Abstract
Amphiregulin (AREG), an epidermal growth factor receptor ligand, is implicated in tissue repair and fibrosis but its cellular source and role in regeneration vs. fibrosis remain unclear. In this study we hypothesize that AREG induced in bone marrow derived CD11c+ cells is essential for pulmonary fibrosis. Thus the objectives were to evaluate the importance and role of AREG in pulmonary fibrosis, identify the cellular source of AREG induction and analyze its regulation of fibroblast function and activation. The results showed that lung AREG expression was significantly induced in bleomycin-induced pulmonary fibrosis. AREG deficiency in knockout (KO) mice significantly diminished pulmonary fibrosis. Analysis of AREG expression in major lung cell types revealed induction in fibrotic lungs predominantly occurred in CD11c+ cells. Moreover depletion of bone marrow derived CD11c+ cells suppressed both induction of lung AREG expression and pulmonary fibrosis. Conversely, adoptive transfer of bone marrow-derived CD11c+ cells from BLM-treated donor mice exacerbated pulmonary fibrosis but not if the donor cells were made AREG-deficient prior to transfer. CD11c+ cell conditioned media or co-culture stimulated fibroblast proliferation, activation and myofibroblast differentiation in an AREG dependent manner. Furthermore recombinant AREG induced telomerase reverse transcriptase (TERT) which appeared to be essential for the proliferative effect. Finally AREG significantly enhanced fibroblast motility, which was associated with increased expression of α6 integrin. These findings suggested that induced AREG specifically in recruited bone marrow-derived CD11c+ cells promoted bleomycin induced pulmonary fibrosis by activation of fibroblast TERT dependent proliferation, motility and indirectly, myofibroblast differentiation.
Introduction
Progressive fibrosis in chronic fibroproliferative diseases is characterized by mensenchymal cell recruitment, proliferation, and activation with de novo emergence and persistence of myofibroblasts (1–3). Pathogenesis of some of these diseases, such as idiopathic pulmonary fibrosis (IPF), remains poorly elucidated. Although a myriad of the factors are known to regulate fibroblast proliferation, motility and invasiveness, the identity and role of the specific factor or factors and their cellular origin remain obscure with respect to their significance in pathogenesis of fibrosis. EGF receptor (EGFR) signaling is implicated in renal, hepatic and pulmonary fibrosis, with TGFα being a candidate EGFR ligand (4–8). This has been demonstrated by use of EGFR specific neutralizing antibodies or tyrosine kinase inhibitors (4, 7, 9, 10). AREG is another polypeptide growth factor that belongs to the EGF family, which mediates its biologic function through the EGFR (11, 12). AREG is expressed in multiple cell populations, including epithelial cells, leukocytes, dendritic cells, fibroblasts and keratinocytes, and more recently shown in group 2 innate lymphoid cells (ILC2) and Tregs (13). It is expressed as a transmembrane precursor (Pro-AREG), which is proteolytically cleaved off by ADAM17 to release the mature soluble form or ectodomain. Because the membrane bound Pro-AREG is active on adjacent EGFR, AREG has juxtacrine, in addition to paracrine and autocrine activities (14). It plays an essential role in the pathogenesis of TGFβ1-induced pulmonary fibrosis (15). In addition, AREG knockout (KO) mice exhibited reduced liver fibrosis with suppression of myofibroblast differentiation (8). AREG is induced in certain cancers and is implicated in the promotion of tumor growth and metastasis. Significant upregulation of amphiregulin (AREG) in tumor-infiltrating CD11c+ dendritic cells (DCs) in human lung cancer samples and patients’ sera provides support for a role of AREG in cancer (16). Moreover cancer-derived ATP induced AREG expression in DCs is reported to promote tumorigenesis (17). Interestingly, DCs are implicated in pulmonary fibrosis in humans and animal models (18–22). EGFR signaling is also implicated in tissue repair/regeneration after injury and recovery of organ function. Treg or ILC2-derived AREG is recently shown to be important in recovery from airway injury due to influenza infection (23–25). Another study shows that systemic administration (intraperitoneal injection) of AREG affords some protection from bleomycin (BLM)-induced lung injury (25). Thus while AREG is implicated in both tissue repair and fibrosis, its cellular source and precise function in pulmonary fibrosis remains unclear.
Based on the prior studies, we hypothesized that bone marrow (BM) derived DCs is a key source of induced AREG expression in pulmonary fibrosis and is important in driving fibrosis by inducing fibroblast proliferation and motility. To test this hypothesis the bleomycin model of pulmonary fibrosis was utilized to evaluate AREG expression, its cellular source and role in regulation of fibroblast function and activation. AREG induction resided primarily in BM derived CD11c+ cells with phenotypic properties consistent with DCs. Conditioned media from these cells or co-culture with these cells induced TERT and fibroblast proliferation, which was TERT dependent. AREG promoted fibroblast motility that was associated with induction of α6 integrin. Further studies revealed an indirect role for CD11c+ cell derived AREG in myofibroblast differentiation. Adoptive transfer of CD11c+ cells promoted fibrosis but not if the donor cells were AREG deficient. Thus AREG induction in BM derived CD11c+ cells are of specific importance in pathogenesis of pulmonary fibrosis.
Materials and Methods
Mice
Female CD11c-DTR mice [B6.FVB-Tg (Itgax-DTR/EGFP)57Lan/J] (26) on the C57BL/6J background and littermates (6–8 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). Areg KO mice (6–8 weeks old) on a mixed background of 129 and C57BL/6J strains (27) were gifts from Dr. Susumu Nakae (University of Tokyo, The Institute of Medical Science, Japan). These Areg KO mice had been backcrossed four times with C57BL/6 mice. Mixed background B6129ASF2/J mice were purchased from the Jackson Laboratory, and used as control wild type mice. Pulmonary fibrosis was induced as before (28, 29) by the endotracheal injection of 2.5U/kg body weight BLM (Blenoxane; Mead Johnson, Plainsboro, NJ). CD11c-DTR bone marrow (BM) chimera mice were generated by transplanting BM from CD11c-DTR mice into irradiated wild type (WT) recipients as before (28, 29). Six weeks after BM transplantation, pulmonary fibrosis was induced. Administration of DT (8 ng/g body weight, ip injection) to CD11c-DTR-BM chimera mice every other day selectively depleted BM-derived CD11c+ cells. Where indicated, 5×104 BM-derived CD11c+ cells from BLM-treated mice without or with AREG shRNA pretreatment (see below) were transferred by endotracheal injection into recipient mice that were treated with 1U/kg body weight low dose BLM 2 days prior to cell transfer. Control mice received sterile saline (SAL) alone. All animal studies were reviewed and approved by the University Committee on Use and Care of Animals at the University of Michigan.
Isolation of Broncho-alveolar lavage fluid and lung cells
Broncho-alveolar lavage (BAL) and single lung cell suspension for flow cytometric analysis were prepared as described before (28–30). Fibroblasts and type II alveolar epithelial cells were isolated from freshly dissected lung tissue by mincing and enzymatic digestion as described before (31, 32). Lineage− CD90.2+ ST2+ (eBiosciences, San Diego, CA) lung ILC2 were sort-purified from whole lung cell suspension using BC MoFloAsrios cell sorter (Beckman Coulter Inc. Brea, CA). Where indicated, cells were treated with recombinant AREG (R &D systems, Inc., Minneapolis, MN) diluted in medium supplemented with 0.5% plasma-derived serum and further incubated for 24–72 hours before harvest and analysis.
Flow cytometry
Cell suspensions from freshly digested lung tissue samples were prepared by enzyme digestion as described above and analyzed by flow cytometry as before (28–30). FITC conjugated anti-mouse CD11c antibody or its isotype control (eBioscience) were used. Flow-cytometric analysis was undertaken using an Epics XL-MCL machine (Beckman Coulter Inc., Miami, Florida, USA). Data collected were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Small hairpin RNA (shRNA) transfection
BMDCs were transfected with AREG shRNA lentiviral particles or its control (sc-39413-V, Santa Cruz) in accordance with the manufacturer’s instructions. Transfected cells were cultured for 7 days before transfer to recipient mice.
Quantitative real-time RT-PCR
Primers and probes for mouse AREG, αSMA, procollagen I, TERT, α6 integrin and 18s RNA were purchased from Applied Biosystems. One-step real-time RT-PCR (qRT-PCR) was performed on a GeneAmp 7500 Sequence Detection System (Applied Biosystems). Results were expressed as 2−ΔΔCT as previously described using 18s RNA as the reference, and the respective control group as calibrator (33).
Cell proliferation assay
Cell proliferation was measured in primary mouse lung fibroblasts (MLFs), TERT KO MLF (34), human lung fibroblasts (HLF) and BJ and BJ5ta (human foreskin fibroblast) cell lines (ATCC, Manassas, Virginia) by cell counting with Z2 Coulter count and size analyzer (Beckman Coulter Inc.) or WST-1 assay (Roche) as described before (30).
Induction of CD11c+ cells from mouse bone marrow progenitors
Dendritic cell skewed bone marrow-derived CD11c+ cells were induced by GM-CSF (R &D systems, Inc., Minneapolis, MN) as described before (29).
Cell culture and coculture
MLFs were plated (1.5×105 cells/well) in the 6-well plates until subconfluent. Where indicated after 24 hours of starvation with serum-free medium, MLFs were treated with 100 ng/ml recombinant AREG (R & D) in absence or presence of 5 μM EGFR inhibitor PD153035 HCl (Sigma) for another 16 hours. The MLFs were then harvested for mRNA analysis. For cell coculture experiments 24-well plates with 0.4-μm Pore Polycarbonate Membrane Inserts (Corning Incorporated, Corning, NY) were used. MLFs were plated (1.5×105 cells/well) in the bottom chambers of plates for 24 hours prior to the addition of 1×106 BM-derived CD11c+ cells from WT or Areg KO mice to the upper inserts. The upper chambers containing medium only were used as controls. The co-cultured MLFs were collected for RNA isolation after an additional 48 hours of incubation.
Histological analysis
The lungs were inflated by intratracheal perfusion and fixed for 24 h with 4% paraformaldehyde. Lung tissue was then paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E).
Hydroxyproline assay
Lung hydroxyproline content was measured in whole lung homogenates as previously described (28). The results were expressed as μg of hydroxyproline per lung.
AREG ELISA and Western blotting analysis
AREG levels in BAL fluid from control and BLM-treated mice were detected using RayBio amphiregulin ELISA kit (RayBiotech, Inc., Norcross GA) in accordance with the manufacturer’s instructions. Goat anti-AREG antibodies (R & D) were used for western blotting analysis of AREG in mouse lung tissue samples and CD11c+ cells using a 4–12% gradient polyacrylamide gel. HRP conjugated GAPDH antibodies (Sigma) were used to detect GAPDH, which was used as the loading control. The intensity of protein bands were quantitatively analyzed by ImageQuant TL software (GE Healthcare Life Sciences, Pittsburgh, PA).
Scratch would healing assay
Primary HLF were plated in a 96-well ImageLock plate (Essen Instruments, Ann Arbor, MI). Twenty-four hours later, the HLF monolayers were wounded using WoundMaker following manufacture’s instruction. AREG (200 ng/ml) or buffer only was added into the plate. Images were recorded every 45 minute, and analyzed by an Incucyte Imaging System (Essen Instruments).
Statistics
Differences between means of various treatment and control groups were assessed for statistical significance by ANOVA followed by post-hoc analysis using Scheffé’s test for comparison between any 2 groups. P value <0.05 was considered significant.
Results
AREG was induced in BLM-induced pulmonary fibrosis
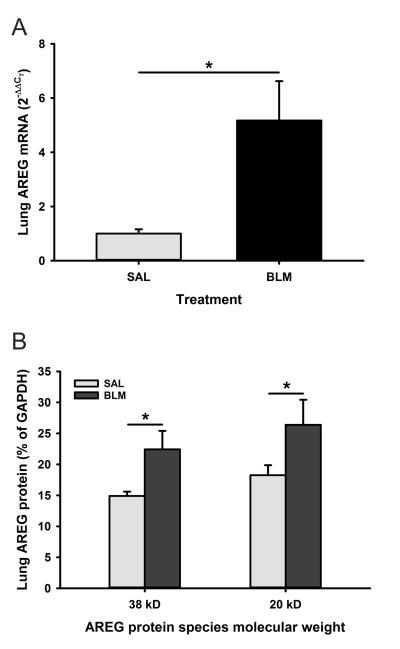
To study the involvement of AREG in BLM-induced pulmonary fibrosis, we first investigated the expression of AREG in the mouse lungs after BLM treatment. The results showed that AREG mRNA expression in BLM treated lungs was highly induced by ~5-fold compared to SAL treated lungs at day 7 after BLM treatment (Figure 1A). This induction was reflected at the protein level as analyzed by Western blotting. Both forms of AREG protein (~38 and 20 kDa) were increased in lung tissue samples from day 21 BLM treated mice (Figure 1B). Thus BLM-induced lung injury caused significant induction of AREG expression in the lung.
Figure 1. AREG was induced in BLM injured lung.
A) AREG mRNA expression level in saline (SAL) or bleomycin (BLM)-treated lung tissues at day 7 were evaluated by qPCR. n=3 animals per group. B) AREG protein levels in SAL or BLM-treated lung tissue samples (30 μg of tissue lysate) were analyzed by Western blotting. The intensity of protein bands were expressed as relative integration units and shown as a percentage of the GAPDH signal used as loading control (n=3 mice/group) Data are shown as the mean ± SD. *P<0.05 between the indicated 2 groups.
BLM-induced pulmonary fibrosis was attenuated in AREG-deficient mice
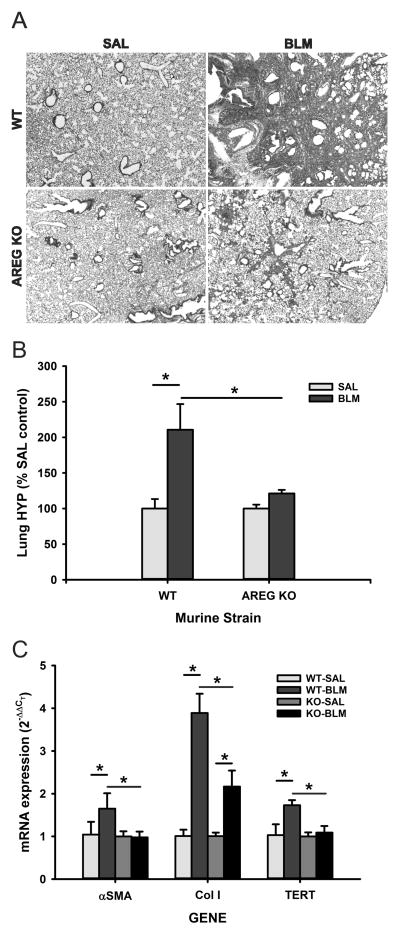
To evaluate the importance of AREG in vivo, BLM-induced lung fibrosis was evaluated in control (B6129SAF2/J) and Areg KO mice. When subjected to BLM-induced lung injury, Areg KO mice showed significantly reduced fibrosis relative to control mice, both morphologically and biochemically by hydroxyproline analysis (Figures 2A and B, respectively). The reduction in fibrosis was accompanied with a significant reduction in BLM-induced increases of αSMA, procollagen I and TERT expression (Figure 2C). These data confirmed an essential role for AREG in BLM induced pulmonary fibrosis, fibroblast activation and myofibroblast differentiation.
Figure 2. BLM-induced pulmonary fibrosis was attenuated in AREG-deficient mice.
The effects of AREG deficiency on BLM-induced pulmonary fibrosis were evaluated in WT or Areg KO mice at day 21 after BLM treatment with respect to (A) histopathology at 20x magnification, (B) lung hydroxyproline content and (C) lung αSMA, collagen I (Col I) and TERT mRNA levels. Data are shown as the mean ± SD with n=5. *P<0.05 between the indicated 2 groups.
AREG was induced in lung CD11c+ cells after BLM treatment
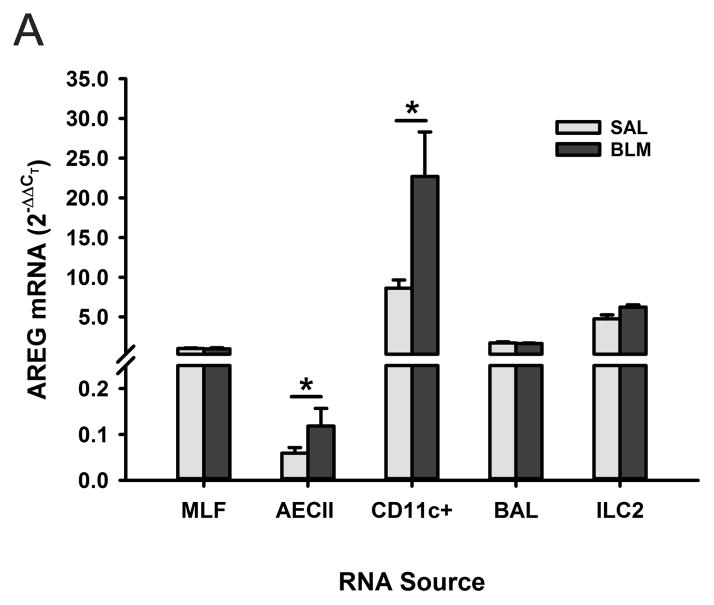
To identify the cellular source of AREG expression in lung tissue, AREG mRNA expression was examined in different lung cell types. The results showed predominant expression in lung CD11c+ cells, with lower levels of expression in lung fibroblasts, ILC2, BAL cells, and minimally in type II alveolar epithelial cells (Figure 3). BLM caused a significant induction of AREG in CD11c+ and type II alveolar epithelial cells only. However the levels of expression in CD11c+ cells were >100-fold higher than that in epithelial cells.
Figure 3. AREG expression in the different lung cell types after BLM treatment.
The indicated lung cell types, including type II alveolar epithelial cells (AECII) and group 2 innate lymphoid cells (ILC2), were isolated from SAL or BLM-treated mice and their AREG mRNA levels were evaluated by qRT-PCR.. Data are shown as the mean ± SD, with n=3 animals per group. *P<0.05 between the indicated 2 groups.
Depletion of BM derived CD11c+ cells reduced lung AREG expression and attenuated pulmonary fibrosis
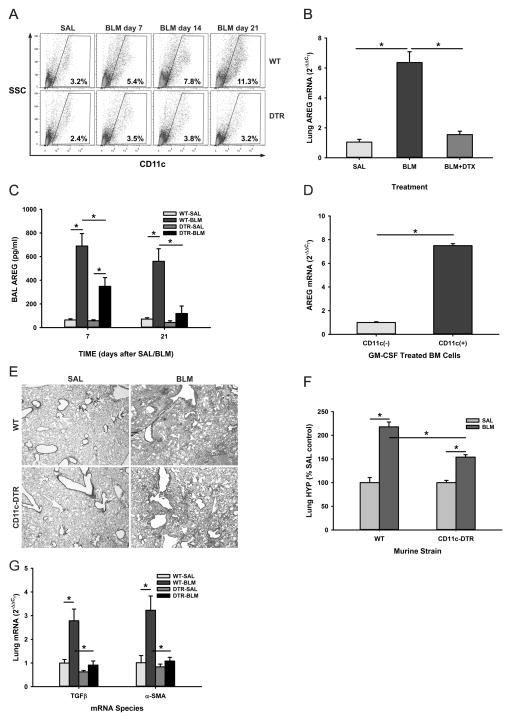
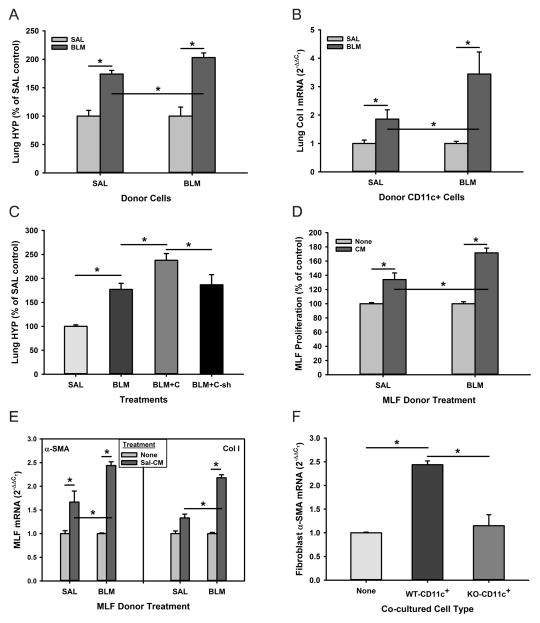
Given the BLM-induced recruitment of BM CD11c+ cells into the lung as reported previously (18, 35), we evaluated the role of these cells in pulmonary fibrosis in vivo. The effect of depleting these cells on the BLM model was examined using CD11c-DTR BM chimera mice. CD11c-DTR mice express a high-affinity diphtheria toxin (DT) receptor under control of the CD11c promoter, which makes the CD11c+ cells selectively susceptible to DT. However, repeated DT injection caused significant number of fatalities (data not shown) or resulted in extra-hematopoietic toxicity (26). Thus, the time window of DT-induced cell ablation was limited to 2–3 injections administered every other day. CD11c-DTR BM chimeric mice were generated through reconstitution of lethally irradiated recipient WT mice with donor CD11c-DTR mouse BM cells. Flow cytometric analysis of the lung cells revealed approximately 3% CD11c+ cells in SAL-treated control lungs, which increased gradually to 11.3% at day 21 after BLM treatment (Figure 4A). This BLM-induced increase in lung CD11c+ cells was absent in DT treated CD11c-DTR BM chimera mice indicating effective suppression of BLM-induced influx of CD11c+ BM cells to the injured lung, which resulted in virtually complete suppression of the BLM-induced increase in lung AREG expression (Figure 4B). Consistent with the BLM-induced increase in lung tissue AREG mRNA, the results showed a remarkable increase (>6-fold) in AREG levels in BAL fluid at day 7, which was sustained at day 21 after BLM treatment. These BLM induced increase in BAL fluid AREG protein levels were significantly reduced in CD11c-DTR BM chimera mice at day 7, and further reduced by day 21 (Figure 4C). Analysis of AREG expression in BM-derived cells treated with GM-CSF to induce CD11c+ cell differentiation confirmed that BM CD11c+ cells represented an inducible source of AREG expression (Figure 4D). Depletion of BM CD11c+ cells by DT treatment of CD11c-DTR BM chimera mice also attenuated BLM-induced fibrosis as revealed by histopathology (Figure 4E) and biochemically by lung tissue hydroxyproline analysis (Figure 4F). This suppression of fibrosis was accompanied by significant inhibition of BLM-induced TGFβ and αSMA expression in the lung (Figure 4G), suggesting that the role of AREG in fibrosis is upstream of that for TGFβ and its stimulatory effect on myofibroblast differentiation (36). The results taken together would be consistent with recruited BM CD11c+ cells as the key cellular source of BLM-induced lung AREG expression, which was essential for the accompanying pulmonary fibrosis.
Figure 4. Depletion of BM derived CD11c+ cells attenuated pulmonary fibrosis.
A) At the indicated time points after SAL or BLM instillation, single cell suspensions from lung tissues of WT or diphtheria toxin treated CD11c-DTR BM chimera (‘DTR’) mice were analyzed for CD11c expression by flow cytometry. The results were expressed as % of total cells. B) Lung tissue samples from these mice were analyzed for AREG mRNA by qRT-PCR. BLM-DTX refers to the BLM treated DTR mice receiving diphtheria toxin. C) The levels of AREG protein in the BAL fluid from SAL or BLM-treated control and DTR mice at days 7 and 21 were analyzed by ELISA. D) Purified CD11c+ and CD11c− cells from GM-CSF treated mouse BM cells were analyzed for AREG mRNA by qRT-PCR. E) Lung tissue sections from mice treated as indicated in (A) were stained with hematoxylin and eosin (images captured at 20x magnification). F) The lung tissue samples were also analyzed for hydroxyproline content. G) Lung TGFβ and αSMA mRNA were also analyzed by qRT-PCR. Data are shown as the mean ± SD, n=3 animals per group. *P<0.05 between the indicated 2 groups.
BM derived CD11c+ cells exacerbated BLM-induced pulmonary fibrosis in an AREG-dependent manner
To further confirm the importance of AREG expression by BM-derived CD11c+ cells in pulmonary fibrosis the effects of transferring BM CD11c+ cells on BLM-induced fibrosis in recipient mice were examined. The results showed that endotracheal transfer of BM CD11c+ cells alone without BLM treatment had no significant effect on lung hydroxyproline or procollagen I mRNA in recipient mice. However, transfer of BM CD11c+ cells from either SAL or BLM treated donor mice significantly exacerbated BLM-induced fibrosis as determined by lung hydroxyproline content and procollagen I mRNA (Figures 5A & B, respectively). The effects of cells from BLM-treated donor mice were significantly greater than that of cells from SAL-treated donor mice. However this exacerbation of fibrosis by cell transfer was not observed when the donor BM CD11c+ cells (from BLM treated donor mice) were depleted of AREG by transfection with AREG shRNA prior to transfer, while cells transfected with control empty vector did exacerbate fibrosis as in the prior experiment (Figure 5C). These findings confirmed that AREG primarily from recruited BM CD11c+ cells played a significant role in driving pulmonary fibrosis.
Figure 5. CD11c+ BM cells from BLM-treated donor mice exacerbated fibrosis and activated fibroblasts in an AREG-dependent manner.
Flow sorted BM-derived CD11c+ cells from SAL or BLM treated donor mice as indicated were transferred endotracheally into recipient mice which had been treated with low dose (1U/kg body weight) BLM or SAL 2 days prior to cell transfer. The effects of this cell transfer on, A) lung hydroxyproline content (n=3), and B) lung Col I mRNA levels are shown (n=3). C) BM-derived CD11c+ cells were transfected with control (‘C’) or AREG shRNA (‘C-sh’), and then transferred endotracheally into recipient mice, which had been treated with SAL or BLM as in (A). The effect of these cell transfers on lung hydroxyproline content of recipient mice are shown (n=3). D) Conditioned media (CM) were prepared from cultures of BM CD11c+ cells isolated from SAL or BLM treated mice as indicated, and their effects on MLF proliferation were measured using the WST-1 cell proliferation assay. The results were expressed as a percentage of the untreated controls (n=5). E) The effects on αSMA and procollagen I (Col I) mRNA levels were evaluated by qRT-PCR in left and right panels, respectively (n=3). F) BM CD11c+ cells from BLM-treated WT or Areg KO (‘KO-CD11c+’) mice were cocultured with MLFs for 48 hours. The MLFs were then harvested for analysis of αSMA mRNA by qRT-PCR (n=3). Data are shown as the mean ± SD. *P<0.05 between the indicated 2 groups.
To further evaluate this role conditioned media from BM CD11c+ cells were evaluated for their effects on MLFs from SAL (SAL MLFs) or BLM (BLM MLFs) treated mice. As shown in Figure 5D, the proliferation of both SAL and BLM MLFs was increased significantly by addition of BM CD11c+ cells conditioned media. Moreover the stimulation of proliferation was accompanied by similar stimulation of αSMA and procollagen I expression in both MLFs (Figure 5E). Notably, the responses of BLM MLFs were significantly higher than those of SAL MLFs, with >2-fold increase over those in SAL MLFs. In addition to the effects of BM CD11c+ cell conditioned media, MLFs exhibited a >2-fold stimulation in αSMA expression when directly co-cultured with BM CD11c+ cells from wild type but not from Areg KO mice (Figure 5F). Surprisingly however, recombinant AREG did not have a significant effect on αSMA expression in MLFs (data not shown). This might suggest CD11c+ cells-derived AREG only indirectly affect myofibroblast differentiation. These findings demonstrated that the AREG derived from BM CD11c+ cells may function as the profibrogenic mediator for the proliferation and activation of fibroblasts, including myofibroblast differentiation.
AREG promoted fibroblast proliferation and motility
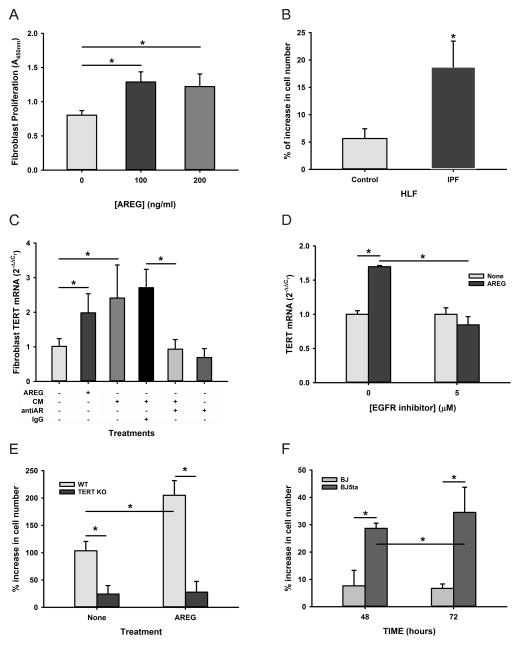
To further elucidate the potential mechanism by which BLM-induced AREG mediated fibroproliferative response in pulmonary fibrosis, the direct effects of AREG on lung fibroblasts were investigated. As shown in Figure 6A, recombinant AREG treatment caused a significant increase in proliferation of MLFs. Moreover human lung fibroblasts (HLFs) from both control and IPF patients also responded to AREG treatment, but the telomerase-expressing IPF cells (37) were >3-fold as responsive as the control telomerase negative cells (Figure 6B). Interestingly, AREG and BM CD11c+ cell conditioned media induced MLF TERT expression (Figure 6C), and in the case of the conditioned media the stimulatory effect was abrogated by pre-incubation with anti-AREG antibodies but not by control IgG. Since telomerase is induced in lung fibroblasts isolated from BLM-treated mice and promotes cell proliferation (38, 39), these in vitro observations on AREG induction of TERT might be of in vivo significance in pathogenesis of fibrosis. Indeed in contrast to wild type mice TERT induction was absent in Areg KO mice after BLM treatment (Figure 2C). To further evaluate the role of AREG-EGFR signaling in this AREG induction of TERT, the effect of a pharmacological inhibitor PD153035 HCl of EGFR on TERT mRNA expression was analyzed. The results showed that AREG induced significant induction in TERT mRNA (1.7-fold) as expected, whereas this induction was completely diminished by the addition of EGFR inhibitor (Figure 6D). Furthermore the effect of AREG on proliferation depended on TERT induction since TERT deficient MLF proliferated poorly basally and in response to AREG treatment, especially when compared to that in wild type MLFs (Figure 6E). Conversely, greater proliferation response to AREG was noted in human BJ5ta fibroblasts with overexpressed TERT relative to that in control human BJ cells (Figure 6F). Thus AREG promotion of fibroblast proliferation might be mediated in part by the up-regulation of TERT expression.
Figure 6. AREG promoted fibroblast proliferation.
A) Effect of recombinant AREG at the indicated doses on MLF proliferation (WST-1 absorbance at 450nm) are shown (n=5). B) The effects of AREG on control or IPF human lung fibroblast proliferation are shown as the % increase in cell number (n=5). C) BM CD11c+ cell conditioned media (CM) were pretreated without or with anti-AREG antibody (antiAR) or non-immune IgG as indicated. They were added to MLF cultures to analyze their effects on TERT expression using qRT-PCR and compared to the effect of recombinant AREG (n=3). D) Following serum starvation for 48 hours mouse lung fibroblasts were treated with the EGFR inhibitor, PD153035 (5 μM) for 1 hour and then were treated with 100 ng/ml of AREG for another 16 hours. Fibroblast TERT mRNA was analyzed by qRT-PCR (n=3). E) The effects of AREG on WT or TERT KO MLF proliferation are shown as % increase in cell number (n=5). F) The effects of AREG on BJ or BJ5ta human fibroblast cell lines are shown as % increase in cell number (n=5). All data are shown as the mean ± SD. *P<0.05 between the indicated 2 groups.
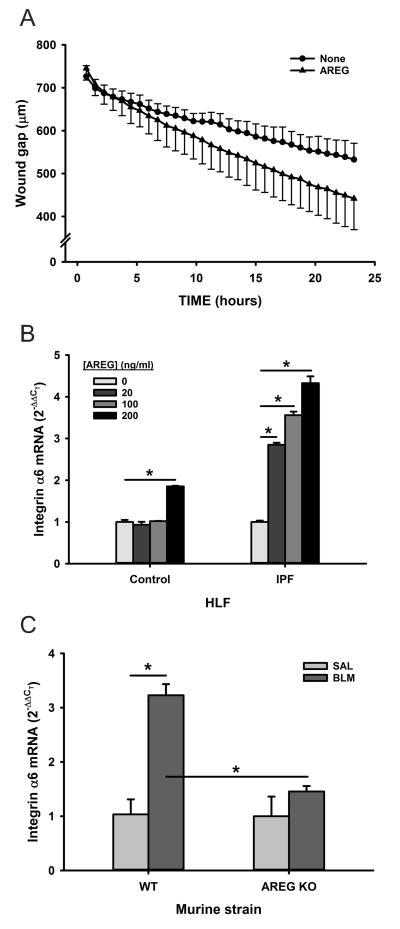
AREG activity on MLF motility was then tested with a scratch wound healing assay. The residual wound gap was measured after human lung fibroblast (HLF) monolayers were scratch wounded. The results showed that AREG treatment significantly enhanced the rate and extent of wound gap reduction relative to those of untreated control cells (Figure 7A), indicating AREG significantly enhanced lung fibroblast motility. The possible involvement of α6 integrin in AREG-induced fibroblast motility was also evaluated since AREG promotes α6β1 integrin dependent enhancement of chondrosarcoma cell motility (40). Indeed, α6 integrin expression was significantly induced in IPF HLF by AREG treatment in a dose-dependent manner but only at the maximal dose in control HLF (Figure 7B). Similar AREG induction of α6 integrin expression was also observed in MLF (data not shown). Finally, to confirm in vivo significance analysis of lung α6 integrin mRNA levels revealed significant induction (>3-fold) in BLM induced pulmonary fibrosis, which was completely abolished in Areg KO mice (Figure 7C). Expression of α6 integrin expression was also decreased in MLF isolated from Areg KO mice relative to that from WT mice (data not shown). These findings suggested that AREG induced α6 integrin expression might play a role in promoting fibroblast motility and invasiveness.
Figure 7. Effect of AREG on lung fibroblast motility.
A) Control human lung fibroblasts monolayers in a 96-well ImageLock plate were scratch wounded using WoundMaker, and treated with AREG or buffer only, as indicated. Images were recorded every 45 minute to measure residual would gap in this wound healing assay. B) Human lung fibroblasts from control or IPF patients were treated with the indicated dose of AREG for 24 hours and analyzed for α6 integrin mRNA by qRT-PCR. C) WT or Areg KO mice were treated with BLM or SAL, and analyzed for lung α6 integrin mRNA by qRT-PCR. All results were expressed as mean ± SD, with n=12 in the wound healing assay and n=3 for the α6 integrin mRNA analysis. *P<0.05 between the indicated 2 groups.
Discussion
EGFR signaling in fibrosis has been described in a variety of tissues, including skin, kidney, liver and lung (4, 7, 9, 10). AREG, an EGFR ligand is recently implicated in repair and fibrosis but its functional importance and cellular source remain unclear, especially vis-à-vis its precise role in the pathogenesis of pulmonary fibrosis. The present study provides novel in vivo and in vitro evidence of AREG induction predominantly in infiltrating lung BM-derived CD11c+ cells upon BLM treatment, which played an essential role in the pulmonary fibrosis associated with this animal model in part by its ability to induce TERT and α6 integrin to promote fibroblast proliferation and motility, respectively. First, AREG was highly induced in BLM-induced pulmonary fibrosis and found to be predominantly due to infiltrating BM-derived CD11c+ cells. Second, AREG deficient mice exhibited diminished fibrosis that was accompanied by abrogation of TERT and α6 integrin induction. Third, depletion of BM CD11c+ cells also impaired pulmonary fibrosis associated with the ablation of lung AREG induction; while transfer of these cells exacerbated fibrosis but not if they were AREG deficient. Finally conditioned media from BM CD11c+ cells stimulated fibroblast proliferation, motility, TERT and α6 integrin expression, as well as differentiation to myofibroblast.
A profibrotic role for AREG is consistent with previous studies of fibrosis in the liver. Thus impaired CCL4-induced liver fibrosis in AREG-deficient mice has been reported (8). AREG expression is enhanced in human non-alcoholic steatohepatitis, and it stimulates human hepatic stellate cell proliferation as well as type I collagen expression via EGFR and PI3K signaling pathways (41). In the lung, TGFβ1 overexpression-induced pulmonary fibrosis is accompanied by significant stimulation of AREG expression, whose silencing by siRNA or chemical inhibition of EGFR signaling significantly attenuates pulmonary fibrosis (15). Since fibrosis resembles an exaggerated or abnormal healing response that does not resolve, it is noteworthy that AREG expressed by diverse cell types can also afford protection from injury.
In these studies exogenous administration of AREG protects from injury such as from infections and other injurious stimuli (25) (23). These findings also suggest that the cellular location of AREG expression and/or selectivity of its target cells may be important factors in determining its role in vivo. Indeed in the lung, both epithelial and mesenchymal cells express AREG but differentially respond to AREG stimulation during lung branching morphogenesis (42).
In the current study the recruited BM CD11c+ cell was shown to be the predominant cellular source of AREG, especially in the BLM-injured lung with much lower levels of expression in fibroblasts, and lower still in alveolar epithelial cells. This is consistent with a previous report showing that human and mouse DCs, which express CD11c, are a source of AREG (17), while human lung tumor-associated DCs secrete high levels of AREG and promote cancer progression (16). Recently BM-derived cells recruited to BLM injured lung are found to be significantly increased in number, and they are mostly MHCII+ CD11c+ cells (28, 35). Notably, adoptive transfer of these cells exacerbates pulmonary fibrosis. Sorted cells attach to the culture plate and show similar dendritic morphology, suggesting that DCs may comprise some of these mobilized BM-derived cells in the injured lung upon BLM treatment (35). Although alternatively activated or M2 macrophages cannot be excluded from this lung CD11c+ cell population, they are unlikely to be a source for AREG since M2 macrophages do not express this EGFR ligand (43). Additionally studies are emerging that suggest a role for DCs in fibrosis in multiple tissues, including the lung (18–22). In the BLM model of scleroderma, CD11c+ cells accumulate in the deep dermis along with the accumulated αSMA+ myofibroblasts, suggesting the possibility of direct cross-talk between the two cell types (20). In a model of liver fibrosis, hepatic CD11c+ cells expand 5-fold and modulate proinflammatory mediator expression and hepatic stellate cell activity (19). Furthermore pharmacological inactivation of DCs by VAG539, a molecular that inhibits upregulation of co-stimulatory molecular on DCs, attenuated the fibrotic sequelae of BLM treatment (18). In the current study a similar profibrotic role for BM-derived CD11c+ cells was suggested that was dependent on their expression of AREG. While there is suggestive evidence that these cells are consistent with DCs, a more precise identification of the cells comprising this BM-derived CD11c+ cell population requires further study.
While there is evidence for a fibrotic role for AREG, based on its known activity and as a ligand for EGFR the assumption is that its importance in fibrosis is likely due to stimulation of fibroblast or mesenchymal cell proliferation. The proximity of AREG-secreting CD11c+ cells to lung fibroblasts may favor their activation by the induced AREG with consequent promotion of fibroproliferation and overall pulmonary fibrosis. DCs are indeed reported to potentially regulate mesenchymal cells (20, 44, 45). In the current study BM CD11c+ cells were shown to stimulate fibroblast proliferation and αSMA gene expression in an AREG-dependent manner. However while treatment with recombinant AREG promoted mouse lung fibroblast proliferation, it did not significantly affect myofibroblast differentiation (αSMA induction). This indicated that the CD11c+ cell-derived AREG dependent effect on myofibroblast differentiation was indirect, requiring mediation by some other factor(s) that was induced by AREG itself. Given that AREG deficiency resulted in reduced TGFβ expression, the possibility that the myofibroblast differentiation effect could be mediated by induction of TGFβ in these cells merits future investigation.
AREG is known to modulate cell proliferation, apoptosis and migration in different cell types (46). A key novel finding in this current study is that AREG could induce TERT in fibroblasts, which mediate in part the effect of AREG on cell proliferation consistent with previous evidence of decreased fibroblast proliferation potential in TERT deficient mice (39). Additionally induction of lung TERT expression in BLM treated mice was essentially abolished in Areg KO mice, indicating the in vivo importance of induction of TERT by AREG in pulmonary fibrosis. Interestingly, EGFR inhibitor PD153035 was able to diminish the AREG-induced TERT expression in mouse lung fibroblasts, suggesting a potential target of the treatment using EGFR inhibitor. While the correlation between TERT levels and proliferative effects suggests the potential importance of TERT in mediating these AREG-induced effects in both murine and human fibroblasts, further work needs to be done to establish that the induction of TERT is indeed a cause for the noted proliferative effect.
Fibroblast invasiveness is reported to be essential for pulmonary fibrosis and IPF fibroblasts exhibit greater motility than control cells (47, 48). AREG is known to regulate cell motility, invasiveness and metastasis in the cancer literature (46), and in chondrosarcoma cells it promotes α6β1 integrin dependent enhancement of cell motility (40). The current findings indicated that the induction of α6 integrin in HLF was associated with AREG promotion of cell motility. Interestingly, the induction of lung α6 integrin expression was also observed in BLM treated mice, which was almost abolished in Areg KO mice confirming in vivo that AREG regulated α6 integrin induction in fibrosis with consequent effects on fibroblast motility and invasiveness. These findings revealed the mechanism of fibroblast activation by which AREG promoted cell proliferation dependent on TERT, and cell motility with potential α6 integrin involvement. In summary, the findings from the current study suggest induced AREG expression in recruited BM-derived CD11c+ cells contributes to pulmonary fibrosis by AREG-mediated paracrine stimulation of fibroblast proliferation and motility/invasiveness directly, and myofibroblast differentiation indirectly. Induction of TERT by AREG mediated in part the effects on cell proliferation, while association with α6 integrin and TGFβ induction suggest their involvement in fibroblast motility and differentiation to myofibroblast, respectively. These novel findings uncover multiple potential therapeutic targets for future study.
Acknowledgments
Areg-deficient (Areg KO) mice were generous gifts from Dr. Susumu Nakae (University of Tokyo, The Institute of Medical Science, Japan). Primary human lung fibroblasts were kind gifts from Dr. Henke A. Craig (University of Minnesota, Minneapolis, MN). We thank Lisa Riggs for the excellent technical assistance in the lung tissue section preparation and the H & E staining.
Funding: This study was supported by the National Institutes of Health (NIH; Grant HL 052285 and HL112880).
Abbreviations
- αSMA
alpha smooth muscle actin
- AREG
amphiregulin
- BLM
bleomycin
- BMDC
bone marrow derived dendritic cell
- DC
dendritic cell
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
- IPF
idiopathic pulmonary fibrosis
- MLF
mouse lung fibroblasts
- SAL
saline
- TERT
telomerase reverse transcriptase
References
- 1.Phan SH. Genesis of the myofibroblast in lung injury and fibrosis. Proc Am Thorac Soc. 2012;9:148–152. doi: 10.1513/pats.201201-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans JN, Kelley J, Low RB, Adler KB. Increased contractility of isolated lung parenchyma in an animal model of pulmonary fibrosis induced by bleomycin. Am Rev Respir Dis. 1982;125:89–94. doi: 10.1164/arrd.1982.125.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Zhuang S, Liu N. EGFR signaling in renal fibrosis. Kidney Int Suppl (2011) 2014;4:70–74. doi: 10.1038/kisup.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinelli M, Pacilli AM, Rivetti S, Lauriola M, Fasano L, Carbonara P, Mattei G, Valentini I, Scapoli L, Solmi R. A role for epidermal growth factor receptor in idiopathic pulmonary fibrosis onset. Mol Biol Rep. 2011;38:4613–4617. doi: 10.1007/s11033-010-0594-0. [DOI] [PubMed] [Google Scholar]
- 6.Korfhagen TR, Swantz RJ, Wert SE, McCarty JM, Kerlakian CB, Glasser SW, Whitsett JA. Respiratory epithelial cell expression of human transforming growth factor-alpha induces lung fibrosis in transgenic mice. J Clin Invest. 1994;93:1691–1699. doi: 10.1172/JCI117152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardie WD, Davidson C, Ikegami M, Leikauf GD, Le Cras TD, Prestridge A, Whitsett JA, Korfhagen TR. EGF receptor tyrosine kinase inhibitors diminish transforming growth factor-alpha-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1217–1225. doi: 10.1152/ajplung.00020.2008. [DOI] [PubMed] [Google Scholar]
- 8.Perugorria MJ, Latasa MU, Nicou A, Cartagena-Lirola H, Castillo J, Goni S, Vespasiani-Gentilucci U, Zagami MG, Lotersztajn S, Prieto J, Berasain C, Avila MA. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology. 2008;48:1251–1261. doi: 10.1002/hep.22437. [DOI] [PubMed] [Google Scholar]
- 9.Vallath S, Hynds RE, Succony L, Janes SM, Giangreco A. Targeting EGFR signalling in chronic lung disease: therapeutic challenges and opportunities. Eur Respir J. 2014;44:513–522. doi: 10.1183/09031936.00146413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galuppo M, Esposito E, Mazzon E, Di Paola R, Paterniti I, Impellizzeri D, Cuzzocrea S. MEK inhibition suppresses the development of lung fibrosis in the bleomycin model. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:21–37. doi: 10.1007/s00210-011-0637-7. [DOI] [PubMed] [Google Scholar]
- 11.Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- 12.Kimura H, Fischer WH, Schubert D. Structure, expression and function of a schwannoma-derived growth factor. Nature. 1990;348:257–260. doi: 10.1038/348257a0. [DOI] [PubMed] [Google Scholar]
- 13.Zaiss DM, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willmarth NE, Ethier SP. Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J Biol Chem. 2006;281:37728–37737. doi: 10.1074/jbc.M606532200. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Lee JY, Lee CM, Cho WK, Kang MJ, Koff JL, Yoon PO, Chae J, Park HO, Elias JA, Lee CG. Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-beta-induced pulmonary fibrosis. J Biol Chem. 2012;287:41991–42000. doi: 10.1074/jbc.M112.356824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu YL, Huang MS, Cheng DE, Hung JY, Yang CJ, Chou SH, Kuo PL. Lung tumor-associated dendritic cell-derived amphiregulin increased cancer progression. J Immunol. 2011;187:1733–1744. doi: 10.4049/jimmunol.1100996. [DOI] [PubMed] [Google Scholar]
- 17.Bles N, Di Pietrantonio L, Boeynaems JM, Communi D. ATP confers tumorigenic properties to dendritic cells by inducing amphiregulin secretion. Blood. 2010;116:3219–3226. doi: 10.1182/blood-2010-01-265611. [DOI] [PubMed] [Google Scholar]
- 18.Bantsimba-Malanda C, Marchal-Somme J, Goven D, Freynet O, Michel L, Crestani B, Soler P. A role for dendritic cells in bleomycin-induced pulmonary fibrosis in mice? Am J Respir Crit Care Med. 2010;182:385–395. doi: 10.1164/rccm.200907-1164OC. [DOI] [PubMed] [Google Scholar]
- 19.Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, Pachter HL, Bar-Sagi D, Frey AB, Miller G. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119:3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu TT. Dendritic cells: novel players in fibrosis and scleroderma. Curr Rheumatol Rep. 2012;14:30–38. doi: 10.1007/s11926-011-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, Herve P, Emilie D, Simonneau G, Humbert M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J. 2007;29:462–468. doi: 10.1183/09031936.00094706. [DOI] [PubMed] [Google Scholar]
- 22.Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med. 2005;172:530–551. doi: 10.1164/rccm.200410-1384SO. [DOI] [PubMed] [Google Scholar]
- 23.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukumoto J, Harada C, Kawaguchi T, Suetsugu S, Maeyama T, Inoshima I, Hamada N, Kuwano K, Nakanishi Y. Amphiregulin attenuates bleomycin-induced pneumopathy in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L131–138. doi: 10.1152/ajplung.90576.2008. [DOI] [PubMed] [Google Scholar]
- 26.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Baek HA, Yu H, Lee HJ, Park BH, Ullenbruch M, Liu J, Nakashima T, Choi YY, Wu GD, Chung MJ, Phan SH. FIZZ2/RELM-beta induction and role in pulmonary fibrosis. J Immunol. 2011;187:450–461. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding L, Dolgachev V, Wu Z, Liu T, Nakashima T, Ullenbruch M, Lukacs NW, Chen Z, Phan SH. Essential role of stem cell factor-c-Kit signalling pathway in bleomycin-induced pulmonary fibrosis. J Pathol. 2013;230:205–214. doi: 10.1002/path.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol. 2003;170:2083–2092. doi: 10.4049/jimmunol.170.4.2083. [DOI] [PubMed] [Google Scholar]
- 32.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Yu H, Ding L, Wu Z, Gonzalez De Los Santos F, Liu J, Ullenbruch M, Hu B, Martins V, Phan SH. Conditional Knockout of Telomerase Reverse Transcriptase in Mesenchymal Cells Impairs Mouse Pulmonary Fibrosis. PLoS One. 2015;10:e0142547. doi: 10.1371/journal.pone.0142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakashima T, Liu T, Yu H, Ding L, Ullenbruch M, Hu B, Wu Z, Oguro H, Phan SH. Lung bone marrow-derived hematopoietic progenitor cells enhance pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:976–984. doi: 10.1164/rccm.201303-0479OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003;29:397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Ullenbruch M, Young Choi Y, Yu H, Ding L, Xaubet A, Pereda J, Feghali-Bostwick CA, Bitterman PB, Henke CA, Pardo A, Selman M, Phan SH. Telomerase and telomere length in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2013;49:260–268. doi: 10.1165/rcmb.2012-0514OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nozaki Y, Liu T, Hatano K, Gharaee-Kermani M, Phan SH. Induction of telomerase activity in fibroblasts from bleomycin-injured lungs. Am J Respir Cell Mol Biol. 2000;23:460–465. doi: 10.1165/ajrcmb.23.4.3958. [DOI] [PubMed] [Google Scholar]
- 39.Liu T, Chung MJ, Ullenbruch M, Yu H, Jin H, Hu B, Choi YY, Ishikawa F, Phan SH. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest. 2007;117:3800–3809. doi: 10.1172/JCI32369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JC, Chen YJ, Lin CY, Fong YC, Hsu CJ, Tsai CH, Su JL, Tang CH. Amphiregulin enhances alpha6beta1 integrin expression and cell motility in human chondrosarcoma cells through Ras/Raf/MEK/ERK/AP-1 pathway. Oncotarget. 2015;6:11434–11446. doi: 10.18632/oncotarget.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKee C, Sigala B, Soeda J, Mouralidarane A, Morgan M, Mazzoccoli G, Rappa F, Cappello F, Cabibi D, Pazienza V, Selden C, Roskams T, Vinciguerra M, Oben JA. Amphiregulin activates human hepatic stellate cells and is upregulated in non alcoholic steatohepatitis. Sci Rep. 2015;5:8812. doi: 10.1038/srep08812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuger L, Johnson GR, Gilbride K, Plowman GD, Mandel R. Amphiregulin in lung branching morphogenesis: interaction with heparan sulfate proteoglycan modulates cell proliferation. Development. 1996;122:1759–1767. doi: 10.1242/dev.122.6.1759. [DOI] [PubMed] [Google Scholar]
- 43.Meng C, Liu G, Mu H, Zhou M, Zhang S, Xu Y. Amphiregulin may be a new biomarker of classically activated macrophages. Biochem Biophys Res Commun. 2015;466:393–399. doi: 10.1016/j.bbrc.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 44.Kaissling B, Hegyi I, Loffing J, Le Hir M. Morphology of interstitial cells in the healthy kidney. Anat Embryol (Berl) 1996;193:303–318. doi: 10.1007/BF00186688. [DOI] [PubMed] [Google Scholar]
- 45.Kaissling B, Le Hir M. Characterization and distribution of interstitial cell types in the renal cortex of rats. Kidney Int. 1994;45:709–720. doi: 10.1038/ki.1994.95. [DOI] [PubMed] [Google Scholar]
- 46.Berasain C, Avila MA. Amphiregulin. Semin Cell Dev Biol. 2014;28:31–41. doi: 10.1016/j.semcdb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 47.White ES, V, Thannickal J, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med. 2003;168:436–442. doi: 10.1164/rccm.200301-041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]