Abstract
Vigilance and avoidance of threat are observed in anxious adults during laboratory tasks, and are posited to have real-world clinical relevance, but data are mixed in anxious youth. We propose that vigilance-avoidance patterns will become evident in anxious youth through a focus on individual differences and real-world strategic avoidance. Decreased functional connectivity between the amygdala and prefrontal cortex (PFC) could play a mechanistic role in this link. 78 clinically anxious youth completed a dot-probe task to assess vigilance to threat while undergoing fMRI. Real-world avoidance was assessed using Ecological Momentary Assessment (EMA) of self-reported suppression and distraction during negative life events. Vigilance toward threat was positively associated with EMA distraction and suppression. Functional connectivity between a right amygdala seed region and dorsomedial and right dorsolateral PFC regions was inversely related to EMA distraction. Dorsolateral PFC-amygdalar connectivity statistically mediated the relationship between attentional vigilance and real-world distraction. Findings suggest anxious youth showing attentional vigilance toward threat are more likely to use suppression and distraction to regulate negative emotions. Reduced PFC control over limbic reactivity is a possible neural substrate of this pattern. These findings lend ecological validity to laboratory vigilance assessments and suggest PFC-amygdalar connectivity is a neural mechanism bridging laboratory and naturalistic contexts.
Keywords: Imaging, Anxiety, Information processing, Attention, Emotion regulation
1. Introduction
Anxious youth, like anxious adults, display a pattern of vigilant attention toward threat-relevant cues in the laboratory (Bar-Haim et al., 2007). Biased attention toward threat is posited to contribute to the maintenance of anxiety over time (MacLeod et al., 1986), providing persistent opportunities for both physiological reactions and anxiety-promoting beliefs (e.g., the world is full of danger) to be rehearsed. When present in youth, threat vigilance may therefore represent a key mechanism through which life-long trajectories of affective psychopathology (Pine et al., 1998) are initiated and maintained.
An excessive focus on threat may promote anxious physiological responding and maladaptive cognitions, but clinical manifestations of anxiety are largely characterized by persistent avoidance of threat. Mogg et al. (2004) proposed a vigilance-avoidance model of cognitive bias in anxiety, suggesting that following early attentional vigilance to threat cues, anxious individuals strategically direct attention away from threat in an attempt to decrease anxiety elicited by aversive stimuli (i.e., avoidance is used as an emotion regulation strategy). Avoidant attentional patterns during late stages of stimulus presentation (i.e., >1500 ms post-onset) have been observed in at least some studies of anxious adults (e.g., Bogels and Mansell, 2004)—although avoidance is not strictly confined to late time points in the adult literature, but has also been observed at early time points, particularly when threat severity is strong (Wald et al., 2011, Shechner et al., 2012). In youth, vigilance-avoidance findings are decidedly mixed. While vigilance to threat is relatively well-established in pediatric samples (Bar-Haim et al., 2007)—although there are clear exceptions, e.g., in the fMRI scanner (Monk et al., 2006)—avoidance findings are quite inconsistent, ranging from the hypothesized vigilance-avoidance pattern (In-Albon et al., 2010), to avoidance at early timepoints only (Gamble and Rapee, 2009), to early vigilance with no later group differences (Shechner et al., 2013), to consistent attentional patterns across anxious and non-anxious youth at both early and later timepoints (Price et al., 2013). One possibility is that subsets of anxious youth differ in their attentional patterns (Salum et al., 2012); but the vigilance-avoidance hypothesis implies that the same individuals would be prone to both early vigilance and later avoidance. An additional possibility is that attention measured on the time-course of seconds in the laboratory (even at stimulus presentations ≥2 s) represents relatively “automatic” (i.e., involuntary, routinized) forms of avoidance (Najmi et al., 2010, Buetti et al., 2012) that may not be fully developed in youth. If this were the case, we might more readily see avoidance in a less automatic form, i.e., deployed as a strategic (i.e., voluntary, effortful) emotion regulation response to real-world stressors. However, previous attentional bias studies have been limited to laboratory assessments of both vigilance and avoidance, leaving this potential pattern uninvestigated.
Ecological Momentary Assessment (EMA) is an ecologically valid method for characterizing emotional reactivity and emotion regulation through repeated in vivo sampling. It entails the gathering of representative real-time data on emotion and behavior in natural environments through the use of signaling devices (Silk et al., 2011). EMA is designed to overcome several limitations of laboratory-based assessments. It accesses emotions experienced in real-world contexts and circumvents memory biases associated with delayed retrospective recall. EMA studies in adults have found that compared to non-anxious individuals, those with social anxiety report higher rates of a specific type of avoidance, experiential avoidance, which is defined as efforts to escape, avoid, alter, or conceal undesirable emotions or thoughts (Kashdan et al., 2013). Conversely, in the only EMA study to date to assess avoidance in anxious youth, anxious and control youth used avoidance with comparable frequency (Tan et al., 2012). A focus on individual differences on an EMA measure of avoidance would enable a direct examination of the hypothesis that laboratory-assessed vigilance in anxious youth is associated with strategic, real-world avoidance. This focus on individual differences may be critical to understanding the role of vigilance in anxiety if, for example, a subset of anxious patients exhibit a high degree of attentional bias, and also show a greater degree of real-world maladaptive avoidance.
Neuroimaging studies have pointed to prefrontal-limbic functional connectivity as a key neural mechanism that is altered during attentional bias tasks in both anxious youth (Price et al., 2014, Monk et al., 2008) and young adults with a childhood history of behavioral inhibition (Hardee et al., 2013). In a previous group comparison of anxious and healthy youth using an overlapping dataset (Price et al., 2014), we reported that anxious youth as a group, particularly the most severely anxious among the clinical sample, were characterized by reduced integration of response across prefrontal (rostrodorsal anterior cingulate) and limbic (hippocampal/parahippocampal) regions during an attentional bias (dot-probe) task. Decreased communication and synchronization between bottom-up limbic regions (reacting to threat) and top-down prefrontal regions (controlling attention) are posited to contribute to anxiety and threat hypervigilance (Bishop, 2008), resulting in reduced capacity to override threat orienting and attentional capture. Because amygdala-prefrontal connectivity subserves both automatic and controlled processing, it may be mechanistically important in both vigilance and avoidance. Specifically, reduced integration in this circuit during threat processing could foster both hyperactive early/automatic responses to threat (vigilance) and overreliance on maladaptive forms of voluntary emotion regulation (e.g., avoidance) in the real world—particularly given that adaptive alternatives for strategic emotion regulation (e.g., cognitive restructuring) may rely on functional integration within the same circuit (Wager et al., 2008). Current analyses provide novel information regarding the extent to which attentional vigilance and real-world avoidance are linked to one another, and to functional integration within this threat processing circuit.
In summary, the current study assessed individual differences in attentional vigilance, related neural substrates, and real-world use of avoidance as an emotion regulation strategy in a sample of 78 clinically anxious youth. We predicted that (a) greater attentional vigilance in the lab and (b) reduced PFC-amygdalar functional connectivity would be associated with greater real-world engagement in avoidance following a negative event, assessed via EMA. To further understand the extent to which observed neural function might be mechanistically important, we tested whether PFC-amygdalar connectivity statistically mediated the relationship between vigilance and avoidance. While primary analyses and conclusions pertain to the sample of anxious youth, we used available data from a small sample of control youth (n = 20) to conduct preliminary explorations of whether observed mechanistic relationships were specific to clinical anxiety or might generalize across the full spectrum of anxious and non-anxious youth.
2. Methods
2.1. Participants
Seventy-eight unmedicated youth (ages 9–14) with DSM-IV diagnoses of Generalized Anxiety Disorder, Separation Anxiety Disorder, and/or Social Phobia were recruited through a larger treatment outcome study (see Table 1 for further details). These three prevalent diagnoses were included to allow investigation of transdiagnostic biobehavioral patterns, a growing priority in psychiatric research (e.g., Research Domain Criteria initiative; Insel et al., 2010). A comparison sample of 20 youth with no lifetime history of DSM-IV-TR disorders also completed all measures (see Table S1a). The larger study was designed to have an uneven allocation due to the primary focus on mechanisms of treatment for pediatric anxiety (treatment outcomes are presented in Silk et al. (in press)). The present analyses therefore focused on the anxious sample, where power was sufficient to permit an individual differences approach. The control sample was used in sensitivity analyses only, allowing us to preliminarily assess the specificity of any observed links to anxious youth.
Table 1.
Descriptive characteristics of the sample.
| Anxious youth (n = 78) | |
|---|---|
| Age | 10.7 (1.4) |
| Female (%) | 56.4 |
| Caucasian (%) | 85.9 |
| Head of household education, median | Standard college degree |
| Household income, median | $80–90,000 |
| Current diagnosisa (%) | |
| Separation anxiety disorder | 20.5 |
| Social phobia | 24.4 |
| Generalized anxiety disorder | 73.1 |
| Specific phobia | 15.4 |
| Major depressive disorder | 1.3 |
| Attention deficit/hyperactivity disorder | 2.6 |
| Pediatric Anxiety Rating Scale | 20.7 (4.6) |
| EMA suppression use, % of negative events | 71.8% (29.5%) |
| EMA distraction use, % of negative events | 43.4% (25.7%) |
| Mean RT bias | 14.2 (114.9) |
Note. Data presented as mean (SD) unless otherwise noted. EMA = Ecological Momentary Assessment; RT = reaction time.
Diagnostic groups are partially overlapping due to inclusion of comorbid patients. Primary/principle diagnoses were not designated, meaning that percentages for the 3 diagnostic inclusion groups will not sum to 100.
Qualifying youth completed a baseline assessment that included, among other measures, a dot-probe task completed in the fMRI scanner and an EMA protocol. Of 141 youth with available fMRI data at baseline, 20 youth (14.2%; 14 anxious, 6 controls) were excluded for excessive head motion (see Supplement for criteria). Of the remaining 121 participants, 23 (19%; 12 anxious, 11 controls) were excluded due to not having at least 3 sampling points meeting EMA criteria below. Participants included/excluded did not differ on any demographic/clinical variable in Table 1 (p's >.10; see Table S1b). Informed consent from parents and informed assent from youth were appropriately obtained; study procedures were approved by the University of Pittsburgh's Institutional Review Board.
2.2. Ecological Momentary Assessment (EMA)
We used a cellphone methodology developed for collecting EMA data on youths’ real-world emotional processes (see Silk et al., 2011 for additional detail). A brief interview assessed, among other things, youths’ emotional, cognitive, and behavioral responses to a self-nominated negative emotional event. Specifically, youth were asked to describe the time in the past hour when they felt the worst, or the most negative; hence a single index negative event was assessed per call. Responses were later categorized into types of events by trained coders (e.g., school, peer concerns, family conflict, having to do something you don’t want to do, transient pain, etc.; see Supplement for further detail, as well as anxious vs. non-anxious group differences). The one hour time window was intended to maximize the chances of assessing naturally occurring emotional experiences while minimizing recall bias (Silk et al., 2003). Youth were called twice between the hours of 4 p.m. and 9:30 p.m. on weekdays (Thursday, Friday and Monday) and four times between the times of 11 a.m. and 9:30 p.m. on Saturday and Sunday, totaling 14 calls or sampling events. Phone calls were included in the analyses if youth were able to identify a negative event in the past hour eliciting a rating ≥3 (on a 5-point scale) on one or more negative emotions (sad, angry, nervous, and upset), allowing for assessment of emotion regulation strategies employed in response to moderate-to-severe distress. The mean number of calls included was 7.82 (SD = 3.12).
Youth reported whether or not they used a number of emotion regulation strategies in response to the negative event (Silk et al., 2003). Youth could endorse multiple strategies for the same event. Here, we focused on suppression (“Did you try not to think about it or try to forget all about it?”) and distraction (“Did you keep your mind off of the problem by doing something else?”). Both strategies are consistent with strategic attentional avoidance. Youth were also queried about their use of rumination, cognitive restructuring, acceptance, and problem solving. In addition, somatic responses indicative of physiological arousal (e.g., sweating, fast heart rate) were separately classified as physiological responding. For the current analyses, a single score was calculated for each participant on each of the two a priori avoidance indices (suppression and distraction). These were calculated as the percentage of eligible phone calls (i.e., those containing a negative event in the past hour that was rated as at least moderately distressing) for which the youth responded ‘Yes’ in response to the corresponding ‘Yes/No’ emotion regulation question, creating a continuous variable for analysis across participants, ranging from 0 to 100%.
2.3. Dot-probe task
Participants completed the dot-probe task in an fMRI scanner using a slow event-related design, as previously described (Price et al., 2014). After an initial fixation cross presented in the middle of the screen (500 ms), a fearful and a neutral face were presented simultaneously on the top and bottom of the screen for either a short (200 ms) or long (2000 ms) interval, followed by a probe (dot) replacing either face. Participants completed a total of 64 fearful-neutral trials in a randomized order. Additional control trials (presenting pairs of blank ovals; n = 16) were presented but not used in primary analyses (see Supplement for exploratory analyses). To reduce multiple comparisons and preserve power by using all available threat-relevant trials, primary analyses were performed collapsing across the two durations, as stimulus duration did not robustly moderate behavioral or neural effects in previously published analyses (Price et al., 2014). Exploratory post hoc analyses were then used to probe the impact of face duration. The dot remained on-screen for the remainder of the trial (8.8–10.6 s; each trial = 11.3 s total), allowing brain responses to dissipate prior to the next trial onset. Faces were grayscale conversions of the well-validated NimStim battery (Tottenham et al., 2009), half male and half female, with the same actor presented in both images in each pair. Participants were instructed to respond as quickly as possible to the probe, indicating its location on the screen by pressing a key for top or bottom. For each subject, bias scores were calculated as mean reaction time (RT) to incongruent – congruent trials, creating an attentional bias score with larger values indicating greater vigilance to threat. To improve psychometrics (Price et al., 2015) and reduce the influence of outliers while preserving all data points, RT outliers were rescaled using a Winsorizing procedure (see Supplement).
2.4. fMRI analysis
In a previous group comparison of anxious and healthy youth conducted in the current sample (Price et al., 2014), we reported aberrant neural disengagement patterns in anxious youth in limbic (hippocampal/parahippocampal) regions during the dot-probe task. The current analysis is novel and distinct in the following ways: (a) the use of EMA data to extend analyses beyond the laboratory and in to the real world; (b) the dimensional approach taken to characterize individual differences within the anxious sample; (c) the use of novel fMRI functional connectivity data using a distinct, a priori anatomical region (the amygdala; this was specifically done to reduce analytic overlap and negate circularity/“double-dipping” concerns that arise when functionally defined regions of interest are used (Kriegeskorte et al., 2010, Vul et al., 2009), given that the prior analyses were performed in an overlapping sample and included connectivity analyses focusing on functionally defined hippocampal/parahippocampal regions); and (d) the application of a statistical mediation approach to further characterize the potential role of brain function in mediating the vigilance-avoidance hypothesis.
Functional images were acquired during the dot-probe by a 3 T head-only Siemens Trio, using a posterior-to-anterior T2*-weighted echoplanar imaging sequence (TR = 1.67 s, TE = 29 ms, FOV = 205 × 205, flip = 75°, 3.2 mm isotropic voxels, 32 axial slices). Functional volumes were preprocessed as previously described (Price et al., 2014) using our laboratory's standard methods (see Supplement).
2.4.1. Connectivity maps
Functional connectivity maps were generated per-participant and used for all analyses in this paper. The right and left amygdalae were selected as seed regions for functional connectivity analysis due to the amygdala's widely recognized role in threat processing and vigilance (Bishop, 2008, Zald, 2003) and demonstrated alterations in amygdalar-PFC connectivity in two previous anxious samples during the dot-probe (Monk et al., 2008, Hardee et al., 2013). Whole-brain connectivity maps were generated for each participant, in a single step, utilizing all trials that included a fearful-neutral face pair (after excluding trials with excessive motion). For each amygdalar region (right and left amygdala taken from a standard MNI atlas; (Tzourio-Mazoyer et al., 2002)), single-subject, trial-by-trial BOLD signal values were extracted for the 4th TR (∼5 s into each trial, roughly corresponding to the observed peak hemodynamic response function, HRF; see Supplement for validation of this decision to use the 4th TR, as well as sensitivity analyses exploring the impact of this decision). For each amygdalar region (right/left), amygdalar signal at the 4th TR was then regressed on single-subject, trial-by-trial whole-brain maps of BOLD signal at the 4th TR, to create functional connectivity maps (as in Rissman et al., 2004) for each subject.
This connectivity approach is implemented in a validated functional connectivity toolbox (Zhou et al., 2009) and has been used previously to assess connectivity in psychiatric samples during cognitive tasks (e.g., Aizenstein et al., 2009). It is particularly appropriate for slow event-related designs, where the full HRF can be captured and returned to baseline on every trial, taking full advantage of the ability to quantify patterns in trial-to-trial variation without the need to calculate contrasts (i.e., other than the change in signal from the start of each trial) or to deconvolve data and make related assumptions regarding the HRF. This approach was selected because it requires no assumptions about the influence of specific trial features (e.g., stimulus duration, congruence), but instead automatically takes into account variability due to both experimental trial features (i.e., stimulus duration, congruence) and participant-level factors (e.g., waxing and waning concentration, variable affective response to specific face stimuli) by quantifying the degree of consistency in brain response from one trial to the next. For example, if an individual consistently has a larger activation value for all congruent trials (compared to incongruent trials), across both amygdalar and PFC regions, the resulting connectivity index will reflect this coupling with a higher value. If another individual's activation is not robustly influenced by trial type, but their amygdalar and PFC values cohere because both regions respond when the participant is attentive to certain face presentations (and not to others), their connectivity index will also be high. The analyses therefore remain agnostic regarding the sources of trial-to-trial variability in the data, but quantify the coherence of response across brain regions in the context of the task (here, during processing of neutral-fearful face pairs and subsequent responses to the probe). See Supplement for additional post hoc analyses exploring the impact of specific experimental trial features (psychophysiological interaction analyses).
2.4.2. EMA-fMRI regressions
Resulting connectivity maps were Winsorized and regressed on EMA scores (suppression and distraction) across all subjects using AFNI's 3dRegAna command to identify clusters where amygdalar connectivity was significantly related to EMA measures. One participant's connectivity data was excluded due to a technical error affecting data preprocessing. Type I error for voxelwise tests was controlled using contiguity thresholds based on the autocorrelation of the statistical maps (AFNI's 3dClustSim with smoothing estimated via 3dFWHM; voxel-wise threshold of p < .001, cluster thresholds of 35–62 voxels, map-wise false positive detection at p < .05).
2.5. Mediation analysis
Neural substrates of vigilance-avoidance patterns were further explored in mediation analyses designed to further characterize the nature of observed interrelationships between behavioral vigilance, EMA avoidance, and brain regions identified in primary regression analyses. Although neural connectivity and behavioral vigilance data were collected concurrently, the neural measure was selected as the mediator (and behavioral vigilance as the independent variable) based on: (a) our a priori theoretical interest in brain mechanisms mediating the link between vigilant and avoidant behaviors, which tests the neural conditions necessary for laboratory vigilance to later translate into real-world avoidance and (b) previous findings where prefrontal and amygdalar neural responses were found to mediate relationships between behavioral vigilance in the lab and self-reported symptoms in daily life (Price et al., 2011). The same whole-brain, trial-to-trial connectivity maps were used as described above; however, to improve power, we constrained the voxel-wise search space for mediation analyses to those clusters where connectivity was related to EMA measures (in the EMA-fMRI regressions described above), as these were the regions we hoped to further characterize with the mediation analysis. Within this search space, we conducted unbiased, voxel-wise tests of amygdalar connectivity at each voxel as a mediator of the relationship between RT measures of attentional vigilance and EMA scores using a standard 3-variable path model (Baron and Kenny, 1986) (see Fig. 2 legend). Bootstrapping was used to test the significance of each path using the Multilevel Mediation and Moderation Toolbox (http://wagerlab.colorado.edu/tools). Results were cluster-corrected to hold map-wise false positive detection at p < .05, as above.
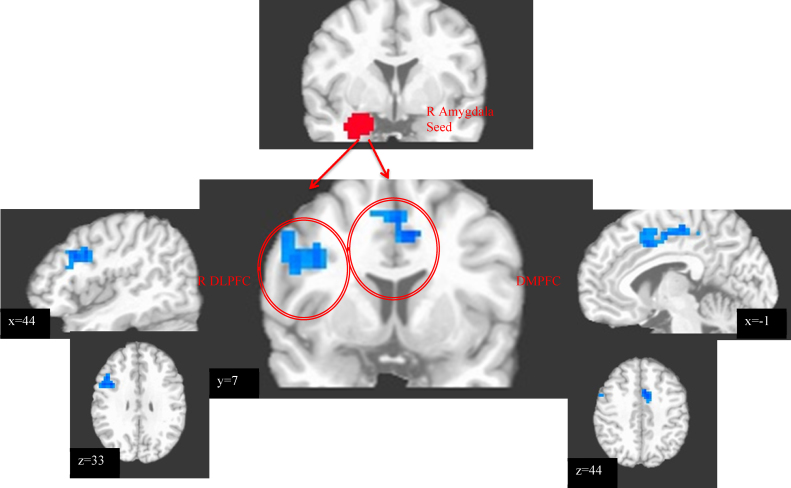
Fig. 2.
Regions in which lesser functional connectivity with the right amygdalar seed region (pictured) was associated with greater real-world distraction use (expressed as % of negative events for which distraction was endorsed). Images in radiological convention (left = right).
3. Results
3.1. Behavioral findings
3.1.1. Primary hypotheses
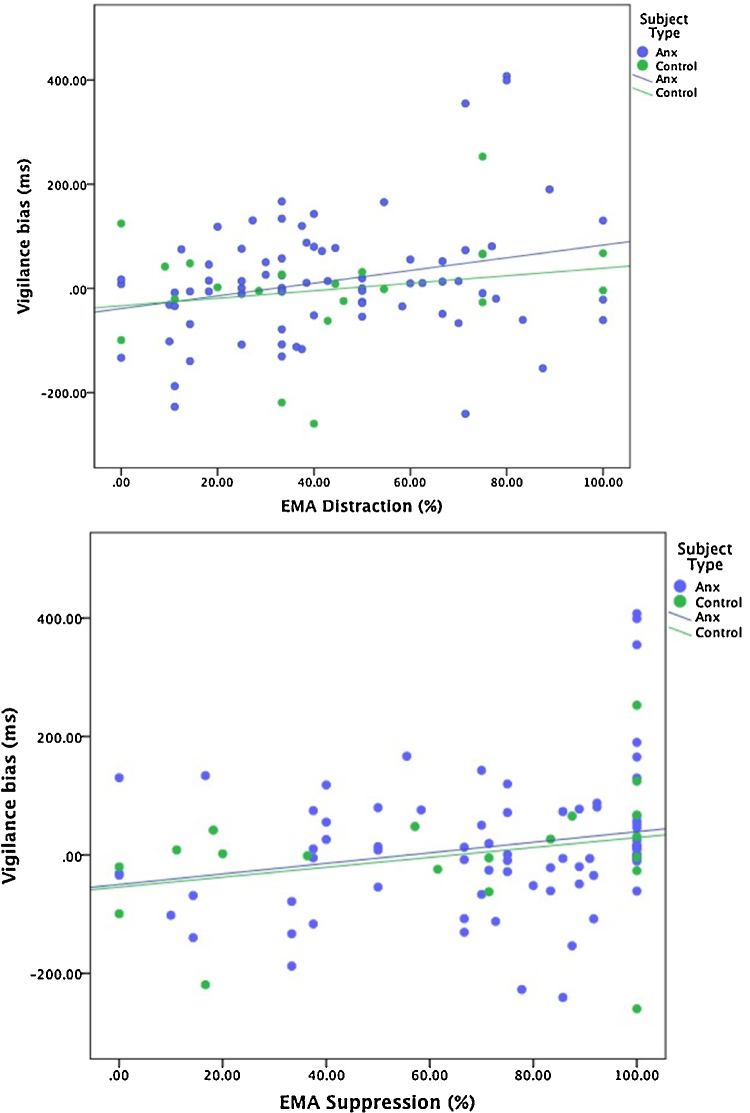
Across the primary sample of anxious youth, greater attentional vigilance (RT bias score) was correlated with both greater EMA distraction (r = .27, p = .02) and suppression (r = .23, p = .04) (Fig. 1).
Fig. 1.
Scatterplots of the relationships between RT vigilance and EMA measures of avoidance (distraction and suppression). The vigilance bias data displayed have been rescaled (Winsorized) to adjust outliers.
3.1.2. Sensitivity analyses: anxious and non-anxious youth
In a sensitivity analysis using an expanded sample that included 20 additional healthy control participants, greater vigilance (RT bias score) remained correlated with greater EMA distraction (r = .26, p = .01) and suppression (r = .25, p = .01; Fig. 1), even after controlling for patient status (p's < .01). These exploratory results suggest the vigilance-avoidance relationships we observed could be equally pertinent to both anxious and non-anxious youth, provided a sufficiently negative event has occurred in daily life. However, as expected, non-anxious youth in our sample reported lower levels of nervousness in response to negative life events (F1,111 = 4.83, p = .03) and less frequent use of suppression as an emotion regulation strategy (F1,111 = 4.86, p = .03) across all calls (i.e., not restricted to those with a negative emotion rating of at least 3). Thus, the circumstances that provoke avoidance in daily life appear to be less frequent overall in non-anxious youth; however, among youth higher on vigilance, even non-anxious youth may endorse avoidance when a sufficiently negative life event has occurred.
3.2. fMRI findings
3.2.1. Primary hypotheses
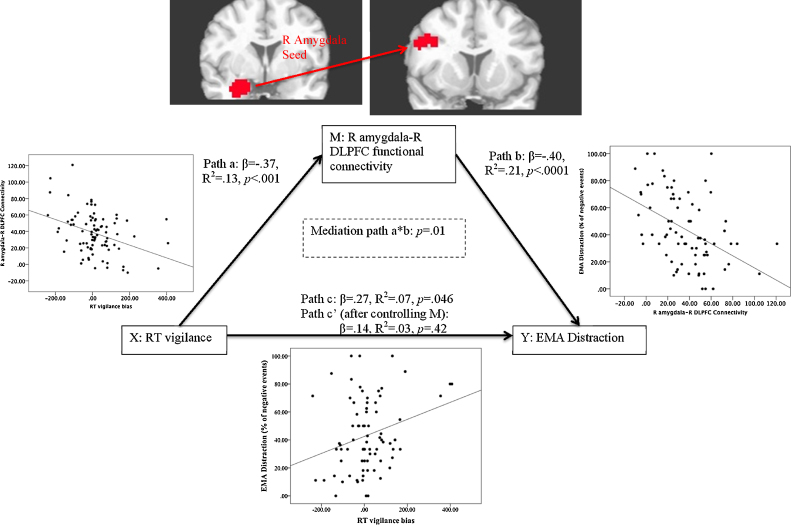
Lesser positive connectivity between the right (R) amygdala seed region and two dorsal PFC clusters (Table 2; Fig. 2) was associated with greater EMA distraction. No significant clusters were identified using a left amygdala seed region, nor when regressing EMA suppression. Furthermore, the relationship between vigilance and EMA distraction was significantly mediated by functional connectivity between R amygdala and R dorsolateral PFC (DLPFC) (Table 2; Fig. 3). See Table S2 for a correlation matrix characterizing interrelationships among all variables.
Table 2.
Regions for which functional connectivity with the right amygdala seed region is related to real-world distraction.
| Region | Location of centroid voxel | Brodmann's areas | x | y | z | Cluster extent (mm3) | r, p, R2* |
|---|---|---|---|---|---|---|---|
| Regression analysis | |||||||
| R DLPFC | R Middle Frontal Gyrus | 9 | 45 | 11 | 33 | 2748 | r = −.46, p < .001, R2 = .21 |
| DMPFC | L Medial Frontal Gyrus | 6, 24, 32 | −3 | −6 | 48 | 3159 | r = −.50, p < .001, R2 = .25 |
| Mediation analysis | |||||||
| R DLPFC | R Inferior Frontal Gyrus | 9 | 45 | 8 | 32 | 1140 | p = .01 (see Fig. 2) |
Note. Coordinates for each cluster's center-of-mass are presented in Talairach space. Regression findings are from unrestricted whole brain analysis with voxel-wise p < .001; map-wise p values are reported above based on Monte Carlo simulations for observed cluster extent. Mediation findings are for brain regions statistically mediating the relationship between reaction time vigilance and real-world distraction, with search-space-wise error rate p < 05. DMPFC = dorsomedial prefrontal cortex; DLPFC = dorsolateral prefrontal cortex.
Cluster-level descriptive statistics (r, R2) derived using mean of all suprathreshold (p < .001) voxels.
Fig. 3.
Statistical mediation analysis results for functional connectivity mediating the relationship between reaction time (RT) vigilance bias and Ecological Momentary Assessment (EMA) distraction. M is considered a mediator of the relationship between X and Y if: (1) X is related to Y (path c); (2) X is related to M (path a); (3) M is related to Y, controlling for X (path b); and (4) the effect of X on Y controlling for M (path c′) is significantly different from the direct effect of X on Y (mediation path a*b). Map-wise error rate held at p < .05 for search space limited to clusters identified as having a bivariate relationship with real-world distraction (Fig. 1). Reported p-values obtained via bootstrapping. Scatterplots depict bivariate relationships between pairs of variables.
3.2.2. Sensitivity analyses: anxious and non-anxious youth
In a sensitivity analysis using an expanded sample that included 20 additional healthy control participants, amygdalar-PFC connectivity remained correlated with EMA distraction in both ROIs (R DLPFC: r = −.35, p < .001; DMPFC r = −.31, p = .002; Table S3). After controlling for patient status, the relationship between amygdala-R DLPFC connectivity and distraction remained robust (p < .01). The relationship between amygdala-DMPFC connectivity and EMA distraction was reduced to a statistical trend after controlling for group (r = −.15, p = .10). These exploratory results suggest the biobehavioral dimensions we assessed could be equally pertinent to both anxious and non-anxious youth.
3.3. Additional sensitivity analyses
Controlling for overall anxiety severity (Pediatric Anxiety Rating Scale) (The Research Units on Pediatric Psychopharmacology Anxiety Study Group, 2002) and self-reported nervousness and upset ratings during the index negative event (EMA variables) did not alter the significance of any behavioral or fMRI finding above. Controlling for presence/absence of each of the three inclusionary anxiety diagnoses also did not alter the significance of any findings, suggesting relationships were independent of diagnosis. All EMA distraction findings above (RT vigilance, PFC-amygdalar connectivity) were robust after controlling for gender, age, gender*age, and total number of negative events identified across the 14 EMA calls. The relationship between RT bias score and EMA suppression dropped slightly to a non-significant level after controlling for age (p = .06) and number of negative events (p = .05), but was robust controlling for gender and gender*age.
3.4. Additional EMA analyses supporting specificity of vigilance-avoidance relationships
In order to further assess whether EMA measures were broadly linked to global clinical features (as opposed to specifically linked to vigilance), we conducted additional analyses within the primary sample of anxious youth. Anxiety severity as assessed by the Pediatric Anxiety Rating Scale was unrelated to frequency of both suppression (r77 = −.02, p = .89) and distraction use (r77 = −.17, p = .15). Anxiety severity was also unrelated to the number of calls in which a moderate to severely distressing negative event was reported (r77 = −.15, p = .19). Last, presence vs. absence of specific anxiety disorder diagnoses was also unrelated to the number of calls in which a negative event was reported: Separation Anxiety Disorder (t76 = .17, p = .88), Social Phobia (t76 = 1.05, p = .30), and Generalized Anxiety Disorder (r76 = .83, p = .41). See Supplement (p. S10) for additional analyses showing that neither vigilance to threat nor anxiety severity were related to any of the other emotion regulation strategies that were assessed. In sum, these null findings suggest the relationships we observed in primary analyses were highly specific to the hypothesized vigilance-avoidance mechanism, rather than being proxies for global anxiety severity or symptomatology.
3.5. Other supplemental analyses
See Supplement for additional analyses of stimulus duration, moderation of connectivity by trial type (psychophysiological interactions; PPI), overall regional fMRI activation levels, types of negative events endorsed, and differences between EMA calls placed on school days vs. weekends.
4. Discussion
In a transdiagnostic sample of anxious youth, greater laboratory-assessed vigilance to threat was associated with greater real-world use of avoidance (distraction and suppression) in the wake of negative events. Given the high clinical relevance of real-world avoidance symptoms, this link provides support for the validity of psychopathological models emphasizing vigilance to threat as a factor in the etiology and maintenance of anxiety (MacLeod et al., 1986). However, our data provide a preliminary suggestion that the same mechanistic relationships may apply more broadly to both anxious and non-anxious youth; specifically, even non-anxious youth who are more vigilant in the lab appear more likely to avoid in real life, if and when they experience a sufficiently negative emotional response to life events. The present study addresses several gaps in the literature by: (a) focusing on individual differences in order to parse heterogeneity within anxious youth, thereby testing mechanistic links on a continuum; (b) testing theoretical links between laboratory-assessed vigilance and clinically relevant behaviors, through examination of real-world emotion regulation strategy use; and (c) linking real-world (EMA) outcomes to brain function during laboratory-assessed vigilance, including the finding that PFC-amygdalar connectivity mediates the link between vigilance and avoidance.
Past research suggests that in anxious youth, vigilance (Bar-Haim et al., 2007) may be more reliably observed than avoidance (Gamble and Rapee, 2009, Shechner et al., 2013, Price et al., 2013). Current findings suggest that avoidance is evident in vigilant youth when assessed as a strategic emotion regulation strategy in real-world settings, while a typical laboratory timeframe (e.g., a few seconds) may be insufficient for youth to switch from vigilant to avoidant attentional allocation. Indeed, we did not find a robust vigilance-avoidance pattern during laboratory assessment in these patients (Supplement), but instead found a relationship spanning laboratory and ecological contexts that was robust enough to withstand considerable method variance (i.e., RT vs. EMA measures). Notably, the distribution of bias scores suggests a subset of youth in our sample did display relatively automatic avoidance patterns on the dot-probe (i.e., negative values; Fig. 1); these “automatic avoiders” were then less likely to report strategic real-world avoidance, potentially due to reduced compensatory need.
Functional connectivity between right amygdala and prefrontal (R DLPFC, DMPFC) regions was further associated with real-world distraction, and amygdalar-DLPFC connectivity statistically mediated the vigilance-avoidance relationship. Individuals with greater RT vigilance and real-world distraction had little apparent functional integration across regions (Fig. 2), while those with low levels of vigilance and distraction had positive amygdalar-PFC connectivity, possibly reflecting flexible recruitment of the PFC when limbic threat response is high. Consistent with neuroanatomical models of emotional disorders at large (Bishop, 2007, Davidson et al., 1999), inefficient top-down regulation of bottom-up threat responses may therefore underlie both excessive attentional capture by threat and subsequent over-reliance on strategic avoidant strategies (such as distraction) in an effort to regulate distress in vivo. Given that neural connectivity was specifically measured during an attention bias task, the observed relationship to real-world distraction suggests that neural patterns underlying control of attention to threat are relevant in a distinct, but theoretically linked, context: when individuals encounter a stressor (a ‘threat’) in their daily life and must select a voluntary strategy to cope. Data suggest that vigilant attention promotes subsequent selection of avoidant strategies in these real world contexts, but only when decreased prefrontal-amygdalar connectivity is also characteristic of threat processing. fMRI data have rarely been externally validated using behaviors measured outside the laboratory. Findings support the real-world relevance of fMRI-assessed functional connectivity when using a task designed to capture key, clinically relevant aspects of information processing.
Current findings are broadly convergent with, yet also clearly distinct from, our previous report (drawn from an overlapping sample) (Price et al., 2014), in which global measures of anxiety (presence of clinical anxiety; greater overall anxiety severity) were linked to decreased integration of response across distinct prefrontal (rostrodorsal anterior cingulate) and limbic (hippocampal/parahippocampal) regions. Our current focus on a more specific behavior (avoidance in the real world) and use of an a priori anatomical region (amygdala, the limbic region most widely linked to anxiety and threat responding) (Bishop, 2008, Zald, 2003) revealed decreased integration with two more dorsal regions of the PFC. While we (Price et al., 2014) and others (Etkin et al., 2011) have suggested that rostrodorsal anterior cingulate activity is tied to fear expression (rather than regulation), the PFC regions observed in the present analysis have been widely implicated in voluntary emotion regulation (Ochsner et al., 2002) and cognitive control over threat processing (Price et al., 2011). Decreased top-down control by these regions appears to be specifically tied to greater reliance on avoidance as a maladaptive emotion regulation strategy, while decreased integration across (cortical and subcortical) fear-responsive regions may be a biomarker of anxiety more globally.
Ours is the third study to implicate right-lateralized amygdalar-lateral PFC connectivity as a clinically relevant neural underpinning of dot-probe task performance, although the precise nature and direction of effects has varied. As a group, anxious youth showed weaker negative (inverse) right amygdalar-ventrolateral PFC connectivity compared to controls during a subliminal dot-probe task (Monk et al., 2008), while adults with a childhood history of behavioral inhibition showed the opposite pattern—stronger negative (inverse) right amygdalar-DLPFC connectivity—during a supraliminal dot-probe (Hardee et al., 2013). Both findings were specific to trials containing a threatening face, akin to the trials we included in our analysis. However, in contrast to these previous studies, we found that decreased positive connectivity conferred risk of real-world avoidance. More consistent with previous findings, this was observed in the context of inverse patterns of activation at the group level, across all trials (i.e., PFC activations coupled with amygdalar deactivations; see Supplement). This overarching context of amygdalar deactivation suggests that the cognitive/attentional demands of the task (and concomitant PFC activations) reduced amygdalar activity in the sample overall (compared to baseline); yet these two opposing responses were not tightly coupled with one another in the most avoidant participants. Compared to previous studies, our focus on dimensional relationships within an anxious sample may have allowed a distinct pattern to emerge, with the less avoidant subset of anxious youth exhibiting positive connectivity. Furthermore, our analysis was model-free and thus requires few of the assumptions usually present in fMRI (e.g., estimation/specification of the expected HRF; modeling based on hypothetical neuronal influences of trial features); this could allow for a more precise and data-driven characterization of relationships across regions, although, like all fMRI analysis, it is limited by the use of a proxy measure (HRF) for neuronal activity.
Notably, functional connectivity correlates were identified for distraction but not suppression. In the present analyses, distraction in response to a negative life event was defined in behavioral terms (engagement in a distracting activity), whereas suppression was defined in more cognitive terms (e.g., thought suppression). It is therefore plausible that decreased PFC-amygdalar connectivity is most relevant to behavioral forms of avoidance—i.e., reduced regulatory capacity for threat responses may create a compensatory need to actively and strategically engage in a distracting task, rather than relying on mental suppression. Indeed, the vigilance-avoidance hypothesis describes avoidance as the strategic directing of attention away from threat, a definition that may fit better with the concept of distraction. Alternatively, behavioral coping is more concrete than cognitive efforts, which may make it easier to be recalled (Stone et al., 1998), and in turn, its assessment more valid. Although distraction is sometimes construed as an adaptive form of emotion regulation, it may be most adaptive when the stressor is beyond one's control (Weisz et al., 1994). In the context of anxiety it suffers from the same long-term consequence as avoidance: the prevention of habituation to feared stimuli and consequent maintenance of anxiety over time (Thiruchselvam et al., 2011).
Findings are relevant to both intervention targets (e.g., personalized medicine) and intervention mechanisms. For example, an emerging literature suggests that targeted interventions remediating attentional vigilance (‘ABM’ procedures) have a broad impact on clinical anxiety symptoms (Hakamata et al., 2010). However, initial support for this approach has recently been called into question as studies emerge showing limited clinical benefit (Cristea et al., 2015). Our study suggests that inconsistent findings are not due to lack of a mechanistic link between attentional vigilance and real-world symptomatology; rather, more robust methods for modifying attentional patterns may be needed (MacLeod and Clarke, 2015). Furthermore, the present findings could help to clarify the mechanism by which ABM works. Reduced vigilance and improved prefrontal regulation of threat responses may translate into reduced real-world avoidance, enabling exposure to feared stimuli and resultant habituation processes to occur, a key mechanism in gold-standard, cognitive-behavioral treatments for anxiety (Foa and Kozak, 1986). Future ABM studies would benefit from inclusion of EMA measures throughout the course of treatment to allow for time-course analysis of critical intervention mechanisms.
Notably, vigilance-avoidance relationships were largely robust after controlling for a variety of potential explanatory variables including anxiety severity, degree of nervousness and upset during indexed real-world negative events, number of negative events identified, anxiety disorder diagnosis, age, and gender. These findings suggest that the links across laboratory and ecological settings are not better explained by third variables but instead may represent a fundamental mechanistic link. Exploratory results suggest that the vigilance-avoidance link may even be applicable to non-anxious youth as well, at least when moderate to severely distressing real-world events are encountered. Whether the subset of non-anxious youth who lie at the higher end of the vigilant and avoidant distributions is at elevated risk of de novo anxiety onset remains an open question for future research.
4.1. Limitations
Effect sizes were small for observed relationships, which may reflect both suboptimal psychometric properties of RT attention bias measures (Price et al., 2015) and restricted range for EMA measures, particularly for suppression which was endorsed on 100% of analyzed calls by many participants (Fig. 1). Vigilance itself was not assessed via EMA, due to expected difficulties with patient insight into the construct of ‘vigilance to threat,’ particularly among youth. Indeed, to our knowledge, no validated self-report measure of threat vigilance exists, which may reflect inherent difficulties measuring this relatively automatic pattern without reliance on computer-based methods. Although our design creates method variance and contextual heterogeneity across the vigilance and avoidance assessments, leaving open the possibility of unassessed intervening or explanatory mechanisms, it also suggests that laboratory patterns are relevant to real-world behavior, as predicted by attentional theories of anxiety. To reduce method/context variance, future studies could incorporate mobile vigilance assessment in ecological settings by capitalizing on current mobile technologies, as recently demonstrated in patients with nicotine dependence (Waters et al., 2012, Waters et al., 2014). Similarly, distraction may not be the most valid construct to capture clinically relevant forms of avoidance. Future studies would be improved by inclusion of an EMA variable that assesses one's proactive avoidance of anxiety-provoking situations. Our dot-probe task design differed from previous studies in anxious youth in several respects (slow event-related design, fearful rather than angry faces), reducing comparability across studies. In particular, our study used fearful faces as a threat cue because they very reliably activate fear-processing regions of the brain (Whalen et al., 2001) and have transdiagnostic relevance to fear perception through the implication that a generic, unspecified threat is present. This design decision stands in contrast to many studies using angry or disgusted faces to connote a social form of threat directed at the participant; however, there is evidence that fearful and angry faces elicit comparable attentional bias patterns during the dot-probe (Mogg et al., 2007). Functional connectivity analyses also differed from previous studies due to the task design (slow event-related design, yielding a small number of trials per condition). Findings were nevertheless consistent with hypotheses and the extant literature (see (Price et al., 2014) for detailed discussion of current task design decisions). While there was tentative evidence that connectivity relationships were specific to trials with fearful-neutral face pairs (see Supplement), the model-free analysis approach limits conclusions regarding the influence of specific task conditions (e.g., congruence). Observed connectivity patterns might therefore not be specific to the dot-probe or other attentional tasks. In mediation analyses, the predictor did not temporally precede the mediator. EMA data are subject to many of the same limitations as traditional self-report measures (e.g., social desirability bias). Last, the small control sample did not allow for a full exploration of whether observed vigilance-avoidance relationships were moderated by the presence of clinical anxiety. Patterns observed suggest possible relevance of the same mechanisms to both anxious and non-anxious youth. A larger control sample and additional indices of clinical course would allow for a better understanding of the clinical significance of these biobehavioral dimensions.
4.2. Conclusions
Real-world measures of avoidance enable a more ecologically valid test of the vigilance-avoidance hypothesis of attentional bias in anxious youth, and lend further credibility to laboratory assessments of attentional vigilance. The most vigilant anxious youth exhibited the largest degree of real-world avoidance, and PFC-amygdalar connectivity emerged as a key mechanism linking these maladaptive attentional patterns. Increased understanding of the processes through which attentional vigilance results in clinically relevant anxiety across the lifespan will refine treatment and/or prevention targets and ideally lead to the development of personalized interventions.
Acknowledgment
This work was supported by National Institutes of Health grants MH080215, MH082998, K23MH100259, and MH018269.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2016.03.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Aizenstein H.J., Butters M.A., Wu M., Mazurkewicz L.M., Stenger V.A., Gianaros P.J., Becker J.T., Reynolds C.F., 3rd, Carter C.S. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am. J. Geriatr. Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van IJzendoorn M.H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn. Sci. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. Neural mechanisms underlying selective attention to threat. Ann. N. Y. Acad. Sci. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bogels S.M., Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clin. Psychol. Rev. 2004;24(7):827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Buetti S., Juan E., Rinck M., Kerzel D. Affective states leak into movement execution: automatic avoidance of threatening stimuli in fear of spider is visible in reach trajectories. Cogn. Emotion. 2012;26(7):1176–1188. doi: 10.1080/02699931.2011.640662. [DOI] [PubMed] [Google Scholar]
- Cristea I.A., Kok R.N., Cuijpers P. Efficacy of cognitive bias modification interventions in anxiety and depression: meta-analysis. Br. J. Psychiatry. 2015;206(1):7–16. doi: 10.1192/bjp.bp.114.146761. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Abercrombie H., Nitschke J.B., Putnam K. Regional brain function, emotion and disorders of emotion. Curr. Opin. Neurobiol. 1999;9:228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E.B., Kozak M.J. Emotional processing of fear: exposure to corrective information. Psychol. Bull. 1986;99(1):20–35. [PubMed] [Google Scholar]
- Gamble A.L., Rapee R.M. The time-course of attentional bias in anxious children and adolescents. J. Anxiety Disord. 2009;23(7):841–847. doi: 10.1016/j.janxdis.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Hakamata Y., Lissek S., Bar-Haim Y., Britton J.C., Fox N.A., Leibenluft E., Ernst M., Pine D.S. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol. Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee J.E., Benson B.E., Bar-Haim Y., Mogg K., Bradley B.P., Chen G., Britton J.C., Ernst M., Fox N.A., Pine D.S., Perez-Edgar K. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol. Psychiatry. 2013;74(4):273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In-Albon T., Kossowsky J., Schneider S. Vigilance and avoidance of threat in the eye movements of children with separation anxiety disorder. J. Abnorm. Child Psychol. 2010;38(2):225–235. doi: 10.1007/s10802-009-9359-4. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kashdan T.B., Farmer A.S., Adams L.M., Ferssizidis P., McKnight P.E., Nezlek J.B. Distinguishing healthy adults from people with social anxiety disorder: evidence for the value of experiential avoidance and positive emotions in everyday social interactions. J. Abnorm. Psychol. 2013;122(3):645–655. doi: 10.1037/a0032733. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N., Lindquist M.A., Nichols T.E., Poldrack R.A., Vul E. Everything you never wanted to know about circular analysis, but were afraid to ask. J. Cereb. Blood Flow Metab. 2010;30:1551–1557. doi: 10.1038/jcbfm.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C., Clarke P.J. The Attentional bias modification approach to anxiety intervention. Clin. Psychol. Sci. 2015;3(1):58–78. [Google Scholar]
- MacLeod C., Mathews A., Tata P. Attentional bias in emotional disorders. J. Abnorm. Psychol. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P., Miles F., Dixon R. Time course of attentional bias for threat scenes: testing the vigilance-avoidance hypothesis. Cognit. Emotion. 2004;18(5):689–700. [Google Scholar]
- Mogg K., Garner M., Bradley B.P. Anxiety and orienting of gaze to angry and fearful faces. Biol. Psychol. 2007;76:163–169. doi: 10.1016/j.biopsycho.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., Nelson E.E., McClure E.B., Mogg K., Bradley B.P., Leibenluft E., Blair R.J., Chen G., Charney D.S., Ernst M., Pine D.S. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am. J. Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., Bradley B.P., Mai X., Louro H.M., Chen G., McClure-Tone E.B., Ernst M., Pine D.S. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch. Gen. Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmi S., Kuckertz J.M., Amir N. Automatic avoidance tendencies in individuals with contamination-related obsessive-compulsive symptoms. Behav. Res. Ther. 2010;48(10):1058–1062. doi: 10.1016/j.brat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Cohen P., Gurley D., Brook J., Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch. Gen. Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Price R.B., Eldreth D.A., Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Transl. Psychiatry. 2011;1:e46. doi: 10.1038/tp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.B., Siegle G., Silk J.S., Ladouceur C.D., McFarland A., Dahl R.E., Ryan N.D. Sustained neural alterations in anxious youth performing an attentional bias task: a pupilometry study. Depress. Anxiety. 2013;30:22–30. doi: 10.1002/da.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.B., Siegle G.J., Silk J.S., Ladouceur C.D., McFarland A., Dahl R.E., Ryan N.D. Looking under the hood of the dot-probe task: an fMRI study in anxious youth. Depress. Anxiety. 2014;31:178–187. doi: 10.1002/da.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.B., Kuckertz J.M., Siegle G.J., Ladouceur C.D., Silk J.S., Ryan N.D., Dahl R.E., Amir N. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychol. Assess. 2015;27(2):365–376. doi: 10.1037/pas0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J., Gazzaley A., D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Salum G.A., Mogg K., Bradley B.P., Gadelha A., Pan P., Tamanaha A.C., Moriyama T., Graeff-Martins A.S., Jarros R.B., Polanczyk G., do Rosário M.C., Leibenluft E., Rohde L.A., Manfro G.G., Pine D.S. Threat bias in attention orienting: evidence of specificity in a large community-based study. Psychol. Med. 2012:1–13. doi: 10.1017/S0033291712001651. [DOI] [PubMed] [Google Scholar]
- Shechner T., Pelc T., Pine D.S., Fox N.A., Bar-Haim Y. Flexible attention deployment in threatening contexts: an instructed fear conditioning study. Emotion. 2012;12(5):1041–1049. doi: 10.1037/a0027072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Jarcho J.M., Britton J.C., Leibenluft E., Pine D.S., Nelson E.E. Attention bias of anxious youth during extended exposure of emotional face pairs: an eye-tracking study. Depress. Anxiety. 2013;30:14–21. doi: 10.1002/da.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J.S., Steinberg L., Morris A.S. Adolescents’ emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Dev. 2003;74(6):1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Silk J.S., Forbes E.E., Whalen D.J., Jakubcak J.L., Thompson W.K., Ryan N.D., Axelson D.A., Birmaher B., Dahl R.E. Daily emotional dynamics in depressed youth: a cell phone ecological momentary assessment study. J. Exp. Child Psychol. 2011;110(2):241–257. doi: 10.1016/j.jecp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J.S., Tan P.Z., Ladouceur C.D., Meller S.M., Siegle G.J., McMakin D.L., Forbes E.E., Dahl R.E., Kendall P.C., Mannarino A., Ryan N.D. A randomized clinical trial comparing individual cognitive behavioral therapy and child-centered therapy for child anxiety disorders. J. Clin. Child Adolesc. Psychol. 2016 doi: 10.1080/15374416.2016.1138408. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A.A., Schwartz J.E., Neale J.M., Shiffman S., Marco C.A., Hickcox M., Paty J., Porter L.S., Cruise L.J. A comparison of coping assessed by ecological momentary assessment and retrospective recall. J. Pers. Soc. Psychol. 1998;74(6):1670–1680. doi: 10.1037//0022-3514.74.6.1670. [DOI] [PubMed] [Google Scholar]
- Tan P.Z., Forbes E.E., Dahl R.E., Ryan N.D., Siegle G.J., Ladouceur C.D., Silk J.S. Emotional reactivity and regulation in anxious and nonanxious youth: a cell-phone ecological momentary assessment study. J. Child Psychol. Psychiatry. 2012;53(2):197–206. doi: 10.1111/j.1469-7610.2011.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Research Units on Pediatric Psychopharmacology Anxiety Study Group The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(9):1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Thiruchselvam R., Blechert J., Sheppes G., Rydstrom A., Gross J.J. The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biol. Psychol. 2011;87(1):84–92. doi: 10.1016/j.biopsycho.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B.J., Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vul E., Harris C., Winkielman P., Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect. Psychol. Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald I., Lubin G., Holoshitz Y., Muller D., Fruchter E., Pine D.S., Charney D.S., Bar-Haim Y. Battlefield-like stress following simulated combat and suppression of attention bias to threat. Psychol. Med. 2011;41(4):699–707. doi: 10.1017/S0033291710002308. [DOI] [PubMed] [Google Scholar]
- Waters A.J., Marhe R., Franken I.H. Attentional bias to drug cues is elevated before and during temptations to use heroin and cocaine. Psychopharmacology (Berl.) 2012;219(3):909–921. doi: 10.1007/s00213-011-2424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A.J., Szeto E.H., Wetter D.W., Cinciripini P.M., Robinson J.D., Li Y. Cognition and craving during smoking cessation: an ecological momentary assessment study. Nicotine Tob. Res. 2014;16(Suppl. 2):S111–S118. doi: 10.1093/ntr/ntt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J.R., McCabe M.A., Dennig M.D. Primary and secondary control among children undergoing medical procedures: adjustment as a function of coping style. J. Consult. Clin. Psychol. 1994;62(2):324–332. doi: 10.1037//0022-006x.62.2.324. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Shin L.M., McInerney S.C., Fischer H., Wright C.I., Rauch S.L. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Zald D.H. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Brain Res. Rev. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zhou D., Thompson W.K., Siegle G. MATLAB toolbox for functional connectivity. Neuroimage. 2009;47(4):1590–1607. doi: 10.1016/j.neuroimage.2009.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.