Abstract
Early neuroimaging studies suggested that adolescents show initial development in brain regions linked with emotional reactivity, but slower development in brain structures linked with emotion regulation. However, the increased sophistication of adolescent brain research has made this picture more complex. This review examines functional neuroimaging studies that test for differences in basic emotion processing (reactivity and regulation) between adolescents and either children or adults. We delineated different emotional processing demands across the experimental paradigms in the reviewed studies to synthesize the diverse results. The methods for assessing change (i.e., analytical approach) and cohort characteristics (e.g., age range) were also explored as potential factors influencing study results. Few unifying dimensions were found to successfully distill the results of the reviewed studies. However, this review highlights the potential impact of subtle methodological and analytic differences between studies, need for standardized and theory-driven experimental paradigms, and necessity of analytic approaches that are can adequately test the trajectories of developmental change that have recently been proposed. Recommendations for future research highlight connectivity analyses and non-linear developmental trajectories, which appear to be promising approaches for measuring change across adolescence. Recommendations are made for evaluating gender and biological markers of development beyond chronological age.
Keywords: Adolescence, Development, Emotion regulation, Emotional reactivity, Neuroimaging
1. Background
In 1904, psychologist Stanley G. Hall first characterized adolescence as a time of “storm and strife,” effectively laying the foundation for the popular conception of this period that persists to this day (Hall, 1904). Scores of popular books and movies depict teenagers as emotional, volatile, highly influenced by their social circle, and prone to risky behavior and poor decision-making. In contrast to this image, most researchers who study adolescence reject the notion that adolescence is a fundamentally tumultuous time, highlighting both strengths and difficulties during this developmental stage. From this line of research, new knowledge continues to emerge about the similarities and differences between adolescents and the adults that they ultimately become.
A core idea underlying the notion of “storm and strife” is that adolescents experience mood disruptions in daily life that differ from adults, particularly with regards to strong and volatile emotional experiences (Arnett, 1999). This is perhaps one of the most ubiquitously noted characteristics of adolescence and is a challenge to many parents and teachers. Furthermore, adolescence is a period of vulnerability for the initial onset of numerous mental illnesses, particularly illnesses that have emotion dysregulation as a core feature (Kessler et al., 2005). The societal cost of mental illnesses is significant (Murray and Lopez, 1996); therefore, research exploring adolescent emotion processing a vital and urgent priority. Despite the clear importance of understanding emotions and emotion regulation during this developmental stage, the nature of emotional changes during adolescence, particularly in terms of underlying biological mechanisms, remains poorly understood. We conducted a systematic review of neuroimaging research on emotion in the adolescent brain and sought to better characterize: (1) what components of emotion processing have been found to differ during adolescence from other developmental stages; (2) what trajectories of change have been theorized and empirically tested; (3) what questions remain unaddressed; and (4) what might be promising directions for future research.
1.1. Delineating emotional processes
In adolescent neuroimaging research, a multitude of experimental paradigms have been used to examine emotional reactivity and regulation. In trying to develop a cohesive picture of the state of this literature, it is clear that using a theoretical framework to integrate these disparate experimental approaches is an important first step.
Theoretical frameworks of emotion that delineate discrete emotional processes, range widely from theories positing that each emotion is a distinct state, elicited by discrete triggers, and manifested in unique biological and behavioral responses (Buck, 1999, LeDoux, 2000) to theories that consider emotions to be wholly social and cultural constructions that do not innately exist but are rather the product of ongoing socialization (Mesquita, 2010; for reviews of theoretical perspectives on emotion processing, see Cole et al., 2004 and Gross and Barrett, 2011). However, the application of these types of theories to adolescent development, particularly as the conceptual basis for neuroimaging studies, has remained limited. Gross and Thompson (2009) propose an intermediate model of emotion processing, whereby: (1) individuals are placed in a given situation; (2) attention is directed to a particular stimulus; (3) they appraise, or interpret the emotional meaning of the stimulus; and (4) they engage in a response. Of all the theoretical models of emotion regulation that have been proposed, this framework is particularly helpful for contextualizing experimental studies of emotion regulation in adolescence (and more generally) because it clearly implicates several points at which emotion regulation can occur: situation selection, situation modification, attentional deployment, reappraisal/cognitive change, and response modification. Furthermore, these delineated steps can be translated into different types of experimental (particularly neuroimaging) paradigms (e.g., those that manipulate attention to emotion, those that involve a cognitive strategy to change an emotional reaction).
In the present review, the experimental methodologies used in each study are parsed into different components of emotion regulation as defined in the Gross and Thompson (2009) model. Specifically, as shown in Fig. 1, we examine emotional reactivity (unregulated emotional experience) as well as emotion regulation through attentional deployment and cognitive change strategies. This delineation allows for comparisons across the diverse experimental methodologies that have been used in the field by highlighting groups of studies that are tapping common underlying emotional demands. Throughout this review, we use “emotion processing” as a general term to refer to the processes of both emotional reactivity and emotion regulation, as both are foci of this review. By mapping experimental paradigms onto a relevant theoretical model, we hope to begin parsing the complex neuroimaging literature on adolescent emotion.
Fig. 1.
Depiction of paradigm types delineated within this review.
1.2. Delineating models of change
Another dimension to examine in the disparate studies within this body of literature is method of analysis used to assess change over time and change trajectories. A model proposed by Casey (2013) is helpful for defining different types of linear and non-linear change trajectories. In this framework, adolescent nonspecific changes are those that begin in childhood and continue to develop at a steady, linear pace through adolescence. Adolescent emergent processes are those that develop from childhood to adolescence and then remain largely stable into adulthood. Finally, adolescent specific changes are those that emerge uniquely during adolescence, but are not present in either child- or adulthood.
The primary analytic approaches used in the reviewed studies involved testing between-group differences or linear changes associated with age. Furthermore, some of the studies reviewed, captured only a segment of the developmental trajectory (e.g., children and adolescents, but not adults). Thus, most of the analytic methods in the reviewed studies do not perfectly map onto these theorized change trajectories. For example, a between-group difference between children and adolescents could be consistent with either an adolescent nonspecific or adolescent emergent process since the adult end of the trajectory was not tested. Structural studies of the developing brain indicate that brain growth is complex and non-linear (Giedd et al., 1999, Gogtay et al., 2004). Thus, the analytic decisions made in a given study (e.g., testing only for linear changes from child- to adulthood) could have a substantial impact on the study’s reported results.
1.3. Delineating key brain regions
A final unifying dimension across the studies reviewed is brain regions of interest. Studies of functional brain activity during emotion processing in adolescence have focused on three primary regions: (1) the amygdala; (2) the medial prefrontal cortex and adjacent anterior cingulate cortex; and (3) the lateral prefrontal cortex. The amygdala was an early focus of studies on adolescent emotion processing (Baird et al., 1999), primarily because of its strong response to fear-inducing stimuli (LeDoux, 2000). The amygdala has also been linked to the rapid processing of both positively and negatively valenced stimuli (for a review, see Zald, 2003), making its role in emotion processing potentially more complex. The medial prefrontal cortex and the anterior cingulate cortex also have been foci of adolescent emotion studies because of their links to the appraisal, expression, and regulation of emotions (Damasio et al., 1996, Etkins et al., 2010), processes that appear challenging during the adolescent period. Finally, the lateral prefrontal cortex, is thought to subserve cognitive functions such as working memory, attention, and response inhibition that may be engaged during the active, conscious regulation of emotion (Kalisch, 2009, Ochsner and Gross, 2008, Perlman and Pelphrey, 2011).
Focusing primarily on these regions, structural and functional imaging studies initially indicated that the development of emotionally reactive responses in subcortical regions preceded development of inhibitory and regulating regions such as the prefrontal cortex (Casey et al., 2008), effectively creating an emotional neural “imbalance.” This notion of neural imbalance fit well with the perception that adolescents experience strong emotions that they are not yet equipped to control and was strongly emphasized in the popular press (e.g., Donovan, 2008). However, further research has produced variable results in support of this theory, indicating that the nature of emotional differences between adolescents and adults may be more nuanced (Casey and Caudle, 2013, Pfeifer and Allen, 2012). In this review, we focus primarily on results across the reviewed studies in these three key regions.
1.4. Goals of current review
Towards the goal of further characterizing emotional changes during adolescence, this review examines basic emotion processing studies (i.e., reactivity and regulation) using functional neuroimaging paradigms. To highlight changes across development, not just a snapshot of this developmental period, we only include studies that draw direct comparisons between adolescents and other age groups and/or evaluate age as a predictor of neural activity.
Additionally, as described above, results of the reviewed studies are presented in terms of three unifying dimensions − emotion processing demands, analytic approach/change trajectory, and brain region of interest. No previous reviews have systematically parsed this body of literature in these ways.
We additionally explore the role of gender and pubertal timing. Structural brain differences between boys and girls (De Bellis et al., 2001, Durston et al., 2001) as well as differences in brain development linked to changes in pubertal hormones (Blakemore et al., 2010, Giedd et al., 2006, Ladouceur, 2012, Moore et al., 2012) have been identified in the literature.
Finally, we examine results from the smaller number of studies that employed functional connectivity methodologies. By exploring the degree to which different parts of the brain are interconnected, these studies have pursued new directions towards a more complex and comprehensive picture of emotion development.
Overall, this review aims to identify what is known about differences at the neural level in basic emotion processing between adolescents and both children and adults. To this end, we did not review studies that focused primarily on complex social emotions (e.g., self-consciousness, Somerville et al., 2013) or adolescents who were experiencing psychopathology (e.g., mood disorders). Such topics have been the subject of several excellent reviews (e.g., Blakemore, 2008, Ladouceur, 2012, Nelson et al., 2005) but are beyond the scope of the present review. In contrast, here we hope to develop a clearer picture of basic emotion processing with the perspective that these basic processes are fundamental building blocks underlying more complex emotional experiences (Pfeifer et al., 2011, Steinberg, 2008).
2. Selection of studies
Studies included in the present review met the following criteria: (1) included at least one fMRI paradigm; (2) fMRI paradigms evaluated emotional reactivity and/or emotion regulation of basic emotions, including at least one negative emotion; (3) analyses of functional imaging data included contrasts that quantified neural activation during negative emotions relative to other conditions (e.g., rest, fixation, neutral); and (4) results included either (a) between-group comparisons between children and/or adults and adolescents or (b) associations between fMRI data and chronological age in a sample incorporating adolescent participants.
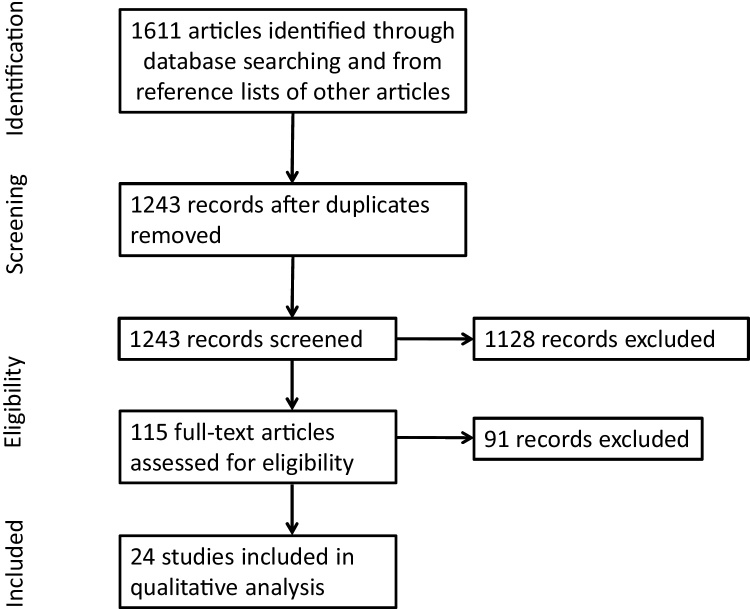
Studies were identified from searches of the literature through December 2015 using PsycINFO, Google Scholar and PubMed using the following terms: “adolescence,” “adolescent,” “development,” “MRI,” “fMRI,” “neuroimaging”, “functional neuroimaging ” “emotion,” “emotion regulation,” and “emotional reactivity.” We also examined those studies included in the reference lists of other studies gathered using the above methods. A summary of the search process for the reviewed articles is presented in Fig. 2.
Fig. 2.
Flowchart of study selection for the systematic review.
2.1. Experimental paradigms
In order to understand how studies differed in their measurement of emotion, analyses were broken into four distinct categories based on the model proposed by Gross and Thompson (2009) as shown in Fig. 1. The first category, Emotional Reactivity (hereafter referred to as “reactivity”), involved passive viewing of emotional faces without any specific direction with regards to the amount of attention that should be given to the emotion displayed. In the second category, Emotion Regulation: Attentional Deployment—Concentration (hereafter referred to as “concentration”), participants also viewed emotional faces, but were specifically instructed to attend to the emotions displayed on the faces. Concentration studies included studies where participants were asked to specifically rate the magnitude of an emotion displayed on a face or the magnitude of an emotion they themselves were experiencing as well as studies where participants were only asked to monitor for the presence of a particular emotion (e.g., neutral) and respond with a button press or label an emotional face. This category also included studies where participants were shown images and asked to identify what emotion they were feeling when viewing the image. The third category was Emotion Regulation: Attentional Deployment—Distraction (hereafter referred to as “distraction”). In these paradigms participants were instructed to attend to something other than the emotion displayed on a face (e.g., gender, age, nose width). Studies where participants viewed masked emotional faces that diminished their conscious awareness of the emotion were also included in this category. Finally, in the fourth category, Emotion Regulation: Cognitive Change (hereafter referred to as “cognitive change”), participants were taught strategies to cognitively and consciously change their reactions to emotional stimuli (e.g., by thinking that the presented situation will improve with time). It should be noted that many studies included conditions in multiple categories (e.g., a passive viewing condition and a gender classifying condition). Therefore, analyses within the reviewed studies are reported separately with consideration for the emotional processes that they elicit.
There were few comparable analyses of positive stimuli reported across the reviewed studies. Therefore, to facilitate comparisons, only analyses that focused on negative emotions (anger, sadness, fear, and disgust) are discussed in the summary of findings. Additionally, only one study (Pitskel et al., 2011) included a condition where participants were asked to use cognitive strategies to actively increase a negative emotion, so this analysis is also not included in the discussion of results.
2.2. Cohort and analysis approach
For classification purposes, cohort age groups were classified as follows: participants under the age of 11 were considered to be children, participants between the ages of 11 and 19 were considered to be adolescents, and participants ages 20 and older were considered to be adults. Definitions of adolescence vary widely and, particularly for studies that employ between-group analyses, having a large age range within an “adolescent” sample can introduce a problematic amount of variability. However, the above age delineation allowed for the inclusion of the greatest number of studies. This range is also theoretically consistent with research indicating that pubertal onset occurs as early as 11 years of age in approximately 40% of youth, making this a reasonable boundary for the transition from childhood to adolescence (Vizmanos and Marti-Henneberg, 2000, Vizmanos et al., 2001). Several studies did not report the age ranges for participants. In these cases, the range was assumed to be +/− 1.5 SD from the reported mean age for classification purposes.
Studies in this review employed several methods for identifying relationships between neural activity and chronological age—between-group, linear, and non-linear analyses. Several studies analyzed relationships with age using multiple approaches (e.g., between-group and linear); for those studies, both analyses are reported. Due to the small number of studies that used a non-linear approach and the complexity of these results, non-linear analyses are discussed separately. Similarly, a small group of studies related connectivity between several brain regions to age and these findings are also discussed separately.
2.3. Brain region of interest delineation
To synthesize findings across studies, we primarily focus on brain regions that have most frequently been linked in previous literature with emotion processing in adolescents and adults—the amygdala, the medial prefrontal cortex/anterior cingulate cortex (mPFC/ACC), and the lateral prefrontal cortex (lPFC). For purposes of classifying results reported within the different studies, findings that were reported to be in the orbitofrontal cortex, ventromedial prefrontal cortex, medial frontal gyrus, medial portion of the superior frontal gyrus, and anterior cingulate cortex were included under the mPFC/ACC findings. Results reported as being within the dorsolateral prefrontal cortex, inferior frontal gyrus, lateral portion of the superior frontal gyrus, and middle frontal gyrus were included under the lPFC findings.
3. Findings of emotion processing fMRI studies in adolescence
Twenty-four studies met the inclusion criteria for this review and are summarized in Table 1. Their sample sizes ranged from 16 to 242. All studies in this review included adolescents, but other participants ranged in age from as young as 4 years (Gee et al., 2013) to as old as 79 years (Williams et al., 2006). Of the 24 studies, nine included an emotional reactivity paradigm, nine studies employed an emotion regulation paradigm using concentration as an attentional deployment strategy, nine studies included an emotion regulation paradigm using distraction as an attentional deployment strategy, and three studies required participants to actively engage in emotion regulation using a cognitive change strategy. Several studies included conditions from multiple categories. Specifically, all three cognitive change studies also included an emotional reactivity condition, one distraction study also included an emotional reactivity condition, and a second distraction study included a concentration condition.
Table 1.
Summary of study characteristics.
| Article | Task | A priori ROIs/Whole Brain | Age & Gender Analyses | Outcome variables | Contrast(s) | Age Analysis Results in Key ROIs |
|---|---|---|---|---|---|---|
| 1. Deeley et al. (2008) N = 40 Ages: 8–59 (M = 24) |
Paradigms −Regulation: Attentional Deployment—Distraction Emotions Disgust, fearful, neutral Conditions −Identify gender of each face shown |
−Whole brain | −Linear −Males Only |
−BOLD signal −Behavioral: RT and accuracy |
−Fear > Neutral −Disgust > Neutral |
−Amygdala: ns −mPFC/ACC: ↓L↑R −lPFC: ↓R −Amygdala: ns −mPFC/ACC: ↓B −lPFC: ↓R |
| 2. Gee et al. (2013) N = 45 Ages: 4.0–22.3 (M = 13.2) |
Paradigms −Regulation: Attentional Deploymen—Concentration Emotions Fearful, happy, neutral Conditions −View fearful and neutral faces, press button for neutral faces −View happy and neutral faces, press button for neutral faces |
−Whole brain −Amygdala |
−Between-group −Linear −No gender analyses |
−BOLD signal −PPI Connectivity −SCARED anxiety measure −Behavioral: RT and emotion rating accuracy |
−Fear > fixation | −Amygdala: ↓R −mPFC/ACC: ns −lPFC: ns |
| 3. Guyer et al. (2008) Adults (n = 30): Ages: 21–40 (M = 31.1) Children/Adolescents (n = 31): Ages 9–17 (M = 14.2) |
Paradigms −Reactivity Emotions Angry, fearful, happy, neutral Conditions −View each neutral face −View each emotional face |
−ACC −Amygdala −Fusiform gyrus −Hippocampus −OFC |
−Between-group −Linear −Quadratic −Cubic −Gender differences tested |
−BOLD signal −Functional connectivity −Behavioral: RT and ratings −Eye tracking: total time looking at eye, nose, and mouth regions |
−Fear > Neutral (Between-Group | Linear) |
−Amygdala: ↓B | ns −mPFC/ACC: ns | N/A −lPFC: N/A | N/A |
| 4. Hare et al. (2008) Adults (n = 24): Ages 19–32 (M = 23.9) Adolescents (n = 24): Ages 13–18 (M = 16.0) Children (n = 12): Ages 7–12 (M = 9.1) |
Paradigms −Regulation: Attentional Deployment—Concentration Emotions Calm, fearful, happy Conditions −Press button for one emotion, don’t press for other emotions (affective go-nogo) |
−Whole brain | −Between-group −Gender differences tested |
−BOLD Signal −Functional connectivity −Behavioral: RT correlation between BOLD and RT |
−All Emotions > rest (Children vs. Adolescents) −All Emotions > rest (Adolescents vs. Adults) |
−Amygdala: ↑B −mPFC/ACC: ns −lPFC: ns −Amygdala: ↓B −mPFC/ACC: ns −lPFC: ns |
| 5. Killgore et al. (2001) N = 19 Ages 9–17: (M = 13.5) |
Paradigms −Regulation: Attentional Deployment —Concentration Emotions Fearful Conditions −Passive viewing of nonsense images −Label fearful faces |
−Amygdala −dlPFC |
−Linear −Gender differences tested |
−BOLD signal −Differences in activation between amygdala and dlPFC |
−Fear > rest | −Amygdala: ↓L −mPFC/ACC: N/A −lPFC: ns |
| 6. Killgore and Yurgelun-Todd (2007) Adults (n = 12): Age M = 23.7 (SD = 2.1) Children/Adolescents (n = 10): Ages 9–17 (M = 12.3) |
Paradigms −Regulation: Attentional Deployment—Distraction Emotions Fearful, happy Conditions −View masked-face images of each emotion |
−Whole brain −Amygdala −Anterior −Cingulate gyrus |
−Between-group −No gender analyses |
−BOLD signal | −Sadness > fixation | −Amygdala: ↓R −mPFC/ACC: ns −lPFC: ns |
| 7. Lindstrom et al. (2009) N = 37 Ages 9–40: (M = 21.5) |
Paradigms −Regulation: Attentional Deployment—Distraction Emotions Angry, happy, neutral Conditions −Dot probe task |
−Whole brain | −Between-group −Linear −No gender analyses |
−BOLD signal −Behavioral: RT and attention bias |
−Angry Incongruent > Angry Congruent (Between-Group | Linear) |
−Amygdala: ns | ns −mPFC/ACC: ns | ns −lPFC: ns | ns |
| 8. McClure et al. (2004) Adults (n = 17): Ages 25–36 (M = 30.8) Children/Adolescents (n = 17): Ages 9–17 (M = 13.1) |
Paradigms −Regulation: Attentional Deployment—Concentration Emotions Angry, happy, fearful, neutral Conditions −Rate how hostile the face looks |
−ACC −Amygdala −OFC |
−Between-group −Gender differences tested |
−BOLD signal −Behavioral: hostility of face ratings |
−Anger > Neutral −Anger > Fear |
−Amygdala: ns −mPFC/ACC: ns −lPFC: ns −Amygdala: ns −mPFC/ACC: ns −lPFC: ns |
| 9. McRae et al. (2012) N = 38 Ages: 10–22 (M = 16.5) |
Paradigms −Reactivity −Regulation: Cognitive Change Emotions Negative, Neutral Conditions −Look at neutral image −Look at negative image—Actively decrease negative feelings by thinking (a) it is not real; (b) things will get better over time or; (c) things are not as bad as they appear |
−Whole brain | −Linear −Quadratic −Gender differences tested |
−BOLD Signal −Behavioral: affect ratings |
−Look Negative > Look Neutral −Decrease Negative > Look Negative |
−Amygdala: ns −mPFC/ACC: ↑L −lPFC: ns −Amygdala: ns −mPFC/ACC: ns −lPFC: ↑L |
| 10. Monk et al. (2003) Adults (n = 17): Ages 25–36 (M = 30.8) Children/Adolescents (n = 17): Ages 9–17 (M = 13.1) |
Paradigms −Reactivity −Regulation: Attentional Deployment—Distraction Emotions Angry, happy, fearful, neutral Conditions −Passive viewing −Rate width of nose −Rate how afraid you feel |
−ACC −Amygdala −OFC |
−Between-group −No gender analyses |
−BOLD signal −Behavioral: RT, reported fear to image, and width of nose ratings |
−Passive Fear > Passive Neutral −Direct Fear > Indirect Fear −Indirect Fear > Indirect Neutral −Direct Anger > Indirect Anger |
−Amygdala: ↓R −mPFC/ACC: ↓B −lPFC: N/A −Amygdala: ns −mPFC/ACC: ↑R −lPFC: N/A −Amygdala: ns −mPFC/ACC: ↓R −lPFC: N/A −Amygdala: ns −mPFC/ACC: ↓B −lPFC: N/A |
| 11. Nelson et al. (2003) Adults (n = 20): Ages 25–35 (M = 30.8) Children/Adolescents (n = 23): Ages 9–17 (M = 13.1) |
Paradigms −Regulation: Attentional Deployment—Distraction Emotions Angry, happy, fearful, neutral Conditions −Passive viewing −Rate width of nose −Rate how afraid you feel −Rate how hostile the face looks |
−Whole brain −ACC −Amygdala −Hippocampus (anterior and posterior) −OFC |
−Between-group −Gender differences tested |
−BOLD signal −Behavioral: Post-scan memory test accuracy |
−Fear Correct > Fear Incorrect −Anger Correct > Anger Incorrect |
−Amygdala: ns −mPFC/ACC: ns −lPFC: ↓R −Amygdala: ns −mPFC/ACC: ↓B −lPFC: ns |
| 12. Pagliaccio et al. (2013) N = 52 Ages 7–12 (M = 10.32) |
Paradigms −Regulation: Attentional Deployment—Distraction Emotions Angry, fearful, happy, sad, neutral Conditions −Identify gender of each face shown *Task administered following negative mood induction |
-Whole brain -Amygdala |
−Linear −Gender differences tested |
−BOLD signal −CDI-self depression scores −CDI-parent depression scores −Children’s Emotion Management Scale for Sadness |
−Anger > fixation −Fear > fixation −Sadness > fixation |
−Amygdala: ns −mPFC/ACC: ns −lPFC: ns −Amygdala: ns −mPFC/ACC: ↓L −lPFC: ns −Amygdala: ns −mPFC/ACC: ns −lPFC: ns |
| 13. Passarotti et al. (2009) Adults (n = 10): Ages: M = 30 (SD = 6) Adolescents (n = 10): Ages: M = 14 (SD = 2) |
Paradigms −Regulation: Attentional Deployment—Concentration −Regulation: Attentional Deployment—Distraction Emotions Angry, happy Conditions −Judge age of each person −Judge emotion of each person |
-Whole brain -ACC -Amygdala -Caudate -dlPFC -IPL -Middle temp. gyrus -Parahipp. gyrus -Precuneus -Post. cingulate gyrus -vlPFC |
−Between-group −Linear −No gender analyses |
−BOLD signal −Behavioral: RT and accuracy −Correlations between BOLD and RT and accuracy |
−All Emotions Direct > fixation (Between-Group | Linear) −All Emotions Indirect > All Emotions Direct (Between-Group | Linear) −All Emotions Indirect > fixation (Between-Group | Linear) |
−Amygdala: ns | ns −mPFC/ACC: ↑B | ns −lPFC: ns | ns −Amygdala: ns | ns −mPFC/ACC: ↓B| ns −lPFC: ↓R | ns −Amygdala: ↓R| ns −mPFC/ACC: ↓R| ns −lPFC: ↓R↑R | ns |
| 14. Pfeifer et al. (2011) N = 38 Longitudinal T1: M = 10.0 (SD = 0.6) T2: M = 13.0 (SD = 0.7) Mean lag = 36 months |
Paradigms −Reactivity Emotions Angry, fearful, happy, sad, neutral Conditions −View each neutral face −View each emotional face |
−Whole brain −Amygdala −vmPFC −Ventral striatum |
−Longitudinal changes −No gender analyses |
−BOLD signal −PPI connectivity |
−All Emotions, Longitudinal Change −Sad, Longitudinal Change −Anger, Longitudinal Change −Fear, Longitudinal Change |
−Amygdala: ns −mPFC/ACC: ↑L −lPFC: ns −Amygdala: ↑R −mPFC/ACC: ↑B −lPFC: ns −Amygdala: ns −mPFC/ACC: ns −lPFC: ns −Amygdala: ns −mPFC/ACC: ↑B −lPFC: ns |
| 15. Pine et al. (2001) Adults (n = 10): Ages: 25–38 (M = 28.5) Adolescents (n = 10): Ages: 12–16 (M = 13.9) |
Paradigms −Regulation: Attentional Deployment—Distraction Emotions Fearful, happy Conditions −View masked-face images of each emotion |
−Whole brain −Amygdala |
−Between-group −No gender analyses |
−BOLD signal −Grey matter volume |
−Indirect Fear > fixation −Indirect Fear > Indirect Happy |
−Amygdala: ns −mPFC/ACC: ns −lPFC: ns −Amygdala: ns −mPFC/ACC: ns −lPFC: ns |
| 16. Pitskel et al. (2011) N = 15 Ages: 7–17 (M = 13.0) |
Paradigms −Reactivity −Regulation: Cognitive Change Emotions Disgust, neutral Conditions −Look at gross image −Actively enhance grossness (e.g., “pretend it’s right in front of you”) −Actively decrease grossness (e.g., “pretend it’s fake”) |
−Whole brain −Amygdala −Insula |
−Linear −No gender analyses |
−BOLD signal −Disgust ratings |
−Look Disgust > Look Neutral −Decrease Disgust > Look Disgust |
−Amygdala: ns −mPFC/ACC: ↓B −lPFC: ns −Amygdala: ↓R −mPFC/ACC: ↓B −lPFC: ↓R |
| 17. Rahko et al. (2010) N = 27 Ages: 11.6–17.3 (M = 14.5) |
Paradigms −Reactivity Emotions Fearful, happy, neutral Conditions −View dynamic mosaics of scrambled face stimuli −View face stimuli transitioning between fearful and happy images |
−Whole brain | −Linear −Gender differences tested |
−BOLD Signal −Post-scan recognition of dynamic and static expressions |
−Fear > Happy | −Amygdala: ns −mPFC/ACC: ns −lPFC: ns |
| 18. Silvers et al. (2015) N = 56 Ages 10.5–22.9 (M = 16.5) |
Paradigm −Reactivity −Regulation: Cognitive Change Emotions Negative, neutral Conditions −“Close”: Imagine standing close to scene and focus on emotional details −“Far”: Imagine standing far away from scene and focus on factual details −Passively view images previously in “far condition” |
-Whole brain -Amygdala |
−Linear −No gender analyses |
−BOLD signal −PPI connectivity −Behavioral: self-reported negative affect |
−Negative > Neutral −Negative “Far” > Negative “Close” |
−Amygdala: ns −mPFC/ACC: N/A −lPFC: N/A −Amygdala: ↓B −mPFC/ACC: ns −lPFC: ns |
| 19. Thomas et al. (2001) Adults (n = 6): Ages: M = 24 (SD = 6.6) Children/Adolescents (n = 12): Ages: M = 11 (SD = 2.4) |
Paradigms −Reactivity Emotions Fearful, happy, neutral Conditions −View each neutral face −View each emotional face |
−Amygdala | −Between-group −Gender differences tested |
−BOLD signal | −Fear > Neutral | −Amygdala: ↑L −mPFC/ACC: N/A −lPFC: N/A |
| 20. Vetter et al. (2015) N = 144 Longitudinal T1: M = 14.8 (SD = 0.35) T2: M = 16.6 (SD = 0.36) Mean lag = 24.1 months |
Paradigm −Regulation: Attentional Deployment—Distraction Emotions Negative, neutral, positive Conditions −Compare if two target images are equal |
−Whole brain −Amygdala |
−Between-group −Gender differences tested |
−BOLD signal −PPI Connectivity −Behavioral: RT and accuracy |
−Emotion x Age (age group main effect) | −Amygdala: ns −mPFC/ACC: ↑R −lPFC: ↑B |
| 21. Vink et al. (2014) N = 60 Ages 10–24 (M = 16.7) |
Paradigm −Regulation: Attentional Deployment − Concentration Emotions Negative, neutral, positive Conditions −Choose emotional valence of picture (negative, neutral, or positive) |
−Amygdala −Hippocampus −Ventral striatum −vlPFC −Medial orbitofrontal cortex |
−Linear −Gender differences tested |
−BOLD signal −PPI Connectivity −Behavioral: match of participant valence ratings to standard ratings |
−Negative > Neutral | −Amygdala: ↓R −mPFC/ACC: ns −lPFC: ns |
| 22. Wiggins et al. (2016) Adults (n = 23): Ages: M = 29.3 (SD = 7.5) Children/Adolescents (n = 21): Ages: M = 14.9 (SD = 2.4) |
Paradigm −Regulation: Attentional Deployment − Concentration Emotions Angry, fearful, happy (50%, 75%, and 100% intensity), and neutral Conditions −Label emotion on each face |
−Whole brain −Amygdala |
−Between-group −Linear −Quadratic* −Cubic* *Assoc. with stimulus intensity −No gender analyses |
−BOLD signal −Behavioral: Accuracy |
−Emotion × Age (age group main effect) | −Amygdala: ns −mPFC/ACC: ↓B↑R −lPFC: ↓B↑L |
| 23. Williams et al. (2006) N = 242 Ages: 12–79 (M = 34.8) |
Paradigms −Reactivity Emotions Fearful, happy, neutral Conditions −View each neutral face −View each emotional face |
−Amygdala −Basal ganglia −mPFC |
−Between-group −Linear −No gender analyses |
−BOLD signal −ERP −Gray matter volume |
−Fear > Neutral (Between-Group | Linear) |
−Amygdala: ↑L | ns −mPFC/ACC: ↑B | ↑B −lPFC: N/A | N/A |
| 24. Yurgelun-Todd and Killgore (2006) N = 16 Ages 8–15 (M = 11.6) |
Paradigms −Regulation: Attentional Deployment Concentration Emotions Fearful, happy Conditions −Attend to emotion on each face |
−Amygdala −Multiple medial and lateral prefrontal regions |
−Linear −Gender differences tested |
−BOLD signal | −Fear > fixation | −Amygdala: ns −mPFC/ACC: ↑B −lPFC: ↑B |
ROI = region of interest; ACC = anterior cingulate cortex; mPFC = medial prefrontal cortex; lPFC = lateral prefrontal cortex; OFC = orbitofrontal cortex; dlPFC = dorsolateral prefrontal cortex; IPL = inferior parietal lobule; vlPFC = ventrolateral prefrontal cortex; vmPFC = ventromedial prefrontal cortex.
BOLD = blood oxygen level-dependent; RT = reaction time; PPI = psychophysiological interaction; ERP = event-related potential.
↑: Children < Adolescents, Adolescents < Adults, or positive relationship with age.
↓: Children > Adolescents, Adolescents > Adults, or negative relationship with age.
ns: non-significant age result.
N/A: region not tested.
L: left hemisphere; R: right hemisphere; B: bilateral.
3.1. Key brain region findings
The results from the reviewed studies are shown in Table 2. Study results are presented by brain region, task demands, and analysis type.
Table 2.
Summary of BOLD signal findings for negative emotion contrasts.
| Analysis Type and Quantity | Amygdala | mPFC/ACC | lPFC |
|---|---|---|---|
| Emotional Reactivity | |||
| Adolescent Only | |||
| Linear (1 study, 1 analysis)17 | ns × 1 | ns × 1 | ns × 1 |
| Children vs. Adolescents | |||
| Between-Group (1 study, 4 analyses)14 | ↑ × 1 ns x 3 |
↑ × 3 ns × 1 |
ns × 4 |
| Linear (1 study, 1 analysis)16 | ns × 1 | ↓ × 1 | ns × 1 |
| Adolescents vs. Adults | |||
| Between-Group (1 study, 1 analysis)23 | ↑ × 1 | ↑ × 1 | – |
| Linear (1 study, 1 analysis)23 | ns × 1 | ↑ × 1 | – |
| All Ages | |||
| Between-Group (3 studies, 3 analyses)3,10,19 | ↑ x 1 ↓ x 2 |
↓ × 1 ns × 1 |
– |
| Linear (3 studies, 3 analyses)3,9,18 | ns × 3 | ↓ x 1 | ns × 1 |
| Emotion Regulation: Attentional Deployment—Concentration | |||
| Children vs. Adolescents | |||
| Between-Group (1 study, 1 analysis)4 | ↑ × 1 ↓ × 1 |
ns × 1 | ns × 1 |
| Linear (2 studies, 2 analyses)5,24 | ns x 1 | ↑ x 1 | ↑ x 1 ns x 1 |
| Adolescents vs. Adults | |||
| Between-Group (2 studies, 2 analyses)4,13 | ↓ x 1 ns x 1 |
↑ x 1 ns x 1 |
ns x 2 |
| Linear (1 study, 1 analysis)13 | ns x 1 | ns x 1 | ns x 1 |
| All Ages | |||
| Between-Group (2 studies, 3 analyses)8,22 | ns x 3 | ↑ x 1 ↓ x 1 |
↑ x 1 ↓ x 1 |
| Linear (2 studies, 2 analyses)2,21 | ↓ x 2 |
ns x 2 ns x 2 |
ns x 2 ns x 2 |
| Emotion Regulation: Attentional Deployment—Distraction | |||
| Adolescent Only | |||
| Between-Group (1 study, 1 analysis)20 | ns x 1 | ↑ x 1 | ↑ x 1 |
| Children vs. Adolescents | |||
| Linear (1 study, 3 analyses)12 | ns x 3 | ↓ x 1 ns x 2 |
ns x 3 |
| Adolescents vs. Adults | |||
| Between-Group (2 studies, 4 analyses)13,15 | ↓ x 1 ns x 3 |
↓ x 2 ns x 2 |
↑ x 1 ↓ x 2 ns x 2 |
| Linear (1 study, 2 analyses)13 | ns x 2 | ns x 2 | ns x 2 |
| All Ages | |||
| Between-Group (4 studies, 7 analyses)6,7,10,11 | ↓ x 1 ns x 6 |
↑ x 2 ↓ x 2 ns x 3 |
↓ x 1 ns x 3 |
| Linear (2 studies, 3 analyses)1,7 | ns x 3 | ↑ x 1 ↓ x 2 ns x 1 |
↓ x 2 ns x 1 |
| Emotion Regulation: Cognitive Change | |||
| Children vs. Adolescents | |||
| Linear (1 study, 1 analysis)16 | ↓ x 1 | ↓ x 1 | ↓ x 1 |
| All Ages | |||
| Linear (2 studies, 2 analyses)9,18 | ↓ x 1 ns x 1 |
ns x 2 | ↑ x 1 ns x 1 |
N.B. Subscript numbers denote study citations as numbered in Table 1.
↑ : Children < Adolescents, Adolescents < Adults, or positive relationship with age.
↓ : Children > Adolescents, Adolescents > Adults, or negative relationship with age.
ns: Non-significant age result.
3.1.1. Emotional reactivity
Studies employing emotional reactivity tasks produced variable results and no consistent patterns were found within any of the key brain regions for this type of paradigm. Variability within reactivity paradigms may be due to the fact that participants are not given instructions during these tasks, but rather are asked to respond organically or passively view images, which may introduce more variability in participant responses and, subsequently, study findings.
3.1.2. Emotion regulation—concentration
Concentration paradigms produced the most consistent findings and generally supported linear (adolescent nonspecific) decreases in amygdala activation and increases in mPFC/ACC activation across development. Only two studies within this category found significant age associations within the lPFC and results were variable.
3.1.3. Emotion regulation—distraction
Distraction studies found highly variable results within the amygdala and mPFC/ACC. This variability of results may be attributable to the diversity of paradigms that were included within this category (e.g., tasks where participants were asked to attend to physical characteristics of the emotional faces and tasks where participants saw emotional faces masked by abstract designs). Interestingly, distraction studies generally reported negative associations between lPFC activation and age. This finding could indicate that adolescents engage brain regions linked with cognitive control more robustly than adults when there is explicit instruction to modulate their attention with respect to emotional stimuli.
3.1.4. Emotion regulation—cognitive change
Only three studies utilized a cognitive change paradigm. Given the small number of studies within this category, no age association patterns were evident within any of the key brain regions.
Overall, findings point to highly variable results within the reviewed studies. This variability, which occurred even within task categories, highlights the need to develop more uniform and theoretically-driven experimental manipulations to enable researchers to examine how different components of emotion processing mature across the adolescent period. The variable findings also point to the need for more complex analytic approaches that can better approximate the trajectories of change that have been proposed theoretically in the literature.
3.2. Additional brain regions
Beyond the three above brain regions that were the focus of many studies reviewed, age related associations were frequently reported in several additional brain areas. Activation in several regions of the basal ganglia, which are thought to be involved in the processing of rewards and salient information (Horvitz, 2000, O’Doherty et al., 2003), generally increased with age. These changes were found both from childhood to adolescence (Pfeifer et al., 2011) and across the lifespan (Passarotti et al., 2009, Williams et al., 2006), which is consistent with adolescent nonspecific increases in the salience and value of emotional stimuli. Decreases with age were identified in the insula (Deeley et al., 2008, Pitskel et al., 2011), which has been linked to feelings of disgust (Wicker et al., 2003), as well as visceral emotional experiences (Adolphs et al., 2003), which could indicate enhanced visceral responses to emotional information during adolescence relative to adulthood. Notably, one study also reported greater insula activation for 16-year-olds than 14-year-olds while averting attention away from negative stimuli (Vetter et al., 2015). Decreased activation during adolescence was also observed in the fusiform gyrus (Deeley et al., 2008, Guyer et al., 2008, Passarotti et al., 2009, Wiggins et al., 2016), the primary region of the brain associated with face processing. This is consistent with research indicating that expertise at decoding facial emotions continues to develop through adulthood (Thomas et al., 2007). Finally, regions associated with memory, including the middle temporal gyrus and parahippocampal gyrus (Squire and Zola-Morgan, 1991), were found to have positive associations with age in some studies and negative associations in other studies with similar emotional demands. These findings may indicate that links between emotional stimuli and memory processes may be changing across development, but that the nature of this change is non-linear or more nuanced (e.g., varying by task demands).
3.3. Non-Linear analyses
Only three of the studies reviewed directly tested for non-linear relationships between neural activation and age in addition to between-group or linear analyses. Guyer et al. (2008) reported no significant non-linear associations. However, analyses in this study were restricted to the amygdala, fusiform gyrus, hippocampus, ACC, and orbitofrontal cortex. In contrast, McRae et al. (2012) found multiple significant quadratic associations between neural activation and age. During an emotional reactivity condition, significant non-linear associations with age were identified in the subgyral region, middle frontal gyrus, postcentral gyrus, cingulate gyrus, insula, parahippocampal gyrus, superior parietal lobule, superior temporal gyrus, and several regions of the cerebellum. Non-linear association with age were also identified during an emotion regulation condition in multiple regions of the medial and lateral prefrontal cortex as well as the ACC, caudate, thalamus, angular gyrus, lingual gyrus, fusiform gyrus, inferior parietal lobule, superior temporal gyrus, inferior temporal gyrus, middle temporal gyrus, and several regions of the cerebellum. Finally, Wiggins et al. (2016) found quadratic interactions between emotion displayed (fearful, happy, angry), intensity of the emotion displayed (0%, 50%, 75%, 100%), and age group (adolescent vs. adult) in the superior temporal sulcus, ventrolateral prefrontal cortex, and middle temporal gyrus. Given the variable nature of these results, it is clear that more research utilizing non-linear analytic approaches is needed to identify the developmental trajectories of change in these complex systems.
3.4. Pubertal timing
Three studies examined pubertal stage (measured by parent report of physical development; Tanner, 1955) as a predictor of neural indicators of emotion processing. One study found differences in activation in the lingual and fusiform gyri, cuneus, and middle and inferior occipital gyri between prepubertal and late pubertal participants (Pagliaccio et al., 2013). The second study examined associations between amygdala activation and pubertal stage, but did not report any significant results (Thomas et al., 2001). A third study (Vetter et al., 2015) did not find any associations between neural responses and pubertal stage. Despite these null findings, other studies outside the scope of this review have found links between pubertal hormones and structural brain development (e.g., Giedd et al., 2006) as well as between hormone levels and connectivity across brain regions such as the amygdala and lateral prefrontal cortex (Scherf et al., 2013; for a review, see Ladouceur, 2012). Associations between pubertal timing and functional brain activation have also been identified. Most notably, one study (Moore et al., 2012) that included the same longitudinal participants as Pfeifer et al. (2011) found significant associations between pubertal development (as measured by parent report of physical development) and neural responses that varied between 10 and 13 years of age. Specifically, pubertal development was associated with responses to emotional stimuli in the amygdala, thalamus, and visual cortex at age 10 as well as the temporal pole and both the lateral and medial prefrontal cortices at age 13. These findings are particularly important because studies of development across adolescence have found differential impacts of pubertal development versus chronological age in terms of key functional outcomes such as social abilities and anxiety (Brooks-Gunn et al., 1985). These findings underscore the importance of considering developmental trajectories of emotion processing systems in terms of hormonal maturation, not just chronological age.
3.5. Gender effects
Twelve of the reviewed studies examined gender in conjunction with age analyses and yielded particularly varied results. For example, one study reported that age associations were found bilaterally in the prefrontal cortex for females, but only in the right hemisphere for males (Yurgelun-Todd and Killgore, 2006). Another study found significant associations between prefrontal activation and age for males, but not females (Killgore et al., 2001). A third study reported an interaction between age and gender when predicting neural activation in the orbitofrontal cortex and amygdala (McClure et al., 2004), indicating that adolescent boys and girls had neural activation patterns that were relatively similar to each other whereas adult men and women differed significantly in their neural responses. Finally, one study reported that age-related amygdala activation results were primarily driven by female participants (Killgore et al., 2001).
Neuroimaging studies have indicated that the patterns of structural development differ significantly between males and females (De Bellis et al., 2001, Durston et al., 2001). Neural responses to emotional stimuli have also been found to differ between male and female adolescents in a large cross-sectional study (Schneider et al., 2011). Given the significant structural differences in brain development that are known to exist during adolescence and functional differences that have emerged cross-sectionally, it is surprising that no consistent gender effects emerged in the studies reviewed. Future studies should continue to test for gender differences, but include larger cohorts and test for interactions between gender and pubertal timing. Attention is also needed to gender differences in the context of differential demands of emotion processing paradigms (e.g., reactivity versus cognitive change), as males and females may develop and utilize emotion regulation strategies differently across adolescence.
3.6. Connectivity analyses
Eight studies examined functional connectivity between the amygdala and other brain regions. Functional connectivity was found to increase with age between the amygdala and the hippocampus (Guyer et al., 2008), ventral striatum (Pfeifer et al., 2011), precuneus (Vetter et al., 2015), posterior cingulate cortex (Vetter et al., 2015), and medial (Gee et al., 2013, Vink et al., 2014) and lateral (Silvers et al., 2015, Vink et al., 2014) prefrontal cortices. One study, although not statistically testing connectivity between neural regions, indicated inverse coupling between the amygdala and dorsolateral prefrontal cortex (dlPFC), finding increasing difference between dlPFC and amygdala activation with age (Killgore et al., 2001). Finally, another study reported no associations between prefrontal-amygdala connectivity and age, but reported a complex interaction between age, self-reported anxiety, trial, and emotion that significantly predicted functional connectivity (Hare et al., 2008). These significant connectivity findings are consistent with research indicating that white matter tracts, the communication architecture between regions of the brain, develop significantly during adolescence, particularly between the amygdala and prefrontal cortex (Swartz et al., 2014). This increase in connectivity is one likely explanation for the observed decreases in emotionally-dysregulated behavior across development − as the cross-talk between brain regions interpreting emotional information and those regulating appropriate responses improves, adolescents will be able to respond to emotional challenges in a more adaptive manner.
In a review of adolescent neuroimaging literature, Pfeifer and Allen (2012) noted lack of connectivity analyses as an important shortcoming of much of the neuroimaging research on adolescent functioning. They argued that the current focus on differences in magnitude of neural activation has overshadowed an understanding of how regions of the brain communicate across development, yielding an overly simplistic picture of the adolescent brain. Given the largely consistent increases in connectivity between the amygdala and other prefrontal brain regions reported in the studies reviewed here, continuing to examine changes in connectivity is an important direction for future research.
4. Expanding directions for adolescent emotion processing research
Our systematic review of emotion processing at the neural level during adolescence indicated that more studies, using consistent and theoretically driven methodologies and analytic approaches, are needed to develop a clear picture of emotional changes during this important developmental period. Our review showed variable support for linear and non-linear developmental trajectories within key emotion processing structures as well as more consistently reported increases in connectivity between these structures across the lifespan. This observed variability highlights the need for further characterization of these processes during adolescence with particular attention to task demands, linear and non-linear age trajectories, neural connectivity, pubertal timing and hormone levels, and gender differences. Beyond these basic characterizations, several additional directions warrant exploration in future research to develop a more robust and ecologically-valid depiction of adolescent emotion and the links between emotional processes and key functional outcomes during this unique developmental period.
4.1. Naturalistic emotion processing
One key issue for the future of research on adolescent emotion processing is improving the ecological validity of experimental paradigms. In day-to-day interactions, adolescents do not experience distinct emotion processes in isolation or in a discrete trial-by-trial fashion. Rather, emotion processing, particularly the regulation of emotion, is an ongoing iterative process that occurs with feedback from one’s environment (Gross and Thompson, 2009) and requires flexible use of multiple regulation strategies (Bonanno and Burton, 2013). In contrast, functional neuroimaging paradigms of emotional reactivity and emotion regulation require participants to respond to discrete trials of different emotional stimuli—an experience that is substantially removed from authentic emotion processing. A key future direction for the study of emotional development in adolescence is to create novel neuroimaging tasks that are theory-driven and more closely approximate the ongoing, flexible, and interactive nature of emotion processing.
Another concern with respect to experimental validity of the reviewed studies is that, with the exception of studies employing cognitive change paradigms, one distraction paradigm, and one concentration paradigm, all of the studies examined emotion processing by showing faces of adults who were expressing emotions. This approach holds two problems for understanding emotion processing in adolescence. First, adolescents may have a distinct neural response to the faces of adults relative to the faces of same-age peers who comprise their primary social environment (e.g., Saxbe et al., 2015). Given the recent surge of studies focusing on social cognition and peer interactions during adolescence (Blakemore, 2008, Crone and Dahl, 2012), developing an understanding of emotional reactivity and regulation as elicited with age-appropriate stimuli (e.g., Coffman et al., 2015) is essential. Furthermore, neural responses may differ to same-gender versus opposite-gender faces at certain points in development (Telzer et al., 2015), which may additionally complicate study findings. Second, there has been little attention paid in the emotion processing literature, particularly in the study of adolescence, to creating distinctions between emotions that are perceived versus experienced. For example, a condition where sad faces are displayed might be referred to as “sad” and treated as though participants are simply observing the sad face and thus exhibiting neural patterns linked with perception of sadness. However, in addition to processing the emotion displayed (sadness), participants may also be concurrently experiencing a reactive emotional response to the stimulus, such as concern or compassion and little attention has been paid to disentangling these processes. Granted, it could be argued that the overlapping neural patterns of activation elicited by different basic emotions (van der Gaag et al., 2007) makes this distinction less essential. However, as technology improves and more nuanced neurological distinctions can be made, the idea that multiple distinct emotions may be concurrently perceived and elicited should be considered in the interpretation of neuroimaging results from such studies.
4.2. Beyond basic emotion
In this review, we focus on characterizing the nature of basic emotion processes during adolescence. Within the functional neuroimaging literature, research on social emotions, peer interactions, reward sensitivity, risk-taking, and mental illness have largely taken the forefront. However, we argue that developing a solid understanding of basic emotion is essential as emotion processing underlies all adolescent social experiences (Parkinson, 1996, Pfeifer et al., 2011, Steinberg, 2008). The dysregulation of emotion in adolescence also is closely linked to adverse outcomes, particularly in terms of mental health (Silk et al., 2003, Weinberg and Klonsky, 2009). Thus, pursuing a symbiotic relationship between the investigation of basic emotional processes, social interactions, and mental health is essential for the advancement of the broader field of adolescent development.
4.3. Theoretical versus analytic approaches
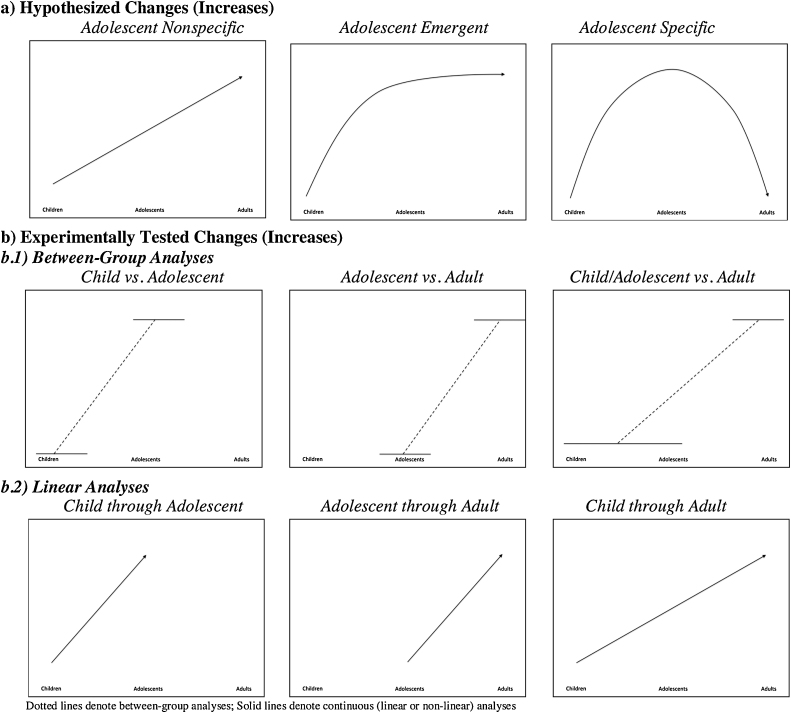
One challenge that is clearly highlighted within the reviewed studies is the mismatch between the hypothesized trajectories of developmental change in the neural networks subserving emotion regulation (Casey, 2013) and how developmental changes are actually being tested experimentally. Fig. 3 shows a comparison of the change models hypothesized (a) and those primarily tested in the different studies reviewed (b). As shown, the tested models are restricted to between-group (b.1) and linear (b.2) age analyses which limit the ability to find support for developmental trajectories other than adolescent nonspecific changes. Fortunately, a small number of studies have begun to directly test more complex non-linear patterns, which will facilitate future experimental support for the complex developmental changes trajectories that have been hypothesized.
Fig. 3.
Depiction of theorized versus empirically-tested models of developmental change.
4.4. Integrating multiple methodologies
In the past two decades, functional neuroimaging (particularly fMRI) has allowed researchers to gain more insight into the internal processes of adolescents than was ever previously possible. However fMRI as a methodology, when used in isolation, has several limitations. In particular, interpretations of statistically significant changes in the blood-oxygen-level dependent (BOLD) signal suffer from the reverse inference problem—observed changes in activation during a specific process are often taken to mean that the brain region activated is responsible for that process in the brain (e.g., a “social emotion center”; Poldrack, 2006). Concerns also have emerged about the stability of neural responses over time. For instance, amygdala responses measured on the same participants using the same paradigm several months apart have been found to vary significantly (van den Bulk et al., 2013). As fMRI data analysis techniques have improved, approaches such as connectivity analysis have offered more nuanced tools for understanding the interconnected components of the brain. Approaches integrating fMRI with other technologies (e.g., simultaneous recording with EEG or ECG) may also help overcome methodological limitations such as relatively poor temporal resolution. Additionally, combined use of fMRI data and genetic analysis has revealed new insight into moderating genetic effects in emotional processes, such as interactions between genotype of the serotonin transporter gene (5-HTT), connectivity between the amygdala and prefrontal cortex, and age (Wiggins et al., 2012). Such studies indicate that imaging genetics is likely a fruitful approach to understanding complex interactional processes underlying emotional development. Finally, research utilizing a multitrait-multimethod framework (Cambell and Fiske, 1959) to test associations between neural findings and other measures extending beyond “the lab” (e.g., measures of real-world adaptive functioning) will be integral to increasing the ecological validity of future neuroimaging studies.
4.5. Individual differences
There has been a general trend in the literature to treat adolescents as a singular group who experience biological changes in concert. However adolescents, just like adults and children, each carry unique risk and resilience factors. Studies on early temperament indicate long-lasting differences in emotion regulation abilities (Calkins et al., 2007) that may persist through adolescence and impact emotion processing and behavior (e.g., behavioral inhibition; Hirshfeld et al., 1992, Fox et al., 2005). Such behavioral differences may additionally be linked to differences in neural responses. For example, adolescents with greater amygdala activation have been found to report greater interpersonal anxiety (Killgore and Yurgelun-Todd, 2005) and such individual differences may obscure age group differences that might otherwise emerge if such factors are not measured and accounted for. Unfortunately, these types of individual differences are understudied in the neuroimaging literature. Furthermore, adolescents do not exist in isolation, but rather navigate multiple emotion-evoking contexts. Research is needed to not only better understand the development of emotion during adolescence, but also how emotional development and competence may vary across different social contexts (family, school, community).
5. Conclusion
Adolescence is a unique time. It is a period when individuals develop autonomy and distinct changes in emotion are frequently noted. Understanding these changes in emotion are essential as difficulties in emotional processes have been intimately linked to maladaptive outcomes including increased incidence of psychopathology (Garnefski et al., 2005, Silk et al., 2003) and risky behaviors (e.g., drug use, school dropout; Archambault et al., 2009, Cooper et al., 1995) − outcomes that have dramatic societal impact and cost. Furthermore, understanding which components of emotion regulation are more effortful or challenging during the adolescent period could lead to novel adolescent-adapted clinical interventions. Despite the importance of understanding emotional processes during this critical period, the neural correlates of these changes have not been well-characterized. Our review highlights the variable nature of the studies within this body of literature on multiple dimensions—experimental task demands, cohort characteristics, and analytic approaches. This variability likely obscured the ability to find many common results across studies. Few studies reviewed tested for relationships with puberty or gender effects and results were highly variable. However, supporting literature suggests that puberty and gender effects may impact emotion processing and further study is warranted. Overall, the studies reviewed, indicate that more complex analytic approaches are essential for developing a clear picture of emotion regulation during adolescence. In particular, testing non-linear developmental patterns (Casey, 2013) and functional connectivity (Pfeifer and Allen, 2012) will likely produce a more comprehensive understanding of developmental changes in the emotional adolescent brain.
Acknowledgements
This research was supported in part by a NSF Graduate Research Fellowship awarded to the first author and NIH NICHD Grants R01HD046807 and R21HD072170 awarded to the last author.
References
- Adolphs R., Tranel D., Damasio A.R. Dissociable neural systems for recognizing emotions. Brain Cogn. 2003;52:61–69. doi: 10.1016/s0278-2626(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Archambault I., Janosz M., Morizot J., Pagani L. Adolescent behavioral, affective, and cognitive engagement in school: relationship to dropout. J. Sch. Health. 2009;79(9):408–415. doi: 10.1111/j.1746-1561.2009.00428.x. [DOI] [PubMed] [Google Scholar]
- Arnett J.J. Adolescent storm and stress, reconsidered. Am. Psychol. 1999;54(5):317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Baird A.A., Gruber S.A., Fein D.A., Maas L.C., Steingard R.J., Renshaw P.F. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38(2):195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bonanno G.A., Burton C.L. Regulatory flexibility: an individual differences perspective on coping and emotion regulation. Perspect. Psychol. Sci. 2013;8(6):591–612. doi: 10.1177/1745691613504116. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J., Petersen A.C., Eichorn D. The study of maturational timing effects in adolescence. J. Youth Adolesc. 1985;14(3):149–161. doi: 10.1007/BF02090316. [DOI] [PubMed] [Google Scholar]
- Buck R. The biological affects: a typology. Psychol. Rev. 1999;106(2):301–336. doi: 10.1037/0033-295x.106.2.301. [DOI] [PubMed] [Google Scholar]
- Calkins S.D., Graziano P.A., Keane S.P. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biol. Psychol. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambell D.T., Fiske D.W. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol. Bull. 1959;56(2):81–105. [PubMed] [Google Scholar]
- Casey B.J., Caudle K. The teenage brain: self-control. Curr. Dir. Psychol. Sci. 2013;22(2):82–87. doi: 10.1177/0963721413480170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008 doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J. The teenage brain: an overview. Curr. Dir. Psychol. Sci. 2013;22(2):80–81. doi: 10.1177/0963721413480170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman M.C., Trubanova A., Richey J.A., White S.W., Kim-Spoon J., Ollendick T.H., Pine D.S. Validation of the NIMH-ChEFS adolescent face stimulus set in an adolescent, parent, and health professional sample. Int. J. Methods Psychiatr. Res. 2015;24(4):275–286. doi: 10.1002/mpr.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P.M., Martin S.E., Dennis T.A. Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Dev. 2004;75(2):317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cooper M.L., Frone M.R., Russell M., Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J. Pers. Soc. Psychol. 1995;69(5):990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Damasio A., Everitt B.J., Bishop D. The somatic marker hypothesis and the possible functions of the prefrontal cortex [and discussion]: Philosophical Transactions of the Royal Society of London. Ser. B: Biol. Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Beers S.R., Hall J., Frustaci K., Masalehdan A. Sex differences in brain maturation during childhood and adolescence. Cereb. Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Deeley Q., Daly E.M., Azuma R., Surguladze S., Giampietro V., Brammer M.J. Changes in male brain responses to emotional faces from adolescence to middle age. NeuroImage. 2008;40(1):389–397. doi: 10.1016/j.neuroimage.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Donovan, S., (2008, February 26) Teen angst linked to brain development. ABC News. Retrieved from http://www.abc.net.au/news/2008-02-26/teen-angst-linked-to-brain-development/1053812.
- Durston S., Hulshoff Pol H.E., Casey B.J., Giedd J.N., Buitelaar J.K., van Engeland H. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Etkins A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2010;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Marshall P.J., Nichols K.E., Ghera M.M. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu. Rev. Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Garnefski N., Kraaij V., van Etten M. Specificity of relations between adolescents cognitive emotion regulation strategies and Internalizing and Externalizing psychopathology. J. Adolesc. 2005;28(5):619–631. doi: 10.1016/j.adolescence.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Humpreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zljendobs A. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Clasen L.S., Lenroot R., Greenstein D., Wallace G.L., Ordaz S. Puberty-related influences on brain development. Mol. Cell. Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J., Barrett L.F. Emotion generation and emotion regulation: one or two depends on your point of view. Emotion Rev. 2011;3(1):8–16. doi: 10.1177/1754073910380974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J., Thompson R.A. Emotion regulation: conceptual foundations. In: Gross J.J., editor. Handbook of Emotion Regulation (3–24) Guilford Press; New York, NY: 2009. [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., Nelson E.E., Roberson-Nay R., Adler A.D. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G.S. vols. 1–2. Appleton; New York: 1904. (Adolescence: Its Psychology and Its Relation to Physiology, Anthropology, Sociology, Sex, Crime, Religion, and Education). [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld D.R., Rosenbaum J.F., Biederman J., Bolduc E.A., Faraone S.V., Snidman N. Stable behavioral inhibition and its association with anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1992;31(1):103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Horvitz J.C. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci. Biobehav. Rev. 2009;33(8):1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. JAMA Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Yurgelun-Todd D.A. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. http://journals.lww.com/neuroreport/Abstract/2005/10170/Social_anxiety_predicts_amygdala_activation_in.12.aspx (Retrieved from) [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Yurgelun-Todd D.A. Unconscious processing of facial affect in children and adolescents. Soc. Neurosci. 2007;2(1):28–47. doi: 10.1080/17470910701214186. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Oki M., Yurgelun-Todd D.A. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12(2):427–433. doi: 10.1097/00001756-200102120-00047. (Retrieved from) [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Front. Integr. Neurosci. 2012;6(65):1–11. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lindstrom K.M., Guyer A.E., Mogg K., Bradley B.P., Fox N.A., Ernst M. Normative data on development of neural and behavioral mechanisms underlying attention orienting toward social–emotional stimuli: an exploratory study. Brain Res. 2009;1292:61–70. doi: 10.1016/j.brainres.2009.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E.B., Monk C.S., Nelson E.E., Zarahn E., Leibenluft E., Bilder R.M. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol. Psychiatry. 2004;55(11):1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D. The developmental of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents, and young adults. Soc. Cogn. Affect. Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita B. Emoting: a contextualized process. In: Mesquita B., Barrett L.F., Smith E., editors. The Mind in Context. Guilford; New York, NY: 2010. pp. 83–104. [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., Zarahn E., Bilder R.M., Leibenluft E. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moore W.E., Pfeifer J.H., Masten C.L., Mazziotta J.C., Iacoboni M., Dapretto M. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Soc. Cogn. Affect. Neurosci. 2012;7(1):35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J., Lopez A.D. Evidence-based health policy—lessons from the global burden of disease study. Science. 1996;274(5288):740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., McClure E.B., Monk C.S., Zarahn E., Leibenluft E., Pine D.S., Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J. Child Psychol. Psychiatry. 2003;44(7):1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- O’Doherty J.P., Dayan P., Friston K., Critchley H., Dolan R.J. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr. Dir. Psychol. Sci. Gross. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Gaffrey M.S., Belden A.C., Botteron K.N., Harms M.P., Barch D.M. Functional brain activation to emotional and nonemotional faces in healthy children: evidence for developmentally undifferentiated amygdala function during the school-age period. Cogn. Affect. Behav. Neurosci. 2013 doi: 10.3758/s13415-013-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson B. Emotions are social. Br. J. Psychol. 1996;87(4):663–683. doi: 10.1111/j.2044-8295.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc. Cogn. Affect. Neurosci. 2009;4:387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Pelphrey K.A. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 2011;108(3):607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Allen N.B. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn. Sci. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Moore W.E., Oswald T.M., Mazziotta J.C., Iacoboni M., Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D.S., Grun J., Zarahn E., Fyer A., Koda V., Li W. Cortical brain regions engaged by masked emotional faces in adolescents and adults: an fMRI study. Emotion. 2001;1(2):137–147. doi: 10.1037/1528-3542.1.2.137. [DOI] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Kaiser M.D., Crowley M.J., Pelphrey K.A. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev. Cogn. Neurosci. 2011;1(3):324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Rahko J., Paakki J.-J., Starck T., Nikkinen J., Remes J., Hurtig T. Functional mapping of dynamic happy and fearful facial expression processing in adolescents. Brain Imaging Behav. 2010;4:164–176. doi: 10.1007/s11682-010-9096-x. [DOI] [PubMed] [Google Scholar]
- Saxbe D.E., Del Piero L.B., Immordino-Yang M.H., Kaplan J.T., Margolin G. Neural correlates of adolescents’ viewing of parents’ and peers’ emotions: Associations with risk-taking behavior and risky peer affiliations. Soc. Neurosci. 2015;10(6):592–604. doi: 10.1080/17470919.2015.1022216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Smyth J.M., Delgado M.R. Horm. Behav. 2013;64:298–313. doi: 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S., Peters J., Brassen S., Menz M.M., Miedl S.F. Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage. 2011;56:1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Silk J.S., Steinberg L., Morris A.S. Adolescents emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Dev. 2003;74(6):1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., Shu J., Hubbard A.D., Weber J., Ochsner K.N. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev. Sci. 2015;18(5):771–784. doi: 10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci. 2013;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L.R., Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk- taking. Dev. Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz J.R., Carrasco M., Wiggins J.L., Thomason M.E., Monk C.S. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage. 2014 doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. Blackwell Scientific; Oxford, UK: 1955. Growth at Adolescence. [Google Scholar]
- Telzer E.H., Flannery J., Humphreys K.L., Goff B., Gabard-Durman L., Gee D.G., Tottenham N. The cooties effect: amygdala reactivity to opposite-versus same-sex faces declines from childhood to adolescence. J. Cogn. Neurosci. 2015;27(9):1685–1696. doi: 10.1162/jocn_a_00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K.M., Drevets W.C., Whalen P.J., Eccard C.H., Dahl R.E., Ryan N.D., Casey B.J. Amygdala response to facial expressions in children and adults. Biol. Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thomas L.A., De Bellis M.D., Graham R., LaBar K.S. Development of emotional facial recognition in late childhood and adolescence. Dev. Sci. 2007;10(5):547–558. doi: 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Vetter N.C., Pilhatsch M., Weigelt S., Ripke S., Smolka M.N. Mid-adolescent neurocognitive development of ignoring and attending emotional stimuli. Dev. Cogn. Neurosci. 2015;14:23–31. doi: 10.1016/j.dcn.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Derks J.M., Hoogendam J.M., Hillegers M., Kahn R.S. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Vizmanos B., Marti-Henneberg C. Puberty begins with a characteristic subcutaneous body fat mass in each sex. Eur. J. Clin. Nutr. 2000;54(3):203–208. doi: 10.1038/sj.ejcn.1600920. [DOI] [PubMed] [Google Scholar]
- Vizmanos B., Marti-Henneberg C., Cliville R., Moreno A., Ballart J.F. Age of pubertal onset affects the intensity and duration of pubertal growth peak but not final height. Am. J. Hum. Biol. 2001;13:409–416. doi: 10.1002/ajhb.1065. [DOI] [PubMed] [Google Scholar]
- van den Bulk B.G., Koolschijn P.C., Meens P.H., van Lang N.D., van der Wee N.J., Rombouts S.A. How stable is activation in the amygdala and prefrontal cortex in adolescence? A study of emotional face processing across three measurements. Dev. Cogn. Neurosci. 2013;4:65–76. doi: 10.1016/j.dcn.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag C., Minderaa R.B., Keyser C. Facial expressions: what the mirror neuron system can and cannot tell us. Soc. Neurosci. 2007;2(3–4):179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Klonsky E.D. Measurement of emotion dysregulation in adolescents. Psychol. Assess. 2009;21(4):616–621. doi: 10.1037/a0016669. [DOI] [PubMed] [Google Scholar]
- Wicker B., Keysers C., Plailly J., Royet J.-P., Gallese V., Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wiggins J.L., Bedoyan J.K., Carrasco M., Swartz J.R., Martin D.M., Monk C.S. Age-related effect of serotonin transporter genotype on amygdala and prefrontal cortex function in adolescence. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J.L., Adleman N.E., Kim P., Oakes A.H., Hsu D., Reynolds R.C. Developmental differences in the neural mechanisms of facial emotion labeling. Soc. Cogn. Affect. Neurosci. 2016 doi: 10.1093/scan/nsv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Brown K.J., Palmer D., Liddell B.J., Kemp A.H., Olivieri G. The mellow years?: Neural basis of improving emotional stability over age. J. Neurosci. 2006;26(24):6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd D.A., Killgore W.D.S. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neurosci. Lett. 2006;406(3):194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Zald D.H. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]