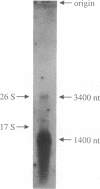
Abstract
Although the nonapeptide hormones vasopressin, oxytocin, and related peptides from vertebrates and some nonapeptides from invertebrates share similarities in amino acid sequence, their evolutionary relationships are not clear. To investigate this issue, we cloned a cDNA encoding a vasopressin-related peptide, Lys-conopressin, produced in the central nervous system of the gastropod mollusc Lymnaea stagnalis. The predicted preproconopressin has the overall architecture of vertebrate preprovasopressin, with a signal peptide, Lys-conopressin, that is flanked at the C terminus by an amidation signal and a pair of basic residues, followed by a neurophysin domain. The Lymnaea neurophysin and the vertebrate neurophysins share high sequence identity, which includes the conservation of all 14 cysteine residues. In addition, the Lymnaea neurophysin possesses unique structural characteristics. It contains a putative N-linked glycosylation site at a position in the vertebrate neurophysins where a strictly conserved tyrosine residue, which plays an essential role in binding of the nonapeptide hormones, is found. The C-terminal copeptin homologous extension of the Lymnaea neurophysin has low sequence identity with the vertebrate counterparts and is probably not cleaved from the prohormone, as are the mammalian copeptins. The conopressin gene is expressed in only a few neurons in both pedal ganglia of the central nervous system. The conopressin transcript is present in two sizes, due to alternative use of polyadenylylation signals. The data presented here demonstrate that the typical organization of the prohormones of the vasopressin/oxytocin superfamily must have been present in the common ancestors of vertebrates and invertebrates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie D. M., McCormick W. M., Chaiken I. M. Photoaffinity labeling of the hormone binding site of neurophysin. J Biol Chem. 1982 Mar 10;257(5):2274–2281. [PubMed] [Google Scholar]
- Acher R. Molecular evolution of biologically active polypeptides. Proc R Soc Lond B Biol Sci. 1980 Oct 29;210(1178):21–43. doi: 10.1098/rspb.1980.0116. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Gainer H. Peptide regulation of bursting pacemaker activity in a molluscan neurosecretory cell. Science. 1974 Jun 28;184(4144):1371–1373. doi: 10.1126/science.184.4144.1371. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Ifshin M. S., Gainer N. Studies on bursting pacemaker potential activity in molluscan neurons. III. Effects of hormones. Brain Res. 1975 Feb 14;84(3):501–513. doi: 10.1016/0006-8993(75)90768-4. [DOI] [PubMed] [Google Scholar]
- Burman S., Wellner D., Chait B., Chaudhary T., Breslow E. Complete assignment of neurophysin disulfides indicates pairing in two separate domains. Proc Natl Acad Sci U S A. 1989 Jan;86(2):429–433. doi: 10.1073/pnas.86.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Q., Rose J. P., Breslow E., Yang D., Chang W. R., Furey W. F., Jr, Sax M., Wang B. C. Crystal structure of a bovine neurophysin II dipeptide complex at 2.8 A determined from the single-wavelength anomalous scattering signal of an incorporated iodine atom. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4240–4244. doi: 10.1073/pnas.88.10.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L. J., de Santos V., Zafaralla G. C., Ramilo C. A., Zeikus R., Gray W. R., Olivera B. M. Invertebrate vasopressin/oxytocin homologs. Characterization of peptides from Conus geographus and Conus straitus venoms. J Biol Chem. 1987 Nov 25;262(33):15821–15824. [PubMed] [Google Scholar]
- Devi L. Consensus sequence for processing of peptide precursors at monobasic sites. FEBS Lett. 1991 Mar 25;280(2):189–194. doi: 10.1016/0014-5793(91)80290-j. [DOI] [PubMed] [Google Scholar]
- Dirks R. W., Van Gijlswijk R. P., Vooijs M. A., Smit A. B., Bogerd J., van Minnen J., Raap A. K., Van der Ploeg M. 3'-end fluorochromized and haptenized oligonucleotides as in situ hybridization probes for multiple, simultaneous RNA detection. Exp Cell Res. 1991 Jun;194(2):310–315. doi: 10.1016/0014-4827(91)90370-a. [DOI] [PubMed] [Google Scholar]
- Figueroa J., Morley S. D., Heierhorst J., Krentler C., Lederis K., Richter D. Two isotocin genes are present in the white sucker Catostomus commersoni both lacking introns in their protein coding regions. EMBO J. 1989 Oct;8(10):2873–2877. doi: 10.1002/j.1460-2075.1989.tb08435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heierhorst J., Mahlmann S., Morley S. D., Coe I. R., Sherwood N. M., Richter D. Molecular cloning of two distinct vasotocin precursor cDNAs from chum salmon (Oncorhynchus keta) suggests an ancient gene duplication. FEBS Lett. 1990 Jan 29;260(2):301–304. doi: 10.1016/0014-5793(90)80129-7. [DOI] [PubMed] [Google Scholar]
- Hyodo S., Kato Y., Ono M., Urano A. Cloning and sequence analyses of cDNAs encoding vasotocin and isotocin precursors of chum salmon, Oncorhynchus keta: evolutionary relationships of neurohypophysial hormone precursors. J Comp Physiol B. 1991;160(6):601–608. doi: 10.1007/BF00571256. [DOI] [PubMed] [Google Scholar]
- Ifshin M. S., Gainer H., Barker J. L. Peptide factor extracted from molluscan ganglia that modulates bursting pacemaker activity. Nature. 1975 Mar 6;254(5495):72–74. doi: 10.1038/254072a0. [DOI] [PubMed] [Google Scholar]
- Lazure C., Saayman H. S., Naudé R. J., Oelofsen W., Chrétien M. Complete amino acid sequence of a VLDV-type neurophysin from ostrich differs markedly from known mammalian neurophysins. Int J Pept Protein Res. 1987 Nov;30(5):634–645. doi: 10.1111/j.1399-3011.1987.tb03374.x. [DOI] [PubMed] [Google Scholar]
- Michel G., Chauvet J., Chauvet M. T., Acher R. One-step processing of the amphibian vasotocin precursor: structure of a frog (Rana esculenta) "big" neurophysin. Biochem Biophys Res Commun. 1987 Dec 16;149(2):538–544. doi: 10.1016/0006-291x(87)90401-3. [DOI] [PubMed] [Google Scholar]
- Morley S. D., Schönrock C., Heierhorst J., Figueroa J., Lederis K., Richter D. Vasotocin genes of the teleost fish Catostomus commersoni: gene structure, exon-intron boundary, and hormone precursor organization. Biochemistry. 1990 Mar 13;29(10):2506–2511. doi: 10.1021/bi00462a011. [DOI] [PubMed] [Google Scholar]
- Nagy G., Mulchahey J. J., Smyth D. G., Neill J. D. The glycopeptide moiety of vasopressin-neurophysin precursor is neurohypophysial prolactin releasing factor. Biochem Biophys Res Commun. 1988 Feb 29;151(1):524–529. doi: 10.1016/0006-291x(88)90625-0. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton D., Sardana V., Breslow E. Application of peptide-mediated ring current shifts to the study of neurophysin-peptide interactions: a partial model of the neurophysin-peptide complex. Biochemistry. 1987 Mar 24;26(6):1518–1525. doi: 10.1021/bi00380a004. [DOI] [PubMed] [Google Scholar]
- Proux J. P., Miller C. A., Li J. P., Carney R. L., Girardie A., Delaage M., Schooley D. A. Identification of an arginine vasopressin-like diuretic hormone from Locusta migratoria. Biochem Biophys Res Commun. 1987 Nov 30;149(1):180–186. doi: 10.1016/0006-291x(87)91621-4. [DOI] [PubMed] [Google Scholar]
- Rehbein M., Hillers M., Mohr E., Ivell R., Morley S., Schmale H., Richter D. The neurohypophyseal hormones vasopressin and oxytocin. Precursor structure, synthesis and regulation. Biol Chem Hoppe Seyler. 1986 Aug;367(8):695–704. doi: 10.1515/bchm3.1986.367.2.695. [DOI] [PubMed] [Google Scholar]
- Reich G. A new peptide of the oxytocin/vasopressin family isolated from nerves of the cephalopod Octopus vulgaris. Neurosci Lett. 1992 Jan 6;134(2):191–194. doi: 10.1016/0304-3940(92)90514-8. [DOI] [PubMed] [Google Scholar]
- Robinson B. G., Frim D. M., Schwartz W. J., Majzoub J. A. Vasopressin mRNA in the suprachiasmatic nuclei: daily regulation of polyadenylate tail length. Science. 1988 Jul 15;241(4863):342–344. doi: 10.1126/science.3388044. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer W. H., Deyrup-Olsen I., Martin A. W. Immunological and biological characteristics of the vasotocin-like activity in the head ganglia of gastropod molluscs. Gen Comp Endocrinol. 1984 Apr;54(1):97–108. doi: 10.1016/0016-6480(84)90204-1. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil E., Jackson J. F., Bouwmeester T., Smit A. B., Van Minnen J., Van Heerikhuizen H., Klootwijk J., Joosse J. Isolation, characterization, and evolutionary aspects of a cDNA clone encoding multiple neuropeptides involved in the stereotyped egg-laying behavior of the freshwater snail Lymnaea stagnalis. J Neurosci. 1988 Nov;8(11):4184–4191. doi: 10.1523/JNEUROSCI.08-11-04184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren R. E., Smit A. B., de With N. D., van Minnen J., Dirks R. W., van der Schors R. C., Joosse J. A vasopressin-related peptide in the mollusc Lymnaea stagnalis: peptide structure, prohormone organization, evolutionary and functional aspects of Lymnaea conopressin. Prog Brain Res. 1992;92:47–57. doi: 10.1016/s0079-6123(08)61164-4. [DOI] [PubMed] [Google Scholar]