Abstract
Objectives
To establish sessile serrated adenoma/polyps (SSA/Ps) as direct precursors of CRC, we identified colon carcinomas (CC) arising from SSA/Ps and examined molecular features of the serrated neoplasia pathway.
Methods
Thirty-three CCs arising from SSA/Ps were identified among 2,646 CCs included in the surgical pathology database at Mayo Clinic (2006–2012). Mutant BRAF (V600E) and MLH1 protein expression were analyzed by immunohistochemistry.
Results
Among patients with SSA/P-associated CCs, median patient age was 75 yrs., 24 (73%) developed in women, 31 (94%) were located in the proximal colon, and 23 (69%) were TNM stage I or II cancers. Furthermore, 31 (94 %) CCs expressed mutant BRAF of which 26 (79%) also showed MLH1 loss indicating deficient DNA mismatch repair (dMMR) of sporadic origin. Twenty-two (67%) were interval cancers that were more common in women and did not differ significantly by TNM stage, BRAF, or MLH1 status.
Conclusions
Direct histopathological evidence of colon carcinoma arising from SSA/P with frequent dysplasia was found. Cancers arising from SSA/Ps were predominantly right-sided and had mutated BRAF and MLH1 loss which supports the SSA/P as the predominant precursor of sporadic colon cancers with dMMR.
Keywords: Sessile serrated polyps, interval colorectal cancer, microsatellite instability, DNA mismatch repair
INTRODUCTION
Approximately 20% of colon cancers (CCs) develop via a serrated neoplasia pathway with the precursor lesion being the sessile serrated adenoma/polyp (SSA/P).1, 2 While CCs commonly contain adenomatous polyp tissue, direct histopathological evidence for CCs arising from SSA/Ps is sparse. Compared to adenomas, SSA/Ps typically lack dysplasia and have different molecular features.3 An initiating molecular event in this pathway is mutational activation of the BRAF oncogene that is detected in approximately 80% of SSA/Ps.4 SSA/Ps also display frequent hypermethylation at CpG islands in the promoter regions of cancer-associated genes that is termed CIMP (CpG island methylator phenotype.2 The subset of sporadic CCs with deficient DNA mismatch repair (dMMR) show hypermethylation of the MLH1 MMR gene and are enriched in BRAFV600E mutations, suggesting that SSA/Ps may be precursor lesions of dMMR cancers and potentially, tumors with mutated BRAF and proficient (p) MMR.5
Given the predilection of SSA/Ps for the right colon and difficulty in their endoscopic detection due to flat morphology, it has been speculated that SSA/Ps may contribute to the reduced effectiveness of colonoscopy to protect against right versus left sided colon cancers.6 Furthermore, SSA/Ps are potential precursors of interval colon cancers, defined as cancers that are detected between recommended intervals for screening or surveillance colonoscopy.7
We sought to establish SSA/Ps as direct precursors of CC by identifying colon cancers arising from SSA/Ps, and by examining key molecular features of the serrated neoplasia pathway.
Materials and Methods
Study Population
From surgical pathology database at the Mayo Clinic (2006–2012), colorectal (CRC) cases (N=2,646) were identified and those with SSA/P and CRC were selected and re-reviewed histopathologically by a GI pathologist (T.C.S.) to confirm those with adenocarcinomas that was contiguous/adjacent to SSA/P tissue (N=33). Patient records were reviewed for demographics, tumor TNM, indications for and details of prior colonoscopic exams, and time between screening or surveillance colonoscopy to CRC diagnosis. Tumor location was categorized as proximal to the splenic flexure or distal which included the splenic flexure or distal to it. Interval cancers were defined as those diagnosed after a negative screening or surveillance colonoscopy for CRC and before the next recommended exam.7 The study was approved by the Institutional Review Board of the Mayo Clinic.
Biomarker Analysis
Formalin-fixed, paraffin embedded (FFPE) tissue sections were analyzed by immunohistochemistry (IHC). Staining for BRAF was performed with a pan-BRAF antibody (pBR1 clone; Spring Bioscience, Inc., Pleasanton, CA) diluted 1:100 at 37°C for 16 min. We then used an anti- BRAFV600E mouse monoclonal antibody (VE1 clone; Spring Bioscience, Inc.) raised against an immunogenic synthetic peptide derived from the internal region of the BRAFV600E protein.8 Tissue sections were incubated with the VE1 antibody (diluted 1:45) at 37°C for 16 min. Proteins were detected using the OptiView DAB Detection Kit (Ventana). For immunohistochemistry to detect MLH1, antigen retrieval was performed and slides were incubated with the primary antibody (MLH1; dilution 1:10) for 1 hr at room temperature, as previously described.9 For the VE1 antibody, the slides were assessed for cytoplasmic staining only, and any nuclear staining or weak staining of interspersed cells was scored as negative. MLH1 expression was defined as absent when nuclear staining of tumor cells was not detected in the presence of positive staining in surrounding cells. Loss of MLH1 expression was interpreted to indicate dMMR. Immunostained slides were evaluated independently by two pathologists (T.C.S., A.J.).
RESULTS
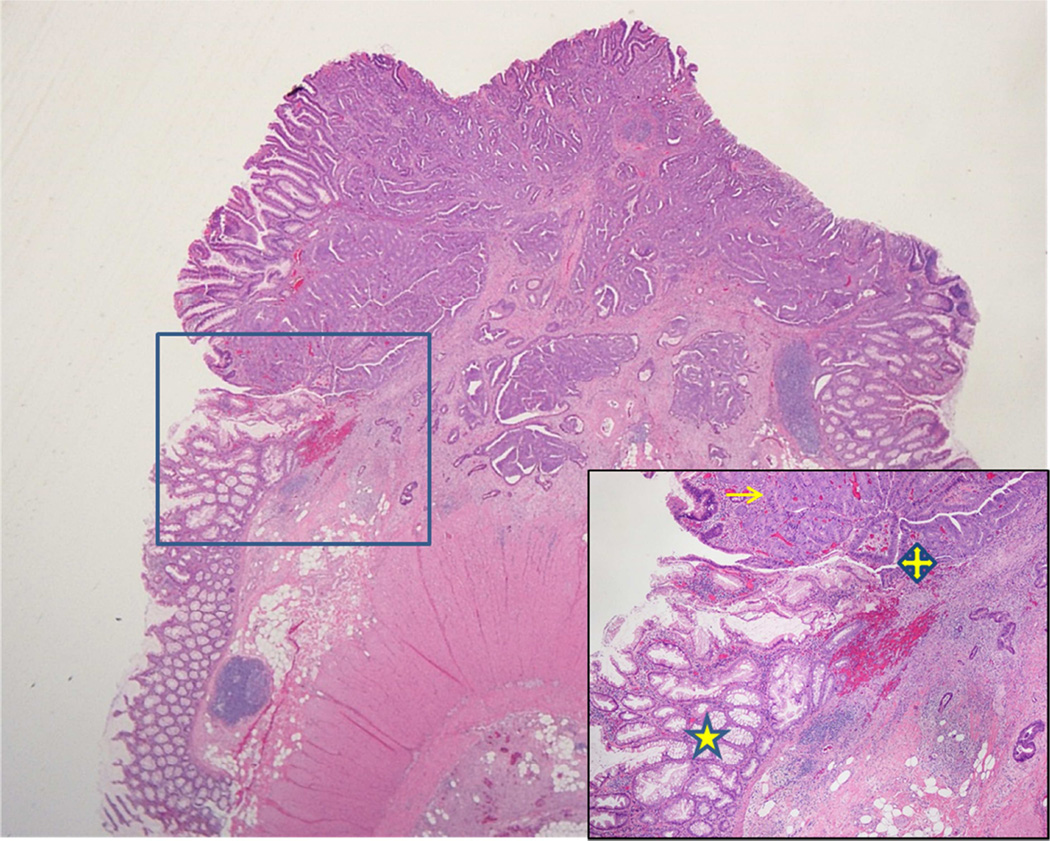
Of the 2,646 CRC cases contained in the Mayo Clinic surgical pathology database over the time period of 2006–2012, we selected and reviewed all cases with both CRC and SSA/P. We identified 33 colon adenocarcinomas that were confirmed to arise within or adjacent to an SSA/P and served as the study cohort. Within these SSA/Ps, we detected a subset [16 (48%)] that contained dysplasia in addition to the associated carcinoma, which indicates a SSA/P to dysplasia to carcinoma sequence (Figure 1). The median age of patients with SSA/P-associated cancers was 75 years (range: 64–92 years) with 73% of cancers occurring in women. Most of the cancers were early stage: TNM stage of 0–I (36%), II (33%), III (27%), and IV (3%), and the majority (94%) of cancers were located proximal to the splenic flexure.
Figure 1.
Carcinoma arising within an SSA/P. Inset shows serrated polyp to dysplasia to carcinoma pathway (star indicates SSA/P, quad arrow shows dysplastic component, arrow labels carcinoma).
Serrated Neoplasia Pathway
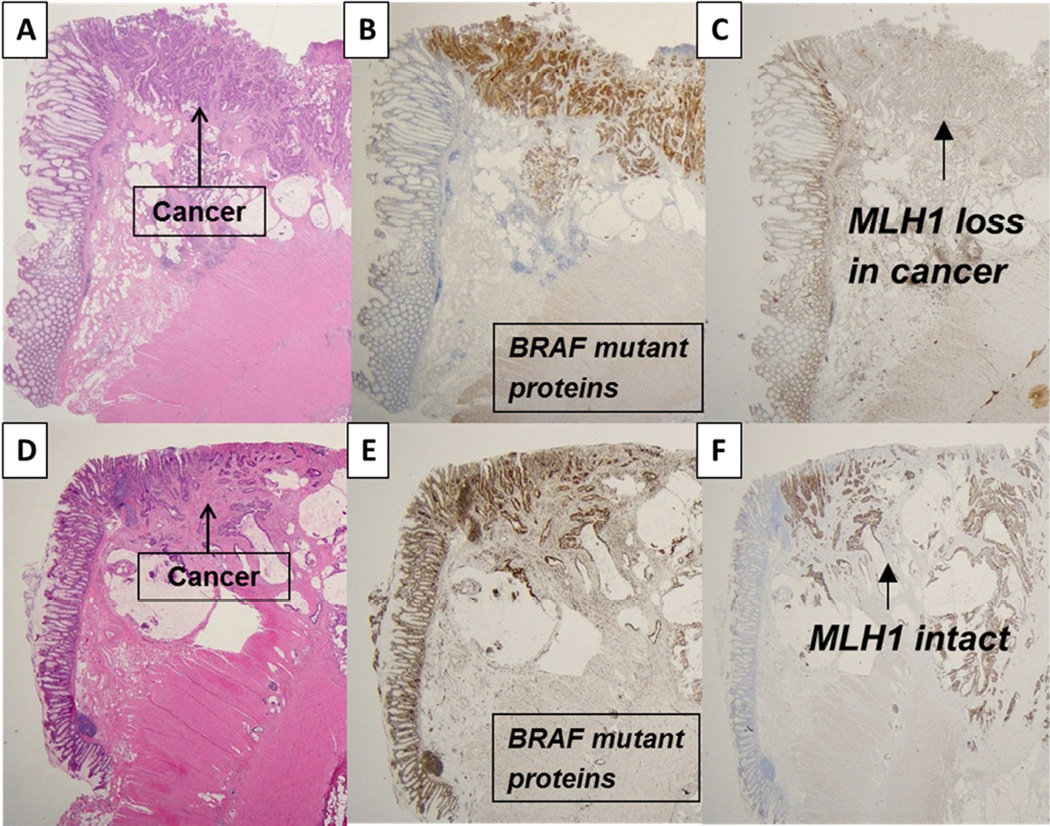
We analyzed mutant BRAF protein expression that results from the c.1799T>A p.V600E BRAF point mutation in exon 15. Immunohistochemical detection of BRAFV600E mutant protein using the VE1 antibody in CRC has been shown to be concordant with molecular testing in most10–13 but not all14 studies. Mutant BRAFV600E protein expression was detected in all SSA/Ps and in 31 of 33 (94 %) SSA/P associated carcinomas. All cases were analyzed for MLH1 protein expression and among cancers expressing mutant BRAFV600E proteins, MLH1 loss was detected in 26 (79%) indicating dMMR of sporadic origin. Figure 2 A–C shows adenocarcinoma arising within an SSA/P with expression of mutant BRAFV600E and loss of MLH1. While the majority of colon cancers associated with SSA/Ps showed dMMR, 21% of cancers with mutant BRAF proteins showed intact MLH1 indicating proficient MMR (pMMR) as shown in Figure 2 D–F. In addition, there were two cancers with wild-type BRAF proteins and intact MLH1 expression also consistent with pMMR.
Figure 2.
A–C shows MSI cancer arising in SSA/P with BRAF mutant protein expression in SSA/P and cancer with MLH1 loss. D–F shows a microsatellite stable cancer arising in an SSA/P that expresses mutant BRAF and MLH1 proteins.
Interval vs Non Interval Cancers
Two-thirds of colon cancer cases [22/33 (67%)] were diagnosed between the recommended colonoscopic screening or surveillance interval, thus meeting the definition of interval cancers.7 The mean interval from prior colonoscopy to CC diagnosis was 42 months (range: 12–96 months). The majority of interval cancers were found in the proximal [21/22 (95%)] vs distal colon as previously reported.6 Indications for colonoscopy that detected cancer were screening (2/33; 6%), polyp surveillance (9/33; 27%), or evaluation of symptoms (22/33; 67%). Patients with interval vs non-interval cancers were more likely women, but no significant differences were found by TNM stage or by BRAF or MMR status.
DISCUSSION
Limited histopathological evidence exists to indicate that SSA/Ps can serve as precursor lesions of CRC.15, 16 We found 33 CCs that arose within or contiguous to an SSA/P, and this progression was accompanied by dysplasia in approximately 50% of these SSA/Ps. Furthermore, we identified molecular features of the serrated neoplasia pathway whereby all SSA/Ps expressed mutant BRAF proteins and 79% of SSA/P-associated cancers showed MLH1 loss indicating sporadic MMR deficiency. Together, these data support histologic progression from SSA/P to dysplasia to carcinoma.
The initiating molecular event in the development of SSA/Ps is believed to be an activating point mutation (V600E) in the BRAF oncogene.1 Hypermethylation of MLH1 has been shown to coincide with the development of both dysplasia and dMMR with MSI in SSA/P.17 In addition to near uniform BRAF mutations in SSA/P-associated cancers, we found loss of MLH1 in 79% of tumors in this cohort suggesting that SSA/Ps are precursor lesions primarily for sporadic dMMR/MSI colon cancers.
In contrast to colon cancer associated SSA/Ps described in this report, SSA/Ps detected at colonoscopy infrequently contain dysplasia. In a large retrospective study of SSA/Ps removed at colonoscopy and not associated with cancer, the overall prevalence of dysplasia was 5%.18 We detected a high rate of dysplasia (48%) in SSA/Ps associated with cancer which supports a dysplasia to carcinoma sequence within SSA/Ps. SSA/Ps are believed to have the potential for rapid growth once dysplasia develops.19 Potentially, this may account for the paucity of colon cancers that arise within SSA/Ps compared to conventional adenomas due to rapid replacement of the SSA/P tissue by carcinoma. The relatively low prevalence of dysplasia in SSA/Ps without associated cancer suggests that only a small subset of these lesions have the potential for progression to cancer. This is supported by longitudinal observations on 23 large SSA/P found at screening colonoscopy that were left in situ and did not progress to cancer over a median of 11 years.20 This finding suggests that epigenetic silencing of MLH1 may be the critical step for SSA/P to progress to dysplasia and subsequent cancer.17. We acknowledge that our series of cancer-associated SSA/Ps represent a highly selected subset for which additional research is needed to identify factors associated with cancer predisposition. In addition, a limitation of our study was the requirement for contiguous SSA/P and carcinoma that may select for earlier stage cancers.
Within our patient cohort, two-thirds of CRC cases [22/33 (67%)] met the definition of interval cancers.7 The majority of SSA/P-associated interval cancers had dMMR which is consistent with a large, observational study where interval vs non interval colon cancers had a significantly higher rate of MSI/dMMR21. Our data suggest that SSA/Ps give rise to sporadic dMMR colon cancers that may disproportionately contribute to interval cancers. In our series, nearly all (95%) interval colon cancers occurred in the right colon where SSA/Ps and dMMR tumors preferentially occur.
In conclusion, our data provide direct histopathological evidence that SSA/Ps are precursor lesions of colon carcinomas. When associated with cancer, SSA/Ps were found to frequently contain dysplasia, consistent with an SSA/P to dysplasia to carcinoma sequence via the serrated neoplasia pathway. The finding of mutant BRAF combined with loss of MLH1 in the majority of SSA/P-associated cancers indicates that SSA/Ps are precursor lesions of sporadic dMMR/MSI colon cancers. Two-thirds of SSA/P-associated cancers were suspected interval malignancies of the proximal colon. Taken together, our data provide histopathological proof of the association of SSA/Ps with colon cancer and provide supportive evidence for SSA/Ps as precursors of sporadic dMMR/MSI colon cancers.
Acknowledgments
GRANT SUPPORT: This work was supported, in part, by a National Cancer Institute Senior Scientist Award (Grant number K05CA-142885) to F.A.S.
ABBREVIATIONS USED
- CRC
colorectal cancer
- DAB
diaminobenzidine
- dMMR
DNA mismatch repair
- MMR
mismatch repair
- MSI
microsatellite instability
- pMMR
proficient mismatch repair
- SSA/P
sessile serrated adenomas/polyp
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: No disclosures to report.
WRITING ASSISTANCE: None
AUTHOR CONTRIBUTIONS
Seth Sweetser – Design, acquisition of data, and drafting of manuscript; Andrea Jones-Design, acquisition of data, and drafting of manuscript; Thomas C. Smyrk– acquisition of data, and drafting of manuscript; Frank A. Sinicrope – design, drafting and critical revision of the manuscript for important intellectual content.
REFERENCES
- 1.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 2.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Sweetser S, Smyrk TC, Sinicrope FA. Serrated colon polyps as precursors to colorectal cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:760–767. doi: 10.1016/j.cgh.2012.12.004. quiz e54–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 5.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 6.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–1705. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Sanduleanu S, le Clercq CM, Dekker E, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64:1257–1267. doi: 10.1136/gutjnl-2014-307992. [DOI] [PubMed] [Google Scholar]
- 8.Capper D, Preusser M, Habel A, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122:11–19. doi: 10.1007/s00401-011-0841-z. [DOI] [PubMed] [Google Scholar]
- 9.Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31:3664–3672. doi: 10.1200/JCO.2013.48.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuan SF, Navina S, Cressman KL, et al. Immunohistochemical detection of BRAF V600E mutant protein using the VE1 antibody in colorectal carcinoma is highly concordant with molecular testing but requires rigorous antibody optimization. Human pathology. 2014;45:464–472. doi: 10.1016/j.humpath.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Sinicrope FA, Smyrk TC, Tougeron D, et al. Mutation-specific antibody detects mutant BRAFV600E protein expression in human colon carcinomas. Cancer. 2013;119:2765–2770. doi: 10.1002/cncr.28133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day F, Muranyi A, Singh S, et al. A mutant BRAF V600E-specific immunohistochemical assay: correlation with molecular mutation status and clinical outcome in colorectal cancer. Targeted oncology. 2015;10:99–109. doi: 10.1007/s11523-014-0319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan S, Arnason T, Drucker A, et al. The utility of BRAFV600E mutation-specific antibody for colon cancers with microsatellite instability. Applied immunohistochemistry & molecular morphology : AIMM / official publication of the Society for Applied Immunohistochemistry. 2014;22:e8–e13. doi: 10.1097/PAI.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 14.Estrella JS, Tetzlaff MT, Bassett RL, Jr, et al. Assessment of BRAF V600E Status in Colorectal Carcinoma: Tissue-Specific Discordances between Immunohistochemistry and Sequencing. Mol Cancer Ther. 2015;14:2887–2895. doi: 10.1158/1535-7163.MCT-15-0615. [DOI] [PubMed] [Google Scholar]
- 15.Boparai KS, Dekker E, Polak MM, et al. A serrated colorectal cancer pathway predominates over the classic WNT pathway in patients with hyperplastic polyposis syndrome. Am J Pathol. 2011;178:2700–2707. doi: 10.1016/j.ajpath.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettington M, Walker N, Rosty C, et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2015 doi: 10.1136/gutjnl-2015-310456. [DOI] [PubMed] [Google Scholar]
- 17.Sheridan TB, Fenton H, Lewin MR, et al. Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions "caught in the act". Am J Clin Pathol. 2006;126:564–571. doi: 10.1309/C7JE8BVL8420V5VT. [DOI] [PubMed] [Google Scholar]
- 18.Yang JF, Tang SJ, Lash RH, et al. Anatomic distribution of sessile serrated adenoma/polyp with and without cytologic dysplasia. Arch Pathol Lab Med. 2015;139:388–393. doi: 10.5858/arpa.2013-0523-OA. [DOI] [PubMed] [Google Scholar]
- 19.Oono Y, Fu K, Nakamura H, et al. Progression of a sessile serrated adenoma to an early invasive cancer within 8 months. Dig Dis Sci. 2009;54:906–909. doi: 10.1007/s10620-008-0407-7. [DOI] [PubMed] [Google Scholar]
- 20.Holme O, Bretthauer M, Eide TJ, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut. 2015;64:929–936. doi: 10.1136/gutjnl-2014-307793. [DOI] [PubMed] [Google Scholar]
- 21.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]