Abstract
Background and Aims
Hyperglycemia is implicated as a major risk factor for delayed gastric emptying in diabetes mellitus and vice versa. However, the extent to which hyperglycemia can affect gastric emptying and vice versa and the implications for clinical practice are unclear. We systematically reviewed the evidence for this bi-directional relationship and the effects of pharmacotherapy for diabetes on gastric emptying.
Methods
Full-length articles investigating the relationship between diabetes mellitus and gastroparesis were reviewed primarily to quantify the relationship between blood glucose concentrations and gastrointestinal sensorimotor functions, particularly gastric emptying, and gastrointestinal symptoms. The effects of drugs and hormones, which affect glycemia, on gastrointestinal sensorimotor functions were also evaluated.
Results
Acute severe hyperglycemia delayed gastric emptying relative to euglycemia in type 1 diabetes; the corresponding effects in type 2 diabetes are unknown. Limited evidence suggests that even mild hyperglycemia (8 mmol/L) can delay gastric emptying in type 1 diabetes. Long-term hyperglycemia is an independent risk factor for delayed gastric emptying in type 1 diabetes. There is little evidence that delayed gastric emptying causes hypoglycemia in diabetes and no evidence that improved control of glycemia improves gastric emptying or vice versa. Glucagonlike peptide-1 agonists but not dipeptidyl peptidase 4 inhibitors given acutely delay gastric emptying but tachyphylaxis may occur.
Conclusions
While acute severe and chronic hyperglycemia can delay gastric emptying, there is limited evidence that delayed gastric emptying is an independent risk factor for impaired glycemic control or hypoglycemia in diabetes. The impact of improved glycemic control on gastric emptying and vice versa in diabetes is unknown.
Keywords: DPP-4, GLP-1, treatment, vomiting
INTRODUCTION
Diabetes mellitus has been associated with delayed, and to a lesser extent, rapid gastric emptying (GE).1, 2 Delayed GE is associated with upper gastrointestinal symptoms, impaired quality of life, poor nutrition, and increased morbidity and mortality. It has also been suggested that GE disturbances may impair glycemic control in diabetes.3 Several abnormalities, including autonomic neuropathy, enteric neuropathy involving excitatory and inhibitory nerves, abnormalities of interstitial cells of Cajal (ICC), acute fluctuations in blood glucose, and other considerations, such as incretin-based medications used to normalize postprandial blood glucose and psychosomatic factors, may be responsible for gastric motor dysfunction in diabetes.2 Studies in animal models of diabetes mellitus suggest that hyperglycemia affects the enteric nervous system and GE. For example, compared to normoglycemia (5 mM glucose), hyperglycemia (20 mM glucose) increased apoptosis of rodent enteric neurons in culture.4 Spontaneously diabetic non-obese diabetic (NOD) mice and streptozotocin-diabetic mice developed reduced neuronal nitric oxide synthase expression and delayed gastric emptying of liquids.5 In NOD mice, hyperglycemia for approximately two months was also associated with depletion of the interstitial cells of Cajal.6
However, these effects of hyperglycemia on enteric neurons4 or GE in (NOD) mice and streptozotocin-diabetic mice5 were not reversed by restoring normoglycemia, albeit for a brief duration. In contrast, treatment with insulin or sildenafil5 or hemin7 restored GE in NOD mice. Together, these data indicate that hyperglycemia is but one factor that affects the enteric nervous system in diabetes. Restoring normoglycemia may not be sufficient to interrupt or reverse the signaling cascades that are altered in diabetes.
Much attention has focused on the impact of hyperglycemia on GE in humans. Fewer studies have evaluated the contribution of delayed GE to poor glycemic control.3 Prompted in part by recent studies,8, 9 this review, which is geared towards gastroenterologists, focuses on the bidirectional relationship between hyperglycemia and gastric functions, the effects of pharmacological therapy for diabetes on gastric emptying, and the implications of these findings to clinical practice.
METHODS
A literature search using OVID (Wolters Kluwer Health, New York, NY, USA) to examine the MEDLINE database from its inception up to May 2015 was performed by combining the search terms “gastric emptying” or “gastroparesis” with “glycemic control,” and separately with “diabetes mellitus.” In addition, the PubMed database was searched for gastroparesis. Reference lists and personal libraries of the authors were used to identify supplemental information. Articles were reviewed in full text. While studies in healthy people are discussed as appropriate, the emphasis is on patients with diabetes. The 26 original studies cited in this review which evaluated gastric emptying used a variety of techniques, such as scintigraphy (i.e., solid [12 studies], liquid [1 study] or mixed solid and liquid meal [7 studies]), ultrasound (1 study), marker technique (phenol red, 1 study), or a 13C breath test (4 studies). The caloric intake of the meal varied among studies.
The results will consider the relationships between acute hyperglycemia and GE and subsequently gastrointestinal motility and sensation, between long-term control of glycemia and GE and separately upper GI symptoms, the effects of hormones and pharmacotherapy for diabetes on glycemia and gastric emptying, and of GE disturbances on glycemic control in diabetes. Unless specified otherwise, the results refer to GE of solids.
Acute Effects of Hyperglycemia on Gastric Emptying in Healthy People and Diabetes Mellitus
In 1956, Stunkard et al. observed that intravenous glucose abolished gastric “hunger-contractions” in healthy people,10 providing perhaps the earliest demonstration that acute hyperglycemia can affect gastric motor activity in humans. A preliminary study in 196211 and a definitive report in 1976 demonstrated that acute hyperglycemia delayed GE in healthy people.12 Thereafter, 2 studies, with a total of 18 patients, comparing marked hyperglycemia (blood glucose of 16–20 mmol/L) to euglycemia (blood glucose of 4–8 mmol/L) in type 1 diabetes observed that the GE half-time of solids was prolonged by an average of 17 minutes (increased from 124 to 141 minutes) and 31 minutes (increased from 74 to 105 minutes) (Table).13, 14 Hyperglycemia also prolonged the GE of nutrient liquids in one of these 2 studies. Even mild hyperglycemia (8 mmol/L vs. 4 mmol/L), facilitated by an insulin clamp, was associated with significantly greater gastric retention of solids and liquids in 8 healthy people and 9 patients with in type 1 diabetes.15 However, among patients with type 1 diabetes, the difference in gastric retention at 100 minutes was small, (44% at a blood glucose level of 8 mmol/L and 36% at 4 mmol/L). In this study and in the studies that evaluated the effects of severe acute hyperglycemia on GE,13, 14 GE was evaluated during normoglycemia and hyperglycemia on 2 separate days. Because patients were not randomized to normoglycemia or hyperglycemia during the second study, the intra-individual day-to-day variability in GE during normoglycemia is unclear. In one study, the intra-individual coefficient of variation for GE assessed on 2 separate days in 14 patients with insulin-dependent diabetes was 29%.16 Mean fasting blood glucose concentrations were not significantly different between the 2 days. Hence, additional studies to determine whether effects of “mild” hyperglycemia on GE are greater than the day-to-day variability in GE in patients with normoglycemia are necessary.
Table.
Summary of the Evidence the Relationship Between Delayed Gastric Emptying and Hyperglycemia in Diabetes
| Concept | Evidence |
|---|---|
| Acute hyperglycemia delays gastric emptying in diabetes | Type 1 diabetes: Marked hyperglycemia (blood glucose of 16–20 mmol/L) delayed gastric emptying in diabetes.13, 14 The magnitude of delay varied between studies. Modest hyperglycemia (8 mmol/L) also delayed gastric emptying15; however, the effects were small and not greater than the intra-individual day-to-day coefficient of variation for gastric emptying in type 1 diabetes.16 Type 2 diabetes: Not assessed. |
| If the blood glucose is greater than 275 mg/dL on the morning of the gastric emptying study, the glucose should be lowered with insulin to 275 mg/dL before commencing the test.18 | Type 1 diabetes: This recommendation is based on the finding that acute hyperglycemia delayed gastric emptying.13, 14 However, there is no evidence that reducing blood glucose improves GE in patients with severe hyperglycemia. Type 2 diabetes: An inverse correlation between fasting glucose concentration and gastric emptying was observed.8 Among 9 of 90 GE studies preceded by a blood glucose concentration >275 mg/dL, 2 had normal, 2 had delayed, and 5 had rapid GE. |
| Improved long-term control of glycemia may improve GE in diabetes | Type 1 diabetes: Not assessed. Type 2 diabetes: Acute and long-term (6 month) improvement in glycemia did not affect GE in one study8 but markedly reduced GE in another study of patients with poorly-controlled type 2 diabetes and delayed GE.36 |
| Delayed gastric emptying predisposes to hypoglycemia in diabetes | While delayed GE was a risk factor for hypoglycemia in 1 study, patients with delayed GE also had other complications of diabetes.72 There is no evidence that delayed GE is an independent risk factor for unexplained hypoglycemia. The effect of accelerating GE on glycemic control has not been evaluated. |
Abbreviations: GE, gastric emptying
The effects of acute hyperglycemia on GE in type 2 diabetes have not been studied. However, in a cross-sectional study of 20 patients with type 2 diabetes, the GE half-time of liquids and the lag time, but not the half-time, for GE of solids were correlated with plasma glucose.17 In contrast, among 30 patients with poorly-controlled type 2 diabetes who underwent GE studies on three occasions (at baseline, after overnight randomization to insulin or saline, and 6 months after optimizing treatment of diabetes), there was an inverse correlation between fasting blood glucose concentrations and GE during the baseline and second assessment,8 i.e., higher fasting blood glucose concentrations were associated with shorter gastric emptying halftimes. Hence, the effects of hyperglycemia on GE in type 2 diabetes are contradictory; further studies are necessary.
In diabetic patients with marked hyperglycemia and delayed GE, it is conceivable that hyperglycemia is at least partly responsible for delayed GE. Therefore, measures to restore normoglycemia seem sensible. The American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine Consensus guidelines for GE scintigraphy recommend that blood glucose exceeding 275 mg/dL on the morning of the test, should be lowered with insulin before commencing the test.18 However, there is no evidence that this approach improves GE. In a study of 30 patients with type 2 diabetes, 9 of 90 GE studies were preceded by a blood glucose concentration greater than 275 mg/dL. Among these patients, 2 had normal, 2 had delayed, and 5 had rapid GE. Moreover, a comparison of GE before and after randomization to insulin or saline, indicated that, contrary to conventional wisdom, among patients with delayed GE at baseline, a smaller reduction in fasting blood glucose concentration was associated with a more pronounced reduction in GE between baseline and second visits.
In contrast to hyperglycemia, acute hypoglycemia can accelerate the GE of liquids and solids. In a study of 8 healthy people, the GE half-time for solids was 43.0 ± 20.0 min during normoglycemia, compared with 16.3 ± 6.6 min during medically-induced hypoglycemia.19
Acute Effects of Hyperglycemia on Gastrointestinal Motility and Sensation
Decreased gastric motility, perhaps mediated by neurohumoral mechanisms, may explain delayed GE during hyperglycemia. Compared to euglycemia (blood glucose of 5–8 mmol/L), hyperglycemia (blood glucose ≥11 mmol/L) reduced antral motility in healthy people.20, 21 More severe hyperglycemia (blood glucose of 16–19 mmol/L) reduced antral motility and GE in 8 patients with type 1 diabetes.14 Since antral contractility is responsible for triturating food into smaller particles, it is conceivable that hyperglycemia-induced inhibition of antral motility is at least partly responsible for delayed GE. Surprisingly, the correlation between antral motility and GE of solids was not significant. One possibility is that hyperglycemia has other effects on gastrointestinal motor functions that may affect GE. For example, hyperglycemia also increased gastric compliance during distention in healthy people22 and in people with type 1 diabetes and reduced duodenal pressure activity, which may facilitate GE.14 Two studies with a total of 18 patients observed that hyperglycemia (15 mmol/L) increased proximal gastric compliance during gastric distention and the perception of fullness, nausea, and bloating during distention relative to euglycemia (6 mmol/L) in type 1 diabetes.23, 24 Likewise, gastric relaxation and the perception of nausea and fullness induced by duodenal infusion of triglycerides was greater during hyperglycemia (blood glucose of approximately 15 mmol/L) than euglycemia (blood glucose of 6 mmol/L).25 These effects of hyperglycemia contrast with impaired postprandial gastric accommodation, possibly due to a vagal neuropathy, which is postulated to explain early satiety in diabetes.26, 27
While increased perception may explain more severe symptoms (e.g., nausea) during hyperglycemia, greater postprandial relaxation would not. Finally, hyperglycemia (mean blood glucose 15.9 mmol/L [range 10.7–17.5]) is also associated with a shorter length (mean of 88 minutes versus 158 minutes) of the migrating motor complex cycle.28
Relationship Between Long-Term Control of Glycemia and Gastric Emptying in Diabetes
Four of 5 studies observed no significant correlation between glycated hemoglobin (HbA1c) and GE in diabetes. Among 83 patients with type 1 diabetes, delayed gastric emptying was associated with an autonomic neuropathy but not with the HbA1c.29 In a study of 250 consecutive patients with type 1 or 2 diabetes, HbA1c (mean 7.9% ± 2.2%) and GE were not correlated.30 A third study reported that HbA1c was not significantly different among 3 cohorts: 1) 94 diabetic (type 1 and type 2) patients each with classic symptoms of gastroparesis and delayed GE; 2) classic symptoms of gastroparesis but normal gastric emptying: and 3) diabetes without symptoms of gastroparesis.31 Parenthetically, in this study patients with diabetic gastroparesis were more likely to have cardiovascular disease, hypertension, and retinopathy, as well as more hospitalizations and office visits, than patients in the other groups. Likewise, the HbA1c was not significantly associated with gastric emptying in 30 patients with poorly-controlled type 2 diabetes.8
However, the HbA1c was correlated with delayed GE in a recent study from the Epidemiology of Diabetes Intensive Complications (EDIC) cohort.9 The EDIC is a prospective, longitudinal, and observational follow-up study of the long-term effects of improved glycemic control of among participants in the Diabetes Control and Complications Trial (DCCT).32 The DCCT demonstrated that intensive vs. conventional therapy, resulting in mean HbA1c levels of 7% and 9%, respectively, over an average 6.5 years, reduced the risk of diabetic retinopathy, nephropathy, and peripheral and cardiac autonomic neuropathy by 40%–60% in type 1 diabetes.33 Of 1,441 participants in the DCCT, 1,375 (96%) agreed to participate in the annual follow-up evaluations for the EDIC study, which began in 1994. Recently, GE was evaluated with a 13C-spirulina breath test among 78 patients with type 1 diabetes in this cohort 27 years after they were enrolled in the DCCT, i.e., 6.5 years in the DCCT and 20 years in the EDIC.9 These participants were studied. GE was normal in 50%, delayed in 47%, and rapid in 3%. Prior to the GE study, the latest HbA1c was not significantly different in patients with normal (average of 7.6%) and delayed GE (average of 7.9%); these values are equivalent to average blood glucose concentrations of 171 mg/dL and 180 mg/dL, respectively. However, DCCT baseline HbA1c and years of diabetes prior to DCCT entry and mean HbA1c during DCCT-EDIC were independently associated with delayed GE. These findings demonstrate that even in the era of intensive therapy, delayed GE is remarkably common in patients with longstanding type 1 diabetes, and demonstrate that even greater glycemic exposure approximately 27 years previously is an independent risk factor for delayed GE in diabetes.
With one exception, there is no evidence that improved long-term control of glycemia improves GE in diabetes. Among 20 of 86 diabetic patients, the HbA1c was significantly, albeit modestly, lower (8.4% ± 2.3% vs. 7.6% ± 1.3%) after an average follow-up period of 12.3 years; however, GE of solids or liquids and upper gastrointestinal symptoms were not significantly different.34 A decade later, only 53 of the original 86 patients were alive. Among 13 of these patients, GE of neither liquids nor solids had changed significantly over a 25-year period.35 In another study, neither an acute improvement in glycemia with an overnight insulin infusion, nor long-term improvement from a HbA1c of 10.6% ± 0.3% at baseline to 9% ± 0.4% at 6-months with approved therapies improved GE in 30 patients with poorly-controlled type 2 diabetes.8 It is conceivable that more prolonged improvement in control of glycemia is necessary to improve GE. Finally, a study from India observed that improved glycemic control normalized GE in 30 asymptomatic women with newly-diagnosed diabetes, marked hyperglycemia (average HbA1c of 10.5%) and markedly delayed GE at baseline.36
Relationship Between Long-Term Control of Glycemia and Upper GI Symptoms in Diabetes
The relationship between long-term hyperglycemia and GI symptoms in diabetes is unclear. In a study of 1,101 people with diabetes, of whom HbA1c was measured in 436 people, poor glycemic control was an independent, albeit weak, risk factor for dysmotility-like dyspepsia (OR 1.32; 95% CI, 1.08–1.60) and ulcer-like dyspepsia (OR 1.36; 95% CI, 1.06–1.75).37 In contrast, the prevalence of GI symptoms was inversely associated with HbA1c levels in 722 diabetics within a larger cohort of 7,700 patients in Taiwan who had endoscopy during a medical examination.38 Moreover, higher HbA1c levels were associated with a higher prevalence of endoscopic abnormalities, e.g., peptic ulcer disease and colonic neoplasia. In a longitudinal population-based assessment of gastrointestinal symptoms among 136 mainly type 2 diabetes patients, the association between worsening GI symptoms and self-reported glycemic control was weak.39
Effect of Hormones and Pharmacotherapy for Diabetes Mellitus on Glycemia and Gastric Emptying
Some medications (e.g., GLP-1 agonists) that are used to treat glycemia also affect gastrointestinal sensorimotor functions. Glucagon-like peptide (GLP-1) is released from specialized enteroendocrine cells in the small intestine in response to a nutrient load and accounts for approximately 50% of postprandial insulin release.40 GLP-1 reduces food intake in rats,41 induces satiety in humans,42 and inhibits gastrointestinal secretion,43, 44 gastric motility, and emptying in humans (Figure 1). These effects are at least partly mediated by efferent vagal inhibition, as evidenced by blockade of the plasma pancreatic polypeptide response to feeding during GLP-1 infusion.41, 42 Endogenous GLP-1 is normally degraded by dipeptidyl peptidase (DPP)-4 and has a half-life of 1–2 minutes.45 The synthetic GLP-1 agonists (exenatide, liraglutide, lixisenatide, albiglutide and dulaglutide) are resistant to DPP-4 and improve control of glycemia.46
Figure 1.
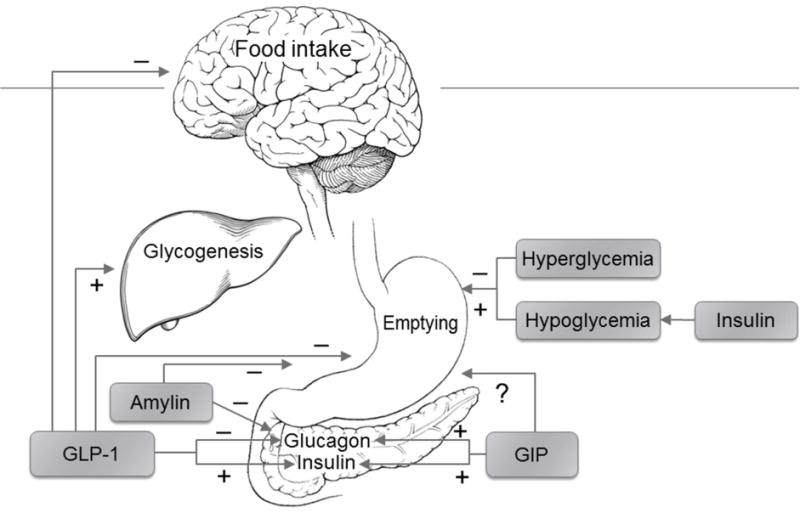
Key Effects of Glucoregulatory Hormones That Affect Gastric Emptying.
The earlier studies suggested that delayed GE, increased insulin, and decreased glucagon secretion were independent determinants of the effect of GLP-1 on glycemia.43, 47–49 Also, GLP-1-induced delayed GE may explain nausea, vomiting, and weight loss, which were common side effects of exenatide.50, 51 However, the effects of GLP-1 agonists on GE and the contribution of delayed GE to gastrointestinal symptoms in diabetes have been questioned. A second dose or a prolonged infusion of GLP-1 had lesser effects on GE than the initial dose.52 Likewise, the GLP-1-induced delay in GE assessed with acetaminophen was preserved when GLP-1 was administered twice daily but not when given as a long-acting preparation, both for 14 weeks.53 These studies have prompted the inference that there is tachyphylaxis to the effects of long-acting GLP-1 agonists on GE. The evidence for a correlation between GI symptoms and delayed GE is mixed.54 The effects of using GLP-1 agonists in patients with normal versus delayed GE have not been compared. Nonetheless, it would seem reasonable to assess GE before starting a GLP-1 agonist in patients with upper GI symptoms and weigh the issue thoughtfully before proceeding with treatment.
The DPP-4 inhibitors (i.e., sitagliptin, saxagliptin, linagliptin, alogliptin, and vildagliptin) improve glycemic control by inhibiting the metabolism of endogenous GLP-1 and glucose-dependent insulinotropic peptide (GIP).55 However, these drugs increase GLP-1 to a lesser extent than GLP-1 agonists, which is probably why they do not significantly affect GE, satiation, or gastric volumes in people with type 2 diabetes and are less likely to cause GI side effects than GLP-1 agonists.55–57
GIP also stimulates insulin secretion in a glucose-dependent manner. In healthy volunteers, GIP did not affect GE in one study58 but accelerated GE in another.59 Insulin-induced hypoglycemia, but not euglycemic hyperinsulinemia, accelerates GE of solids and liquids in diabetes.60, 61 Pharmacological concentrations of amylin, as with the use of the amylin analog pramlintide, can delay GE.49, 62 Conversely, impaired hyperglycemia-induced release of amylin, which delays GE, may contribute to rapid GE in diabetes.63 Moreover, while a subset of patients with type 2 diabetes have increased plasma glucagon concentrations, higher glucagon concentrations in isolation are unlikely to explain rapid GE of liquids in these patients.64, 65
Effects of Gastric Emptying Disturbances on Glycemic Control in Diabetes
The stomach normally empties nutrients into the small intestine at a tightly-regulated rate averaging 2–3 kcal per minute.66, 67 The postprandial increase in blood glucose concentration is significantly correlated with the GE rate in healthy people68, 69 and in people with diabetes.70
There is little evidence that delayed GE contributes to hypoglycemia in diabetes. Among 11 patients with type 1 diabetes on an insulin pump, postprandial insulin requirements were lower for the first 120 minutes and greater between 180–240 minutes in 5 patients with gastroparesis than in 6 patients without.71 Blood glucose values during the postprandial period were not provided. It is unclear to what extent these different insulin requirements were explained by gastroparesis per se rather than other associated complications (i.e., nephropathy, vagal neuropathy). In another study, GE was delayed in more patients with hypoglycemia (25 of 31 [81%]) than without (3 of 18 [17%]); 1 patient in each group had accelerated GE.72 Based on these observations, it has been suggested that gastroparesis may lead to otherwise unexplained hypoglycemia, particularly early in the postprandial period (so-called gastric hypoglycemia).73 While delayed GE is a plausible explanation for hypoglycemia, another possible explanation is that shared risk factors, such as autonomic neuropathy, are responsible for hypoglycemia and delayed GE.
These studies did not directly evaluate the effect of accelerating GE on glycemic control. In a study of 9 patients with type 2 diabetes, the peak postprandial plasma glucose was lower after morphine delayed GE and greater after erythromycin accelerated GE.74 In summary, while it is conceivable that GE disturbances contribute to poor glycemic control and hypoglycemia, further studies that directly evaluate the effects of accelerating GE on glycemic control in diabetes are necessary.
Summary
There is evidence that acute, severe hyperglycemia delays GE in health and type 1 diabetes mellitus. Suboptimal, long-term control of glycemia is associated with delayed GE in type 1 diabetes. Mild hyperglycemia in the “physiological range” has also been attributed to delayed GE in type 1 diabetes. However, the effects of mild hyperglycemia are small and within the normal coefficient of variation in GE in diabetes. There is no evidence that improved control of glycemia improves GE in diabetes and very little evidence that delayed GE is an independent risk factor for hypoglycemia in diabetes mellitus. GLP-1 agonists but not DPP-4 inhibitors administered acutely delay GE; however, the long-term effects of GLP-1 agonists on GE in type 2 diabetes deserve further study. Further studies are also necessary to: (i) clarify the effects of mild hyperglycemia on GE in type 1 diabetes and even severe hyperglycemia on GE in type 2 diabetes; (ii) ascertain the effects of improving glycemic control on GE in type 1 and 2 diabetes; and (iii) understand the contribution of delayed GE to hypoglycemia in diabetes.
Acknowledgments
Grant Support: This study was primarily supported by grant R01 DK068055 from the United States Public Health Service National Institutes of Health.
Abbreviations
- CI
confidence interval
- DCCT
Diabetes Control and Complications Trial
- DPP
dipeptidyl peptidase
- EDIC
Epidemiology of Diabetes Intensive Complications
- GE
gastric emptying
- GIP
glucose-dependent insulinotropic peptide
- GLP-1
glucagon-like peptide-1
- HbA1c
glycated hemoglobin
- ICC
interstitial cells of Cajal
- OR
odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
Author contributions: Dr. Magnus Halland–literature review, drafting and critical review of manuscript; Dr. Adil E Bharucha–literature review, drafting and critical review of manuscript
References
- 1.Bharucha AE, Camilleri M, Forstrom LA, et al. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2009;70:415–420. doi: 10.1111/j.1365-2265.2008.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. quiz e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips LK, Deane AM, Jones KL, et al. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11:112–128. doi: 10.1038/nrendo.2014.202. [DOI] [PubMed] [Google Scholar]
- 4.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373–384. doi: 10.1172/JCI8273. [erratum appears in J Clin Invest 2000 Sep;106(6):803] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ordog T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 7.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme Oxygenase-1 Protects Interstitial Cells of Cajal From Oxidative Stress and Reverses Diabetic Gastroparesis. Gastroenterology. 2008;135:2055–2064. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharucha AE, Kudva YC, Basu A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. 2014;13:466–476.e461. doi: 10.1016/j.cgh.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharucha AE, Batey-Schaefer B, Cleary PA, et al. Delayed Gastric Emptying is Associated with Early and Long-Term Hyperglycemia in Type 1 Diabetes Mellitus. Gastroenterology. 2015;149:330–339. doi: 10.1053/j.gastro.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stunkard AJ, Wolff HG. Studies on the physiology of hunger. I. The effect of intravenous administration of glucose on gastric hunger contractions in man. J Clin Invest. 1956;35:954–963. doi: 10.1172/JCI103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aylett P. Gastric emptying and change of blood glucose level, as affected by glucagon and insulin. Clin Sci. 1962;22:171–178. [PubMed] [Google Scholar]
- 12.MacGregor IL, Gueller R, Watts HD, et al. The effect of acute hyperglycemia on gastric emptying in man. Gastroenterology. 1976;70:190–196. [PubMed] [Google Scholar]
- 13.Fraser RJ, Horowitz M, Maddox AF, et al. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675–680. doi: 10.1007/BF00400569. [DOI] [PubMed] [Google Scholar]
- 14.Samsom M, Akkermans LM, Jebbink RJ, et al. Gastrointestinal motor mechanisms in hyperglycaemia induced delayed gastric emptying in type I diabetes mellitus. Gut. 1997;40:641–646. doi: 10.1136/gut.40.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schvarcz E, Palmer M, Aman J, et al. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60–66. doi: 10.1016/s0016-5085(97)70080-5. [DOI] [PubMed] [Google Scholar]
- 16.Lartigue S, Bizais Y, Des Varannes SB, et al. Inter- and intrasubject variability of solid and liquid gastric emptying parameters. A scintigraphic study in healthy subjects and diabetic patients. Dig Dis Sci. 1994;39:109–115. doi: 10.1007/BF02090069. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz M, Harding PE, Maddox AF, et al. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151–159. doi: 10.1007/BF00265086. [DOI] [PubMed] [Google Scholar]
- 18.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 19.Schvarcz E, Palmer M, Aman J, et al. Atropine inhibits the increase in gastric emptying during hypoglycemia in humans. Diabetes Care. 1995;18:1463–1467. doi: 10.2337/diacare.18.11.1463. [DOI] [PubMed] [Google Scholar]
- 20.Barnett JL, Owyang C. Serum glucose concentration as a modulator of interdigestive gastric motility. Gastroenterology. 1988;94:739–744. doi: 10.1016/0016-5085(88)90248-x. [erratum appears in Gastroenterology 1988 Jul;95(1):262] [DOI] [PubMed] [Google Scholar]
- 21.Hasler WL, Soudah HC, Dulai G, et al. Mediation of hyperglycemia-evoked gastric slow-wave dysrhythmias by endogenous prostaglandins. Gastroenterology. 1995;108:727–736. doi: 10.1016/0016-5085(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 22.Hebbard GS, Sun WM, Dent J, et al. Hyperglycaemia affects proximal gastric motor and sensory function in normal subjects. Eur J Gastroenterol Hepatol. 1996;8:211–217. doi: 10.1097/00042737-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Rayner CK, Verhagen MA, Hebbard GS, et al. Proximal gastric compliance and perception of distension in type 1 diabetes mellitus: effects of hyperglycemia. Am J Gastroenterol. 2000;95:1175–1183. doi: 10.1111/j.1572-0241.2000.02006.x. [DOI] [PubMed] [Google Scholar]
- 24.Samsom M, Salet GA, Roelofs JM, et al. Compliance of the proximal stomach and dyspeptic symptoms in patients with type I diabetes mellitus. Dig Dis Sci. 1995;40:2037–2042. doi: 10.1007/BF02208676. [DOI] [PubMed] [Google Scholar]
- 25.Hebbard GS, Samsom M, Sun WM, et al. Hyperglycemia affects proximal gastric motor and sensory function during small intestinal triglyceride infusion. Am J Physiol. 1996;271:G814–819. doi: 10.1152/ajpgi.1996.271.5.G814. [DOI] [PubMed] [Google Scholar]
- 26.Bredenoord AJ, Chial HJ, Camilleri M, et al. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2003;1:264–272. [PubMed] [Google Scholar]
- 27.Kumar A, Attaluri A, Hashmi S, et al. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil. 2008;20:635–642. doi: 10.1111/j.1365-2982.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 28.Oster-Jørgensen E, Qvist N, Pedersen SA, et al. The influence of induced hyperglycaemia on the characteristics of intestinal motility and bile kinetics in healthy men. Scand J Gastroenterol. 1992;27:285–288. doi: 10.3109/00365529209000076. [DOI] [PubMed] [Google Scholar]
- 29.Merio R, Festa A, Bergmann H, et al. Slow gastric emptying in type I diabetes: relation to autonomic and peripheral neuropathy, blood glucose, and glycemic control. Diabetes Care. 1997;20:419–423. doi: 10.2337/diacare.20.3.419. [DOI] [PubMed] [Google Scholar]
- 30.Reddy S, Ramsubeik K, Vega KJ, et al. Do HbA1C Levels Correlate With Delayed Gastric Emptying in Diabetic Patients? J Neurogastroenterol Motil. 2010;16:414–417. doi: 10.5056/jnm.2010.16.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyett B, Martinez FJ, Gill BM, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology. 2009;137:445–452. doi: 10.1053/j.gastro.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 32.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilpatrick ES, Rigby AS, Atkin SL. The Diabetes Control and Complications Trial: the gift that keeps giving. Nat Rev Endocrinol. 2009;5:537–545. doi: 10.1038/nrendo.2009.179. [DOI] [PubMed] [Google Scholar]
- 34.Jones KL, Russo A, Berry MK, et al. A longitudinal study of gastric emptying and upper gastrointestinal symptoms in patients with diabetes mellitus. Am J Med. 2002;113:449–455. doi: 10.1016/s0002-9343(02)01228-7. [DOI] [PubMed] [Google Scholar]
- 35.Chang J, Russo A, Bound M, et al. A 25-year longitudinal evaluation of gastric emptying in diabetes. Diabetes Care. 2012;35:2594–2596. doi: 10.2337/dc12-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laway BA, Malik TS, Khan SH, et al. Prevalence of abnormal gastric emptying in asymptomatic women with newly detected diabetes and its reversibility after glycemic control-a prospective case control study. J Diabetes Complications. 2013;27:78–81. doi: 10.1016/j.jdiacomp.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Bytzer P, Talley NJ, Hammer J, et al. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–611. doi: 10.1111/j.1572-0241.2002.05537.x. [DOI] [PubMed] [Google Scholar]
- 38.Tseng P-HH, Lee Y-CC, Chiu H-MM, et al. Association of diabetes and HbA1c levels with gastrointestinal manifestations. Diabetes Care. 2012;35:1053–1060. doi: 10.2337/dc11-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quan C, Talley NJ, Jones MP, et al. Gastrointestinal symptoms and glycemic control in diabetes mellitus: a longitudinal population study. Eur J Gastroenterol Hepatol. 2008;20:888–897. doi: 10.1097/MEG.0b013e3282f5f734. [DOI] [PubMed] [Google Scholar]
- 40.Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 41.Tang-Christensen M, Larsen PJ, Goke R, et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 42.Gibbons C, Caudwell P, Finlayson G, et al. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J Clin Endocrinol Metab. 2013;98:E847–855. doi: 10.1210/jc.2012-3835. [DOI] [PubMed] [Google Scholar]
- 43.Schirra J, Kuwert P, Wank U, et al. Differential effects of subcutaneous GLP-1 on gastric emptying, antroduodenal motility, and pancreatic function in men. Proc Assoc Am Physicians. 1997;109:84–97. [PubMed] [Google Scholar]
- 44.Wank U, Schirra J, Arnold R, et al. Effects of GLP-1 on proximal gastric motor and sensory function in human. Gastroenterology. 1998;114:A1190. [Google Scholar]
- 45.Deacon CF, Nauck MA, Toft-Nielsen M, et al. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 46.Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011:CD006423. doi: 10.1002/14651858.CD006423.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981–988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 48.Schirra J, Leicht P, Hildebrand P, et al. Mechanisms of the antidiabetic action of subcutaneous glucagon-like peptide-1(7–36)amide in non-insulin dependent diabetes mellitus. J Endocrinol. 1998;156:177–186. doi: 10.1677/joe.0.1560177. [DOI] [PubMed] [Google Scholar]
- 49.Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2719–2725. doi: 10.1210/jc.2003-030049. [DOI] [PubMed] [Google Scholar]
- 50.Sekine Y, Yumioka T, Yamamoto T, et al. Modulation of TLR4 signaling by a novel adaptor protein signal-transducing adaptor protein-2 in macrophages. J Immunol. 2006;176:380–389. doi: 10.4049/jimmunol.176.1.380. [DOI] [PubMed] [Google Scholar]
- 51.Zinman B, Hoogwerf BJ, Duran Garcia S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477–485. doi: 10.7326/0003-4819-146-7-200704030-00003. [DOI] [PubMed] [Google Scholar]
- 52.Nauck MA, Kemmeries G, Holst JJ, et al. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60:1561–1565. doi: 10.2337/db10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 54.Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008;151:123–129. doi: 10.1016/j.regpep.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- 56.Vella A, Bock G, Giesler PD, et al. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes. 2007;56:1475–1480. doi: 10.2337/db07-0136. [DOI] [PubMed] [Google Scholar]
- 57.Vella A, Bock G, Giesler PD, et al. The effect of dipeptidyl peptidase-4 inhibition on gastric volume, satiation and enteroendocrine secretion in type 2 diabetes: a double-blind, placebo-controlled crossover study. Clin Endocrinol (Oxf) 2008;69:737–744. doi: 10.1111/j.1365-2265.2008.03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meier JJ, Goetze O, Anstipp J, et al. Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am J Physiol Endocrinol Metab. 2004;286:E621–625. doi: 10.1152/ajpendo.00499.2003. [DOI] [PubMed] [Google Scholar]
- 59.Edholm T, Degerblad M, Gryback P, et al. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol Motil. 2010;22:1191–1200. doi: 10.1111/j.1365-2982.2010.01554.x. [DOI] [PubMed] [Google Scholar]
- 60.Kong MF, King P, Macdonald IA, et al. Euglycaemic hyperinsulinaemia does not affect gastric emptying in type I and type II diabetes mellitus. Diabetologia. 1999;42:365–372. doi: 10.1007/s001250051164. [DOI] [PubMed] [Google Scholar]
- 61.Russo A, Stevens JE, Chen R, et al. Insulin-induced hypoglycemia accelerates gastric emptying of solids and liquids in long-standing type 1 diabetes. J Clin Endocrinol Metab. 2005;90:4489–4495. doi: 10.1210/jc.2005-0513. [DOI] [PubMed] [Google Scholar]
- 62.Vella A, Lee JS, Camilleri M, et al. Effects of pramlintide, an amylin analogue, on gastric emptying in type 1 and 2 diabetes mellitus. Neurogastroenterol Motil. 2002;14:123–131. doi: 10.1046/j.1365-2982.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 63.Woerle HJ, Albrecht M, Linke R, et al. Impaired hyperglycemia-induced delay in gastric emptying in patients with type 1 diabetes deficient for islet amyloid polypeptide. Diabetes Care. 2008;31:2325–2331. doi: 10.2337/dc07-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frank JW, Saslow SB, Camilleri M, et al. Mechanism of accelerated gastric emptying of liquids and hyperglycemia in patients with type II diabetes mellitus. Gastroenterology. 1995;109:755–765. doi: 10.1016/0016-5085(95)90382-8. [DOI] [PubMed] [Google Scholar]
- 65.Frank JW, Camilleri M, Thomforde GM, et al. Effects of glucagon on postprandial carbohydrate metabolism in nondiabetic humans. Metabolism. 1998;47:7–12. doi: 10.1016/s0026-0495(98)90185-8. [DOI] [PubMed] [Google Scholar]
- 66.Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640–658. doi: 10.1053/j.gastro.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 67.Chang J, Rayner CK, Jones KL, et al. Diabetic gastroparesis and its impact on glycemia. Endocrinol Metab Clin North Am. 2010;39:745–762. doi: 10.1016/j.ecl.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Horowitz M, Edelbroek MA, Wishart JM, et al. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993;36:857–862. doi: 10.1007/BF00400362. [DOI] [PubMed] [Google Scholar]
- 69.Mourot J, Thouvenot P, Couet C, et al. Relationship between the rate of gastric emptying and glucose and insulin responses to starchy foods in young healthy adults. Am J Clin Nutr. 1988;48:1035–1040. doi: 10.1093/ajcn/48.4.1035. [DOI] [PubMed] [Google Scholar]
- 70.Stevens JE, Gilja OH, Gentilcore D, et al. Measurement of gastric emptying of a high-nutrient liquid by 3D ultrasonography in diabetic gastroparesis. Neurogastroenterol Motil. 2011;23:220. doi: 10.1111/j.1365-2982.2010.01630.x. [DOI] [PubMed] [Google Scholar]
- 71.Ishii M, Nakamura T, Kasai F, et al. Altered postprandial insulin requirement in IDDM patients with gastroparesis. Diabetes Care. 1994;17:901–903. doi: 10.2337/diacare.17.8.901. [DOI] [PubMed] [Google Scholar]
- 72.Lysy J, Israeli E, Strauss-Liviatan N, et al. Relationships between hypoglycaemia and gastric emptying abnormalities in insulin-treated diabetic patients. Neurogastroenterol Motil. 2006;18:433–440. doi: 10.1111/j.1365-2982.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 73.Horowitz M, Jones KL, Rayner CK, et al. ‘Gastric’ hypoglycaemia–an important concept in diabetes management. Neurogastroenterol Motil. 2006;18:405–407. doi: 10.1111/j.1365-2982.2006.00804.x. [DOI] [PubMed] [Google Scholar]
- 74.Gonlachanvit S, Hsu CW, Boden GH, et al. Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type-II diabetic patients. Dig Dis Sci. 2003;48:488–497. doi: 10.1023/a:1022528414264. [DOI] [PubMed] [Google Scholar]


