Abstract
Objective
The Look AHEAD trial was a multi-center, randomized controlled trial, to determine whether weight loss reduces cardiovascular morbidity and mortality in overweight individuals with type 2 diabetes. The objective of this study was to evaluate the incidence of severe hypoglycemia in patients enrolled in Look AHEAD.
Research Design and Methods
5,145 subjects were randomized to diabetes support and education (DSE) or intensive lifestyle intervention (ILI). Instances of severe hypoglycemia were recorded. Regression analysis was used to compare the development of severe hypoglycemia between groups.
Results
Over the entire study, the severe hypoglycemia rate was not different between ILI and DSE groups (0.49 ILI, 0.51 DSE/100 person years, Rate Ratio=1.12, p=0.41), but was greater in ILI during year 1 (p=0.008 for year by intervention interaction). During follow-up, severe hypoglycemia risk was higher with insulin, sulfonylurea or glitinide use at baseline (p<0.0001). The intervention effect differed by post-randomization insulin use (ILI to DSE HR=1.45 in insulin users versus HR=0.71 in non-users, p=0.009). Insulin use reduced by 3% in ILI in year 1. Compared to DSE, ILI participants in the lower 50% of weight loss in year 1 had similar percent insulin use and incident hypoglycemia, but ILI participants in the upper 50% of weight loss had lower percent insulin use and incident hypoglycemia.
Conclusions
Reduction in insulin is necessary during intensive weight loss to avoid episodes of hypoglycemia. Although limited by self-reported evaluation of hypoglycemia, greater weight loss in ILI during year 1 was associated with reduced insulin use and lower rates of hypoglycemia later in the trial.
Keywords: Diabetes, Hypoglycemia, Weight regulation and obesity, Insulin therapy, Diabetes education
Introduction
Eight percent of hospital admissions due to adverse drug events have been reported to involve hypoglycemia, generally severe (1). Hypoglycemia is more prevalent in individuals over 65 years of age, and hospital admissions for Medicare Beneficiaries from 1999 to 2011 increased 12% for hypoglycemia while declining 39% for hyperglycemia (2). Severe hypoglycemia impairs glucose counter-regulation, and is associated with morbidity and increased mortality (3; 4). Type 2 diabetes is a chronic disease associated with progressive reduction in insulin secretion, with rates of severe hypoglycemia and people taking insulin or insulin secretagogues being greater after 15 years of the disease (5; 6). A population-based 1-year study from Scotland reported severe hypoglycemia in 7.3 and 0.8/100 patient-years in individuals with type-2 diabetes treated with insulin and sulfonylureas, respectively (7).
Lifestyle change, calorie reduction and increased physical activity, are recommended treatments for overweight and obese people with type 2 diabetes (8). The acute reduction in calorie intake or increased physical activity in individuals with diabetes taking insulin or insulin secretogogues can cause episodes of hypoglycemia, but the risk is not well-defined (9; 10). The Look AHEAD trial (Action for Health in Diabetes, NCT00017953) trial enrolled 5,145 overweight or obese volunteers with type-2 diabetes in 16 study centers. Volunteers were randomly assigned to an intensive lifestyle intervention (ILI) or diabetes support and education (DSE) to determine whether weight loss reduces cardiovascular events. The ILI promoted weight loss through diet and physical activity; DSE provided a control (11). The Look AHEAD trial primary endpoint was composite death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina pectoris (11). With no significant difference in cardiovascular events between the ILI and DSE groups, the trial intervention was stopped for futility at an average follow-up of 9.6 years. Hypoglycemia rates in Look AHEAD have not yet been reported, but hypoglycemia was a concern in the ILI group due to calorie restriction and increased physical activity. Risk for hypoglycemia might be increased if insulin or insulin secretogogues were not reduced sufficiently; conversely, risk might be reduced if weight loss reduced the need for diabetes medications associated with hypoglycemia much as weight loss slowed the progression to diabetes in the Diabetes Prevention Program (DPP). (12)
This report presents incidence rates and the relative risk of severe hypoglycemia in the ILI and DSE groups of the Look AHEAD study to inform the risks and benefits of intensive lifestyle treatment in overweight/obese individuals with type 2 diabetes.
Research Design and Methods
Participants
The Look AHEAD trial design, methods and study cohort baseline characteristics were previously described (13; 14). Eligible participants were 45–76 years of age and had a body mass index (BMI) >25 kg/m2 or >27 kg/m2, if taking insulin. Informed consent approved by participating clinics’ Institutional Review Boards (IRB) was obtained.
Interventions
Participants were randomized to diabetes support and education (DSE) or intensive lifestyle intervention (ILI) consisting of diet, behavior modification with goals for moderate physical activity of 175 minutes per week and ≥7% initial body weight loss (15; 16). In the initial year, the ILI group met frequently with the clinic intervention teams, and meal replacement products were prescribed. After the first 6 months, those not at goal weight could use orlistat and/or intensified behavior strategies until year 4. All participants received diabetes education before randomization on how to avoid hypoglycemia. The DSE group received 3 additional education sessions during the first year.
Clinical Care
All diabetes and medical care was provided by the participants’ own clinicians, except the temporary reduction in glucose lowering therapy by Look AHEAD staff using a pre-specified algorithm (Figure B.1) during intensive weight loss. Adjustments in insulin, sulfonylureas or glitinide doses were based on twice daily self-monitored blood sugars to reduce the risk of severe hypoglycemia. Healthcare providers received results of the study assessments including glucose and HbA1c values. Blood glucose lowering medications could be readjusted after the period of intensive calorie restriction by study staff (17).
Ascertainment of Hypoglycemia
Look AHEAD hypoglycemic events were collected with the following schedules and methods: (1) At every 6-month outcomes assessment, participants reported hospitalizations, emergency room visits or outpatient clinic visits due to hypoglycemic events; (2) Annual self-report questionnaires asking about hypoglycemic episodes in the past 3 months and whether they required hospitalization, an emergency room visit or assistance by another party; and (3) Serious adverse event (SAE) reports completed by study staff when made aware of a serious hypoglycemic event through the reports above or an ad hoc report by a participant. A severe hypoglycemia SAE was defined as “loss of consciousness, seizure, or a glucose <70 mg/dL that prevented self-treatment, and required assistance of another person”. This report focuses on SAE events; however, since the ILI group had more frequent contact with study staff which could have biased SAE ascertainment, a sensitivity analysis was conducted using the study outcomes data ascertained by self-report every 6 months, in both the ILI and DSE groups. A drawback of using these self-reported events as the outcome variable in a sensitivity analysis was a lack of specific criteria for the severity of the events and the number of events was not collected; thus, severe hypoglycemia by SAE report was selected as the primary outcome variable. This report focuses on follow up for hypoglycemia through the termination of the intervention on September 14, 2012.
Statistical Methods
Tabulations of those having no events, one event, two events, and three or more events were created according to intervention group. To examine the influence of ILI on development of severe hypoglycemia compared to the DSE arm, the rates per 100 person years (100 PY) of the occurrence of the initial reports of severe hypoglycemia were compared between the ILI and DSE groups. Overall rates that allowed for multiple events per participant were also obtained and compared between ILI and DSE groups using Poisson regression with log-transformed follow-up time as an offset and allowing for an over-dispersion parameter when analyzing overall rates. GEE Poisson regression was used to explore rate differences by year of follow-up and heterogeneity of treatment effects by follow-up year was determined by testing for the interaction between the year effect and the treatment effect.
Analysis of time to first instance of an SAE of severe hypoglycemia was performed using Cox proportional hazards regression. Participants with no hypoglycemia SAEs were censored at the time of their last study outcomes form, last adverse events form if applicable, or date of death, whichever came last. Similar to the approach used to analyze the cardiovascular primary outcome in the Look AHEAD trial, the initial model only included clinical site to stratify the underlying hazard since randomization was stratified by site. Subgroup analyses focused on prior CVD, gender, race/ethnicity, pre-randomization classes of diabetes medication, duration of diabetes, age, and history of retinopathy, nephropathy, and neuropathy. Ascertainment of insulin use at baseline primarily relied on whether any insulin was brought to the randomization visit (96% of participants) and, in the absence of this information (4% of participants), used self-report of insulin use. For participants who did not bring their medication to the visit, other classes of diabetes medication could not be ascertained and are missing in the diabetes severity variable, which incorporated the use of medications. Tests of homogeneity of intervention effects among subgroups were performed by entering the main effect for the subgroup and the interaction term for the subgroup by intervention into the base model containing effects for clinic and intervention group.
To examine potential baseline correlates of incident severe hypoglycemia across both intervention groups pooled, baseline variables including age, gender, pre-randomization diabetes treatment, duration of diabetes, retinopathy, nephropathy, neuropathy, race/ethnicity and history of CVD were entered into an initial Cox proportional hazards regression model. A second model added a factor representing the intervention group and used clinic as a stratifying factor for the underlying hazard, in addition to the terms contained in the initial model. Finally, to explore the effect of post-randomization concurrent insulin use on incident severe hypoglycemia, insulin use was entered into this second model as a time-dependent covariate. We further explored the interaction between the intervention group and concurrent insulin use, thus directly addressing the question of whether the effect of the intervention differed depending on the use of insulin.
Results
At baseline, the ILI and DSE groups were well matched (17). The ILI group lost more weight (8.6% vs. 0.7% at 1 year and 6.0% vs. 3.5% at end of study), had greater improvements in fitness and cardiovascular risk factors other than low-density-cholesterol, and had lower HbA1c level (11). A comparison of the incident rates of severe hypoglycemia per 100 PY of follow-up revealed no significant difference between the ILI and the DSE groups (see Table 1) with rates per 100 PY of follow-up of 0.49 and 0.51 for ILI and DSE, respectively (Incidence Density Ratio=0.96; p=0.71). When allowing multiple events per participant, the event rate per 100 PY was 0.67 in the ILI group and 0.60 in the DSE group (Rate Ratio= 1.12; p=0.41). The sensitivity analyses performed using self-reported hypoglycemia from the study outcomes form provided an increased number of events. The incidence rate per 100 PY of hypoglycemic events using the study outcomes data was 2.76 in ILI vs. 3.12 in DSE (Incidence Density Ratio=0.88; p=0.02), with corresponding event rates of 3.93 vs. 4.68 per 100 PY in the ILI and DSE groups, respectively (Rate Ratio=0.84; p<0.0001) when allowing for multiple events per participant.
Table 1.
Comparison of the severe hypoglycemia rates per 100 person years in the ILI and DSE groups using SAE data over an average 9.6 years of follow-up.
| Hypoglycemic events | DSE | ILI | P-Value for Comparison | ||
|---|---|---|---|---|---|
| Frequency N (%) | Events per 100 Person Years | Frequency N (%) | Events per 100 Person Years | ||
| None | 2459(95.5) | Incidence=0.51 Overall=0.60 |
2458 (95.6) | Incidence=0.49 Overall=0.67 |
0.7051 0.4038 |
| One | 101 (3.9) | 86 (3.4) | |||
| Two | 9 (0.4) | 17 (0.7) | |||
| Three or more | 6 (0.2) | 9 (0.4) | |||
| Total with 1+ event | 116 (4.5) | 112 (4.4) | |||
Comparison of the hazard ratios of severe hypoglycemia using the SAE report data for the subgroups of interest showed no significant differences in ILI compared to the DSE. (See Figure 1). Since interaction tests often have low statistical power to detect small yet meaningful effects, two subgroup comparisons of potential interest for future study in other samples are those based on diabetes treatment (p=0.12 for interaction) and a history of prior CVD (p=0.11 for interaction). The trend toward a difference in the intervention effect among the CVD subgroups may be explained by a higher rate of post-randomization insulin use in ILI subjects with prior cardiovascular disease (28% vs. 18%; p<0.0001).
Figure 1.
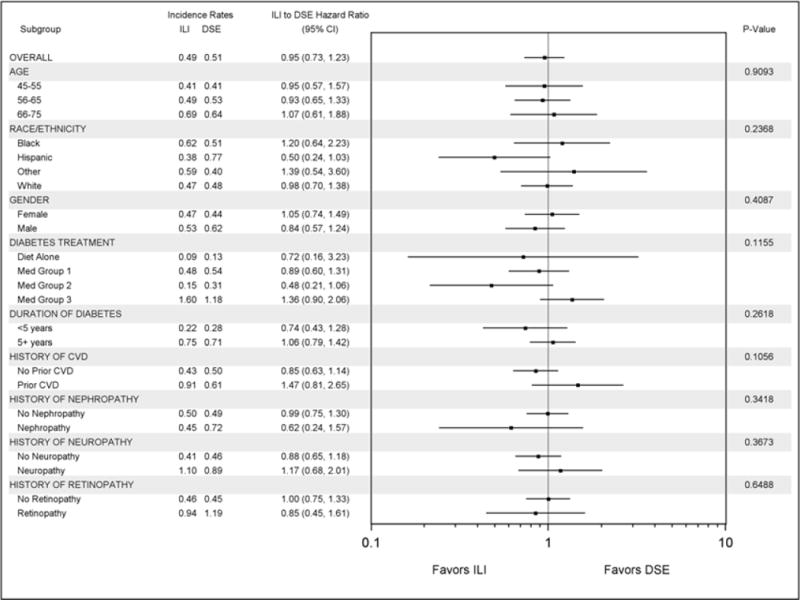
Forest plot comparing ILI and DSE groups on rates of incident severe hypoglycemia across subgroups of interest in the Look AHEAD trial. Incidence rates are expressed per 100 participant years of follow-up. Med Group 1 = Diabetes treated with medications other than insulin, sulfonylureas or glitinides; Med Group 2 = Diabetes treated with sulfonylureas or glitinides with or without other medications; Med Group 3 = Treated with insulin, with or without other medications.
Given the lack of an interaction effect between the intervention groups and subgroups of interest (see Figure 1), a Cox model for incident severe hypoglycemia was fit using both intervention groups (see Table 2). A single model is presented incorporating stratification of the hazard by clinic and the main effects of variables of interest as well as the intervention group. Descriptive statistics on demographics and medication use for the Look AHEAD cohort is contained in Table B.1. Compared to the diet only group, the incidence of severe hypoglycemia was significantly greater in those treated with sulfonylureas or glitinides, and those treated with insulin with or without other medications. The incidence of severe hypoglycemia was significantly greater for those with longer duration of diabetes and a history of neuropathy. To this model, we added a time-dependent indicator of post-randomization insulin use and the insulin by intervention interaction. The effect of the intervention (ILI vs. DSE) on severe hypoglycemia varied depending on whether or not the participant was on insulin (HR= 1.45, 95% CI 1.02 to 2.07 when on insulin; HR=0.72, 95% CI 0.48 to 1.06 when not on insulin; p=0.009 for interaction) (data not shown).
Table 2.
Multivariable model of incident severe hypoglycemia showing the main effects of treatment assignment and other variables of interest in the Look AHEAD trial.
| Characteristic | Subgroup | HR (95% CI) | P-value |
|---|---|---|---|
| Treatment assignment | ILI | 0.986 (0.759, 1.282) | 0.916 |
| DSE | 1.00 | ||
| Age | 45–55 | 0.646 (0.435, 0.961) | 0.094 |
| 56–65 | 0.758 (0.538, 1.068) | ||
| 66–75 | 1.00 | ||
| Race/Ethnicity | Black | 1.049 (0.708, 1.555) | 0.696 |
| Hispanic | 1.352 (0.743, 2.461) | ||
| Other | 1.301 (0.682, 2.483) | ||
| White | 1.00 | ||
| Gender | Male | 1.091 (0.826, 1.440) | 0.540 |
| Female | 1.00 | ||
| Diabetes Treatment | Diet only | 1.00 | <0.001 |
| Diabetes controlled on medications other than insulin, sulfonylureas or glitinides | 2.00 (0.869, 4.614) | ||
| Diabetes controlled on sulfonylureas or glitinides with or without other medications | 3.686 (1.688, 8.051) | ||
| Insulin with or without other medications | 8.021 (3.583, 17.954) | ||
| Duration of Diabetes | <5 years | 1.00 | 0.017 |
| 5+ years | 1.521 (1.079, 2.146) | ||
| Prior CVD | Yes | 1.230 (0.872, 1.736) | 0.239 |
| No | 1.00 | ||
| Nephropathy | Yes | 1.154 (0.707, 1.884) | 0.567 |
| No | 1.00 | ||
| Neuropathy | Yes | 1.570 (1.128, 2.184) | 0.008 |
| No | 1.00 | ||
| Retinopathy | Yes | 1.371 (0.951, 1.977) | 0.091 |
| No | 1.00 |
Since a time by treatment interaction was significant (p=0.008) when looking at all hypoglycemic events and severe hypoglycemia risk may be increased during the intensive weight loss period, the rate of severe hypoglycemia based on serious adverse events was evaluated by year. As expected, there was a significantly higher rate of severe hypoglycemia in the first year of the trial in the ILI group compared to the DSE group (0.74 vs. 0.20 events/100 PY, p=0.023) despite use of the hypoglycemia algorithm in ILI subjects. This ILI group increase was primarily driven by the rates in those on insulin, in whom the rate was 3.2 events per 100 PY (11 events/339 PY); for those not on insulin (regardless of sulfonylureas or glitinides use), the hypoglycemia rate in the ILI group was 0.4 events per 100 PY (8 events/2108 PY). Beginning in the eighth year of follow-up, hypoglycemic events consistently were more frequent in the DSE group, but the by-year rates were not statistically significantly different between groups. The drop-off in person years of follow-up starting at year 10 due to termination of the trial intervention should be noted (Table 3).
Table 3.
Comparison of the yearly rates of severe hypoglycemic events in the Look AHEAD trial in the ILI and DSE groups.
| Years of Follow-up | DSE | ILI | P-value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Events | Person Years of Follow Up | Rate/100 Person Years | Events | Person Years of Follow Up | Rate/100 Person Years | ||
| 1 | 5 | 2558.09 | 0.20 | 19 | 2552.86 | 0.74 | 0.0230 |
| 2 | 5 | 2524.08 | 0.20 | 13 | 2521.62 | 0.52 | 0.0691 |
| 3 | 14 | 2490.03 | 0.56 | 8 | 2495.84 | 0.32 | 0.3716 |
| 4 | 14 | 2464.32 | 0.57 | 6 | 2477.05 | 0.24 | 0.0806 |
| 5 | 13 | 2433.55 | 0.53 | 14 | 2454.52 | 0.57 | 0.8583 |
| 6 | 9 | 2401.62 | 0.37 | 19 | 2428.37 | 0.78 | 0.0689 |
| 7 | 12 | 2370.07 | 0.51 | 23 | 2391.65 | 0.96 | 0.0962 |
| 8 | 23 | 2327.21 | 0.98 | 18 | 2352.05 | 0.77 | 0.4058 |
| 9 | 19 | 2028.95 | 0.94 | 16 | 2053.37 | 0.78 | 0.6987 |
| 10 | 21 | 1210.33 | 1.74 | 15 | 1224.60 | 1.22 | 0.4604 |
| 11 | 4 | 244.65 | 1.63 | 4 | 249.04 | 1.61 | 0.9799 |
|
| |||||||
| TOTAL | 139 | 23052.9 | 0.60 | 155 | 23201.0 | 0.67 | 0.4038 |
Since hypoglycemia was associated with insulin use, the yearly percent use of insulin in the DSE and ILI groups for the first 10 years of the trial was determined and revealed an overall increased use over time in both groups (DSE: Year 1=16.5%, Year 11=41.2%; ILI: Year 1=15.4%, Year 11=37.5%). However, during the rapid weight loss phase in the first year of the trial, the percent use of insulin in the ILI group declined by 3% compared to the DSE group, and the two groups maintained a 3 to 6.2 percent relative difference in insulin use throughout the first 10 years of the trial (see Figure 2). To determine the relationship of insulin use to weight loss during the study within the ILI group, insulin use was evaluated in two groups defined by the top and bottom 50% of weight loss in the first 12-months. ILI participants in the bottom 50% of weight loss in Year 1 had similar rates of insulin use to those in the DSE group throughout the remainder of the study, with overall hypoglycemia rates of 0.58 (DSE) and 0.52 events (ILI) per 100 PY. Those in the ILI group in the top 50% of weight loss in the first year had a lower rate of insulin use throughout the remainder of the study, with an overall lower rate of hypoglycemic events (0.32) per 100 PY.
Figure 2.
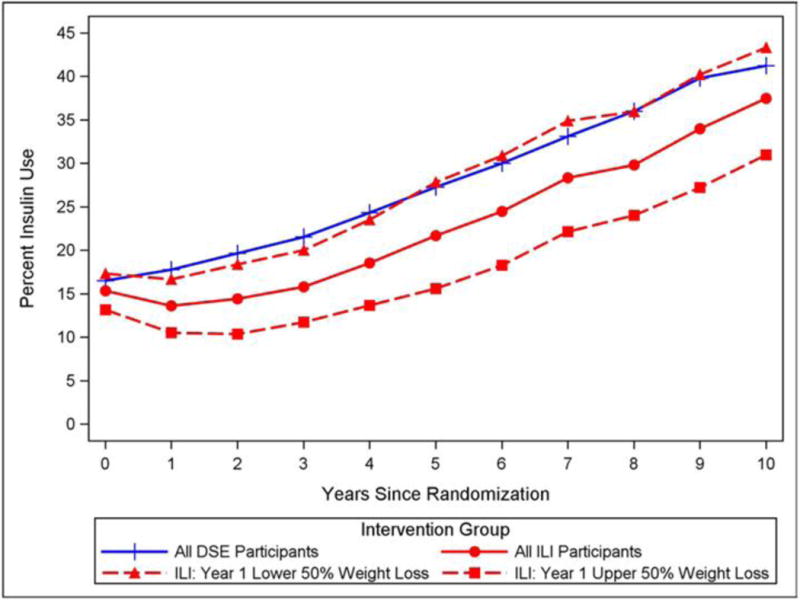
Percent of the DSE and ILI groups taking insulin by year in the Look AHEAD trial. Percentages are shown for the ILI group as a whole and for those in the ILI group whose weight loss during the first year of the trial was in the top and bottom 50%.
Discussion
The incidence of self-reported severe hypoglycemia adverse events over the course of the Look AHEAD trial was low and not different in the ILI and DSE groups as assessed by the Look AHEAD measure confirmed by interview, serious adverse events. A sensitivity analysis used self-reported outcome data collected in both groups every 6 months which showed both the incidence of hypoglycemia and the total number of hypoglycemic events were significantly greater in the DSE group than in the ILI group.
The rate of severe hypoglycemia was significantly higher in the ILI group during the first year of the trial, as anticipated, but event rates in the two groups were not significantly different after this time point. Although the percent insulin use increased in both groups over the trial, it declined 3% in the ILI group during the first year of the trial, and a difference in insulin use between groups was maintained throughout the trial. Among those in the ILI group who were in the lower 50% of weight loss during the first year, the percent of participants using insulin and the rate of incident hypoglycemia throughout the remainder of the trial was similar to the DSE group, but for ILI participants in the upper 50% of weight loss during the first year, the percent using insulin and the rate of incident hypoglycemia were lower than those in the DSE. Since insulin secretion declines with time in people with type 2 diabetes, these findings are consistent with the hypothesis that weight loss drives preservation of insulin secretion, delaying the need for exogenous insulin and therefore reducing the risk of insulin therapy mediated severe hypoglycemia (5).
Although the size, retention, and long-term follow-up of the Look AHEAD cohort are strengths of the study, the serious adverse events may have been biased by more frequent study staff contact with the ILI group. For this reason, a sensitivity analysis was performed on self-reported outcome assessments taken at 6 month intervals in both groups. Theses outcome reports relied upon self-report, and the number of events was not recorded. The incidence of severe hypoglycemia can be compared with other large trials of people with diabetes although these trials had different treatment modalities and definitions of severe hypoglycemia, making comparative interpretations challenging. In Look AHEAD, the rate of severe hypoglycemia was 0.49 vs. 0.51 events per 100 persons per year in the ILI and DSE groups respectively. Standard group rates of severe hypoglycemia in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) were 1.46 events per 100 PY (18). In the Action in Diabetes and Vascular Disease (ADVANCE) study, rates in the less-intensive group were 0.4 events per 100PY (19). In the Veterans Affairs Diabetes Trial (VADT), 9.9% of participants experienced severe hypoglycemia over a median follow-up of 5 years in the standard glucose control group (20). The percentage taking insulin at baseline was 15%, 35%, 1.5% and 52% in Look AHEAD, ACCORD, ADVANCE and VADT, respectively. The rate of severe hypoglycemia in Look AHEAD was at or below the rate observed in the range of standard glucose control in large trials evaluating a variety of glucose control interventions on cardiovascular outcomes in people with diabetes. The overall incidence of severe hypoglycemia in Look AHEAD was associated with medication treatments known to cause hypoglycemia, was significantly greater in participants whose diabetes was treated with various types of antidiabetic medications compared to those treated with diet alone. Severe hypoglycemia was increased in those with longer duration of diabetes or who had diabetic neuropathy. An analysis that examined insulin-use as a time-dependent covariate and included an intervention by insulin use interaction effect showed that severe hypoglycemia was greater in the ILI group than in the DSE group when participants were using insulin but 28% lower than DSE when ILI participants were not using insulin. This is important, since it indicates that greater care is needed to adjust insulin during intensive weight loss (21).
Conclusions
We confirmed that greater severe hypoglycemia adverse events occurred in the ILI group during the intensive weight loss period, but by the eighth year of the trial, severe hypoglycemia was less in the ILI group explaining no over-all difference over the course of the trial. Since the incidence of self-reported severe hypoglycemia adverse events and percent insulin use was lower in the top 50% of weight losers in the ILI group during the first year of the trial, weight loss may reduce the risk of severe hypoglycemia by reducing the need for exogenous insulin treatment. These observations suggest that clinicians should exercise care in adjusting insulin during intensive weight loss, but also provide greater confidence in the long-term safety of weight loss in overweight and obese participants with type 2 diabetes.
Acknowledgments
Funding and Support
Funded by the National Institutes of Health through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Abbreviations
- ILI
Intensive Lifestyle Intervention
- DSE
Diabetes Support and Education
- DPP
Diabetes Prevention Program
- SAE
Serious Adverse Event
- PY
Person Years
- CVD
Cardiovascular Disease
Appendix A: Look AHEAD Research Group at End of Intervention
Clinical Sites
The Johns Hopkins University
Frederick L. Brancati, MD, MHS1; Jeanne M. Clark, MD, MPH1 (Co-Principal Investigators); Lee Swartz2; Jeanne Charleston, RN3; Lawrence Cheskin, MD3; Kerry Stewart, EdD3; Richard Rubin, PhD3; Jean Arceci, RN; Susanne Danus; David Bolen; Danielle Diggins; Sara Evans; Mia Johnson; Joyce Lambert; Sarah Longenecker; Kathy Michalski, RD; Dawn Jiggetts; Chanchai Sapun; Maria Sowers; Kathy Tyler
Pennington Biomedical Research Center
George A. Bray, MD1; Allison Strate, RN2; Frank L. Greenway, MD1; Donna H. Ryan, MD3; Donald Williamson, PhD3; Timothy Church, MD3 ; Catherine Champagne, PhD, RD; Valerie Myers, PhD; Jennifer Arceneaux, RN; Kristi Rau; Michelle Begnaud, LDN, RD, CDE; Barbara Cerniauskas, LDN, RD, CDE; Crystal Duncan, LPN; Helen Guay, LDN, LPC, RD; Carolyn Johnson, LPN, Lisa Jones; Kim Landry; Missy Lingle; Jennifer Perault; Cindy Puckett; Marisa Smith; Lauren Cox; Monica Lockett, LPN
The University of Alabama at Birmingham
Cora E. Lewis, MD, MSPH1; Sheikilya Thomas, MPH2; Monika Safford, MD3; Stephen Glasser, MD3; Vicki DiLillo, PhD3; Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Sara Hannum, MA; Anne Hubbell, MS; Jane King, MLT; DeLavallade Lee; Andre Morgan; L. Christie Oden; Janet Wallace, MS; Cathy Roche, PhD,RN, BSN; Jackie Roche; Janet Turman
Harvard Center
Massachusetts General Hospital
David M. Nathan, MD1; Enrico Cagliero, MD3; Kathryn Hayward, MD3; Heather Turgeon, RN, BS, CDE2; Valerie Goldman, MS, RD2; Linda Delahanty, MS, RD3; Ellen Anderson, MS, RD3; Laurie Bissett, MS, RD; Virginia Harlan, MSW; Theresa Michel, DPT, DSc, CCS; Mary Larkin, RN; Christine Stevens, RN
Joslin Diabetes Center
Edward S. Horton, MD1; Sharon D. Jackson, MS, RD, CDE2; Osama Hamdy, MD, PhD3; A. Enrique Caballero, MD3; Sarah Bain, BS; Elizabeth Bovaird, BSN, RN; Barbara Fargnoli, MS,RD; Jeanne Spellman, BS, RD; Kari Galuski, RN; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; Kerry Ovalle, MS, RCEP, CDE
Beth Israel Deaconess Medical Center
George Blackburn, MD, PhD1; Christos Mantzoros, MD, DSc3; Ann McNamara, RN; Kristina Spellman, RD
University of Colorado Anschutz Medical Campus
James O. Hill, PhD1; Marsha Miller, MS RD2; Holly Wyatt, MD3, Brent Van Dorsten, PhD3; Judith Regensteiner, PhD3; Debbie Bochert; Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Loretta Rome, TRS; Terra Thompson, BA; Kirstie Craul, RD,CDE; Cecilia Wang, MD
Baylor College of Medicine
John P. Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Molly Gee, MEd, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Chu-Huang Chen, MD, PhD3; Peter Jones, MD3; Michele Burrington, RD, RN; Allyson Clark Gardner,MS, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Sarah Lee; Sarah Lane Liscum, RN, MPH; Susan Cantu-Lumbreras; Julieta Palencia, RN; Jennifer Schmidt; Jayne Thomas, RD; Carolyn White
The University of Tennessee Health Science Center
University of Tennessee East
Karen C. Johnson, MD, MPH1; Carolyn Gresham, RN2; Mace Coday, PhD; Lisa Jones, RN; Lynne Lichtermann, RN, BSN; J. Lee Taylor, MEd, MBA; Beate Griffin, RN; Donna Valenski
University of Tennessee Downtown
Abbas E. Kitabchi, PhD, MD1; Ebenezer Nyenwe, MD3; Helen Lambeth, RN, BSN2; Moana Mosby, RN; Amy Brewer, MS, RD,LDN; Debra Clark, LPN; Andrea Crisler, MT; Gracie Cunningham; Debra Force, MS, RD, LDN; Donna Green, RN; Robert Kores, PhD; Renate Rosenthal, PhD; Elizabeth Smith, MS, RD, LDN
University of Minnesota
Robert W. Jeffery, PhD1; Tricia Skarphol, MA2; Carolyn Thorson, CCRP2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Richard S. Crow, MD3; Kerrin Brelje, MPH, RD; Carolyne Campbell; Lisa Hoelscher, MPH, RD, CHES; Melanie Jaeb, MPH, RD; LaDonna James; Patti Laqua, BS, RD; Vicki A. Maddy, BS, RD; Therese Ockenden, RN; Birgitta I. Rice, MS, RPh, CHES; Ann D. Tucker, BA; Mary Susan Voeller, BA; Cara Walcheck, BS,RD
St. Luke’s Roosevelt Hospital Center
Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Carmen Pal, MD3; Lynn Allen, MD;Janet Crane, MA, RD, CDN; Lolline Chong, BS, RD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; Michelle Horowitz, MS, RD
University of Pennsylvania
Thomas A. Wadden, PhD 1; Barbara J. Maschak-Carey, MSN, CDE2; Robert I. Berkowitz, MD 3; Seth Braunstein, MD, PhD 3 ; Gary Foster, PhD 3; Henry Glick, PhD 3; Shiriki Kumanyika, PhD, RD, MPH 3; Stanley S. Schwartz, MD 3 ; Yuliis Bell, BA; Raymond Carvajal, PsyD; Helen Chomentowski; Renee Davenport; Anthony Fabricatore, PhD; Lucy Faulconbridge, PhD; Louise Hesson, MSN, CRNP; Nayyar Iqbal, MD; Robert Kuehnel, PhD; Patricia Lipschutz, MSN; Monica Mullen, RD, MPH
University of Pittsburgh
John M. Jakicic, PhD1; David E. Kelley, MD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Lewis H. Kuller, MD, DrPH3; Andrea Kriska, PhD3; Amy D. Rickman, PhD, RD, LDN3; Lin Ewing, PhD, RN3; Mary Korytkowski, MD3; Daniel Edmundowicz, MD3; Rose Salata, MD3; Rebecca Danchenko, BS; Tammy DeBruce; Barbara Elnyczky; David O. Garcia, MS; Patricia H. Harper, MS, RD, LDN; Susan Harrier, BS; Dianne Heidingsfelder, MS, RD, CDE, LDN; Diane Ives, MPH; Juliet Mancino, MS, RD, CDE, LDN; Lisa Martich, MS, RD; Tracey Y. Murray, BS; Karen Quirin; Joan R. Ritchea; Susan Copelli, BS, CTR
The Miriam Hospital/Brown Medical School
Rena R. Wing, PhD1; Renee Bright, MS2; Vincent Pera, MD3; John Jakicic, PhD3; Deborah Tate, PhD3; Amy Gorin, PhD3; Kara Gallagher, PhD3; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; Jane Tavares, BS
The University of Texas Health Science Center at San Antonio
Steven M. Haffner, MD1; Helen P. Hazuda, PhD1; Maria G. Montez, RN, MSHP, CDE2; Carlos Lorenzo, MD3; Charles F. Coleman, MS, RD; Domingo Granado, RN; Kathy Hathaway, MS, RD; Juan Carlos Isaac, RC, BSN; Nora Ramirez, RN, BSN
VA Puget Sound Health Care System / University of Washington
Steven E. Kahn, MB, ChB1; Anne Kure, BS2; Robert Knopp, MD3; Edward Lipkin, MD, PhD3; Dace Trence, MD3; Elaine Tsai, MD3; Basma Fattaleh, BA; Diane Greenberg, PhD; Brenda Montgomery, RN, MS, CDE; Ivy Morgan-Taggart; Betty Ann Richmond, MEd; Jolanta Socha, BS; April Thomas, MPH, RD; Alan Wesley, BA; Diane Wheeler, RD, CDE
Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico
William C. Knowler, MD, DrPH1; Paula Bolin, RN, MC2; Tina Killean, BS2; Cathy Manus, LPN3; Jonathan Krakoff, MD3; Jeffrey M. Curtis, MD, MPH3; Sara Michaels, MD3; Paul Bloomquist, MD3; Peter H. Bennett, MB, FRCP3; Bernadita Fallis RN, RHIT, CCS; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Christina Morris, BA; Julie Nelson, RD; Carol Percy, RN; Patricia Poorthunder; Sandra Sangster; Leigh A. Shovestull, RD, CDE; Miranda Smart; Janelia Smiley; Teddy Thomas, BS; Katie Toledo, MS, LPC
University of Southern California
Anne Peters, MD1; Siran Ghazarian, MD2; Elizabeth Beale, MD3; Kati Konersman, RD, CDE; Brenda Quintero-Varela; Edgar Ramirez; Gabriela Rios, RD; Gabriela Rodriguez, MA; Valerie Ruelas MSW, LCSW; Sara Serafin-Dokhan; Martha Walker, RD
Coordinating Center
Wake Forest University
Mark A. Espeland, PhD1; Judy L. Bahnson, BA, CCRP3; Lynne E. Wagenknecht, DrPH3; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain G. Bertoni, MD, MPH3; Wei Lang, PhD3; Michael S. Lawlor, PhD3; David Lefkowitz, MD3; Gary D. Miller, PhD3; Patrick S. Reynolds, MD3; Paul M. Ribisl, PhD3; Mara Vitolins, DrPH3; Daniel Beavers, PhD3; Haiying Chen, PhD, MM3; Dalane Kitzman, MD3; Delia S. West, PhD3; Lawrence M. Friedman, MD3; Ron Prineas, MD3; Tandaw Samdarshi, MD3;Kathy M. Dotson, BA2; Amelia Hodges, BS, CCRP2; Dominique Limprevil-Divers, MA, MEd2; Karen Wall2; Carrie C. Williams, MA, CCRP2; Andrea Anderson, MS; Jerry M. Barnes, MA; Mary Barr; Tara D. Beckner; Cralen Davis, MS; Thania Del Valle-Fagan, MD; Tamika Earl, Melanie Franks, BBA; Candace Goode; Jason Griffin, BS; Lea Harvin, BS; Mary A. Hontz, BA; Sarah A. Gaussoin, MS; Don G. Hire, BS; Patricia Hogan, MS; Mark King, BS; Kathy Lane, BS; Rebecca H. Neiberg, MS; Julia T. Rushing, MS; Valery Effoe Sammah; Michael P. Walkup, MS; Terri Windham
Central Resources Centers
DXA Reading Center, University of California at San Francisco
Michael Nevitt, PhD1; Ann Schwartz, PhD2; John Shepherd, PhD3; Michaela Rahorst; Lisa Palermo, MS, MA; Susan Ewing, MS; Cynthia Hayashi; Jason Maeda, MPH
Central Laboratory, Northwest Lipid Metabolism and Diabetes Research Laboratories
Santica M. Marcovina, PhD, ScD1; Jessica Hurting2; John J. Albers, PhD3, Vinod Gaur, PhD4
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
Elsayed Z. Soliman MD, MSc, MS1; Charles Campbell 2; Zhu-Ming Zhang, MD3; Mary Barr; Susan Hensley; Julie Hu; Lisa Keasler; Yabing Li, MD
Diet Assessment Center, University of South Carolina, Arnold School of Public Health, Center for Research in Nutrition and Health Disparities
Elizabeth J Mayer-Davis, PhD1; Robert Moran, PhD1
Hall-Foushee Communications, Inc
Richard Foushee, PhD; Nancy J. Hall, MA
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases
Mary Evans, PhD; Barbara Harrison, MS; Van S. Hubbard, MD, PhD; Susan Z. Yanovski, MD
National Heart, Lung, and Blood Institute
Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR; Mario Stylianou, PhD
Centers for Disease Control and Prevention
Edward W. Gregg, PhD; Ping Zhang, PhD
1 Principal Investigator
2 Program Coordinator
3 Co-Investigator
All other Look AHEAD staffs are listed alphabetically by site
Appendix B
Figure B.1.
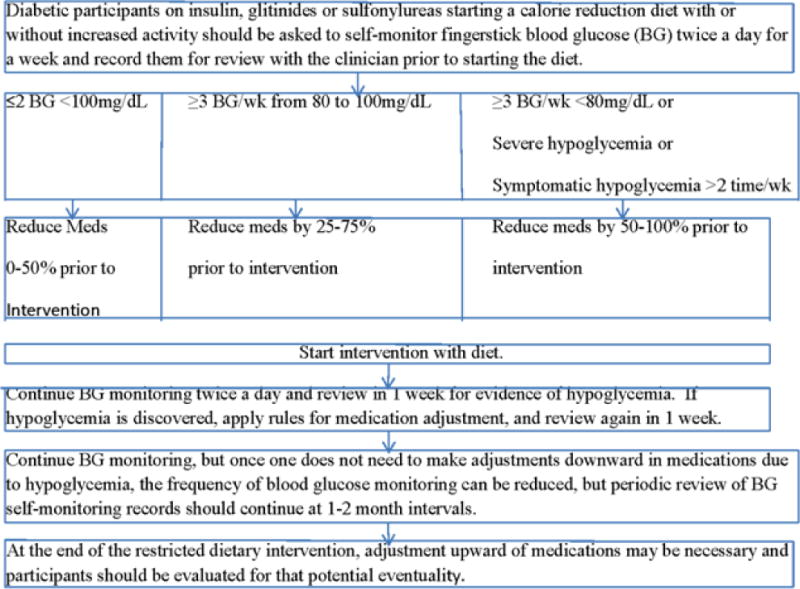
Look AHEAD protocol for adjusting insulin, sulfonylurea and glitinide medications in the ILI group during intensive weight loss.
Table B.1.
Participant Characteristics
| N (%) | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | Level | Overall | ILI | DSE |
| Age | 45–55 | 1620 (31.5%) | 824 (32.1%) | 796 (30.9%) |
| 56–65 | 2651 (51.5%) | 1333 (51.9%) | 1318 (51.2%) | |
| 66–75 | 874 (17.0%) | 413 (16.1%) | 461 (17.9%) | |
| Race/Ethnicity | Black | 804 (15.6%) | 400 (15.6%) | 404 (15.7%) |
| Hispanic | 680 (13.2%) | 340 (13.2%) | 340 (13.2%) | |
| Other | 408 (7.9%) | 208 (8.1%) | 200 (8.1%) | |
| White | 3252 (63.2%) | 1621 (63.1%) | 1631 (63.3%) | |
| Gender | Male | 2082 (40.5%) | 1044 (40.6%) | 1038 (40.3%) |
| Female | 3063 (59.5%) | 1526 (59.4%) | 1537 (59.7%) | |
| Diabetes Treatment | Diet only | 681 (13.5%) | 345 (13.7%) | 336 (13.4%) |
| Diabetes controlled on medications other than insulin, sulfonylureas or glitinides | 2243 (44.6%) | 1132 (45.0%) | 1111 (44.2%) | |
| Diabetes controlled on sulfonylureas or glitinides with or without other medications | 1313 (26.1%) | 655 (26.1%) | 658 (26.2%) | |
| Insulin with or without other medications | 792 (15.8%) | 382 (15.2%) | 410 (16.3%) | |
| Ever on insulin* | 1988 (38.6%) | 891 (35.4%) | 1097 (43.5%) | |
| Ever on insulin secretagogues* | 3238 (62.9%) | 1502 (59.6%) | 1736 (68.9%) | |
| Ever on both insulin and insulin secretagogues* | 1000 (19.4%) | 257 (10.2%) | 348 (13.8%) | |
| Duration of Diabetes | <5 years | 2768 (54.2%) | 1357 (53.3%) | 1411 (55.1%) |
| 5+ years | 2337 (45.8%) | 1189 (47.7%) | 1148 (44.9%) | |
| Prior CVD | Yes | 714 (13.9%) | 366 (14.2%) | 348 (13.5%) |
| No | 4431 (86.1%) | 2204 (85.8%) | 2227 (86.5%) | |
| Nephropathy | Yes | 367 (7.1%) | 174 (6.8%) | 193 (7.5%) |
| No | 4778 (92.9%) | 2396 (93.2%) | 2382 (92.5%) | |
| Neuropathy | Yes | 624 (12.1%) | 326 (12.7%) | 298 (11.6%) |
| No | 4519 (87.8%) | 2243 (87.3%) | 2276 (88.4%) | |
| Retinopathy | Yes | 427 (8.3%) | 199 (7.7%) | 228 (8.9%) |
| No | 4716 (91.7%) | 2371 (92.3%) | 2345 (91.1%) | |
Participants can appear in multiple rows
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Writing Group
Frank L. Greenway, MD (chair), Michael E. Miller, PhD, Sarah Gaussoin, MS; Judy Bahnson, BA, CCRP; George Blackburn, MD; Jeanne M. Clark MD, MPH; Ed Horton, MD; Karen C. Johnson, MD, MPH; Steven E. Kahn, MB, ChB; Anne Peters, MD; Xavier Pi-Sunyer, MD; Bruce Redmond, MD.
As chair of the writing group, Dr. Frank Greenway is the guarantor of this work.
Authors
Frank L. Greenway, MD; Judy L. Bahnson, BA, CCRP; Alain Bertoni, MD, MPH; George Blackburn, MD, PhD; Frederick L. Brancati, MD, MHS; George A. Bray, MD; Jeanne M. Clark, MD, MPH; Jeffrey M. Curtis, MD, MPH; Mary Evans, PhD; Michelle Fisher, RN, CDOE; John Foreyt, PhD; Sarah A. Gaussoin, MS; Siran Ghazarian, MD; Stephen Glasser, MD; Helen P. Hazuda, PhD; James O. Hill, PhD; Edward S. Horton, MD; Van S. Hubbard, MD, PhD; John M. Jakicic, PhD; Robert W. Jeffery, PhD; Karen C. Johnson, MD, MPH; Steven E. Kahn, MB, ChB; Abbas Kitabchi, PhD, MD; William C. Knowler, MD, Dr PH; Mary Korytkowski, MD; Anne Kure, BS; Cora E. Lewis, MD, MSPH; Barbara Maschak-Carey, RN, BSN, CDE; Maria Meacham, BSN, RN, CDE; Michael E. Miller, PhD; Maria G. Montez, RN, MSHP, CDE; David M. Nathan, MD; Ebenezer Nyenwe, MD; Jennifer Patricio, MS; Anne Peters, MD; Xavier Pi-Sunyer, MD; Henry Pownall, PhD; Bruce Redmon, MD; Brent Van Dorsten, PhD; Thomas A. Wadden, PhD; Rena R. Wing, PhD; Susan Z. Yanovski, MD; Ping Zhang, PhD
References
- 1.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296:1858–1866. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 2.Lipska KJ, Ross JS, Wang Y, Inzucchi SE, Minges K, Karter AJ, Huang ES, Desai MM, Gill TM, Krumholz HM. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174:1116–1124. doi: 10.1001/jamainternmed.2014.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noh RM, Graveling AJ, Frier BM. Medically minimising the impact of hypoglycaemia in type 2 diabetes: a review. Expert Opin Pharmacother. 2011;12:2161–2175. doi: 10.1517/14656566.2011.589835. [DOI] [PubMed] [Google Scholar]
- 4.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klarenbach S, Cameron C, Singh S, Ur E. Cost-effectiveness of second-line antihyperglycemic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. CMAJ. 2011;183:E1213–1220. doi: 10.1503/cmaj.110178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U. K. Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 7.Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, Frier BM, Morris AD, Collaboration DM. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 9.Aziz KM. Effect of fasting ramadan in diabetes control status - application of extensive diabetes education, serum creatinine with HbA1c statistical ANOVA and regression models to prevent hypoglycemia. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:233–251. doi: 10.2174/18715303113139990010. [DOI] [PubMed] [Google Scholar]
- 10.Zisser H, Gong P, Kelley CM, Seidman JS, Riddell MC. Exercise and diabetes. Int J Clin Pract Suppl. 2011:71–75. doi: 10.1111/j.1742-1241.2010.02581.x. [DOI] [PubMed] [Google Scholar]
- 11.Look AHEAD Research Group. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research G Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Look AHEAD Research Group. Bray G, Gregg E, Haffner S, Pi-Sunyer XF, WagenKnecht LE, Walkup M, Wing R. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3:202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ, Look ARG. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 15.Look AHEAD Research Group. Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, Kumanyika S. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wesche-Thobaben JA. The development and description of the comparison group in the Look AHEAD trial. Clin Trials. 2011;8:320–329. doi: 10.1177/1740774511405858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Accord Study Group. Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH, Jr, Byington RP, Rosenberg YD, Friedewald WT. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Advance Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 20.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, Investigators V. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 21.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362. doi: 10.1001/jamainternmed.2014.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]


