Abstract
The C-type lectin receptor (CLR) Dectin-2 can trigger the leukotriene C4 synthase (LTC4S)-dependent generation of cysteinyl leukotrienes (cys-LTs) and the CARD9- and NF-κB-dependent generation of cytokines, such as IL-23, IL-6, and TNF-α, to promote Th2 and Th17 immunity, respectively. Dectin-2 activation also elicits the type 2 cytokine IL-33, but the mechanism by which Dectin-2 induces these diverse innate mediators is poorly understood. Here we identify a common upstream requirement for phosphoinositide 3-kinase delta (PI3Kδ) activity for the generation of each Dectin-2-dependent mediator elicited by the house dust mite species, Dermatophagoides farinae (Df), using both pharmacologic inhibition and siRNA knockdown of PI3Kδ in bone marrow-derived dendritic cells (BMDCs). PI3Kδ activity depends on Spleen tyrosine kinase (Syk) and regulates the activity of protein kinase Cδ, indicating that PI3Kδ is a proximal Syk-dependent signaling intermediate. Inhibition of PI3Kδ also reduces cys-LTs and cytokines elicited by Dectin-2 cross-linking, confirming the importance of this molecule in Dectin-2 signaling. Using an adoptive transfer model, we demonstrate that inhibition of PI3Kδ profoundly reduces the capacity of BMDCs to sensitize recipient mice for Th2 and Th17 pulmonary inflammation in response to Df. Furthermore, administration of a PI3Kδ inhibitor during the sensitization of WT mice prevents the generation of Df-induced pulmonary inflammation. These results demonstrate that PI3Kδ regulates Dectin-2 signaling and its DC function.
Introduction
Dectin-2 is a myeloid C-type lectin receptor (CLR) with well-described roles in anti-fungal immunity (1, 2). Upon activation with hyphae from Candida albicans, Dectin-2 triggers the generation of NF-κB-dependent inflammatory cytokines IL-6, IL-10, IL-23, and TNF-α and the development of T helper 17 (Th17) immunity (3). Dectin-2 has a short cytoplasmic tail, but pairs with the ITAM-bearing Fc receptor gamma-chain (FcRγ), to initiate SH2 domain-containing protein tyrosine phosphatase (SHP-2) and Spleen tyrosine kinase (Syk)-dependent signaling (3-5). The downstream activation and nuclear translocation of NF-κB by Dectin-2, and by several other CLRs, requires the protein kinase C delta (PKCδ)-dependent phosphorylation of the adaptor protein caspase-associated recruitment domain (CARD9) (6) and its assembly with B-cell lymphoma/leukemia 10 and Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Malt1) (7). Inhibition of Malt1 abrogates Th17 immunity to Candida (8), which is in keeping with the importance of the Dectin-2/CARD9 pathway in the generation of Th17 immunity.
Dectin-2 activation can also elicit T helper 2 (Th2)-dependent pulmonary inflammation through the immediate generation of pro-inflammatory lipid mediators, cysteinyl leukotrienes (cys-LTs). Cys-LTs are derived from membrane arachidonate through the serial actions of cytosolic phospholipase A2, 5-lipoxygenase in the presence of the 5-lipoxygenase activating protein, and leukotriene C4 synthase (LTC4S) (9-11). Dectin-2-dependent cys-LT generation is elicited by glycans from common aeroallergens including Aspergillus fumigatus and the house dust mite (HDM) species Dermatophagoides farinae (Df) and Dermatophagoides pteronyssinus (Dp) (12). Dectin-2-dependent arachidonate metabolism in macrophages can also be triggered by Candida albicans (13), suggesting it is a central feature of Dectin-2 signaling. Whereas Dectin-2 signaling is required for the generation of both Th2 and Th17 allergic pulmonary inflammation elicited by HDM (14-16), the Dectin-2/LTC4S pathway in dendritic cells (DCs) is required only for Th2 immunity to HDM through the autocrine actions of cys-LTs at the type 1 cys-LT receptor, CysLT1R (14).
HDM-elicited allergic pulmonary inflammation also requires the type 2 cytokine IL-33, which promotes sensitization to HDM through the IL-1 receptor like 1 (ST2)-dependent upregulation of OX40L on lung DCs (17, 18). Notably, HDM can elicit IL-33 generation in BMDCs through a Dectin-2 and PI3K-dependent pathway (19), suggesting that IL-33 production may be a second Dectin-2-dependent autocrine signal that conditions DCs for Th2 immunity. However, the mechanism by which Dectin-2 controls the generation of these diverse mediators remains poorly understood.
Class I PI3Ks catalyze the phosphorylation of phosphatidylinositol at the 3-position to generate second messengers in response to transmembrane signaling (20). While the upstream signaling that activates PI3Ks is incompletely understood, YXXM-bearing receptors, G protein–coupled receptors, and Rat Sarcoma-dependent receptors have been implicated (21). The PI3K p110δ isoform (PI3Kδ) is highly expressed in hematopoietic cells and plays a key role in lymphocyte activation through the TCR and the BCR (22). PI3Kδ is also activated by the high affinity receptor for IgE, FcεR1, and mediates the phosphorylation of protein kinase B (Akt) and degranulation in mast cells (23, 24). As FcεR1 and Dectin-2 each use the FcRγ chain to initiate ITAM/Syk-dependent cys-LT generation, we sought to determine whether PI3Kδ is required for Dectin-2-dependent cys-LT generation, and whether it may be critical for Dectin-2 signaling more broadly.
Here we find that Df stimulation of BMDCs elicits phosphorylation of Akt, a PI3K-dependent kinase, and inhibition of PI3K reduces Df–elicited cys-LTs, IL-23, and IL-33, suggesting that PI3K activity regulates Dectin-2 signaling. PI3Kδ is the most abundant class I PI3K isoform in BMDCs and both pharmacologic inhibition and siRNA knockdown of PI3Kδ reduces Df-elicited cys-LTs, IL-23, and IL-33. Inhibition of PI3Kδ also reduces cys-LTs and cytokines elicited by Dectin-2 cross-linking, demonstrating that PI3Kδ is an important signaling intermediate in the Dectin-2 pathway. While Df-elicited PI3Kδ activity and the generation of each inflammatory mediator depends on Syk, inhibition of PI3Kδ has no effect on Syk phosphorylation but reduces the phosphorylation of protein kinase C delta (PKCδ), indicating that PI3Kδ is a proximal Syk-dependent signaling intermediate. Inhibition of PI3Kδ potently reduces DC-mediated sensitization, as WT mice sensitized with PI3Kδ-treated Df-pulsed BMDCs have a dramatic attenuation in allergic pulmonary inflammation and Th2/Th17 cytokine production after Df challenge. Furthermore, selective treatment of WT mice with a PI3Kδ inhibitor during sensitization attenuates the generation of Df-induced pulmonary inflammation. These findings demonstrate that PI3Kδ regulates divergent Dectin-2-dependent signaling pathways to promote both Th2 and Th17 immunity. Thus, strategies to inhibit the mucosal activation of PI3Kδ may have therapeutic efficacy in allergen-induced pulmonary inflammation.
Materials and Methods
Mice
C57BL/6 wild-type (WT) mice were purchased from Charles River Laboratories and ROSA26-EGFP transgenic mice (Tg(Gt(ROSA)26Sor-EGFP)I1Able) were purchased from Jackson Laboratory. Ltc4s−/− mice were generated and maintained in our laboratory (25). Clec4n−/− mice (2) and Card9−/− mice (26) were generated as previously described. IL-33-deficient (Il33−/−) mice were provided by Pfizer Research (Cambridge, MA). All mice were on C57BL/6 background and were 8-12 wk old for in vivo experiments. All animal studies were approved and in accordance of the Animal Care and Use Committee of the Dana-Farber Cancer Institute.
Reagents
Df extracts (Greer Laboratories, Lenoir, NC) were reconstituted in PBS. LPS from E. coli 055:B5 was obtained from Sigma-Aldrich (St. Louis, MO). Curdlan (Wako Pure Chemical Industries, Osaka, Japan) was dissolved in DMSO. Rat anti-mouse Dectin-2 IgG2a (clone D2.11E4, AbD Serotec, Raleigh, NC) and goat anti-rat IgG2a (Jackson Immunoresearch, West Grove, PA) were used for Dectin-2 cross-linking. Pan-PI3K inhibitor (Ly294002; EMD Millipore, Billerica, MA), PI3K p110δ inhibitors (CAL-101 and IC87114; Selleckchem, Houston, TX), PI3K p110β inhibitor (TGX-221; Selleckchem), Syk inhibitor II (Santa Cruz Biotechnology, Dallas, TX), and Syk inhibitor (R406; Selleckchem) were dissolved in DMSO.
BMDC generation and Dectin-2 activation
BMDCs were generated with GM-CSF according to Lutz et al (27) and as previously described (28). Briefly, bone marrow was harvested from the femur, washed, and plated in petri dishes at 4 × 105 cells/ml in complete media consisting of RPMI 1640 with 10% heat-inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 5 μM 2-mercaptoethanol, and 40 ng/ml recombinant mouse GM-CSF (PeproTech, Rocky Hill, NJ). This suspension was cultured at 37°C in a 5% CO2 incubator. On day 3, 10 ml of complete media was added. On day 6, 10 ml of complete media was exchanged. Harvested cells on day 7 were washed and counted for stimulation. Cells were plated at 1 × 106 cells/ml and stimulated with Df for the indicated times.
Dectin-2 Cross-linking Assay
Tissue culture plates were incubated with goat Fab2 anti-rat IgG2a at 4°C overnight. The next day, the plates were washed with PBS and incubated with rat anti-mouse Dectin-2 IgG2a dissolved in PBS with 1% bovine serum albumin (BSA) at indicated doses for 2 h. The plate was washed again with PBS, and BMDCs (2.5 - 5 ×105 cells) were added and incubated in 37°C in a 5% CO2 incubator for indicated times. Supernatants and cell lysates were collected for cytokine analysis.
Cys-LT and Cytokine Measurements
Cys-LTs in the cultured supernatants at 30 min. were analyzed by ELISA according to the manufacturer's protocol (Cayman Chemical, Ann Arbor, MI). IL-23 and TNF-α in the cultured supernatants and IL-33 in the cell lysates after freeze and thaw were measured by ELISA (eBioscience, San Diego, CA) at an 8 h time point, unless otherwise indicated. Lymph node cells were cultured at 4 × 106 cells/ml, stimulated with 20 μg/ml Df for 72 h, and IL-4, IL-5, IL-13, IL-17A, and IFN-γ were measured by ELISA (eBioscience) in the cultured supernatants.
Quantitative PCR
RNA was extracted from BMDCs with TRIzol reagent, and cDNA was generated using first-strand kit (Thermo Scientific, Waltham, MA). Transcripts were measured using Mx3006P Real-Time PCR systems (Agilent Technologies, Santa Clara, CA) with PI3Kβ, PI3Kδ, IL-23, IL-33, 18S and GAPDH primers (SA Biosciences, Frederick, MD).
Western Blot
After treatment with or without Df in the presence or absence of PI3K inhibitor for the indicated times, the cells were lysed in NP-40 lysis buffer (Boston BioProducts, Ashland, MA). The total cellular protein concentrations were then quantified using a bicinchoninic acid assay (Pierce, Rockford, IL, USA). After quantification, 10 μg of denatured protein was loaded onto 10 or 12% SDS-PAGE gels (Bio-Rad Laboratories, Hercules, CA). The protein was then transferred to a polyvinylidene difluoride membrane (Bio-Rad) and blocked in TBS-T containing BSA or non-fat milk (1× TBS, 5% BSA or non-fat milk, 0.05% Tween-20) depending on the primary antibody for 60 minutes. The blocked membranes were then incubated overnight at 4°C with antibodies to phosphorylated Akt Ser-473, phosphorylated Syk Tyr-525/526, phosphorylated PKCδ Tyr-311 (dilution: 1:1000; Cell Signaling Technology, Beverly, MA) or PI3Kδ (dilution: 1:500; Santa Cruz Biotechnology). The following day, membranes were washed 3 times with TBS-T wash buffer (1 × TBS, 0.05% Tween 20). Membranes were incubated for 60 min with HRP-conjugated anti-rabbit secondary antibody (Santa Cruz Biotechnology; 1: 10,000 dilution) and washed 3 times in TBS-T wash buffer. The membrane was developed using SuperSignal West Femto (Thermo Scientific). For total Akt, the same membrane were stripped with Restore PLUS Western Blot Stripping Buffer reagent (Thermo Scientific) according to the manufacturer's instruction, followed by TBS wash and blocked in TBS-T containing NFM (1× PBS, 5% non-fat milk, 0.05% Tween-20) for 60 minutes. The blocked membrane was then incubated at 4°C overnight with Akt or GAPDH (dilution: 1:1000- 1:5000; Cell Signaling Technology) antibody for subsequent analysis.
PI3Kδ siRNA knockdown
Day 7 WT BMDCs were transfected with PI3Kδ or non-targeting control siRNA (GE Dharmacon, Lafayette, CO) using Amaxa Dendritic Cell Transfection Kit reagent (Invitrogen) according to manufacturer's protocol. After transfection, BMDCs were stimulated with 200 μg/ml Df or 100 ng/ml of LPS for 30 minutes for cys-LTs and 6 h for cytokines and analyzed by qPCR or ELISA.
Adoptive transfer protocol
Day 7 BMDCs were stimulated with PBS or Df in the presence or absence of CAL-101 at the concentration of 10 nM for 8 hrs and washed twice and resuspended in PBS. WT mice were sensitized with 104 cells in 20 μl PBS intranasally on day 0, challenged with 3 μg Df on days 11 and 13, and killed on day 15 with pentobarbital overdose. BAL fluid and MDLN cells were collected for analysis. For adoptive transfer of BMDC from transgenic GFP mice, 1 × 106 cells in 20 μl PBS were administered intranasally into WT recipients, MDLN cell were collected the following day for analysis.
Flow cytometry
For live cell/dead cell discrimination, Zombie Aqua dye (BD Biosciences, San Jose, CA) was used according to manufacturer's protocol. Isolated MDLN cells were first blocked with 1% mouse IgG (Sigma-Aldrich) and 1% anti-mouse CD16/CD32 (BD Biosciences), then stained with anti-mouse MHCII-Alexa Fluor 627 (clone M5/114.15.2; BD Biosciences) and anti-mouse CD11c-allophycocyanin-Cy7 (clone N418; BD Biosciences), according to the manufacturer's protocol. Analyses were performed on a FACSCanto flow cytometer (BD Biosciences), and data were analyzed with the FlowJo 7.5.
Direct sensitization and challenge protocol
On day 0, WT mice were sensitized with 3 μg Df intranasally. PI3Kδ inhibitor IC87114 (30 mg/kg) (29, 30) or vehicle control (PEG-400) was administered orogastrically at 1 h before and 8 h after Df administration. Mice were then challenged intranasally with 3 μg Df on days 11 and 13, and killed on day 15. BAL fluid was collected as described below.
BAL and lymph node isolation
BAL fluid was collected via three instillations of 0.75 ml PBS + 1 mM EDTA, and cells were pelleted for counting (14). 4 × 105 cells in 200 μl were centrifuged and counted after staining with 3 Stain Set (Thermo Scientific). A total of 200 cells per slide were counted and discriminated based on morphological differences. Isolated mediastinal lymph node cells were filtered through 70-μm strainers, washed, and plated at 4 × 106 cells/ml. Cells were restimulated with Df 20 μg/ml for 72 h and supernatants were collected for cytokine analysis.
Histology
The left lung was removed and fixed in 4% paraformaldehyde solution. Histology sections were prepared as previously described (28). They were stained with H&E for general morphology characterization and periodic acid-Schiff for mucus and goblet cell identification.
Statistical analysis
Results were expressed as means ± SEM. Unpaired Student's t tests were used for the statistical analysis unless stated otherwise. To compare between multiple genotypes or stimuli, one-way analysis of variance tests were used. To compare multiple genotypes over doses or time, two-way analysis of variance tests were used with Bonferroni post-tests. A P value <0.05 was considered significant.
Results
HDM stimulation elicits PI3K activity and the generation of cys-LTs, IL-23, and IL-33 in a PI3K-dependent manner
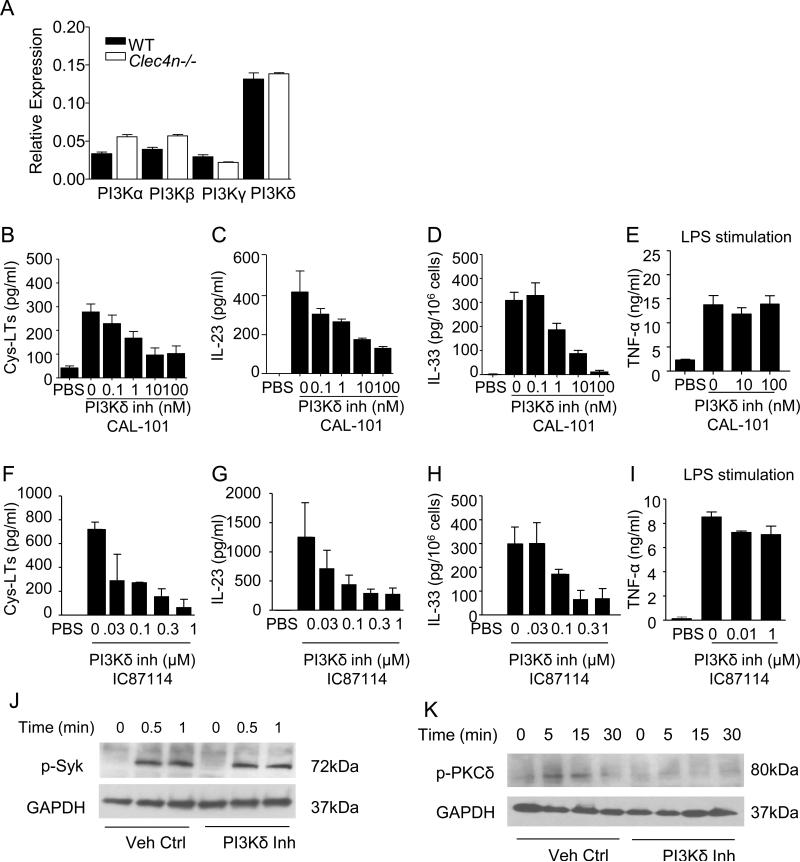
To determine whether PI3K plays a central role in Dectin-2 signaling, we first assessed BMDC activation in response to Df. Phosphorylation of Akt, a PI3K-dependent serine-threonine kinase, was detected in WT BMDCs stimulated with Df at 1 minute (Fig. 1A). This induction was absent in Df-stimulated BMDCs treated with the pan-PI3K inhibitor Ly294002. To determine whether PI3K activity was required for the production of each Dectin-2-dependent inflammatory mediator, we stimulated WT BMDCs with Df in the presence of Ly294002. Df elicited cys-LTs at 30 min, and IL-23 and IL-33 at 8 h in a dose-dependent manner, which was absent in Clec4n−/− BMDCs (Fig. 1B-D). The specificity of IL-33 protein measurement was verified by the absence of detection in Df-stimulated Il33−/− BMDCs (data not shown, dns). PI3K inhibition reduced production of Df-induced cys-LTs, IL-23, and IL-33 with IC50 values of 0.7 μM, 0.9 μM, and 0.5 μM, respectively (Fig. 1E-G). PI3K inhibition had no effect on LPS-induced TNF-α generation (Fig. 1H), demonstrating the specificity of PI3K inhibition.
FIGURE 1.
PI3K activity is required for the generation of each Dectin-2 mediator. (A) Representative Western blot from one of two independent experiments. Phosphorylation of Akt in WT BMDCs stimulated with 0 (−) or 100 μg/ml Df (+) for 1 min in the presence of 3 μM Ly294002 (Pan-PI3K inhibitor) or vehicle control. (B-D) Day 7 BMDCs from WT and Clec4n−/− mice were stimulated with increasing doses of Df. Cys-LTs and IL-23 were measured in supernatants and IL-33 in the cell lysates by ELISA at 30 min, 8 h, and 8 h, respectively. P = 0.0004 for cys-LTs, P < 0.0001 for IL-23 and IL-33. (E-H) WT BMDCs were stimulated with PBS or (E-G) 100 μg/ml Df or (H) 100 ng/ml LPS in the presence of Ly294002 at indicated doses. P < 0.01 for cys-LTs and IL-23, P < 0.05 for IL-33, and not significant for TNF-α. Results are means ± SEM pooled from 3 independent experiments. Significance was determined with two-way ANOVA.
Df elicits cys-LTs through Dectin-2, the ITAM-bearing FcRγ chain, and the downstream activation of Syk. To determine whether PI3K activity depends on Syk, we assessed the phosphorylation of Akt in the presence of the Syk inhibitor R406. Df stimulation of WT BMDCs triggered phosphorylation of Akt at 1 minute (Fig. 2A) which was reduced by 88 ± 2% in the presence of R406. In keeping with the role of Syk as an upstream mediator of the Dectin-2 pathway, blockade of Syk with two different inhibitors, Syk II and R406, significantly reduced the production of Df-elicited cys-LTs, IL-23, and IL-33 in a dose-dependent manner (Fig. 2B-G). LPS-induced TNF-α generation was intact in the presence of each Syk inhibitor, indicating the specificity of inhibition (Fig. 2H). These findings demonstrate that both PI3K and Syk are required for production of each Dectin-2-dependent mediator in response to Df and that PI3K activity depends on Syk.
FIGURE 2.
Df-elicited PI3K activity, cys-LTs, and cytokines depend on Syk. (A) Phosphorylation of Akt in WT BMDCs stimulated with 100 μg/ml Df (+) at various time points in the presence of 300 nM R406 (Syk inhibitor) or vehicle control. (B-G) WT BMDCs were stimulated with PBS or 100 μg/ml of Df or (H) 100 ng/ml LPS in the presence of specific Syk inhibitors, (B-D) Syk II inhibitor P < 0.0001 for cys-LTs, P < 0.05 for IL-23 and IL-33, or (E-G) R406 P < 0.05 for cys-LTs, IL-23, and IL-33. Results are means ± SEM pooled from 3 independent experiments. Significance was determined with one-way ANOVA.
PI3K p110δ isoform regulates Dectin-2 signaling
Next we investigated which isoform of PI3K is required for Dectin-2 signaling elicited by Df. WT BMDCs expressed each class I PI3K isoform, with PI3Kδ being the most abundant (Fig. 3A). There was no difference in PI3K isoform expression between WT and Clec4n−/− BMDCs. Two isoforms of class I PI3K, p110β (PI3Kβ) and p110δ (PI3Kδ) have been linked to the function of FcRγ chain-dependent receptors in myeloid cells (24, 31). To assess their role in Dectin-2 signaling, we first used available inhibitors. Inhibition of PI3Kδ with CAL-101 reduced Df-induced cys-LTs, IL-23, and IL-33 with IC50 values of 0.5 nM, 1.63 nM, and 1.67 nM, respectively (Fig. 3B-D). The inhibition was specific, as BMDCs treated with higher levels of inhibitor had no reduction in LPS-induced TNF-α (Fig. 3E). Similarly, use of another highly-specific PI3Kδ inhibitor, IC87114, also significantly reduced Df-induced cys-LTs, IL-23, and IL-33 (Fig. 3F-H), with intact LPS-induced TNF-α (Fig. 3I). By contrast, treatment with a PI3Kβ inhibitor at doses specific for the p110β isoform had no effect on the levels of Df-induced cys-LTs, IL-23, or IL-33), and no effect on LPS-induced TNF-α (Fig. S1). At 100 nM of PI3Kβ inhibitor, a dose previously reported to also block PI3Kδ (32), there was a small trend to reduced cys-LTs and IL-33 that was not significant.
FIGURE 3.
Pharmacologic inhibition suggests PI3Kδ is required for cysLTs, cytokines, and activation of PKCδ. (A) PI3Kα, PI3Kβ, PI3Kγ and PI3Kδ transcript expression relative to GAPDH in WT and Clec4n−/− BMDCs. (B-I) WT BMDCs were stimulated with PBS or 100 μg/ml Df or 100 ng/ml LPS in the presence of (B-E) CAL-101 or (F-I) IC87114. For CAL-101, P < 0.001 for cys-LTs and for IL-23, P < 0.0001 for IL-33. For IC87114, P < 0.05 for cys-LTs, IL-23, and IL-33. Results are means ± SEM pooled from 3 independent experiments. Significance was determined with one-way ANOVA. (J) Phosphorylation of Syk in WT BMDCs stimulated with 100 μg/ml Df at various time points in the presence of 100 nM CAL-101 (PI3Kδ inh) or vehicle control (Veh Ctrl). (K) Phosphorylation of PKCδ in WT BMDCs stimulated with 100 μg/ml Df at various time points in the presence of 100 nM CAL-101 or vehicle control.
In keeping with our prior data suggesting that PI3K activity is downstream of Syk (Fig. 2A), PI3Kδ inhibition had no effect on Syk phosphorylation (Fig. 3J). PKCδ, another downstream Syk-dependent signaling molecule in the Dectin-2 pathway, phosphorylates CARD9 to facilitate NF-κB activation and the generation of Dectin-2-dependent cytokines elicited by Candida albicans (2, 6). To determine whether PI3Kδ is upstream of PKCδ and CARD9 activation, we assessed the effect of PI3Kδ inhibition on Df-elicited PKCδ phosphorylation. Df stimulation of WT BMDCs induced phosphorylation of PKCδ, which was detected at 5 and 15 min (Fig. 3K). PI3Kδ inhibition reduced PKCδ phosphorylation by 90 ± 3 percent at 15 min, indicating that PI3Kδ is proximal to PKCδ and CARD9 signaling. In keeping with a downstream role for CARD9 in Df-elicited signaling, we did find that Df-stimulated Card9−/− BMDCs had reduced generation of both IL-23 and IL-33, but preserved cysLT generation (Fig. S2).
To confirm a requirement for PI3Kδ in Dectin-2 signaling, we used a knockdown approach. WT BMDCs were harvested on day 7, treated with PI3Kδ siRNA (PI3Kδ siRNA) or non-targeting siRNA control (control siRNA) by nucleofection, and stimulated with Df 24 h later. At 24 h, PI3Kδ siRNA-treated BMDCs had a significant reduction in PI3Kδ transcript (Fig. 4A), but no reduction in transcript for PI3Kα, β, or γ isoforms (Fig. 4B-D). Western blotting confirmed a reduction in PI3Kδ protein in siRNA-treated BMDCs relative to controls (Fig. 4E), and the reduction averaged 64 ± 4 % at 24 h (Fig. 4F). Accordingly, Df-induced cys-LTs, IL-23, and IL-33 were significantly reduced (Fig. 4G-I), as compared to control siRNA-treated BMDCs. The inhibition was specific, as PI3Kδ siRNA-treated BMDCs had no reduction in LPS-elicited TNF-α (Fig. 4J). These results demonstrate that PI3Kδ mediates Df-elicited Dectin-2 signaling.
FIGURE 4.
PI3Kδ knockdown confirms a role for PI3Kδ in Dectin-2 signaling. Day 7 WT BMDCs were transfected with non-targeting control siRNA or PI3Kδ siRNA. (A-D) PI3Kδ, PI3Kα, PI3Kβ, and PI3Kγ transcript expression relative to 18s in non-targeting control siRNA or PI3Kδ siRNA group. (E) Representative Western blots of PI3Kδ and GAPDH in BMDCs transfected with either non-targeting control siRNA or PI3Kδ siRNA. (F) Densitometric analysis of PI3Kδ proteins relative to GAPDH on Western blots in control (set as 1) and PI3Kδ siRNA transfected groups. (G-I) Df-elicited cys-LTs at 30 min, and IL-23 and IL-33 expression at 6 h, as compared to control siRNA-treated BMDCs. (J) LPS-elicited TNF-α at 6h, as measured by ELISA. Results are means ± SEM pooled from 3 independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.0001, as compared to control siRNA.
To verify that PI3Kδ activity contributes specifically to Dectin-2 signaling, BMDCs were activated with plate-bound Dectin-2 antibody overnight in the presence of 0, 10, or 100 nM PI3Kδ inhibitor. Dectin-2 cross-linking elicited cys-LTs and IL-33, each of which was absent in Clec4n−/− BMDCs (Fig. 5A-B). IL-23 was also detected, but high levels were elicited from plate-bound IgG controls (data not shown). TNF-α, another Dectin-2- and CARD9-dependent cytokine, was also elicited by Dectin-2 cross-linking and significantly reduced in Clec4n−/− BMDCs (Fig. 5C). Treatment with a PI3Kδ inhibitor reduced Dectin-2-elicited cys-LTs, IL-33, and TNF-α in a dose-dependent fashion (Fig. 5A-C). These results further demonstrate that PI3Kδ is an essential component of Dectin-2 signaling and mediates the generation of both cys-LTs and cytokines.
FIGURE 5.
PI3Kδ inhibition reduces Dectin-2 signaling elicited by antibody-mediated cross-linking. WT and Clec4n−/− BMDCs were stimulated with plate-bound Dectin-2 antibody overnight in the presence or absence of CAL-101. The concentrations of cys-LTs and TNF-α in the supernatants and of IL-33 in the cell lysates were measured by ELISA. P<0.001 (cys-LTs), P<0.01 (TNF-α), P<0.05 (IL-33). Results are means ± SEM pooled from 3 independent experiments. Significance was determined with one-way ANOVA.
PI3Kδ is required for Dectin-2-mediated pulmonary inflammation in vivo
To understand whether PI3Kδ activity is essential for Df-induced pulmonary inflammation, we used an adoptive transfer model in mice (14). WT BMDCs were pulsed with either PBS (PBS-DC) or Df in the presence of the PI3Kδ inhibitor CAL-101 (PI3Kδ-Df-DC) or vehicle control (Df-DC) for 8 h, washed, and transferred intranasally into naïve WT C57BL/6 recipient mice on day 0. All recipients were challenged intranasally with 3 μg Df on days 11 and 13, and killed on day 15. WT mice sensitized with Df-DCs showed inflammation in the bronchoalveolar lavage (BAL) fluid after Df challenge, as compared to mice sensitized with PBS-DCs (Fig. 6A). This included an influx of macrophages, neutrophils, and eosinophils. Mice sensitized with PI3Kδ-Df-DCs, had significant and marked reductions in total cell counts of 80%, neutrophils of 91%, and eosinophils of 96%, as compared to mice sensitized with vehicle control-treated Df-DCs. PI3Kδ treatment of BMDCs did not influence BMDC survival, as PI3Kδ-Df-DCs cultured for an additional 48 h demonstrated no difference in staining with a non-permeant amine-reactive fluorescent viability dye compared to vehicle control-treated Df-DCs (Fig. S3A). Furthermore, EGFP+ PI3Kδ-Df-DCs were detected in the mediastinal lymph nodes (MDLNs) of recipient mice at 24 h after adoptive transfer in comparable numbers to EGFP+ vehicle control-treated Df-DCs (Fig. S3B).
FIGURE 6.
PI3Kδ is required for Dectin-2-mediated pulmonary inflammation in vivo. (A-C) WT mice were sensitized with 104 PBS- or Df-pulsed BMDCs in the presence or absence of PI3Kδ inhibitor CAL-101 at 10 nM (PBS-DC, Df-DC, PI3Kδ-Df-DC), challenged with 3 μg Df on day 11 and 13, and killed on day 15. (A) Total and differential cell counts for BAL fluid macrophages, neutrophils, eosinophils, and lymphocytes. (B) H&E and PAS staining of lung tissue from each group. (C) MDLN cells were isolated and restimulated with 20 μg/ml Df for 72 h. Cytokines were measured in the supernatants. Results are means ± SEM (n= 8, 13, 15 mice for PBS-DC, Df-DC, PI3Kδ-Df-DC groups, respectively) from 3 experiments. (D) On day 0, WT mice were sensitized with 3 μg Df intranasally. PI3Kδ inhibitor IC87114 (30 mg/kg) or vehicle control (PEG-400) was administered orogastrically at 1 h before and 8 h after Df administration. Mice were then challenged intranasally with 3 μg Df on days 11 and 13, and killed on day 15. Total and differential cell counts for BAL fluid macrophages, neutrophils, eosinophils, and lymphocytes. Results are means ± SEM (n= 8 and 10 mice for Df-vehicle and Df- PI3Kδ treated groups, respectively) pooled from 3 independent experiments * P < 0.05, ** P < 0.01.
Histologic analysis of the lung stained with H&E showed that mice sensitized with Df-DCs had dense cellular infiltrates around the bronchovascular bundles that were not present in mice sensitized with PBS-DCs (Fig. 6B, H&E). These infiltrates were markedly diminished in mice sensitized with PI3Kδ-Df-DCs. Goblet cell metaplasia and mucus production, as detected by periodic acid-Schiff (PAS) staining, were also present in mice sensitized with Df-DCs, but was absent in mice sensitized with either PBS-DCs or PI3Kδ-Df-DCs (Fig. 6B, PAS). Thus, PI3Kδ is critical for the capacity of BMDCs to elicit Df-induced pulmonary inflammation.
To understand whether PI3Kδ-Df-DCs elicited an altered T cell cytokine response, we harvested the MDLN cells from each cohort at day 15, restimulated them with 20 μg/ml Df for 72 h, and assayed for T cell cytokines in cultured supernatants (14, 33). MDLN cell cultures from WT mice sensitized with Df-DCs generated high levels of IL-4, IL-5, IL-13, and IL-17A, as compared to those sensitized with PBS-DCs (Fig. 6C). MDLN cell cultures from WT mice sensitized with PI3Kδ-Df-DCs generated significantly less IL-4, IL-5, IL-13, and IL-17A. MDLN cell cultures from each group generated high levels of IFN-γ, consistent with an antigen-independent response to DC adoptive transfer previously reported (14). Thus, PI3Kδ is critical for the capacity of BMDCs to generate pulmonary inflammation and Th2 and Th17 immunity to Df.
To verify that PI3Kδ was important in priming through the mucosal route, we treated WT mice with 3 μg intranasal Df on days 0, 10, and 12, and with the PI3Kδ inhibitor IC87114 or vehicle control by oral gavage on day 0. IC87114 was chosen based on its established use in murine models of inflammation (29, 30, 34). The BAL fluid at day 14 revealed pulmonary inflammation in WT mice sensitized and challenged with Df that included both eosinophils and neutrophils (Fig. 6D). Mice treated with the PI3Kδ inhibitor on day 0 showed attenuated development of Df-elicited pulmonary inflammation with significant reductions in total BAL fluid cell counts, macrophages, neutrophils, and eosinophils, as compared to mice treated with vehicle control. These results further suggest that PI3Kδ plays an important role in the generation of Df-elicited allergic pulmonary inflammation.
Discussion
Our study demonstrates that PI3Kδ is an important signaling intermediate in the DC Dectin-2 signaling pathway elicited either by Df or by antibody-mediated cross-linking. We found that PI3Kδ is Syk-dependent and regulates PKCδ activation and the generation of both cys-LTs and cytokines elicited by Df. Accordingly, two models of Df-elicited Dectin-2-dependent allergic pulmonary inflammation were significantly attenuated by PI3Kδ inhibition, indicating the importance of Dectin-2 and PI3Kδ in the immune response triggered by this common allergen.
To our knowledge, this is the first study to identify PI3Kδ in CLR signaling. However, because several members of the myeloid CLR family use a shared Syk signaling pathway (1), PI3Kδ may regulate the signaling of additional receptors. Indeed, we have found that the Dectin-1 agonist, curdlan, also induces cys-LTs in a manner that partially depends on PI3Kδ (data not shown). As PI3Kδ inhibitors are in clinical trials for B cell malignancies, further identifying such roles will be important to understand the possible risks associated with systemic PI3Kδ inhibition.
PI3Kδ is an upstream mediator of several membrane-bound receptors that use ITAM/Syk-dependent signaling, including the BCR and the TCR (22). Ligation of the BCR causes tyrosine kinase-mediated phosphorylation of Igα and Igβ, and recruitment of PI3K through phosphorylation of the YXXM-bearing CD19 coreceptor and the B cell adaptor for PI3K (BCAP) (35, 36). TCR activation of PI3K also depends on tyrosine kinase signaling and has been variably attributed to the YXXM-bearing adaptor protein TCR interacting molecule (37) or to phosphorylation of additional non-ITAM tyrosine-containing proteins such as the Linker of Activated T cells (LAT) and Src homology 2 (SH2) domain-containing leukocyte protein of 76kDa (SLP-76) (38, 39). Our finding that Df-elicited PI3K activity depends on Syk is in keeping with these studies. However, neither Dectin-2, FcRγ, nor Dectin-3, which dimerizes with Dectin-2 (40), contain the tyrosine motifs that support PI3Kδ recruitment. As such, our study suggests that Dectin-2 uses an additional adaptor protein(s) as the docking site for the SH2 domain-containing p85 regulatory subunit of PI3Kδ. Notably, the tyrosine-containing adaptor protein Linker for Activation of B cells/Non-T cell Activating Linker (LAB/NTAL) was recently reported to be phosphorylated after Dectin-2 activation (41), but whether Dectin-2 utilizes this protein or an as yet unidentified YXXM-bearing adaptor protein to support PI3Kδ activation remains to be determined.
The generation of cys-LTs is unique to CLRs among pattern recognition receptor (PRR) families, likely due to the sustained calcium flux that is required to translocate cytosolic phospholipase A2 from the cytosol to the nuclear membrane to release arachidonate from membrane phospholipids (10, 42). PI3K plays a key role in mobilizing calcium and activating PKCs in response to FcεR1 signaling or BCR signaling by activating phospholipase Cγ (PLCγ) (24, 43, 44). Recent studies on Dectin-1 and Dectin-2 have also demonstrated that PLCγ is required for eliciting a calcium flux and for PKCδ- and CARD9-dependent cytokine generation (6, 45, 46). Moreover, our data demonstrate that inhibition of PI3Kδ impairs PKCδ phosphorylation elicited by Df. Taken together, these studies indicate that PI3Kδ is a proximal mediator of Dectin-2-dependent arachidonate metabolism and cytokine generation and likely acts by triggering activation of PLCγ.
We found that Dectin-2, PI3Kδ, and CARD9 were required for Df-elicited IL-33 generation in DCs. IL-33 generation in antigen-presenting cells can be induced by multiple stimuli such as influenza virus (18), Nippostrongylus Brasiliensis (47), Histoplasma capsulatum (48), and TLR agonists (49), and prior studies have demonstrated roles for both interferon regulatory factors (IRFs) and NF-κB in the transcriptional regulation of IL-33. IRF3 is required for TLR3 and TLR4-induced IL-33 in murine macrophages (49), and IRF4 is required for both Dectin-1 and Dectin-2-induced IL-33 in macrophages and DCs, respectively (48, 50). However, NF-κB is required for TLR5-induced IL-33 generation from murine DCs (51) and for TLR3 and TLR5-induced IL-33 from human epithelial cells (52). In keeping with these findings, our results show that Df-induced IL-33 generation is reduced in Card9−/− BMDCs but more significantly attenuated with PI3Kδ inhibition, suggesting that PI3Kδ signaling can promote IL-33 induction through both CARD9-dependent NF-κB activation and CARD9-independent IRF4 activation.
We and others have identified a role for Dectin-2 in the generation of both Th2 and Th17-dependent allergic pulmonary inflammation to HDM (14, 15, 19, 50). Here we demonstrate that PI3Kδ signaling in DCs is required for this function, as the adoptive transfer of PI3Kδ-inhibited Df-DC had a reduced capacity to generate Th2 and Th17-dependent allergic pulmonary inflammation. We have previously demonstrated a role for the Dectin-2/LTC4S pathway in the CysLT1R-dependent conditioning of DCs to promote Th2 sensitization (14), but our findings here suggest that an additional Dectin-2 mediator, IL-33, may participate in this process. IL-33 promotes Th2 immunity through several actions including the upregulation of DC costimulatory molecules and the polarization of naive T cells to Th2 (18). Although IL-33 is widely expressed (18), it is bound and neutralized by circulating soluble ST2 (18), suggesting that anatomic and temporal control of IL-33 generation may regulate its function and that the DC-specific generation of IL-33 in response to allergen may contribute to local polarization in the lymph node.
We found that inhibition of PI3Kδ solely on the day of Df sensitization prevented allergic pulmonary inflammation elicited by Df challenge 2 weeks later. Because of the short half life of the PI3Kδ inhibitor used (53), the results of PI3Kδ inhibition in vivo are likely due to proximal effects on DC-mediated sensitization which occurs over the first 24 - 72 h (54), but additional effects on the activation of T cells or B cells cannot be excluded. Several previous studies have demonstrated a role for PI3Kδ in the effector phase of antigen-induced allergic pulmonary inflammation. In these studies, the airway administration of a PI3Kδ inhibitor during the OVA challenge of previously sensitized mice reduced BAL eosinophilia, Th2 and Th17 lung cytokine production, airway hyperresponsiveness, and OVA-specific IgE (29, 34). Furthermore, PI3KδD910A/D910A mice, which express a catalytically inactive form of PI3Kδ, were also protected from BAL eosinophilia and Th2 cytokine production in models of Th2 pulmonary inflammation using sensitization and challenge to OVA (55) or to uric acid (56). Although the current study demonstrates a key role for PI3Kδ in DC function during the sensitization phase, the same Dectin-2 pathway contributes to pulmonary inflammation in effector phase responses (33). Thus, use of a well-tolerated and highly-specific PI3Kδ inhibitor such as CAL-101 (also known as idelalisib), with recently reported efficacy in lymphoma (57), may be a rational therapeutic strategy to prevent the mucosal activation of both lymphoid and myeloid cells that participate in type 2 pulmonary inflammation.
Supplementary Material
Acknowledgements
We thank K. Frank Austen for his critical reading of the manuscript; Karl Nocka and Peter Symanowicz for providing reagents, and Li Li and Juying Lai for technical assistance.
This work was supported by National Institutes of Health Grants T32 AI007306-27, U19 AI095219, R21 AI107425, R01 HL120952, K08 AI 080948, and by generous contributions from the Vinik Family and the Kaye Family.
The abbreviations used in this paper
- Akt
protein kinase B
- BAL
bronchoalveolar lavage
- BMDC
bone marrow-derived dendritic cell
- CARD9
caspase-associated recruitment domain
- CLR
C-type lectin receptor
- cys-LT
cysteinyl leukotriene
- DC
dendritic cell
- Df
extracts from Dermatophagoides farinae
- Dp
extracts from Dermatophagoides pteronyssinus
- EGFP
enhanced green fluorescent protein
- FcRγ
Fc receptor gamma-chain
- HDM
house dust mite
- IRFs
interferon regulatory factors
- LAB/NTAL
Linker for Activation of B cells/Non-T cell Activating Linker; Linker of Activated T cells
- LT
leukotriene
- LTC4S
leukotriene C4 synthase
- Malt1
Mucosa-associated lymphoid tissue lymphoma translocation protein 1
- MDLN
mediastinal lymph node
- PAS
Periodic acid-Schiff
- PH domain
plekstrin homology domain
- PLCγ
phospholipase Cγ
- PRR
pattern recognition receptor
- ST2
IL-1 receptor like 1
- Syk
spleen tyrosine kinase
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Reis e Sousa C. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng Z, Ma S, Zhou H, Zang A, Fang Y, Li T, Shi H, Liu M, Du M, Taylor PR, Zhu HH, Chen J, Meng G, Li F, Chen C, Zhang Y, Jia XM, Lin X, Zhang X, Pearlman E, Li X, Feng GS, Xiao H. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal T17 responses. Nat Immunol. 2015 doi: 10.1038/ni.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD, Jr., Ariizumi K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 6.Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, Brown GD, Leitges M, Ruland J. Syk kinase-coupled C-type lectin receptors engage protein kinase C-delta to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi L, Gojestani S, Wu W, Hsu YM, Zhu J, Ariizumi K, Lin X. CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans. J Biol Chem. 2010;285:25969–25977. doi: 10.1074/jbc.M110.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gringhuis SI, Wevers BA, Kaptein TM, van Capel TMM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TBH. Selective C-Rel Activation via Malt1 Controls Anti-Fungal Th17 Immunity by Dectin-1 and Dectin-2. PLoS Pathog. 2011;7:e1001259. doi: 10.1371/journal.ppat.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brock TG, McNish RW, Peters-Golden M. Translocation and leukotriene synthetic capacity of nuclear 5-lipoxygenase in rat basophilic leukemia cells and alveolar macrophages. J Biol Chem. 1995;270:21652–21658. doi: 10.1074/jbc.270.37.21652. [DOI] [PubMed] [Google Scholar]
- 10.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 11.Lam BK, Penrose JF, Freeman GJ, Austen KF. Expression cloning of a cDNA for human leukotriene C4 synthase, an integral membrane protein conjugating reduced glutathione to leukotriene A4. Proc Natl Acad Sci U S A. 1994;91:7663–7667. doi: 10.1073/pnas.91.16.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett NA, Maekawa A, Rahman OM, Austen KF, Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suram S, Gangelhoff TA, Taylor PR, Rosas M, Brown GD, Bonventre JV, Akira S, Uematsu S, Williams DL, Murphy RC, Leslie CC. Pathways regulating cytosolic phospholipase A2 activation and eicosanoid production in macrophages by Candida albicans. J Biol Chem. 2010;285:30676–30685. doi: 10.1074/jbc.M110.143800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, Kanaoka Y. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke DL, Davis NH, Campion CL, Foster ML, Heasman SC, Lewis AR, Anderson IK, Corkill DJ, Sleeman MA, May RD, Robinson MJ. Dectin-2 sensing of house dust mite is critical for the initiation of airway inflammation. Mucosal Immunol. 2014;7:558–567. doi: 10.1038/mi.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norimoto A, Hirose K, Iwata A, Tamachi T, Yokota M, Takahashi K, Saijo S, Iwakura Y, Nakajima H. Dectin-2 promotes house dust mite-induced T helper type 2 and type 17 cell differentiation and allergic airway inflammation in mice. Am J Respir Cell Mol Biol. 2014;51:201–209. doi: 10.1165/rcmb.2013-0522OC. [DOI] [PubMed] [Google Scholar]
- 17.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131:187–200. e181–188. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Makrinioti H, Toussaint M, Jackson DJ, Walton RP, Johnston SL. Role of interleukin 33 in respiratory allergy and asthma. Lancet Respir Med. 2014;2:226–237. doi: 10.1016/S2213-2600(13)70261-3. [DOI] [PubMed] [Google Scholar]
- 19.Tjota MY, Hrusch CL, Blaine KM, Williams JW, Barrett NA, Sperling AI. Signaling through FcRgamma-associated receptors on dendritic cells drives IL-33-dependent TH2-type responses. J Allergy Clin Immunol. 2014;134:706–713. e708. doi: 10.1016/j.jaci.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 21.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 22.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 23.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 24.Kuehn HS, Swindle EJ, Kim MS, Beaven MA, Metcalfe DD, Gilfillan AM. The phosphoinositide 3-kinase-dependent activation of Btk is required for optimal eicosanoid production and generation of reactive oxygen species in antigen- stimulated mast cells. J Immunol. 2008;181:7706–7712. doi: 10.4049/jimmunol.181.11.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated Zymosan-induced Peritoneal Vascular Permeability and IgE-dependent Passive Cutaneous Anaphylaxis in Mice Lacking Leukotriene C4 Synthase. J. Biol. Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 26.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin X. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 27.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 28.Barrett NA, Fernandez JM, Maekawa A, Xing W, Li L, Parsons MW, Austen KF, Kanaoka Y. Cysteinyl Leukotriene 2 Receptor on Dendritic Cells Negatively Regulates Ligand-Dependent Allergic Pulmonary Inflammation. J Immunol. 2012;189:4556–4565. doi: 10.4049/jimmunol.1201865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J. 2006;20:455–465. doi: 10.1096/fj.05-5045com. [DOI] [PubMed] [Google Scholar]
- 30.Ali K, Camps M, Pearce WP, Ji H, Ruckle T, Kuehn N, Pasquali C, Chabert C, Rommel C, Vanhaesebroeck B. Isoform-specific functions of phosphoinositide 3-kinases: p110 delta but not p110 gamma promotes optimal allergic responses in vivo. J Immunol. 2008;180:2538–2544. doi: 10.4049/jimmunol.180.4.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leverrier Y, Okkenhaug K, Sawyer C, Bilancio A, Vanhaesebroeck B, Ridley AJ. Class I phosphoinositide 3-kinase p110beta is required for apoptotic cell and Fcgamma receptor-mediated phagocytosis by macrophages. J Biol Chem. 2003;278:38437–38442. doi: 10.1074/jbc.M306649200. [DOI] [PubMed] [Google Scholar]
- 32.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, Sturgeon SA, Prabaharan H, Thompson PE, Smith GD, Shepherd PR, Daniele N, Kulkarni S, Abbott B, Saylik D, Jones C, Lu L, Giuliano S, Hughan SC, Angus JA, Robertson AD, Salem HH. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 33.Parsons MW, Li L, Wallace AM, Lee MJ, Katz HR, Fernandez JM, Saijo S, Iwakura Y, Austen KF, Kanaoka Y, Barrett NA. Dectin-2 regulates the effector phase of house dust mite-elicited pulmonary inflammation independently from its role in sensitization. J Immunol. 2014;192:1361–1371. doi: 10.4049/jimmunol.1301809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SJ, Lee KS, Kim SR, Min KH, Moon H, Lee MH, Chung CR, Han HJ, Puri KD, Lee YC. Phosphoinositide 3-kinase delta inhibitor suppresses interleukin-17 expression in a murine asthma model. Eur Respir J. 2010;36:1448–1459. doi: 10.1183/09031936.00106609. [DOI] [PubMed] [Google Scholar]
- 35.Tuveson DA, Carter RH, Soltoff SP, Fearon DT. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993;260:986–989. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- 36.Okada T, Maeda A, Iwamatsu A, Gotoh K, Kurosaki T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 2000;13:817–827. doi: 10.1016/s1074-7613(00)00079-0. [DOI] [PubMed] [Google Scholar]
- 37.Bruyns E, Marie-Cardine A, Kirchgessner H, Sagolla K, Shevchenko A, Mann M, Autschbach F, Bensussan A, Meuer S, Schraven B. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR CD3-zeta complex, recruits intracellular signaling proteins to the plasma membrane. J Exp Med. 1998;188:561–575. doi: 10.1084/jem.188.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shim EK, Moon CS, Lee GY, Ha YJ, Chae SK, Lee JR. Association of the Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD (SLP-76) with the p85 subunit of phosphoinositide 3-kinase. FEBS Lett. 2004;575:35–40. doi: 10.1016/j.febslet.2004.07.090. [DOI] [PubMed] [Google Scholar]
- 39.Shim EK, Jung SH, Lee JR. Role of two adaptor molecules SLP-76 and LAT in the PI3K signaling pathway in activated T cells. J Immunol. 2011;186:2926–2935. doi: 10.4049/jimmunol.1001785. [DOI] [PubMed] [Google Scholar]
- 40.Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X. C-Type Lectin Receptors Dectin-3 and Dectin-2 Form a Heterodimeric Pattern- Recognition Receptor for Host Defense against Fungal Infection. Immunity. 2013;39:324–334. doi: 10.1016/j.immuni.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Orr SJ, Burg AR, Chan T, Quigley L, Jones GW, Ford JW, Hodge D, Razzook C, Sarhan J, Jones YL, Whittaker GC, Boelte KC, Lyakh L, Cardone M, O'Connor GM, Tan C, Li H, Anderson SK, Jones SA, Zhang W, Taylor PR, Trinchieri G, McVicar DW. LAB/NTAL facilitates fungal/PAMP-induced IL-12 and IFN-gamma production by repressing beta-catenin activation in dendritic cells. PLoS Pathog. 2013;9:e1003357. doi: 10.1371/journal.ppat.1003357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buczynski MW, Stephens DL, Bowers-Gentry RC, Grkovich A, Deems RA, Dennis EA. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem. 2007;282:22834–22847. doi: 10.1074/jbc.M701831200. [DOI] [PubMed] [Google Scholar]
- 43.Kurosaki T, Tsukada S. BLNK: connecting Syk and Btk to calcium signals. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- 44.Cho SH, Woo CH, Yoon SB, Kim JH. Protein kinase Cdelta functions downstream of Ca2+ mobilization in FcepsilonRI signaling to degranulation in mast cells. J Allergy Clin Immunol. 2004;114:1085–1092. doi: 10.1016/j.jaci.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 45.Xu S, Huo J, Lee KG, Kurosaki T, Lam KP. Phospholipase Cgamma2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem. 2009;284:7038–7046. doi: 10.1074/jbc.M806650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorjestani S, Yu M, Tang B, Zhang D, Wang D, Lin X. Phospholipase Cgamma2 (PLCgamma2) is key component in Dectin-2 signaling pathway, mediating anti-fungal innate immune responses. J Biol Chem. 2011;286:43651–43659. doi: 10.1074/jbc.M111.307389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, Lewis L, Finkelman FD, Smith DE, Bryce PJ, Kurt-Jones EA, Wang TC, Sivaprasad U, Hershey GK, Herbert DR. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209:607–622. doi: 10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verma A, Kroetz DN, Tweedle JL, Deepe GS., Jr. Type II cytokines impair host defense against an intracellular fungal pathogen by amplifying macrophage generation of IL-33. Mucosal Immunol. 2014;8:380–389. doi: 10.1038/mi.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polumuri SK, Jayakar GG, Shirey KA, Roberts ZJ, Perkins DJ, Pitha PM, Vogel SN. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J Immunol. 2012;189:50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, Sciammas R, Sperling AI. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su Z, Lin J, Lu F, Zhang X, Zhang L, Gandhi NB, de Paiva CS, Pflugfelder SC, Li DQ. Potential autocrine regulation of interleukin-33/ST2 signaling of dendritic cells in allergic inflammation. Mucosal Immunol. 2013;6:921–930. doi: 10.1038/mi.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Lu R, Zhao G, Pflugfelder SC, Li DQ. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. Int J Biochem Cell Biol. 2011;43:1383–1391. doi: 10.1016/j.biocel.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thappali SR, Varanasi KV, Veeraraghavan S, Vakkalanka SK, Mukkanti K. Simultaneous quantitation of IC87114, roflumilast and its active metabolite roflumilast N-oxide in plasma by LC-MS/MS: application for a pharmacokinetic study. J Mass Spectrom. 2012;47:1612–1619. doi: 10.1002/jms.3103. [DOI] [PubMed] [Google Scholar]
- 54.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Nashed BF, Zhang T, Al-Alwan M, Srinivasan G, Halayko AJ, Okkenhaug K, Vanhaesebroeck B, Hayglass KT, Marshall AJ. Role of the phosphoinositide 3-kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol. 2007;37:416–424. doi: 10.1002/eji.200636401. [DOI] [PubMed] [Google Scholar]
- 56.Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, Rogers N, Osorio F, Reis e Sousa C, Hammad H, Lambrecht BN. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Fruman DA, Cantley LC. Idelalisib--a PI3Kdelta inhibitor for B-cell cancers. N Engl J Med. 2014;370:1061–1062. doi: 10.1056/NEJMe1400055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.