Abstract
Respiratory syncytial virus causes a significant public health burden, particularly in very young infants and the frail elderly. The legacy of enhanced RSV disease (ERD) from a whole formalin-inactivated RSV vaccine, and the complex biology of the virus and the neonate have delayed the development of effective vaccines. However, new insights into factors associated with ERD and breakthroughs in understanding the antigenic structure of the fusion (F) glycoprotein have increased optimism that vaccine development is possible. This has led to investment of time and resources by industry, regulatory authorities, governments, and nonprofit organizations to develop the infrastructure needed to make the advanced clinical development of RSV vaccine candidates a reality.
Keywords: RSV, immunization, vaccination, structure-based vaccine design, fusion, neutralization, vaccine-enhanced illness, bronchiolitis, pneumonia, infants, elderly, Th2, eosinophilia, subunit vaccine, vaccine vector, wheezing, asthma, protein conformation, epitope
Epidemiology
Respiratory syncytial virus (RSV) is the most common cause of hospitalization in children under 5 years of age (1). In developing countries RSV also causes substantial mortality in children under 1 year of age (2). All children are infected by the age of 3 and people are repeatedly infected throughout life (3). In otherwise healthy children over 5 years of age and in adults, RSV typically causes an upper respiratory syndrome sometimes complicated by sinusitis and otitis media (4, 5). In individuals with T cell deficiencies like Severe Combined Immunodeficiency (SCID) or following allogenic bone marrow transplantation or lung transplantation, RSV can cause a life-threatening progressive pneumonia (6, 7). In addition, RSV infection in the frail elderly is associated with excess mortality at frequencies comparable to influenza virus infection (8). Infections tend to be seasonal in temperate climates, but in tropical climates can be detected throughout the year (9).
Approximately 20 per 1000 infants less than six months of age are hospitalized with severe RSV illness, and in the institutionalized elderly about 1–2 per 1000 (1, 8, 10, 11). Hospitalized children have a higher frequency of wheezing during childhood than their counterparts with milder disease. This predisposition to wheezing subsides during adolescence (12, 13). In children prophylactically treated with palivizumab (a neutralizing monoclonal antibody specific for antigenic site II on the RSV fusion glycoprotein) the frequency of subsequent wheezing is diminished (14). Conversely, there is also genetic, clinical, and experimental evidence that a pre-existing tendency towards allergic inflammation is associated with more severe disease (15, 16).
Pathology
RSV infects ciliated epithelium in the upper and lower respiratory tract. Bronchiolar epithelium is especially susceptible to infection, and type I pneumocytes in the alveoli are also commonly infected resulting in high frequency (~20%) of children with hypoxemia, even in those without other significant symptoms. In children with severe RSV disease, it is thought that a major feature of pathology is airway obstruction. The few autopsy studies of fatal RSV infection show that small airways can be obstructed by sloughed epithelium and inflammatory cells combined with mucus and fibrin (17). The airways can also become hyper-reactive contributing to the signs of wheezing and retractions characteristic of infants with severe disease. RSV is one of the first pathogens encountered by young infants and in premature infants who are especially susceptible to severe disease, the impact of RSV on the developing lung and airways is not well understood. One of the important consequences of having a successful vaccine for RSV would be the opportunity to ask whether diminishing the severity of RSV disease in early childhood would reduce the frequency of childhood asthma and airway hypersensitivity.
Goals of vaccination
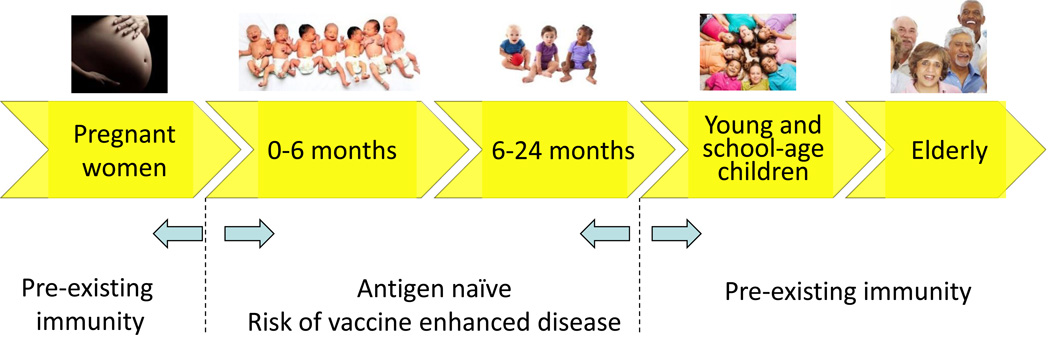
The primary clinical goal for an RSV vaccine is to prevent severe lower respiratory tract disease in young infants. Endpoints would include the prevention of hospitalization or medically attended lower respiratory tract infection (MALRI) in industrialized countries, prevention of mortality and hospitalization or MALRI in developing countries, and if possible, development of a clinical severity index as a continuous variable for disease severity. Secondary goals are to: 1) prevent medically attended lower respiratory tract infection in young children, 2) prevent hospitalization and mortality in the elderly, and 3) reduce childhood wheezing, otitis media, and overall morbidity associated with RSV infection in children and adults. To achieve these goals RSV vaccines have been considered for use in five main populations including 1) pregnant women, 2) infants <6 months of age, 3) infants and children >6 months to 2 years, 4) young (2–5 years old) and school-age children, and 5) individuals >60 years of age (Figure 1). The critical distinction between target populations is whether the subject is RSV antigen naïve and vaccination will be the first inductive priming event or the subject has already experienced natural infection and vaccination will be boosting pre-existing immunity. This will influence which vaccine approach is selected for a given population.
Figure 1.
Potential target populations for RSV vaccines include 1) pregnant women, 2) infants <6 months of age, 3) infants and children >6 months to 2 years, 4) young (2–5 years old) and school-age children, and 5) individuals >60 years of age. Children less than 2 years of age and the elderly would derive the most direct benefit from an effective vaccine. Since all children are infected early in life, anyone over 2 years of age is likely to have experienced natural infection, so vaccination of older children and adults is designed to boost pre-existing immunity. Vaccination in children under 2 years of age could be the primary immunization event.
While protecting infants prior to the peak of hospitalization at 2 months of age by direct vaccination would be ideal, there are several factors that make this challenging including presence of maternal antibody, lack of significant capacity for somatic mutation and affinity maturation of antibody, Th2-biased immune response patterns. In addition, there are more idiosyncratic events like apnea in neonates, which complicates the interpretation of adverse events temporally related to vaccination. Immunization of pregnant women is proposed as a way of protecting neonates by boosting maternal antibody that is acquired by the infant transplacentally. The main objective is to delay the time of first RSV infection until the child is at least 6 months old when airways are more fully developed and have larger diameters to reduce the risk of obstruction from mucus, inflammatory debris, and airway hypersensitivity. Children between 6 and 24 months still have significant morbidity from RSV and would directly benefit from a preventive vaccine. It would also allow the vaccination event to be the first exposure to RSV antigen in many cases, providing the opportunity to induce more effective priming than natural infection. Immunization of young and school-age children would be done in the presence of pre-existing immunity from prior natural infection. Therefore, the likelihood of adverse events would be lower, but the direct benefit to the individual would also be lower, since by age 5 most people have achieved a level of immunity that prevents serious lower airway disease unless immunity is compromised by disease or aging. Immunization of this population would be primarily to reduce transmission to neonates and the elderly, based on RSV transmission dynamics studies (18, 19) showing that most neonatal infections come from older siblings, and the precedent in influenza showing that immunization of school-age children is more cost-effective than immunizing the elderly (20). Boosting pre-existing immunity in the elderly may reduce RSV-related disease, but immunity and pathogenesis in this population is complex, making the definition of clinical endpoints short of hospitalization difficult. Typically viral shedding is limited in this population making pathogen-specific diagnosis more challenging, and disease is often manifest as multi-organ failure rather than confined to the respiratory tract. Deficiencies in both T cell- and antibody-mediated immunity may contribute to the higher susceptibility to disease, and may require alternative antigen designs, formulations, and delivery approaches than younger populations. Better definitions of severe lower respiratory tract disease in young infants and the elderly would facilitate the use of more discriminating clinical endpoints in future vaccine trials (21).
Virology
RSV is a pneumovirus in the Paramyxoviridae family. It has a single-stranded, negative-sense RNA genome of about 15 kilobases with 10 genes separated by stop/start sequences that encode 11 known proteins. There are two major subtypes of RSV that are distinguished largely by variation in the G glycoprotein (22). The subtypes tend to alternate in dominance from year to year, but co-circulate and are not exclusive in any given season. While there is genetic variation between strains characteristic of any RNA virus, it is not extreme and does not seem to explain the susceptibility to reinfection. The first two genes at the 5’ end of the genome are NS1 and NS2 (non-structural) that are primarily devoted to evading the induction and effector functions of type I interferons (IFN). The genetic capital invested in this function suggests it is a critical element of the RSV life cycle. RSV is a pleomorphic enveloped virus about 100–200 nanometers in diameter. The virus enters and buds from the apical surface of polarized epithelial cells. The replication cycle is entirely cytoplasmic. During infection, RhoA is activated and produces filopodia in which the virus assembles emerging from the cell initially as filamentous particles that can be up to 10 microns in length (23). The filamentous projections from cells can mediate syncytium formation with adjacent cells or produce budding particles. As the matrix protein that underlies the viral envelope fragments the particles become pleomorphic and then round.
Surface proteins
There are three proteins in the lipid envelope exposed on the surface of virions including SH (small hydrophobic), G, and F (fusion) (24). SH is a 64–65 amino acid long pentameric ion channel analogous to the M2 protein of influenza virus. G is a heavily glycosylated 298 amino acid long type II integral membrane protein with a molecular weight of about 90 kilodaltons. It major features include a high serine/threonine content of close to 30% resulting in a high level of O-glycosylation, and about 10% proline residues, giving it a chemical composition resembling mucins. The F glycoprotein is a type I integral membrane protein that is 574 amino acids long and mediates viral entry as a class I fusion machine. It has 25 amino acid signal peptide and 2 endoproteolytic cleavage sites resulting in F2 (26–109) and F1 (137–574) subcomponents and a liberated 27 amino acid glycopeptide of unknown function. F2 has 2 N-linked glycans at aa27 and aa70 and F1 has a single N-linked glycan at aa500, while the 27aa peptide has 2 or 3 N-linked glycosylation sites. The native F is a trimer of heterodimers with the F2 and F1 subcomponents connected by 2 disulfide bonds. F1 has a cysteine-rich region between the 2 heptad repeats that form a series of loops in the assembled molecule.
Most of the neutralizing activity in human serum is directed against the F glycoprotein (25, 26). F has several antigenic sites associated with neutralizing monoclonal antibodies (mAbs) (27–33). To date, all these antibodies work through fusion inhibition, meaning that they do not prevent attachment and can prevent virus entry when added after virus has attached to the cell. There is one known antigenic site associated with neutralization on the G glycoprotein near the cysteine noose in the central conserved domain (34–36). MAbs to this site can prevent virus attachment, particularly when evaluated on primary human airway epithelium (37). SH is not required for virus entry and antibodies to SH do not neutralize RSV. However, similar to antibodies specific for the M2 protein of influenza, anti-SH antibodies can reduce viral load and protect against illness in animal models using antibody Fc-mediated immune functions like ADCC (38).
Correlates of immunity
The basis for frequent RSV reinfection is not known. The fact that there is relatively little genetic variation suggests that it is based on other mechanisms of immune avoidance. One possibility is that the induction of immunity during the initial priming event is ineffective and permanently alters the ability to achieve sustained immunity. The initial exposure often occurs in very young children in the presence of maternal antibody and in hosts with a tendency towards lower IFN activity. These factors could result in a suboptimal specificity and function of B and T cell precursors. In this scenario, it is possible that vaccination as the primary RSV antigen exposure for most infants could significantly alter lifetime RSV immunity and reduce the frequency of reinfection. Alternatively, RSV could be so prevalent and contagious that infection of the superficial epithelium in the upper airways can occur because the gradient of antibody between serum and mucosal secretions in the upper airway is steep, and local mucosal responses are not sustained. Therefore, finding immune correlates of protection in serum may be challenging. It is also possible that there are more nuanced antigenic differences between subtypes and between strains from year to year that are not yet understood.
Neutralizing antibody has long been regarded as a correlate of protection against severe RSV disease based on maternal-infant pairs (39); passive protection studies in animal models (40); and clinical studies with passively administered high titer polyclonal serum (41) or monoclonal antibodies specific for antigenic site II on the F glycoprotein (42, 43). However, the correlation of neutralizing activity and protection from infection has not been as clear. There are trends or marginally significant differences in pre-existing serum NT activity in naturally infected elderly patients (44) or experimentally infected young adults (45, 46). There has been an association between pre-existing RSV-specific nasal antibody titers and susceptibility to infection (44, 45, 47), but there is not yet an analysis of epitope-specific antibody responses and susceptibility, and the role of ADCC and other Fc-mediated immune functions on antibody are not established in humans. Mapping all the antibody epitopes on RSV surface proteins, and using new reagents and serological assay platforms to define epitope-specific antibody functions, may provide more clarity on the basis for antibody-mediated protection in the future.
RSV-specific T cells have been associated with both viral clearance and immunopathology. One study showed a paucity of CD8 T cells in lung tissue in children with fatal infection (48). Particularly in persons with Severe Combined Immunodeficiency (SCID), allogenic bone marrow and lung transplant recipients, RSV can cause a fatal pneumonia with widespread syncytia formation and epithelial destruction (6, 7, 49–51). In one patient with SCID and life-threatening RSV infection, passively administered neutralizing mAb did not impact viral load, but a syngeneic bone marrow transplantation and expansion of CD8 T cells was temporally correlated with a drop in viral load and a worsening in respiratory function (7). It has also been shown that HIV-infected infants can shed RSV for months (52), although the same type of lethal cytopathic process does not occur in this type of T cell deficiency. In murine models it has been shown that CD8 T cells are required for normal viral clearance, but are also positively correlate with disease severity in this setting where a large virus inoculum is required to establish infection (53). In humans, pre-existing CD8 T cell memory is likely to be useful in rapidly clearing virus-infected cells, as suggested by human challenge studies where pre-existing intraepithelial (tissue-resident) CD8 T cells are negatively correlated with disease severity (54). Overall, the weight of evidence suggests vaccine approaches that can induce CD8 T cell responses in antigen-naïve infants would have value for virus clearance and for producing IFN-gamma to promote a Th1-biased CD4 T cell response. In persons with pre-existing immunity, particularly in the elderly where there is some evidence to suggest waning CD8 T cell immunity (55, 56), boosting CD8 T cell responses may also have value.
Vaccine-enhanced disease
A vaccine-enhanced disease syndrome was associated with immunizing antigen-naïve infants with whole formalin-inactivated RSV (FI-RSV) formulated in alum (57–60). Although the exact mechanism underlying the FI-RSV vaccine-enhanced RSV disease (ERD) is not known, there are two major immunological phenomena associated with this syndrome based on evaluation of pathology from the original cases (61, 62) and extensive studies in mice, cotton rats, nonhuman primates (NHP), and cattle (63–66). First, the original vaccine induced antibody responses measured by complement fixation or ELISA, but induced poor functional antibody responses measured by neutralizing (67) or fusion-inhibition assays (68). This was associated with evidence of immune complex formation and complement deposition in small airways of the affected infants (69). Secondly, studies in animal models have consistently shown that immunizing antigen-naïve animals with FI-RSV intramuscularly followed by airway challenge with RSV results in a Th2-biased CD4 T cell response and alveolitis (66, 70, 71). This is consistent with the finding of eosinophils in the lungs of infants who died (60). A similar disease syndrome has been demonstrated in NHP models of measles virus attempting to reproduce the atypical pneumonia caused by measles in recipients of the whole-inactivated measles virus vaccine when neutralizing antibody titers waned (72). The FI-RSV ERD presents a difficult safety concern for vaccines intended for antigen-naïve infants. The major difficulty is the lack of adequate animal models to insure that the FI-RSV ERD will not happen. The rodent and even the NHP models are semi-permissive for RSV and therefore too easy to protect. To demonstrate the Th2-biased T cell response and alveolitis pathology, vanishingly low doses of vaccine and/or long intervals between vaccination and challenge are needed to preserve susceptibility to infection. Without active virus replication in lung, the pathological effects cannot be evaluated. The bovine model using bovine RSV probably recapitulates the pathogenesis of natural RSV infection better than the animal models for human RSV, but still requires a large intra-tracheal inoculum for consistent infection and disease severity is highly variable (64). This calls into question whether the risk of vaccine-induced ERD can ever be excluded solely by results from currently available animal models. However, the collective experience evaluating this syndrome does provide some useful guidance for regulators. There is abundant evidence that live-attenuated virus vaccines are not associated with this syndrome whether given mucosally or parenterally. Therefore, vaccines based on live-attenuated, live chimeric, replication-defective vectors, or nucleic acid delivery that express native proteins and induce CD8 T cells and Th1-biased responses should be safe in antigennaïve infants. Also, there is significant evidence that once memory responses have been established by live virus infection, FI-RSV immunization does not result in ERD. Therefore, only vaccines that induce strong functional antibody responses relative to total binding antibody, and those that induce Th1-biased CD4 T cell responses should be considered for direct immunization of antigen-naïve infants. For persons with pre-existing actively-induced RSV immunity, boosting with any vaccine platform should be relatively safe from ERD, but induction of functional antibody responses in proportion to binding antibody responses would be preferred.
Vaccine approaches
Since the FI-RSV vaccine episode, only live-attenuated or chimeric live vector vaccine approaches given intranasally have been advanced in antigen-naïve infants. However, over the last few years, there has been a large surge in the number and variety of RSV candidate vaccines (Table 1). Live-attenuated and chimeric live vectors have not been associated with ERD, and have generally been safe and well tolerated (73). Some of the recent designs have demonstrated higher levels of immunogenicity despite greater attenuation and are still being advanced (74–76). An earlier study showed that delivery of live virus intramuscularly was also safe in this age group, but poor immunogenicity curtailed development (77). Directly immunizing antigen-naïve infants is challenging because of pre-existing maternal antibody and the idiosyncratic events that may occur when infecting the airway of young infants, and currently efforts are focused on infants older than 6 months of age. As noted above, it is thought that gene-based replication-defective approaches using viral vectors (like adenovirus) (78, 79) or nucleic acid (80) would also be safe in this target population because antigen presentation will recapitulate live virus infection with induction of CD8 T cell responses as well as antibody and CD4 T cell responses. These types of approaches are currently in early phase clinical testing in adults.
Table 1.
RSV vaccine approaches in development*
| Live-attenuated RSV |
| Deletion of M2-2 (delta-M2-2) |
| Modification or deletion of NS1 and/or NS2 |
| Codon deoptimization |
| Selected cold-adapted or temperature sensitive mutations |
| Deletion of G (delta-G) |
| Live chimeric vectors |
| Chimeric parainfluenza viruses – expressing F and/or G |
| Sendai virus – expressing F |
| BCG – expressing N |
| Replication-defective vectors |
| Various recombinant adenovirus vectors expressing F or F/M2-1/N |
| Recombinant alphavirus vectors expressing F |
| Recombinant MVA expressing F and/or G or F/M2-1/N |
| Nucleic acid delivery |
| mRNA |
| DNA |
| Particle-based vaccines |
| Membraned virus-like particles (VLP) based on RSV, Newcastle Disease Virus, or Influenza |
| Bacterium-like particles displaying RSV F |
| RNA bacteriophage displaying RSV peptides |
| Alfalfa mosaic virus VLP displaying RSV G peptide |
| Multilayer polypeptide nanofilms displaying RSV peptides |
| Protein subunits |
| Stabilized prefusion F trimer |
| Postfusion F either as single trimer or an aggregate rosetted on fusion peptide |
| SH delivered as a pentameric complex |
| Various versions of G glycoprotein |
| Various peptide formulations |
| Whole-inactivated RSV |
| Virosomes |
| Oil-in-water emulsion |
Adapted from: http://sites.path.org/vaccinedevelopment/files/2015/07/RSV-snapshot-July2015.pdf and reference 21.
Vaccine approaches that rely on phagocytosis and obligate MHC class II presentation pathways like subunit proteins or protein-based nanoparticles are currently only being proposed for immunization of antigen-experienced individuals, particularly pregnant women and the elderly. The approaches are designed to primarily induce antibody-mediated protection, and are primarily based on presenting the F glycoprotein. However, some approaches focus on ADCC responses that can be induced against SH (38) or anti-G antibodies that may be able to protect through neutralizing virus in vivo (37) or through preventing some of the immunomodulatory effects of the secreted G (81, 82).
Delivery of multiple RSV antigens through virus-like particles (83), virosomes (84), and other complex formulations (85) including modern adjuvants will engage antigen processing machinery and activate innate immunity in a variety of ways. Therefore, the antigen content and formulation will need to be considered on a product-by-product basis. They may or may not induce protective CD8 T cell responses in addition to antibody-mediated immunity.
F structure and implications for vaccine development
Solving the crystal structure of the F glycoprotein trimer in its pre-triggered (prefusion) conformation is a recent breakthrough that has energized RSV vaccine development (29, 86, 87). The prefusion F (pre-F) surfaces are targeted by antibodies from multiple distinct binding competition groups of which five are currently published (27, 28). Only two of these sites are present on the shared surface that remains on the postfusion F (post-F) trimer, which is the end-product of a massive molecular rearrangement, the process designed to mediate membrane fusion and allow entry of the viral nucleocapsid into the host cell. Unlike other class I fusion proteins like the hemagglutinin (HA) of influenza, the pre-F conformation is very unstable. Soluble trimers of HA can be expressed and the molecule remains in the pre-triggered state until pH is lowered. In contrast, RSV F expressed as a soluble protein spontaneously rearranges into the highly stable post-F, 6-helix antiparallel bundle form. This rearrangement or “flipping” occurs even in the virus membrane (88), and may be part of the viral strategy for evading neutralization. The post-F form is about 16 nm tall, while the pre-F form is only about 11 nm tall. When the virus is assembled in RhoA-induced filopodia and emerges from the infected cell as long filamentous structures, the matrix layer is highly ordered (89), and in those structures, the F trimer is more often in the pre-F conformation (88). However, when virus buds and becomes asymmetric and then round, the matrix layer becomes fragmented and the F tends to flip into the post-F conformation. Therefore, most transmitted virus has an abundance of post-F on its surface that may shield access to the untriggered pre-F. The major implications for vaccine development are: 1) post-F is not a fusion-competent molecule, so antibodies targeting post-F will not neutralize virus, and 2) the neutralization epitopes specific for pre-F are much more neutralization-sensitive than epitopes shared with post-F (27–29, 62, 90). Therefore, using pre-F (the functional form of the molecule) as the vaccine antigen will induce significantly more neutralizing activity than post-F antigens as a primary immunogen in antigen-naïve children. Also, because in pre-immune individuals virtually all neutralizing activity is attributable to antibody that recognizes pre-F surfaces (25), it is expected that preserving those features in an immunogen will boost neutralizing activity in older children and adults better than post-F.
Guided by the crystal structure of pre-F, stabilizing mutations have been identified that allow pre-F to be produced as a soluble protein (91, 92). F antigens that preserve the pre-F surfaces have superior immunogenic properties than F antigens in the post-F conformation whether presented as subunit proteins (91, 92) or expressed from a vaccine vector (75). The pre-F molecule also has significantly different antigenic properties than post-F because of its unique surfaces, and because it is the functional form of the molecule. Therefore, using pre-F as a reagent in serological assays instead of post-F will allow the measurement of different antibody specificities and functions (25). The insights provided by the stabilized pre-F trimer will improve our understanding of the immune correlates of protection and improve the efficacy of vaccines designed to induce antibody-mediated protection.
Conclusions
RSV is a major cause of morbidity and mortality and is a high priority for vaccine development. Despite the fact that humans are frequently reinfected by RSV despite a lack of significant genetic variation and the legacy of FI-RSV ERD, there is optimism that a vaccine solution for preventing severe disease is possible. This is based on new insights into virus morphology, atomic-level structure of the F glycoprotein in the pre-F and post-F conformational states, mechanisms of virus neutralization, serological responses to natural infection, methods of virus attenuation, and immunological factors associated with FI-RSV ERD. These events have resulted in new reagents, ideas, and an accelerated pace of basic research into the virology, immunology, and pathogenesis of RSV. In addition, the possibility of a vaccine intervention has in part motivated more detailed studies of epidemiology, transmission dynamics, and clinical assessment of disease severity that are providing the information needed for clinical trial design and to establish efficacy endpoints. Importantly, there is now significant involvement of regulatory authorities, and investment by both large and small pharmaceutical companies, to create the clinical development pathways and manufacturing approaches needed to achieve licensed vaccine products.
Acknowledgments
This work was supported by intramural funding from the National Institute of Allergy and Infectious Diseases. I thank Jason Gall and Kaitlyn Morabito for thoughtful editorial comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 4.Hall CB, Long CE, Schnabel KC. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33(6):792–796. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 5.Hall WJ, Hall CB, Speers DM. Respiratory syncytial virus infection in adults: clinical, virologic, and serial pulmonary function studies. Ann Intern Med. 1978;88(2):203–205. doi: 10.7326/0003-4819-88-2-203. [DOI] [PubMed] [Google Scholar]
- 6.Hertz MI, Englund JA, Snover D, Bitterman PB, McGlave PB. Respiratory syncytial virus-induced acute lung injury in adult patients with bone marrow transplants: a clinical approach and review of the literature. Medicine (Baltimore) 1989;68(5):269–281. doi: 10.1097/00005792-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 7.El Saleeby CM, Suzich J, Conley ME, DeVincenzo JP. Quantitative effects of palivizumab and donor-derived T cells on chronic respiratory syncytial virus infection, lung disease, and fusion glycoprotein amino acid sequences in a patient before and after bone marrow transplantation. Clin Infect Dis. 2004;39(2):e17–e20. doi: 10.1086/421779. [DOI] [PubMed] [Google Scholar]
- 8.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 9.Hall CB, Simoes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Current topics in microbiology and immunology. 2013;372:39–57. doi: 10.1007/978-3-642-38919-1_2. [DOI] [PubMed] [Google Scholar]
- 10.Boyce TG, Mellen BG, Mitchel EF, Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137(6):865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 11.Zhou H1, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis. 2012;54(10):1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J. 2003;22(2 Suppl):S76–S82. doi: 10.1097/01.inf.0000053889.39392.a7. [DOI] [PubMed] [Google Scholar]
- 13.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 14.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368(19):1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 15.Miyairi I, DeVincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008;21(4):686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi EH, Lee HJ, Chanock SJ. Human genetics and respiratory syncytial virus disease: current findings and future approaches. Current topics in microbiology and immunology. 2013;372:121–137. doi: 10.1007/978-3-642-38919-1_6. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2007;20(1):108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 18.Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR., Jr Respiratory syncytial virus infections within families. N Engl J Med. 1976;294(8):414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- 19.Munywoki PK, Koech DC, Agoti CN, Lewa C, Cane PA, Medley GF, et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis. 2014;209(11):1685–1692. doi: 10.1093/infdis/jit828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baguelin M, Flasche S, Camacho A, Demiris N, Miller E, Edmunds WJ. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013;10(10):e1001527. doi: 10.1371/journal.pmed.1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modjarrad K, Giersing B, Kaslow DC, Smith PG, Moorthy VS Group WRVCE. WHO consultation on Respiratory Syncytial Virus Vaccine Development Report from a World Health Organization Meeting held on 23–24 March 2015. Vaccine. 2016;34(2):190–197. doi: 10.1016/j.vaccine.2015.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Current topics in microbiology and immunology. 2013;372:3–38. doi: 10.1007/978-3-642-38919-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gower TL, Pastey MK, Peeples ME, Collins PL, McCurdy LH, Hart TK, et al. RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J Virol. 2005;79(9):5326–5336. doi: 10.1128/JVI.79.9.5326-5336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Current topics in microbiology and immunology. 2013;372:83–104. doi: 10.1007/978-3-642-38919-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med. 2015;7(309):309ra162. doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, et al. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A. 2012;109(8):3089–3094. doi: 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilman MS, Moin SM, Mas V, Chen M, Patel NK, Kramer K, et al. Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein. PLoS Pathog. 2015;11(7):e1005035. doi: 10.1371/journal.ppat.1005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLellan JS. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr Opin Virol. 2015;11:70–75. doi: 10.1016/j.coviro.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLellan JS, Chen M, Chang JS, Yang Y, Kim A, Graham BS, et al. Structure of a major antigenic site on the respiratory syncytial virus fusion glycoprotein in complex with neutralizing antibody 101F. J Virol. 2010;84(23):12236–12244. doi: 10.1128/JVI.01579-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat Struct Mol Biol. 2010;17(2):248–250. doi: 10.1038/nsmb.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Barreno B, Palomo C, Penas C, Delgado T, Perez-Brena P, Melero JA. Marked differences in the antigenic structure of human respiratory syncytial virus F and G glycoproteins. J Virol. 1989;63(2):925–932. doi: 10.1128/jvi.63.2.925-932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beeler JA, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989;63(7):2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez I, Dopazo J, Melero JA. Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J Gen Virol. 1997;78(Pt 10):2419–2429. doi: 10.1099/0022-1317-78-10-2419. [DOI] [PubMed] [Google Scholar]
- 35.Plotnicky-Gilquin H, Goetsch L, Huss T, Champion T, Beck A, Haeuw JF, et al. Identification of multiple protective epitopes (protectopes) in the central conserved domain of a prototype human respiratory syncytial virus G protein. J Virol. 1999;73(7):5637–5645. doi: 10.1128/jvi.73.7.5637-5645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, et al. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol. 2009;183(10):6338–6345. doi: 10.4049/jimmunol.0901373. [DOI] [PubMed] [Google Scholar]
- 37.Johnson SM, McNally BA, Ioannidis I, Flano E, Teng MN, Oomens AG, et al. Respiratory Syncytial Virus Uses CX3CR1 as a Receptor on Primary Human Airway Epithelial Cultures. PLoS Pathog. 2015;11(12):e1005318. doi: 10.1371/journal.ppat.1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schepens B, Sedeyn K, Vande Ginste L, De Baets S, Schotsaert M, Roose K, et al. Protection and mechanism of action of a novel human respiratory syncytial virus vaccine candidate based on the extracellular domain of small hydrophobic protein. EMBO molecular medicine. 2014;6(11):1436–1454. doi: 10.15252/emmm.201404005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98(5):708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 40.Prince GA, Horswood RL, Chanock RM. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55(3):517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groothuis JR, Simoes EA, Hemming VG. Respiratory syncytial virus (RSV) infection in preterm infants and the protective effects of RSV immune globulin (RSVIG). Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics. 1995;95(4):463–467. [PubMed] [Google Scholar]
- 42.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–537. [PubMed] [Google Scholar]
- 43.O'Brien KL, Chandran A, Weatherholtz R, Jafri HS, Griffin MP, Bellamy T, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15(12):1398–1408. doi: 10.1016/S1473-3099(15)00247-9. [DOI] [PubMed] [Google Scholar]
- 44.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190(2):373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 45.Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, et al. Impaired Antibodymediated Protection and Defective IgA B-Cell Memory in Experimental Infection of Adults with Respiratory Syncytial Virus. Am J Respir Crit Care Med. 2015;191(9):1040–1049. doi: 10.1164/rccm.201412-2256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 47.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE, Jr, Boyce TG, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 48.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195(8):1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wendt CH. Community respiratory viruses: organ transplant recipients. Am J Med. 1997;102(3A):31–36. doi: 10.1016/s0002-9343(97)80008-3. discussion 42–3. [DOI] [PubMed] [Google Scholar]
- 50.Englund JA, Sullivan CJ, Jordan MC, Dehner LP, Vercellotti GM, Balfour HH., Jr Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med. 1988;109(3):203–208. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- 51.Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315(2):77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 52.King JC, Jr, Burke AR, Clemens JD, Nair P, Farley JJ, Vink PE, et al. Respiratory syncytial virus illnesses in human immunodeficiency virus- and noninfected children. Pediatr Infect Dis J. 1993;12(9):733–739. doi: 10.1097/00006454-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88(3):1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Wang Y, Gilmore X, Xu K, Wyde PR, Mbawuike IN. An aged mouse model for RSV infection and diminished CD8(+) CTL responses. Exp Biol Med (Maywood) 2002;227(2):133–140. doi: 10.1177/153537020222700208. [DOI] [PubMed] [Google Scholar]
- 56.Malloy AM, Falsey AR, Ruckwardt TJ. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. Current topics in microbiology and immunology. 2013;372:211–231. doi: 10.1007/978-3-642-38919-1_11. [DOI] [PubMed] [Google Scholar]
- 57.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 58.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 59.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89(4):405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 60.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 61.Polack FP. Atypical measles and enhanced respiratory syncytial virus disease (ERD) made simple. Pediatr Res. 2007;62(1):111–115. doi: 10.1203/PDR.0b013e3180686ce0. [DOI] [PubMed] [Google Scholar]
- 62.Graham BS, Modjarrad K, McLellan JS. Novel antigens for RSV vaccines. Curr Opin Immunol. 2015;35:30–38. doi: 10.1016/j.coi.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Swart RL, Kuiken T, Timmerman HH, van Amerongen G, Van Den Hoogen BG, Vos HW, et al. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J Virol. 2002;76(22):11561–11569. doi: 10.1128/JVI.76.22.11561-11569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor G. Bovine model of respiratory syncytial virus infection. Current topics in microbiology and immunology. 2013;372:327–345. doi: 10.1007/978-3-642-38919-1_16. [DOI] [PubMed] [Google Scholar]
- 65.Blanco JC, Boukhvalova MS, Perez DR, Vogel SN, Kajon A. Modeling Human Respiratory Viral Infections in the Cotton Rat () J Antivir Antiretrovir. 2014;6:40–42. doi: 10.4172/jaa.1000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151(4):2032–2040. [PubMed] [Google Scholar]
- 67.Murphy BR, Prince GA, Walsh EE, Kim HW, Parrott RH, Hemming VG, et al. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24(2):197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26(8):1595–1597. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polack FP, Teng MN, Collins PL, Prince GA, Exner M, Regele H, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196(6):859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Connors M, Kulkarni AB, Firestone CY, Holmes KL, Morse HC, 3rd, Sotnikov AV, et al. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSVimmunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66(12):7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Connors M, Collins PL, Firestone CY, Sotnikov AV, Waitze A, Davis AR, et al. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia--RSV recombinants or RSV. Vaccine. 1992;10(7):475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 72.Polack FP, Hoffman SJ, Moss WJ, Griffin DE. Altered synthesis of interleukin-12 and type 1 and type 2 cytokinesin rhesus macaques during measles and atypical measles. J Infect Dis. 2002;185(1):13–19. doi: 10.1086/338009. [DOI] [PubMed] [Google Scholar]
- 73.Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Current topics in microbiology and immunology. 2013;372:259–284. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karron RA, Luongo C, Thumar B, Loehr KM, Englund JA, Collins PL, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med. 2015;7(312):312ra175. doi: 10.1126/scitranslmed.aac8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang B, Surman S, Amaro-Carambot E, Kabatova B, Mackow N, Lingemann M, et al. Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate. J Virol. 2015;89(18):9499–9510. doi: 10.1128/JVI.01373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russell CJ, Hurwitz JL. Sendai virus as a backbone for vaccines against RSV and other human paramyxoviruses. Expert Rev Vaccines. 2015:1–12. doi: 10.1586/14760584.2016.1114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belshe RB, Van Voris LP, Mufson MA. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982;145(3):311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- 78.Johnson TR, Rangel D, Graham BS, Brough DE, Gall JG. Genetic vaccine for respiratory syncytial virus provides protection without disease potentiation. Mol Ther. 2014;22(1):196–205. doi: 10.1038/mt.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor G, Thom M, Capone S, Pierantoni A, Guzman E, Herbert R, et al. Efficacy of a virus-vectored vaccine against human and bovine respiratory syncytial virus infections. Sci Transl Med. 2015;7(300):300ra127. doi: 10.1126/scitranslmed.aac5757. [DOI] [PubMed] [Google Scholar]
- 80.Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, et al. Nonviral delivery of selfamplifying RNA vaccines. Proc Natl Acad Sci U S A. 2012;109(36):14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chirkova T, Boyoglu-Barnum S, Gaston KA, Malik FM, Trau SP, Oomens AG, et al. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J Virol. 2013;87(24):13466–13479. doi: 10.1128/JVI.01741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson TR, McLellan JS, Graham BS. Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2. J Virol. 2012;86(3):1339–1347. doi: 10.1128/JVI.06096-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, et al. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol. 2010;84(2):1110–1123. doi: 10.1128/JVI.01709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamphuis T, Shafique M, Meijerhof T, Stegmann T, Wilschut J, de Haan A. Efficacy and safety of an intranasal virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice and cotton rats. Vaccine. 2013;31(17):2169–2176. doi: 10.1016/j.vaccine.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 85.Van Braeckel-Budimir N, Haijema BJ, Leenhouts K. Bacterium-like particles for efficient immune stimulation of existing vaccines and new subunit vaccines in mucosal applications. Front Immunol. 2013;4:282. doi: 10.3389/fimmu.2013.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.2013 Runners-Up. In vaccine design, looks do matter. Science. 2013;342(6165):1442–1443. doi: 10.1126/science.342.6165.1442-a. [DOI] [PubMed] [Google Scholar]
- 87.Cohen J. Vaccines. Structural biology triumph offers hope against a childhood killer. Science. 2013;342(6158):546–547. doi: 10.1126/science.342.6158.546-a. [DOI] [PubMed] [Google Scholar]
- 88.Liljeroos L, Krzyzaniak MA, Helenius A, Butcher SJ. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proc Natl Acad Sci U S A. 2013;110(27):11133–11138. doi: 10.1073/pnas.1309070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiss G, Holl JM, Williams GM, Alonas E, Vanover D, Lifland AW, et al. Structural analysis of respiratory syncytial virus reveals the position of M2-1 between the matrix protein and the ribonucleoprotein complex. J Virol. 2014;88(13):7602–7617. doi: 10.1128/JVI.00256-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501(7467):439–443. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- 91.Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJ, et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun. 2015;6:8143. doi: 10.1038/ncomms9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]