Abstract
Aims
Cardiovascular autonomic neuropathy (CAN) predicts clinical diabetic nephropathy (DN). We investigated the relationship between DN structural lesions and CAN.
Methods
Sixty three Pima Indians with type 2 diabetes underwent kidney biopsies following a 6-year clinical trial testing the renoprotective efficacy of losartan vs. placebo. CAN was assessed a median 9.2 years later. CAN variables included expiration/inspiration ratio (E/I), standard deviation of the normal RR interval (sdNN), and low and high frequency signal power and their ratio (LF, HF, LF/HF); lower values reflect more severe neuropathy. Associations of CAN with renal structural variables were assessed by linear regression adjusted for age, sex, diabetes duration, blood pressure, HbA1c, glomerular filtration rate, and treatment assignment during the trial.
Results
Global glomerular sclerosis was negatively associated with sdNN (partial r = −0.35, p = 0.01) and LF (r = −0.32, p = 0.02); glomerular basement membrane width was negatively associated with all measures of CAN except for LF/HF (r = −0.28 to −0.42, p<0.05); filtration surface density was positively associated with sdNN, LF, and HF (r = 0.31 to 0.38, p<0.05); and cortical interstitial fractional volume was negatively associated with HF (r = −0.27, p = 0.04).
Conclusions
CAN associates with DN lesions.
Keywords: Type 2 Diabetes, Cardiovascular Autonomic Neuropathy, Diabetic Nephropathy, American Indians
1. Introduction
Cardiovascular autonomic neuropathy (CAN) and diabetic nephropathy (DN) are common complications of diabetes with major impacts on morbidity and mortality (1). CAN is the consequence of damage to and loss of the small, unmyelinated nerve fibers that innervate the heart and blood vessels, resulting in abnormalities of heart rate control and vascular dynamics; the pathophysiology is similar to that of peripheral neuropathy. Presence of CAN, which may be documented by abnormal cardiovascular reflex tests and reduction in heart rate variability (HRV), increases the risk of all-cause mortality 3-fold in those with diabetes. DN is the leading cause of end-stage renal disease worldwide (2, 3), and its interrelationship with CAN remains unclear. Persons with either type 1 or type 2 diabetes who also have CAN have a faster rate of renal function decline than those who do not (4–7), suggesting an association between these complications (4–13) which may be attributable to CAN-induced changes in renal hemodynamics (7, 9, 11, 12). The structural underpinnings of this relationship have not been assessed.
In the present study, we examined the association between CAN and preceding DN lesions in Pima Indians with type 2 diabetes using standard measures of CAN and morphometry of kidney tissue obtained via protocol research biopsy.
2. Subjects
From 1965–2007, Pima Indians from the Gila River Indian Community participated in a longitudinal study of diabetes and its complications. We selected 169 adults with type 2 diabetes from this population to participate in a randomized clinical trial testing the renoprotective efficacy of losartan in early DN (ClinicalTrials.gov number, NCT00340678). Ninety-one subjects had normal urinary albumin excretion (albumin/creatinine ratio [ACR] <30 mg/g) at baseline and 78 had microalbuminuria (ACR=30–299 mg/g). Glomerular filtration rate (GFR) was measured annually throughout the trial, and the pre-specified primary endpoint was a decline in GFR to ≤60 ml/min or to half the baseline value in subjects who entered the study with a GFR <120 ml/min. At the end of the six-year clinical trial, 111 of the subjects underwent kidney biopsy (60 with normoalbuminuria and 51 with microalbuminuria at baseline) to determine whether treatment was associated with preservation of kidney structure (14). Among subjects with microalbuminuria at baseline, those who received losartan had lower mesangial fractional volume on average than those who received placebo, suggesting that losartan preserved some aspects of normal glomerular structure in early DN, although losartan did not significantly affect the primary outcome, i.e., decline in GFR.
We assessed CAN under a separate protocol a median of 9.3 years (range = 6.9–12.3 years) after kidney biopsy by cardiovascular reflex testing and heart rate variability studies in 63 participants. Subjects who died (n=11), developed end-stage renal disease (n=15), were lost to follow-up (n=20) or did not have full clinical data available at both the biopsy and the CAN evaluation (n=2) were excluded from the analysis.
Clinical data were collected within a median of 46 days (range = 7–216 days) of the kidney biopsy and again at the time of the CAN examination. At the exam nearest the kidney biopsy, 17 (49%) of the participants in the normoalbuminuria group and 16 (57%) in the microalbuminuria group were receiving losartan as part of the clinical trial. Upon completion of the clinical trial, participants received all medical care at the direction of their primary physicians.
This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each subject signed an informed consent document.
3. Materials and Methods
3.1 Clinical and Anthropometric Measures
BMI was defined as weight divided by the square of height (kg/m2). Blood pressure was measured while the subject was resting in the seated position; mean arterial pressure (MAP) was calculated as (2*diastolic blood pressure + systolic blood pressure)/3. HbA1c was measured by HPLC (Tosoh, Tokyo, Japan). HPLC was also used to measure urinary clearance of non-radioactive iothalamate for GFR determination (Waters, Milford, Massachusetts) (15). Urinary albumin concentration was measured by nephelometric immunoassay and urinary creatinine by a modified Jaffé reaction (Siemens, Erlangen, Germany) (16, 17). Urinary albumin concentration below the detection limit of the assay (≤6.8 mg/L) was set to 6.8 mg/L in the analyses. Inflammatory factors such as C-reactive protein, interleukin-6, and TNF-α were not measured in study participants.
3.2 Morphometric Measures of DN
We estimated renal structural parameters using quantitative morphometric methods on digital images obtained by masked unbiased random sampling of light and electron microscopy sections (14, 18). Parameters included in this study were mean glomerular volume, glomerular basement membrane (GBM) width, mesangial fractional volume, glomerular filtration surface density, total filtration surface per glomerulus, cortical interstitial fractional volume, percent globally sclerotic glomeruli, number of podocytes per glomerulus, podocyte foot process width, percent podocyte detachment, and percentage of normally fenestrated endothelium (14, 19–22). An equation that takes into account the smaller diameter of sclerotic glomeruli, and the consequent difference in the probability of encountering a sclerotic or nonsclerotic glomerulus in a random cross-section, was used to calculate the percentage of sclerotic glomeruli (23). Glomerular variables were calculated for each individual as the mean of all glomeruli evaluated (3±1) for that individual.
3.3 CAN Measures
We performed standardized CAN evaluations (deep breathing test and heart rate variability) on all subjects after an overnight fast as described previously (24). Subjects were asked to avoid vigorous exercise for 24 hours and caffeine and tobacco products for 8 hours prior to testing, and to hold any medicines on the day of testing until the evaluation was completed. The median fasting plasma glucose concentration at time of testing was 191 mg/dL (range=94 – 422 mg/dL). None of the subjects experienced a hypoglycemic episode within 24 hours prior to testing. Electrocardiogram (ECG) recordings were obtained after a 20-minute rest in the supine position using a physiologic monitor (Nightingale PPM2, Zoe Medical Inc., Topsfield, MA). Data were collected during a resting study (5 minutes) and a standard deep breathing test paced at 6 breaths/min (5 seconds of inspiration and 5 seconds of expiration), which is one of the most sensitive cardiovascular reflex tests for CAN assessment (24). We analyzed heart rate variability studies according to current guidelines using the continuous wavelet transform methods with the ANX 3.1 (ANSAR Inc., Philadelphia, PA) (25). This method incorporates respiratory activity in the formula and is considered superior for the analysis of nonstationary signals compared with the Fourier transform as it performs a time-frequency decomposition of the signal (26).
The following measures of CAN were predefined and analyzed as outcomes of interest: a) the expiration/inspiration (E/I) ratio, calculated as the longest R-R interval (in milliseconds) during expiration divided by the shortest R-R interval during inspiration, averaged for the six respiration cycles, and b) time- and frequency-domain measures of heart rate variability (HRV) using recordings during rest. These HRV measures included the standard deviation of the normal R-R interval (sdNN), low frequency signal power (LF, 0.04–0.15 Hz), high frequency signal power (HF, 0.15–0.40 Hz), and the LF/HF ratio.
The E/I ratio assesses the magnitude of sinus arrhythmia, a physiologic response mediated predominantly by the parasympathetic nervous system (25). The sdNN, a measure of overall heart rate variability, and the HF are largely mediated by parasympathetic activity. LF is affected by both sympathetic and parasympathetic modulation (27). Generally, increased sympathetic outflow leads to a reduction of both LF and HF. It is important to note that LF and HF are not necessarily indicative of autonomic activity, but rather autonomic regulation.
There are currently no established, validated cut-points on the measures of HRV to define the presence of CAN, but lower values reflect more advanced autonomic impairment. Therefore, we used continuous measures of CAN in the analyses.
3.4 Statistical Analysis
Data are presented as mean ± standard deviation or median (IQR). Since the clinical variables were recorded at the kidney biopsy and again during the CAN assessment, the clinical characteristics were reported as the mean (geometric mean for ACR) of these two measures and the means were used in the multivariable analyses. Associations of CAN measures with clinical and morphometric measures were examined by Spearman’s correlation. Differences in morphometric variables between subjects included in the study vs. those not included were evaluated by a Student’s t-test when the variances were equivalent and a Welch’s t-test when they were not. Linear regression models were used to examine the association between measures of CAN and morphometric variables adjusted for potential confounders. Three models were considered. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, diabetes duration, HbA1c, MAP, GFR, and treatment assignment during the clinical trial. Treatment assignment was included as this was an important exposure in the six years leading up to the present observational study. Model 3 was adjusted for all covariates in Model 2, plus ACR. Because ACR is highly correlated with the underlying lesions, Model 2 provides the best estimate of the association between measures of CAN and the structural lesions. Percent globally sclerotic glomeruli, percent denuded glomerular basement membrane, and all CAN variables were log-transformed due to non-normality (Shapiro-Wilk test). Regression model fit was assessed for normality and leverage with Studentized residuals. Multicollinearity was assessed with eigenvalues and the condition index (28). Each CAN variable was also tested for interaction with treatment assignment. Associations between CAN and morphometric measures were illustrated graphically and by partial Pearson correlation coefficients.
We performed three sensitivity analyses. In the first, we included clinical measures from both the biopsy exam and the CAN evaluation as covariates in the regression analyses, rather than the average of the two measures. In the second, we also adjusted for use of beta-blockers and renin-angiotensin-system inhibitors at the time of CAN evaluation. Lastly, to assess if beta-blockers or inhibitors of the renin-angiotensin system (RAS) affected the relationship between CAN and DN, we added an interaction term between each morphometric variable and use of each of these drugs. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
4. Results
The mean-averaged age of the 63 subjects (17 men, 46 women) was 51±9 years, HbA1c was 9.3±1.5% (78 mmol/mol), and GFR was 141±46 ml/min. The median diabetes duration was 18 years (IQR=16–25) and ACR was 45 mg/g (IQR=14–129). Fifty-eight (92%) of the participants reported taking RAS inhibitors for at least part of the time between the kidney biopsy and the CAN assessment. At the time of CAN assessment, 15 subjects reported taking beta-blockers and 40 subjects reported taking reninangiotensin system inhibitors. Clinical, morphometric, and CAN variables for the subjects are shown in Table 1 according to tertiles of the E/I ratio. Lower E/I ratio was associated with male sex, greater duration of diabetes, diastolic blood pressure, ACR, and serum creatinine concentration. Lower E/I ratio was also associated with a higher proportion of globally sclerotic glomeruli, greater GBM width and mesangial fractional volume, and reduced podocyte number per glomerulus. We observed similar associations with sdNN, LF, and HF. Participants who underwent kidney biopsy but were not included in the present study because of death, end-stage renal disease, loss to follow-up, or missing data had more severe structural lesions, including greater GBM width and lower filtration surface density and total filtration surface per glomerulus, than those who were included (Supplemental Table 1).
Table 1.
Subject characteristics according to tertiles of E/I ratio. Clinical measures are presented as the average values from the two clinical exams, and are given as either mean ± standard deviation or as median [interquartile range]. P-values are shown for Spearman’s correlations, and a chi-square test for sex.
| Characteristic | Tertile 1 n=24 |
Tertile 2 n=22 |
Tertile 3 n=17 |
p-value |
|---|---|---|---|---|
| Clinical Measures | ||||
| Age, years | 53.3 ± 8.6 | 48.0 ± 9.3 | 49.8 ± 9.7 | 0.17 |
| Sex, male (%) | 10 (42%) | 5 (22.7%) | 2 (11.8%) | 0.09 |
| Diabetes duration, years | 21.0 [18.3–27.1] | 17.0 [15.2–18.9] | 17.8 [15.5–24.5] | <0.01 |
| BMI, kg/m2 | 35.0 ± 6.5 | 38.4 ± 9.7 | 34.2 ± 7.4 | 0.96 |
| Systolic blood pressure, mmHg | 126 ± 12 | 121 ± 12 | 117 ± 10 | <0.01 |
| Diastolic blood pressure, mmHg | 78 ± 8 | 73 ± 7 | 73 ± 5 | 0.01 |
| Fasting plasma glucose, mg/dL (mmol/L) | 160 [191–226] (8.9) | 213 [166–260] (11.8) | 187 [154–219] (10.4) | 0.84 |
| HbA1c, % (mmol/mol) | 9.6 ± 1.6 (81) | 9.5 ± 1.3 (80) | 8.8 ± 1.4 (73) | 0.11 |
| Albumin/creatinine ratio, mg/g | 75.1 [23.5–385.2] | 48.7 [24.1–336.9] | 20.1 [8.5–36.6] | <0.01 |
| Serum creatinine, mg/dL | 82.2 ± 28.3 | 70.7 ± 15.9 | 63.7 ± 8.0 | <0.01 |
| GFR, ml/min | 136.1 ± 45.8 | 147.4 ± 45.2 | 140.8 ± 47.8 | 0.63 |
| Morphometric Measures | ||||
| Mean glomerular volume, ×106µ3 | 5.5 [5.0–6.8] | 5.7 [4.8–6.4] | 5.6 [4.7–6.7] | 0.99 |
| Globally sclerotic glomeruli, % | 13.1 [0.0–21.7] | 0.0 [0.0–9.1] | 0.0 [0.0–10.5] | 0.02 |
| Glomerular filtration surface density, µ2 µ3 | 0.07 [0.06–0.08] | 0.08 [0.07–0.10] | 0.08 [0.08–0.10] | 0.06 |
| Total filtration surface per glomerulus, ×105µ2 | 3.9 [3.4–5.1] | 4.0 [3.7–6.0] | 5.1 [4.1–6.9] | 0.11 |
| GBM width, nm | 521.8 [459.5–611.7] | 454.6 [423.5–526.8] | 402.5 [378.7–516.9] | <0.01 |
| Mesangial fractional volume, % | 19.6 [15.5–26.2] | 17.7 [14.3–21.3] | 14.1 [11.1–17.1] | <0.01 |
| Cortical interstitial fractional volume, % | 29.0 [25.0–32.5] | 29.4 [24.4–32.1] | 25.5 [20.3–30.1] | 0.15 |
| Podocyte number per glomerulus | 587.9 [506.0–687.1] | 667.5 [510.5–751.6] | 730.6 [695.2–974.8] | 0.06 |
| Foot process width, nm | 496.5 [408.5–546.0] | 436.1 [399.4–453.7] | 428.0 [398.3–452.0] | 0.01 |
| Podocyte detachment, % | 0.0 [0.0–0.4] | 0.0 [0.0–0.3] | 0.0 [0.0–0.6] | 0.13 |
| Fenestrated endothelium, % | 24.4 [21.1–32.3] | 27.9 [21.6–31.2] | 31.7 [28.8–35.2] | 0.04 |
| CAN Measures | ||||
| sdNN, ms | 11 [10–14] | 26 [17–28] | 27 [21–38] | <0.01 |
| Low frequency signal power, ms2 | 17 [11–25] | 69 [55–121] | 213 [80–502] | <0.01 |
| High frequency signal power, ms2 | 16.5 [10.5–27.0] | 61.5 [26–195] | 95 [65–236] | <0.01 |
| LF/HF Ratio | 1.05 [0.51–1.91] | 0.98 [0.62–2.63] | 1.77 [1.01–3.08] | 0.03 |
GBM, glomerular basement membrane; GFR, glomerular filtration rate; E/I ratio, expiratory/inspiratory ratio; sdNN, standard deviation of the normal R-R interval; LF, low frequency signal power; HF, high frequency signal power.
All CAN measures, except for HF and LF/HF, correlated with each other, and all except for LF/HF ratio correlated inversely with ACR, diabetes duration, and MAP (Supplemental Table 2). All measures of heart rate variability, except for LF/HF ratio, correlated with the proportion of globally sclerotic glomeruli, GBM width, mesangial fractional volume, and glomerular filtration surface density; E/I ratio, LF, and LF/HF ratio correlated with the percentage of fenestrated endothelium; sdNN, LF, and HF correlated with foot process width. One subject had a foot process width value 6.5 standard deviations above the mean. When this influential data point was removed from subsequent parametric analyses, foot process width was no longer associated with any CAN measures. Mean glomerular volume, number of podocytes per glomerulus, and percent podocyte detachment were also not significantly associated with any CAN measures.
Adjusted for sex, treatment assignment, and mean-averaged age, diabetes duration, HbA1c, MAP, and GFR, global glomerular sclerosis was negatively associated with sdNN (r=−0.35, p=0.01) and LF (r=− 0.32, p=0.02); GBM width was negatively associated with all measures of CAN except for LF/HF (r=− 0.28 to −0.42, p<0.05); glomerular filtration surface density was positively associated with sdNN, LF, and HF (r=0.31 to 0.38, p<0.05); cortical interstitial fractional volume was negatively associated with HF (r=− 0.27, p=0.04); and total filtration surface was positively, but not significantly, associated with HF (r=0.26, p=0.06) (Supplemental Table 3–5). The inclusion of sex in the model strengthened many of the relationships between the morphometric measures and CAN. Additional adjustment for ACR weakened several of the relationships, although global glomerular sclerosis and GBM width remained negatively associated with sdNN, LF, and HF, and glomerular filtration surface density remained positively associated with sdNN and HF, and showed a trend with LF (p=0.084). Partial Pearson correlation plots of the statistically significant adjusted correlations of the CAN measures with the morphometric measures are shown in Figure 1. For illustration, Figure 2 shows electron micrographs of the differences in GBM width between a subject with mild CAN and another with severe CAN. Figure 3 shows a partial residual regression plot of GBM width with several CAN measures.
Figure 1.
Partial Pearson correlation coefficients for morphometric variables and CAN measures. Model 1 is adjusted for age and sex. Model 2 is adjusted for age, sex, diabetes duration, HbA1c, mean arterial pressure, glomerular filtration rate, and treatment assignment during the clinical trial. Model 3 is adjusted for Model 2+ albumin/creatinine ratio. Asterisks denote a p-value < 0.05.
GS, globally sclerotic glomeruli; Sv, filtration surface density; TFS, total filtration surface per glomerulus; GBM, glomerular basement membrane width; VvInt, cortical interstitial fractional volume.
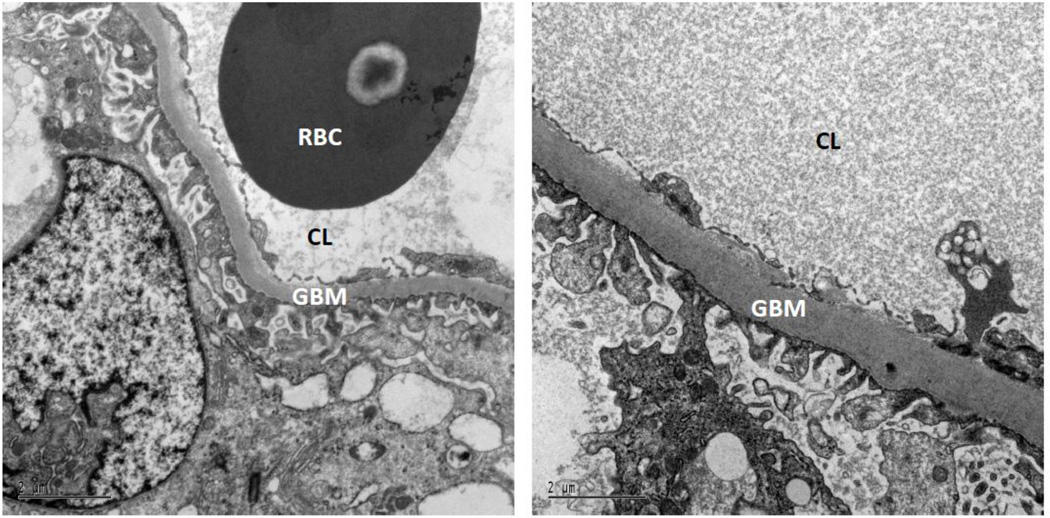
Figure 2.
GBM width was greater with increased severity of CAN. The left panel shows an individual with less severe CAN and GBM width = 301 nm, E/I ratio = 1.13, sdNN = 37 ms, ACR = 11 mg/g, and GFR = 115 ml/min. The right panel shows an individual with more severe CAN and GBM width = 848 nm, E/I ratio = 1.01, sdNN = 5 ms, ACR = 54 mg/g, and GFR = 193 ml/min. Both scale bars are equal to 2 µm.
ACR, albumin/creatinine ratio; CL, capillary lumen; GBM, glomerular basement membrane; GFR, glomerular filtration rate; RBC, red blood cell.
Figure 3.
Partial regression plots showing the relationship of GBM width with CAN measures after adjustment for age, sex, diabetes duration, HbA1c, mean arterial pressure, glomerular filtration rate, albumin/creatinine ratio, and treatment assignment during the clinical trial. GBM width was inversely associated with measures of heart rate variability, including the standard deviation of the normal R-R interval and low and high frequency power.
GBM, glomerular basement membrane; LF, low frequency signal power; HF, high frequency signal power; sdNN, standard deviation of the normal R-R interval.
In sensitivity analyses, our conclusions were unchanged when we included clinical measures from both the biopsy exam and the CAN evaluation as covariates, rather than the average of the two measures, or when we added treatment with beta-blockers or renin-angiotensin-system inhibitors to the model. We observed no interaction between treatment with beta-blockers or RAS inhibitors at the time of CAN evaluation and morphometric variables for any CAN measure, indicating that the relationships between morphometric lesions and CAN were not modified by these medications. We also observed no interactions between treatment assignment during the clinical trial and most morphometric variables. Losartan treatment assignment during the clinical trial did modify the relationship between GBM width and sdNN (p=0.04 for Model 2) or LF (p=0.03 for Model 2).
5. Discussion
In this cohort of Pima Indians with type 2 diabetes, we found significant associations between DN lesions and measures of CAN. Subjects with more advanced structural lesions, including greater GBM width, higher cortical interstitial fractional volume, higher proportion of globally sclerotic glomeruli, and lower glomerular filtration surface density and total filtration surface per glomerulus had more severe indices of CAN, as documented by a lower E/I ratio and lower indices of HRV. GBM width was the structural lesion most strongly and consistently associated with the various measures of CAN, followed by globally sclerotic glomeruli and filtration surface density. These associations remained significant after adjustment for potential confounders, including HbA1c, mean arterial pressure, and GFR. As expected, findings were somewhat attenuated in models that adjusted for ACR, since ACR is highly correlated with the underlying structural lesions. Neither treatment with beta-blockers nor RAS inhibitors at the time of CAN measurement modified the association between CAN and the structural lesions. Treatment assignment during the clinical trial may have modified the association between sdNN or LF and GBM width. Planned follow-up of these study participants may help us determine whether CAN indices will enhance prediction of kidney disease progression.
Functional measures of DN are related to CAN in both type 1 and type 2 diabetes (9, 11, 13). For example, higher 24-hr urinary albumin excretion and proteinuria were found in patients with type 1 diabetes who had reduced cardiac vagal activity (9, 13). In addition, among normotensive patients with type 1 diabetes, individuals with CAN had higher nocturnal urinary albumin excretion attributed to an attenuation of nocturnal blood pressure dipping, which is a risk factor for progression of kidney disease (11, 29, 30). In a multiethnic cohort of 204 European and South Asian adults with type 2 diabetes, patients with CAN had lower estimated GFR and higher urinary albumin excretion (5). Similarly, we found negative associations between ACR and cardiac vagal function (E/I, sdNN, HF ratio) in the Pima Indians. The relationship between albuminuria and DN, however, is inconsistent. Normal urinary albumin excretion is often observed in patients with chronic kidney disease (CKD) related to diabetes, and regression of microalbuminuria occurs in both types of diabetes, suggesting that elevated urinary albumin excretion does not always predict progressive DN (31–33). Glomerular structural changes may precede the appearance of microalbuminuria and progress even in the absence of elevated albuminuria (34–36). Thus, combining ACR with sensitive measures of GFR and morphometric measures of renal structure allows for more accurate characterization of the association between DN and CAN in this cohort.
Both CAN and DN are serious complications of diabetes with significant impacts on mortality, and each has several common pathogenic mechanisms driven by hyperglycemia and other risk factors (1–3, 37). Hemodynamic changes play an important role in the progression of DN. Glomerular hyperfiltration facilitates production of mesangial matrix and thickening of the glomerular basement membrane, and also induces localized release of cytokines (38). Impairment of cardiac vagal function associated with CAN may influence the overall sympathovagal balance, with relative increase in sympathetic tone and subsequent activation of the renin-angiotensin-aldosterone system. This may contribute to changes in glomerular hemodynamics and the circadian rhythms of blood pressure, leading to impairment of nocturnal blood pressure dipping which has been associated with a more rapid decline in serum creatinine clearance and increasing albuminuria (11, 29, 30). These mechanisms may be common links between CAN and DN, as demonstrated in animal models and in human studies (4, 12, 39). Interestingly, a denervated transplant kidney is not associated with accelerated development of diabetic glomerular lesions among transplant recipients with type 1 diabetes compared to individuals without transplanted kidneys, suggesting a limited direct neurogenic role in the progression of diabetic kidney disease (40). However, pathological denervation found in diabetic neuropathy may not be comparable to denervation in a transplanted kidney.
CAN predicts DN in several longitudinal studies of type 1 or 2 diabetes. Among 35 patients with type 1 diabetes, those with CAN had a significant decline in GFR (−22 ± 4 ml/min/1.73m2), measured by 51Cr-EDTA clearance, over a 10-year period, whereas those without CAN experienced almost no change (−8 ± 5 ml/min/1.73m2) (6). Similarly, in another study of 26 patients with type 1 diabetes, CAN predicted a rise in serum creatinine concentration over a one-year follow-up period (12). A subset of the First Joslin Kidney Study, which included 204 normoalbuminuric patients with type 1 diabetes and 166 with microalbuminuria, underwent a baseline CAN assessment and were followed for a median of 14 years. CAN was strongly associated with both early GFR loss, defined by a loss of cystatin C clearance of more than 3.3%/year, and advanced CKD, defined by eGFR <60 ml/min/1.73m2 (7). Similar associations have been reported in patients with type 2 diabetes. Among the multiethnic cohort of adults with type 2 diabetes described earlier, eGFR declined more rapidly over 2.5 years in those with CAN (9.0% decline) than in those without (3.3% decline) (5). Likewise, in a cohort of 1,117 Korean patients with type 2 diabetes and no CKD (eGFR ≥ 60 ml/min/1.73m2) at baseline, CAN increased the risk for developing CKD (eGFR < 60 ml/min/1.73m2) by over 2.6-fold during nearly 10 years of follow-up (4).
The relationship between DN and CAN may also be bidirectional (5). For example, DN may contribute to progression of CAN via modulation of leptin signaling in the hypothalamus which, in turn, may have direct effects on sympathetic tone and function (41). Clearly, the complex relationship between DN and CAN in diabetes is yet to be fully understood. In the present study, the temporal relationship between CAN and DN cannot be assessed because of the study design.
Strengths of this study include the use of unbiased structural measures of DN obtained by protocol biopsy, the measured GFR, and the standardized and comprehensive measures of CAN. Limitations include the relatively small sample size and the lag time between the kidney biopsy and CAN evaluation. Because of this temporal difference, we used an average of the clinical covariates measured at both time points in the regression models, but we were unable to control for medicine usage between the two examinations. The conclusions of the study were unchanged when clinical covariates at both time points were substituted for the mean values in the regression models. Another potential limitation is survival bias, as CAN evaluations were not done in those who died or developed end-stage renal disease. The relationship between DN lesions and CAN may be different in individuals with more severe DN.
We did not adjust for multiple comparisons in our analyses because many of the variables were correlated with each other, which would lead to overcorrection by the Bonferroni method. As this was an exploratory study, we did not want to mask potential relationships. It is noteworthy that greater structural damage by several morphometric measures was consistently associated with more severe measures of CAN.
5.1 Conclusions
Our findings indicate that in Pima Indians with type 2 diabetes, measures of CAN are associated with DN lesions. Planned follow-up of these patients with repeat kidney biopsies and serial measures of GFR and CAN will help establish the temporal relationship between CAN and the progression of diabetic kidney disease.
Supplementary Material
Acknowledgments
The authors thank the participants of the losartan clinical trial and the doctors, nurses, and support staff for their role in collecting and processing the data.
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and by the American Diabetes Association (Clinical Science Award 1-08-CR-42), and in part by R01-HL-102334 and the American Diabetes Association 1-14-MN-02 to R.P.-B. The funding sources played no role in data collection, study design, or drafting of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work were presented at the American Diabetes Association 75th Scientific Sessions (Diabetes 2015;64[S1]:A150) and the 51st EASD Annual Meeting (Diabetologia 2015;58[S1]:S504).
References
- 1.Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 4.Yun JS, Ahn YB, Song KH, Yoo KD, Kim HW, Park YM, Ko SH. The association between abnormal heart rate variability and new onset of chronic kidney disease in patients with type 2 diabetes: A ten-year follow-up study. Diabetes Res Clin Pract. 2015;108:31–37. doi: 10.1016/j.diabres.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 5.Tahrani AA, Dubb K, Raymond NT, Begum S, Altaf QA, Sadiqi H, Piya MK, Stevens MJ. Cardiac autonomic neuropathy predicts renal function decline in patients with type 2 diabetes: a cohort study. Diabetologia. 2014;57:1249–1256. doi: 10.1007/s00125-014-3211-2. [DOI] [PubMed] [Google Scholar]
- 6.Sundkvist G, Lilja B. Autonomic neuropathy predicts deterioration in glomerular filtration rate in patients with IDDM. Diabetes Care. 1993;16:773–779. doi: 10.2337/diacare.16.5.773. [DOI] [PubMed] [Google Scholar]
- 7.Orlov S, Cherney DZ, Pop-Busui R, Lovblom LE, Ficociello LH, Smiles AM, Warram JH, Krolewski AS, Perkins BA. Cardiac Autonomic Neuropathy and Early Progressive Renal Decline in Patients with Nonmacroalbuminuric Type 1 Diabetes. Clin J Am Soc Nephrol. 2015;10:1136–1144. doi: 10.2215/CJN.11441114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maguire AM, Craig ME, Craighead A, Chan AKF, Cusumano JM, Hing SJ, Silink M, Howard NJ, Donaghue KC. Autonomic Nerve Testing Predicts the Development of Complications A 12-year follow-up study. Diabetes Care. 2007;30:77–82. doi: 10.2337/dc06-0793. [DOI] [PubMed] [Google Scholar]
- 9.Molgaard H, Christensen PD, Sorensen KE, Christensen CK, Mogensen CE. Association of 24-h cardiac parasympathetic activity and degree of nephropathy in IDDM patients. Diabetes. 1992;41:812–817. doi: 10.2337/diab.41.7.812. [DOI] [PubMed] [Google Scholar]
- 10.Moran A, Palmas W, Field L, Bhattarai J, Schwartz JE, Weinstock RS, Shea S. Cardiovascular autonomic neuropathy is associated with microalbuminuria in older patients with type 2 diabetes. Diabetes Care. 2004;27:972–977. doi: 10.2337/diacare.27.4.972. [DOI] [PubMed] [Google Scholar]
- 11.Spallone V, Gambardella S, Maiello MR, Barini A, Frontoni S, Menzinger G. Relationship between autonomic neuropathy, 24-h blood pressure profile, and nephropathy in normotensive IDDM patients. Diabetes Care. 1994;17:578–584. doi: 10.2337/diacare.17.6.578. [DOI] [PubMed] [Google Scholar]
- 12.Weinrauch LA, Kennedy FP, Gleason RE, Keough J, D'Elia JA. Relationship between autonomic function and progression of renal disease in diabetic proteinuria: clinical correlations and implications for blood pressure control. Am J Hypertens. 1998;11:302–308. doi: 10.1016/s0895-7061(97)00472-x. [DOI] [PubMed] [Google Scholar]
- 13.Zander E, Schulz B, Heinke P, Grimmberger E, Zander G, Gottschling HD. Importance of cardiovascular autonomic dysfunction in IDDM subjects with diabetic nephropathy. Diabetes Care. 1989;12:259–264. doi: 10.2337/diacare.12.4.259. [DOI] [PubMed] [Google Scholar]
- 14.Weil EJ, Fufaa G, Jones LI, Lovato T, Lemley KV, Hanson RL, Knowler WC, Bennett PH, Yee B, Myers BD, Nelson RG. Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes. 2013;62:3224–3231. doi: 10.2337/db12-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers BD, Nelson RG, Tan M, Beck GJ, Bennett PH, Knowler WC, Blouch K, Mitch WE. Progression of overt nephropathy in non-insulin-dependent diabetes. Kidney Int. 1995;47:1781–1789. doi: 10.1038/ki.1995.246. [DOI] [PubMed] [Google Scholar]
- 16.Vasquez B, Flock EV, Savage PJ, Nagulesparan M, Bennion LJ, Baird HR, Bennett PH. Sustained reduction of proteinuria in type 2 (non-insulin-dependent) diabetes following dietinduced reduction of hyperglycaemia. Diabetologia. 1984;26:127–133. doi: 10.1007/BF00281119. [DOI] [PubMed] [Google Scholar]
- 17.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol. 1960;30:207–212. [PubMed] [Google Scholar]
- 18.Weibel ER. Stereological Methods: Vol.: 1.: Practical Methods for Biological Morphometry. Academic press; 1979. [Google Scholar]
- 19.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weil EJ, Lemley KV, Mason CC, Yee B, Jones LI, Blouch K, Lovato T, Richardson M, Myers BD, Nelson RG. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82:1010–1017. doi: 10.1038/ki.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes. 1994;43:1358–1364. doi: 10.2337/diab.43.11.1358. [DOI] [PubMed] [Google Scholar]
- 23.Tan JC, Busque S, Workeneh B, Ho B, Derby G, Blouch KL, Sommer FG, Edwards B, Myers BD. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010;78:686–692. doi: 10.1038/ki.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaiswal M, McKeon K, Comment N, Henderson J, Swanson S, Plunkett C, Nelson P, Pop-Busui R. Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2014;37:2616–2621. doi: 10.2337/dc14-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardi L, Spallone V, Stevens M, Hilsted J, Frontoni S, Pop-Busui R, Ziegler D, Kempler P, Freeman R, Low P, Tesfaye S, Valensi P. Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev. 2011;27:654–664. doi: 10.1002/dmrr.1224. [DOI] [PubMed] [Google Scholar]
- 26.Pichot V, Gaspoz JM, Molliex S, Antoniadis A, Busso T, Roche F, Costes F, Quintin L, Lacour JR, Barthelemy JC. Wavelet transform to quantify heart rate variability and to assess its instantaneous changes. J Appl Physiol (1985) 1999;86:1081–1091. doi: 10.1152/jappl.1999.86.3.1081. [DOI] [PubMed] [Google Scholar]
- 27.Reyes del Paso GA, Langewitz W, Mulder LJ, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- 28.Belsley DA, Kuh E, Welsch RE. Regression diagnostics: Identifying influential observations and sources of collinearity. Hoboken, New Jersey: John Wiley and Sons; 1980. [Google Scholar]
- 29.Farmer CK, Goldsmith DJ, Quin JD, Dallyn P, Cox J, Kingswood JC, Sharpstone P. Progression of diabetic nephropathy--is diurnal blood pressure rhythm as important as absolute blood pressure level? Nephrol Dial Transplant. 1998;13:635–639. doi: 10.1093/ndt/13.3.635. [DOI] [PubMed] [Google Scholar]
- 30.Knudsen ST, Laugesen E, Hansen KW, Bek T, Mogensen CE, Poulsen PL. Ambulatory pulse pressure, decreased nocturnal blood pressure reduction and progression of nephropathy in type 2 diabetic patients. Diabetologia. 2009;52:698–704. doi: 10.1007/s00125-009-1262-6. [DOI] [PubMed] [Google Scholar]
- 31.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 32.Marshall SM. Natural history and clinical characteristics of CKD in type 1 and type 2 diabetes mellitus. Adv Chronic Kidney Dis. 2014;21:267–272. doi: 10.1053/j.ackd.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Pavkov ME, Mason CC, Bennett PH, Curtis JM, Knowler WC, Nelson RG. Change in the distribution of albuminuria according to estimated glomerular filtration rate in Pima Indians with type 2 diabetes. Diabetes Care. 2009;32:1845–1850. doi: 10.2337/dc08-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M. The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes. 2005;54:2164–2171. doi: 10.2337/diabetes.54.7.2164. [DOI] [PubMed] [Google Scholar]
- 35.Perrin NE, Torbjornsdotter T, Jaremko GA, Berg UB. Risk markers of future microalbuminuria and hypertension based on clinical and morphological parameters in young type 1 diabetes patients. Pediatr Diabetes. 2010;11:305–313. doi: 10.1111/j.1399-5448.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- 36.Caramori ML, Parks A, Mauer M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol. 2013;24:1175–1181. doi: 10.1681/ASN.2012070739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 38.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 39.Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;8:405–416. doi: 10.1038/nrendo.2012.21. [DOI] [PubMed] [Google Scholar]
- 40.Chang S, Caramori ML, Moriya R, Mauer M. Having one kidney does not accelerate the rate of development of diabetic nephropathy lesions in type 1 diabetic patients. Diabetes. 2008;57:1707–1711. doi: 10.2337/db07-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasrallah MP, Ziyadeh FN. Overview of the physiology and pathophysiology of leptin with special emphasis on its role in the kidney. Semin Nephrol. 2013;33:54–65. doi: 10.1016/j.semnephrol.2012.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.