Abstract
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase that is expressed in the brain and implicated in alcohol abuse in humans and behavioral responses to ethanol in mice. Previous studies have shown an association of human ALK with acute responses to alcohol and alcohol dependence. In addition, Alk knockout (Alk −/−) mice consume more ethanol in a binge-drinking test and show increased sensitivity to ethanol sedation. However, the function of ALK in excessive drinking following the establishment of ethanol dependence has not been examined. In this study, we tested Alk −/− mice for dependence-induced drinking using the chronic intermittent ethanol-two bottle choice drinking (CIE-2BC) protocol. We found that Alk −/− mice initially consume more ethanol prior to CIE exposure, but do not escalate ethanol consumption after exposure, suggesting that ALK may promote the escalation of drinking after ethanol dependence. To determine the mechanism(s) responsible for this behavioral phenotype we used an electrophysiological approach to examine GABA neurotransmission in the central nucleus of the amygdala (CeA), a brain region that regulates alcohol consumption and shows increased GABA signaling after chronic ethanol exposure. GABA transmission in ethanol-naïve Alk −/− mice was enhanced at baseline and potentiated in response to acute ethanol application when compared to wild-type (Alk +/+) mice. Moreover, basal GABA transmission was not elevated by CIE exposure in Alk −/− mice as it was in Alk +/+ mice. These data suggest that ALK plays a role in dependence-induced drinking and the regulation of presynaptic GABA release in the CeA.
Keywords: addiction, ALK, amygdala, chronic intermittent ethanol, GABA, synaptic
1. Introduction
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase that acts as an oncogene in various human cancers, including anaplastic large cell lymphoma (for which it is named), neuroblastoma, and lung cancer (Hallberg and Palmer, 2013). ALK is expressed in the developing and adult mammalian nervous system (Iwahara et al., 1997; Vernersson et al., 2006) and Alk knockout (Alk −/−) mice display behavioral phenotypes related to psychiatric disorders such as depression and anxiety. For instance, Alk −/− mice show less immobility in the tail suspension and Porsolt swim tests, increased novel object recognition, enhanced retention of spatial memory, and reduced anxiety in the elevated zero maze (Bilsland et al., 2008; Weiss et al., 2012). ALK also regulates behaviors related to alcohol abuse. Alk −/− mice are more sensitive to ethanol-induced sedation and consume more ethanol in a limited-access drinking test (Lasek et al., 2011), phenotypes consistent with ALK modulating acute responses to alcohol. In humans, polymorphisms in ALK are associated with acute ethanol-induced body sway and subjective measures of ethanol-induced euphoria (Lasek et al., 2011). Interestingly, a meta-analysis of genome-wide association studies has found that polymorphisms in human ALK are also associated with alcohol dependence (Wang et al., 2011). However, Alk −/− mice have not been investigated for their role in behaviors related to chronic ethanol exposure or dependence.
Here, we tested Alk −/− mice for dependence-induced ethanol consumption using the chronic intermittent ethanol-two bottle choice ethanol consumption (CIE-2BC) protocol. In this paradigm, animals exhibit increased alcohol intake in a limited-access ethanol/water choice after chronic passive exposure to ethanol vapor. This procedure has been successfully used in rats (Vendruscolo and Roberts, 2014) and C57BL/6 mice (Becker and Lopez, 2004; Finn et al., 2007; Griffin et al., 2009) to model the motivational aspects of ethanol dependence and excessive ethanol drinking associated with the addicted state. We found that Alk −/− mice drink more ethanol prior to CIE, but do not escalate ethanol consumption after CIE in comparison to Alk +/+ mice.
As the central nucleus of the amygdala (CeA) promotes excessive ethanol consumption, particularly in ethanol-dependent animals (Koob et al., 2014), and Alk −/− mice drink more ethanol than Alk +/+ mice and do not escalate drinking after CIE, we hypothesized that the physiology of CeA neurons might be altered in Alk −/− mice. Specifically, the CeA is critically involved in the development of the negative emotional state that drives dependence-induced alcohol consumption (Koob and Volkow, 2010), and basal CeA GABA neurotransmission is augmented by acute ethanol and in ethanol-dependent animals (Roberto et al., 2012; Roberto et al., 2004). Since GABA neurotransmission in the CeA appears to play a critical role in ethanol intake (Hyytia and Koob, 1995; Roberts et al., 1996), we examined GABA neurotransmission in ethanol-naïve, ethanol non-dependent, and ethanol-dependent Alk −/− and +/+ mice using whole-cell recordings from CeA slices. We observed increased basal GABA neurotransmission in ethanol-naïve Alk −/− mice. In addition, CIE treatment did not further augment GABA transmission in Alk −/− mice as it did in Alk +/+ mice. These alterations in GABA release are consistent with the ethanol consumption phenotypes observed in Alk −/− mice prior and subsequent to chronic ethanol exposure. Our results suggest that ALK regulates GABA release in the CeA and provide a novel link between ALK, GABA neurotransmission, and excessive ethanol consumption.
2. Material and methods
2.1 Experimental animals
Alk −/− mice have been described (Lasek et al., 2011) and were previously backcrossed to C57BL/6J mice for 4 generations. Heterozygote breeding was used to produce Alk +/+ and homozygous Alk knockout (−/−) littermates for this study. For the CIE-2BC experiments, 71 mice were used, with 67 completing the study. Mice were tested starting at 11–13 weeks of age in 3 cohorts. Cohort 1 (n = 35) consisted of 11 male Alk +/+, 11 male Alk −/−, 6 female Alk +/+, and 7 female Alk −/− mice. Cohort 2 (n = 19) consisted of 6 male Alk +/+, 7 male Alk −/−, 2 female Alk +/+, and 4 female Alk −/− mice. Finally, Cohort 3 consisted of 9 female Alk +/+ and 8 female Alk −/− mice. Two Alk −/− mice from each of cohorts 2 & 3 that were in the ethanol vapor exposure groups were removed from the study due to ill health. For the electrophysiology experiments, we used 13 male Alk +/+ and 11 male Alk −/− mice. Mice were housed in groups of 2–4 except during the daily 2 h 2BC testing of ethanol consumption. Mice had access to food and water ad libitum. Efforts were taken to minimize suffering and to reduce the number of animals used. Lights were on a 12 h light/dark cycle with lights off at 8 am. All experimental approaches were approved by The Scripps Research Institute (TSRI) Institutional Animal Care & Use Committee and performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 CIE-2BC procedure
For the first 15 days of testing (5 days per week for 3 weeks), 30 min before the lights went off (7:30 am), mice were individually housed for 2 h with access to 2 drinking tubes, one containing 15% ethanol and the other containing water. Ethanol and water consumption after the 2 h period were recorded. Following this baseline period of drinking, mice were divided by genotype and sex, based on equal ethanol and water consumption, into 2 balanced treatment groups that were exposed to intermittent ethanol vapor or air in identical chambers. Chambers consisted of standard plastic mouse-sized shoebox cages containing up to 4 mice per chamber (La Jolla Alcohol Research Inc., La Jolla, CA, USA). Ethanol vapor was created by dripping 95% ethanol (Pharmco-AAPER, Brookfield, CT, USA) into a 2 l Erlenmeyer vacuum flask kept at 50°C on a warming tray. Air was blown over the bottom of the flask at a rate of 11 l/min. Concentrations of ethanol vapor were adjusted by varying the rate at which the ethanol was pumped into the flask, which in turn, was based on the blood alcohol levels of the mice. Ethanol vapor was independently introduced into each sealed chamber through a stainless steel manifold. Air was pumped into identical chambers for controls. The ethanol vapor group was injected with 1.75 g/kg ethanol and 68.1 mg/kg pyrazole (an alcohol dehydrogenase inhibitor) and placed in the chambers to receive intermittent vapor for 4 days (16 h vapor on, 8 h off). The control group was injected with 68.1 mg/kg pyrazole in 0.9% saline and placed in chambers delivering air for the same periods as the ethanol vapor group and received 2BC testing at the same time as the vapor group. Following each 16 h bout of ethanol vapor exposure, mice were removed and on the 2nd and 4th day, tail blood was sampled for blood alcohol levels. Blood was collected in capillary tubes and emptied into 1.5 ml centrifuge tubes containing evaporated heparin and kept on ice. Samples were centrifuged and plasma was decanted into fresh 1.5 ml centrifuge tubes. The plasma was injected into an oxygen-rate alcohol analyzer (Analox Instruments, Lunenburg, MA, USA) for blood alcohol determination. Five pairs of ethanol standards (0.5 – 3.0 mg/ml) were analyzed prior to the samples. Target blood alcohol levels were 200–250 mg%. Following the fourth day of exposure, mice were allowed 72 h of undisturbed time in their home cages. The mice were then given 5 days of access to 2 bottles containing 15% ethanol or water for 2 h to measure ethanol drinking and preference. The 4 days of vapor or air exposure and 5 days of 2 bottle choice testing were repeated for a total of 4 rounds, designated CIE1-CIE4.
2.3 Electrophysiology
CeA slices were prepared as previously described (Roberto et al., 2010) from male Alk +/+ and Alk −/− mice (25–30g). We chose to do these experiments in male mice since we did not find any sex differences in ethanol consumption after CIE (see Results). Mice from behavioral testing (after 4 rounds of CIE-2BC) were used for electrophysiology and were either exposed to air and subjected to 2BC ethanol consumption, or were exposed to ethanol vapor and subjected to 2BC ethanol consumption. Additional groups of ethanol naïve mice were used to measure baseline GABA transmission. Mice were anesthetized with isoflurane (3%) and decapitated. Brains were rapidly removed and placed into oxygenated (95% O2 and 5% CO2; pH 7.3) ice-cold high-sucrose solution containing (in mM): sucrose 206; KCl 2.5; NaH2PO4 1.2; MgCl2 7; CaCl2 0.5; NaHCO3 26; glucose 5; HEPES 5. Transverse slices 300 µm thick were cut on a Vibratome (Leica VT1000S, Leica Microsystems, Buffalo Grove, IL, USA) and transferred into oxygenated artificial cerebrospinal fluid (ACSF) of the following composition (in mM): NaCl, 130; KCl, 3.5; NaH2PO4, 1.25; MgSO4·7H2O, 1.5; CaCl2, 2.0; NaHCO3, 24; glucose, 10. Slices were incubated for 30 min at 35–37°C and then kept at room temperature for the remainder of the experiment. Individual slices were transferred to a recording chamber mounted on the stage of an upright microscope (Olympus BX50WI, Tokyo, Japan) for each experiment. Recordings were performed in continuously oxygenated ACSF perfused at a rate of 2–3 ml/min, 1–8 h after slice preparation. Drugs were added to the ACSF from stock solutions to obtain known concentrations in the superfusate.
Recordings were performed in the whole cell configuration from 48 neurons in the medial subdivision of the CeA using voltage clamp mode. Neurons were visualized using infrared differential interference contrast (IR-DIC) optics and a CCD camera (EXi Aqua and ROLERA-XR, QImaging, Surrey, BC, Canada). Recordings were performed in gap-free acquisition mode (sample rate 10 kHz) and low-pass filtered (10kHz), using a Multiclamp 700B amplifier, Digidata 1440A and pClamp 10 software (Molecular Devices, Sunnyvale, CA, USA). Patch pipettes (impedance range 3–7MΩ) were pulled from borosilicate glass (Warner Instruments, Hamden, CT, USA) and filled with KCl internal solution (in mM): KCl 145; EGTA 5; MgCl2 5; HEPES 10; Na-ATP 2; Na-GTP 0.2. Pharmacologically-isolated GABAA receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in the presence of 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 µM, from Tocris, Ellisville, MO, USA), DL-2-amino-5-phosphonovalerate (AP-5, 30 µM, from Sigma) and CGP 55845A (1 µM, from Sigma) to block AMPA, NMDA and GABAB receptors. Cells were held at −60 mV. Experiments with a series resistance >15 MΩ or a >20% change in series resistance, as monitored with a 10 mV pulse, were excluded from the final data set. The sIPSC frequency, amplitude and kinetics were analyzed with Mini Analysis software (Synaptosoft Inc., Fort Lee, NJ, USA), with sIPSCs <5 pA excluded from the final data set and the average sIPSC measures derived from a minimum time interval of 3 minutes. Ethanol was purchased from Remet (La Miranda, CA, USA).
2.4 Data analysis and statistics
For the CIE-2BC experiments, a four-way ANOVA was performed for CIE episodes (CIE1-4) and treatments (air or vapor) with between subjects factors of genotype, sex and treatment, and the within-subjects factor of CIE episode. Data was further analyzed using two-way ANOVA for each genotype and CIE episode with the between subjects factors of treatment and sex using Prism 6.0g software for Mac (GraphPad, San Diego, CA, USA). For the electrophysiology experiments, MiniAnalysis 5.1 software was used for data analysis and Prism 5.0 software (GraphPad) was used for statistical analyses. The electrophysiology results were evaluated with cumulative probability analysis, and significance determined with the Kolmogorov-Smirnov nonparametric two-sample test. T-test analyses were used for individual means comparisons, and within-subject one-way repeated measures ANOVAs were used to compare sIPSCs within a group. When appropriate, the Bonferroni post hoc comparisons were used to assess significance between treatments. Data are presented as the mean ± SEM.
3. Results
3.1 Alk −/− mice do not escalate ethanol consumption after CIE
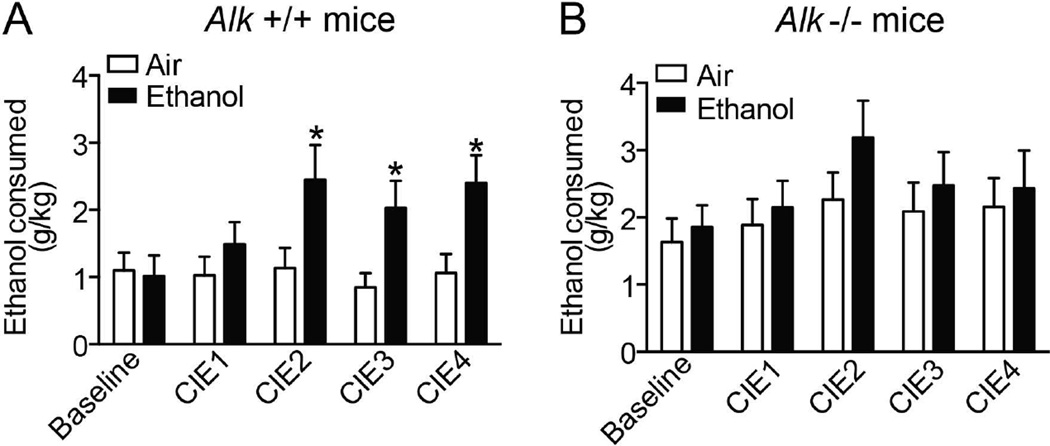
We previously discovered that Alk −/− mice consume more ethanol in a limited-access ethanol consumption test (Lasek et al., 2011). To determine if Alk −/− mice exhibit altered ethanol consumption after chronic ethanol exposure, we tested them for 2BC ethanol consumption after CIE. Prior to CIE (baseline ethanol consumption), Alk −/− mice consumed 63% more ethanol than Alk +/+ controls (two-way ANOVA, genotype effect: F1,63 = 4.7, p = 0.03), consistent with our previous results. Female mice drank slightly more than male mice; however, the effect of sex and the genotype by sex interaction were not significant. Fig. 1 shows the CIE-2BC data with the male and female data combined within each genotype. Analysis of the data across all 4 CIE exposures, using a four-way repeated measures ANOVA, indicated that there were significant effects of genotype (F1,59 = 4.9, p = 0.03), treatment (F1,59 = 4.8, p = 0.03) and CIE episode (F3,177 = 6.0, p = 0.0007). Again, although female mice of both genotypes drank slightly more ethanol than males, there were no significant sex effects. We next performed two-way ANOVAs for the 2BC drinking after each CIE episode for Alk +/+ and Alk −/− mice separately, with the between subject factors of treatment and sex. In Alk +/+ mice, there were no effects of sex even when examining these unitary tests. However, there were significant effects of treatment in CIE2 (116% increase in ethanol vapor-exposed compared to air control, F1,30 = 4.6, p = 0.04), CIE3 (140% increase in ethanol vapor-exposed compared to air control, F1,30 = 6.2, p = 0.02), and CIE4 (125% increase in ethanol vapor-exposed compared to air control, F1,30 = 6.5, p = 0.02), demonstrating that Alk +/+ mice escalated their ethanol consumption after the second round of ethanol vapor exposure (Fig. 1A). For Alk −/− mice, there was a significant effect of sex in CIE2 (F1,29 = 5.3, p = 0.03), with female mice drinking more than males. However, there were no significant effects of treatment or a treatment by sex interaction in any of the ethanol consumption tests, indicating that Alk −/− mice do not escalate ethanol drinking after repeated intermittent ethanol vapor exposure (Fig. 1B).
Fig. 1.
Alk −/− mice do not escalate ethanol consumption after CIE. Alk +/+ (A) and Alk −/− (B) mice were subjected to 4 rounds of CIE-2BC (CIE1-4) with air (as a control) or ethanol vapor exposure. Ethanol consumption was measured before the CIE protocol (baseline) and after each round of CIE and is expressed as g ethanol consumed per kg body weight. Data from male and female mice were combined within each genotype since no significant sex differences were observed. Alk +/+ mice escalated ethanol drinking after the second round of CIE, while Alk −/− mice did not. Data are expressed as mean ± SEM, *p < 0.05.
3.2 Increased basal GABA transmission in the CeA of Alk −/− mice
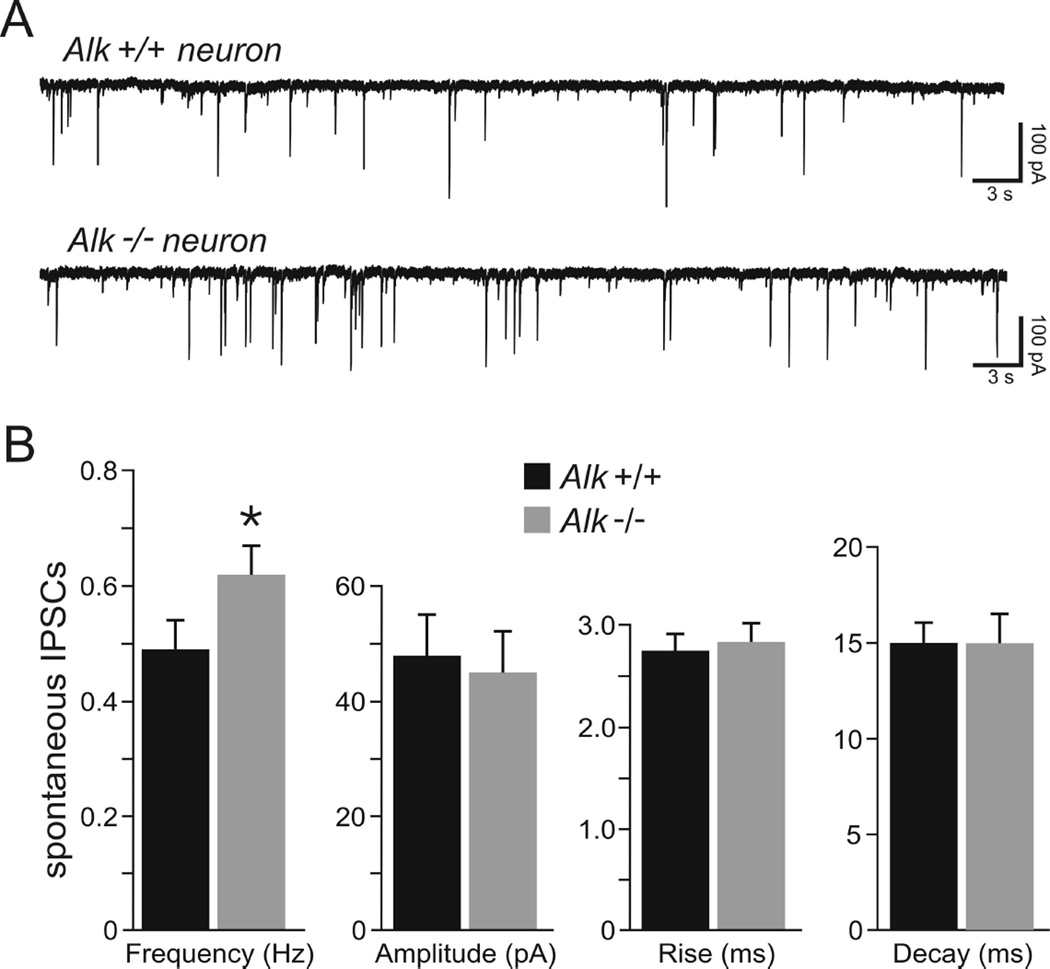
The CeA is implicated in excessive ethanol consumption, particularly in ethanol-dependent animals (Koob et al., 2014). Since Alk −/− mice drink more ethanol than Alk +/+ mice and do not escalate drinking after CIE, we hypothesized that the physiology of CeA neurons might be altered in Alk −/− mice. To test this, we recorded from medial CeA neurons using intracellular voltage-clamp in the whole-cell configuration and pharmacologically isolated GABAA-mediated spontaneous inhibitory postsynaptic currents (sIPSCs). Basal GABAergic activity was significantly increased from 0.49 ± 0.05 Hz in Alk +/+ CeA neurons (n = 8) to 0.62 ± 0.05 Hz in Alk −/− CeA neurons (n = 8), representing an overall increase of 27% (Fig. 2, p < 0.05). The average sIPSC amplitude and rise and decay times were not significantly different in neurons from Alk +/+ compared to Alk −/− mice (Fig. 2). The higher occurrence of spontaneous GABA activity in the CeA of Alk −/− mice indicates that Alk deletion leads to increased inhibitory transmission in the CeA. Since increased sIPSC frequency is indicative of a higher probability of GABA release (De Koninck and Mody, 1994), the increased frequency of sIPSCs in CeA neurons from Alk −/− mice suggests that the effect occurs at a presynaptic site to alter GABA release, rather than an alteration of GABAA receptor function.
Fig. 2.
Basal spontaneous GABA transmission is higher in neurons from Alk −/− mice. (A) Representative whole-cell current recordings of sIPSCs in CeA neurons obtained from an Alk +/+ (top trace) and an Alk −/− (bottom trace) mouse. A higher frequency of sIPSCs was observed in neurons from Alk −/− mice compared to neurons from Alk +/+ animals. (B) Mean sIPSC frequency in CeA neurons from Alk +/+ and Alk −/− mice, showing an increase in sIPSC frequency in Alk −/− mice. Mean sIPSC amplitude, rise time, and decay time in Alk +/+ and Alk −/− mice are also shown, showing no significant differences between Alk +/+ and Alk −/− mice in these measures. Data are expressed as mean ± SEM, *p < 0.05.
3.3 Enhancement of GABA transmission by acute ethanol is amplified in Alk −/− mice
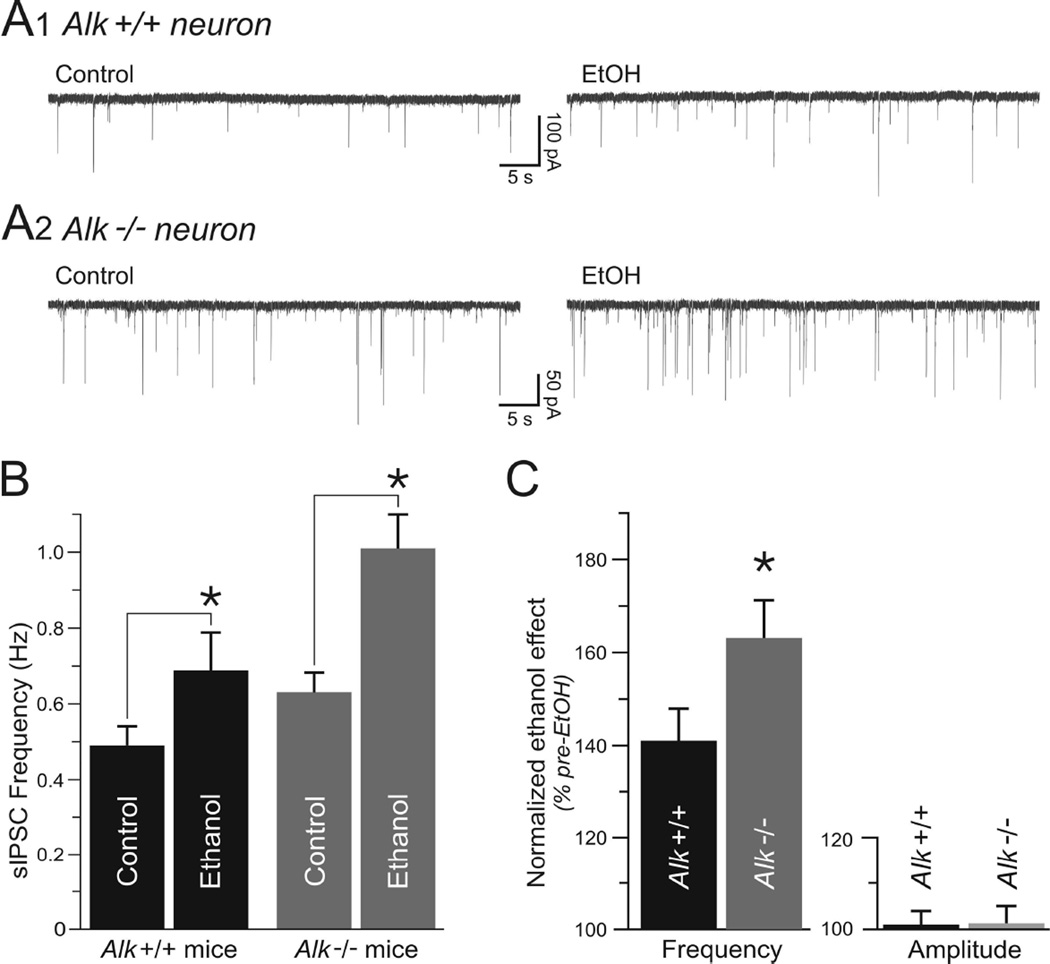
We next examined the effect of acute ethanol treatment on GABA transmission in Alk +/+ and Alk −/− mice by superfusing 44 mM ethanol onto CeA slices. This concentration of ethanol has been shown to elicit an optimum enhancement of GABAergic transmission in the CeA (Roberto et al., 2003). Application of ethanol for 8–10 minutes onto CeA neurons from Alk +/+ mice increased the sIPSC frequency from 0.49 ± 0.05 Hz to 0.69 ± 0.10 Hz (Fig. 3, n = 8). CeA neurons from Alk −/− mice also displayed an increase of sIPSCs from 0.62 ± 0.05 Hz in control condition to 1.01 ± 0.08 Hz upon superfusion of ethanol (n = 8). A two-way ANOVA demonstrated that there were significant main effects of ethanol, genotype, and a significant ethanol by genotype interaction (ethanol: F1, 14 = 58.51, p < 0.0001; genotype: F1, 14 = 5.49, p = 0.034; ethanol by genotype interaction: F1, 14 = 5.74, p = 0.031). Ethanol caused a significantly greater enhancement of sIPSC frequency in neurons from Alk −/− mice compared to neurons from Alk +/+ mice (Fig. 3C, p < 0.05, 63 ± 8% increase in Alk −/− vs. 41 ± 7% increase in Alk +/+ neurons). The sIPSC amplitude was not significantly affected by ethanol in CeA neurons from Alk +/+ (101 ± 3%; n = 8) or Alk −/− (101 ± 4%; n = 8) mice (Fig. 3C), indicative of a selective effect of ethanol on GABA release in both genotypes. In addition, sIPSC kinetics (rise and decay) were not significantly altered (data not shown). After washout of ethanol for 10–20 minutes, sIPSC frequency returned to pre-ethanol levels in neurons from both Alk +/+ and Alk −/− mice (0.48 ± 0.05 Hz for Alk +/+ and 0.67 ±0.06 Hz for Alk −/−; data not shown). Together, these data indicate that homozygous Alk deletion enhances GABA transmission in CeA neurons in response to acute ethanol to a greater extent than in neurons expressing normal levels of Alk.
Fig. 3.
Enhancement of GABA transmission by acute ethanol is amplified in Alk −/− mice. (A) Representative whole-cell current recordings of sIPSCs in CeA neurons from an Alk +/+ (top traces) and an Alk −/− (bottom traces) mouse in the absence of ethanol (control, left traces) and after exposure of slices to 44 mM ethanol (ethanol, right traces). Ethanol significantly increased sIPSC frequency. (B) Mean sIPSC frequency in CeA neurons from Alk +/+ and Alk −/− mice in the absence and presence of ethanol. (C) Normalized effect of ethanol on sIPSC frequency in Alk +/+ and Alk −/− mice, expressed as the percentage of pre-ethanol sIPSC frequency. The increase in sIPSC frequency by acute ethanol application was more pronounced in Alk −/− mice. Mean sIPSC amplitude in Alk +/+ and Alk −/− mice is also shown, showing no significant difference between genotypes. Data are expressed as mean ± SEM, *p < 0.05.
3.4 CIE augments basal GABA transmission in neurons from Alk +/+ but not Alk −/− mice
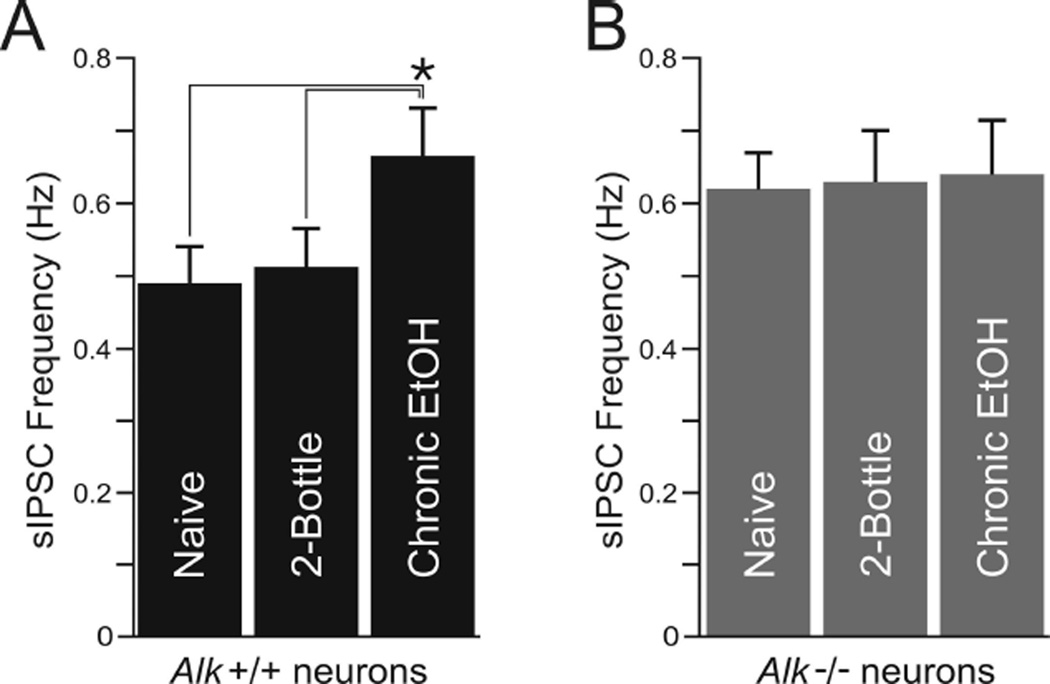
Since Alk −/− mice do not escalate ethanol drinking after CIE, we next tested for differences in GABA transmission after CIE in CeA neurons from Alk +/+ and Alk −/− mice. Alk +/+ ethanol-naïve mice (control) and mice that were exposed to air and underwent 2BC showed sIPSC frequencies that were not significantly different (0.49 ± 0.05 Hz and 0.51 ± 0.05 Hz respectively, n = 8; Fig. 4A), indicating that 2BC ethanol consumption did not effect basal GABA transmission. However, in Alk +/+ mice that were chronically exposed to ethanol vapor, the basal sIPSC frequency was 0.66 ± 0.06 Hz (n = 9; Fig. 4A), a 35% (p < 0.05) and 29% (p < 0.05) augmentation compared to ethanol-naïve and air-exposed mice, respectively. The sIPSC amplitudes were comparable in neurons from all three groups of Alk +/+ mice (control, 48 ± 7 pA, n = 8; air, 52 ± 6 pA, n = 8; ethanol vapor, 46 ± 5 pA, n = 9). In contrast to Alk +/+ mice, CIE treatment did not change basal GABAergic transmission in CeA neurons from Alk −/− mice (Fig. 4B). Note that the higher sIPSC frequency obtained in Alk +/+ neurons upon CIE treatment (Fig. 4A, right column) is comparable to the sIPSC frequency obtained in control Alk −/− neurons (Fig. 4B), suggesting that Alk deletion elicits an effect similar to CIE treatment. Finally, as in Alk +/+ mice, the sIPSC amplitude and kinetics remained unchanged after CIE in neurons from Alk −/− mice (data not shown).
Fig. 4.
CIE augments GABA transmission in neurons from Alk +/+ but not Alk −/− mice. Mean sIPSC frequency in CeA neurons from Alk +/+ (A) and Alk −/− (B) mice that were ethanol-naïve, underwent air exposure and 2BC (2-bottle), or CIE-2BC (chronic EtOH). Alk +/+ mice showed increased sIPSC frequency after CIE, whereas Alk −/− mice did not. Note that the sIPSC frequency in Alk −/− mice was higher than Alk +/+ mice under all conditions and compared to the sIPSC frequency in ethanol-vapor treated Alk +/+ mice. Data are expressed as the mean ± SEM. *p < 0.05.
3.5 CIE does not modify the effect of acute ethanol on GABA transmission
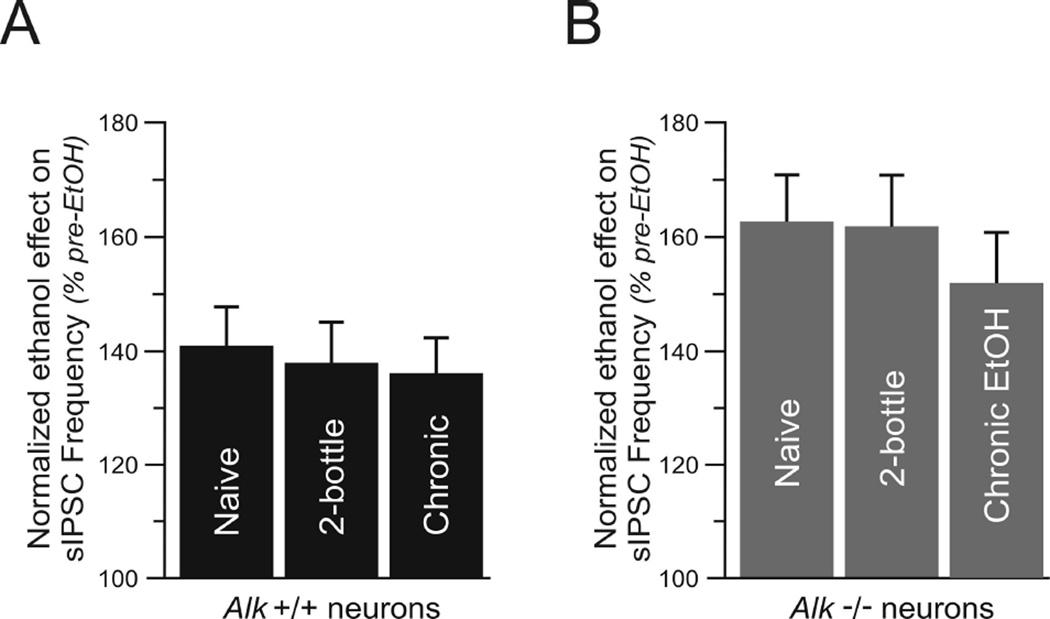
After assessing the influence of CIE on basal GABAergic transmission, we next determined if CIE would affect the response to acute ethanol in Alk −/− mice. In Alk +/+ mice, superfusion of 44 mM ethanol increased sIPSC frequency by 41 ± 7% in neurons from ethanol-naïve mice (n = 8), 38% ± 7 in neurons from mice exposed to air-2BC (n = 7), and 36 ± 6% in neurons from mice exposed to CIE-2BC (n = 8) (Fig. 5A, no significant differences). After washout of ethanol for 10–20 minutes, sIPSC frequencies in all groups returned to control levels (data not shown). In addition, sIPSC amplitudes were not affected by ethanol in all groups (data not shown). We observed a similar pattern in neurons obtained from Alk −/− mice. Although application of acute ethanol elicited a larger effect on GABAergic transmission overall in CeA neurons from Alk −/− compared to Alk +/+ mice (as in Fig. 3), CIE did not alter the extent of the increase in sIPSC frequency by acute ethanol. Superfusion of 44 mM ethanol onto CeA slices from Alk −/− mice augmented sIPSC frequency by 63 ± 8% in ethanol-naïve neurons (n = 8), 62 ± 9% in neurons from mice exposed to air (n = 6), and 52 ± 9% in neurons from mice exposed to ethanol vapor (Fig. 5B, n = 8). After ethanol washout, sIPSC frequencies returned to control levels in all groups and the amplitude and kinetics of sIPSCs were not affected by ethanol (data not shown). These results indicate that the effect of acute ethanol remains unchanged after CIE and that chronic ethanol exposure does not induce tolerance in neurons from Alk +/+ or Alk −/− mice.
Fig. 5.
CIE does not modify the effect of acute ethanol on GABA transmission. Normalized sIPSC frequency in neurons from Alk +/+ (A) and Alk −/− (B) mice. CeA slices were treated with 44 mM ethanol. Data shows the normalized ethanol effect, expressed as the percentage increase in sIPSC frequency prior to ethanol treatment. Comparisons are made between neurons from ethanol-naïve mice, mice that underwent air exposure and 2BC (2-bottle), and mice that received CIE-2BC (chronic EtOH). CIE did not alter the ability of acute ethanol to increase sIPSC frequency in CeA neurons from Alk +/+ and Alk −/− mice. Note that the acute effect of ethanol was greater in neurons from Alk −/− compared to Alk +/+ mice. Data are expressed as the mean ± SEM.
4. Discussion
In this study we provide evidence that ALK regulates alcohol consumption and GABA neurotransmission in the CeA. To our knowledge, this is the first study that shows that ALK may regulate dependence-induced drinking and GABA neurotransmission. Our key findings are that Alk −/− mice initially consume more ethanol and have increased basal and ethanol-stimulated GABA release in the CeA when compared to Alk +/+ mice. However, after chronic ethanol exposure, Alk +/+ mice escalate their ethanol consumption, whereas Alk −/− mice do not. Moreover, basal GABA release in Alk −/− mice is not enhanced by CIE as it is in Alk +/+ mice. Thus, it appears that eliminating ALK creates a “dependence-like” phenotype, such that the knockout mice are predisposed to consume more ethanol as a result of increased GABA neurotransmission in the CeA.
We previously found that Alk −/− mice consume more ethanol in a binge-like, limited-access intermittent drinking procedure in which mice were given a single bottle of 20% ethanol for 4 h per day, 3 days per week (Lasek et al., 2011). Here, we reproduced the drinking phenotype prior to chronic ethanol exposure (baseline drinking) in a 2-hour, 2BC procedure in which mice were given a choice between water and 15% ethanol for 5 consecutive days per week. The increased ethanol consumption observed in Alk −/− mice may be due to increased basal GABA neurotransmission, since GABA signaling in the CeA clearly affects ethanol intake (Hyytia and Koob, 1995; Roberts et al., 1996). CeA neurons in Alk −/− mice also respond more robustly to acute ethanol stimulation of GABA release, and this might contribute to the increased drinking behavior in these mice.
It should be noted that increased ethanol consumption exhibited by Alk −/− mice prior to ethanol exposure might be due to developmental compensation, since these mice lack Alk during embryogenesis. We recently treated adult mice with small-molecule ALK inhibitors and found decreased ethanol consumption in a limited access binge-like drinking test (Dutton et al., 2016). Since we observed the opposite phenotype in Alk −/− mice, we suspect that there is compensation occurring. Alk −/− mice have increased striatal levels of phosphorylated mitogen-activated protein kinase kinase (MEK, or MAP2K), a signaling molecule in the Ras/extracellular signal-regulated kinase (ERK) pathway (Lasek et al., 2011), although it is well established that ALK activates the Ras/ERK pathway (Hallberg and Palmer, 2013). In fact, we have found that acute ethanol treatment increases ALK-dependent ERK phosphorylation in a neuroblastoma cell line, and that treatment of C57BL/6J mice with an ALK inhibitor reduces ethanol-stimulated ERK phosphorylation in the CeA (He et al., 2015). Alteration of the Ras/ERK signaling pathway in Alk −/− mice could account for the increased ethanol consumption observed in these mice. Ras/ERK signaling clearly plays a role in alcohol consumption, although the relationship is complicated by the fact that activation and inhibition of this pathway can have the same phenotype (Agoglia et al., 2015; Ben Hamida et al., 2012; Faccidomo et al., 2009; Mulligan et al., 2006; Stacey et al., 2012). This is similar to the learning and memory literature, in which deficits or increases in Ras/ERK signaling are detrimental to learning and memory (Fasano and Brambilla, 2011).
In addition to our observation that ethanol-naïve Alk −/− mice consume more ethanol than control mice, we found that Alk −/− mice do not escalate ethanol consumption after CIE, in contrast to Alk +/+ mice. There are two possible explanations for this effect. The most parsimonious explanation is that the high levels of drinking exhibited by Alk −/− mice are already at a maximum, such that it is difficult to see a further increase in ethanol consumption during the two-hour drinking test (i.e. a ceiling effect). The other possibility is that ALK plays an active role in promoting dependence-induced drinking. We tested Alk mRNA expression levels by quantitative real-time PCR in a small group of wild-type mice (n = 3) exposed to CIE and found that Alk mRNA increases by approximately 47% in the CeA when compared to control mice treated with air (p = 0.057, data not shown). This result suggests that Alk expression is regulated by ethanol and may contribute to the escalation of ethanol consumption during the development of ethanol dependence. The fact that we do not see an escalation in ethanol consumption after CIE in Alk −/− mice is consistent with increased Alk expression after CIE promoting excessive drinking. Future studies will address this possibility by treating mice with small-molecule ALK inhibitors or by using RNA interference to determine if this can reduce escalation of alcohol consumption and GABA neurotransmission induced by chronic ethanol exposure. This experiment would overcome problems with possible developmental compensation in Alk −/− mice.
Interestingly, two other members of the Ras/ERK signaling pathway, neurofibromin (encoded by the Nf1 gene) and K-Ras, have been shown to modulate the escalation of ethanol consumption after chronic ethanol exposure. Heterozygous Nf1 (+/−) and homozygous K-Ras (−/−) mice do not escalate ethanol consumption after CIE (Repunte-Canonigo et al., 2015; Repunte-Canonigo et al., 2010), similar to what we observed in Alk −/− mice. Neurofibromin is a Ras GTPase activating protein and a negative regulator of Ras/ERK signaling. Nf1 +/− mice have higher levels of phosphorylated ERK in the brain and basal GABA release (Cui et al., 2008; Repunte-Canonigo et al., 2015), also similar to what we found in Alk −/− mice. In Drosophila, genetic or pharmacological inhibition of Alk rescues the increased ERK activation and associative learning deficits observed in Nf1 mutants (Gouzi et al., 2011), suggesting that ALK and NF1 function in the same intracellular signaling pathway. These results, combined with the human genetic association studies that implicate the NF1 and ALK genes in alcohol dependence (Repunte-Canonigo et al., 2015; Wang et al., 2011), support the possibility that ALK may play an active role in dependence-induced drinking through regulation of the Ras/ERK signaling pathway.
In addition to the ethanol consumption phenotypes, we observed increased basal and acute ethanol-stimulated GABA release in the CeA of Alk −/− mice, indicating that ALK regulates GABA release. How might this occur? Again, one possibility is through the Ras/ERK pathway. ERK phosphorylates synapsin I, a critical component for vesicular neurotransmitter release (Jovanovic et al., 1996), and neurofibromin regulates GABA release in inhibitory neurons in an ERK-dependent manner in the hippocampus (Cui et al., 2008). We hypothesize that ALK functions in GABA neurons in the CeA to regulate GABA release through phosphorylation of synapsin I via the Ras/ERK pathway. Future studies will test this hypothesis using ALK inhibitors and examine in detail the signaling pathways downstream of ALK that regulate GABA neurotransmission.
CIE has been shown to increase GABA neurotransmission in rats and mice (Repunte-Canonigo et al., 2015; Repunte-Canonigo et al., 2010; Roberto et al., 2004). Although ethanol-naïve Alk −/− mice display increased GABA release, GABA neurotransmission after CIE is not augmented in Alk −/− mice as it is in Alk +/+ mice. This phenotype may be the result of a ceiling effect, which we cannot rule out at this point. However, Alk −/− mice still show acute ethanol-stimulation of GABA release after CIE, similar to Alk +/+ mice, demonstrating that GABA release can still be enhanced despite the high basal levels observed in Alk −/− mice and perhaps arguing against a ceiling effect. To more conclusively demonstrate a causal relationship between ALK and the enhancement of GABA neurotransmission after CIE, further experiments are necessary using ALK inhibitors.
Together, our data implicate ALK in excessive drinking, potentially contributing to the transition to dependence-induced drinking. ALK expression or kinase activity might be modified by chronic ethanol exposure, leading to changes in signaling cascades downstream of ALK that regulate the development of altered GABA neurotransmission observed after CIE. ALK-dependent processes are likely not the only processes regulating GABA release and subsequent increases in drinking in mice, but may play a modulatory role. Increased GABA neurotransmission in the CeA after CIE would promote the negative affective state that drives cycles of excessive alcohol drinking. Future studies will examine in more detail the molecular mechanisms through which ALK signals to regulate GABA release and ethanol consumption.
Mice deficient in the Alk gene consume more ethanol than controls.
Alk deficient mice exhibit enhanced basal GABA release in the amygdala.
Ethanol-potentiated GABA release is higher in Alk deficient mice.
Alk deficient mice do not escalate ethanol drinking after chronic ethanol exposure.
GABA release does not increase after chronic ethanol exposure in Alk deficient mice.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (Integrative Neuroscience Initiative on Alcoholism, AA020912 and AA016654 to A.W.L., AA020893 to A.J.R., AA013498 to M.R., and AA013517 to P.S.). This is manuscript #29266 from The Scripps Research Institute.
Abbreviations
- ALK
anaplastic lymphoma kinase
- CeA
central nucleus of the amygdala
- CIE
chronic intermittent ethanol
- ERK
extracellular signal-regulated kinase
- GABA
gamma aminobutyric acid
- sIPSCs
spontaneous inhibitory post-synaptic currents
- 2BC
two bottle choice
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- Agoglia AE, Sharko AC, Psilos KE, Holstein SE, Reid GT, Hodge CW. Alcohol alters the activation of ERK1/2, a functional regulator of binge alcohol drinking in adult C57BL/6J mice. Alcohol Clin Exp Res. 2015;39:463–475. doi: 10.1111/acer.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Ben Hamida S, Neasta J, Lasek AW, Kharazia V, Zou M, Carnicella S, Janak PH, Ron D. The small G protein H-Ras in the mesolimbic system is a molecular gateway to alcohol-seeking and excessive drinking behaviors. J Neurosci. 2012;32:15849–15858. doi: 10.1523/JNEUROSCI.2846-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland JG, Wheeldon A, Mead A, Znamenskiy P, Almond S, Waters KA, Thakur M, Beaumont V, Bonnert TP, Heavens R, Whiting P, McAllister G, Munoz-Sanjuan I. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2008;33:685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Dutton JW, 3rd, Chen H, You C, Brodie MS, Lasek AW. Anaplastic lymphoma kinase regulates binge-like drinking and dopamine receptor sensitivity in the ventral tegmental area. Addict Biol. 2016 Jan 11; doi: 10.1111/adb.12358. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Besheer J, Stanford PC, Hodge CW. Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl) 2009;204:135–147. doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano S, Brambilla R. Ras-ERK Signaling in Behavior: Old Questions and New Perspectives. Front Behav Neurosci. 2011;5:79. doi: 10.3389/fnbeh.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Gouzi JY, Moressis A, Walker JA, Apostolopoulou AA, Palmer RH, Bernards A, Skoulakis EM. The receptor tyrosine kinase Alk controls neurofibromin functions in Drosophila growth and learning. PLoS Genet. 2011;7:e1002281. doi: 10.1371/journal.pgen.1002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13:685–700. doi: 10.1038/nrc3580. [DOI] [PubMed] [Google Scholar]
- He D, Chen H, Muramatsu H, Lasek AW. Ethanol activates midkine and anaplastic lymphoma kinase signaling in neuroblastoma cells and in the brain. J Neurochem. 2015;135:508–521. doi: 10.1111/jnc.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci U S A. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, Jr, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek AW, Lim J, Kliethermes CL, Berger KH, Joslyn G, Brush G, Xue L, Robertson M, Moore MS, Vranizan K, Morris SW, Schuckit MA, White RL, Heberlein U. An evolutionary conserved role for anaplastic lymphoma kinase in behavioral responses to ethanol. PLoS One. 2011;6:e22636. doi: 10.1371/journal.pone.0022636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Herman MA, Kawamura T, Kranzler HR, Sherva R, Gelernter J, Farrer LA, Roberto M, Sanna PP. Nf1 regulates alcohol dependence-associated excessive drinking and gamma-aminobutyric acid release in the central amygdala in mice and is associated with alcohol dependence in humans. Biol Psychiatry. 2015;77:870–879. doi: 10.1016/j.biopsych.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, van der Stap LD, Chen J, Sabino V, Wagner U, Zorrilla EP, Schumann G, Roberts AJ, Sanna PP. Genome-wide gene expression analysis identifies K-ras as a regulator of alcohol intake. Brain Res. 2010;1339:1–10. doi: 10.1016/j.brainres.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010;35:1962–1972. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR. The central amygdala and alcohol: role of gamma-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med. 2012;2:a012195. doi: 10.1101/cshperspect.a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Stacey D, Bilbao A, Maroteaux M, Jia T, Easton AC, Longueville S, Nymberg C, Banaschewski T, Barker GJ, Buchel C, Carvalho F, Conrod PJ, Desrivieres S, Fauth-Buhler M, Fernandez-Medarde A, Flor H, Gallinat J, Garavan H, Bokde AL, Heinz A, Ittermann B, Lathrop M, Lawrence C, Loth E, Lourdusamy A, Mann KF, Martinot JL, Nees F, Palkovits M, Paus T, Pausova Z, Rietschel M, Ruggeri B, Santos E, Smolka MN, Staehlin O, Jarvelin MR, Elliott P, Sommer WH, Mameli M, Muller CP, Spanagel R, Girault JA, Schumann G, Consortium I. RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release. Proc Natl Acad Sci U S A. 2012;109:21128–21133. doi: 10.1073/pnas.1211844110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernersson E, Khoo NK, Henriksson ML, Roos G, Palmer RH, Hallberg B. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr Patterns. 2006;6:448–461. doi: 10.1016/j.modgep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Wang KS, Liu X, Zhang Q, Pan Y, Aragam N, Zeng M. A meta-analysis of two genome-wide association studies identifies 3 new loci for alcohol dependence. J Psychiatr Res. 2011;45:1419–1425. doi: 10.1016/j.jpsychires.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Weiss JB, Xue C, Benice T, Xue L, Morris SW, Raber J. Anaplastic lymphoma kinase and leukocyte tyrosine kinase: functions and genetic interactions in learning, memory and adult neurogenesis. Pharmacol Biochem Behav. 2012;100:566–574. doi: 10.1016/j.pbb.2011.10.024. [DOI] [PubMed] [Google Scholar]