Abstract
Synthetic cathinones produce dysregulation of monoamine systems, but their effects on the glutamate system and the influence of glutamate on behavioral effects related to cathinone abuse are unknown. A principal regulator of glutamate homeostasis is glutamate transporter subtype 1 (GLT-1), an astrocytic protein that clears glutamate from the extracellular space and influences behavioral effects of established psychostimulants. We hypothesized that repeated administration of the synthetic cathinone, MDPV (3,4-methylenedioxypyrovalerone), would affect GLT-1 expression in the corticolimbic circuit, and that a GLT-1 activator (ceftriaxone, CTX) would reduce rewarding and locomotor-stimulant effects of MDPV in rats. GLT-1 protein expression in the nucleus accumbens (NAcc), but not prefrontal cortex (PFC), was decreased following withdrawal (2, 5 and 10 days) from repeated MDPV treatment, but not immediately after the last MDPV injection. CTX (200 mg/kg) pretreatment did not affect acute locomotor activation produced by MDPV (0.5, 1, 3 mg/kg). However, CTX (200 mg/kg) administered during a 7-day MDPV treatment paradigm attenuated the development of MDPV-induced sensitization of repetitive movements in rats challenged with MDPV following 11 days of drug abstinence. Pretreatment with CTX (200 mg/kg) during a 4-day MDPV (2 mg/kg) conditioned place preference (CPP) paradigm reduced the development of place preference produced by MDPV. The present data demonstrate dysregulation of corticolimbic glutamate transport systems during withdrawal from chronic MDPV exposure, and show that a GLT-1 transporter activator disrupts behavioral effects of MDPV that are related to synthetic cathinone abuse.
Keywords: MDPV, ceftriaxone, GLT-1, behavioral sensitization, conditioned place preference, glutamate, bath salts, cathinone
1. Introduction
Synthetic cathinones, commonly referred to as “bath salts”, are a popular class of new psychoactive substances emerging on the illicit drug scene. According to the United Nations Global Synthetic Drugs Assessment from 2013, a total of 348 new psychoactive substances have been identified from worldwide clinical presentations and drug seizures, 97 of which were new to the year 2013, and 25% of the total number of new psychoactive substances were identified as synthetic cathinones (United Nations, 2014). Synthetic cathinone abuse has increased in the past decade, both in the number of synthetic cathinone exposures, and the number of unique compounds identified in illicit drug preparations as synthetic cathinones (DEA Public Affairs, 2011; Elliott and Evans, 2014). In response to this increase, at least 10 synthetic cathinones and 19 positional isomers of synthetic cathinones have been designated Schedule I in the United States (DEA Office of Diversion Control, 2015).
3,4-methylenedioxypyrovalerone (MDPV) is one of the more prevalent Schedule I synthetic cathinones. MDPV was the most commonly abused “bath salt” compound in 2011, and while abuse of MDPV decreased following its scheduling in 2013, it is still abused and available on illicit drug markets (NMS Labs, 2015; Seely et al., 2013). Additionally, MDPV has served as a pharmacological template for designing second-generation, pyrrolidine ring-substituted synthetic cathinones like α-pyrrolidinopentiophenone (α-PVP). Considering the persistence of MDPV abuse, and its role as a progenitor for replacement cathinones, defining the neuropharmacological profile of MDPV neuropharmacology and characterizing its abuse liability are necessary steps in better understanding the public health concern of synthetic cathinones.
The mechanism of action and abuse liability of MDPV have been investigated almost entirely in the context of monoamine neurotransmitters. MDPV is a highly selective and potent reuptake inhibitor at monoamine transporters, with greater activity at the dopamine (DAT) and norepinephrine (NET) transporters, compared to weaker activity at serotonin transporters (Baumann et al., 2013). In vitro experiments have shown that MDPV is a more potent reuptake inhibitor at DAT and NET compared to cocaine, while in vivo studies have found that MDPV has a 10-fold greater potency at increasing extracellular dopamine compared to cocaine and the synthetic cathinone methylone (Schindler et al., in press). Moreover, MDPV robustly increases locomotion, distance traveled and stereotypic movements in rats and mice, and has a longer duration of ambulation compared to cocaine and methamphetamine (Aarde et al., 2013; Baumann et al., 2013; Fantegrossi et al., 2013; Gatch et al., 2013; Marusich et al., 2014). In drug reward assays, MDPV is more efficacious at producing conditioned place preference (CPP) than amphetamine and mephedrone in mice, and produces dose-dependent CPP effects in both male and female rats (Karlsson et al., 2014; King et al., 2015a; King et al., 2015b). Intracranial self-stimulation (ICSS) experiments have also characterized MDPV reward, with acute MDPV producing a greater reduction in ICSS thresholds than mephedrone and methylone (Bonano et al., 2014; Watterson et al., 2014). MDPV is highly reinforcing in self-administration paradigms, where MDPV displays greater potency as a reinforcer than cocaine or methamphetamine in fixed-ratio acquisition schedules (Aarde et al., 2013; Watterson et al., 2014; Schindler et al., in press). Taken together, these data describe MDPV as a highly potent, abuse-liable psychomotor stimulant.
No studies to date have reported effects of a synthetic cathinone on the glutamate system. Dysregulation of corticolimbic glutamate signaling, including decreased basal extracellular glutamate in the nucleus accumbens (NAcc) during cocaine abstinence (Baker et al., 2003; McFarland et al., 2003), contributes to psychostimulant reinforcement and relapse (Kalivas and Volkow, 2005; Kalivas et al., 2009; Knackstedt and Kalivas, 2009). One glutamatergic target of interest in the context of psychostimulant abuse is glutamate transporter subtype-1 (GLT-1), an astrocytic protein responsible for approximately 90% of glutamate reuptake in the mammalian forebrain (O’Shea, 2002). GLT-1 expression and functionality is decreased in the NAcc following cocaine self-administration, both in short- and long-access paradigms, and in short- and long-term withdrawal (Knackstedt et al., 2010; Fischer-Smith et al., 2012). The β-lactam antibiotic ceftriaxone (CTX), when administered repeatedly, increases both GLT-1 protein expression and glutamate reuptake, and decreases extracellular glutamate in the NAcc of rats (Rothstein et al., 2005; Lee et al., 2008; Miller et al., 2008; Rasmussen et al., 2011). CTX pretreatment blocks development of behavioral sensitization to cocaine and amphetamine (Rasmussen et al., 2011; Sondheimer and Knackstedt, 2011; Tallarida et al., 2013). Repeated CTX is also efficacious in self-administration models, where it reduces acquisition of cocaine self-administration in mice, and attenuates cue- and cocaine-induced relapse of drug seeking in rats (Ward et al., 2011; Sari et al., 2009; Knackstedt et al., 2010; Sondheimer and Knackstedt, 2011). These findings suggest that GLT-1 transporters contribute to the rewarding, reinforcing, stimulant and drug-seeking effects of cocaine, and provide the basis for our proposed studies with MDPV.
2. Materials and Methods
2.1. Animals and drugs
Male Sprague-Dawley rats weighing between 270–300 g (N = 212; Harlan Laboratories, Indianapolis, IN, USA) were housed two per cage and maintained on a 12-h light/dark cycle. Food and water were provided ad libitum, except during locomotor testing and during CPP conditioning and testing. Animal use procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and Temple University Guidelines for the Care of Animals. (±)-MDPV was synthesized in accordance with previously published methods (Abiedalla et al., 2012; Kolanos et al., 2015) by Dr. Allen Reitz. Briefly, 1-Benzo[1,3]dioxol-5-yl-pentan-1-one was prepared via Friedel Crafts acylation of benzo[1,3]dioxole with pentanoyl chloride and tin (IV) chloride. Subsequent bromination of 1-benzo[1,3]dioxol-5-yl-pentan-1-one followed by displacement with pyrrolidine and salt formation provided MDPV HCl. MDPV and ceftriaxone sodium (APP Pharmaceuticals, Schaumburg, IL) were dissolved in physiological saline (0.9%) and injected intraperitoneally (ip).
2.2. GLT-1 protein expression assays
To determine the effect of repeated, intermittent administration of MDPV on GLT-1 protein expression, experimentally-naïve rats (N = 6–7 per group) were injected with saline or MDPV (Days 1 and 7: 0.5 mg/kg MDPV and Days 2–6: 1 mg/kg MDPV) for 7 days. This paradigm was adapted from a behavioral sensitization paradigm we employed with cocaine and the synthetic cathinone mephedrone (Gregg et al., 2014; Gregg et al., 2013a, Gregg et al., 2013b; Kalivas and Duffy, 1998). Rats that were housed together did not receive the same treatment, but instead, always comprised one rat receiving MDPV and another receiving saline, which were paired for their dissection time. Rats were euthanized by CO2 asphyxiation and decapitation either 2 h (Withdrawal Day 0), 2 days (Withdrawal Day 2), 5 days (Withdrawal Day 5), or 10 days (Withdrawal Day 10) after the last saline/MDPV injection. The brains were extracted and rapidly dissected on ice to isolate the NAcc and prefrontal cortex (PFC) based on coordinates in a brain atlas (Paxinos and Watson, 2007). The tissue extractions were then processed for Western blotting.
Tissues were prepared by sonicating tissue in a 1% (w/v) sodium dodecyl sulfate solution containing 1mM sodium fluoride and 1mM sodium vanadate, boiled for 5 minutes after proper homogenization, and stored at −80°C. Equal amounts of protein (20 μg per loading sample, as determined using a Thermo Scientific NanoDrop 2000 spectrometer) were loaded onto 7.5% Mini Protean Precast gels (Bio-Rad, Hercules, CA) for separation and transferred onto nitrocellulose membranes (Life Technologies, Carlsbad, CA). Membranes were blocked for 1 hour at room temperature in Odyssey Blocking Buffer (Li-Cor Biosciences, Lincoln, NE), followed by overnight incubation with the GLT-1 primary antibody at 4°C (1:100,000, polyclonal guinea pig, Millipore, Billerica, MA). The following day, membranes were washed with Tween-Tris Buffered Saline (TTBS) 3 times for 8 minutes, followed by a 90-min incubation with β-tubulin loading control primary antibody at room temperature (1:800,000, monoclonal mouse, Cell Signaling, Danvers, MA). Membranes were then washed again 3 times for 8 minutes with TTBS, and incubated for 1 hour at room temperature with IRDye 800-conugated donkey anti-guinea pig and IRDye 680-conjugated goat anti-mouse secondary antibodies (1:10,000 each, Li-Cor Biosciences). After secondary antibody incubation, membranes were washed 3 times with TTBS and proteins were detected and quantified using the Odyssey infrared imaging system (Li-Cor Biosciences). Relative density of each sample (GLT-1/β-tubulin optical densities) were determined and averaged for each group.
2.3. Acute and repeated dosing paradigms for locomotor experiments
Rats were placed individually into activity chambers and allowed to acclimate for 60 min. Basal locomotor activity was recorded for at least 30 min prior to the first injection in both acute and repeated dosing paradigms, followed by recording of locomotor activity for 90 min after saline or MDPV injection. Activity was recorded using a Digiscan DMicro System (Accuscan, Inc., Columbus, OH) (Gregg et al., 2013a; Gregg et al., 2013b; Gregg et al., 2014). Activity chambers consisted of transparent plastic boxes (45 cm (L) × 20 cm (W) × 20 cm (H)) set inside metal frames equipped with 16 infrared light emitters and detectors spaced 2.5 cm apart. The number of photocell beam breaks was recorded by a computer interface and expressed as counts. Locomotor activity was divided into two specific types of activity. Ambulatory activity was recorded as consecutive beam breaks resulting from horizontal movement. Non-ambulatory activity resulting in repetitive beam breaks was recorded as repetitive movements, a measure indicative of a stationary animal engaged in a repetitive, stereotypic behavior.
Separate groups of rats were used for the acute and repeated dosing experiments. For acute locomotor activity experiments, rats (N = 8 per group) were treated with saline or CTX (200 mg/kg) for 4 days prior to test day. On test day, rats were injected with saline or CTX (200 mg/kg) 30 min before receiving saline or MDPV (0.5, 1, or 3 mg/kg). After saline or MDPV injection, ambulatory activity and repetitive movements were recorded for 90 min.
Repeated, intermittent administration paradigms were divided into 4 phases: pretreatment with CTX, repeated MDPV treatment, abstinence, and MDPV challenge. Injections were performed in home cages except on challenge day (injections in activity chambers). For pretreatment, rats were injected with saline or CTX (200 mg/kg) daily for 4 days. The following day, rats were injected with saline or CTX (200 mg/kg) 30 min prior to saline or MDPV (using variable-dose administration paradigm) (see section 2.2) for 7 days. All rats then underwent an 11-day abstinence period during which they received no injections. On the final day of the abstinence period, rats were placed into activity chambers for 60 min to acclimate and then challenged with either saline or MDPV (0.5 mg/kg), and ambulatory activity and repetitive movements were recorded for 90 min. The 4 treatment groups (N = 8 per group) for the repeated dosing experiment were as follows: 1) Saline/Saline + Saline (“Saline”), 2) Saline/Saline + MDPV (“Acute MDPV”), 3) Saline/MDPV + MDPV (“Repeated MDPV”) and 4) CTX/MDPV + MDPV (“Repeated MDPV + CTX”).
2.4. Conditioned place preference (CPP) experiments
CPP experiments (N = 8–12 per group) were conducted in experimentally naïve rats using a 4-day biased design (Gregg et al., 2014). CPP chambers consisted of two compartments (each 45 cm (L) × 20 cm (W) × 20 cm (H)) separated by a removable door. Each compartment was environmentally distinguishable, with one compartment consisting of black walls and textured floor, and the other having white walls with vertical black stripes and a smooth floor. Prior to starting conditioning, rats were injected with either saline or CTX (50 or 200 mg/kg) daily for 4 days. Sixty min after the last saline or CTX injection, the initial CPP compartment preference of each rat was determined in a 30-min pre-test, during which rats were allowed access to both compartments in a drug-free state. The time spent in each compartment was recorded and manually scored at a later time by a technician blinded to individual animal treatments. A rat was considered to have entered a compartment when all forelimbs crossed the compartment threshold. The compartment identified as the ‘least-preferred compartment’ during the pre-test was designated as the drug-paired compartment for the subsequent conditioning phase.
The day after the pre-test, rats began the 4-day conditioning paradigm in which rats were subjected to two conditioning sessions per day. The first session involved an injection of saline/CTX 30 min prior to an injection of saline (for Saline + Saline and CTX + Saline groups) or MDPV (for Saline + MDPV and CTX + MDPV groups), administered immediately before a 30-min confinement in the drug-paired compartment. The second session involved an injection of saline prior to confinement for 30 min in the preferred compartment. Conditioning sessions were conducted 4 h apart and rats in the saline control group received saline in both compartments. Rats underwent a post-conditioning test one day after the last conditioning session, where place preference was evaluated by allowing free exploration of both compartments in a drug-free state for 30 min, during which time in each compartment was recorded and manually scored after the test by an experimenter blinded to treatment conditions. Data are presented as a difference score, calculated by taking the total time spent in the drug-paired compartment during the post-conditioning test minus the time spent in the drug-paired (least-preferred) compartment during the pre-conditioning test.
A 30-min confinement time was chosen to ensure that a sufficient concentration of MDPV was available in vivo based on the rapid onset of MDPV levels in the brain following systemic administration and an elimination half-life of 61 min ex vivo (Novellas et al., 2015). A dose of 2 mg/kg MDPV was selected based on both pilot experiments in our lab, and previously published results with a similar CPP paradigm (King et al., 2015b). In a follow-up experiment (N = 7 per group), we also tested a higher dose of MDPV (5 mg/kg) in combination with CTX (200 mg/kg). To minimize animal usage, this experiment tested the Saline + MDPV (5 mg/kg) and CTX (200 mg/kg) + MDPV (5 mg/kg) groups, and did not include the Saline + Saline and CTX + Saline groups that had been tested in the first CPP experiment.
2.4. Statistical analyses
Statistical significance for all assays was set at p<0.05. The relative sample densities of saline and MDPV-treated animals in the GLT-1 expression assays were expressed as a percentage of the saline control values, and then compared using a Student’s t-test. Behavioral data from the acute locomotor experiment were analyzed using a two-way ANOVA with the between-subject factors of CTX pretreatment and MDPV dose, followed by Bonferroni post-hoc tests to compare treatment groups on total activity. For the repeated dosing locomotor study, data were analyzed using a mixed model two-way ANOVA with the between-subject factor of treatment, and the within-subject repeated measures factor of time. Bonferroni post-hoc tests were then used to compare treatment groups on locomotor activity at each time point. Data from the first CPP experiment were analyzed using a two-way ANOVA with the between-subject factors of CTX pretreatment and MDPV treatment, followed by Bonferroni post-hoc tests to compare treatment groups on the difference score. For the CTX dose-response, data were expressed as a percentage of the MDPV (2 mg/kg) CPP values, and analyzed with a one-way ANOVA with the between-subject factor of CTX dose. The groups were then compared using Dunnett’s Multiple Comparison Tests.
3. Results
3.1. Withdrawal from repeated MDPV decreases GLT-1 expression in the NAcc, but not PFC
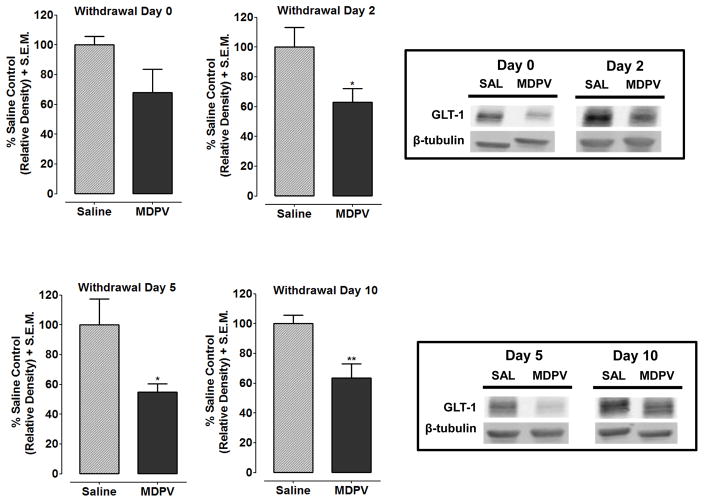
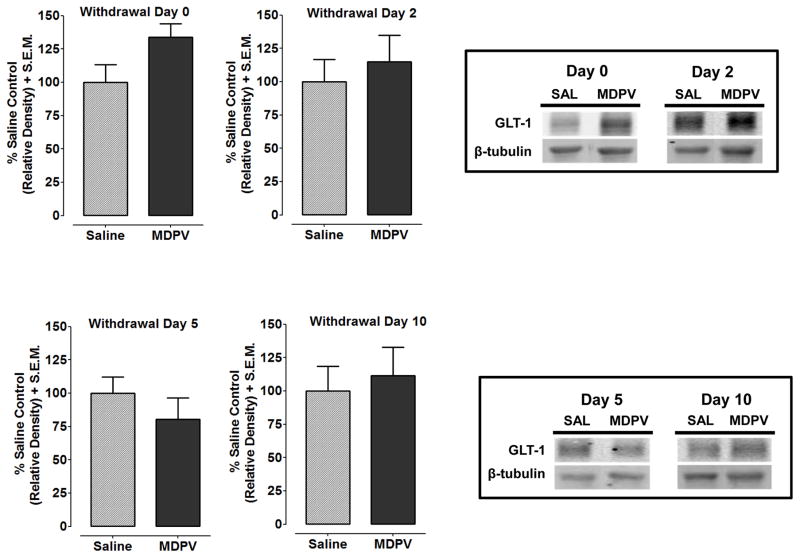
Fig. 1 shows GLT-1 protein expression following withdrawal from repeated MDPV or saline treatment for the NAcc (see Fig. 1A–D) and PFC (Fig. 2A–D). MDPV-treated rats, relative to saline-treated controls, showed decreased GLT-1 expression in the NAcc at Withdrawal Day 2 (p<0.05), Withdrawal Day 5 (p<0.05) and Withdrawal Day 10 (p<0.01), but not Withdrawal Day 0 (p>0.05). In the PFC, GLT-1 expression was not significantly different between MDPV and saline treatments at any time point (Withdrawal Day 0, p>0.05).
Fig. 1. GLT-1 protein expression in the NAcc is decreased following withdrawal from repeated MDPV exposure.
Rats were treated with saline or MDPV for 7 days. Expression of GLT-1 was measured in the NAcc at 2 h, 2 days, 5 days and 10 days after the last MDPV injection. GLT-1 (50 kDa) expression was normalized compared to a β-tubulin (52 kDa) control protein to obtain a relative density. Data are shown as a percentage of saline control values + S.E.M. MDPV-treated rats showed decreased GLT-1 expression at 2 days (p<0.05), 5 days (p<0.05) and 10 days (p<0.01) of withdrawal, while no significant changes in GLT-1 expression were observed 2 h (p>0.05) after the last MDPV injection. Boxes: Representative protein bands from treatment groups at different withdrawal time points.
Fig. 2. GLT-1 protein expression in the PFC was not affected following withdrawal from repeated MDPV exposure.
Expression of GLT-1 was measured in the PFC at 2 h, 2 days, 5 days and 10 days after the last MDPV injection. GLT-1 (50 kDa) expression was normalized compared to a β-tubulin (52 kDa) control protein to obtain a relative density. Data are shown as a percentage of saline control values + S.E.M. No significant changes in GLT-1 expression were observed at any withdrawal time point. Boxes: Representative protein bands from treatment groups at different withdrawal time points.
3.2. CTX does not affect locomotor activation produced by acute MDPV exposure
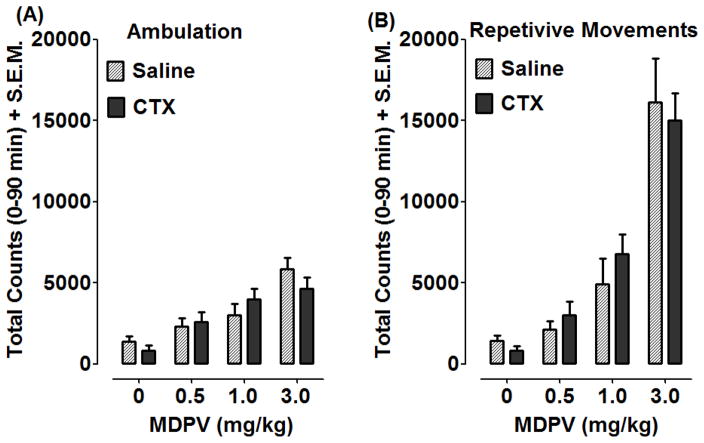
Effects of CTX pretreatment on ambulatory activity and repetitive movements produced by acute MDPV exposure are presented in Fig. 3A and 3B. CTX pretreatment prior to a single injection of MDPV (0–3 mg/kg) was recorded as counts and summated over 90 min. Two-way ANOVA and Bonferonni post-hoc tests on acute MDPV total ambulatory activity (Fig. 3A) revealed a significant effect of MDPV dose [F(3,56)=17.68, p<0.001], but no effect of CTX pretreatment [F(1, 56)=0.08, p>0.05] and no differences between pretreatment groups with post-hoc tests. A similar result was found with total repetitive movements (Fig. 3B), with a significant effect of MDPV dose [F(3,56)= 43.83, p<0.001] but no CTX pretreatment [F(1,56)= 0.07, p>0.05] or differences between pretreatment groups.
Fig. 3. CTX did not affect locomotor activation produced by acute MDPV exposure.
Rats were treated with saline or CTX (200 mg/kg) for 4 days. The following day, rats were injected with either saline or CTX (200 mg/kg) 30 min prior to MDPV (0.5, 1, 3 mg/kg). Ambulatory activity (A) and repetitive movements (B) were recorded for 90 min, and data are presented as mean total counts + S.E.M. Total counts were analyzed with two-way ANOVA and Bonferroni post-hoc tests, and no differences were observed between the treatment groups.
3.3. CTX attenuates behavioral sensitization produced by repeated MDPV exposure
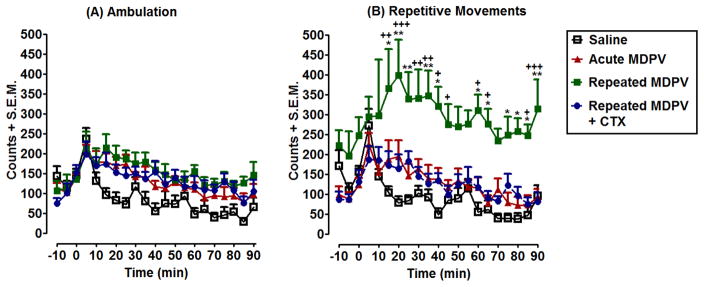
Time courses of ambulatory activity and repetitive movements produced by a challenge injection of MDPV given following 11 days of abstinence from repeated MDPV dosing is shown in Fig. 4A–B. Two-way ANOVA on ambulatory activity (Fig. 4A) revealed a significant effect of treatment [F(3,588)= 29.00, p<0.001] and time [F(20,588)= 7.99, p<0.001]. Post-hoc tests did not reveal any differences between Repeated MDPV and Repeated MDPV+CTX groups (p>0.05). Two-way ANOVA on repetitive movements (Fig. 4B) found a significant effect of treatment [F(3,588)= 103.83, p<0.0001] and time [F(20,588)= 3.80, p<0.0001]. Post-hoc tests revealed that MDPV produced significant sensitization of repetitive movements, with differences between acute MDPV and repeated MDPV detected at 15min (p<0.05), 20 min (p<0.01), 25 min (p<0.01), 30 min (p<0.05), 35 min (p<0.01), 40 min (p<0.05), 60 min (p<0.05), 65 min (p<0.05), 75 min (p<0.05), 80 min (p<0.05), 85 min (p<0.05), and 90 min (p<0.01). CTX pretreatment decreased sensitization of repetitive movements produced by repeated MDPV as measured by Bonferonni post-hoc tests (Repeated MDPV vs Repeated MDPV+CTX), at 15 min (p<0.01), 20 min (p<0.001), 30–35 min (p<0.01), 40–45 min (p<0.05), 60–65 min (p<0.05), 85 min (p<0.05), and 90 min (p<0.001).
Fig. 4. CTX attenuates the development of repeated MDPV-induced sensitization of repetitive movements.
Rats were pretreated for 4 days with either saline or CTX (200 mg/kg), prior to 7 days of saline or a variable-dose MDPV administration. After 11 days of drug abstinence, rats were challenged with saline or MDPV (0.5 mg/kg) and activity counts were recorded for 90 min. Time-course data are expressed as ambulatory activity (A) and repetitive movements (B) (mean + S.E.M.) in 5 min intervals. * p<0.05, **p<0.01 compared to Acute MDPV and +p<0.05, ++p<0.01, +++p<0.001 compared to Repeated MDPV+CTX.
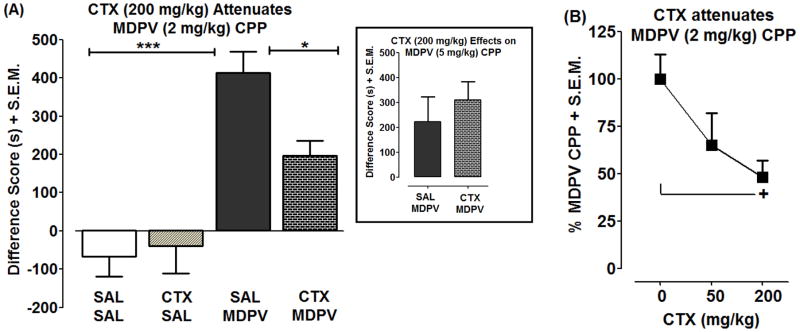
3.4. CTX attenuates MDPV conditioned place preference
Fig. 5A presents the effect of CTX pretreatment (200 mg/kg) on the CPP produced by MDPV (2 mg/kg). A significant main effect was observed with MDPV treatment [F(1,36)=39.37, p<0.0001] and a non-significant MDPV treatment by CTX pretreatment interaction trend was observed [F(1,36)=4.00, p>0.05], while no overall effect was observed with CTX pretreatment [F(1,36)=2.23, p>0.05]. Post-hoc tests revealed that MDPV produced a significant place preference relative to both saline and CTX alone controls (p<0.0001), and that CTX pretreatment significantly decreased the place preference produced by MDPV (p<0.05). CTX pretreatment (200 mg/kg) did not significantly affect the CPP produced by a higher dose of MDPV (5 mg/kg) (p>0.05; Fig. 5A inset).
Fig. 5. CTX attenuates development of MDPV conditioned place preference (CPP).
Rats were pretreated with saline or CTX (50 or 200 mg/kg) for 4 days prior to conditioning. During conditioning, rats were pretreated with saline or CTX 30 min prior to conditioning with saline or MDPV (2 or 5 mg/kg) for 4 days. Data are presented as a difference score (difference in time spent in drug-paired compartments between post-conditioning and pre-conditioning phases) (s + S.E.M.) (A and inset) or as a percentage of CPP produced by MDPV (2 mg/kg) (% + S.E.M.) (B). ***p<0.001 or *p<0.05 compared to SAL + MDPV (A) and +p<0.05 compared to CTX (0 mg/kg) (i.e., SAL + MDPV) (B).
The dose-related effects of CTX (50 and 200 mg/kg) on the place preference produced by 2 mg/kg MDPV are shown in Fig. 5B. There was a significant main effect of CTX dose [F(2,29)= 3.38, p<0.05]. Post-hoc tests showed that CTX significantly reduced the MDPV-induced place preference at 200 mg/kg (p<0.05), but not at 50 mg/kg (p>0.05).
4. Discussion
Our studies are the first to investigate a role for the glutamate system in the neurochemical and behavioral effects of a synthetic cathinone. We showed that GLT-1 expression is decreased in the NAcc during withdrawal from repeated MDPV exposure. The GLT-1 activator CTX disrupted behavioral effects of MDPV that are related to psychostimulant abuse. CTX pretreatment attenuated rewarding effects of MDPV in the CPP assay and inhibited MDPV-induced sensitization of repetitive movements in a locomotor assay, but had no effects against acute locomotor activity produced by MDPV. Our data suggest similarities and differences in the profiles of MDPV and established psychostimulants as related to GLT-1 transporters.
Similar to cocaine, MDPV decreased GLT-1 expression in the NAcc following chronic exposure (Knackstedt et al., 2010, Sondheimer and Knackstedt, 2011, Fischer-Smith et al., 2013). Here, we detected decreases in GLT-1 expression in the NAcc, but not in the PFC, following a 7-day, non-contingent MDPV administration paradigm in rats. In mice, decreased GLT-1 expression is also detected 30 days following withdrawal from 14 days of chronic cocaine injections, and CTX administered during the forced cocaine-abstinence interval normalizes this GLT-1 dysregulation (Kovalevich et al., 2012). While no current studies have evaluated GLT-1 expression in rats after systemic, non-contingent dosing, several operant cocaine self-administration studies have identified both dosing- and withdrawal interval-specific effects on GLT-1 expression. Following self-administration of cocaine, GLT-1 expression is decreased in the NAcc, with greater decreases detected following long-access (6 h) compared to short-access (2 h) self-administration, as well as decreased expression at longer withdrawal intervals (i.e., 40–45 days) versus short-term withdrawal times (1-day) (Fischer-Smith et al., 2012). Decreases in both expression and functionality (i.e., reduced glutamate uptake) of GLT-1 were also detected in the NAcc 3 weeks following discontinuation of cocaine self-administration (Knackstedt et al., 2010). Compared to our injection paradigm with MDPV that investigated withdrawal effects at a maximum of 11 days, the studies with cocaine involved rats that received higher concentrations of drug and more prolonged withdrawal intervals. Future studies will evaluate GLT-1 expression following an MDPV self-administration paradigm, and will extend the withdrawal time course to determine if MDPV-induced reductions in GLT-1 expression extend further into withdrawal.
The increased locomotor activity observed following MDPV administration was due to robust increases in repetitive movements rather than increased ambulatory activity in both the acute and repeated paradigms. This finding parallels our published findings with mephedrone (Gregg et al., 2014), which acts as a monoamine transporter substrate compared to MDPV that functions as a transporter blocker (Baumann et al., 2013). In the present experiments, pretreatment with CTX prior to varying acute doses of MDPV did not affect MDPV-induced increases in total repetitive movements or ambulatory activity. In contrast, pretreatment with CTX attenuates both acute cocaine- and amphetamine-induced increases in ambulatory activity and stereotypy in rats, and decreases cocaine-induced total locomotor activity in mice (Rassmussen et al., 2011, Sondheimer and Knackstedt, 2011, Tallarida et al., 2013, Barr et al., 2015). This difference in CTX efficacy against the acute locomotor effects of MDPV and cocaine may be related to the total number of CTX treatments, as the Tallarida et al. and Barr et al. studies pretreated rats for 10 days with CTX versus 5 days in our studies. Although 5 days of CTX can normalize glutamate homeostasis in rats with a prior history of cocaine self-administration, and decrease total distance traveled following acute cocaine exposure (Sondheimer et al., 2011, Trantham-Davidson et al., 2012), efficacies observed with CTX in our sensitization and place preference experiments occurred after 11 or 8 treatments with CTX. This may indicate that CTX efficacy against MDPV-evoked behaviors requires a greater frequency of CTX treatments.
For our behavioral sensitization paradigm, treatment with CTX attenuated MDPV-induced sensitization of repetitive movements. Sensitization was limited to repetitive movements, again paralleling observations we observed previously with mephedrone (Gregg et al., 2013a). The challenge dose of MDPV (0.5 mg/kg) was a sub-threshold dose, displaying no differences in repetitive movements or ambulatory activity when administered acutely compared to saline. CTX pretreatment paradigms similar to the one used in our current study attenuate cocaine sensitization of total distance traveled at Withdrawal Day 0 in rats (i.e., increases in total distance traveled at Day 1 and Day 7 of injection) and locomotor activity at Withdrawal Day 30 in mice (Sondheimer et al., 2011, Tallarida et al., 2013). CTX treatment prior to cocaine also attenuates the effects of repeated cocaine exposure on synaptic glutamate release and glutamate clearance kinetics produced by a cocaine challenge after 10 days of withdrawal (Parikh et al., 2014). It has been hypothesized that CTX, by increasing the capacity for cellular glutamate uptake via increased GLT-1 expression, attenuates behavioral sensitization to cocaine by normalizing elevations in extracellular glutamate produced by a cocaine challenge (for a review on neuroadaptations observed with cocaine behavioral sensitization, see Vanderschuren and Kalivas, 2000). While the relatively similar efficacies of CTX against behavioral sensitization produced by cocaine and MDPV suggest related normalizations of withdrawal-induced glutamate dysregulation, further studies are necessary to elucidate the nature of glutamate dysregulation during abstinence, such as possible changes in extracellular glutamate and cystine-glutamate exchange.
CTX treatment attenuated the development of place preference produced by MDPV. The place conditioning effects of MDPV that we observed were consistent with previous data showing that MDPV produces rewarding effects in a CPP assay (King et al., 2015b) and reinforcing effects in self-administration assays (Aarde et al., 2013, Watterson et al., 2014, Aarde et al., 2015, King et al., 2015a, Schindler et al., in press). Information about the effects of CTX on place preference produced by psychostimulants is limited, but it has been shown that CTX blocks reinstatement of methamphetamine seeking in a CPP paradigm (Abulseoud et al., 2012). In our experiments, CTX produced a dose-related reduction of MDPV-induced CPP, with the higher CTX dose (200 mg/kg) producing a 52% inhibition and the lower dose (50 mg/kg) producing a 35% inhibition. This dose-related feature of CTX is likely due to its limited brain penetrability, a pharmacokinetic factor that requires the use of a dosing paradigm in which CTX is administered repeatedly at a dose of at least 200 mg/kg to achieve CNS efficacy (Rothstein et al., 2005; Rasmussen et al., 2011). Doses of CTX greater than 200 mg/kg were not investigated because of concerns about adverse effects, especially diarrhea that can occur with β-lactam antibiotics, and increasing the dose of CTX beyond 200 mg/kg does not result in further inhibition of morphine physical dependence in rats (Rawls et al., 2010). In an additional experiment, we also found that 5 mg/kg MDPV produced a place preference, although the magnitude of the CPP was less than that observed with 2 mg/kg MDPV and not affected by CTX pretreatment (200 mg/kg). This suggests that the 5 mg/kg dose of MDPV is on the downward slope of the dose-response curve, and perhaps engages additional neurotransmitter systems that are not affected by CTX pretreatment.
The most parsimonious explanation for our data, and the one most consistent with the literature, is that CTX reduced MDPV-induced CPP through pharmacological activation of GLT-1. However, it is important to note that we did not test whether the MDPV dosing regimen employed in the CPP test actually alters GLT-1 expression, and this should be examined in future research. An alternative possibility is that the efficacy of CTX in our CPP assay was related to interactions with neurotransmitter systems other than glutamate. Recent findings indicate that CTX induces alterations in dopaminergic signaling (Barr et al., 2015). Specifically, CTX pretreatment decreases cocaine-evoked extracellular dopamine levels through a non-GLT-1 transporter-mediated mechanism and decreases tyrosine hydroxylase and phosphorylated α-synuclein expression in the NAcc (Barr et al., 2015). Therefore, since MDPV produces robust elevations in extracellular dopamine levels in the NAcc (Baumann et al., 2013, Schindler et al., in press), the observed reduction in MDPV-induced CPP by CTX may have been related to its ability to blunt the rise in extracellular dopamine produced by MDPV. Signaling contributions from both glutamate and dopamine are likely to contribute to the overall development of CPP, as both glutamatergic and dopaminergic signaling are involved in the development of cocaine place preference (Cervo et al., 1995). Additional investigations separating the contributions of dopamine and glutamate in the rewarding effects of MDPV are necessary to fully understand the underlying mechanism.
In conclusion, our studies are the first to demonstrate a role for the glutamate system in the context of synthetic cathinone neurochemistry and behavior. Our studies demonstrate an interaction between MDPV and GLT-1, and suggest a role for GLT-1 transporters in the rewarding and locomotor-stimulant effects of MDPV. We have also identified both similarities and differences in the effects of CTX on cocaine versus MDPV, suggesting differences in the mechanism of action of synthetic cathinones versus established psychostimulants.
Highlights.
No studies have evaluated a role for glutamate in synthetic cathinone pharmacology.
MDPV withdrawal reduces GLT-1 expression in the nucleus accumbens.
GLT-1 activator ceftriaxone attenuates MDPV-induced behavioral sensitization.
GLT-1 activator ceftriaxone attenuates MDPV-induced CPP.
Acknowledgments
Funding Sources: National Institute on Drug Abuse grants R01DA039139 and P30DA013429
Footnotes
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: Self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology. 2015;232:1867–1877. doi: 10.1007/s00213-014-3819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abiedalla YFH, Abdel-Hay K, DeRuiter J, Clark CR. Synthesis and GC–MS analysis of a series of homologs and regioisomers of 3,4-methylenedioxypyrovalerone (MDPV) Forensic Sci Int. 2012;223:189–197. doi: 10.1016/j.forsciint.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res. 2012;1456:14–21. doi: 10.1016/j.brainres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holly M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse limiting effects of methcathinone and the synthetic cathinone “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- DEA Office of Diversion Control. All Controlled Substances in Alphabetical Order [Report] Department of Justice; 2015. Retrieved from http://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf. [Google Scholar]
- DEA Public Affairs. Chemicals Used In “Bath Salts” Now Under Federal Control and Regulation [Press Statement] 2011 Retrieved from http://www.dea.gov/pubs/pressrel/pr102111.html.
- Elliott S, Evans J. A 3-year review of new psychoactive substances in casework. Forensic Sci Int. 2014;243:55–60. doi: 10.1016/j.forsciint.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith KD, Houston AC, Rebec GV. Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience. 2012;210:333–339. doi: 10.1016/j.neuroscience.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz A, McCurdy C, Rawls SM. Mephedrone (4-methylmethcathinone), a principal component in psychoactive bath salts, produces behavioral sensitization in rats. Drug Alcohol Depend. 2013a;133:746–750. doi: 10.1016/j.drugalcdep.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz AB, Rawls SM. Mephedrone interactions with cocaine: prior exposure to the ‘bath salt’ constituent enhances cocaine-induced locomotor activation in rats. Behav Pharmacol. 2013b;24:684–688. doi: 10.1097/FBP.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Baumann MH, Partilla JS, Bonano JS, Vouga A, Tallarida CS, Velvadapu V, Smith GR, Peet MM, Reitz AB, Negus SS, Rawls SM. Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioral and neurochemical effects in rats. Br J Pharmacol. 2014;172:883–894. doi: 10.1111/bph.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, methylone and 3,4-methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clin Pharmacol Toxicol. 2014;115:411–416. doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- King HE, Wakeford A, Taylor W, Wetzell B, Rice KC, Riley AL. Sex differences in 3,4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences. Pharmacol Biochem Behav. 2015a;137:16–22. doi: 10.1016/j.pbb.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL. An assessment of MDPV-induced place preference in adult Sprague-Dawley rats. Drug Alcohol Depend. 2015b;146:116–119. doi: 10.1016/j.drugalcdep.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Partilla J, Baumann M, Hutsell B, Banks M, Negus S, Glennon R. Stereoselective actions of methylenedioxypyrovalerone (MDPV) to inhibit dopamine and norepinephrine transporters and facilitate intracranial self-stimulation in rats. ACS Chem Neurosci. 2015;6:771–777. doi: 10.1021/acschemneuro.5b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Corley G, Yen W, Rawls SM, Langford D. Cocaine-induced loss of white matter proteins in the adult mouse nucleus accumbens is attenuated by administration of a β-lactam antibiotic during cocaine withdrawal. Am J Pathol. 2012;181:1921–1927. doi: 10.1016/j.ajpath.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expresssion increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NMS Labs. Designer Drugs: The Trends Report 2015. DesignerDrugTrends.org; 2015. pp. 8–9. Retrieved from http://designerdrugtrends.org/documents/trendsreport2015_3.pdf. [Google Scholar]
- Novellas J, López-Arnau R, Carbó ML, Pubill D, Camarasa J, Escubedo E. Concentrations of MDPV in rat striatum correlate with the psychostimulant effect. J Psychopharmacol. 2015;29:1209–1218. doi: 10.1177/0269881115598415. [DOI] [PubMed] [Google Scholar]
- O’Shea RD. Roles and regulation of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol. 2002;29:1018–1023. doi: 10.1046/j.1440-1681.2002.03770.x. [DOI] [PubMed] [Google Scholar]
- Parikh V, Naughton SX, Shi X, Kelley LK, Yegla B, Tallarida CS, Rawls SM, Unterwald EM. Cocaine-induced neuroadaptations in the dorsal striatum: glutamate dynamics and behavioral sensitization. Neurochem Int. 2014;75:54–65. doi: 10.1016/j.neuint.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Academic Press; San Diego, CA: 2007. [Google Scholar]
- Rasmussen B, Unterwald EM, Rawls SM. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug Alcohol Depend. 2011;118:484–488. doi: 10.1016/j.drugalcdep.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Baron DA, Kim J. β-Lactam antibiotic inhibits development of morphine physical dependence in rats. Behav Pharmacol. 2010;21:161–164. doi: 10.1097/FBP.0b013e328337be10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes-Hoberg M, Vidensky S, Chung DS, Toan SV, Brujin LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antiboitics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology. doi: 10.1007/s00213-015-4057-0. in press. in press. [DOI] [PMC free article] [PubMed]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, Radominska-Pandya A, Endres GW, Channell KB, Smith NH, McCain KR, James LP, Moran JH. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int. 233:416–422. doi: 10.1016/j.forsciint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behav. Brain Res. 2011;225:252–258. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida CS, Corley G, Kovalevich J, Yen W, Langford D, Rawls SM. Ceftriaxone attenuates locomotor activity induced by acute and repeated cocaine exposure in mice. Neurosci Lett. 2013;556:155–159. doi: 10.1016/j.neulet.2013.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. 2014 Global Synthetic Drugs Assessment [Report] Vienna, Austria: 2014. Retrieved from https://www.unodc.org/documents/scientific/2014_Global_Synthetic_Drugs_Assessment_web.pdf. [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, Rawls SM. Beta-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]