–532. doi: 10.1007/s11255-006-0093-3. [DOI] [PubMed] [Google Scholar]
- Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995;153(2):342–344. doi: 10.1097/00005392-199502000-00013. [DOI] [PubMed] [Google Scholar]
- Karoil R, Bhat S, Fatima J, Priya S. A study of bladder dysfunction in women with type 2 diabetes mellitus. Indian J Endocrinol Metab. 2014;18(4):552–557. doi: 10.4103/2230-8210.137518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Nagesh A, Leena M, Shravani G, Chandrasekar V. Incidence of metabolic syndrome and its characteristics of patients attending a diabetic outpatient clinic in a tertiary care hospital. J Nat Sci Biol Med. 2013;4(1):57–62. doi: 10.4103/0976-9668.107261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Chien CT, Yu HJ, Lee SW. Bladder dysfunction in rats with metabolic syndrome induced by long-term fructose feeding. J Urol. 2008;179(6):2470–2476. doi: 10.1016/j.juro.2008.01.086. [DOI] [PubMed] [Google Scholar]
- Lee WC, Wu HP, Tai TY, Yu HJ, Chiang PH. Investigation of urodynamic characteristics and bladder sensory function in the early stages of diabetic bladder dysfunction in women with type 2 diabetes. J Urol. 2009;181(1):198–203. doi: 10.1016/j.juro.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Liu G, Daneshgari F. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R837–R843. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- Liu G, Daneshgari F. Diabetic bladder dysfunction. Chin Med J (Engl) 2014;127(7):1357–1364. [PMC free article] [PubMed] [Google Scholar]
- Liu G, Daneshgari F. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol. 2005;288(6):F1220–F1226. doi: 10.1152/ajprenal.00449.2004. [DOI] [PubMed] [Google Scholar]
- Liu G, et al. Bladder function in mice with inducible smooth muscle-specific deletion of the manganese superoxide dismutase gene. Am J Physiol Cell Physiol. 2015;309(3):C169–C178. doi: 10.1152/ajpcell.00046.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Lin YH, Yamada Y, Daneshgari F. External urethral sphincter activity in diabetic rats. Neurourol Urodyn. 2008;27(5):429–434. doi: 10.1002/nau.20543. [DOI] [PubMed] [Google Scholar]
- Liu RT, Chung MS, Lee WC, Chang SW, Huang ST, Yang KD, Chancellor MB, Chung YC. Prevalence of overactive bladder and associated risk factors in 1359 patients with type 2 diabetes. Urology. 2011;78(5):1040–1045. doi: 10.1016/j.urology.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Pawlyk B, Damaser M, Li T. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol. 2006;168(2):519–528. doi: 10.2353/ajpath.2006.050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Kihara K, Saito K, Matsuoka Y, Yoshida S, Chancellor MB, de Groat WC, Yoshimura N. Reactive oxygen species mediate detrusor overactivity via sensitization of afferent pathway in the bladder of anaesthetized rats. BJU Int. 2008;101(6):775–780. doi: 10.1111/j.1464-410X.2007.07310.x. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obesity Research & Clinical Preactice. 2013;7:e330–e341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Moller CF. Diabetic cystopathy.I: A clinical study of the frequency of bladder dysfunction in diabetics. Dan Med Bull. 1976;23(6):267–278. [PubMed] [Google Scholar]
- Oger-Roussel S, Behr-Roussel D, Caisey S, Kergoat M, Charon C, Audet A, Bernabe J, Alexander L, Giuliano F. Bladder and erectile dysfunctions in the Type 2 diabetic Goto-Kakizaki rat. Am J Physiol Regul Integr Comp Physiol. 2014;306(2):R108–R117. doi: 10.1152/ajpregu.00033.2013. [DOI] [PubMed] [Google Scholar]
- Rahman NU, Phonsombat S, Bochinski D, Carrion RE, Nunes L, Lue TF. An animal model to study lower urinary tract symptoms and erectile dysfunction: the hyperlipidaemic rat. BJU Int. 2007;100(3):658–663. doi: 10.1111/j.1464-410X.2007.07069.x. [DOI] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R534–R547. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai HC, Chung SD, Ho CH, Tai TY, Yang WS, Tseng CH, Wu HP, Yu HJ. Metabolic syndrome components worsen lower urinary tract symptoms in women with type 2 diabetes. J Clin Endocrinol Metab. 2010;95(3):1143–1150. doi: 10.1210/jc.2009-1492. [DOI] [PubMed] [Google Scholar]
- Torimoto K, Fraser MO, Hirao Y, de Groat WC, Chancellor MB, Yoshimura N. Urethral dysfunction in diabetic rats. J Urol. 2004;171(5):1959–1964. doi: 10.1097/01.ju.0000121283.92963.05. [DOI] [PubMed] [Google Scholar]
- Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N. alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol. 2005;173(3):1027–1032. doi: 10.1097/01.ju.0000146268.45662.36. [DOI] [PubMed] [Google Scholar]
- Ueda T, Tamaki M, Kageyama S, Yoshimura N, Yoshida O. Urinary incontinence among community-dwelling people aged 40 years or older in Japan: prevalence, risk factors, knowledge and self-perception. Int J Urol. 2000;7(3):95–103. doi: 10.1046/j.1442-2042.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Uzun H, Zorba OU. Metabolic syndrome in female patients with overactive bladder. Urology. 2012;79(1):72–75. doi: 10.1016/j.urology.2011.08.050. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. Inhibition of TNF-alpha improves the bladder dysfunction that is associated with type 2 diabetes. Diabetes. 2012;61(8):2134–2145. doi: 10.2337/db11-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Xiao N, Huang Y, Kavran M, Elrashidy RA, Liu G. Short-term diabetes-and diuresis-induced alterations of the bladder are mostly reversible in rats. Int J Urol. 2015;22(4):410–415. doi: 10.1111/iju.12695. [DOI] [PubMed] [Google Scholar]
- Xiao N, Wang Z, Huang Y, Daneshgari F, Liu G. Roles of polyuria and hyperglycemia in bladder dysfunction in diabetes. J Urol. 2013;189(3):1130–1136. doi: 10.1016/j.juro.2012.08.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Dolber PC, Fraser MO. Diabetic urethropathy compounds the effects of diabetic cystopathy. J Urol. 2007;178(5):2213–2219. doi: 10.1016/j.juro.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005;95(6):733–738. doi: 10.1111/j.1464-410X.2005.05392.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


