Abstract
Past research has demonstrated links between cortical activity, measured via EEG power, and cognitive processes during infancy. In a separate line of research, family socioeconomic status (SES) has been strongly associated with children’s early cognitive development, with socioeconomic disparities emerging during the second year of life for both language and declarative memory skills. The present study examined associations among resting EEG power at birth, SES, and language and memory skills at 15-months in a sample of full-term infants. Results indicate no associations between SES and EEG power at birth. However, EEG power at birth was related to both language and memory outcomes at 15-months. Specifically, frontal power (24–48 Hz) was positively correlated with later Visual Paired Comparison (VPC) memory scores. Power (24–35 Hz) in the parietal region was positively correlated with later PLS-Auditory Comprehension language scores. These findings suggest that SES disparities in brain activity may not be apparent at birth, but measures of resting neonatal EEG power are correlated with later memory and language skills independently of SES.
Keywords: EEG, Socioeconomic status, Language, Memory, Infancy
1. Introduction
Research using electroencephalography (EEG) as a measure of neurophysiology during infancy and early childhood has increased in recent years and studies have utilized EEG techniques to examine both generalities and individual differences in early cognitive development. In typically developing children there is a developmental decrease in EEG power of low-frequency rhythms (e.g., delta and theta) and an increase in high-frequency rhythms (e.g., beta and gamma) across age (Matousek and Petersen, 1973, Harmony et al., 1990). Relative to typically developing children, children with learning or attention disorders often demonstrate higher levels of low-frequency power and lower levels of high-frequency power (Barry et al., 2003). This atypical EEG profile has also been found in children who were previously institutionalized (Marshall et al., 2004) and children growing up in economically disadvantaged environments (Harmony et al., 1990, Otero et al., 2003, Tomalski et al., 2013).
Growing up in a socioeconomically disadvantaged environment is associated with substantially worse health and impaired psychological, cognitive, and emotional development throughout the lifespan (McLoyd, 1998, Bradley and Corwyn, 2002). Childhood socioeconomic status (SES), typically characterized by parental educational attainment, family income and parental occupation (McLoyd, 1998), is strongly associated with later cognitive development and academic achievement (Bradley et al., 2001, Brooks-Gunn and Duncan, 1997, Evans, 2004, Hoff, 2003, McLoyd, 1998). In contrast to investigations examining associations between childhood SES and general intelligence or global measures of cognitive development, a number of more recent studies have adopted a cognitive neuroscience approach to understanding SES differences in cognition (Hackman and Farah, 2009, Brito and Noble, 2014). These studies measured associations between SES and specific neurocognitive systems and have reported SES-related differences in child language (Farah et al., 2006; Noble et al., 2007, Noble et al., 2005), memory (Farah et al., 2006; Noble et al., 2007, Noble et al., 2005), and executive functions (Farah et al., 2006; Kishiyama et al., 2009, Lipina et al., 2005, Stevens et al., 2009).
Recently, Noble and colleagues (2015) reported socioeconomic disparities in both language and declarative memory emerging between 15 and 21 months of age. Both language and memory show individual differences in developmental trajectories in the first two years of life (Barr et al., 1996, Halle et al., 2009) and these skills are predictive of later cognitive development (Halle et al., 2009, Hoff, 2003). Findings from Noble et al. (2015) were consistent with past work demonstrating socioeconomic disparities in early language skills by the age of two (Fernald et al., 2013, Halle et al., 2009, Hoff, 2003, Rowe and Goldin-Meadow, 2009), and extended work on SES disparities to declarative memory skills during infancy.
Although behavioral paradigms assessing specific cognitive skills have reported SES disparities in the second year of life, SES differences in resting EEG have been reported as early as 6–9 months of age (Tomalski et al., 2013). In a sample of 45 full-term infants, Tomalski and colleagues measured resting EEG power of infants from higher and lower-SES households based on family income and parental occupation. Parental education was not used as a predictor as the sample was relatively well educated. Infants were split into SES groups by median family income and occupational group status. Researchers examined two gamma frequencies (21–30 Hz and 31–45 Hz) from four scalp areas (frontal, left temporal, right temporal, and occipital) and reported significantly reduced frontal low-gamma (21–30 Hz) power in infants from lower SES families. The associations between EEG power and SES were not explained by infant sex, age at testing, parental education, breastfeeding, exposure to smoke, or quality of infant sleep (Tomalski et al., 2013). In children, gamma power increases across age, particularly in the frontal regions of the brain (Takano and Kgawa, 1998), and differences in frontal gamma power have been related to language and cognitive skills in toddlers (Benasich et al., 2008) and preschoolers (Gou et al., 2011). In a sample of 63 toddlers, Benasich and colleagues (2008) found associations between individual differences in the distribution of resting frontal gamma power (31–50 Hz) and both concurrent language (Preschool Language Scale: PLS-3) and cognitive scores (Bayley Scales of Infant Development: BSID-II; Stanford-Binet Intelligence Scale—4) during the 2nd year of life (Benasich et al., 2008).
Two recent prospective studies have investigated the association between resting EEG power and later cognitive development. In a follow-up to the Benasich et al. (2008) study, children with resting EEG data at 16, 24, and 36 months of age were tested on measures of general cognitive ability and language skills at 4 and 5 years of age. Results indicated significant correlations between resting EEG gamma power and individual differences in language and cognition during preschool years (Gou et al., 2011). Additionally, Williams et al. (2012) reported significant correlations between resting EEG power at birth and 18-month Bayley cognitive scores (BSDI-III) in 13 full-term infants born with congenital heart disease (CHD). Although Bayley cognitive scores were below average in this high-risk group, higher power in the frontal regions in the beta (12–24 Hz), low-gamma (24–35 Hz), and higher-gamma (36–48 Hz) frequencies were significantly associated with higher cognitive scores at 18-months of age (Williams et al., 2012).
Given that resting EEG power has been associated with both SES disparities during the first year of life and neurocognitive skills in the second year of life, the current prospective study examined the associations between neonatal EEG power, family SES, and neurocognitive skills at 15-months of age. As SES differences in resting EEG power have been reported as early as 6–9 months (Tomalski et al., 2013), it was hypothesized that SES disparities would explain differences in neonatal EEG power and that these differences in early electrocortical activity would be associated with both language and declarative memory skills during the second year of life.
2. Method
2.1. Participants
All participants were selected from a subset of infants participating in a large, longitudinal study investigating the relation between prenatal exposures and birth outcomes (http://safepassagestudy.org; Dukes et al., 2014). The present study took place at a single participating clinic site in an urban Midwest community. Children were enrolled without regard to prenatal exposures. The present study was not powered to detect effects of these exposures; further, at the time of this writing, investigators remained blind to these exposures, as data collection and analysis in the larger study was ongoing. Women were excluded from the study if they carried three or more fetuses in pregnancy, planned abortion, planned to move out of the area before delivery, were unable to provide consent, or if their health care provider advised against participation. All parents provided written informed consent for their family’s participation in this study. Research procedures were approved by the Columbia University Medical Center IRB and the Sanford Health IRB.
In order to be included in the analysis, infants were required to have artifact free EEG data from at least one of the four cortical sites. The final sample included 66 full-term infants (29 males; gestational age at birth M: 39.5 weeks, SD: 1.2). The majority of children were Caucasian (n = 62), with the remaining children of mixed race (n = 4). Infants were tested 12–96 h after birth, then again at 15 months of age (M: 15.43 months, SD: 0.40). Participants were excluded from participating in the present study on the basis of major neurological or developmental deficits, birth before 37 weeks gestation, multiple births, or maternal age under 18 years.
2.2. Measures
2.2.1. Socioeconomic status
Information on family socioeconomic status was collected via sociodemographic questionnaire and included items pertaining to educational attainment (total years of education for mother and father separately), household composition (number of adults and children in household), and family income (estimated gross annual income). An income-to-needs (ITN) ratio for each family was calculated by dividing reported annual income by the federal poverty level for a family of that size in the year the data were collected. We asked that the child’s primary caregiver provide responses for the entire questionnaire; mothers comprised 95% of respondents. In single-mother households, only maternal demographic information was obtained.
2.2.2. Neonatal EEG
Electroencephalogram (EEG) is a sensitive measure of cortical function, reflecting local synchronous depolarization of neurons; total EEG activity is typically quantified as EEG power. Resting EEG was acquired at 1000 samples/sec for 10 min using a BioPac EEG100c and electrocap; all EEG data reported were collected during active sleep. EEG activity was collected from 4 scalp locations − left frontal (F3), right frontal (F4), left parietal (P3), and right parietal (P4). Data were excluded if contaminated by movement-related artifact or other sources of artifact from noncortical electrical activity. The standard deviation of voltage was computed for every 30-second epoch for each lead and data were excluded for each lead within each epoch if standard deviation exceeded 50 μV. Additionally, data were excluded for entire epochs if more than 25% of the leads exceeded the standard deviation criterion during the epoch. Data for an entire study were excluded if more than 80% of the epochs failed to pass these criteria. Data from each lead were then re-referenced in each 30-second epoch to the average of the electrodes without artifact. The natural log (ln) of EEG power was used in all analyses and any data point more than two standard deviations above the mean were winsorized, that is, replaced with values exactly two standard deviations from the mean. After initial screening for artifact, EEG power was computed using one-second fast Fourier transformations (FFTs) for each of the leads. Total EEG activity (power) was partitioned into different frequency bands ranging from 1 to 48 Hz. EEG power was averaged across right and left frontal leads and across right and left parietal leads. Based on previous studies (Benasich et al., 2008, Tomalski et al., 2013, Williams et al., 2012) analyses focused on the low-gamma (24–35 Hz) and higher-gamma (36–48 Hz) frequencies. Additional exploratory analyses were carried out in three other spectral bands: delta (1–3 Hz), theta (4–6 Hz), alpha (7–9 Hz), and beta (12–23 Hz).
2.2.3. 15-month language skills
The Preschool Language Scale-4 (PLS) is a standardized language assessment, normed for children from birth to age 6 (Zimmerman & Castilleja, 2005). This measure assesses children’s receptive and expressive language development through a series of interactive items designed to elicit desired language skills. The Auditory Comprehension subscale measures receptive language skills by examining a child’s ability to comprehend and respond to spoken language. Individual items assess skills like following directions, vocabulary knowledge, and spatial understanding. The Expressive Communication subscales measures expressive language skills by assessing a child’s ability to produce verbal language and respond to questions. Items assess language skills such as phoneme production, social communication, and sentence complexity. This measure yields standard scores (M = 100, SD = 15) for both subscales. Children at 15-months sat with their parent and the experimenter at a small Table or on the floor of a well-lit room. Parents were instructed not to reply or help their child unless specifically instructed to do so. Due to fussiness during testing, 4 children are missing responses for the auditory subscale and 2 children are missing responses to the expressive subscale.
2.2.4. 15-month Memory Skills
Memory was measured using the Visual Paired Comparison (VPC: Morgan and Hayne, 2006) and the Deferred Imitation (DI: Barr et al., 1996; Herbert and Hayne, 2000) tasks. The Visual Paired Comparison (VPC) task assesses the degree to which children remember a familiar visual stimulus by comparing looking time to the familiar stimulus vs. a novel stimulus. This task has been used in past studies of short-term and long-term visual recognition memory (Pascalis and de Haan, 2003). In this brief task, children were seated on their parents’ laps 40 in. (101.6 cm) away from two 20 in. (50.8 cm) monitors situated 33 in. (83.8 cm) apart at their centers. A video camera was situated between the monitors to capture the participant’s gaze. Parents were told to close their eyes or look directly between the monitors so as not to influence the child’s response. First, to orient the participant toward the monitors, each screen displayed an identical spinning ball for 13s. During the 10s familiarization block, each screen displayed an identical blue, mailbox shaped face. This was followed by the first 10s novelty preference block in which one of the blue faces was replaced by a circular yellow face. In the second 10s novelty preference block, the yellow face was replaced by the familiar blue face, and the other screen displayed a square red face. Responses from 6 children are missing due to child fussiness (n = 2), experimenter error (n = 2), the child not attending to stimuli (n = 1), and computer error (n = 1).
For the VPC task, coders reviewed the videos frame-by-frame to establish total looking time for each block. At every 200 ms interval, the coder determined whether the child was attending to the left monitor, right monitor, or neither. This enabled calculation of the ratio of novel looking time (i.e., attending to red or yellow faces) to total looking time (i.e., attending to any face). Ratios above 0.5 indicate greater looking time for novel relative to the familiar stimuli. Reliability checks were run on 20% of the scores. Inter-rater reliability was greater than 95%.
In Deferred Imitation (DI) tasks, infants observe a series of simple actions and are given the opportunity to imitate the actions after a delay. The DI paradigm has been a useful tool in examining age-related changes in declarative memory processing and is considered a reliable measures of declarative memory among preverbal children (Barr and Hayne, 2000; Meltzoff, 1995). Stimuli and task administration were based on the puppet (Barr et al., 1996) and rattle (Herbert and Hayne, 2000) tasks.
2.2.4.1. DI stimuli
Puppet task stimuli consisted of a handheld puppet (gray mouse) 12 in. (30.5 cm) in height. The puppet was fashioned with a removable felt mitten that fit over the right hand of the puppet and matched the color of the puppet. A jingle bell was attached to the inside of the mitten to create a noise when shaken. The rattle task consisted of a 5 in. (12.7 cm) green wooden stick attached to a plastic lid with a Velcro underside. This handle could be attached to or detached from a clear plastic cup 3 in. (7.6 cm) in height with a 0.5 in (1.3 cm) diameter opening in the Velcro top. A green wooden bead 0.5 in. (1.3 cm) in diameter fit through the hole.
2.2.4.2. DI demonstration phase
Parents were asked to sit on a chair and hold their child on their lap. Parents were asked to refrain from touching, pointing to, or speaking about the stimuli. The experimenter knelt on the floor in front of the participant and held the puppet at the child’s eye level, approximately 32 in. (81.3 cm) away from the child. After the child oriented to the puppet, the experimenter removed the mitten from the puppet’s hand, shook the mitten 3 times to ring the bell inside, then replaced the mitten on the puppet’s right hand. The experimenter repeated these steps twice more for a total of three demonstrations. The puppet demonstration was immediately followed by the rattle demonstration. Parents were asked to sit on the floor with the child on their lap. The experimenter then placed the pieces of the rattle in a line on the floor. After the child oriented to the rattle pieces, the experimenter picked up the bead, pushed it through the opening of the cup, attached the handle to the top of the cup, and shook the constructed rattle. The experimenter dismantled the rattle and repeated the demonstration twice more for a total of three demonstrations. The demonstration phase was followed by an approximately 40 min delay during which the child completed other neurocognitive tasks.
2.2.4.3. DI test phase
After the delay, the puppet and rattle tests were initiated. Testing took place in a small, well-lit room. Parents were asked to refrain from touching, pointing to, or speaking about the stimuli. At test, the bell was removed from the puppet’s mitten. The experimenter knelt in front of the seated participants and held the puppet within reach of the child and encouraged the child to interact with the puppet. After the child touched the puppet, they were given 90s from the time the puppet was first touched to imitate the previously demonstrated actions. For the rattle test, the participants were seated on the floor and the experimenter placed the rattle pieces within reach of the child. The child was again given 60s to imitate the previously demonstrated actions from the time they first touched any of the stimuli. Responses from 14 children were omitted due to child fussiness (n = 10), child unwillingness to play with stimuli (n = 2), and experimenter error (n = 2).
2.2.4.4. DI coding
A digital video camera was used to record all DI tasks. Coders reviewed the videos frame-by-frame to score participants’ attention to the demonstration and performance during testing. For both the puppet and the rattle tests, behavior was coded from the time of first touch of the experimental items. Memory was evaluated by determining the number of individual target behaviors the child imitated during the test session. For the puppet task, participants were awarded 1 point for exhibiting each of the following target actions: removing the mitten from the puppet’s hand, shaking the mitten, attempting to replace the mitten on either hand. For the rattle task, participants were awarded 1 point for each of the following target actions: placing the ball in the cup, attaching the lid to the cup, shaking the rattle with the ball inside. Scores were summed across the puppet and rattle tasks; participants could score between 0 and 6 points for their imitation of the target actions. Reliability checks were run on 20% of the scores to ensure the target actions had been scored properly. Inter-rater reliability was greater than 95%.
3. Results
3.1. Socioeconomic status and neonatal EEG
Average parental educational attainment was 15 years (SD = 1.5, range = 11.0 to 17.0 years), with half the sample obtaining a college degree or higher. No significant differences were found between maternal and paternal education, therefore average parental education was used. Family income in this sample was widely distributed, with incomes ranging from below the poverty line to many times above it (M = 78,486, SD = 52, 284, range = $6500 to $300,000). To account for family size in relation to family income, income-to-needs (ITN) was calculated, defined as the total family income divided by the poverty line for a family of that size (M = 3.8, SD = 3.1, range = 0.29 to 19.73).
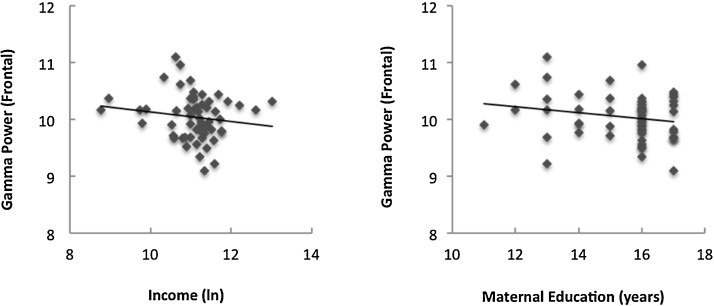
Sex differences were found in neonatal EEG, with females demonstrating more parietal higher-gamma power, t(59) = −3.05, p = 0.003 than males; no other sex differences in neonatal EEG were found. Examining associations between socioeconomic status and resting EEG at birth, no significant correlations (p’s > 0.12) were found between any of the SES variables of interest (average parental education, family income, or family income-to-needs) and neonatal EEG in any of the frequency bands for either region (frontal or parietal), see Fig. 1. Results remained non-significant (p’s > 0.24) even after controlling for sex and gestational age.
Fig. 1.
No correlation between SES, family income (left panel) or parental education (right panel), and neonatal EEG power.
3.2. Neonatal EEG and 15-Month Cognitive Skills
Overall, infants in this sample scored within normal ranges for language measures and memory scores reflected typical ranges reported from past studies of VPC and DI (Morgan and Hayne, 2006, Morgan and Hayne, 2011, Barr et al., 1996), see Table 1. No significant associations were found between 15-month memory or language skills and gestational age at birth (p’s > 0.12) or sex (p’s > 0.10). Additionally, no significant correlations were found between any of the SES variables of interest and any of the 15-month memory or language measures (p’s > 0.06).
Table 1.
Descriptive statistics on 15-month cognitive skills.
| VPC | DI | PLS-A | PLS-E | |
|---|---|---|---|---|
| N | 60 | 52 | 62 | 64 |
| M | 0.63 | 4.08 | 103.40 | 116.70 |
| SD | 0.15 | 1.56 | 10.71 | 6.84 |
| Range | 0.13–0.93 | 0–6 | 90–135 | 99–135 |
Note: VPC = Visual Paired Comparison, DI = Deferred Imitation, PLS-A = Preschool Language Scale Auditory Comprehension, PLS-E = Preschool Language Scale Expressive Communication.
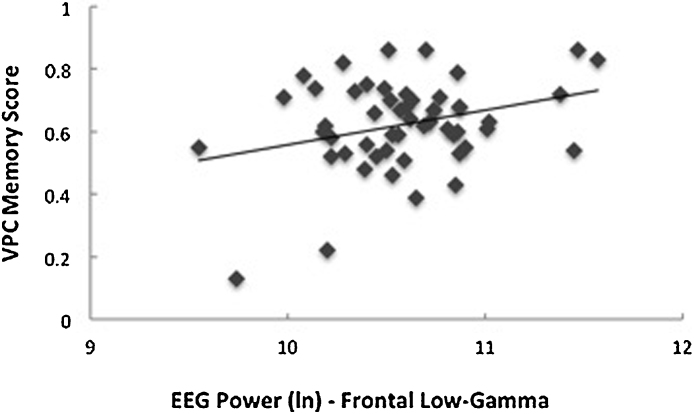
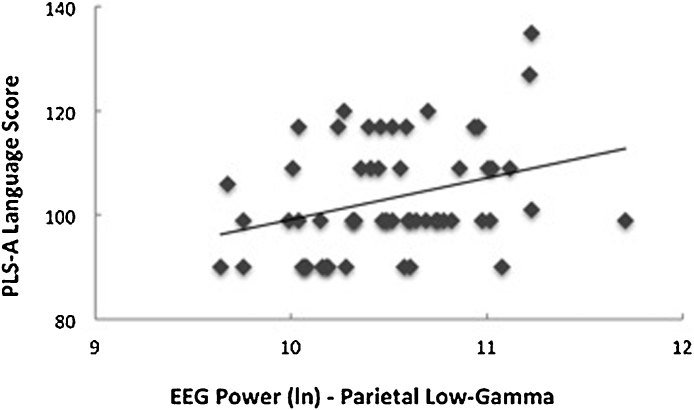
Examining our frequency bands of interest (i.e., low-gamma and higher-gamma), analyses yielded significant associations between both frontal low-gamma (β = 0.32, p = 0.03, R2 = 0.12) and frontal higher-gamma (β = 0.34, p = 0.02, R2 = 0.13) power and VPC memory scores (Fig. 2). No significant associations were found between neonatal EEG power (in any of the frequency bands of interest for either region) and DI memory scores. Analyses yielded significant correlations between parietal low-gamma (β = 0.30, p = 0.03, R2 = 0.12) and PLS Auditory Comprehension language scores (Fig. 3). Association between parietal higher-gamma power and PLS Auditory Comprehension language scores approached significance, controlling for both sex and gestational age (β = 0.25, p = 0.09, R2 = 0.09). No significant associations were found between neonatal EEG power (in any of the frequency bands of interest for either region) and PLS Expressive Communication language scores. All analyses included sex, gestational age at birth, and SES1 in the regression models, but significant correlations between EEG power and cognitive skills were found without controlling for infant characteristics, see Table 2. Exploratory analyses yielded no significant associations between neonatal EEG power in other frequencies (i.e., delta, theta, alpha, and beta) and memory or language scores.
Fig. 2.
Association between frontal low-gamma EEG power at birth and later Visual Paired Comparison (VPC) memory scores (β = 0.32, p = .03, R2 = .12). Plots were similar for both low-gamma and higher-gamma.
Fig. 3.
Association between parietal low-gamma EEG power at birth and later preschool language scale (PLS) auditory comprehension scores (β = 0.30, p = .03, R2 = .12).
Table 2.
Unadjusted correlations between EEG power and 15-month cognitive skills.
| VPC | DI | PLS-A | PLS-E | |
|---|---|---|---|---|
| Low-gamma (24–35 Hz) | ||||
| Frontal | 0.31* | −0.15 | 0.23 | 0.10 |
| Parietal | −0.09 | −0.16 | 0.32* | 0.19 |
| Higher-Gamma (36–48 Hz) | ||||
| Frontal | 0.30* | −0.02 | 0.17 | 0.08 |
| Parietal | −0.09 | −0.08 | 0.28# | 0.13 |
Note: VPC = Visual Paired Comparison, DI = Deferred Imitation, PLS-A = Preschool Language Scale Auditory Comprehension, PLS-E = Preschool Language Scale Expressive Communication. *p < 0.05, ** p < 0.01, *** p < 0.001. # = No longer significant after controlling for sex and gestational age.
4. Discussion
Our aim was to investigate whether differences in infants’ socioeconomic background were associated with resting EEG power at birth, and if those differences were correlated with later memory and language performance at 15 months of age. We found no correlations between resting EEG power at birth and any of our SES variables (i.e., parental educational attainment, family income, family income-to-needs). Had we found differences in resting EEG at birth, it would have suggested that these differences were likely the result of socioeconomic differences in the prenatal environment (e.g., maternal diet, stress) and/or genetic differences (Monk et al., 2013, Turkheimer et al., 2003). Of course, this study cannot rule out those possibilities. However, in the context of previous work reporting SES disparities in resting EEG power as early as 6–9 months in a similar sample size of infants (Tomalski et al., 2013), the present null findings suggest the possibility that differences in postnatal experience lead to the emergence of these disparities in the first year of life. A longitudinal study assessing both the prenatal and postnatal environments would be necessary to formally test this hypothesis.
Furthermore, no significant correlations were found between family SES and our memory and language outcomes of interest at 15 months. A recent study by Noble and colleagues (2015) reported socioeconomic disparities in both language and declarative memory emerging between 15 and 21 months of age; significant correlations between SES and language scores were reported at 15 months (r = 0.18), but significant correlations between SES and memory scores were not reported until 21 months of age (r = 0.31). The lack of a correlation between SES and memory is consistent with this past study, and our smaller sample size (N = 66 compared to N = 179) may have restricted our ability to find SES correlations with language.
Consistent with previous research examining infants with congenital heart disease (Williams et al., 2012), we found significant associations between neonatal EEG power and later cognitive abilities, specifically declarative memory and auditory comprehension, in our sample of typically developing full-term infants. These individual differences in EEG power at birth could be due to a host of variables including genetics (Zietsch et al., 2007), developmental programming (Pluess and Belsky, 2011), differences in the prenatal environment (Monk et al., 2013), placental function (Schulkin et al., 2005), or hypoxia (Murray et al., 2009). As with past studies examining EEG power and early global cognitive development (Benasich et al., 2008; Gou et al., 2011), these associations between EEG power and later cognitive activity were limited to the low-gamma (24–35 Hz) and higher-gamma frequency ranges (36–48 Hz).
Past work with adults has also reported associations between gamma oscillations and specific perceptual and cognitive processes. For example, in adults the gamma rhythm (30–100 Hz) has been linked to object perception (Gruber and Muller, 2005; Gruber et al., 2002), attention (Muller et al., 2005; Ray et al., 2008), memory (Miltner et al., 1999, Hermann et al., 2010) and language-related processes (Eulitz et al., 1996, Pulvermuller et al., 1996). In both adults and infants, gamma oscillations have been proposed to reflect the active maintenance of object representations in memory (Kaufman et al., 2005, Tallon-Baudry et al., 1998) and may be closely tied to attention capabilities as positive correlations between gamma activity and attention measures have been reported (Muller et al., 2000, Reid et al., 2007). Gamma power has even been correlated with a parent report measure of concurrent attention (Toddler Behavior Assessment Questionnaire: TBAQ) at 24-months of age (Benasich et al., 2008). Our results contribute to this literature by extending findings between resting EEG activity in the gamma frequency collected in the newborn period and specific neurocognitive processes (i.e., declarative memory and language).
Interestingly, our significant associations between neonatal EEG power and neurocognitive processes were specific to memory via visual paired comparison (VPC) paradigm and auditory comprehension language scores only. Compared to deferred imitation (DI), which involves memory recall after a delay and requires some motoric ability, VPC is a passive perceptual task assessing novelty object preference. Although, like all cognitive functions, both DI and VPC require attention, past studies have linked individual differences in VPC to both infants’ ability to distribute attention between stimuli during behavioral tasks (Rose et al., 2003) and infants’ neural responses of attention measured by event-related potentials (de Haan, 2007). Additionally, as the auditory environment is vital to the development of efficient native language processing (Fernald et al., 2013, Kuhl, 2007, Melvin et al., 2016), a recent study has reported that both active and passive non-linguistic acoustic experience during the first year of life impacts prelinguistic acoustic brain mapping. Using ERPs, these researchers demonstrated that this experience increases perceptual attention to environmental acoustic stimuli (Benasich et al., 2014), which suggests that nonlinguistic acoustic experience during early development may help the infant process later linguistically relevant environmental cues.
There are several limitations to the current study that should be addressed in future work. Although the sample size in the current study was larger than most studies reporting associations between resting EEG and cognitive performance (Benasich et al., 2008; Gou et al., 2011; Williams et al., 2012) or SES (Tomalski et al., 2013) during infancy, the socioeconomic range, although distributed, did not oversample families in the lowest extremes. It is possible that a study with a larger sample of families under the poverty line may find SES disparities in neonatal EEG or in cognitive skills at 15-months of age, as socioeconomic disparities in child development are often nonlinear, with the greatest differences found at the low end of the income distribution (Duncan and Magnuson, 2012). Additionally, we cannot say with certainty whether the associations between neonatal EEG and cognitive skills at 15-months reported here are indicative of causal relations. Nonetheless, it is plausible that some underlying level of neural activity at birth, particularly in the high-frequency bands often linked to attentional abilities, would continue to be correlated with tasks that tap into sensory or perceptual processes related to memory and language. We also note that, although significant variation in 15-month cognitive skills could be explained by neonatal EEG power, effect sizes were small (R2’s = 0.12). Future studies using high-density EEG recordings and 128 lead multi-electrode nets are needed to replicate the EEG power results. Increasing the number of electrodes improves spatial resolution, making it possible to record cerebral activities that are more difficult to localize; resulting in genuine source localization of neonatal EEG (Odabaee et al., 2013). Additionally, spatial sampling error for infants, in comparison to adults, is much larger − a spatial sampling error of less than 10% for an adult can be obtained with a 64-electrode array, but a 256-electrode array is needed for an infant to achieve the same level of error (Grieve et al., 2004). Improving measurement accuracy of EEG spatial properties and extending to findings of functional connectivity, via EEG coherence, (Myers et al., 2015) would strengthen our current results.
Our results demonstrate that measures of EEG power at birth are associated with memory and language skills at 15 months of age, independently of socioeconomic status. These findings suggest that early variations in high frequency oscillatory activity may contribute to individual differences in cognitive trajectories. More attention to environmental correlates of EEG power during infancy may contribute to our understanding of early brain maturation and help to identify markers for later cognitive and learning disorders. Understanding how early experiences influence neurocognitive trajectories has implications for the design and timing of targeted screenings and interventions.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
The authors would like to acknowledge and thank the PASS Research Network for providing data for this work. We also gratefully acknowledge Laura Engelhardt and Luke Mack who made significant contributions to this research. This publication was supported by NIH Grants UL1TR000040, R37HD032773 and U01HD045935.
Footnotes
Income-To-Needs (ITN) was added to all regression models; results are unchanged when adding family income or parental education as the SES variable.
Contributor Information
Natalie H. Brito, Email: nhb2111@cumc.columbia.edu.
William P. Fifer, Email: wpf1@cumc.columbia.edu.
Michael M. Myers, Email: mmm3@cumc.columbia.edu.
Amy J. Elliott, Email: amy.elliott@sanfordhealth.org.
Kimberly G. Noble, Email: kgn2106@tc.columbia.edu.
References
- Barr R., Dowden A., Hayne H. Developmental changes in deferred imitation by 6-to 24-month-old infants. Infant Behav. Dev. 1996;19(2):159–170. [Google Scholar]
- Barry R.J., Clarke A.R., Johnstone S.J. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophysiol. 2003;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Benasich A.A., Gou Z., Choudhury N., Harris K.D. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav. Brain Res. 2008;195(2):215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich A.A., Choudhury N.A., Realpe-Bonilla T., Roesler C.P. Plasticity in developing brain: active auditory exposure impacts prelinguistic acoustic mapping. J. Neurosci. 2014;34(40):13349–13363. doi: 10.1523/JNEUROSCI.0972-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J., Duncan G.J. The effects of poverty on children. Future Child. 1997:55–71. [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F. Socioeconomic status and child development. Annu. Rev. Psychol. 2002;53(1):371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F., Burchinal M., McAdoo H.P., García Coll C. The home environments of children in the United States Part II: relations with behavioral development through age thirteen. Child Dev. 2001;72(6):1868–1886. doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]
- Brito N.H., Noble K.G. Socioeconomic status and structural brain development. Front. Neurosci. 2014;8:276. doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M. Visual attention and recognition memory in infancy. In: de Haan M., editor. Infant EEG and Event-related Potentials. Psychology Press; New York: 2007. pp. 101–143. [Google Scholar]
- Duncan G.J., Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3(3):377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Dukes K.A., Burd L., Elliott A.J., Fifer W.P., Folkerth R.D., Hankins G.D., Signore C. The safe passage study: design, methods, recruitment, and follow‐up approach. Paediatr. Perinat. Epidemiol. 2014;28(5):455–465. doi: 10.1111/ppe.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulitz C., Maess B., Pante C., Friederici A.D., Feige B., Elbert T. Oscillatory neuro-magnetic activity induced by language and non-language stimuli. Cogn. Brain Res. 1996;4:121–132. [PubMed] [Google Scholar]
- Evans G.W. The environment of childhood poverty. Am. Psychol. 2004;59(2):77. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Fernald A., Marchman V.A., Weisleder A. SES differences in language processing skill and vocabulary are evident at 18 months. Dev. Sci. 2013;16(2):234–248. doi: 10.1111/desc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve P.G., Emerson R.G., Isler J.R., Stark R.I. Quantitative analysis of spatial sampling error in the infant and adult electroencephalogram. NeuroImage. 2004;21:1260–1274. doi: 10.1016/j.neuroimage.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Gruber T., Muller M.M., Keil A. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb. Cortex. 2002;15:109–116. doi: 10.1093/cercor/bhh113. [DOI] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J. Socioeconomic status and the developing brain. Trends Cogn. Sci. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle T., Forry N., Hair E., Perper K., Wandner L., Wessel J., Vick J. Child Trends; Washington, DC: 2009. Disparities in Early Learning and Development: Lessons from the Early Childhood Longitudinal Study–Birth Cohort (ECLS-B) pp. 1–7. [Google Scholar]
- Harmony T., Marsoi E., Diaz de Leon A.E., Becker J., Fernandez T. Effect of sex: psychosocial disadvantages and biological risk factors on EEG maturation. Electroencephalogr. Clin. Neurophysiol. 1990;75:482–491. doi: 10.1016/0013-4694(90)90135-7. [DOI] [PubMed] [Google Scholar]
- Hermann C.S., Frund I., Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci. Biobehav. Rev. 2010;34:981–992. doi: 10.1016/j.neubiorev.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Hoff E. The specificity of environmental influence: socioeconomic status affects early vocabulary development via maternal speech. Child Dev. 2003;74(5):1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Csibra G., Johnson M.H. Oscillatory activity in the infant brain reflects object maintenance. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15271–15274. doi: 10.1073/pnas.0507626102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishiyama M.M., Boyce W.T., Jimenez A.M., Perry L.M., Knight R.T. Socioeconomic disparities affect prefrontal function in children. J. Cogn. Neurosci. 2009;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Kuhl P.K. Is speech learning ‘gated’ by the social brain? Dev. Sci. 2007;10(1):110–120. doi: 10.1111/j.1467-7687.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Lipina S.J., Martelli M.I., Colombo J.A. Performance on the A-not-B task of Argentinean infants from unsatisfied and satisfied basic needs homes. Int. J. Psychol. 2005;39(1):49–60. [Google Scholar]
- Marshall P.J., Fox N., The Core Group A.B.I.E.P. A comparison of the electroencephalogram (EEG) between institutionalized and community children in Romania. J. Cogn. Neurosci. 2004;16:1327–1338. doi: 10.1162/0898929042304723. [Erratum, 19, 1173-1174] [DOI] [PubMed] [Google Scholar]
- Matousek M., Petersen I. Frequency analysis of the EEG in normal children and in normal adolescents. In: Kellaway P., Petersen I., editors. Automation of Clinical EEG. Raven Press; New York: 1973. pp. 75–102. [Google Scholar]
- McLoyd V.C. Socioeconomic disadvantage and child development. Am. Psychol. 1998;53(2):185. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Meltzoff A.N. What infant memory tells us about infantile amnesia: long-term recall and deferred imitation. J. Exp. Child Psychol. 1995;59(3):497–515. doi: 10.1006/jecp.1995.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin S.A., Brito N.H., Mack L.J., Engelhardt L.E., Fifer W.P., Elliott A.J., Noble K.G., in collaboration with the PASS Network Contributions of the home environment to early disparities in language development. Infancy. 2016 [Google Scholar]
- Miltner W.H., Braun C., Arnold M., Witte H., Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- Monk C., Georgieff M.K., Osterholm E.A. Maternal prenatal distress and poor nutrition—mutually influencing risk factors affecting infant neurocognitive development. J. Child Psychol. Psychiatry. 2013;54(2):115–130. doi: 10.1111/jcpp.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K., Hayne H. Age-related changes in memory reactivation by 1- and 2-year-old human infants. Dev. Psychobiol. 2006;48:48–57. doi: 10.1002/dev.20110. [DOI] [PubMed] [Google Scholar]
- Morgan K., Hayne H. Age-related changes in visual recognition memory during infancy and early childhood. Dev. Psychobiol. 2011;53(2):157–165. doi: 10.1002/dev.20503. [DOI] [PubMed] [Google Scholar]
- Muller M.M., Gruber T., Keil A. Modulation of induced gamma band activity in the human EEG by attention and visual information processing. Int. J. Psychophysiol. 2000;38:283–299. doi: 10.1016/s0167-8760(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Murray D.M., Boylan G.B., Ryan C.A., Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124(3):459–467. doi: 10.1542/peds.2008-2190. [DOI] [PubMed] [Google Scholar]
- Myers M.M., Grieve P.G., Stark R.I., Isler J.R., Hofer M.A., Yang J., Ludwig R.J., Welch M.G. Family Nurture Intervention in preterm infants alters frontal cortical functional connectivity assessed by EEG coherence. Acta Paediatr. 2015;104(7):670–677. doi: 10.1111/apa.13007. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Norman M.F., Farah M.J. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Engelhardt L.E., Brito N.H., Mack L.J., Nail E.J., Angal J., Barr R., Fifer W.P., Elliott A.J., in collaboration with the PASS Network Socioeconomic disparities in neurocognitive development in the first two years of life. Dev. Psychobiol. 2015;57(5):535–551. doi: 10.1002/dev.21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odabaee M., Freeman W.J., Colditz P.B., Ramon C., Vanhatalo S. Spatial patterning of the neonatal EEG suggests a need for a high number of electrodes. NeuroImage. 2013;68:229–235. doi: 10.1016/j.neuroimage.2012.11.062. [DOI] [PubMed] [Google Scholar]
- Otero G.A., Pliego-Rivero F.B., Fernandez T., Ricardo J. EEG development in children with sociocultural disadvantages: a follow-up study. Clin. Neurophysiol. 2003;114:1918–1925. doi: 10.1016/s1388-2457(03)00173-1. [DOI] [PubMed] [Google Scholar]
- Pluess M., Belsky J. Prental programming of postnatal plasticity? Dev. Psychopathol. 2011;23:29–38. doi: 10.1017/S0954579410000623. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F., Eulitz C., Pantev C., Mohr B., Feige B., Lutzenberger W., Birbaumer N. High-frequency cortical responses reflect lexical processing: an MEG study. Electroencephalogr. Clin. Neurophysiol. 1996;98:76–85. doi: 10.1016/0013-4694(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Ray S., Niebur E., Hsiao S.S., Sinai A., Crone N.E. High-frequency gamma activity (80–150 Hz) is increased in human cortex during selective attention. Clin. Neurophysiol. 2008;119:116–133. doi: 10.1016/j.clinph.2007.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid V.M., Csibra G., Belsky J., Johnson M.H. Neural correlates of the perception of goal-directed action in infants. Acta Psychol. (Amst.) 2007;124:129–138. doi: 10.1016/j.actpsy.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Rose S.A., Feldman J.F., Jankowski J.J. Infant visual recognition memory: independent contributions of speed and attention. Dev. Psychol. 2003;39(3):563. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rowe M.L., Goldin-Meadow S. Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science. 2009;323(5916):951–953. doi: 10.1126/science.1167025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J., Schmidt L., Erickson K. Birth, Distress, and Disease: Placenta-brain Interactions. Cambridge University Press; Cambridge: 2005. Glucocorticoid facilitation of corticotropin-releasing hormone in the placenta and the brain: functional impact on birth and behavior; pp. 235–268. [Google Scholar]
- Stevens C., Lauinger B., Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Dev. Sci. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Kgawa T. Characterization of developmental changes in EEG gamma-band activity during childhood using the autoregressive model. Acta Paediatric Japan. 1998;40:446–452. doi: 10.1111/j.1442-200x.1998.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O., Peronnet F., Pernier J. Induced γ-band activity during the delay of a visual short-term memory task in humans. J. Neurosci. 1998;18(11):4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P., Moore D.G., Ribeiro H., Axelsson E.L., Murphy E., Karmiloff-Smith A., Kushnerenko E. Socioeconomic status and functional brain development—associations in early infancy. Dev. Sci. 2013;16(5):676–687. doi: 10.1111/desc.12079. [DOI] [PubMed] [Google Scholar]
- Turkheimer, Haley, Waldron, D’Onofrio, Gottesman Socioeconomic status modifies heritability of IQ in young children. Psychol. Sci. 2003;14(6):623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Williams I.A., Tarullo A.R., Grieve P.G., Wilpers A., Vignola E.F., Myers M.M., Fifer W.P. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet. Gynecol. 2012;40(3):304–309. doi: 10.1002/uog.11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietsch B.P., Hansen J.L., Hansell N.K., Geffen G.M., Martin N.G., Wright M.J. Common and specific genetic influences on EEG power bands delta, theta, alpha, and beta. Biol. Psychol. 2007;75(2):154–164. doi: 10.1016/j.biopsycho.2007.01.004. [DOI] [PubMed] [Google Scholar]