Abstract
Behavioral inhibition (BI) is a biologically-based temperament characterized by vigilance toward threat. Over time, many children with BI increasingly fear social circumstances and display maladaptive social behavior. BI is also one of the strongest individual risk factors for developing social anxiety disorder. Although research has established a link between BI and anxiety, its causal mechanism remains unclear. Attention biases may underlie this relation. The current study examined neural markers of the BI-attention-anxiety link in children ages 9–12 years (N = 99, Mean = 9.97, SD = 0.97). ERP measures were collected as children completed an attention-bias (dot-probe) task with neutral and angry faces. P2 and N2 amplitudes were associated with social anxiety and attention bias, respectively. Specifically, augmented P2 was related to decreased symptoms of social anxiety and moderated the relation between BI and social anxiety, suggesting that increasing attention mobilization may serve as a compensatory mechanism that attenuates social anxiety in individuals with high BI. The BI by N2 interaction found that larger N2 related to threat avoidance with increasing levels of BI, consistent with over-controlled socio-emotional functioning. Lastly, children without BI (BN) showed an augmented P1 to probes replacing angry faces, suggesting maintenance of attentional resources in threat-related contexts.
Keywords: Anxiety, Attention bias, Behavioral inhibition, Dot-probe, ERP, Temperament
1. Introduction
Behavioral inhibition (BI) is a biologically-based temperament characterized by fear and avoidance in response to novelty. Coll et al. (1984) first coined the term BI to describe children initially, assessed at age two, who were extremely shy and withdrawn when experiencing novel situations or interacting with unfamiliar people. Longitudinal studies have noted a relatively stable profile of BI across childhood (see Clauss and Blackford, 2012; for review). As behaviorally inhibited children mature, they can increasingly fear social circumstances and can display poorly regulated social behavior, which potentially leads to peer rejection, internalizing problems, and poor social competence (Fox et al., 2005; Henderson et al., 2015).
Children with stable and extreme BI are more likely to develop anxiety disorders (Fox and Kalin, 2014, Fox and Pine, 2012, Pérez-Edgar and Fox, 2005). Specifically, BI is one of the strongest individual risk factors for the development of social anxiety disorder (Clauss and Blackford, 2012). Indeed, the pattern of anxious behaviors, social withdrawal, and negative affect observed in children with BI often parallels the set of symptoms used to diagnose anxiety disorders (Pérez-Edgar and Guyer, 2014). Anxiety, however, is additionally characterized by chronic impairment in functioning and atypical interpretation of and reaction to social threats. Thus, anxiety may represent an extreme end of an individual differences spectrum that is rooted in temperament (McNaughton and Corr, 2004, Pérez-Edgar and Guyer, 2014).
Most children with BI, however, do not develop anxiety disorders, prompting efforts to delineate specific mechanisms that underlie the heterogeneity in risk and resilience. One factor that may moderate the stability of BI, as well as the association between BI and anxiety, is attention bias to threat. In a longitudinal study by Pérez-Edgar et al. (2011), children with a BI profile at age 2–3 years exhibited social withdrawal by age 5. Importantly, this relation was moderated by attention bias, such that the relation from BI to social withdrawal was only significant in children who displayed a threat bias. White et al. (2016, in press) also showed that BI assessed during toddlerhood was associated with anxiety most strongly for children who exhibited a concurrent threat bias at age 7. However, there was no predictive relation between attention bias at age 5 and anxiety at age 7. Pérez-Edgar et al. (2010) found that adolescents with a history of BI during childhood showed increased levels of threat bias at age 15. Threat bias again moderated the trajectory of childhood BI to concurrent social withdrawal. Taken together, the link between BI and anxiety-related outcomes may be sustained by displays of heightened attention biases toward threat, in line with research indicating that threat bias plays a role in the emergence and maintenance of anxiety (Bar-Haim et al., 2007, Linetzky et al., 2015).
Given the increased vulnerability for anxiety among individuals with BI through the moderating role of attention bias, the present study aims to examine the BI-attention-anxiety link by delineating the electrophysiological correlates of threat bias in an at-risk sample. The dot-probe task, a standard measure for capturing threat bias, has been criticized for being an indirect measure of covert orienting of attention since inferences must be made from response time performance (Brown et al., 2014). One way to obtain a more direct, physiological measure of attention is to examine event-related potentials (ERPs) during this task. The ability to capture changes in brain activity within milliseconds make ERPs a powerful and sensitive candidate to study early attentional processes associated with the behaviorally observed threat bias. ERPs can therefore offer insight into primary processes involved in attention to threat and their relations to social anxiety.
Early, automatic attentional processes can be captured via the P1 and N1 components. The P1 reflects allocation of attention to stimuli, is maximal in the occipital region (Mangun, 1995, Mangun and Buck, 1998), and is sensitive to face stimuli, particularly novel and emotional faces (Eimer and Holmes, 2007, Halit et al., 2000, Itier and Taylor, 2004). The N1 emerges during visual processing and reflects perceptual facilitation of attended inputs, such as target enhancement and discrimination (Luck, 2014, Mangun, 1995). There is some evidence that the N1 may be particularly sensitive to face processing (Eimer and Holmes, 2007).
To date, a growing literature has used ERPs during the administration of the dot-probe task (or related variants) to track the chronometry of attention processing to threatening and nonthreatening face stimuli in adults (see Table 1). The extant findings were equivocal: two studies found greater P1 amplitude time-locked to face displays in clinically socially anxious adults (Mueller et al., 2009) or adults with reported high fear of evaluation (Rossignol et al., 2013). On the other hand, studies reported no significant modulation of the P1 in non-selected samples (Pourtois et al., 2004; Santesso et al., 2008) and participants high in self-report trait anxiety (Eldar et al., 2010). These discrepancies in findings could stem from the distinct samples being studied. Finally, one dot-probe study reported no difference in N1 amplitude between high and low trait anxious adults (Eldar et al., 2010).
Table 1.
A summary of dot-probe ERP studies in adults. To our knowledge, there are no published dot-probe ERP studies in youths.
| Study | N | Age | Measure of Anxiety | Face Pairs | Significant ERP Effects |
|---|---|---|---|---|---|
| Pourtois et al. (2004) | 12 | 20–25 | None | Fearful/Neutral Happy/Neutral |
Participants showed an increased P1 to probes replacing fearful faces |
| Fox et al. (2008) | 28 | 23–38 | State-Trait Anxiety Inventory (STAI) trait scale | Angry/Neutral Happy/Neutral |
Anxious group revealed enhanced N2pc time-locked to angry faces. All participants revealed enhanced P1 to probes replacing angry faces |
| Santesso et al. (2008) | 16 | 17–26 | None | Angry/Neutral Happy/Neutral |
Participants showed a greater P1 to probes replacing angry faces |
| Mueller et al. (2009) | 27 | 20–42 | Anxiety Disorders Interview Schedule for DSM-IV: Lifetime Version (ADIS-IV-L) | Angry/Neutral Happy/Neutral |
Socially anxious participants showed increased P1 amplitudes to angry-neutral face pairs and decreased P1 to probes replacing angry and happy faces |
| Eldar and Bar-Haim (2010)a | 60 | 20–26 | STAI trait scale | Angry/Neutral Neutral/Neutral |
Following threat avoidance training, anxious group showed attenuated P2 and P3 amplitudes, and enhanced N2 amplitude |
| Eldar et al. (2010) | 46 | 21–24 | STAI trait scale | Angry/Neutral Happy/Neutral Neutral/Neutral |
Anxious group showed greater P2 amplitude to faces across emotions |
| O’Toole and Dennis (2012) a | 49 | 18–38 | STAI state scale | Angry/Happy Happy/Happy |
Following threat avoidance training, participants showed reduced P1 to all faces. Regardless of training condition (toward or away from threat), participants showed increased P2, decreased N170 and N2 to all faces |
| Rossignol et al. (2013) | 26 | 18–24 | Fear of Negative Evaluation Scale | Angry/Neutral Happy/Neutral Disgust/Neutral Fear/Neutral |
Anxious participants showed increased P1 to all faces and increased P2 to angry-neutral face pairs. Anxious participants also showed increased P1 to probes replacing emotional faces |
Attention bias modification training study using the dot-probe task.
Higher-order and more controlled attention processes can be captured via the P2 and N2 components. The P2 component has been associated with sustained perceptual processing (Schupp et al., 2004, Schupp et al., 2003), greater mobilization of attentional resources on salient stimuli (Bar-Haim et al., 2005; Eldar et al., 2010), and the initial processing of emotion evaluation (Carretié et al., 2001; Huang and Luo, 2006). Face displays in the dot-probe task elicited higher P2 amplitudes in anxious adults or adults with high fear of negative evaluation (Eldar et al., 2010, Rossignol et al., 2013).
The N2 is thought to be involved in top-down executive function, specifically to signal the need to inhibit a prepotent response to allow for the execution of subdominant behavior. The N2 component also reflects attention control (Van Veen and Carter, 2002) and conflict monitoring (Yeung and Cohen, 2006) relating to efforts at diverting attention away from threat (Dennis and Chen, 2007, Dennis and Chen, 2009). An ERP study on attention training found that the N2 is augmented following training of attention away from threatening faces (Eldar and Bar-Haim, 2010). Further, recent functional magnetic resonance imaging (fMRI) data suggest that behaviorally inhibited children (Fu et al., in press) and healthy adults who were behaviorally inhibited as children (Jarcho et al., 2013, Jarcho et al., 2014) exhibit perturbed functioning in brain regions underlying cognitive control processes. High levels of cognitive control – in the context of BI – has also been associated with increases in anxiety (Henderson et al., 2015, White et al., 2011) and is marked by an increased N2 during task performance (Lamm et al., 2014).
The N170 is a face-specific component (unrelated to spatial attention) that may provide an index of rapid structural encoding of faces (Eimer and Holmes, 2007). Although emotion-face variants of the dot-probe task are theoretically predicated on variations in face processing, previous studies have failed to detect modulations of the N170 (Pourtois et al., 2004, Rossignol et al., 2013, Santesso et al., 2008).
Finally, the assessment of the ERP response to the target (i.e., the probe) may allow researchers to differentiate enhanced vigilance toward threat-cued location at a later stage of cognitive processing from initial orientating toward face stimuli. Comparing cued (congruent) and non-cued (incongruent) locations may also help tease apart individual differences in attention disengagement. Several dot-probe studies have specifically observed enhanced P1 amplitude for probes replacing threatening/angry cues in high trait-anxious (Fox et al., 2008), socially anxious participants (Rossignol et al., 2013), and non-selected samples (Pourtois et al., 2004, Santesso et al., 2008). One study (Mueller et al., 2009) found reduced P1 amplitudes for probes cued by threatening faces in adults with clinical social anxiety. Another study did not detect any modulations of the P1 time-locked to probes (Eldar et al., 2010). While findings are mixed, there is a general pattern for an augmented P1 to probes replacing angry or threat trials. There is less support in the literature for other probe-locked components (e.g., N2 and P3, Eldar and Bar-Haim, 2010, O’Toole and Dennis, 2012, Rossignol et al., 2013).
Despite both basic and translational importance, the electrophysiological correlates of attention bias in children have largely been unexplored. The present study is the first to fill this developmental gap and extend an investigation to individual differences by examining these relations in generally healthy children varying in temperamental risk for anxiety. Specifically, we aim to: (1) document the electrophysiological correlates of attention to salient (i.e., threatening) face stimuli in children, and (2) examine whether the electrophysiological correlates of face processing moderate the relation between BI and behavioral threat bias as well as social anxiety. In addition, we aim to examine the ERP response to the targets that follow trials involving angry faces, differentiating between cued and uncued locations. Addressing these aims will provide insights into the neurocognitive mechanisms that influence individual differences in temperament before the emergence of psychopathology, which may highlight specific targets for intervention. We predict that the electrophysiological correlates of face processing will be associated with individual variation in both attention biases and social anxiety, particularly for individuals at temperamental risk. However, given mixed findings in the adult literature, we do not have strong directional hypotheses.
2. Methods
All methods for recruitment and study procedures were approved by the institutional review board (IRB) of The Pennsylvania State University.
2.1. Participants
The sample consisted of 112 9–12 year-olds, drawn from a larger, ongoing study on temperament, attention, and anxiety. Participants were recruited using the university’s database of families interested in participating in research studies, community outreach, and word-of-mouth throughout Central Pennsylvania and neighboring areas. 216 participants were screened using parental report on the Behavioral Inhibition Questionnaire (BIQ; Bishop et al., 2003). Children who met BI cutoff scores (≥119 total score or ≥60 social novelty subscale) were identified and oversampled, such that although only 18% of the children met BI criteria, they represent 36% of the current study sample. Cutoff scores were based on a previous study of extreme temperament in children 4–15 years of age (Broeren and Muris, 2010). The sample was 82% Caucasian, 2% African American, 3% Hispanic, 2% biracial, and 11% declined to respond. All parents and children provided written consent/assent. Participants received monetary compensation for participating in the study.
Nine participants were excluded for poor performance on the dot-probe task ( < 75% accuracy). One participant was excluded due to significant artifacts (see EEG data reduction section for details). Finally, three participants from sibling pairs were randomly excluded to eliminate influences due to shared genetic and environmental factors (Gregory and Eley, 2007). Thus, 99 participants represented our final sample, providing both behavioral and ERP data. Included (N = 99, 49 males, Mage = 9.97, SD = 0.97) and excluded (N = 13, 5 males, Mage = 9.69, SD = 0.85) participants did not differ in age, gender, IQ, Total BIQ, attention bias, social anxiety symptoms, or ERP amplitudes (t’s < 1.66, p’s > 0.11, d’s < 0.30). Table 2 provides descriptive statistics for included participants.
Table 2.
Demographic characteristics and descriptive statistics (mean and standard deviation) of main study variables.
| Variable | |
|---|---|
| N | 99 |
| Gender | 49M/50F |
| Age | 9.97 (0.97) |
| IQ | 110.87 (13.12) |
| Total BIQ | 93.27 (29.71) |
| Attention Bias | 0.49 (18.56) |
| Social Anxiety Symptoms | 1.61 (2.91) |
| P1 amplitude | 3.42 (2.13) |
| N1 amplitude | −2.62 (1.73) |
| N170 amplitude | 2.90 (2.74) |
| P2 amplitude | 2.32 (3.20) |
| N2 amplitude | −3.48 (2.88) |
| P1-probe amplitude | 0.31 (1.85) |
| N2-probe amplitude | −2.00 (2.56) |
| P3-probe amplitude | 1.37 (3.96) |
2.2. Measures
To assess IQ, participants completed the vocabulary and block design subtests of the Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV; Wechsler, 2003). IQ scores were computed from the sum of the subtests’ scaled scores.
To assess behavioral inhibition, parents completed the BIQ (Bishop et al., 2003), a 30-item questionnaire consisting of BI-linked behavior in the domains of social and situational novelty. Parents rated their children’s behavior in response to novelty on a 7-point Likert scale ranging from 1 (“Hardly Ever”) to 7 (“Almost Always”). The questionnaire has adequate internal consistency and validity in differentiating children with or without BI (Bishop et al., 2003) and parental reports on the BIQ are correlated with laboratory observations of BI (Dyson et al., 2011). Furthermore, maternal reports of childhood BI have been shown to predict social anxiety disorder into adolescence (Chronis-Tuscano et al., 2009). In the present study, the BIQ had good internal consistency (Cronbach’s α = 0.86). Continuous total BIQ scores were used for our analyses of face-locked ERPs (Range = 34–159).
To assess social anxiety symptoms, the computer-assisted Diagnostic Interview Schedule for Children version IV (C-DISC 4; Shaffer et al., 2000) was administered to primary caregivers. A trained research assistant conducted the semi-structured interview, in which parents judged DSM-IV symptoms as either present (“yes”) or absent (“no”). “Yes” responses were tallied to obtain a total symptom score. Total symptom scores from the present study ranged from 0 to 11. To reflect changes implemented with the DSM-5, we use the term ‘social anxiety’ throughout the manuscript, rather than the term ‘social phobia’, as originally used in the C-DISC 4.
2.2.1. Dot-probe task
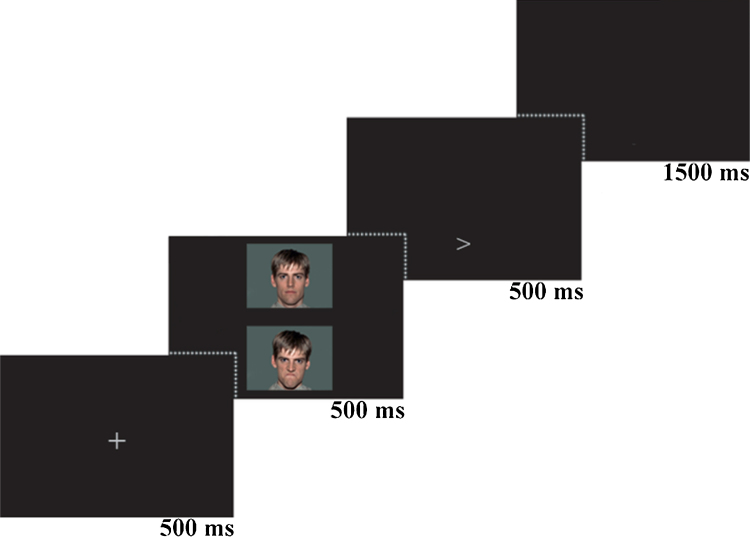
To assess attention bias, participants performed the dot-probe task (Fig. 1), first designed by MacLeod et al. (1986). The current version of the task was modified from Abend et al. (2014). Each trial began with a 500 ms fixation at the center of the screen, followed by a face pair displayed on the top and bottom of the fixation point for 500 ms. The faces were then replaced by an arrow probe (< or >) presented for 500 ms in the location of one of the preceding faces. Participants indicated as quickly and accurately as possible whether the arrow pointed left or right using a mouse click (response recorded for 2000 ms). Trials advanced regardless of accuracy in response (1500 ms inter-trial interval).
Fig. 1.
Illustration of the dot-probe task, presenting a congruent cue trial.
Face stimuli were comprised of colored photographs of 10 different actors (5 males) taken from the NimStim stimulus set (Tottenham et al., 2009). Participants were presented with pairs of faces (angry-neutral or neutral-neutral) of the same actor. The face photographs (5 cm by 4 cm) were presented equidistant from the fixation cross. The visual angle for the face stimuli was 5.3° (H) × 6.2° (V).
There were a total of 180 trials divided into three equal blocks. There were 60 trials for each of the 3 conditions: (1) angry-neutral congruent trials in which the probe replaced the position of the angry face; (2) angry-neutral incongruent trials in which the probe replaced the position opposite of the angry face; and (3) neutral–neutral trials in which the probe appeared either on top or bottom. Trials were counterbalanced across emotion face location, probe location, probe orientation, and gender of face. The task was administered using the E-Prime software package version 2.0 (Psychology Software Tools, Pittsburgh, PA).
Invalid trials including nonresponses, inaccurate responses, RTs outside a 150–2000 ms window, and/or RTs ± 2 standard deviations of the individual’s mean were removed before analyses. Attention bias to the angry faces was calculated for each participant by subtracting the mean RT for congruent trials from the mean RT for incongruent trials. Positive scores denote a bias toward threat whereas negative scores indicate bias away from threat.
2.2.2. Electrophysiological recording and reduction
Electroencephalogram (EEG) activity was recorded continuously during performance of the dot-probe task using a 128-channel geodesic sensor net (Electrical Geodesics Inc., Eugene, Oregon). The EEG signal from each channel was digitized at a 1000 Hz sampling rate. EEG channels were collected with reference to Cz and, after acquisition, re-referenced to the average of the left and right mastoids. Vertical eye movements were recorded from electrodes placed approximately 1 cm above and below each eye. Horizontal eye movements were monitored with electrodes placed approximately 1 cm at the outer canthi of each eye. Impedances were kept below 50 kΩ.
All data preparation and processing after recording were conducted using Brain Vision Analyzer (Brain Products GmbH, Germany). Data were filtered with a high-pass frequency of 0.1 Hz and a low-pass frequency of 40 Hz. Ocular artifacts from eye blinks and horizontal eye movements were corrected using the Gratton method (Gratton et al., 1983). Data time-locked to face displays were segmented into epochs from 100 ms before baseline to 500 ms after display onset, with a 100 ms baseline correction. EEG signals with artifacts exceeding ±100 μV were removed. Trials with incorrect response, no response, or latencies faster than 150 ms or exceeding 2000 ms were excluded from analyses. All included participants provided at least 30 artifact-free segments with at least 10 segments per condition.
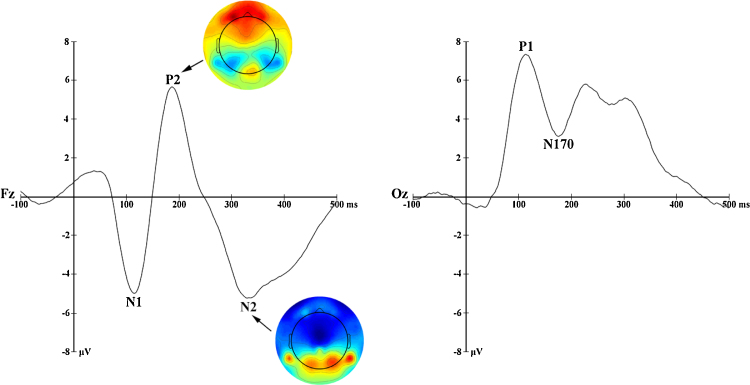
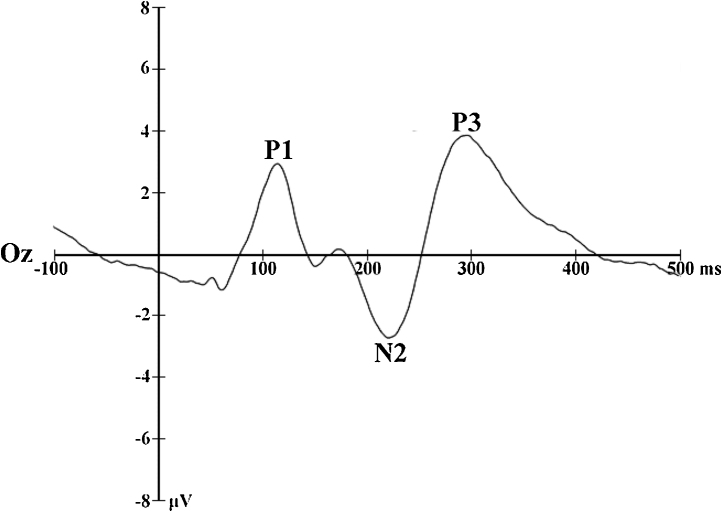
Following the inspection of the grand average ERP’s, and in accordance with recent literature on the electrophysiological correlates of spatial attention (i.e. Eldar et al., 2010, Mueller et al., 2009, Rossignol et al., 2013), ERP analyses focused on the mean amplitudes of the P1, N1, N170, P2, and N2 elicited by face pairs (Fig. 2) and P1, N2, and P3 evoked by the probe display (Fig. 3). The P1 (40–140 ms), P1-probe (60–140 ms), N170 (120–220 ms), N2-probe (180–260 ms), and P3-probe (260–380 ms) components were quantified as the average mean amplitude over occipital electrodes (65, 66, 69, 70, 71, 74, 76, 82, 83, 84, 89, 90). The N1 (60–140 ms), P2 (140–240 ms), and N2 (260–360 ms) components were derived from the average mean amplitudes over fronto-central electrodes (3, 4, 5, 9, 10, 11, 12, 16, 18, 19, 20, 22, 23, 24, 27, 28, 33, 117, 118, 122, 123, 124).
Fig. 2.
Grand average waveforms and scalp topographies for components time-locked to all face displays.
Fig. 3.
Grand average waveform time-locked to probe displays.
2.2.3. Model testing
To assess the relations between BI, behavioral attention bias, ERP correlates of attention bias, and social anxiety, we tested a moderated mediation model (Hayes, 2015, Preacher et al., 2007) separately for each ERP component: P1, N1, N170, P2, and N2 (see Table 3; and Fig. 4, Fig. 5). Using SPSS (Version 22; Chicago, IL, USA) macro PROCESS model 59 (Hayes, 2012), BI was entered as the predictor, attention bias as mediator, and social anxiety as outcome, with each ERP component serving in turn as the moderator. Predictor, mediator, and moderator variables were mean centered prior to analysis. Significant conditional indirect effects were determined using 95% bootstrap bias corrected confidence intervals (CIs) based on 1000 bootstrap samples.
Table 3.
Bivariate correlations for Total BIQ, attention bias (AB), social anxiety, and ERP components.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavioral Measures | 1. Total BIQ | 1.00 | ||||||||||
| 2. AB | 0.01 | 1.00 | ||||||||||
| 3. Social Anxiety | 0.52** | −0.10 | 1.00 | |||||||||
| ERPs to faces | 4. P1 amp | −0.03 | 0.13 | −0.04 | 1.00 | |||||||
| 5. N1 amp | 0.07 | 0.05 | −0.11 | −0.20* | 1.00 | |||||||
| 6. N170 amp |
−0.02 | 0.11 | 0.04 | 0.71** | −0.17+ | 1.00 | ||||||
| 7. P2 amp | −0.04 | 0.16 | -0.22* | 0.26* | 0.39** | 0.01 | 1.00 | |||||
| 8. N2 amp | 0.02 | 0.19+ | 0.04 | 0.18+ | 0.24* | 0.08 | 0.41** | 1.00 | ||||
| ERPs to probe | 9. P1 amp | 0.00 | 0.03 | −0.06 | 0.25* | −0.07 | 0.21* | 0.03 | 0.07 | 1.00 | ||
| 10. N2 amp | 0.09 | −0.05 | 0.07 | −0.26** | −0.09 | −0.08 | −0.24* | −0.07 | 0.49** | 1.00 | ||
| 11. P3 amp | −0.06 | 0.01 | −0.13 | −0.20* | 0.01 | −0.12 | −0.12 | −0.12 | 0.42** | 0.75** | 1.00 |
p < 0.10.
p < 0.05.
p < 0.01.
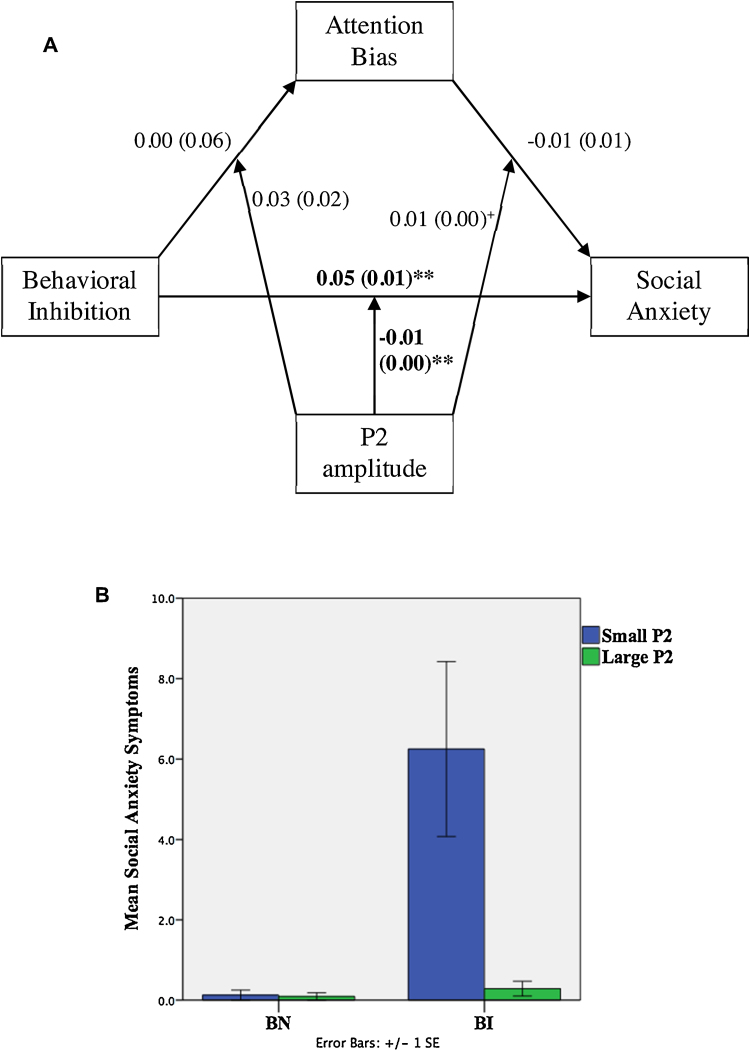
Fig. 4.
(A) Path results for the moderated mediation model illustrating the relation between BI, attention bias, social anxiety, and P2 amplitude. Noted are the effect coefficients with standard errors in parentheses; +p < 0.10; *p < 0.05; **p < 0.01. (B) P2B moderates the relation between behavioral inhibition and social anxiety symptoms.
Fig. 5.
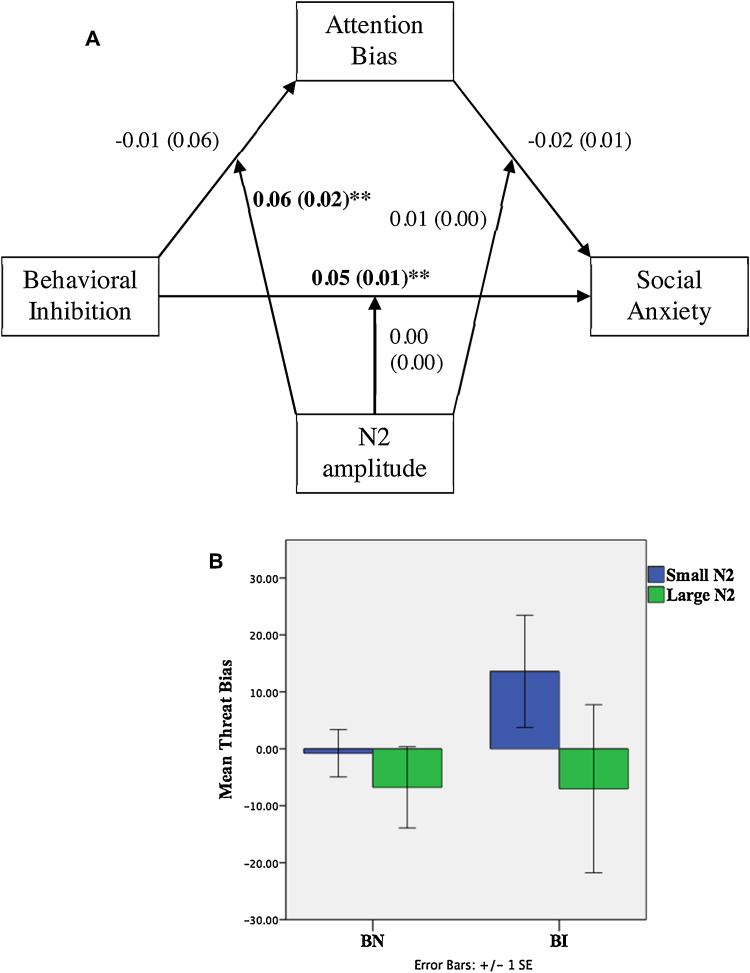
(A) Path results for the moderated mediation model illustrating the relation between BI, attention bias, social anxiety, and N2 amplitude. Noted are the effect coefficients with standard errors in parentheses; +p < 0.10; *p < 0.05; **p < 0.01. (B) BI interacts with N2 to predict attention bias.
For ERPs evoked by the probe displays, repeated measures ANCOVA was performed on the mean amplitude of each component with Trial Type (angry congruent, angry incongruent) as a within-subject factor and Group (BI, BN) as a between-subjects factor. Social anxiety symptoms were entered as a covariate.
Each set of analyses initially included gender as a potential between-subjects variable. However, we found no systematic effects of gender. As such, the variable was removed for parsimony and the analyses re-run. Results were not corrected for multiple comparisons.
3. Results
3.1. Behavioral results
The one-sample t-test versus zero examining attention bias to angry faces was non-significant, t(98) = 0.27, p = 0.79, d = 0.05. As shown in Table 3, attention bias did not correlate with total BIQ scores, r(97) = 0.01, p = 0.96, or social anxiety symptoms, r(97) = −0.10, p = 0.33. As expected, total BIQ scores and social anxiety symptoms were highly correlated, r(97) = 0.52, p < 0.001.
3.2. Electrophysiological results
3.2.1. ERPs evoked by face displays
Paired samples t-tests revealed no significant differences in amplitude between angry-neutral and neutral–neutral conditions for any of the five ERP components, t’s < 1.40, p’s > 0.16, d’s < 0.28. We therefore present results for ERPs time-locked to all face displays, which were pooled in Brain Vision Analyzer across angry-neutral and neutral–neutral trials and then exported to SPSS for analysis.
Attention bias was marginally correlated with N2 amplitude, r(97) = 0.19, p = 0.06, Table 3. Social anxiety was significantly correlated with P2 amplitude, r(97) = −0.22, p = 0.03.
3.2.2. Moderated mediation models
Path results for the P1, N1, and N170 moderated mediation models revealed a significant association only between BI and social anxiety (β’s > 0.05, p’s < 0.01; Table 3). As such, we fully present the results for the P2 and N2 models only.
3.2.3. Relation between BI and social anxiety moderated by P2
P2 amplitude was negatively associated with social anxiety (β = −0.25, p = 0.002; Table 4). Further, P2 moderated the conditional direct effect of BI on social anxiety (−0.02 ≤ CI95% ≤ −0.01). Probing the interaction at ±1 standard deviation of mean P2, we found that at decreasing levels of P2, the relation between BI and social anxiety strengthened (β = 0.08, p = 0.00, 0.06 ≤ CI95% ≤ 0.11; Fig. 4). Finally, there was a marginal interaction of P2 by attention bias in predicting social anxiety (β = 0.01, p=0.09, Table 4).
Table 4.
Path results for the moderated mediation models for P1, N1, N170, P2, and N2 components. BI was entered as the predictor, attention bias as mediator, social anxiety as outcome, and ERP component as moderator.
|
β (SE) |
t |
β (SE) |
t |
|
|---|---|---|---|---|
| Mediator: Attention Bias | Dependent Variable: Social Anxiety | |||
| P1 | 0.90 (0.93) | 0.97 | −0.01 (0.13) | −0.10 |
| BI | 0.00 (0.06) | 0.03 | 0.05 (0.01) | 6.02** |
| P1 × BI | 0.02 (0.03) | 0.73 | 0.00 (0.00) | −0.06 |
| AB | −0.02 (0.01) | −1.16 | ||
| P1 × AB | 0.00 (0.01) | −0.56 | ||
| N1 | 0.62 (1.11) | 0.65 | −0.22 (0.15) | −1.52 |
| BI | 0.00 (0.06) | 0.06 | 0.05 (0.01) | 6.19** |
| N1 × BI | −0.01 (0.03) | −0.43 | 0.00 (0.00) | −0.60 |
| AB | −0.01 (0.01) | −1.02 | ||
| N1 × AB | 0.00 (0.01) | −0.07 | ||
| N170 | 0.79 (0.70) | 1.13 | 0.06 (0.10) | 0.59 |
| BI | 0.01 (0.06) | 0.11 | 0.05 (0.01) | 6.00** |
| N170 × BI | −0.01 (0.03) | −0.42 | 0.00 (0.00) | 0.03 |
| AB | −0.02 (0.01) | −1.44 | ||
| N170 × AB | −0.01 (0.01) | −0.95 | ||
| P2 | 0.94 (0.58) | 1.61 | −0.25 (0.08) | −3.15** |
| BI | 0.00 (0.06) | −0.02 | 0.05 (0.01) | 6.65** |
| P2 × BI | 0.03 (0.02) | 1.33 | −0.01 (0.00) | −4.03** |
| AB | −0.01 (0.01) | −0.43 | ||
| P2 × AB | 0.01 (0.00) | 1.72+ | ||
| N2 | 1.59 (0.64) | 2.48* | 0.09 (0.09) | 1.02 |
| BI | −0.01 (0.06) | −0.17 | 0.05 (0.01) | 5.56** |
| N2 × BI | 0.06 (0.02) | 2.73** | 0.00 (0.00) | 1.60 |
| AB | −0.02 (0.01) | −1.30 | ||
| N2 × AB | 0.01 (0.00) | 1.57 | ||
Note. AB = Attention Bias. BI = Behavioral Inhibition.
p < 0.10.
p < 0.05.
p < 0.01.
3.2.4. Relation between BI and attention bias moderated by N2
N2 amplitude was significantly associated with attention bias (β = 1.59, p = 0.01; Table 4). In addition, there was a BI by N2 interaction predicting attention bias (0.02 ≤ CI95% ≤ 0.10). Probing the interaction at ±1 standard deviation of mean N2, we found that increasing levels of N2 mitigated the BI-to-Attention Bias relation (β = 0.04, p = 0.01, 0.01 ≤ CI95% ≤ 0.06, Fig. 5).
3.2.5. ERPs evoked by probe displays
The repeated measures ANCOVA for each ERP component (P1-probe, N2-probe, and P3-probe) found a significant interaction effect for Bias × Group for the P1-probe component, F(1,96) = 4.9, p = 0.03, η2p = 0.05. Follow-up paired-samples t-tests revealed that for the BN group, there was a greater P1 to probes replacing angry faces, t(60) = 2.11, p = 0.04, d = 0.54. The relation was not significant for the BI group, t(37) = −0.87, p = 0.39, d = 0.29. No significant effects were found for the N2 and P3 components time-locked to probe onset, p’s > 0.38.
4. Discussion
The current study identified electrophysiological markers associated with attentional factors known to impact the emergence of social anxiety. Specifically, we found that higher-order, more cognitively tinged ERP components were associated with core factors of the study during face processing.
P2 amplitude was related to decreased symptoms of social anxiety. P2 further moderated the positive relation between BI and social anxiety, such that the relation between BI and social anxiety symptoms weakened with increases in P2. This suggests that an augmented P2, reflecting greater mobilization of attentional resources and heightened evaluative processing of faces, may serve as a compensatory mechanism that attenuates social anxiety in individuals high in BI.
Indeed, a heightened P2 component may indicate more top-down, sustained perceptual processing (Schupp et al., 2004, Schupp et al., 2003). Specifically, the P2 reflects later stages of face processing compared to the N170, such as recognition or decision-making within the context of the task (Halit et al., 2000, Itier and Taylor, 2004, Latinus and Taylor, 2005). Fronto-central modulations by face expressions may reflect rapid representation of emotional significance in prefrontal regions (Eimer and Holmes, 2007) as well as greater attentional mobilization (Bar-Haim et al., 2005), which may offset initial reactive responses. This, in turn, may function as a compensatory mechanism in some individuals with high levels of BI.
In contrast, the N2 was associated with attention bias. Here, bias toward threat was associated with smaller N2, and threat avoidance with larger N2. The N2 component reflects the recruitment of cognitive control (van Veen and Carter, 2002) and conflict monitoring (Dennis and Chen, 2007, Dennis and Chen, 2009) processes, which may reflect efforts at diverting attention away from threat. In our sample, children with a bias away from threat recruited greater neural activation to support cognitive control processes presumed to regulate the processing of threatening stimuli, while those biased toward threat failed to recruit such regulatory resources. This interpretation is consistent with attention training work in anxious adults, who showed increased N2 following attention training away from threat (Eldar and Bar-Haim, 2010). For the sample as a whole, an augmented N2 may have assisted participants in gaining better control over their attentional resources.
Moreover, BI moderated the relation between N2 amplitude and threat bias. For participants who demonstrated higher levels of BI, a larger N2 predicted bias away from threat. Previous research demonstrates that behaviorally inhibited individuals with enhanced response monitoring, marked by the error-related negativity (ERN), are at increased risk for anxiety disorders (McDermott et al., 2009). In addition, adolescents with childhood BI and increased N2 also show an increased likelihood of having anxiety (Henderson et al., 2015, Lamm et al., 2014, White et al., 2011). This finding may also help explain recent behavioral data linking extreme early temperament to both attention bias to threat and attention avoidance.
Recent work suggests that young children with a history of dysregulated fear exhibit biases away from threat (Morales et al., 2015). Dysregulated fear, in turn, is linked to increased risk for social anxiety symptoms (Buss et al., 2013). This is in line with research suggesting that attention avoidance in response to real-world threat predicts increases in PTSD (Buckley et al., 2002; Wald et al., 2011). In addition, recent research conducted with children in community and clinical samples finds that attention bias varies across anxiety disorders, where threat bias is associated with generalized anxiety disorders and threat avoidance is associated with fear disorders (including social anxiety and specific phobias) (Salum et al., 2013, Waters et al., 2014). Finally, we recently reported a study of 9–12-year-olds who completed two related attention bias tasks (Morales et al., in press). Children with inconsistent bias patterns across tasks were low in anxiety. In contrast, children with stable patterns of attention, either toward or away, showed elevated levels of anxiety. Together, these data suggest that bias patterns related to threat are complex, and subject to a number of moderating factors. Additional work will be needed in order to identify the relevant factors determining directionality across time and populations. Our findings suggest that ERPs linked to cognitive control, such as the N2, are potential candidates in teasing apart the relation between threat avoidance and threat vigilance in BI or anxious populations.
Finally, children without BI elicited heightened P1 mean amplitude for angry congruent versus angry incongruent probes, suggesting vigilance toward threat-cued location. Probes replacing threatening cues have been shown to modulate spatial attention in healthy, non-selected adults (Pourtois et al., 2004, Santesso et al., 2008). It may be that BN children are able to build on information derived from face processing to then modulate subsequent target processing. It appears that children high in BI do not show the same processing sequence, which may reflect (1) a heightened or overwhelming response to the initial face cue or (2) a general inflexibility to incorporate new information for the next stimulus. Clearly, more work is needed in order to separate these potential mechanisms.
We chose face stimuli as our task parameters because core features of social anxiety include fear of negative social evaluation and heightened detection of negative emotions in others. Faces convey significant social information (Adolphs, 2002, Bradley et al., 1997, Ekman, 1993). Our findings did not differentiate between angry versus neutral faces. The lack of an emotion effect has been reported in the literature for multiple components, including the P1 (Eldar et al., 2010, Helfinstein et al., 2008; Rossignol et al., 2013), N1 (Eldar et al., 2010, Helfinstein et al., 2008), P2 (Eldar et al., 2010, Kolassa and Miltner, 2006), and N2 (Hum et al., 2013). ERP modulation to emotion faces (fearful, happy, and angry) has been reported in two dot-probe studies (Mueller et al., 2009, Rossignol et al., 2013). Specifically, Mueller et al. (2009) found increased P1 to angry-neutral face pairs compared to happy-neutral face pairs. Rossignol et al. (2013) found heightened P2 to angry-neutral pairs, but no emotion effect was found for the P1 component. Given these discrepant findings, which may be due to task variations, additional research is needed to elucidate the exact nature of the attention-emotion interaction.
Our findings suggest that early components of attention processes, as indexed by the P1 and N1, and the early-emerging face-specific N170 component, may be relatively insensitive to the dot-probe task. In our sample, children do not show an early bias in perceptual processing of faces, regardless of their temperament or social anxiety symptoms. Eldar and Bar-Haim (2010) and Kolassa and Miltner (2006) also reported null findings for these early components in their trait anxious and socially anxious samples. Further, the N170 reflects the encoding of structural properties of facial stimuli (Bentin et al., 1996) and modulation of this component in response to the presentation of pairs of faces has rarely been studied. Perhaps the requirement of encoding two faces simultaneously, as in the dot-probe task, reduced the sensitivity of this component to their emotional load. Taken together, the lack of an emotion effect and engagement in early processing of social stimuli appear to be consistent across studies of children and adults with either risk for, or diagnoses of, anxiety disorders.
Findings from the current study should be interpreted in light of three core limitations. First, we did not detect significant threat bias using reaction time data from the dot-probe task. This limits our ability to interpret the present data as a function of individual patterns of attention bias. Perhaps having only 10 face models shown repeatedly throughout the study impedes significant findings and more trial-unique stimuli are needed. Several studies have found that the dot-probe paradigm failed to elicit a behavioral threat bias in behaviorally inhibited samples (Broeren and Muris, 2010, Cole et al., 2016; Morales et al., in press; Pérez-Edgar et al., 2011; White et al., 2016, in press). Further, traditional reaction time data measured by the dot-probe task may have poor internal reliability, poor test-retest reliability, and may not be associated with anxiety (Kappenman et al., 2014). However, using neural correlates, we did note individual differences in ERP modulations of attention processing. Other studies (e.g., Bar-Haim et al., 2005, Kappenman et al., 2015; Mueller et al., 2009; Sun et al., 2012) have also found that ERP measures can capture the chronometry of attention processes, despite the absence of behavioral effects. Similar patterns have been noted for fMRI studies of attention bias (Britton et al., 2012; Fu et al., in press; Hardee et al., 2013, Price et al., 2014). While behavioral response time reflects a global index of task performance, encompassing influences from cognitive, affective, and motor processes, modulation of specific ERP components may reflect more refined and specific stages of processing.
Second, due to the nature of our cross-sectional design, we cannot make causal inferences. This snapshot in time cannot explain how these temperament-linked relations unfold over the course of development. The trajectory and development of BI into divergent outcomes – adaptive versus maladaptive – should be examined longitudinally to more fully understand the mechanisms of risk. For example, White et al. (2016, in press) showed that although children do not exhibit stable patterns of attention biases across development, BI predicted anxiety in children with concurrent attention bias to threat or attention bias away from happy faces.
Third, this study is an initial attempt to examine the variety of potential ERP-based markers of the BI-attention-anxiety link noted in the broader literature. As such, we examined a large number of ERP components, time-locked to both faces and probes. As with many ERP studies, this led to a number of statistical analyses. This leaves our findings vulnerable to Type I error. As such, it is important to regard the findings presented here as preliminary in nature and in strong need for replication.
In conclusion, our study is the first to offer direct evidence of electrophysiological markers of heightened attention processing of faces in the dot-probe task among healthy children at temperamental risk for anxiety. Overall, our results capture distinct mechanisms of attention bias and social anxiety that can be differentiated at the neural level. Early, automatic and pre-attentive processes indexed by the P1, N1, and N170 components were unrelated to temperament, attention bias, or social anxiety. Later, more controlled, processes indexed by the P2 and N2 components differentially related to social anxiety and attention bias, each varying as a function of temperament. This set of findings suggests that top-down, controlled processes, such as attention shifting and evaluative approaches, may modulate attention bias and social anxiety, particularly among behaviorally inhibited children. These preliminary results support the need for more nuanced investigations of selective attention in conjunction with temperament and anxiety. Further research is needed to better distinguish the distinct roles between early and later components in influencing attention biases and social anxiety. The current results confirm the feasibility of using ERP in children to examine the neurophysiological underpinnings that differentially modulate levels of threat bias and social anxiety associated with perceptual processing of face stimuli presented in the context of an attention task.
Acknowledgements
This work is supported by a grant from the National Institutes of Health [BRAINS R01 MH094633] to KPE. The authors would like to thank the Pennsylvania State University Social, Life, & Engineering Sciences Imaging Center (SLEIC) Human Electrophysiology Facility for supporting data collection, the TAU/NIMH ABMT Initiative for providing the task toolkit, and the many individuals who contributed to data collection and data processing. We would especially like to thank the parents of the children who participated and continue to participate in our studies.
References
- Abend R., Pine D.S., Bar-Haim Y. 2014. The TAU-NIMH Attention Bias Measurement Toolbox.http://people.socsci.tau.ac.il/mu/anxietytrauma/research/ Retrieved from. [Google Scholar]
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behav. Cogn. Neurosci. Rev. 2002;1(1):21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Glickman S. Attentional bias in anxiety: a behavioral and ERP study. Brain Cogn. 2005;59(1):11–22. doi: 10.1016/j.bandc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., Van Ijzendoorn M.H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 2007;133(1):1. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bentin S., Allison T., Puce A., Perez E., McCarthy G. Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G., Spence S.H., McDonald C. Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Dev. 2003:1899–1917. doi: 10.1046/j.1467-8624.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- Bradley B.P., Mogg K., Millar N., Bonham-Carter C., Fergusson E., Jenkins J., Parr M. Attentional biases for emotional faces. Cogn. Emot. 1997;11(1):25–42. [Google Scholar]
- Britton J.C., Bar-Haim Y., Carver F.W., Holroyd T., Norcross M.A., Detloff A., Pine D.S. Isolating neural components of threat bias in pediatric anxiety. J. Child Psychol. Psychiatry. 2012;53(6):678–686. doi: 10.1111/j.1469-7610.2011.02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeren S., Muris P. A psychometric evaluation of the behavioral inhibition questionnaire in a non-clinical sample of Dutch children and adolescents. Child Psychiatry Hum. Dev. 2010;41(2):214–229. doi: 10.1007/s10578-009-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H., Eley T., Broeren S., MacLeod C., Rinck M., Hadwin J., Lester K. Psychometric properties of reaction time based experimental paradigms measuring anxiety-related information-processing biases in children. J. Anxiety Disord. 2014;28(1):97–107. doi: 10.1016/j.janxdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Buckley T.C., Blanchard E.B., Hickling E.J. Automatic and strategic processing of threat stimuli: a comparison between PTSD, panic disorder, and nonanxiety controls. Cogn. Ther. Res. 2002;26(1):97–115. [Google Scholar]
- Buss K.A., Davis E.L., Kiel E.J., Brooker R.J., Beekman C., Early M.C. Dysregulated fear predicts social wariness and social anxiety symptoms during kindergarten. J. Clin. Child Adolesc. Psychol. 2013;42(5):603–616. doi: 10.1080/15374416.2013.769170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L., Mercado F., Tapia M., Hinojosa J.A. Emotion, attention, and the negativity bias, studied through event-related potentials. Int. J. Psychophysiol. 2001;41(1):75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A., Degnan K.A., Pine D.S., Perez-Edgar K., Henderson H.A., Diaz Y., Fox N.A. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(9):928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J.A., Blackford J.U. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(10):1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C., Zapp D.J., Fettig N., Pérez-Edgar K.E. Impact of attention biases to threat and effortful control on individual variations in negative affect and social withdrawal in very young children. J. Exp. Child Psychol. 2016;141:210–221. doi: 10.1016/j.jecp.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll C.G., Kagan J., Reznick J.S. Behavioral inhibition in young children. Child Dev. 1984:1005–1019. [Google Scholar]
- Dennis T.A., Chen C.-C. Neurophysiological mechanisms in the emotional modulation of attention: the interplay between threat sensitivity and attentional control. Biol. Psychol. 2007;76(1):1–10. doi: 10.1016/j.biopsycho.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T.A., Chen C.-C. Trait anxiety and conflict monitoring following threat: an ERP study. Psychophysiology. 2009;46(1):122–131. doi: 10.1111/j.1469-8986.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson M.W., Klein D.N., Olino T.M., Dougherty L.R., Durbin C.E. Social and non-social behavioral inhibition in preschool-age children: differential associations with parent-reports of temperament and anxiety. Child Psychiatry Hum. Dev. 2011;42(4):390–405. doi: 10.1007/s10578-011-0225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M., Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45(1):15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. Facial expression and emotion. Am. Psychol. 1993;48(4):384–392. doi: 10.1037//0003-066x.48.4.384. [DOI] [PubMed] [Google Scholar]
- Eldar S., Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychol. Med. 2010;40(04):667–677. doi: 10.1017/S0033291709990766. [DOI] [PubMed] [Google Scholar]
- Eldar S., Yankelevitch R., Lamy D., Bar-Haim Y. Enhanced neural reactivity and selective attention to threat in anxiety. Biol. Psychol. 2010;85(2):252–257. doi: 10.1016/j.biopsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Fox A.S., Kalin N.H. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am. J. Psychiatry. 2014;171:1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.A., Pine D.S. Temperament and the emergence of anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(2):125–128. doi: 10.1016/j.jaac.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Marshall P.J., Nichols K.E., Ghera M.M. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu. Rev. Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox E., Derakshan N., Shoker L. Trait anxiety modulates the electrophysiological indices of rapid spatial orienting towards angry faces. Neuroreport. 2008;19(3):259–263. doi: 10.1097/WNR.0b013e3282f53d2a. [DOI] [PubMed] [Google Scholar]
- Fu, X., Taber-Thomas, B.C., Pérez-Edgar, K., in press. Frontolimbic functioning duringthreat-related attention: relations to early behavioral inhibition and anxiety inchildren. Biol. Psychol. 10.1016/j.biopsycho.2015.08.010. [DOI] [PMC free article] [PubMed]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gregory A.M., Eley T.C. Genetic influences on anxiety in children: what we’ve learned and where we’re heading. Clin. Child Fam. Psychol. Rev. 2007;10(3):199–212. doi: 10.1007/s10567-007-0022-8. [DOI] [PubMed] [Google Scholar]
- Halit H., de Haan M., Johnson M.H. Modulation of event-related potentials by prototypical and atypical faces. Neuroreport. 2000;11(9):1871–1875. doi: 10.1097/00001756-200006260-00014. [DOI] [PubMed] [Google Scholar]
- Hardee J.E., Benson B.E., Bar-Haim Y., Mogg K., Bradley B.P., Chen G. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol. Psychiatry. 2013;74(4):273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. 2012. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper]http://www.afhayes.com/public/process2012.pdf Retrieved from. [Google Scholar]
- Hayes A.F. An index and test of linear moderated mediation. Multivar. Behav. Res. 2015;50(1):1–22. doi: 10.1080/00273171.2014.962683. [DOI] [PubMed] [Google Scholar]
- Helfinstein S.M., White L.K., Bar-Haim Y., Fox N.A. Affective primes suppress attention bias to threat in socially anxious individuals. Behav. Res. Ther. 2008;46(7):799–810. doi: 10.1016/j.brat.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson H.A., Pine D.S., Fox N.A. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2015;40(1):207–224. doi: 10.1038/npp.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-X., Luo Y.-J. Temporal course of emotional negativity bias: an ERP study. Neurosci. Lett. 2006;398(1):91–96. doi: 10.1016/j.neulet.2005.12.074. [DOI] [PubMed] [Google Scholar]
- Hum K.M., Manassis K., Lewis M.D. Neural mechanisms of emotion regulation in childhood anxiety. J. Child Psychol. Psychiatry. 2013;54(5):552–564. doi: 10.1111/j.1469-7610.2012.02609.x. [DOI] [PubMed] [Google Scholar]
- Itier R.J., Taylor M.J. Face recognition memory and configural processing: a developmental ERP study using upright, inverted, and contrast-reversed faces. J. Cogn. Neurosci. 2004;16(3):487–502. doi: 10.1162/089892904322926818. [DOI] [PubMed] [Google Scholar]
- Jarcho J.M., Fox N.A., Pine D.S., Etkin A., Leibenluft E., Shechner T., Ernst M. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol. Psychol. 2013;92(2):306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho J.M., Fox N.A., Pine D.S., Leibenluft E., Shechner T., Degnan K.A., Ernst M. Enduring influence of early temperament on neural mechanisms mediating attention–emotion conflict in adults. Depression Anxiety. 2014;31(1):53–62. doi: 10.1002/da.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman E.S., Farrens J.L., Luck S.J., Proudfit G.H. Behavioral and ERP measures of attentional bias to threat in the dot-probe task: poor reliability and lack of correlation with anxiety. Front Psychol. 2014;5:1368. doi: 10.3389/fpsyg.2014.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman E.S., MacNamara A., Proudfit G.H. Electrocortical evidence for rapid allocation of attention to threat in the dot-probe task. Social Cogn. Affect. Neurosci. 2015;10:577–583. doi: 10.1093/scan/nsu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I.-T., Miltner W.H. Psychophysiological correlates of face processing in social phobia. Brain Res. 2006;1118(1):130–141. doi: 10.1016/j.brainres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Lamm C., Walker O.L., Degnan K.A., Henderson H.A., Pine D.S., McDermott J.M., Fox N.A. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Dev. Sci. 2014;17(5):667–681. doi: 10.1111/desc.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinus M., Taylor M.J. Holistic processing of faces: learning effects with Mooney faces. J. Cogn. Neurosci. 2005;17(8):1316–1327. doi: 10.1162/0898929055002490. [DOI] [PubMed] [Google Scholar]
- Linetzky M., Pergamin-Hight L., Pine D.S., Bar-Haim Y. Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depress. Anxiety. 2015;32(6):383–391. doi: 10.1002/da.22344. [DOI] [PubMed] [Google Scholar]
- Luck S.J. MIT press; 2014. An introduction to the event-related potential technique. [Google Scholar]
- MacLeod C., Mathews A., Tata P. Attentional bias in emotional disorders. J. Abnorm. Psychol. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mangun G.R., Buck L.A. Sustained visual-spatial attention produces costs and benefits in response time and evoked neural activity. Neuropsychologia. 1998;36(3):189–200. doi: 10.1016/s0028-3932(97)00123-1. [DOI] [PubMed] [Google Scholar]
- Mangun G.R. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32(1):4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- McDermott J.M., Perez-Edgar K., Henderson H.A., Chronis-Tuscano A., Pine D.S., Fox N.A. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol. Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N., Corr P.J. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004;28(3):285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Morales S., Perez-Edgar K., Buss K. Attention biases towards and away from threat mark the relation between early dysregulated fear and the later emergence of social withdrawal. J. Abnorm. Child Psychol. 2015;43:1067–1078. doi: 10.1007/s10802-014-9963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, S., Taber-Thomas, B., Pérez-Edgar, K., in press. Patterns of attention to threatacross tasks in behaviorally inhibited children at risk for anxiety. Dev. Sci. 10.1111/desc.12391. [DOI] [PMC free article] [PubMed]
- Mueller E., Hofmann S., Santesso D., Meuret A., Bitran S., Pizzagalli D.A. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychol. Med. 2009;39(07):1141–1152. doi: 10.1017/S0033291708004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole L., Dennis T.A. Attention training and the threat bias: an ERP study. Brain Cogn. 2012;78(1):63–73. doi: 10.1016/j.bandc.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Fox N.A. Temperament and anxiety disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2005;14(4):681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K.E., Guyer A.E. Behavioral inhibition: temperament or prodrome? Curr. Behav. Neurosci. Rep. 2014;1(3):182–190. doi: 10.1007/s40473-014-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Bar-Haim Y., McDermott J.M., Chronis-Tuscano A., Pine D.S., Fox N.A. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10(3):349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Reeb-Sutherland B.C., McDermott J.M., White L.K., Henderson H.A., Degnan K.A., Fox N.A. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. J. Abnorm. Child Psychol. 2011;39(6):885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G., Grandjean D., Sander D., Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cereb. Cortex. 2004;14(6):619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Rucker D.D., Hayes A.F. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivar. Behav. Res. 2007;42(1):185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Price R.B., Siegle G.J., Silk J.S., Ladouceur C.D., McFarland A., Dahl R.E., Ryan N.D. Looking under the hood of the dot-probe task: an fMRI study in anxious youth. Depress. Anxiety. 2014;31(3):178–187. doi: 10.1002/da.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol M., Campanella S., Bissot C., Philippot P. Fear of negative evaluation and attentional bias for facial expressions: an event-related study. Brain Cogn. 2013;82(3):344–352. doi: 10.1016/j.bandc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Salum G., Mogg K., Bradley B., Gadelha A., Pan P., Tamanaha A. Threat bias in attention orienting: evidence of specificity in a large community-based study. Psychol. Med. 2013;43(04):733–745. doi: 10.1017/S0033291712001651. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Meuret A.E., Hofmann S.G., Mueller E.M., Ratner K.G., Roesch E.B., Pizzagalli D.A. Electrophysiological correlates of spatial orienting towards angry faces: a source localization study. Neuropsychologia. 2008;46(5):1338–1348. doi: 10.1016/j.neuropsychologia.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H.T., Junghöfer M., Weike A.I., Hamm A.O. Attention and emotion: an ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003;14(8):1107–1110. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Öhman A., Junghöfer M., Weike A.I., Stockburger J., Hamm A.O. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4(2):189. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Shaffer D., Fisher P., Lucas C.P., Dulcan M.K., Schwab-Stone M.E. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sun J., Sun B., Wang B., Gong H. The processing bias for threatening cues revealed by event-related potential and event-related oscillation analyses. Neuroscience. 2012;203:91–98. doi: 10.1016/j.neuroscience.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen V., Carter C.S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002;77(4):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Wald I., Shechner T., Bitton S., Holoshitz Y., Charney D., Muller D., Bar-Haim Y. Attention bias away from threat during life threatening danger predicts PTSD symptoms at one-year follow-up. Depress. Anxiety. 2011;28(5):406–411. doi: 10.1002/da.20808. [DOI] [PubMed] [Google Scholar]
- Waters A.M., Bradley B., Mogg K. Biased attention to threat in paediatric anxiety disorders (generalized anxiety disorder, social phobia, specific phobia, separation anxiety disorder) as a function of distress versus feardiagnostic categorization. Psychol. Med. 2014;44(03):607–616. doi: 10.1017/S0033291713000779. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio TX: 2003. Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV) [Google Scholar]
- White L.K., McDermott J.M., Degnan K.A., Henderson H.A., Fox N.A. Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. J. Abnorm. Child Psychol. 2011;39(5):735–747. doi: 10.1007/s10802-011-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L., Henderson H., Pérez-Edgar K., Walker O., Degnan K., Shechner T., Fox N. Developmental relations between behavioral inhibition, anxiety, and attention biases to threat and positive information. Child Dev. 2016 doi: 10.1111/cdev.12696. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N., Cohen J.D. The impact of cognitive deficits on conflict monitoring predictable dissociations between the error-related negativity and N2. Psychol. Sci. 2006;17(2):164–171. doi: 10.1111/j.1467-9280.2006.01680.x. [DOI] [PubMed] [Google Scholar]