Abstract
Exogenous brain-derived neurotrophic factor (BDNF), acting through TrkB, is known to promote neurite formation and branching. This response to BDNF was eliminated by inhibition of TrkB kinase and by specific inhibition of the GEF1 domain of Kalirin, which activates Rac1. Neurons from Kalrn knockout mice were unable to activate Rac1 in response to BDNF. BDNF-triggered neurite outgrowth was abolished when Kalrn expression was reduced using shRNA that targets all of the major Kalrn isoforms, and reduced in neurons from Kalrn knockout mice. The Kalrn isoforms expressed early in development also include a GEF2 domain that activates RhoA. However, BDNF-stimulated neurite outgrowth in Kalrn knockout neurons was rescued by expression of Kalirin-7, which includes only the GEF1 domain but lacks the GEF2 domain. Dendritic morphogenesis, which requires spatially restricted, coordinated changes in the actin cytoskeleton and in the organization of microtubules, involves essential contributions from multiple Rho GEFs. Since Tiam1, another Rho GEF, is also required for BDNF-stimulated neurite outgrowth, an inhibitory fragment of Tiam1 (PHn-CC-EX) was tested and found to interfere with both Kalirin and Tiam1 GEF activity. The prolonged TrkB activation observed in response to BDNF in Kalrn knockout neurons and the altered time course and extent of ERK, CREB and Akt activation observed in the absence of Kalrn would be expected to alter the response of these neurons to other regulatory factors.
Keywords: Rho GEF, Kalrn knockout, TrkB kinase, TrkB phosphorylation, ERK, Akt, CREB
1. Introduction
The architectural and functional integrity of neural circuity requires proper formation of dendritic arbors (Craig and Banker, 1994; Jan and Jan, 2010; Wong and Ghosh, 2002). Dendrite initiation and branching start with filopodial formation, a process that is regulated by actin organization/polymerization and microtubule organization (Galic et al., 2014; Sainath and Gallo, 2015). The early development of dendrites is regulated by intrinsic genetic programs, which define the competence of neurons in response to extrinsic cues (Barnes and Polleux, 2009; Craig and Banker, 1994). Extrinsic cues in dendritic morphogenesis include membrane-bound proteins and soluble components such as neurotrophins (Konur and Ghosh, 2005; Landgraf and Evers, 2005).
Brain derived neurotrophic factor (BDNF) plays crucial roles in regulating neuronal survival, differentiation and morphology (Huang and Reichardt, 2003; Park and Poo, 2013). BDNF promotes dendritic arbor growth (Ozdinler and Macklis, 2006; Reichardt, 2006; Wang et al., 2015) and axonal branching (Panagiotaki et al., 2010) by binding to TrkB (Bramham, 2008; Park and Poo, 2013), a receptor tyrosine kinase (Cohen-Cory and Fraser, 1995; McAllister et al., 1999). The binding of BDNF stimulates trans-phosphorylation of multiple Tyr residues, which triggers multiple downstream pathways, including activation of the small Rho family GTPase, Rac1 (Huang and Reichardt, 2003; Minichiello, 2009). A key feature of growth factor signaling is timing; receptor inactivation occurs after the internalized ligand-receptor complex is removed from the cell surface (Assaife-Lopes et al., 2014; Ji et al., 2010; Lai et al., 2012; Mitchell et al., 2012; Philippidou et al., 2011).
Rho family GTPases are critical regulators of actin cytoskeletal dynamics and neuronal morphogenesis (Nakayama et al., 2000; Tada and Sheng, 2006). Guanine-nucleotide exchange factors (GEFs) activate Rho proteins in a spatially and temporally controlled manner and are essential for neuronal process outgrowth and branching (Jaffe and Hall, 2005; Miller et al., 2013). Kalirin, a multifunctional Rho GEF, influences neuronal morphology in different ways at different stages of development (Ma et al., 2003; May et al., 2002; Shioda et al., 2011; Sommer and Budreck, 2009).
Kalirin has been shown to play a central role in signaling pathways triggered by several receptor tyrosine kinases, including EphB2, ErbB4, TrkA and PDGFR-β (Cahill et al., 2013; Chakrabarti et al., 2005; Penzes et al., 2003; Wu et al., 2013). In pheochromocytoma cells, an interaction between Kalirin and TrkA contributes to neurite outgrowth (Chakrabarti et al., 2005), suggesting a role for Kalirin in neurotrophin actions. However, whether Kalirin plays a role in BDNF/TrkB signaling in neurons is unknown.
Interestingly, Tiam1, another neuronal Rho GEF, is phosphorylated by TrkB in vitro and in cortical neurons; phosphorylation of Tiam1 is required for activation of Rac1 downstream of BDNF/TrkB signaling, and TrkB phosphorylation of Tiam1 is required for BDNF-induced neurite outgrowth in neurons (Lai et al., 2012; Miyamoto et al., 2006). In the current study, we set out to explore the hypothesis that endogenous Kalirin is also required for the ability of exogenous BDNF, acting through TrkB, to stimulate dendritic morphogenesis.
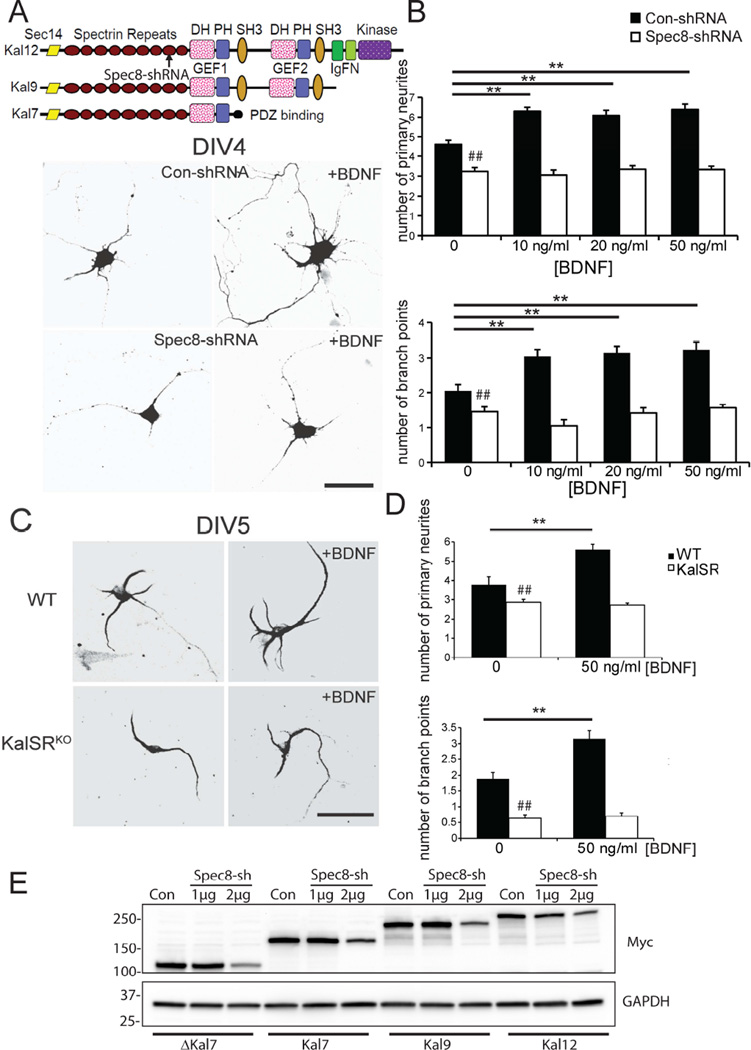
The Kalrn gene undergoes alternative splicing. Kalirin-9 (Kal9) and Kalirin-12 (Kal12) include two Rho GEF domains and are the only isoforms present in early development (Yan et al., 2015). Kal9 and Kal12 are required for normal dendritic development. Kalirin-7 (Kal7), which includes only one GEF domain and is absent in early development, becomes the major isoform in the adult brain [Fig.1A].
Fig 1. Exogenous BDNF mediated neurite outgrowth requires Kalrn.
A. Top - diagram showing major Kalirin isoforms and site targeted by Spec8 shRNA. Rat hippocampal neurons transfected at the time of plating with control or Spec8 shRNA were maintained in the absence or presence of 50 ng/ml BDNF from the time of plating until they were fixed at DIV4. Morphological measurements of transfected neurons used DsRed; representative neurons are shown. Scale bar, 50 µm. B. The concentration of BDNF added to the culture medium was varied from 10 to 50 ng/ml; cultures were fixed at DIV2 or DIV4 for quantification of primary neurites and branch points. Data shown are for DIV4; 10 ng/ml BDNF gave a maximal response. Spec8 shRNA had the expected effect on neurite number and branch points. **p<0.01 between Con and Spec8 shRNAs. C. Hippocampal neurons from P0 WT or KalSRKO animals were maintained in culture medium lacking or containing 50 ng/ml BDNF from 24 hr until DIV5; fixed cells were stained with antibody to MAP2 for quantification. Representative cells are shown. Scale bar, 50 µm. D. Quantification of effect of exogenous BDNF on number of primary neurites and branch points at DIV5. Exogenous BDNF failed to enhance neurite outgrowth or branching in KalSRKO neurons. **p<0.01. ## p<0.05 between WT and KalSRKO groups. Three separate experiments were performed using rat hippocampal neurons; two separate experiments were performed using mouse hippocampal neurons. For each experiment, 12–18 neurons per group were analyzed. E. HEK-293 (PEAK) cells were transfected with the four indicated myc-tagged Kalirin plasmids plus two amounts of Spec8 shRNA vector, and harvested 24 h later. Western was developed using myc and GAPDH antibodies. The experiment was repeated 5 separate times with similar results.
In this study, we identified Kalirin as a key component of the downstream signaling by TrkB that leads to BDNF-induced neurite outgrowth and branching in hippocampal neurons. Kal GEF1 mediated Rac1 activation was required for BDNF-induced neuronal morphogenesis. The fact that Kal7, which lacks a GEF2 domain, stimulated neurite formation and rescued the ability of young KalSRKO neurons to respond to BDNF, confirmed the essential role of Rac activation. Our findings demonstrate extensive crosstalk between two key players in the formation of dendrites early in development, Kalirin, a Rho GEF, and TrkB, a receptor tyrosine kinase.
2. Materials and Methods
2.1 Antibodies
Commercial antibodies included: TrkB (#610101, mouse, BD Biosciences, San Jose, CA); phospho-TrkB Tyr515 (#9141, rabbit, Cell Signaling, Danvers, MA) and Tyr705/706 (#4621, rabbit, Cell Signaling), phospho-Akt (#4051, mouse, Cell Signaling), Akt (#4691, rabbit, Cell Signaling), phospho-CREB (#9198, rabbit, Cell Signaling), CREB (#9104, mouse, Cell Signaling), phospho-MAPK (Erk1/2) (#9106, mouse, Cell Signaling), ERK (#sc-154, rabbit, Santa Cruz, Santa Cruz, CA), FLAG (#F7425, rabbit, Sigma), GAPDH (MAB374, mouse, EMD Millipore) and MAP2 (AB15452, chicken, EMD Millipore). The Myc monoclonal 9E10 was from spent hybridoma medium (Borjigin and Nathans, 1994). Rabbit antibody to the Kalirin Sec14 domain has been described and validated (CT302) (Yan et al., 2015).
2.2 Plasmids
To reduce expression of endogenous Kalirin in rat hippocampal cultures, shRNA targeted to all major isoforms of rat and mouse Kalirin (Spec8 shRNA) was expressed using pSiren-DsRed (Francone et al., 2010). The sequences targeted were: Scramble shRNA, AATGCACGCTCAGCACAAGC, which corresponds to no rodent cDNAs in Genbank; Spec8 shRNA, GCAGGAACAATGTGAGCATG. The efficacy of the Spec8 shRNA was validated previously using the shorter isoform ΔKal7 (Yan et al., 2015). Further testing shRNA efficacy, pEAK.His-myc-ΔKal7, -Kal7, -Kal9 and -Kal12 were transfected into pEAK cells (hEK-293 derivative) (Ma et al., 2014; May et al., 2002) along with Spec8 shRNA, using TransIT-2020 (Mirus); cells were harvested into SDS lysis buffer (Yan et al., 2015) 24 h post transfection for western blot analysis. The Kal7-shRNA-rescue plasmid was produced by making seven silent mutations in the target sequence of the Spec8 shRNA and verified by DNA sequencing (CGC AAT AAC GTC TCC for RNNVS). PEAK-kGEF1 was described previously (Ma et al., 2014; May et al., 2002). Plasmids expressing two FLAG-tagged fragments of Tiam 1, pCMV5.C1199 (Tiam1[393–1591]) and pCMV5.PHnCCEX (Tiam1[393–841]) (Miyamoto et al., 2006), were kindly provided by Dr. Yuki Miyamoto (National Institute of Child Health Development, Tokyo). The Rac1 biosensor plasmid (Das et al., 2015a) was kindly provided by Dr. Yi Wu, UConn Health.
2.3 Experimental Treatment of Neuronal Cultures
Cultures of hippocampal neurons were prepared from embryonic day 18 Sprague Dawley rat embryos and cultures of hippocampal neurons were prepared from postnatal day 1 WT or KalSRKO mice (Yan et al., 2015). To evaluate the effect of BDNF on neurite outgrowth, BDNF (10–50 ng/ml) (Alomone, Jerusalem, Israel) was added 24 h after plating and remained in the medium for the next 3 days; BDNF was reconstituted in H2O as a sterile 10 µg/ml stock. For shRNA knock-down experiments, scramble or Kalirin shRNAs were introduced into hippocampal neurons by electroporation. For overexpression experiments, full length Kalirin vectors (7 µg DNA per 106 neurons) were introduced into hippocampal neurons from rat or KalSRKO animals by electroporation at the time of plating (Yan et al., 2015); transfection efficiency was around 10%. To examine the effects of K252a (Tocris) and ANA-12 (Tocris), stocks (10 mM K252a in DMSO; 25 mM ANA-12 in DMSO) were diluted into growth medium to yield 100 nM K252a and 10 µM ANA-12. DMSO was used in the vehicle group. K252a or ANA-12 was pre-incubated for 1h before BDNF was applied to the neurons and remained on the cells until harvest. To examine the effects of ITX3 (Sigma), a 1000x stock of ITX3 dissolved in DMSO (100 mM) was diluted into maintenance medium containing BDNF (final ITX3 concentration 100 µM) or not and kept on the cells until fixation. For the time course study, hippocampal cultures were plated into 12-well plates (106 neurons per well); no growth factor or BDNF (50 ng/ml) was applied and cultures were harvested at the times indicated into 150 µl SDS-P lysis buffer [50 mM Tris pH7.6, 1% SDS, 130mM NaCl, 5 mM EDTA, 50 mM NaF, 10 mM sodium pyrophosphate, 1 mM PMSF, P8340 (Sigma, 1:100), phosphatase cocktail inhibitor I (CalBiochem, 1:100), 1 mM orthovanadate and PhosSTOP (Roche, 1 tablet in 10 ml)]. The concentration of protein was determined using the bicinchoninic acid assay (Pierce, Rockford, IL) and 20 µg of the total protein was loaded onto each lane as described (Yan et al., 2015). Samples were heated at 95°C for 5 min and centrifuged at 15,000 × g for 30 sec before loading onto gels.
2.4 Pull down assay
Hippocampal cultures from WT and KalSRKO mice were maintained until DIV21, and 50 ng/ml BDNF was added in maintenance medium for 15 min. Neurons were extracted in 0.3 ml ice-cold MLB buffer (25 mM HEPES, pH7.5, 150 mM NaCl, 1% NP-40, 10 mM MgCl2, 1 mM EDTA and 2% glycerol, 0.3 mg/ml PMSF, proteinase inhibitors (Schiller et al., 2008)). Cell lysates were incubated with GST-Pak-CRIB agarose beads slurry at 4°C for 1 hr with gentle agitation. Beads were washed 3 times and bound proteins were released by heating at 95°C in SDS-loading buffer for 5 min. Extracts (a pool from all samples, 100 µl) were incubated with 10 mM EDTA and 0.2 mM GTPγS (positive control) or 2 mM GDP (negative control) for 30 min at 30 °C, cooled and brought to 60 mM MgCl2 prior to incubation with GST-Pak-CRIB beads.
2.5 Immunocytochemistry and Image Acquisition
Immunocytochemical staining of neuronal cultures was performed as described (Ma et al., 2014). Images were taken using a Zeiss LSM 510 Meta confocal microscope and a 40× objective. A single image per neuron was taken to visualize the dendritic arbor. For each neuron, neurite number and branching were measured from the same image.
2.6 Western Blot Analysis
Cortices and hippocampi collected from P7 mice were sonicated into SDS lysis buffer, heated for 5 min at 95°C and centrifuged to remove insoluble debris. The concentration of protein in the supernatant was determined using the bicinchoninic acid assay (Pierce, Rockford, IL) with bovine serum albumin as a standard; 20 µg of total protein was loaded onto each lane. For analysis of the effect of BDNF on TrkB phosphorylation in neuronal cultures, phospho-specific antibodies were used first; PVDF membranes were stripped using low pH buffer and TrkB antibody was then applied.
2.7 FRET assay of GEF activity
HEK-293 (pEAK) cells were transfected in a glass-bottom 24-well plate in duplicate. All wells except the non-transfected wells received the Rac1-biosensor vector (Das et al., 2015a). The total amount of vector per well was kept constant using empty pEAK10 vector. DNA was mixed with TransIT-2020 and fluorescence emission spectra were obtained in DMEM without phenol red, pyruvate or vitamins (US Biological) at 30 h after transfection (Das et al., 2015a), using an EnSpire 2300 Multilabel Plate Reader (Perkin Elmer). FRET signal was normalized to the 475 nm signal as described (Das et al., 2015a).
3. Results
3.1 BDNF mediated neurite outgrowth requires Kal9 and Kal12
BDNF is known to play a critical role in dendritic growth and morphological specialization. Well before birth, hippocampal and cortical neurons in the brain express BDNF and TrkB (Behar et al., 1997; Mioranzza et al., 2014). Exogenous BDNF increases the number of primary dendrites in an activity dependent manner through TrkB in specific cortical layers (McAllister et al., 1997; McAllister et al., 1995). We first asked whether Kalirin is involved in BDNF-induced neurite outgrowth. During the first 7 days in vitro (DIV), Kal9 and Kal12 play an essential role in neurite formation and branching in hippocampal and cortical neurons (Yan et al., 2015). Based on the literature, we expected exogenous BDNF to increase the number of primary neurites and branch points produced by hippocampal neurons (Cheung et al., 2007; Ji et al., 2010). Recombinant BDNF was added to the culture medium 24 h after plating and remained in the medium until the cultures were fixed and examined at DIV4 [Fig.1A]. Using a control shRNA, and doses of BDNF ranging from 10 to 50 ng/ml, it was clear that exposure to exogenous BDNF produced the expected increase in the number of primary neurites and branch points [Fig.1A, B].
Expression of Kal9 and Kal12 was manipulated using an shRNA (Spec8 shRNA) targeted to the spectrin repeat region common to all major isoforms of Kalirin [Fig.1A]. As expected, neurite formation and branching were reduced when expression of Kal9/Kal12 was reduced [Fig.1A, B]. We next asked whether rat hippocampal neurons in which expression of Kal9/Kal12 is reduced could respond to exogenous BDNF in the same manner as neurons expressing the control shRNA. We found that attenuation of Kal9 and Kal12 expression abrogated the ability of exogenous BDNF to stimulate neurite outgrowth; even the highest concentration of BDNF tested was unable to increase the number of primary neurites or branch points in hippocampal neurons expressing the Kalirin Spec8 shRNA [white boxes, Fig.1B].
We next turned to KalSRKO hippocampal neurons as an independent means of determining whether Kalrn plays an essential role in the ability of these neurons to respond to exogenous BDNF. WT and KalSRKO neurons were maintained without or with 50 ng/ml BDNF starting 24 hrs after plating, and quantification of primary neurites and branch points was performed on DIV5 [Fig.1C, D]. As reported previously, the number of neurites and branch points was reduced in KalSRKO neurons (Yan et al., 2015). As expected, the addition of exogenous BDNF increased the number of primary neurites and branch points in WT neurons; in contrast, exogenous BDNF failed to enhance neurite outgrowth in KalSRKO neurons [Fig.1D]. The results obtained using KalSRKO mouse hippocampal neurons were consistent with the results obtained using shRNA to reduce Kalirin expression in rat hippocampal neurons [Fig.1A, B]. The effectiveness of Spec8 shRNA was previously documented using a shorter Kalirin isoform (Yan et al., 2015); here we validated the knockdown efficiency (to 35–40% of control) for all isoforms: ΔKal7, Kal7, Kal9 and Kal12 [Fig.1E]. Taken together, our results on neurons from both rat and mouse demonstrate that Kal9/Kal12 play a crucial role in the ability of exogenous BDNF to stimulate neurite outgrowth and extension.
3.2 Kalirin is a major GEF activating Rac1 downstream BDNF/TrkB signaling
To determine whether the catalytic activity of the GEF1 domain of Kalirin plays an essential role in the morphological response of hippocampal neurons to exogenous BDNF, we took a pharmacological approach. ITX3, a cell permeant inhibitor of Kalirin (and Trio) GEF1-mediated Rac1 activation, specifically blocked the ability of BDNF to stimulate neurite growth and branching [Fig.2A]. Interestingly, ITX3 had no effect on neurite or branch point number in the absence of BDNF at DIV4. These results are consistent with previous observations that BDNF is not required for the development of basal neuronal architecture (Kellner et al., 2014). Inhibiting Kalirin GEF1 activity specifically blocked BDNF-stimulated neurite outgrowth. ITX3 does not inhibit the GEF activity of Tiam1, another Rac GEF known to play an essential role in the response of young neurons to BDNF (Bouquier et al., 2009).
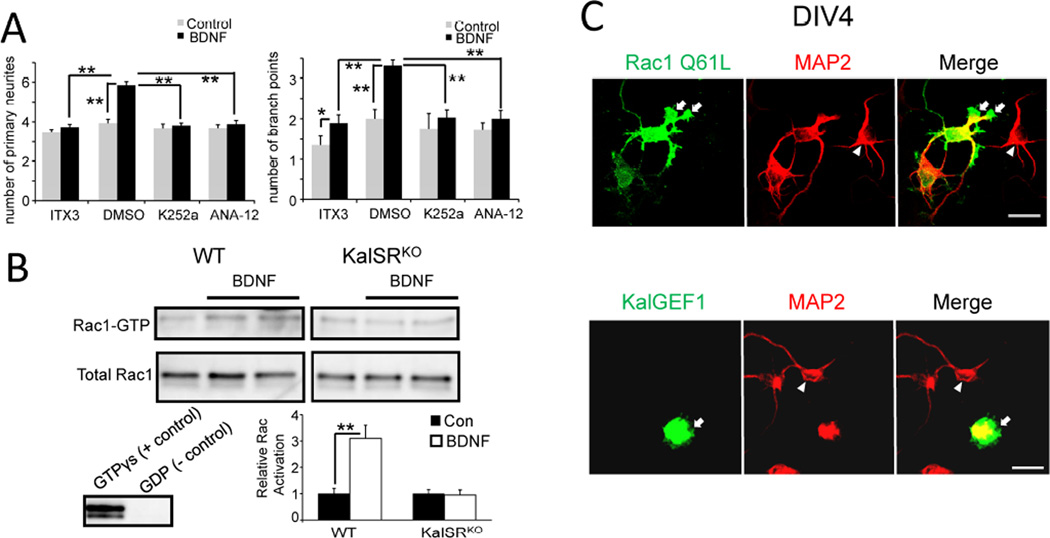
Fig 2. Kalirin GEF1 mediated Rac1 activation is required for BDNF effects.
A. Rat hippocampal neurons were grown in the absence (Control) or presence of exogenous BDNF until DIV4. Before the addition of BDNF, neurons were pre-incubated for 1 h with DMSO (diluted 2500-fold), K252a (100 nM), ANA-12 (10 µM) or ITX-3 (100 µM). Primary neurites and branch points were quantified as described above; data were compared by 2-way ANOVA using drug and BDNF as independent variables. **p<0.001; *p<0.05. B. BDNF (50 ng/ml for 15 min) activated endogenous Rac1 in WT hippocampal cultures at DIV21 but was unable to activate Rac1 in KalSRKO neurons. Four separate experiments were performed; data were compared by 2-way ANOVA using genotype and BDNF as independent variables. **p<0.001. C. Examples of DIV4 hippocampal neurons transfected at plating with GFP-Rac1 Q61L or GFP-Kal GEF1 and stained for MAP2 (Yan et al., 2015). Lamellipodial extensions were indicated by arrows. Non-transfected neurons were indicated by arrowheads. Scale bar: 20 µm.
To compare the effects of inhibiting Kalirin GEF1 to the effects of inhibiting TrkB kinase activity, we included K252a, a selective inhibitor of Trk kinases when used at an appropriate concentration (Ohmichi et al., 1992; Tapley et al., 1992), or ANA-12, a non-competitive TrkB-specific antagonist (Alder et al., 2013) in the culture medium from the time of plating [Fig.2A]. Both 100 nM K252a and 10 µM ANA-12 completely eliminated the ability of exogenous BDNF to promote neurite outgrowth or increase the number of branch points [Fig.2A]. As observed for ITX3, incubation with K252a or ANA-12 did not alter the number of primary neurites or branch points under basal conditions [Fig.2A], suggesting that endogenous TrkB kinase activity is not required for basal neurite architecture development in primary hippocampal cultures during this early stage of development. Consistent with this conclusion, incubating primary hippocampal neurons in BDNF antibody or TrkB-Fc, to remove endogenous secreted BDNF, did not affect the architecture of hippocampal neurites (Kellner et al., 2014). Since the response to exogenous BDNF was abolished in neurons lacking Kalirin or treated with an inhibitor of its first GEF domain [Fig.1], we concluded that this experimental system provides a valid means of evaluating the role of Kalirin GEF1/Rac1 in the response to BDNF.
Rac1 activation downstream of BDNF is thought to mediate process outgrowth in developing neurons (Huang and Reichardt, 2003; Miyamoto et al., 2006); we asked whether Kalirin contributes to BDNF-mediated activation of endogenous Rac1. Hippocampal cultures from WT or KalSRKO neurons (Yan et al., 2015), were exposed to BDNF for 15 min. Activated, GTP-bound Rac1 was detected by its ability to bind to the CRIB domain of Pak (Benard et al., 1999). As expected, BDNF treatment led to a significant increase in Rac1 activation in WT neurons. In contrast, BDNF was unable to increase the amount of activated Rac1 in KalSRKO neurons [Fig.2B]. The simplest interpretation of this experiment is that Kalirin, one of multiple Rac1 GEFs expressed in neurons, catalyzes the activation of a small fraction of the Rac1 in hippocampal neurons exposed to BDNF.
Normal dendritic morphogenesis requires that Rac1 signaling pathways be regulated precisely in time and space (Sainath and Gallo, 2015). To establish the effects of simply increasing the activation of Rac1 in our system, a constitutively active variant [Rac1(Q61L)] was expressed in hippocampal neurons [Fig.2C, upper]. For comparison, the isolated GEF1 domain of Kalirin, which is a more active Rac1 GEF than Kal7, was also expressed [Fig.2C, lower]. Expression of Rac1(Q61L) yielded neurons with massive lamellipodial growth cones while expression of Kal-GEF1 produced lamellipodial extensions from the cell soma; in neither case was there any sign of additional neurite formation. These results are consistent with previous work (Allen et al., 2000; Fournier et al., 2003; Luo et al., 1996): Rac1 is essential for neurite outgrowth, but constitutively active Rac1 does not produce more neurites. The spatiotemporal activation of Rac1 is essential for proper morphogenesis.
3.3 Expression of Kal7 promotes neurite formation and rescues the ability of KalSRKO neurons to respond to BDNF
Kal9 and Kal12 are complex proteins; based on the ability of ITX3, which inhibits only the first GEF domain of Kalirin, to block the actions of BDNF, we hypothesized that Kal7, with its single GEF domain, might be capable of controlling primary neurite formation in rat hippocampal neurons. Whether assessed at DIV2 or DIV4, rat hippocampal neurons expressing exogenous Kal7 had more primary neurites than neurons expressing GFP [Fig.3A]. To verify that Kal9 and Kal12 were the only isoforms functioning this early in development (Yan et al., 2015), we expressed a previously validated Kal7-specific shRNA (Ma et al., 2008) in rat hippocampal neurons and examined their response to BDNF [Fig.3B]. As expected, the Kal7 shRNA did not alter basal dendritic morphology or the ability of the neurons to respond to BDNF [Fig.3B]. To control for any off-target effects of our Spec8 shRNA construct, we created a Kal7 rescue plasmid, in which the coding sequence was mutated to make the rescue protein resistant to the Spec8 shRNA. We expressed Kal7 rescue with Spec8 shRNA in rat neurons in the absence or presence of BDNF [Fig.3C]. Neurons expressing Kal7 rescue and Spec8 shRNA were responsive to BDNF. As predicted by our pharmacological data [Fig.2] (Yan et al., 2015), the actions of exogenous Kal7 mimicked those of endogenous Kal9 and Kal12 in early development(Yan et al., 2015).
Fig 3. Kal7 rescues BDNF responsiveness.
A. Representative images of myc-tagged Kal7 transfected DIV4 neurons. Kal7 was electroporated into rat hippocampal neurons during plating; neurons were fixed at DIV4 and Kal7 was visualized using a myc monoclonal antibody (Ma et al., 2014). Scale bar: 20 µm. Quantification of primary neurite number in GFP- and Kal7-expressing rat neurons at DIV2 and DIV4; GFP-transfected neurons served as the control. Three separate experiments were performed using rat hippocampal neurons. Between 8 and 11 neurons per group per experiment were analyzed. B. Examples of Kal7 shRNA transfected rat hippocampal neurons at DIV4; 20 ng/ml BDNF was added to the medium 24h after plating and was not removed. 13–14 neurons per group per experiment were analyzed. Scale bar: 20 µm. **p<0.001. Four separate experiments were performed. C. Examples of Kal7-rescue and Spec8 co-transfected rat hippocampal neurons at DIV4. 20 ng/ml BDNF was added to the medium 24 h after plating and was not removed. Scale bar: 20 µm. Three separate experiments were performed. D. Representative images of Kal7 transfected KalSRKO neurons at DIV5; 20 ng/ml BDNF was added to the medium 24 hrs after plating and was not removed. Scale bar: 20 µm. Quantification of primary neurite number at DIV5 in the presence/absence of BDNF. Two separate experiments were performed using KalSRKO hippocampal neurons. Between 12 and 18 neurons per group per experiment were analyzed; data were compared by 2-way ANOVA using genotype and BDNF as independent variables. **p<0.001.
We next asked whether exogenous Kal7 (introduced at the time of plating) could rescue the ability of KalSRKO neurons to respond to BDNF, or whether there are permanent deficits from developing for nearly 3 weeks in the absence of Kalirin. Kal7 was expressed in KalSRKO neurons, which were exposed to BDNF (20 ng/ml) 24 hrs after transfection and plating; neuronal morphology was analyzed at DIV5 [Fig.3D]. As expected, KalSRKO neurons did not respond to BDNF [Fig.3D and Fig.1C, D]; consistent with the ability of Kal7 to stimulate neurite formation in rat hippocampal neurons, KalSRKO neurons expressing Kal7 showed an increase in the number of primary neurites. Most importantly, KalSRKO neurons expressing exogenous Kal7 regained the ability to respond to BDNF administration by producing more primary neurites. While multiple Rho GEFs are expected to contribute to BDNF mediated process outgrowth and branching, it is clear that Kalirin plays a major role in this response. The fact that exogenous Kal7 is capable of replacing endogenous Kal9/Kal12 focused our attention on the N-terminal regions common to all three isoforms (Sec14, spectrin repeats, GEF1) [Fig.1A].
3.4 TrkB activation by exogenous BDNF in vitro is altered in the absence of Kalrn
Next we explored whether TrkB activation is altered in the absence of Kalirin. We used hippocampal cultures from WT or KalSRKO mice at DIV7 to compare the ability of exogenous BDNF to activate TrkB [Fig.4A, B]. TrkB dimerizes and the intracellular subunits phosphorylate each other at Tyr515 and Tyr705/706 after binding BDNF (Huang and Reichardt, 2003; Minichiello, 2009). The time course over which TrkB activation is maintained is known to reflect the activity of phosphatases as well as the endocytic trafficking of TrkB and the essential creation of signaling endosomes (Assaife-Lopes et al., 2014; Mitchell et al., 2012; Philippidou et al., 2011). Antisera specific for the phosphorylation of TrkB at Tyr515 and Tyr705/706 were used to evaluate the TrkB activation time course using neurons that had not previously been exposed to exogenous BDNF. The TrkB phospho-Tyr515 antibody used recognizes the corresponding residue in TrkA and TrkC (Cell Signaling #9141), but by far the major Trk receptor in the hippocampus at this time is TrkB (Cheung et al., 2007; French et al., 1999; Fryer et al., 1996). In WT neurons, exogenous BDNF induced maximum phosphorylation at both sites in 5–15 min; phosphorylation at both sites decreased by 60 min [Fig.4A, B], but remained significantly higher than baseline. The time course observed for TrkB activation in WT hippocampal neurons was consistent with published studies monitoring the same sites (Ji et al., 2010; Lai et al., 2012). Retardation of TrkB recycling (via Rab11-positive endosomes) is also seen in Slitkr5 knockout neurons (Song et al., 2015).
Fig 4. BDNF activation of TrkB is altered in KalSRKO neurons.
A. Time course for exogenous BDNF treatment of DIV7 cultures from WT or KalSRKO hippocampi; pTrk515, pTrk705/706, TrkB and βIII tubulin were visualized in lysates prepared after the indicated treatment times. The pTrk515 and 705/706 antibodies crossreacts with TrkA, which are quantitatively minor compared to TrkB, so the blots are labeled Trk515 and Trk705/706. B. The signal for phosphorylated Trk or TrkB was normalized to the total TrkB signal at each time point; the WT ratio for the 5 min sample was set to 100%. $$ p=0.011; ## p=0.025; 2-way ANOVA analysis was performed using time and genotype as independent variables, phosphorylation level as the dependent variable. Six separate experiments were performed for the TrkB activation time course. C. TrkB levels in WT and KalSRKO cultures were normalized to βIII-tubulin levels; no significant difference in expression level was observed. D. Cortices and hippocampi from P7 wildtype and KalSRKO mice were blotted for pTrk515 and total TrkB. E. Quantification of the ratio of pTrk515 to total TrkB. Quantification of TrkB to βIII tubulin ratio; no significant difference in expression level was observed. Six WT animals and 8 KalSRKO animals were analyzed.
TrkB phosphorylation (activation) at both sites was also increased in KalSRKO neurons exposed to exogenous BDNF for 5 min and 15 min. In contrast to WT neurons, TrkB phosphorylation in KalSRKO neurons had not begun to decline 60 min after exposure to exogenous BDNF [Fig.4A, B]. Similar results were obtained using the same approach to evaluate TrkB activation by exogenous BDNF in DIV7 cortical cultures prepared from WT and KalSRKO mice (data not shown). Although continuous exposure of KalSRKO neurons to exogenous BDNF did not increase neurite outgrowth or branching, acute exposure to BDNF resulted in the prolonged activation of TrkB. Total TrkB levels did not differ in DIV7 cultures prepared from WT and KalSRKO mice and maintained in medium lacking exogenous BDNF [Fig.4C]. These results demonstrated that the kinetics of TrkB phosphorylation/dephosphorylation are altered in neurons lacking Kalirin after exposure to BDNF.
We next determined whether Trk activation in vivo differs in WT and KalSRKO mice at this developmental stage. Total TrkB levels did not differ in hippocampal or cortical tissue from postnatal day 7 WT vs. KalSRKO mice [Fig.4D, E]. Our data indicate that Trk family phosphorylation (activation) at Tyr515 does not differ in the hippocampus or cortex of WT vs. KalSRKO mice at this age [Fig.4D, E].
3.5 Activation of TrkB targets is altered in KalSRKO hippocampus and cortex
In response to BDNF, phosphorylated TrkB stimulates the MAPK/ERK, GSK-3β/PI3K/Akt and PLC-γ/CaMK/CREB signaling pathways to control a wide variety of downstream responses (Chao, 2003; Minichiello, 2009). The time course over which these pathways, which respond to many inputs in addition to BDNF, are activated and inactivated is critical to the outcomes observed (Ji et al., 2010; Lai et al., 2012; Marshall, 1995; Park and Poo, 2013). Since our cell culture studies revealed a role for Kalirin in determining the time course over which TrkB is activated in response to BDNF [Fig.4], we reasoned that activation of its downstream targets might be altered at this developmental stage. To test this hypothesis, we used phospho-specific antibodies to evaluate activation of the ERK, Akt and CREB pathways (Chao, 2003; Huang and Reichardt, 2003; Minichiello, 2009) in tissue from WT and KalSRKO mice. Levels of phospho-ERK normalized to total ERK were five-fold lower in KalSRKO cortex and hippocampus at P7 than in WT tissue [Fig.5A, B]. A lesser, but still significant, decrease was observed in Akt activation (phospho-Akt/total Akt) and in CREB activation (phospho-CREB/total CREB) in KalSRKO vs. WT cortex and hippocampus [Fig.5A, B]. While altered TrkB signaling would be expected to contribute to the diminished phosphorylation of ERK, CREB and Akt in KalSRKO neurons, altered signaling by other receptor tyrosine kinases and other signaling pathways would also be expected to contribute.
Fig 5. Activation of downstream targets of TrkB differs in KalSRKO tissue.
A. Activation of ERK, CREB and Akt in the same P7 wildtype and KalSRKO mouse tissue samples analyzed in Fig.4D was evaluated by Western blot analysis using the indicated antisera. Representative blots are shown (n = 7 per genotype). B. Quantification of phosphorylated protein to total protein; ratios for WT were set to 1.00. **p<0.01; *p<0.05.
3.6 Activation of TrkB targets is altered in KalSRKO hippocampal and cortical neurons
To investigate whether BDNF/TrkB signaling is altered due to the absence of Kalirin, we compared the effect of BDNF on activation of ERK, CREB and Akt in hippocampal cultures prepared from WT and KalSRKO mice. ERK, CREB and Akt phosphorylation was evaluated after neurons were treated with BDNF for 5, 15 and 60 min. The time course over which pERK, pCREB and pAkt levels rose and fell in response to BDNF in WT cultures was consistent with the literature (Ji et al., 2010; Lai et al., 2012). The time course for activation of ERK, CREB and Akt was significantly altered in KalSRKO cultures [Fig.6A, B]. Peak activation of ERK in response to BDNF (pERK/ERK) was delayed in KalSRKO neurons. The extent to which CREB and Akt were activated in response to BDNF (pCREB/CREB and pAkt/Akt) was diminished in KalSRKO neurons. TrkB downstream signaling is clearly altered in neurons lacking Kalirin. In addition, at baseline (time 0, before exogenous BDNF was added), ERK, CREB and Akt activation was lower in KalSRKO neuronal cultures than in WT cultures [Fig.6B, right panel]; this result was consistent with our in vivo observations and may reflect altered signaling by multiple growth factors [Fig.5A, B]. TrkB activation kinetics were prolonged in KalSRKO neurons [Fig.4]; altered TrkB dephosphorylation may contribute to the alterations observed in downstream target activation. Decreased activation of these common downstream targets would be expected to contribute to the reduced ability of exogenous BDNF to stimulate the formation and elongation of neurites in KalSRKO neurons [Fig.1C, D].
Fig 6. Activation of downstream targets of TrkB differs in KalSRKO neuronal cultures.
A. Time course for exogenous BDNF treatment of DIV7 cultures prepared from WT or KalSRKO hippocampi; pERK, ERK, pAkt, Akt, pCREB, and CREB were visualized in lysates prepared after the indicated treatment times. B. The signal for phosphorylated protein was normalized to the total protein signal at each time point; the WT ratio for the highest value was set to 100%. ##p<0.03, *p<0.05; 2-way ANOVA was performed using time and genotype as independent variables, with phosphorylation level as the dependent variable. C. Quantification of phosphorylated protein to total protein before BDNF was added (time 0); ratios for WT were set to 1.00. **p<0.01; *p<0.05. Six independent cultures were analyzed (n=3 per genotype) for BDNF time course experiments.
3.7 Tiam PHn-CC-EX inhibits Kalirin GEF1 activation of Rac1
BDNF-stimulated neuronal morphogenesis is known to require Rac1 activation by Tiam1 (Lai et al., 2012; Miyamoto et al., 2006). The PHn-CC-EX region of Tiam1 blocks the ability of Tiam1 to bind to membranes, an essential step in its activation of Rac1 (Stam et al., 1997), blunts its GEF activity in COS-7 cells transfected with TrkB and treated with BDNF and interferes with BDNF-stimulated morphogenesis in neurons (Lai et al., 2012; Miyamoto et al., 2006). We first explored the possibility that this fragment of Tiam1 could be used to distinguish the actions of Tiam1 and Kalirin by expressing PHn-CC-EX alone or with Kal7 at the time of plating. Neuronal morphology was altered significantly by expression of PHn-CC-EX; lamellipodial-like structures appeared at the distal tips of many short, thin processes [Fig.7A]. Co-expression of PHn-CC-EX and Kal7 produced neurons with multiple short processes and lamellipodia [Fig.7B], a notably different morphology from expression of Kal7 alone [Fig.3A].
Fig 7. Evaluation of PHn-CC-EX effects.
Examples of rat hippocampal neurons transfected at the time of plating with PHn-CC-EX alone (A) or with PHn-CC-EX and Kal7 (B) and fixed at DIV4. PHn-CC-EX was visualized using a FLAG antibody and Kal7 with a myc antibody; βIII tubulin staining (A) and MAP2 staining (B) were used to demonstrate the neuronal identity of the cells. Scale bar: 20 µm. Three separate experiments were performed; arrowheads indicate lamellipodial extensions. C and D. Rac1 activation was assessed using HEK-293 (pEAK) cells transfected in a glass-bottom 24-well plate in duplicate. All wells except the non-transfected received 0.1 µg of Rac1-biosensor vector (Das et al., 2015b). Vector amounts were adjusted to maintain cell viability: 0.015 µg pEAK-kGEF1, 0.3 µg pEAK-aKal7, 0.3 µg C1199 and 0.05 and 0.3 µg PHn-CC-EX. The total amount of vector per well was made up to 0.6 µg DNA using empty pEAK10 vector. DNA was mixed with 0.9 µl TransIT-2020 and spectra were obtained 30 h after transfection (Das et al., 2015b) C. Cells were extracted by boiling into SDS lysis buffer (Yan et al., 2015), and 1/10 of each sample was analyzed by SDS-PAGE. PVDF membranes were cut as indicated to enable detection of kGEF1 and Kal7 (myc epitope tagged) and C1199 and PHn-CC-EX (FLAG tagged) on the same membrane (D). C and D are representative of three experiments with hEK-293 cells and two experiments with COS-7 cells, all with similar results.
To try to understand these responses, we turned to a FRET-based bioassay for Rac1 activation in non-neuronal cells (Das et al., 2015a). PHn-CC-EX was expressed alone or with Kal-GEF1, Kal7 or Tiam1 C1199 (a more stable form of Tiam1) (Miyamoto et al., 2006; Stam et al., 1997) and a Rac1 biosensor. Expression of PHn-CC-EX alone had no effect on background FRET [Fig.7C]. As expected, PHn-CC-EX suppressed the ability of Tiam1 C1199 to activate Rac1 [Fig.7C, D]. Importantly, PHn-CC-EX also decreased the ability of Kal-GEF1 and Kal7 to activate Rac1 in a dose-dependent manner. As levels of PHn-CC-EX rose, levels of Kal7 and Tiam1 C1199 proteins fell. Kal7 and Tiam1 share the ability to interact with phosphoinositides and with EphB2, NMDA receptors and spinophilin (Kiraly et al., 2011; Miller et al., 2015; Terawaki et al., 2010). The ability of PHn-CC-EX to bind phosphoinositides and multiple proteins (Joshi et al., 2013; Stam et al., 1997; Terawaki et al., 2010) may account for its ability to disrupt Rac1 activation by Kalirin. Expression of PHn-CC-EX cannot be used to distinguish the effects of these two Rac1 GEFs.
4. Discussion
We have demonstrated an essential role for Kalirin in the ability of neurons to respond to BDNF. Neurite outgrowth is many steps removed from the initial interaction of BDNF with TrkB. Although we demonstrate a role for Kalirin in several intervening steps, we are far from understanding the pathway. Our observations provide compelling evidence that Kalirin, acting downstream of TrkB, is required early in development for neurite outgrowth and branching in response to exogenous BDNF. Kal GEF1 mediated Rac1 activation plays an essential role in the ability of BDNF to stimulate neurite outgrowth, and Kal7, with its single GEF domain, can substitute for the longer isoforms, with their two GEF domains [Fig. 1, 2] (Yan et al., 2015). Kalirin contributes to activation of the ERK, Akt and CREB pathways in vivo and to the ability of hippocampal and cortical neurons to activate these pathways in response to exogenous BDNF in vitro [Fig. 5, 6]. Based on its phosphorylation state, TrkB is activated to a similar extent in tissue from wildtype and KalSRKO tissue. However, the time course over which TrkB is activated and inactivated after exposure to BDNF differs in neurons lacking Kalirin [Fig. 4, 6]. The regulated endocytosis of many receptors, including TrkB, is crucial to normal cortical development (Song et al., 2015; Yap and Winckler, 2015). The role of the natural truncated variant TrkB.T1, which lacks the kinase domain and becomes the dominant TrkB protein in the adult (Fenner, 2012), has not been investigated here, nor have the changes in NMDA signaling that occur within 2 minutes of TrkB activation by BDNF (Levine et al., 1998).
4.1 TrkB signaling in the absence of Kalirin
As described previously, neurons lacking Kal9/Kal12 are unable to produce the normal number of neurites and branch points when maintained in culture (Yan et al., 2015). The current studies used the same approach to elucidate the role of Kalirin in neurite and branch point formation in response to exogenous BDNF. Neither the level of TrkB nor the phosphorylation of TrkB at Tyr515 differed in KalSRKO cortex or hippocampus at postnatal day 7 [Fig.4D, E]. The phosphorylation of TrkB may be different in KalSRKO tissues in older animals, because at postnatal day 7 the brain is still developing. However, activation of three downstream targets of BDNF/TrkB signaling (P-ERK, P-CREB and P-Akt) was diminished in the KalSRKO cortex and hippocampus [Fig.5A, B]. Whether altered in vivo TrkB signaling in the absence of Kalirin is the direct cause of these changes is not yet clear. Baseline activation of ERK, CREB and Akt in KalSRKO neurons was diminished compared to wildtype neurons, and the ability of BDNF to activate Akt and CREB in KalSRKO neurons was suppressed [Fig.6A, B]. Altered ERK, Akt and CREB activation would be expected to contribute to the blunted morphological effects of BDNF seen in the absence of Kalirin [Fig.1], but the exact molecular signaling pathway is not clear at present.
TrkB is rapidly activated by BDNF, even in the absence of Kalirin. The binding of BDNF to full length TrkB induces trans-phosphorylation of specific Tyr residues (Chao, 2003; Segal and Greenberg, 1996), a key step for activation of downstream signaling pathways (Huang and Reichardt, 2001; Minichiello, 2009). Increased phosphorylation of TrkB at Tyr515 and Tyr705/706 was observed within 5 min in neurons expressing or lacking Kal9/Kal12. We documented a striking prolongation in the time course of TrkB phosphorylation at Tyr515 and Tyr705/706 in KalSRKO neurons [Fig.4A, B]. The effects of BDNF/TrkB on the activation of key downstream targets is dependent on sustained signaling from endosomes after internalization of BDNF/TrkB (Cohen et al., 2011; Lazo et al., 2013; Ouyang et al., 2013); normal signaling can be disrupted by prolonged phosphorylation or lack of internalization of TrkB (Assaife-Lopes et al., 2014; Ji et al., 2010; Mitchell et al., 2012). The prolonged time course of TrkB dephosphorylation in the absence of Kal9/Kal12 could reflect changes in endocytic trafficking (Ma et al., 2014; Schiller et al., 2008) or the phosphatases inactivating TrkB. Altered levels of cell surface NR2B in Kal7KO neurons as well as in vitro studies have suggested a role for Kalirin in endocytic trafficking (Kiraly et al., 2011; Schiller et al., 2008).
Several tyrosine phosphatases (LAR, MKP-1 and PTPN12) play an essential role in BDNF/TrkB dependent neurite outgrowth and branching (Ambjorn et al., 2013; Jeanneteau et al., 2010; Yang et al., 2006). Receptor protein tyrosine phosphatase type O (PTPRO) and protein tyrosine phosphatase σ (PTPσ) both dephosphorylate TrkB, providing a negative modulatory effect on the ability of BDNF to stimulate neurite outgrowth and spine morphogenesis (Faux et al., 2007; Gatto et al., 2013; Kurihara and Yamashita, 2012). Like KalSRKO neurons, neurons lacking Slitrk5, a PTPσ interactor, are unable to respond to BDNF (Song et al., 2015). Levels of these phosphatases are controlled by BDNF (and hence also surface TrkB) and affect multiple signaling pathways, such as MAPK, Akt and PLC. The molecular features that determine phosphatase substrate specificity and their effects on TrkB signaling in vivo are not yet fully understood.
4.2 Roles for Multiple Rho GEFs and neurotrophins in neurite outgrowth
Activation of Rho GTPases by neurotrophins is known to play a role in the establishment of dendritic and axonal morphology. Both Rac1 and RhoA are involved in BDNF stimulated dendritic growth (Cheung et al., 2007). Dual Rho GEFs like Kal9 and Kal12 could play a unique role in coordinating the activation of Rac1/RhoG and RhoA, which signal to distinctly different effectors (May et al., 2002; Penzes et al., 2001). Recent studies have demonstrated important roles for additional Dbl family members, PDZ-GEF1, and Dock3, a non-Dbl Rho GEF, in the actin rearrangement and microtubule assembly changes needed for neurite outgrowth downstream of BDNF (Hisata et al., 2007; Namekata et al., 2012). The distinctive features of the different Rho GEFs that link neurotrophin receptors to activation of Rho GTPases may be needed to confer cell type and developmental specificity to the responses. Although Rac1 is essential for proper dendritic morphology, it is clear that expression of constitutively active Rac1 or exogenous KalGEF1 fails to stimulate normal dendritic development [Fig.2C], while expression of dominant negative Rac1 reduces dendritic complexity (Allen et al., 2000; Fournier et al., 2003; Luo et al., 1996). Normal neurite initiation clearly requires spatiotemporal coordination of Rho GTPase activation and inactivation by multiple GEFs and GAPs (Das et al., 2015b; Galic et al., 2014).
Tiam1 is clearly required for the effects of BDNF on dendritic arborization (Miyamoto et al., 2006; Tolias et al., 2007). TrkB both binds to and phosphorylates Tiam1; strikingly, mutation of the Tyr residue in Tiam1 that is phosphorylated by TrkB eliminates the ability of BDNF to affect dendritic morphology. We tried to use the PHn-CC-EX fragment of Tiam1 to distinguish the roles of Tiam1 and Kalirin in BDNF-dependent neurite formation [Fig.7]. The subcellular localization of Tiam1 and Kalirin, which reflects their interaction with phosphoinositides and with multiple different proteins, governs their access to Rho GTPases. Our cell based assay for Rac1 activation demonstrated that PHn-CC-EX diminished the ability of both Tiam and Kalirin to activate Rac1, precluding its use as a tool to distinguish their roles. This result is consistent with the crystal and solution structures of PHn-CC-EX(Joshi et al., 2013; Subramanian et al., 2013; Terawaki et al., 2010), which identified binding sites for phosphoinositides and multiple protein partners shared by these two Rho GEFs.
Earlier studies in PC12 cells demonstrated a role for Kalirin in the ability of NGF/TrkA to promote neurite formation (Chakrabarti et al., 2005). Signaling by other receptor tyrosine kinases (EphB2, ErbB4, PDGFR-β) has also been shown to involve Kalirin (Penzes et al., 2003; Wu et al., 2013). The ability of BDNF, acting through TrkB, to promote neurite outgrowth in early development requires Tiam1 (Tolias et al., 2007; Um et al., 2014), Slitrk5 (Song et al., 2015) and Kalirin. A direct interaction between TrkB and Tiam1 and the essential phosphorylation of a single Tyr in Tiam1 by TrkB place Tiam1 early in this signaling pathway. Although Tiam1 has a PDZ domain and Kal7 terminates with a PDZ binding motif, this type of direct interaction is unlikely to play an important role; Kal9 and Kal12, the isoforms expressed early in development, do not have a PDZ binding motif. In addition, the sequence of the Kal7 PDZ binding motif (−STYV) does not match the established specificity of the PDZ domain in Tiam1 (Shepherd et al., 2011). Additional studies are needed to define the roles of the many other proteins that play essential roles in the complex process of dendrite formation and branching.
Kalirin is required for BDNF-stimulated dendritic outgrowth and branching
BDNF/TrkB activation of Rac1 in neurons is largely mediated by Kalirin
The Tiam1 fragment PHn-CC-EX blocks Tiam1 and Kalirin Rac1 activation
Kalirin isoforms lacking the second GEF domain can rescue BDNF responsiveness
Kalirin affects the time course of BDNF/TrkB pathway activation in neurons
Acknowledgments
We thank Darlene D’Amato, Yanping Wang and Taylor LaRese for their outstanding technical support and Dr. Eric Levine for generously providing ANA-12. This work was supported by grants from the National Institutes of Health (DA015464, DA023082, DK032948), and by the Janice and Rodney Reynolds Endowment and the William Beecher Scoville Endowment. We thank Drs. Yuki Miyamoto and Yi Wu for gifts of vectors.
The funders of the work played no role in the study design or conduct of the research, preparation of the article or the decision to submit the work for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflicts.
References
- Alder J, Kallman S, Palmieri A, Khadim F, Ayer JJ, Kumar S, Tsung K, Grinberg I, Thakker-Varia S. Neuropeptide orphanin FQ inhibits dendritic morphogenesis through activation of RhoA. Dev Neurobiol. 2013;73:769–784. doi: 10.1002/dneu.22101. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Shan X, Murphey RK. A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Mol Cell Neurosci. 2000;16:754–765. doi: 10.1006/mcne.2000.0903. [DOI] [PubMed] [Google Scholar]
- Ambjorn M, Dubreuil V, Miozzo F, Nigon F, Moller B, Issazadeh-Navikas S, Berg J, Lees M, Sap J. A loss-of-function screen for phosphatases that regulate neurite outgrowth identifies PTPN12 as a negative regulator of TrkB tyrosine phosphorylation. PLoS One. 2013;8:e65371. doi: 10.1371/journal.pone.0065371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaife-Lopes N, Sousa VC, Pereira DB, Ribeiro JA, Sebastiao AM. Regulation of TrkB receptor translocation to lipid rafts by adenosine A(2A) receptors and its functional implications for BDNF-induced regulation of synaptic plasticity. Purinergic Signal. 2014;10:251–267. doi: 10.1007/s11302-013-9383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Dugich-Djordjevic MM, Li YX, Ma W, Somogyi R, Wen X, Brown E, Scott C, McKay RD, Barker JL. Neurotrophins stimulate chemotaxis of embryonic cortical neurons. Eur J Neurosci. 1997;9:2561–2570. doi: 10.1111/j.1460-9568.1997.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Borjigin J, Nathans J. Insertional mutagenesis as a probe of rhodopsin’s topography, stability, and activity. J Biol Chem. 1994;269:14715–14722. [PubMed] [Google Scholar]
- Bouquier N, Vignal E, Charrasse S, Weill M, Schmidt S, Leonetti JP, Blangy A, Fort P. A cell active chemical GEF inhibitor selectively targets the Trio/RhoG/Rac1 signaling pathway. Chem Biol. 2009;16:657–666. doi: 10.1016/j.chembiol.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol. 2008;18:524–531. doi: 10.1016/j.conb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Cahill ME, Remmers C, Jones KA, Xie Z, Sweet RA, Penzes P. Neuregulin1 signaling promotes dendritic spine growth through kalirin. J Neurochem. 2013;126:625–635. doi: 10.1111/jnc.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti K, Lin R, Schiller NI, Wang Y, Koubi D, Fan YX, Rudkin BB, Johnson GR, Schiller MR. Critical role for Kalirin in nerve growth factor signaling through TrkA. Mol Cell Biol. 2005;25:5106–5118. doi: 10.1128/MCB.25.12.5106-5118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Chin WH, Chen Y, Ng YP, Ip NY. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 2007;5:e63. doi: 10.1371/journal.pbio.0050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Bas Orth C, Kim HJ, Jeon NL, Jaffrey SR. Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites. Proc Natl Acad Sci U S A. 2011;108:11246–11251. doi: 10.1073/pnas.1012401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Das S, Tin T, Yang Q, Zhang J, Wu Y. Single-molecule tracking of small GTPase Rac1 uncovers spatial regulation of membrane translocation and mechanism for polarized signaling. pnas. 2015a;112:E6067–E6076. doi: 10.1073/pnas.1409667112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Yin T, Yang Q, Zhang J, Wu YI, Yu J. Single-molecule tracking of small GTPase Rac1 uncovers spatial regulation of membrane translocation and mechanism for polarized signaling. Proc Natl Acad Sci U S A. 2015b;112:E267–E276. doi: 10.1073/pnas.1409667112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux C, Hawadle M, Nixon J, Wallace A, Lee S, Murray S, Stoker A. PTPsigma binds and dephosphorylates neurotrophin receptors and can suppress NGF-dependent neurite outgrowth from sensory neurons. Biochim Biophys Acta. 2007;1773:1689–1700. doi: 10.1016/j.bbamcr.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Fenner BM. Truncated TrkB: beyond a dominant negative receptor. Cytokine Growth Factor Rev. 2012;23:15–24. doi: 10.1016/j.cytogfr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francone VP, Ifrim MF, Rajagopal C, Leddy CJ, Wang Y, Carson JH, Mains RE, Eipper BA. Signaling from the secretory granule to the nucleus: Uhmk1 and PAM. Mol Endocrinol. 2010;24:1543–1558. doi: 10.1210/me.2009-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Humby T, Horner CH, Sofroniew MV, Rattray M. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Brain Res Mol Brain Res. 1999;67:124–136. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF. Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol. 1996;374:21–40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Galic M, Tsai FC, Collins SR, Matis M, Bandara S, Meyer T. Dynamic recruitment of the curvature-sensitive protein ArhGAP44 to nanoscale membrane deformations limits exploratory filopodia initiation in neurons. Elife. 2014;3:e03116. doi: 10.7554/eLife.03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto G, Dudanova I, Suetterlin P, Davies AM, Drescher U, Bixby JL, Klein R. Protein tyrosine phosphatase receptor type O inhibits trigeminal axon growth and branching by repressing TrkB and Ret signaling. J Neurosci. 2013;33:5399–5410. doi: 10.1523/JNEUROSCI.4707-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisata S, Sakisaka T, Baba T, Yamada T, Aoki K, Matsuda M, Takai Y. Rap1-PDZ-GEF1 interacts with a neurotrophin receptor at late endosomes, leading to sustained activation of Rap1 and ERK and neurite outgrowth. J Cell Biol. 2007;178:843–860. doi: 10.1083/jcb.200610073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci. 2010;13:1373–1379. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L, Duan S, Lu B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M, Gakhar L, Fuentes EJ. High-resolution structure of the Tiam1 PHn-CC-Ex domain. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013;69:744–752. doi: 10.1107/S1744309113014206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner Y, Godecke N, Dierkes T, Thieme N, Zagrebelsky M, Korte M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front Synaptic Neurosci. 2014;6:5. doi: 10.3389/fnsyn.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Lemtiri-Chlieh F, Levine ES, Mains RE, Eipper BA. Kalirin binds the NR2B subunit of the NMDA receptor, altering its synaptic localization and function. J Neurosci. 2011;31:12554–12565. doi: 10.1523/JNEUROSCI.3143-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konur S, Ghosh A. Calcium signaling and the control of dendritic development. Neuron. 2005;46:401–405. doi: 10.1016/j.neuron.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Kurihara D, Yamashita T. Chondroitin sulfate proteoglycans down-regulate spine formation in cortical neurons by targeting tropomyosin-related kinase B (TrkB) protein. J Biol Chem. 2012;287:13822–13828. doi: 10.1074/jbc.M111.314070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KO, Wong AS, Cheung MC, Xu P, Liang Z, Lok KC, Xie H, Palko ME, Yung WH, Tessarollo L, Cheung ZH, Ip NY. TrkB phosphorylation by Cdk5 is required for activity-dependent structural plasticity and spatial memory. Nat Neurosci. 2012;15:1506–1515. doi: 10.1038/nn.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M, Evers JF. Control of dendritic diversity. Curr Opin Cell Biol. 2005;17:690–696. doi: 10.1016/j.ceb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lazo OM, Gonzalez A, Ascano M, Kuruvilla R, Couve A, Bronfman FC. BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J Neurosci. 2013;33:6112–6122. doi: 10.1523/JNEUROSCI.4630-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci U S A. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- Ma XM, Huang J, Wang Y, Eipper BA, Mains RE. Kalirin, a multifunctional Rho guanine nucleotide exchange factor, is necessary for maintenance of hippocampal pyramidal neuron dendrites and dendritic spines. J Neurosci. 2003;23:10593–10603. doi: 10.1523/JNEUROSCI.23-33-10593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Miller MB, Vishwanatha KS, Gross MJ, Wang Y, Abbott T, Lam TT, Mains RE, Eipper BA. Nonenzymatic domains of Kalirin7 contribute to spine morphogenesis through interactions with phosphoinositides and Abl. Mol Biol Cell. 2014;25:1458–1471. doi: 10.1091/mbc.E13-04-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J Neurosci. 2008;28:711–724. doi: 10.1523/JNEUROSCI.5283-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- May V, Schiller MR, Eipper BA, Mains RE. Kalirin Dbl-homology guanine nucleotide exchange factor 1 domain initiates new axon outgrowths via RhoG-mediated mechanisms. J Neurosci. 2002;22:6980–6990. doi: 10.1523/JNEUROSCI.22-16-06980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Miller MB, Vishwanatha KS, Mains RE, Eipper BA. An N-terminal Amphipathic Helix Binds Phosphoinositides and Enhances Kalirin Sec14 Domain-mediated Membrane Interactions. J. Biol. Chem. 2015;290:13541–13555. doi: 10.1074/jbc.M115.636746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Yan Y, Eipper BA, Mains RE. Neuronal Rho GEFs in synaptic physiology and behavior. Neuroscientist. 2013;19:255–273. doi: 10.1177/1073858413475486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Mioranzza S, Nunes F, Marques DM, Fioreze GT, Rocha AS, Botton PH, Costa MS, Porciuncula LO. Prenatal caffeine intake differently affects synaptic proteins during fetal brain development. Int J Dev Neurosci. 2014;36:45–52. doi: 10.1016/j.ijdevneu.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, Blasier KR, Jeffery ED, Ross MW, Pullikuth AK, Suo D, Park J, Smiley WR, Lo KW, Shabanowitz J, Deppmann CD, Trinidad JC, Hunt DF, Catling AD, Pfister KK. Trk activation of the ERK1/2 kinase pathway stimulates intermediate chain phosphorylation and recruits cytoplasmic dynein to signaling endosomes for retrograde axonal transport. J Neurosci. 2012;32:15495–15510. doi: 10.1523/JNEUROSCI.5599-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci U S A. 2006;103:10444–10449. doi: 10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekata K, Harada C, Guo X, Kimura A, Kittaka D, Watanabe H, Harada T. Dock3 stimulates axonal outgrowth via GSK-3beta-mediated microtubule assembly. J Neurosci. 2012;32:264–274. doi: 10.1523/JNEUROSCI.4884-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmichi M, Decker SJ, Pang L, Saltiel AR. Inhibition of the cellular actions of nerve growth factor by staurosporine and K252A results from the attenuation of the activity of the trk tyrosine kinase. Biochemistry. 1992;31:4034–4039. doi: 10.1021/bi00131a019. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Lizarraga SB, Schmidt M, Yang U, Gong J, Ellisor D, Kauer JA, Morrow EM. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80:97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- Panagiotaki N, Dajas-Bailador F, Amaya E, Papalopulu N, Dorey K. Characterisation of a new regulator of BDNF signalling, Sprouty3, involved in axonal morphogenesis in vivo. Development. 2010;137:4005–4015. doi: 10.1242/dev.053173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Kambampati V, Mains RE, Eipper BA. Distinct roles for the two Rho GDP/GTP exchange factor domains of kalirin in regulation of neurite growth and neuronal morphology. J Neurosci. 2001;21:8426–8434. doi: 10.1523/JNEUROSCI.21-21-08426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidou P, Valdez G, Akmentin W, Bowers WJ, Federoff HJ, Halegoua S. Trk retrograde signaling requires persistent, Pincher-directed endosomes. Proc Natl Acad Sci U S A. 2011;108:852–857. doi: 10.1073/pnas.1015981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainath R, Gallo G. Cytoskeletal and signaling mechanisms of neurite formation. Cell Tissue Res. 2015;359:267–278. doi: 10.1007/s00441-014-1955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller MR, Ferraro F, Wang Y, Ma XM, McPherson CE, Sobota JA, Schiller NI, Mains RE, Eipper BA. Autonomous functions for the Sec14p/spectrin-repeat region of Kalirin. Exp Cell Res. 2008;314:2674–2691. doi: 10.1016/j.yexcr.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Shepherd TR, Hard RL, Murray AM, Pei D, Fuentes EJ. Distinct ligand specificity of the Tiam1 and Tiam2 PDZ domains. Biochemistry. 2011;50:1296–1308. doi: 10.1021/bi1013613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda N, Beppu H, Fukuda T, Li E, Kitajima I, Fukunaga K. Aberrant calcium/calmodulin-dependent protein kinase II (CaMKII) activity is associated with abnormal dendritic spine morphology in the ATRX mutant mouse brain. J Neurosci. 2011;31:346–358. doi: 10.1523/JNEUROSCI.4816-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer JE, Budreck EC. Kalirin-7: linking spine plasticity and behavior. J Neurosci. 2009;29:5367–5369. doi: 10.1523/JNEUROSCI.0235-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Giza J, Proenca CC, Jing D, Elliott M, Dincheva I, Shmelkov SV, Kim J, Schreiner R, Huang SH, Castren E, Prekeris R, Hempstead BL, Chao MV, Dictenberg JB, Rafii S, Chen ZY, Rodriguez-Boulan E, Lee FS. Slitrk5 Mediates BDNF-Dependent TrkB Receptor Trafficking and Signaling. Dev Cell. 2015;33:690–702. doi: 10.1016/j.devcel.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam JC, Sander EE, Michiels F, van Leeuwen FN, Kain HE, van der Kammen RA, Collard JG. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J Biol Chem. 1997;272:28447–28454. doi: 10.1074/jbc.272.45.28447. [DOI] [PubMed] [Google Scholar]
- Subramanian N, Navaneethakrishnan S, Biswas J, Kanwar RK, Kanwar JR, Krishnakumar S. RNAi mediated Tiam1 gene knockdown inhibits invasion of retinoblastoma. PLoS One. 2013;8:e70422. doi: 10.1371/journal.pone.0070422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- Terawaki S, Kitano K, Mori T, Zhai Y, Higuchi Y, Itoh N, Watanabe T, Kaibuchi K, Hakoshima T. The PHCCEx domain of Tiam1/2 is a novel protein- and membrane-binding module. EMBO J. 2010;29:236–250. doi: 10.1038/emboj.2009.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci U S A. 2007;104:7265–7270. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um K, Niu S, Duman JG, Cheng JX, Tu YK, Schwechter B, Liu F, Hiles L, Narayanan AS, Ash RT, Mulherkar S, Alpadi K, Smirnakis SM, Tolias KF. Dynamic control of excitatory synapse development by a Rac1 GEF/GAP regulatory complex. Dev Cell. 2014;29:701–715. doi: 10.1016/j.devcel.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chang X, She L, Xu D, Huang W, Poo MM. Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J Neurosci. 2015;35:8384–8393. doi: 10.1523/JNEUROSCI.4682-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Wu JH, Fanaroff AC, Sharma KC, Smith LS, Brian L, Eipper BA, Mains RE, Freedman NJ, Zhang L. Kalirin promotes neointimal hyperplasia by activating Rac in smooth muscle cells. Arterioscler Thromb Vasc Biol. 2013;33:702–708. doi: 10.1161/ATVBAHA.112.300234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Eipper BA, Mains RE. Kalirin-9 and Kalirin-12 Play Essential Roles in Dendritic Outgrowth and Branching. Cereb Cortex. 2015;25:3487–3501. doi: 10.1093/cercor/bhu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Massa SM, Longo FM. LAR protein tyrosine phosphatase receptor associates with TrkB and modulates neurotrophic signaling pathways. J Neurobiol. 2006;66:1420–1436. doi: 10.1002/neu.20291. [DOI] [PubMed] [Google Scholar]
- Yap CC, Winckler B. Adapting for endocytosis: roles for endocytic sorting adaptors in directing neural development. Front Cell Neurosci. 2015;9:119. doi: 10.3389/fncel.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]