Abstract
Animals vary in their sensitivities to different wavelengths of light. Sensitivity differences can have fitness implications in terms of animals’ ability to forage, find mates and avoid predators. As a result, visual systems are likely selected to operate in particular lighting environments and for specific visual tasks. This review focuses on cichlid vision, as cichlids have diverse visual sensitivities, and considerable progress has been made in determining the genetic basis for this variation. We describe both the proximate and ultimate mechanisms shaping cichlid visual diversity using the structure of Tinbergen’s four questions. We describe 1) the molecular mechanisms that tune visual sensitivities including changes in opsin sequence and expression; 2) the evolutionary history of visual sensitivity across the African cichlid flocks; 3) the ontological changes in visual sensitivity and how modifying this developmental program alters sensitivities among species; and 4) the fitness benefits of spectral tuning mechanisms with respect to survival and mating success. We further discuss progress to unravel the gene regulatory networks controlling opsin expression and suggest that a simple genetic architecture contributes to the lability of opsin gene expression. Finally, we identify unanswered questions including whether visual sensitivities are experiencing selection, and whether similar spectral tuning mechanisms shape visual sensitivities of other fishes.
Keywords: vision, opsin, gene expression, retina
1. Introduction
Organismal phenotypes can vary widely, with diversity accumulating through processes such as neutral drift or adaptation. Understanding diversity is particularly challenging because scientists often do not understand the genetic mechanisms underlying traits. Therefore it is difficult to weigh the possible forces that might be acting to create or limit diversity. There are a few traits where progress has been made to unravel their genetic basis including color patterns (Keys et al. 1999, Brunetti et al. 2001, Nachman et al. 2003, Prud'homme et al. 2006, Manceau et al. 2011) and morphology (Peichel et al. 2001, Frankel et al. 2012). Evolution at the molecular level results from many different processes, including changes in coding sequence and gene expression. The early evolutionary synthesis focused on coding sequence variation, but they realized that changes in gene expression could also shape phenotypic variation between closely related species (Jacob and Monod 1961, Britten and Davidson 1969, King and Wilson 1975). As studies have continued, there has been much debate about the mechanisms that control phenotypic variation and the relative importance of coding sequence changes versus altering gene expression (reviewed in Hoekstra and Coyne 2007, Wray 2007, Carroll 2008, Stern and Orgogozo 2008). The relative importance of different mechanisms may depend on the type of trait, as changes in morphology appear driven largely by cis-regulation changes that alter gene expression (Carroll 2008). However, every gene functions within a larger regulatory network, and its connectivity and placement in the network influences how it evolves (Stern and Orgogozo 2009, Olson-Manning et al. 2012). To continue assessing the relative importance of these different molecular mechanisms, we need studies focused on a greater diversity of traits to see where the commonalities lie.
In this review, we address the evolution of sensory systems and in particular the visual sensitivities of fishes. The visual system is an ideal model for studying the evolution of diversity, on both short and long time scales. First, there is a tight link between genotype and phenotype (Yokoyama 2008). The spectral sensitivity underlying the neural response of a retinal photoreceptor is determined by the visual pigment it contains (Ebrey and Koutalos 2001). Visual pigments are composed of opsin proteins bound to a light absorbing chromophore (typically 11-cis retinal). The amino acids of the opsin protein set the absorption spectrum of the visual pigment through their interactions with the chromophore. Changes in opsin sequence can alter the peak wavelengths absorbed by the visual pigment (Yokoyama 2008). Opsins are under strong selection to maximize object detection and discrimination in particular environments (reviewed in Horth 2007, Davies et al. 2012). Therefore adaptation of opsins to ecological niches is likely. Second, changes in gene expression can modify the photoreceptor opsin compliment over time, both within existing and new retinal cells. A portion of the photoreceptor outer segments are replenished each day (Young 1978). In addition, fish retinas continually grow, generating new photoreceptors as eye size increases (Fernald 1990). Third, the retina’s spectral sensitivity can be modulated by the environment, which could lead to short term spectral shifts as light environments change (Munz and McFarland 1977). These plastic responses might occur because of variation in water quality (e.g. from increased rainfall or land runoff) or shifts in environmental spectra with day length or season.
Cichlids fishes are a great model for examining visual sensitivities and spectral tuning mechanisms. Cichlids are a highly diverse group of freshwater fishes, occupying different light environments in a Gondwanan distribution around the globe from South and Central America to Africa, Madagascar, and India (Kocher 2004, Seehausen 2015). However, much of their diversity is located in the Great Lakes of Africa. Estimates suggest 500 species of cichlids have evolved in Lake Malawi in the last few million years (Wagner et al. 2014), with another 500 species in Lake Victoria (Seehausen 1996), and several hundred in Lake Tanganyika (Salzburger et al. 2005). There is substantial variation in the light environments both within and between these lakes. Because cichlids within each of the lakes are endemic to that basin, these replicate radiations can be compared to see how the visual system evolved in parallel from the common riverine ancestors (Genner et al. 2007). Studies comparing large numbers of cichlid species with new methods to characterize visual sensitivity have identified several ways, some of them novel, in which visual systems can vary. These include differential gene expression, coexpression, intraretinal variation in spectral sensitivity, and covariation of the lens and visual pigments. Studies of cichlids have also added to our understanding of the ecological factors that shape visual sensitivities. As visual sensitivities are examined in other taxa, we will learn whether the molecular mechanisms and ecological impacts identified in cichlids occur more broadly.
In describing cichlid visual sensitivities, we utilize a framework based on the four questions that Nikolaas Tinbergen developed to further the study of animal behavior (Tinbergen 1963). In celebrating the fiftieth anniversary of Tinbergen’s paper, Bateson and Laland (2013) pointed out that these questions are applicable to many different organismal traits in addition to behavior. Nesse (2013) organized these four questions according to whether they addressed proximate versus evolutionary mechanisms and whether they addressed recent versus historical forces (Table 1). These four questions then become: 1) What is the structure of the trait and how does it work? 2) What is the phylogenetic history of the trait? 3) How does the trait develop in an individual? 4) How does fitness, through interactions with the environment, influence and help to explain the trait’s form? In this review, we characterize cichlid vision with an emphasis on spectral sensitivity in the context of these four questions. This helps us interrelate what we have learned with regards to how visual sensitivities vary, how they have evolved, and how they are related to cichlid fitness, diversity and speciation. We also identify questions raised by this work that currently remain unanswered.
Table 1.
Tinbergen’s four questions for understanding trait diversity (after Nesse 2013). The corresponding questions for understanding cichlid visual sensitivity are given.
| What to explain | |||
|---|---|---|---|
| Trait | Trait sequence through time | ||
| How to explain it | Proximate |
Mechanism: What is the trait’s structure? How does it work? Section 2: How do changes in gene sequence, gene expression and coexpression contribute to shaping cichlid visual sensitivities? |
Ontogeny: How does the trait develop in individuals? Section 4: How is the developmental pattern altered to generate cichlid visual diversity? |
| Ultimate |
Adaptive significance: How does the trait influence fitness that helps explain its form? Section 5: What role does cichlid visual tuning play in shaping cichlid diversity and cichlid species? |
Phylogeny: How does the trait vary through evolutionary time Section 3: How variable are cichlid tuning mechanisms across populations, species, genera and lakes? |
|
2. Tinbergen question 1: What is the structure of the trait and how does it work?
Vision begins when the eye detects light. In the natural environment, this light is emitted by the sun, transmitted through the environment (air and /or water), reflected from an object, and transmitted through the environment again before reaching a receiver. The light then passes through the ocular media, including the cornea and lens, and is finally absorbed by the photoreceptors of the retina. The photoreceptors contain visual pigments that preferentially absorb light of different wavelengths. Visual sensitivity can change in several ways. These include altering the transmission of the ocular media, thereby biasing which wavelengths reach the retina, and shifting the absorption spectra of the photoreceptors. The signals detected by the photoreceptors are neurally processed, both in the retina and in the brain. This processing can also modify visual sensitivity before the brain perceives an object.
2.1 The eye and ocular media
As light passes through the cornea, lens, and vitreous humor, pigments located in these optical elements can filter out certain wavelengths and limit visual sensitivity (Siebeck and Marshall 2001). These pigments typically act as long pass filters, absorbing ultraviolet and other short wavelengths of light. New World cichlids often have yellow corneas and lenses, significantly reducing the proportion of shorter wavelength light that reaches the retina (Muntz 1973, Muntz 1982). Since Amazonian waters are high in turbidity and preferentially transmit longer wavelengths, fish lenses may have adapted to block short wavelength light that would be highly scattered and reduce object visibility. By comparison, African cichlids have broadly transmissive corneas, lenses and vitreous humors with no obvious coloration (Hofmann et al. 2010a, O'Quin et al. 2010). African cichlids do vary in lens transmission at the shortest wavelengths (Fig. 1). Cichlid lenses are known to contain either of two pigments, palythine, with a 320 nm peak absorption and palythene, with a 360 nm peak absorption (Thorpe and Douglas 1993, Thorpe et al. 1993). Different concentrations of these pigments alter the rising edge of the transmission spectrum, as characterized by the lens T50 (the wavelength where 50% of the light is transmitted). Lenses of eight species from Lake Victoria contained both pigments and therefore had a longer T50 than two species from Lake Tanganyika, which only had the palythine pigment (Thorpe et al. 1993). Hofmann et al (2010a) studied 65 species from Lake Malawi and found two kinds of lenses. Either the lens transmitted ultraviolet light (UV), having a lens T50 less than 370 nm, or the lens blocked UV light with a T50 above 390 nm. Examining 13 species from Lake Tanganyika, O’Quin et al (2010) found not only UV-transmitting species and UV-blocking species, but several intermediates with T50 in the range of 370–385 nm. Therefore, these Tanganyikan species likely vary in the amount of the 320 and 360 nm absorbing pigments identified by Thorpe et al (1993). Comparisons between lens transmission and photoreceptor sensitivities suggest that because lens transmission can limit short wavelength light reaching the retina, retinal sensitivity is often correlated with lens transmission (see section 2.3.2).
Figure 1.
Lens transmission properties. a) Lens transmission spectra from a typical UV transmitting lens (Mchenga eucinostomus; T50= 355 nm) and a UV blocking lens (Trematocranus placodon; T50=394 nm). b) Distribution of lens T50 for 63 species examined from Lake Malawi (Hofmann et al 2010) and 13 species examined from Lake Tanganyika (O’Quin et al 2010).
Fish lenses are typically spherical. Since the cornea provides little focusing power in an aquatic environment, fish obtain high focusing power with spherical lenses having a high index of refraction (Sivak 2004, Kroger 2013). However, rays of light passing through the periphery and center of a spherical lens focus at different distances, resulting in spherical aberration. To prevent this aberration, cichlids (like other aquatic organisms) use a graded index lens in which the index of refraction is higher at the center and lower towards the periphery (Fernald and Wright 1985, Kroger et al. 1994). An additional optical problem results from the lens’s index of refraction changing with wavelength. Shorter wavelengths are focused closer to the lens, producing chromatic aberration (Kroger et al. 1999). Fishes, including cichlids, compensate by having multifocal lenses, where certain wavelengths are focused by more central parts of the lens and other wavelengths are focused more peripherally (Karpestam et al. 2007, Gagnon et al. 2012).
2.2. Retinal photoreceptors
The cichlid retina is composed of rods for dim light vision and cones for seeing in daylight (Fernald and Liebman 1980). Cones are of two morphological types, double cones, which are two cone cells joined along their inner segments, and single cones. These are arranged in a highly organized pattern. Most species have a square cone mosaic where four pairs of double cones surround each single cone (Fernald 1981, Braekevelt et al. 1998, Dalton et al. 2014). Photoreceptor spectral sensitivity can be characterized by using microspectrophotometry (MSP) to measure the wavelength of maximal absorbance (λmax). Since the early work of Fernald and Liebman (1980) MSP studies have found that cichlids typically have three spectral classes of cones and one rod (reviewed in Carleton 2009). There is significant diversity in both single and double cone pigments with λmax from the ultraviolet to the red end of the spectrum (Parry et al. 2005, Jordan et al. 2006). These MSP studies identified three common combinations of visual pigments in the cone cells (Fig. 2). This includes a UV sensitive single cone with a double cone consisting of one blue-green and one green sensitive member; a violet sensitive single cone with a double cone consisting of the same blue-green and green sensitive members; and a blue sensitive single cone with green and a red sensitive double cones. Figure 2A shows the rather narrow range of λmax found by MSP for species having each of these three palettes. Double cones do show some variation with opposite members sometimes expressing the same pigments, particularly for the blue/ green/ red palette. Both green/green and red/red wavelength sensitive double cones have been observed. The number and distribution of these twin cones varies within species sampled from different habitats as well as spatially (dorsal /ventral) within individual retina (Levine et al. 1979, Carleton et al. 2005)
Figure 2.
a) Typical cichlid visual pigment combinations including the short (top) medium (middle) and long (bottom) wavelength-sensitive palettes. Visual pigment templates calculated using method of Govordovskii et al 2000. Points and error bars are peak absorption determined by MSP giving the mean +/− 2 S.D. for cichlid species from Lakes Malawi, Tanganyika and Victoria (data from Carleton 2009). b) Opsin gene expression as determined by quantitative PCR for species from these three visual palettes including Metriaclima zebra (top), Melanochromis vermivorous (middle), and Trematocranus placodon (bottom). These correspond to expression of gene sets: SWS1, RH2B, and RH2A (short palette), SW2B, RH2B, and RH2A (medium palette) or SWS2A, RH2A, and LWS (long palette). Here gene expression of the RH2A class includes expression of both RH2Aα and β. A typical retinal mosaic for each species is shown in the insets.
2.3 Visual pigment tuning mechanisms
Cichlid visual systems are based on seven different cone opsin genes plus a rod opsin gene, RH1 (Parry et al. 2005, Spady et al. 2006, O'Quin et al. 2011b). The cone opsin genes are members of the four vertebrate cone opsin classes, very short wavelength sensitive (SWS1), short wavelength sensitive (SWS2A, SWS2B), rhodopsin like (RH2Aα, RH2Aβ and RH2B) and long wavelength sensitive (LWS). These occur in just a few genomic locations. The SWS1 gene is located on LG17 while the other six cone opsin genes form two separate tandem arrays on LG5 separated by more than 16 Mb (Figure 3; O'Quin et al. 2011b). One array contains the SWS2A – SWS2B – LWS genes while the second contains the RH2B – RH2Aα– RH2Aβ genes. The SWS2A/B and RH2A/B gene pairs are old gene duplicates that each arose over 100 MY ago (Carleton and Kocher 2001, Parry et al. 2005) and are shared with other Acanthopterygians including medaka (Matsumoto et al. 2006) but differ from gene duplications in Ostaryophysians, such as zebrafish (Chinen et al. 2003). The RH2A α and β gene duplication is more recent (20 MY) and likely cichlid specific. However, it is also possible that this pair of genes is the result of a more ancient duplication followed by a recent gene conversion. Recent reviews confirm the relatively frequent duplication of fish opsin genes (Hofmann and Carleton 2009, Rennison et al. 2012) as well as the prevalence of gene conversion (Cortesi et al. 2015). This supports the idea that gene duplications followed by sequence divergence produced the genes necessary to make seven unique cichlid visual pigments, covering a broad spectral range from ultraviolet to red wavelengths (Fig. 3). The lambda;max has been confirmed by expression in HEK cells for all seven cone opsin genes from the riverine cichlid tilapia, Oreochromis niloticus (Spady et al. 2006) and five of the genes for the Lake Malawi rock dweller cichlid, M. zebra (Parry et al. 2005).
Figure 3.
Cichlid cone opsin genes. a) The visual pigment spectral sensitivities are shown with peak sensitivities as determined through protein expression for Oreochromis niloticus (Spady et al 2006). b) The seven cone opsin genes occur in three locations in the cichlid genome. This includes six of the cone opsin genes in two tandem arrays on LG5 and the SWS1 gene is on LG 17.
Cichlid spectral sensitivities are tuned by several different mechanisms. These include two that were first documented in cichlids: differential expression of the set of opsin genes by different species, and coexpression of multiple opsins in single photoreceptors (Carleton and Kocher 2001, Dalton et al. 2014). Cichlid spectral sensitivities also vary as a result of changes in opsin sequence, the gain or loss of opsin genes, and changes in chromophore. These five different tuning mechanisms are discussed below (sections 2.3.1–2.3.5).
2.3.1 Opsin sequence tuning
The first studies of opsin genes in cichlids from Lakes Malawi and Victoria suggested that their opsin sequences are relatively conserved as might be expected for species less than 1 MY old (Carleton and Kocher 2001, Terai et al. 2002, Carleton et al. 2005). MSP and protein expression suggest that homologous genes produce visual pigments with similar sensitivities, even when considering the more divergent O. niloticus (Fig. 2A; summarized in Table 1 of Carleton 2009)). Preliminarily, this suggests that opsin sequence variation has small effect on spectral tuning. There are a few exceptions, including variation in the LWS opsin in Lake Victorian cichlids (Carleton et al. 2005, Terai et al. 2006) and the SWS1 gene in Lake Malawi species (Smith and Carleton 2010) (see section 3.1). Changes of single amino acids in these opsin classes produce fine scale λmax shifts of 2–15 nm. As discussed in section 5, these spectral shifts are likely important for adapting to different light environments and may ultimately lead to cichlid speciation. However, sequence changes do not explain the most substantial visual pigment differences (30–90 nm spectral shifts) observed between different cichlid species.
2.3.2 Opsin gene expression
MSP studies of cichlid visual pigments suggest that individual species vary widely in their single and double cone λmax (Levine et al. 1979, Fernald and Liebman 1980, van der Meer and Bowmaker 1995, Carleton et al. 2005, Parry et al. 2005, Jordan et al. 2006). Since cichlids have seven cone opsin genes, and sequences of each gene differ little between species, large sensitivity differences can only be explained by different species expressing distinct subsets of the seven cone opsin genes. This differential gene expression has been confirmed by quantitative reverse transcription PCR (qRT-PCR; Carleton and Kocher 2001). In Lakes Malawi and Tanganyika, there are three common combinations of highly expressed opsins, or visual palettes (Hofmann et al. 2009, O'Quin et al. 2010). These include the short (SWS1, RH2B, RH2A), medium (SWS2B, RH2B, RH2A) and long (SWS2A, RH2A, LWS) wavelength palettes (Fig. 2B). For much of this work, the RH2A α and β genes have been analyzed together as they are genetically so similar that the same qRT-PCR primers and probes detect them both (though in situ hybridization studies show they localize to different double cones; see section 2.3.3).
The expression of different cichlid opsin genes has been compared to the lens transmission properties for cichlids from both Lake Malawi and Lake Tanganyika (Hofmann et al. 2010b, O'Quin et al. 2010). These studies found that lens transmission matched opsin expression. If the retina contained UV sensitive opsins, the lens was UV transmitting. If UV opsins were absent, the lens was partially or entirely UV blocking. While there could be a genetic link between absorbance in the lens and cones, it is possible that lens pigments come from the fishes’ diet (Thorpe et al. 1993). Since diet is correlated with expression and λmax of SWS opsins (see section 5.2), it would provide a simple link for cone sensitivity and lens transmission. One observation supporting the link with diet is that if cichlid species having UV blocking lenses in the wild are raised in the lab on commercial food, they have UV transmissive lenses (personal observation). An alternate explanation might be that the more UV transmissive lenses are a response to lower UV light levels in the lab.
2.3.3 Opsin coexpression
In examining opsin expression from different species, it is clear that many species express more than just three cone opsin genes (Hofmann et al. 2009). Often these are expressed at low levels, being less than 5% of the total cone opsin (Fig. 2B), though in some species they are higher. How these additional genes are expressed could have profound implications for cichlid visual capabilities. If they are expressed alone in a cone, they could produce cones with unique spectral sensitivities, making the cichlid tetra- or even penta-chromatic. Evidence for unique cones was observed in some MSP studies. However, they found only one or two actual cones of each type, suggesting these cone types are quite rare (Parry et al. 2005). Pentachromacy was also suggested by electroretinography (ERG) experiments in cichlids (Sabbah et al. 2010). However, support is somewhat equivocal considering the low resolution of the ERG data and the large spectral overlap of cichlid visual pigments. Alternatively, these “extra” opsin genes could be coexpressed with the three primary cone opsin genes in a way that would shift the spectral sensitivity of the single and double cones. It’s not clear whether the spectral absorbance of the coexpressing and non-coexpressing cones would be distinct enough to make cichlids more than trichromatic.
In situ studies to test these alternative modes of expression found that coexpression is by far the more common fate for these extra opsin genes. Dalton et al (2014) studied expression of double cone genes in the Lake Malawi rock-dweller M. zebra. The sensitivities of the visual pigments corresponding to the M. zebra double cone genes are: 488 nm (RH2B), 518 nm (RH2Aβ), and 528 nm (RH2Aα) as determined by protein expression, (Parry et al. 2005) and 567 nm (LWS) as inferred from MSP of other Malawi species (Table 1 in Carleton 2009). Using dual labeling fluorescent in situ hybridization, Dalton et al (2014) found that all four of these genes occurred in double cones, and never in single cones. Previous MSP data suggested that M. zebra expressed just two double cone genes, RH2B and RH2Aα, each in opposite cells of a double cone pair. Dalton et al (2014) confirmed that this pair of genes was expressed in alternate double cone members 99% of the time. However, they found that in some individuals and in some parts of the retina, the other two double cone genes (RH2Aβ and LWS) could be coexpressed with this pair. This coexpression was not random, but rather involved genes that were spectrally nearest neighbors. This resulted in four combinations of opsins expressed in double cones: RH2B only, coexpressed RH2B/RH2Aβ, RH2Aα only, and coexpressed RH2Aα/LWS. There were very few pure RH2Aβ or LWS expressing cones, though such cones did occur, particularly in one individual.
Proof that coexpression affected protein complements and therefore cone sensitivity was confirmed using MSP. The λmax of pure RH2B expressing cones was 488 nm, while the coexpression of RH2Aβ with RH2B shifted the λmax to longer wavelengths, 498 nm for the average coexpressing cone. Similarly, coexpression shifted λmax from 528 nm for pure RH2Aα expressing cones out to 536 nm for cells coexpressing RH2Aα and LWS. Photobleaching was used to test whether two different pigments were present in the same cone. Photobleaching exposes individual cones to high levels of light, thereby converting the 11-cis retinal to all trans retinal and preventing further light absorption. By using a bleaching wavelength that targeted the longer wavelength pigment, these studies showed that this pigment could be separately bleached to shift the cell’s absorption back to that of the pure, shorter wavelength pigment. This demonstrated that there were two viable, spectrally distinct visual pigments contained in the same cone cell and that both contributed to absorption.
An additional finding of this work concerned the spatial distribution of coexpressed pigments, which varied across the retina. Coexpression of LWS occurred primarily in ventral but not in dorsal retina. RH2Aβ was coexpressed in the nasal retina and rarely in the temporal region. Coexpression of these extra genes therefore formed gradients along two orthogonal axes. The RH2AαLWS coexpression occurred in both lab-reared fish and in wild-caught fish in similar retinal locations. The RH2B/RH2Aβ coexpression was only observed in lab reared fish. However, RH2Aβ expression has been detected by qPCR in different wild populations of M. zebra.
2.3.4 Opsin gene loss and duplication
Another way that visual pigment diversity varies in cichlid fishes is through the loss of certain opsin genes. This can result if a particular gene acquires a nonsense mutation that makes it a pseudogene. Pseudogenes have been observed for all three SWS opsins in just one species from Lake Tanganyika (Spady et al. 2005). Nonsense mutations have also been observed for the SWS1, SWS2B, RH2B and RH2Aα genes in a few species from Lake Victoria (Miyagi et al. 2012). These genes may never be expressed and so become pseudogenized. This might occur when lake species adapt from their previous riverine environment, and subsequently acquire mutations in genes that no longer impact their fitness.
Opsin genes have also been lost in the Neotropical cichlid Crenicichla frenata (Weadick et al. 2012). This species lives in the clear rivers of Trinidad, which are relatively shallow. Studies of genomic DNA and expressed mRNA suggest C. frenata has lost the SWS1 gene and has a pseudogene for RH2B. Neotropical cichlids diverged from African cichlids over 80 MY ago. Both lineages appear to contain relatively recent RH2A duplicates, though C. frenata only expresses one of them. This may be the result of separate duplications, or a shared duplication followed by recent gene conversion events in each lineage. The net result is that C. frenata expresses four distinct cone opsin genes (SWS2B, SWS2A, RH2A, LWS) having lost two the seven typically found in African species.
There could be gains in opsin genes as well. Although no cichlids studied to date have duplications other than the previously described RH2A duplication, many species remain to be examined. A recent study suggests that duplications and losses of fish opsins occur quite frequently (Cortesi et al. 2015). Following the SWS2A/B split in Neoteleosts, this work found a second SWS2 duplication in Percomorph fishes. This SWS2A duplication produced two copies of the SWS2A gene, with these two copies then either retained or lost in different fish lineages. Cichlids have a pseudogene for this second SWS2A copy, retaining just a few exons approximately1.5 kb upstream of the functional SWS2A gene (Cortesi et al 2015).
2.3.5 Chromophore tuning
Fish, along with reptiles, are able to alter the chromophore that combines with the opsin protein to produce the visual pigment. In addition to synthesizing 11-cis retinal from vitamin A1, fish can utilize vitamin A2 to make the chromophore 3,4 didehydroretinal. Switching from an A1 to A2 derived chromophore shifts visual pigment absorbance to longer wavelengths (Harosi 1994, Parry and Bowmaker 2000). Chromophore switches typically require several weeks to occur and may be associated with seasonal day length or temperature changes (Munz and McFarland 1977). MSP data suggests that cichlids from clear Lake Malawi utilize 11-cis retinal (Carleton et al. 2000, Parry et al. 2005). This was supported by A1:A2 chromophore ratio measurements of 10:1 by high pressure liquid chromatography (Sugawara et al. 2005). However, Lake Victorian cichlids have been found to use A1 or A1/A2 mixtures (van der Meer 1995, Terai et al. 2006, Miyagi et al. 2012). MSP measurements of the long wavelength sensitive pigments had λmax of 595 nm or longer in some Victorian species. This suggests some contribution of a vitamin A2 derived chromophore, since the longest visual pigments based on A1 chromophore have peak sensitivities of 575 nm (Blatz and Liebman 1973). The riverine O. niloticus also uses A1/A2 chromophore mixtures with variation in A1/A2 ratios among lab reared individuals (Carleton et al. 2008).
3. Tinbergen question 2: What is the phylogenetic history of the trait?
Cichlid visual systems are diverse and data suggests that closely related species can differ in spectral sensitivity (Parry et al. 2005, Jordan et al. 2006). These differences could be the result of any of the five spectral tuning mechanisms, but little is known about coexpression or chromophore switching across this broad set of taxa. No significant phylogenetic differences have been found for gene duplications or losses, other than those described above. Therefore, we will focus on phylogenetic variation in opsin sequence and gene expression.
3.1 Variation in opsin sequences across cichlid lineages
Comparisons of opsin genes among Lake Malawi species identified significant sequence differences in both the LWS and SWS1 opsin genes (Hofmann et al. 2009). The LWS alleles likely cause spectral shifts, since the variable sites are the same as those found to cause 4–15nm shifts in Lake Victoria cichlids (Carleton et al. 2005, Terai et al. 2006). The SWS1 variation is also likely functional as sequence variants correspond to species that have different SWS1 λmax (Parry et al. 2005, Smith and Carleton 2010). These data identified two SWS1 variants, one with shorter wavelength (M. zebra: 368 nm) and the other with longer wavelength sensitivity (Pseudotropheus acei: 378 nm). Interestingly, phylogenetic analyses suggest that the longer wavelength SWS1 variant occurs in Lake Victoria and Lake Malawi and so is likely ancestral, while the shorter wavelength variant arose in the clear Lake Malawi waters (Smith and Carleton 2010).
Comparisons of cone opsin gene sequences from 17 cichlid species have found that the SWS1 and LWS genes are the most variable while the other opsins vary only slightly (Hofmann et al. 2009). While the SWS1 and LWS genes show evidence of functional variation, of the other five genes only SWS2B showed one variable site that might alter peak sensitivity. In cichlids from Lake Tanganyika, sequencing of SWS1 and LWS genes suggests that they might vary functionally in cichlids from this lake as well (O'Quin et al. 2010). Analysis of sites in the retinal binding pocket, determined by homology with sites in the bovine rhodopsin crystal structure (Palczewski et al. 2000), identified five potentially functional sites in SWS1, and one in the LWS gene.
Studies have found evidence for functional variation in the rod RH1 gene when comparing cichlids from lakes with different light environments as well as species that inhabit different depths (Sugawara et al. 2002, Spady et al. 2005). These results further suggest that parallel amino acid changes have occurred in both Lakes Malawi and Tanganyika as species adapt to live at depth (Sugawara et al. 2005, Nagai et al. 2011). Recent studies have examined sequence evolution of the RH1 gene in Neotropical cichlids. Though sequences vary, they are not as variable as those from the African lakes (Schott et al. 2014, Torres-Dowdall et al. 2015).
3.2 Phylogenetic variation in gene expression
African cichlid species show three common combinations of expressed opsins (Hofmann et al. 2009). As mentioned above, these result from expression of the short (SWS1, RH2B, RH2A), medium (SWS2B, RH2B, RH2A) and long (SWS2A, RH2A, LWS) wavelength palettes of opsin genes. However, these palettes are an oversimplification, because additional genes can also be expressed. To display this diversity, we use the fact that MSP and in situ hybridization show expression of SWS genes in single cones and the RH2 and LWS opsins in double cones. Therefore, gene expression for a large number of species can be displayed on a two-dimensional plot whose axes are defined by single cone (SC) and double cone (DC) opsin expression. For each cone type, we calculate the weighted gene expression as:
where fi is the relative expression of each opsin gene and Xi is a value that is assigned to each opsin gene based on their relative sensitivities. In previous papers, the Xi have been chosen as the λmax for the opsin genes from one particular species (e.g. Hofmann et al. 2009). Here, we use a more general form that does not require assumptions of actual λmax values, which might differ between species. In this case, the X values for SWS genes are set so that XSWS1 = 1, XSWS2B = 2 and XSWS2A = 3. This relative ordering agrees with the relative λmax values determined from protein expression values for tilapia: SWS1 λmax = 360 nm, SWS2B λmax = 425 nm, and SWS2A λmax = 456 nm (Spady et al. 2006). Therefore, single cone weighted expression (XSC) increases in proportion to the overall peak sensitivity of the single cones. Similar values are chosen for double cone opsin genes, XRH2B =1, XRH2A = 2 and XLWS = 3. Weighted gene expression makes sense, based on recent evidence that genes are coexpressed in double cones, suggesting opsin expression is a good proxy for overall cone sensitivity (Dalton et al. 2014). Since single cones also coexpress opsins (Dalton personal comm.), XSC is proportional to single cone sensitivity. For double cones, XDC is a bit more complex as it averages the sensitivities of the two double cone members. Figure 4 shows weighted gene expression for single and double cones in over 60 wild caught Lake Malawi species (Hofmann et al. 2009). Clustering analysis of opsin expression across these species produced three major clusters (dotted boxes) that have statistical support. These clusters roughly correspond to the short, medium and long visual palettes. Individual species are represented by different colored symbols according to whether they fall in the short (pink), medium (purple) or long (blue) visual palettes. Considerable variation is evident between and even within these palettes. For example, within the short palette some species express significant LWS, yielding a higher XDC, and some express more SWS2B, increasing XSC.
Figure 4.
Relative expression of opsin genes in single cones versus that in double cones for African cichlid species. For each species, the gene expression in single and double cones is calculated as a weighted average. For the genes expressed in single cones, 1= SWS1, 2=SWS2B and 3=SWS2A expression. For the genes expressed in double cones, 1=RH2B, 2=RH2A and 3=LWS. Here RH2Aα and β are grouped together as RH2A and the double cone expression is the average of the RH2 and LWS cone opsin genes. The three major clusters of opsin expression palettes are shown by dotted lines (short – pink, medium – violet and long – blue). The solid points show expression among 60 Lake Malawi cichlids, colored by palette. Variation for 28 cichlids from Lake Tanganyika (open circles) and 13 cichlids from Lake Victoria (red triangles) are also shown. Finally, the solid black circles show the location of the ideal palettes with only one cone opsin expression in each cone type: short (SWS1, RH2B, RH2A), medium (SWS2B, RH2B, RH2A) and long (SWS2A, RH2A, LWS).
Figure 4 also includes cichlids from Lakes Tanganyika and Victoria which further supports that gene expression is variable and roughly falls into three clusters. The data suggest that opsin expression is just as variable among Tanganyikan species (open circles) and overlaps with that from Lake Malawi (closed circles) (O'Quin et al. 2010). Tanganyika and Malawi are both clear lakes. It makes sense that all three palettes produce functional visual systems, since relatively broad light spectra are found in the clear water, especially in shallow habitat. In addition, there are two unusual opsin combinations expressed by individual species within Tanganyika. In the first, the shortest wavelength sensitive single cone opsin (SWS1) and the longer wavelength sensitive double cone opsins (RH2A/LWS) are expressed (upper left). This makes the single and double cones spectrally further apart than for the three normal palettes. For the second, the longest wavelength sensitive single cone opsin (SWS2A) is expressed in combination with the shorter wavelength sensitive double cone opsins (RH2B/RH2A; lower right). This spectrally compresses the single and double cone sensitivities. It is possible that these unique combinations are less prevalent and require longer evolutionary time to evolve, thereby only occurring in the older Tanganyikan lineages.
Cichlids from Lake Victoria differ quite markedly from those in Lakes Tanganyika and Malawi as all species express the long visual palette (shown in red in Figure 4). Lake Victoria is shallower and quite murky. Due to high chlorophyll concentrations and eutrophication, its waters transmit longer wavelengths best (Seehausen et al. 1997). Visual systems sensitive to longer wavelengths will better match the light environment. Thus, this supports the sensitivity hypothesis that visual systems evolve to match the background light (Munz and McFarland 1977, Loew 1995). Figure 5 shows the varying light spectra in a clear lake like Malawi versus a murkier lake like Victoria and how it can impact the visual palette. Since male signaling colors often evolve to best match the sensitivity of receivers, cichlid colors have also likely evolved differently in these two lakes (Dalton et al. 2010).
Figure 5.
a) A typical scene from Lake Malawi showing the relatively clear waters and downwelling light (Photo: Chris Hofmann). b) The light which penetrates different lakes depends on the spectral properties of the water. Clearer lakes transmit blue to green light best though all wavelengths are transmitted to some degree. Eutrophic lakes containing lots of chlorophyll transmit longer wavelengths best, though light transmission is reduced overall. The net result is cichlids in these different light environments have evolved to have different spectral sensitivities (visual opsin palettes) and to use different colors to communicate.
It is possible to consider at what phylogenetic scale these palettes vary if we use the most conservative assessment of expression variation to assign individual species to one of the three visual palettes. Figure 6 shows the variation of visual palettes mapped onto a taxonomic phylogeny for cichlids from all three great lakes as well as a few riverine lineages. Using ancestral state reconstruction, these data suggest that the riverine ancestor of the flocks most likely had the long palette (O'Quin et al. 2010), similar to the expression pattern of present day riverine species including O. niloticus and A. burtoni. This is also supported by MSP studies of other riverine and west African species (Levine et al. 1979). From this long wavelength sensitive ancestor, the short and medium palettes then evolved multiple times within the cichlid radiations of both Lakes Tanganyika and Malawi. Thus, there is probably considerable lability in opsin expression within cichlids, facilitating changes from one palette to another.
Figure 6.
Variation in opsin expression palette mapped onto a phylogeny. This shows variation among a subset of cichlids from Lake Malawi and Lake Victoria as well as all the Tanganyikan cichlids plotted in Fig. 4. The pie charts at each of the ancestral nodes shows the probability that the ancestor at that node had each of the palettes (short – pink, medium – violet, or long – blue; modified from O’Quin et al 2010).
Opsin gene expression has only been examined in one New World cichlid (Weadick et al. 2012). These data along with a few microspectrophotometry measurements (Levine et al. 1979) suggest that New World cichlids rely on the long visual palette of SWS2A, RH2A and LWS. This may make sense based on their riverine environments, which are often murky for at least some part of the year.
4. Tinbergen question 3: How does the trait develop in an individual?
Studies to date suggest that adult cichlids predominantly express three of the six classes of opsins, with species differing in the expressed set. This raises the question of why all the opsin genes have been retained. With few exceptions, all seven cone opsin genes are present and functional in over 100 species examined to date. It is possible that opsin genes have been retained for expression at different life stages. This motivated several studies that examined how opsin expression varies through development.
Fish eyes form quite early in development and are easily observable after just a few days. In cichlids, eyes are formed by three days post fertilization (dpf) with the retina fully constructed by day seven (Hagedorn and Fernald 1992). A study of opsin expression quantified from whole embryos suggests that opsin expression begins on approximately day four (Carleton et al. 2008). Fish eyes then continue to grow throughout life with new cone photoreceptors being added at the retinal margin (Fernald 1990). The cone mosaic seems to be stable throughout life with no sign of cell death or cell loss through development (Hagedorn and Fernald 1992, van der Meer 1995, Braekevelt et al. 1998). Therefore, the majority of any changes in opsin gene expression in adults must result within existing photoreceptors as the proteins of their outer segments turnover, rather than from the gain or loss of additional cells. Studies in other vertebrates suggest it takes approximately two weeks to replace the outer segment, with the tips of each cone photoreceptor being phagocytosed during the night and new proteins added near the inner segment (Young 1978). Daily regeneration of photoreceptor outer segments results in opsin gene expression throughout life with a circadian rhythm. In A. burtoni, opsin expression fluctuates diurnally for both rods and cones (Korenbrot and Fernald 1989, Halstenberg et al. 2005). These data support the idea that rods have high opsin expression during the day when they are regenerating, while cones have high opsin expression towards the end of the day and into night when they regenerate.
Studies examining developmental opsin expression found that in the Nile tilapia, opsin gene expression changes significantly with life stage (Carleton et al. 2008). Single cones of O. niloticus larvae express SWS1, switch to SWS2B in juveniles, and then express SWS2A in adults. In this way, single cones shift sensitivity from ultraviolet to violet to blue through the first six months of life, when fish become sexually mature (adults). The double cones also shift sensitivity from shorter to longer wavelengths, expressing RH2B in larvae, RH2Aβ in juveniles, low RH2Aα expression at all stages, and predominately LWS in adults. These gene expression patterns are consistent with MSP studies of protein expression in fish of different ages (Carleton et al. 2008). Studies in other fishes suggest that such a change could be related to ontogenetic shifts in foraging from plankton to other food items (Loew and Wahl 1991, Britt et al. 2001). O. niloticus also shift foraging, eating more zooplankton in the larval stage, and switching to other foods as they age (Fattah Ibrahim et al. 2015).
Other cichlid species that have been studied show less developmental variation in opsin expression (Fig. 7). Studies of the riverine A. burtoni found that although the single cones do undergo developmental shifts in opsin expression from SWS1 to SWS2B to SWS2A, the double cones do not (O'Quin et al. 2011a). Double cones only express RH2A and LWS and never RH2B. This suggests that the single and double cone expression patterns are independent of one another. Opsin gene expression in lake cichlids shows less developmental variation than riverine species. Species expressing the long palette (SWS2A, RH2A, LWS) turn on this gene set early in development and keep it on throughout life (Carleton et al. 2005, Carleton et al. 2008). Melanochromis auratus expresses the medium palette (SWS2B, RH2B, RH2A) as adults. Although it does express SWS1 in larvae, it switches to SWS2B expression by 20 dpf (O'Quin et al. 2011a). Species that express the short palette (SWS1, RH2B, RH2A), such as M. zebra, turn this gene set on as larvae and essentially keep it on throughout life. One possible explanation for these patterns is that the lake species are modifying the developmental progression found in O. niloticus. Some species have a retina that directly develops, expressing the longer wavelength gene set expressed in adult tilapia. Other species have a retina that remains neotenic, expressing the shorter wavelength gene sets found in young tilapia. The loss of developmental progression in opsin expression may result from the more stable light environments found in the lakes, where seasonal variation in water clarity might be less than in rivers. Further studies of additional riverine species are needed to confirm whether O. niloticus is representative of the riverine ancestor.
Figure 7.
Developmental progression of opsin expression from larval (L) to juvenile (J) to adult (A) stage. Gene expression changes in O. niloticus in both the single and double cones. Single cones switch from expression of SWS1 (pink) to SWS2B (violet) to SWS2A (blue). The two double cones start expressing RH2B (teal) and RH2A (green) but quickly turn on LWS (red) with adults having a predominance of LWS expression (likely having many red/red double cone pairs as shown by extra red cones). In A. burtoni, single cones switch expression as in O. niloticus, but the double cones obtain the adult expression combination of RH2A / LWS expression from earliest stage. Other species show less variation than these two species. Some species retaining the larval expression pattern (short) while others only express the adult opsin palette (long). The medium palette results from shifts in single cone expression from SWS1 to SWS2B.
All of these developmental series were carried out in the laboratory. In some of the Lake Malawi species adult opsin expression differs between laboratory reared and wild caught fish. This has been examined mostly for species expressing the short palette (SWS1, RH2B, RH2A). In addition to their dominant opsins, lab-reared adults often express more of the longer wavelength sensitive genes in both single (SWS2B) and double (LWS) cones than adults of the same populations sampled in the wild (Fig. 8; Hofmann et al. 2010b). This variation was further examined by splitting individual broods and raising them under different light environments. QPCR studies of Lake Malawi cichlids and electrophysiology in Nile tilapia suggest that rearing conditions do influence the development of opsin expression (Sabbah et al. 2012, Smith et al. 2012, Hornsby et al. 2013). In situ hybridization has demonstrated that environmentally plastic changes in opsin expression can be induced by altering the angle of incidence of the light when fish are reared from eggs to adult (Dalton et al. 2015). Regions of the retina that are illuminated by upwelling fluorescent light respond by increasing LWS expression in the dorsal retina of M. zebra. This agrees with developmental plasticity demonstrated in other fishes such as the black bream (Shand et al. 2008). The only study to examine adult plasticity showed that adult tilapia were not plastic to altered lighting environments (Hornsby et al. 2013). This lack of adult plasticity is similar to studies of the South American species Aequidens pulcher, which found no shifts in photoreceptor sensitivities when fish were reared under different colored lights in the lab (Wagner and Kroger 2005). However, they did find longer outer segments in cones that were not highly stimulated, suggesting an adaptation to increase sensitivity. The lack of plasticity in adult cichlids agrees with the lack of adult plasticity for stickleback opsin expression (Flamarique et al. 2013). However, it differs from guppies that shift opsin expression over developmental time scales (Ehlman et al. 2015) and fresh water adult killifish that show remarkable shifts in opsin gene expression in just a few days in changing light environments (Fuller and Claricoates 2011). Further studies are needed to examine lake cichlids to see if they show any adult plasticity in spectral sensitivity.
Figure 8.
Mean opsin expression for four Lake Malawi cichlids. Open bars are wild-caught and filled bars are lab-reared individuals. Error bars are +/− 1 S.D. Opsin genes that differed significantly are marked: * p<0.01 and ** p<0.001.
Lenses and the wavelengths that they transmit also show developmental variation. Lens transmission during development has been examined for the Nile tilapia, O. niloticus. Several studies have found that lens T50 shifted to longer wavelengths with age (Thorpe and Douglas 1993, Sabbah et al. 2012). This may partially be a path length effect, since as the lens gets larger, the path length through the lens increases, which can cause greater absorption and so a T50 shift. But it may also involve deposition of increasing amounts of UV absorbing pigment in the lens with age.
5. Tinbergen question 4: How does fitness, through interactions with the environment, influence and help to explain the trait’s form?
There are numerous ways in which cichlid visual sensitivities are tuned. These include altered coding sequences, changes in gene expression, and gene coexpression. If visual sensitivity is key to organismal fitness, there should be some evidence that these different tuning mechanisms lead to adaptation. Below, we discuss the evidence for selection.
5.1 Coding sequence variation
The cichlid cone opsin genes that vary the most in sequence produce pigments that are sensitive to the shortest and longest parts of the spectrum (Fig. 9A; Hofmann et al. 2009). This has several possible explanations. First, there is some evidence that visual pigments, and hence opsin genes, evolve to match the illuminating light spectrum (Munz and McFarland 1977, Loew and Lythgoe 1978). Since light absorption by clear water is greatest at the shortest and longest wavelength parts of the spectrum, any changes in lake level and hence depth of cichlid habitat will alter the available light most at the spectral extremes (Fig. 9B). Because there is always light in the middle parts of the spectrum, the opsins producing middle wavelength sensitive visual pigments can not absorb much more light through a change in opsin sequence. However, if the light environment changes, the opsin genes at the ends of the spectrum could shift their absorption spectrum to absorb more light by mutating key-tuning sites. So for example, if water depth increased, mutations that shift SWS1 opsins to longer wavelengths and LWS opsins to shorter wavelengths would result in increased light absorption (Hofmann et al. 2009).
Figure 9.
a) Opsin sequence variation for sites in the retinal binding pocket which change in polarity (after Hofmann et al 2009). Comparisons among 16 species of Lake Malawi (solid bars) show the greatest number of changes for the shortest and longest wavelength genes, while comparisons among 10 species from Lake Victoria (striped bars) show changes primarily in the LWS opsin gene. b) Transmission of light through different depths of water. The transmission data has been normalized to peak intensity to show how the shortest and longest wavelengths are attenuated relative to the middle part of the spectrum. Since cichlids are stenotopic, as lake levels change, the light spectrum reaching a particular rocky out crop will change, causing selection particularly on the shortest and longest wavelength sensitive genes to shift to better capture the available light.
Adaptation is also indicated by studies that show that opsin genes are under positive selection. This demonstration is most definitive for the LWS gene in Lake Victoria cichlids (Terai et al. 2006). The authors identified a 5 kb region containing the regulatory and coding sequence that shows high Fst between populations living at different depths, which have different LWS alleles. Evidence for selection between opsin sequences for cichlids in turbid (rivers and Lake Victoria) versus clear habitats (Lakes Tanganyika and Malawi) has been shown for the RH2 and LWS genes (Spady et al. 2005). Differential selection of the two different RH2A genes has been substantiated using clade based models (Weadick and Chang 2012). Studies of the cichlid rhodopsin gene also suggest that selection has acted. These studies have compared shallow and deep cichlids within the clear African lakes as well as lake versus riverine species in both African and Neotropical cichlids (Sugawara et al. 2002, Sugawara et al. 2005, Weadick and Chang 2012, Schott et al. 2014, Torres-Dowdall et al. 2015). Results of these studies suggest that the peak absorbance of rhodopsin shifts to shorter wavelengths with increasing depth, matching the light environment. Parallel changes in RH1 sequence support this occurrence in both Lakes Malawi and Tanganyika.
Sequence differences can have quite important ramifications. Studies using the optomotor response have confirmed that different LWS alleles contribute to differences in spectral sensitivity (Maan et al. 2006). The species Pundamilia pundamilia has the shorter LWS allele and is more sensitive to blue light, which might correlate with males being blue. Conversely, P. nyererei has the longer LWS allele and is more sensitive to red light. These differences are thought to contribute to speciation (Fig. 10; Seehausen et al. 2008). In the wild, P. nyererei are found at greater depths. Since the light spectrum in the eutrophied waters of Lake Victoria shifts to longer wavelengths with depth, the longer LWS allele will absorb more light. P. pundamilia lives closer to the surface where its shorter wavelength LWS allele matches the shorter wavelength light spectrum. Shifts in the LWS peak sensitivity can increase the apparent brightness of red patches observed a few meters below the surface by 10% (Carleton et al. 2005). The increased brightness could enhance P. nyererei female preference for red males. This suggests that the spectral light gradients in the lake could have led to a shift in visual sensitivity with depth, which then led to differences in female preference, divergence in male mating coloration, and speciation. This pattern has been seen with several rock dwelling cichlid pairs as well as sand dwelling pairs of Lake Victoria (Seehausen et al. 2008, Miyagi et al. 2012). It is the first conclusive proof that evolution of visual system sensitivity can lead to speciation through the process of sensory drive.
Figure 10.
One scenario for how sensory drive might induce speciation in a heterogeneous environment over evolutionary time. a) A population might diverge in response to a habitat gradient, here shown as variation in the light environment with depth. Divergence in population phenotypes (male color pattern) increases with time through sensory drive. b) Selection first acts to alter the sensitivity of visual pigment absorption spectra over time. Here, the shallower individuals shift to shorter wavelengths (blue) sensitive while the deeper individuals become longer wavelength (red) sensitive to match the background light spectrum. These visual sensitivities then select for male signals that best stimulates those sensitivities, causing male colors to shift through time. At the end of such a process, the shallow and deep individuals have different visual sensitivities and visual signals, which then contribute to behavioral isolation and result in two new species (modified from Carleton 2014).
5.2 Gene expression variation
Gene expression varies substantially between cichlid species living in the African Great Lakes. Ancestral state reconstruction of varying opsin gene expression suggests that the cichlid ancestor for the three Great Lakes had the long wavelength palette (Fig. 6). This ancestral state is consistent with opsin expression in riverine species including the sister taxa of O. niloticus as well as Astatotilapia burtoni (Fernald and Liebman 1980, Carleton et al. 2008). Cichlids in Lakes Malawi and Tanganyika have therefore convergently evolved short and medium visual palettes (O'Quin et al. 2010). This implies that it is relatively easy to alter opsin expression.
If gene expression is adaptive, it should be linked to important ecological traits. Some subtle differences in gene expression correlate with the light environment. In Lake Victoria, species sampled from the clearest parts of the lake express both SWS2B and SWS2A. However, the same species sampled from a murkier habitat expresses only the SWS2A gene (Hofmann et al. 2009). If SWS genes are coexpressed in this species, coexpressing more or less SWS2B in an SWS2A-expressing cone would provide a way to shift cone sensitivities to shorter or longer wavelengths in clearer and murkier habitats, respectively. Indeed, the amount of SWS2B expression seems to match well with changes in light spectra as predicted by the quantum catch of the SWS2B receptor in the different environments (Fig. 3 in Hofmann et al 2009). Studies in Lake Malawi cichlids found that gene expression also correlates with light environment. In one species, LWS opsin expression decreased for individuals sampled below 10 m as compared to individuals sampled near the surface (Smith et al. 2011). However, quantum catch calculations did not explain the opsin expression differences as there was still sufficient long wavelength light at depth in spite of there being little LWS expression. This study also found variation in gene expression between populations of M. zebra with essentially the same light environment. This suggests there is more natural variation than can be easily explained by what is currently known about the ecology of these species.
Differences in expression are correlated with foraging. In both Lake Malawi and Tanganyika, algal browsers and zooplantivorous species have increased SWS1 expression compared to fish that are benthic or piscivorous feeders (Fig. 11; Hofmann et al. 2009, O'Quin et al. 2010). In figure 11, we have slightly modified the groups from the previous analyses to include not only species that are thought to be strongly zooplanktivorous, but also those that seem to be opportunistically so and are categorized as consuming small amounts of zooplankton (data in Supp Table S1). In addition, we plot SWS1 expression as a fraction of the SWS opsin genes expressed in single cones (%SWS1 in single cones = SWS1 / (SWS1 + SWS2B + SWS2A)). Sensitivity in the ultraviolet part of the spectrum is thought to enhance detection of zooplankton (Browman et al. 1994). Many of the algal foragers are opportunistic zooplanktivores, which may explain their increased SWS1 expression. Ultraviolet sensitivity may also provide some advantages for algal foraging. Lens transmission in the UV part of the spectrum is also correlated with zooplanktivory, supporting the importance of this spectral range for certain foraging strategies (Hofmann et al. 2010a, O'Quin et al. 2010).
Figure 11.
SWS1 expression values (% of single cone expression) for cichlids in either Lake Malawi or Tanganyika divided by foraging style. Data in supplementary table S1.
Increased opsin expression does impact behavior through increased visual sensitivity. Smith et al (2012) split broods of Metriaclima lombardoi, and raised them to express different amounts of LWS opsin by keeping them in different lighting conditions. They then measured sensitivity at different wavelengths with an optomotor apparatus that displayed a moving bar pattern in which color and brightness were manipulated. Opsin expression was then quantified for each individual. Smith et al (2012) found that when fish expressed more LWS opsin, they were more sensitive and responded to lower levels of red light than fish expressing less LWS opsin (Fig. 12). This suggests that changes in opsin expression do have behavioral implications and benefits.
Figure 12.
Optomotor measurements of visual sensitivities. Metriaclima lombardoi broods were split and raised in either broad spectrum lighting (metal halide bulbs meant to mimic the natural environment), or red shifted lighting (fluorescent bulbs). Melanochromis auratus broods were raised under broad-spectrum lighting. a) Relative LWS opsin expression measured by quantitative PCR. b) Optomotor sensitivity measured as the minimum light intensity (nanowatts) at which fish still followed a black and red striped pattern. Fish with higher LWS expression were on average more sensitive to the optomotor task performed under red lighting. (modified from Smith et al 2012).
Other studies have demonstrated that certain wavelengths contribute to particular behaviors. Jordan et al (2004) showed that if UV illumination is removed, finding prey takes longer in UV sensitive cichlids but not in fish without UV sensitivity. This provides behavioral evidence that expressing the UV sensitive SWS1 gene could enhance foraging for some species. Sensitivity to a broad spectrum of wavelengths (expressing multiple opsin genes) is important for cichlids to correctly choose conspecifics for mating. When full spectrum light is replaced with monochromatic illumination, species indiscriminately choose mates in some (Seehausen and van Alphen 1998, Selz et al. 2014), though not all species (Jordan et al. 2003, Plenderleith et al. 2005).
5.3 Gene coexpression variation
Dalton et al (2014) documented opsin coexpression in the Lake Malawi species, M. zebra, and used modeling to suggest that coexpression impacts fitness. Opsin coexpression is not uniform, but only occurs in certain parts of the retina. Coexpression can increase absorption of the background space light that is incident on that part of the retina (Fig. 13). This would provide higher contrast to better enable a fish to detect a dark shape against a bright background. Typical dark shapes could be predators, such as otters, birds or other fish, and might also include cichlid egg predators that prey on females holding eggs. Coexpression of RH2Aβ with RH2B was shown to increase the absorbance of space light by 7.5%, while coexpression of LWS with RH2Aα increased absorbance of downwelling light by 2% to 5% depending on depth. These differences increase both detection distance and absolute contrast at that distance. Even a few percent improvement in detection distance could improve the chances of surviving a predatory attack, and therefore increase fitness.
Figure 13.
The expression of the four opsin genes expressed in double cones varies across the retina of Metriaclima zebra. For essentially every double cone across the entire retina, RH2B (B) and RH2Aα (α) are expressed in opposite members of each double cone. However, the RH2Aβ (β) and LWS genes are coexpressed with the other genes and occur predominantly in certain parts of the retina. RH2Aβ is coexpressed with RH2B in the more nasal part of the retina. LWS is coexpressed with RH2Aα in the ventral part of the retina. These different retinal regions view different backgrounds with the ventral retina illuminated by the broad downwelling light, the nasal retina illuminated by the background space light and the dorsal retina illuminated by upwelling light bouncing off the substrate. Coexpression may produce visual pigments that absorb more of the different illuminants, improving overall contrast detection (modified from Dalton et al 2014).
Although coexpression of opsins in specific regions of the retina increases sensitivity to the corresponding backgrounds, modeling indicates it may hinder color discrimination. In particular, the coexpression of RH2Aβ/RH2B reduces the difference in color space between male cichlid colors and viewing backgrounds in the lake, while coexpression of RH2Aα/LWS tended to increase these differences (Dalton et al. 2014). Thus coexpression may create a trade-off between visual functions that affects where in the retina it can optimally occur. This agrees with the fact that certain parts of the retina likely perform different functions. The dorso-temporal region looks ahead and down at potential mates or to find food. The naso-ventral region looks up and back, perhaps to detect the rapid motion of predators.
In summary, there are numerous ways in which these tuning mechanisms contribute to fitness. Variation in opsin sequence tunes visual pigments to better match the light environment and can further contribute to speciation through mate choice. Differential opsin expression is correlated with environmental lighting and foraging style, and likely plays a role in mate choice as well. Opsin coexpression tunes visual sensitivities regionally within the retina to better match viewing backgrounds, enhancing contrast detection. Therefore, key elements of fitness, including mating success, foraging, and predator avoidance are impacted by visual sensitivity.
6. Proximate mechanisms and gene regulatory networks
Both opsin sequence and opsin expression contribute to visual sensitivity. The proximate mechanism of opsin sequence is clear. The characteristics of the amino acids of a given opsin protein that are close to the retinal molecule interact with the chromophore’s electron cloud. This alters the energies of the highest filled and lowest unfilled molecular orbitals and modifies the peak of the visual pigment absorption spectrum (Chang et al. 1995, Katayama et al. 2015). The proximate mechanisms controlling opsin expression are more complex. Gene expression is typically controlled by changes in a gene’s promoter. However, in comparing Lake Malawi cichlids that express different opsin palettes, O’Quin et al (2011b) found that opsin cis regulatory regions are quite conserved, with little variation that could alter gene expression. This agrees with studies using genetic crosses made between species that differ in expression. They found that the key regulatory regions are not in cis to the opsin genes but are rather trans factors located elsewhere in the genome (Carleton et al. 2010). This suggests that variation in expression of transcription factors, rather than opsin promoters, is the driving force behind variation in opsin expression. This is different from the cis regulatory changes known to drive the evolution of many morphological traits (reviewed in Carroll 2008). Further, these crosses identified several quantitative trait loci (QTL) of large effect suggesting a relatively simple genetic architecture for the gene regulatory network controlling opsin expression (O'Quin et al. 2012). A simple architecture would contribute to the lability of opsin expression, enabling rapid shifts between closely related species.
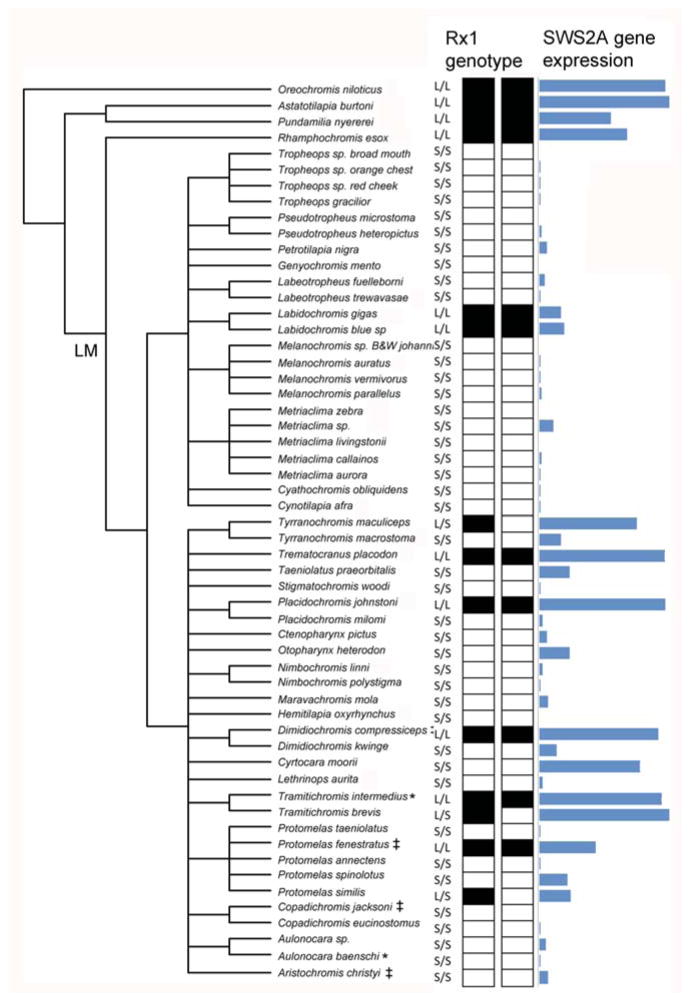
One of the key regulatory factors that controls expression of the blue sensitive SWS2A opsin gene has been mapped using genetic approaches. Schulte et al (2014) utilized a cross between two Lake Malawi species that differed in SW2A expression (Aulonocara baenschi x Tramitichromis intermedius). They identified retinal homeobox 1 (Rx1) as a transcription factor controlling SWS2A opsin expression. The gene was first identified through fine mapping and identification of candidate genes using retinal transcriptomes. Support for the role of Rx1 came from there being four Rx1 binding sites in the SWS2A promoter. Association mapping identified a 413 bp deletion in the Rx1 promoter, several kb upstream of the Rx1 start site that was correlated with SWS2A expression across 53 species of Lake Malawi cichlids (Fig. 14). This deletion likely arose early in the history of the Lake Malawi flock as it is shared across the two major clades of rock dwelling and sand dwelling cichlids that diverged over 1 MY ago. As such, it appears to be an ancestral polymorphism that is sorting through the flock. This study suggests that it is possible to identify the causative mutations underlying the gene regulatory network controlling opsin expression.
Figure 14.
The SWS2A expression levels for cichlids from Lake Malawi in comparison to the genotype of the promoter of the retinal homeobox 1 (Rx1) gene. Genotypes for both alleles are shown for individuals, where the promoter is either intact (black box, L=long allele) or includes a 413-bp deletion (white box, S= short allele). The phylogenetic tree is based on generic relationships of cichlid species.
7. Remaining questions
In terms of visual sensitivities, cichlids are one of the best-studied groups, with variable sensitivities documented across a diverse set of closely related taxa. Comparisons among taxa give us significant insights into understanding both the proximate molecular mechanisms controlling visual system diversity as well as the ultimate evolutionary factors that can shape it. In spite of this progress, there is still much that remains unknown. Below we discuss the unanswered aspects of Tinbergen’s questions concerning cichlid visual sensitivities.
7.1 Molecular mechanisms of visual sensitivity
7.1.1 Which molecular tuning mechanisms are most significant for spectral tuning?
Visual sensitivities in cichlids are variable, with several mechanisms playing a role. Variation, both within and between populations, has been documented for opsin sequence (Terai et al. 2002, Terai et al. 2006, Smith and Carleton 2010), opsin expression (Smith et al. 2011) opsin coexpression (Dalton et al. 2014), and plasticity of opsin expression (Dalton et al 2015). So far, sequence variation seems important for the shortest (SWS1) and longest (LWS) wavelength sensitive cone opsin genes, along with the rod opsin (RH1). Further study is needed to understand how much of the gene sequence variation is functional, particularly for SWS1 opsin. Studies could focus on functionally testing sites in other opsin genes, particularly with regards sequence variants from the older Tanganyikan flock (O'Quin et al. 2010). These studies are necessary to confirm the effect size of opsin sequence differences, which have been suggested to have a relatively small effect on spectral tuning.
Opsin coexpression has been demonstrated for the RH2 and LWS opsin genes expressed in double cones (Dalton et al 2014). Studies are now underway that suggest that the SWS genes are also coexpressed in single cones (Dalton et al unpublished results). Tests for correlations in single cone and double cone coexpression could be informative. If coexpression varies spatially across the retina in a concerted way, with both single and double cones coexpressing longer wavelength genes in the same parts of the retina, this would suggest that spectral spacing might be important for certain particular visual tasks, such as color vision for object recognition.
An additional mechanism for producing longer wavelength sensitive visual pigments is to switch the chromophore from A1 to A2. This has modest effects (10–15 nm shifts) for cones expressing the SWS genes, but larger effects (30–60 nm) for RH2 and LWS expressing cones (Harosi 1994, Parry and Bowmaker 2000). While a few studies have documented chromophore use, all of the individuals were kept in the lab. No studies have yet examined how much chromophore variation there is in wild caught fishes. One prediction would be that fish from murky habitats might utilize significant A2 chromophore to facilitate the longer wavelength sensitivities. Since chromophore shifts for LWS opsin based visual pigments can be quite large, this overlooked mechanism requires further study.
7.1.2 What is the gene regulatory network that directs opsin expression?
QTL studies are starting to unravel the gene regulatory network controlling opsin expression. The network’s genetic architecture seems relatively simple with a few genes of large effect acting in trans to direct expression (O'Quin et al. 2012). Variation in the one transcription factor implicated to control expression for Lake Malawi cichlids results from a deletion in an enhancer region. This suggests that regulatory deletions could play an important role in shaping this network. It would be interesting to know if this transcription factor contributes to variation in Lake Tanganyika, or if differential opsin expression in cichlids from that lake is controlled by variation in other parts of the network. This might provide evidence of convergence in the regulatory network to independently produce all three palettes in both lakes.
7.2 Evolutionary diversity
7.2.1 How do mechanisms vary among species, lineages, and lakes?
Studies have documented significant variation in both opsin gene sequence and gene expression for wild sampled individuals, even among individuals from a single population. Although sequence variation is obviously genetic, it is unclear which mechanisms contribute to variable gene expression. Lab studies have shown that there is a large genetic component to gene expression with single loci explaining 5–50% of the variance (O'Quin et al. 2012). However, other studies have shown that environmental plasticity can play a moderate role (Smith et al. 2012, Hornsby et al. 2013, Dalton et al. 2015). This plasticity could have a genetic basis as demonstrated for killifish (Fuller et al. 2005).
In wild populations, there is the likely effect of random drift. Cichlid populations can be quite small with population sizes estimated to be anywhere from 500 to 10,000 individuals (Won et al. 2005, Husemann et al. 2015). The first studies of the loci controlling opsin expression suggest that they are ancestral polymorphisms that are sorting through the flock (Schulte et al 2014). Alternate fixation due to drift of loci in the gene regulatory network controlling differential expression or environmental plasticity could lead to variable expression within small populations. Further, alternate fixation across lineages would certainly help explain the considerable variation between species and lakes.
7.2.2 When and where did visual sensitivity differences arise?
Many of the studies performed so far have examined the African lake cichlids. These species arose from the African riverine species. Studies of the riverine species have not been extensive enough to know when and where the variants causing diversity within the lakes might have arisen. There is also a Gondwanan distribution of cichlids with species flocks in Central and South America and smaller groups in Madagascar and India. Work on the South and Central American species is just beginning and suggests there is considerable variation in the rod opsin sequence (Schott et al. 2014, Torres-Dowdall et al. 2015). Studies of one cichlid from Trinidad has found it has fewer opsins than its African cousins (Weadick et al. 2012). Further studies are needed to examine the cone opsins of cichlids from the Americas as well as the rivers of Africa, Madagascar and India to understand the evolutionary diversity in cichlid vision.
7.3 Developmental variation
7.3.1 How does environmental plasticity affect opsin expression?
There is a strong genetic program that produces shifts in opsin expression through development in some cichlid species. However, there is evidence that gene expression can be modified by lab rearing environment (Hofmann et al. 2010b, Dalton et al. 2015). Presumably this means that different natural environments could also influence expression. While natural variation in opsin expression has been seen with depth and water clarity, it is not clear if that variation is genetic or environmental (Hofmann et al. 2009, Smith et al. 2011). Only one study has examined plasticity in adult cichlids and found little to no effect (Hornsby et al. 2013). Further studies are necessary to determine if there is a critical developmental period during which plasticity is activated and if adult fish also show plasticity. Environmental plasticity may be important for coexpression as well.
7.3.2 How do the genes underlying QTL fit into the gene regulatory network controlling developmental changes in opsin expression?
The successful identification of a transcription factor underlying opsin expression shows the potential to map out the full gene regulatory network that is controlling visual sensitivity. Studies have suggested that variation in adult opsin gene expression is the result of altering the developmental pattern of progression through the different opsin expression palettes (Carleton et al. 2008). It will therefore be important to determine whether the network is simple or complex, what kinds of genes are contributing and how the developmental pattern is altered to change opsin expression across different species.
7.4 Fitness
7.4.1 Is fitness affected by the large-scale variation in visual sensitivity?
Several ecological factors have been identified that are correlated with variation in opsin sequence including depth, light environment and foraging style (Sugawara et al. 2005, Hofmann et al. 2009, O'Quin et al. 2010, Smith et al. 2011). However, much of the variation in opsin expression does not yet have an ecological explanation. Fish that are essentially swimming next to each other can have drastically different visual sensitivities. One explanation for all the variation is that it results from ecological factors that we don’t yet understand. This might occur if selection acts during particular life stages or times of the year, or even during historical times that have not been considered. The light environment has been characterized at only a few locations during the dry season (July 2005, July 2008; Dalton et al. 2010, Sabbah et al. 2011). Examining the range of light spectra in different habitats (sand, rock, pelagic, rivers), during all seasons, or estimating it for some historical condition may be useful. Alternatively, it is possible that visual sensitivities vary randomly and that any combination of three visual pigments works well enough (Marshall et al. 2015). This variation might result from drift, which could occur in small cichlid populations. One additional hypothesis for the evolution of cichlid diversity is that it results from the hybridization of diverse cichlids that enter the lakes from different rivers (Seehausen 2004). If riverine species did create a hybrid swarm, and if different riverine systems favor different cichlid visual sensitivities driven by particular transcription factors, then alternate fixation of these ancestral polymorphisms could lead to random visual palettes in the lakes.
While ecological factors that correlate with visual sensitivity have been identified, this is not the same as demonstrating that visual sensitivity affects fitness. Few studies have examined the role of different parts of the spectrum in contributing to cichlid behavior. Exceptions include studies that show feeding rate is enhanced with UV light (Jordan et al. 2004) and full spectrum lighting is needed for correct mate choice (Seehausen and van Alphen 1998). Further work to quantify whether visual differences contribute to actual measures of fitness (foraging or mating success) is needed.
7.4.2 Is there evidence for selection acting on gene expression?
Evidence for selection on opsin sequences has been clearly documented for several different opsin genes in both African and New World cichlids. Many of these studies have focused on the LWS and RH1 genes. However, it is less clear whether selection is acting on opsin expression, in spite of its ecological correlations. Proof will require identifying the loci controlling opsin expression and then looking for evidence of selective sweeps. Such sweeps may be difficult to detect if they occurred several million years ago and were then followed by interspecific hybridization (Seehausen 2004). If the exact causative mutations underlying changes in gene expression can be found, as well as when and where they arose (e.g. a murky river versus a clear lake), we might better understand the role of selection in shaping these variants.
7.5 What are the behavioral implications for highly variable cichlid visual sensitivities?
Being more or less sensitive in different parts of the spectrum could have major impacts on the types of visual tasks that fish perform optimally, as well as the types of colors of prospective mates that fish prefer.
7.5.1 How does visual sensitivity affect contrast detection of objects?
Certain visual tasks rely on contrast. Detecting a dark object against a bright background is best accomplished by photoreceptors tuned to match the background spectrum, while detecting a bright object against a dark background works best when photoreceptor sensitivity is spectrally offset from the background (Munz and McFarland 1977, Lythgoe 1979, Loew 1995). While one study has shown that UV light enhances zooplanktivory (Jordan et al. 2004), further behavioral evidence is needed to demonstrate that matching or offset visual sensitivities in cichlids enhance foraging, predator detection, or even navigation.
7.5.2 How does variation in visual sensitivity impact color preferences?
The difference in sensitivities among the cichlid palettes should have major implications for species recognition and speciation. According to the sensory drive theory, potential mates will use colors that optimally stimulate viewers, either potential female partners or potential male rivals. In studies of the Lake Victorian cichlids, calculations indicated that species with the longer wavelength version of the LWS allele would detect a brighter stimulus from a displaying male than those with the shorter wavelength allele (Carleton et al. 2005). These alleles only differed by 4 nm in λmax, suggesting that larger shifts in peak sensitivity could have even large effects under the right conditions. However, no clear links have been found between color and vision in the clearer lakes (Dalton et al. 2010). Modeling to examine different habitats, time of day, and color patterns is needed to examine how tasks such as foraging and mate choice are affected by visual sensitivity shifts. 7.6 What does variation in cichlid visual sensitivities tell us about variation in other fishes?
Work to understand the cichlid visual systems began nearly 40 years ago. From the first studies of visual sensitivities in the riverine Astatotilapia burtoni (Fernald and Liebman 1980) to the identification of opsin genes and the role of differential gene expression (Carleton and Kocher 2001, Hofmann et al. 2009), the work has progressed at a traditional molecular biology pace. However, recent developments in next generation sequencing are enabling more rapid characterization of opsin diversity in other fish groups. Preliminary studies suggest that many of the same mechanisms contributing to cichlid visual diversity are playing a role in shaping visual systems of other fishes. Work on damselfish suggests that variation in opsin sequence plays a role in diversity, with more sequence variation in genes at the short and long wavelength ends of the spectrum (Hofmann et al. 2012). Studies to examine gene expression in this group will help determine whether it contributes to variation. Next generation methods have been used to examine the molecular mechanisms shaping visual sensitivity in twelve labrid species (Phillips et al. 2015). The results suggest that while opsin sequence variation is low at known spectral tuning sites, changes in opsin gene expression and gene duplications are significant. This variation suggests a role for ecology including foraging, but needs further testing. The importance of opsin gene duplications has also been demonstrated in fishes by Cortesi et al (2015). They found far more variation in the actual complement of opsin genes between taxa than had been previously thought. These next generation sequencing studies suggest a framework for moving forward to examine the full range of tuning mechanisms across fishes and other vertebrates as well.
8. Conclusion
In 1963, Tinbergen suggested that to clearly understand a trait and its mechanistic and functional roles requires a diversity of approaches. Here we have tried to address his four questions to show the rich way in which molecular mechanisms have been shaped by evolution to modify developmental patterns and produce diverse cichlid visual sensitivities. Evidence suggests that this diversity is adaptive and likely enhances fitness via both survival and mating success. Work is in progress to document the full genetic network underlying cichlid opsin expression. This will facilitate studies to examine the evolution of this network and how it can be modified to generate diversity. How far these mechanisms extrapolate to other fishes (and perhaps other vertebrates) is not yet clear. However, next generation sequencing methods for simultaneously determining opsin sequence and expression should rapidly push this work forward. Therefore, exciting times seem to be ahead for examining both the proximate mechanisms underlying variable visual sensitivities and the ultimate forces that shape them.
Supplementary Material
Acknowledgments
We would like to thank numerous colleagues and collaborators for making this work possible. First, we thank Russ Fernald for pioneering the study of cichlid visual systems nearly 40 years ago. Numerous cichlid experts generously contributed ideas, specimens, collecting trips, and encouragement including Tom Kocher, Ole Seehausen, Todd Streelman, Darin Hulsey, Craig Albertson, Pat Danley, Michael Kidd, Richard Zatha, Hans Hofmann, and Walter Salzburger. Cichlid vision research has also benefited from numerous vision scientists who took an interest in the cichlid system, helped gather data, and taught us how to think about the system. These include Jim Bowmaker, Tom Cronin, David Hunt, Ellis Loew, Justin Marshall, and Bill Macfarland. Much of the work described here is the result of the enthusiasm and hard work of previous Carleton lab members including Tyrone Spady, Chris Hofmann, Kelly O’Quin, Adam Smith, Jane Schulte, Zil Patel, Jessica Lu, and Conor O’Brien. We also appreciate the encouragement to think outside of the cichlid box by the Marshall and Salzburger labs. Finally, we are grateful for support from NSF IOS-0841270 and NIH 1R01EY024639.
References
- Bateson P, Laland KN. Tinbergen's four questions: an appreciation and an update. Trends Ecol Evol. 2013;28(12):712–718. doi: 10.1016/j.tree.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Blatz PE, Liebman PA. Wavelength regulation in visual pigments. Exp Eye Res. 1973;17(6):573–580. doi: 10.1016/0014-4835(73)90086-9. [DOI] [PubMed] [Google Scholar]
- Braekevelt CR, Smith SA, Smith BJ. Photoreceptor fine structure in Oreochromis niloticus L. (Cichlidae; Teleostei) in light- and dark-adaptation. Anat Rec. 1998;252(3):453–461. doi: 10.1002/(SICI)1097-0185(199811)252:3<453::AID-AR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Britt LL, Loew ER, McFarland WN. Visual pigments in the early life stages of Pacific northwest marine fishes. J Exp Biol. 2001;204(Pt 14):2581–2587. doi: 10.1242/jeb.204.14.2581. [DOI] [PubMed] [Google Scholar]
- Britten RJ, Davidson EH. Gene regulation for higher cells: a theory. Science. 1969;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Browman HI, Novales Flamarique I, Hawryshyn C. Ultraviolet photoreception contributes to prey search behaviour in two species of zooplanktivorous fishes. Journal of Experimental BIology. 1994;186:187–198. doi: 10.1242/jeb.186.1.187. [DOI] [PubMed] [Google Scholar]
- Brunetti CR, Selegue JE, Monteiro A, French V, Brakefield PM, Carroll SB. The generation and diversification of butterfly eyespot color patterns. Curr Biol. 2001;11(20):1578–1585. doi: 10.1016/s0960-9822(01)00502-4. [DOI] [PubMed] [Google Scholar]
- Carleton KL. Cichlid fish visual systems: mechanisms of spectral tuning. Integrative Zoology. 2009;4:75–86. doi: 10.1111/j.1749-4877.2008.00137.x. [DOI] [PubMed] [Google Scholar]
- Carleton KL. Visual photopigment evolution in speciation. In: Hunt DM, Hankins MW, Collin SP, Marshall NJ, editors. Evolution of Visual and Non-visual Pigments. New York, NY: Springer; 2014. pp. 241–267. [Google Scholar]
- Carleton KL, Harosi FI, Kocher TD. Visual pigments of African cichlid fishes: evidence for ultraviolet vision from microspectrophotometry and DNA sequences. Vision Res. 2000;40(8):879–890. doi: 10.1016/s0042-6989(99)00238-2. [DOI] [PubMed] [Google Scholar]
- Carleton KL, Hofmann CM, Klisz C, Patel Z, Chircus LM, Simenauer LH, Soodoo N, Albertson RC, Ser JR. Genetic basis of differential opsin gene expression in cichlid fishes. J Evol Biol. 2010;23(4):840–853. doi: 10.1111/j.1420-9101.2010.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton KL, Kocher TD. Cone opsin genes of african cichlid fishes: tuning spectral sensitivity by differential gene expression. Mol Biol Evol. 2001;18(8):1540–1550. doi: 10.1093/oxfordjournals.molbev.a003940. [DOI] [PubMed] [Google Scholar]
- Carleton KL, Parry JW, Bowmaker JK, Hunt DM, Seehausen O. Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia. Mol Ecol. 2005;14(14):4341–4353. doi: 10.1111/j.1365-294X.2005.02735.x. [DOI] [PubMed] [Google Scholar]
- Carleton KL, Spady TC, Streelman JT, Kidd MR, McFarland WN, Loew ER. Visual sensitivities tuned by heterochronic shifts in opsin gene expression. BMC Biol. 2008;6(1):22. doi: 10.1186/1741-7007-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134(1):25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Chang BS, Crandall KA, Carulli JP, Hartl DL. Opsin phylogeny and evolution: a model for blue shifts in wavelength regulation. Mol Phylogenet Evol. 1995;4(1):31–43. doi: 10.1006/mpev.1995.1004. [DOI] [PubMed] [Google Scholar]
- Chinen A, Hamaoka T, Yamada Y, Kawamura S. Gene duplication and spectral diversification of cone visual pigments of zebrafish. Genetics. 2003;163(2):663–675. doi: 10.1093/genetics/163.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesi F, Musilova Z, Stieb SM, Hart NS, Siebeck UE, Malmstrom M, Torresen OK, Jentoft S, Cheney KL, Marshall NJ, Carleton KL, Salzburger W. Ancestral duplications and highly dynamic opsin gene evolution in percomorph fishes. Proc Natl Acad Sci U S A. 2015;112(5):1493–1498. doi: 10.1073/pnas.1417803112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton BE, Cronin TW, Marshall NJ, Carleton KL. The fish eye view: are cichlids conspicuous? J Exp Biol. 2010;213(Pt 13):2243–2255. doi: 10.1242/jeb.037671. [DOI] [PubMed] [Google Scholar]
- Dalton BE, Loew ER, Cronin TW, Carleton KL. Spectral tuning by opsin coexpression in retinal regions that view different parts of the visual field. Proc Biol Sci. 2014;281(1797):20141980. doi: 10.1098/rspb.2014.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton BE, Lu J, Leips J, Cronin TW, Carleton KL. Variable light environments induce plastic spectral tuning by regional opsin coexpression in the African cichlid fish, Metriaclima zebra. Mol Ecol. 2015;24(16):4193–4204. doi: 10.1111/mec.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WI, Collin SP, Hunt DM. Molecular ecology and adaptation of visual photopigments in craniates. Mol Ecol. 2012;21(13):3121–3158. doi: 10.1111/j.1365-294X.2012.05617.x. [DOI] [PubMed] [Google Scholar]
- Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20(1):49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- Ehlman SM, Sandkam BA, Breden F, Sih A. Developmental plasticity in vision and behavior may help guppies overcome increased turbidity. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015;201(12):1125–1135. doi: 10.1007/s00359-015-1041-4. [DOI] [PubMed] [Google Scholar]
- Fattah Ibrahim ANA, Castilho Noll MSM, Valenti WC. Zooplankton capturing by Nile Tilapia, Oreochromis niloticus (Teleostei: Cichlidae) throughout post-larval development. Zoologia (Curitiba) 2015;32(6):469–475. [Google Scholar]
- Fernald RD. Chromatic organization of a cichlid fish retina. Vision Res. 1981;21:1749–1753. doi: 10.1016/0042-6989(81)90207-8. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Teleost vision: seeing while growing. J Exp Zool Suppl. 1990;5:167–180. doi: 10.1002/jez.1402560521. [DOI] [PubMed] [Google Scholar]
- Fernald RD, Liebman PA. Visual receptor pigments in the African cichlid fish, Haplochromis burtoni. Vision Res. 1980;20(10):857–864. doi: 10.1016/0042-6989(80)90066-8. [DOI] [PubMed] [Google Scholar]
- Fernald RD, Wright SE. Growth of the visual system in the African cichlid fish, Haplochromis burtoni. Optics Vision Res. 1985;25(2):155–161. doi: 10.1016/0042-6989(85)90108-7. [DOI] [PubMed] [Google Scholar]
- Flamarique IN, Cheng CL, Bergstrom C, Reimchen TE. Pronounced heritable variation and limited phenotypic plasticity in visual pigments and opsin expression of threespine stickleback photoreceptors. J Exp Biol. 2013;216(Pt 4):656–667. doi: 10.1242/jeb.078840. [DOI] [PubMed] [Google Scholar]
- Frankel N, Wang S, Stern DL. Conserved regulatory architecture underlies parallel genetic changes and convergent phenotypic evolution. Proc Natl Acad Sci U S A. 2012;109(51):20975–20979. doi: 10.1073/pnas.1207715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RC, Carleton KL, Fadool JM, Spady TC, Travis J. Genetic and environmental variation in the visual properties of bluefin killifish, Lucania goodei. J Evol Biol. 2005;18(3):516–523. doi: 10.1111/j.1420-9101.2005.00886.x. [DOI] [PubMed] [Google Scholar]
- Fuller RC, Claricoates KM. Rapid light-induced shifts in opsin expression: finding new opsins, discerning mechanisms of change, and implications for visual sensitivity. Mol Ecol. 2011;20(16):3321–3335. doi: 10.1111/j.1365-294X.2011.05180.x. [DOI] [PubMed] [Google Scholar]
- Gagnon Y, Soderberg B, Kroger R. Optical advantages and function of multifocal spherical fish lenses. J Opt Soc Am A Opt Image Sci Vis. 2012;29(9):1786–1793. doi: 10.1364/JOSAA.29.001786. [DOI] [PubMed] [Google Scholar]
- Genner MJ, Seehausen O, Lunt DH, Joyce DA, Shaw PW, Carvalho GR, Turner GF. Age of cichlids: new dates for ancient lake fish radiations. Mol Biol Evol. 2007;24(5):1269–1282. doi: 10.1093/molbev/msm050. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Fernald RD. Retinal growth and cell addition during embryogenesis in the teleost, Haplochromis burtoni. J Comp Neurol. 1992;321(2):193–208. doi: 10.1002/cne.903210203. [DOI] [PubMed] [Google Scholar]
- Halstenberg S, Lindgren KM, Samagh SP, Nadal-Vicens M, Balt S, Fernald RD. Diurnal rhythm of cone opsin expression in the teleost fish Haplochromis burtoni. Vis Neurosci. 2005;22(2):135–141. doi: 10.1017/S0952523805222022. [DOI] [PubMed] [Google Scholar]
- Harosi FI. An analysis of two spectral properties of vertebrate visual pigments. Vision Res. 1994;34(11):1359–1367. doi: 10.1016/0042-6989(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Hoekstra HE, Coyne JA. The locus of evolution: evo devo and the genetics of adaptation. Evolution Int J Org Evolution. 2007;61(5):995–1016. doi: 10.1111/j.1558-5646.2007.00105.x. [DOI] [PubMed] [Google Scholar]
- Hofmann CM, Carleton KL. Gene duplication and differential gene expression play an important role in the diversification of visual pigments in fish. Integrative and Comparative Biology. 2009;49(6):630–643. doi: 10.1093/icb/icp079. [DOI] [PubMed] [Google Scholar]
- Hofmann CM, Marshall NJ, Abdilleh K, Patel Z, Siebeck UE, Carleton KL. Opsin evolution in damselfish: convergence, reversal, and parallel evolution across tuning sites. J Mol Evol. 2012;75(3–4):79–91. doi: 10.1007/s00239-012-9525-0. [DOI] [PubMed] [Google Scholar]
- Hofmann CM, O'Quin KE, Justin Marshall N, Carleton KL. The relationship between lens transmission and opsin gene expression in cichlids from Lake Malawi. Vision Res. 2010a;50(3):357–363. doi: 10.1016/j.visres.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Hofmann CM, O'Quin KE, Marshall NJ, Cronin TW, Seehausen O, Carleton KL. The eyes have it: regulatory and structural changes both underlie cichlid visual pigment diversity. PLoS Biol. 2009;7(12):e1000266. doi: 10.1371/journal.pbio.1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann CM, O'Quin KE, Smith AR, Carleton KL. Plasticity of opsin gene expression in cichlids from Lake Malawi. Mol Ecol. 2010b;19(10):2064–2074. doi: 10.1111/j.1365-294X.2010.04621.x. [DOI] [PubMed] [Google Scholar]
- Hornsby MA, Sabbah S, Robertson RM, Hawryshyn CW. Modulation of environmental light alters reception and production of visual signals in Nile tilapia. J Exp Biol. 2013;216(Pt 16):3110–3122. doi: 10.1242/jeb.081331. [DOI] [PubMed] [Google Scholar]
- Horth L. Sensory genes and mate choice: evidence that duplications, mutations, and adaptive evolution alter variation in mating cue genes and their receptors. Genomics. 2007;90(2):159–175. doi: 10.1016/j.ygeno.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Husemann M, Nguyen R, Ding B, Danley PD. A genetic demographic analysis of Lake Malawi rock-dwelling cichlids using spatio-temporal sampling. Mol Ecol. 2015;24(11):2686–2701. doi: 10.1111/mec.13205. [DOI] [PubMed] [Google Scholar]
- Jacob F, Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jordan R, Howe D, Juanes F, Stauffer JR, Loew ER. Ultraviolet radiation enhances zooplanktivory rate in ultraviolet sensitive cichlids. Afr J Ecol. 2004;42:229–231. [Google Scholar]
- Jordan R, Kellogg K, Howe D, Juanes F, Stauffer JR, Loew ER. Photopigment spectral absorbance of Lake Malawi cichlids. J Fish Biology. 2006;68(4):1291–1299. [Google Scholar]
- Jordan R, Kellogg K, Juanes F, Stauffer JR. Evaluation of female mate choice cues in a group of Lake Malawi Mbuna (Cichlidae) Copeia. 2003;2003(1):181–186. [Google Scholar]
- Karpestam B, Gustafsson J, Shashar N, Katzir G, Kroger RH. Multifocal lenses in coral reef fishes. J Exp Biol. 2007;210(Pt 16):2923–2931. doi: 10.1242/jeb.002956. [DOI] [PubMed] [Google Scholar]
- Katayama K, Sekharan S, Sudo Y. Color tuning in retinylidene proteins. In: YH, Kandori H, Koizumi A, editors. Optogenetics: Light sensing proteins and their applications. Japan: Springer; 2015. pp. 89–107. [Google Scholar]
- Keys DN, Lewis DL, Selegue JE, Pearson BJ, Goodrich LV, Johnson RL, Gates J, Scott MP, Carroll SB. Recruitment of a hedgehog regulatory circuit in butterfly eyespot evolution. Science. 1999;283(5401):532–534. doi: 10.1126/science.283.5401.532. [DOI] [PubMed] [Google Scholar]
- King MC, Wilson AC. Evolution at two levels in humans and chimpanzees. Science. 1975;188(4184):107–116. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- Kocher TD. Adaptive evolution and explosive speciation: the cichlid fish model. Nat Rev Genet. 2004;5(4):288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337(6206):454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Kroger RH. Optical plasticity in fish lenses. Prog Retin Eye Res. 2013;34:78–88. doi: 10.1016/j.preteyeres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Kroger RH, Campbell MC, Fernald RD, Wagner HJ. Multifocal lenses compensate for chromatic defocus in vertebrate eyes. J Comp Physiol [A] 1999;184(4):361–369. doi: 10.1007/s003590050335. [DOI] [PubMed] [Google Scholar]
- Kroger RH, Campbell MC, Munger R, Fernald RD. Refractive index distribution and spherical aberration in the crystalline lens of the African cichlid fish Haplochromis burtoni. Vision Res. 1994;34(14):1815–1822. doi: 10.1016/0042-6989(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Levine JS, MacNichol EF, Jr, Kraft T, Collins BA. Intraretinal distribution of cone pigments in certain teleost fishes. Science. 1979;204(4392):523–526. doi: 10.1126/science.432658. [DOI] [PubMed] [Google Scholar]
- Loew ER. Determinants of visual pigment spectral location and photoreceptor cell spectral sensitivity. In: Djamgoz MBA, Archer SN, Vallerga S, editors. Neurobiology and clinical aspects of the outer retina. London: Chapman and Hall; 1995. pp. 57–77. [Google Scholar]
- Loew ER, Lythgoe JN. The ecology of cone pigments in teleost fishes. Vision Res. 1978;18(6):715–722. doi: 10.1016/0042-6989(78)90150-5. [DOI] [PubMed] [Google Scholar]
- Loew ER, Wahl CM. A short-wavelength sensitive cone mechanism in juvenile yellow perch, Perca flavescens. Vision Res. 1991;31(3):353–360. doi: 10.1016/0042-6989(91)90088-m. [DOI] [PubMed] [Google Scholar]
- Lythgoe JN. The ecology of vision. Oxford: Clarendon Press; 1979. [Google Scholar]
- Maan ME, Hofker KD, van Alphen JJ, Seehausen O. Sensory Drive in Cichlid Speciation. Am Nat. 2006;167(6) doi: 10.1086/503532. [DOI] [PubMed] [Google Scholar]
- Manceau M, V, Domingues S, Mallarino R, Hoekstra HE. The developmental role of Agouti in color pattern evolution. Science. 2011;331(6020):1062–1065. doi: 10.1126/science.1200684. [DOI] [PubMed] [Google Scholar]
- Marshall J, Carleton KL, Cronin T. Colour vision in marine organisms. Curr Opin Neurobiol. 2015;34:86–94. doi: 10.1016/j.conb.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Fukamachi S, Mitani H, Kawamura S. Functional characterization of visual opsin repertoire in Medaka (Oryzias latipes) Gene. 2006;371(2):268–278. doi: 10.1016/j.gene.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Miyagi R, Terai Y, Aibara M, Sugawara T, Imai H, Tachida H, Mzighani SI, Okitsu T, Wada A, Okada N. Correlation between nuptial colors and visual sensitivities tuned by opsins leads to species richness in sympatric Lake Victoria cichlid fishes. Mol Biol Evol. 2012;29(11):3281–3296. doi: 10.1093/molbev/mss139. [DOI] [PubMed] [Google Scholar]
- Muntz WR. Yellow filters and the absorption of light by the visual pigments of some Amazonian fishes. Vision Res. 1973;13(12):2235–2254. doi: 10.1016/0042-6989(73)90225-3. [DOI] [PubMed] [Google Scholar]
- Muntz WR. Visual adaptations to different light environments in Amazonian fishes. Rev Can Biol Exp. 1982;41(1):35–46. [PubMed] [Google Scholar]
- Munz FW, McFarland WN. Evolutionary adaptations of fishes to the photic environment. In: Crescitelli F, editor. The visual system in vertebrates. New York: Springer-Verlag; 1977. pp. 194–274. [Google Scholar]
- Nachman MW, Hoekstra HE, D'Agostino SL. The genetic basis of adaptive melanism in pocket mice. Proc Natl Acad Sci U S A. 2003;100(9):5268–5273. doi: 10.1073/pnas.0431157100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Terai Y, Sugawara T, Imai H, Nishihara H, Hori M, Okada N. Reverse evolution in RH1 for adaptation of cichlids to water depth in Lake Tanganyika. Mol Biol Evol. 2011;28(6):1769–1776. doi: 10.1093/molbev/msq344. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Tinbergen's four questions, organized: a response to Bateson and Laland. Trends Ecol Evol. 2013;28(12):681–682. doi: 10.1016/j.tree.2013.10.008. [DOI] [PubMed] [Google Scholar]
- O'Quin KE, Hofmann CM, Hofmann HA, Carleton KL. Parallel evolution of opsin gene expression in African cichlid fishes. Mol Biol Evol. 2010;27(12):2839–2854. doi: 10.1093/molbev/msq171. [DOI] [PubMed] [Google Scholar]
- O'Quin KE, Schulte JE, Patel Z, Kahn N, Naseer Z, Wang H, Conte MA, Carleton KL. Evolution of cichlid vision via trans-regulatory divergence. BMC Evol Biol. 2012;12:251. doi: 10.1186/1471-2148-12-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Quin KE, Smith AR, Sharma A, Carleton KL. New evidence for the role of heterochrony in the repeated evolution of cichlid opsin expression. Evol Dev. 2011a;13(2):193–203. doi: 10.1111/j.1525-142X.2011.00469.x. [DOI] [PubMed] [Google Scholar]
- O'Quin KE, Smith D, Naseer Z, Schulte J, Engel SD, Loh YH, Streelman JT, Boore JL, Carleton KL. Divergence in cis-regulatory sequences surrounding the opsin gene arrays of African cichlid fishes. BMC Evol Biol. 2011b;11:120. doi: 10.1186/1471-2148-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson-Manning CF, Wagner MR, Mitchell-Olds T. Adaptive evolution: evaluating empirical support for theoretical predictions. Nat Rev Genet. 2012;13(12):867–877. doi: 10.1038/nrg3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289(5480):739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Parry JW, Bowmaker JK. Visual pigment reconstitution in intact goldfish retina using synthetic retinaldehyde isomers. Vision Res. 2000;40(17):2241–2247. doi: 10.1016/s0042-6989(00)00101-2. [DOI] [PubMed] [Google Scholar]
- Parry JW, Carleton KL, Spady T, Carboo A, Hunt DM, Bowmaker JK. Mix and match color vision: tuning spectral sensitivity by differential opsin gene expression in Lake Malawi cichlids. Curr Biol. 2005;15(19):1734–1739. doi: 10.1016/j.cub.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Peichel CL, Nereng KS, Ohgi KA, Cole BL, Colosimo PF, Buerkle CA, Schluter D, Kingsley DM. The genetic architecture of divergence between threespine stickleback species. Nature. 2001;414(6866):901–905. doi: 10.1038/414901a. [DOI] [PubMed] [Google Scholar]
- Phillips GA, Carleton KL, Marshall NJ. Multiple Genetic Mechanisms Contribute to Visual Sensitivity Variation in the Labridae. Mol Biol Evol. 2015 doi: 10.1093/molbev/msv213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenderleith M, van Oosterhout C, Robinson RL, Turner GF. Female preference for conspecific males based on olfactory cues in a Lake Malawi cichlid fish. Biol Lett. 2005;1(4):411–414. doi: 10.1098/rsbl.2005.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme B, Gompel N, Rokas A, Kassner VA, Williams TM, Yeh SD, True JR, Carroll SB. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature. 2006;440(7087):1050–1053. doi: 10.1038/nature04597. [DOI] [PubMed] [Google Scholar]
- Rennison DJ, Owens GL, Taylor JS. Opsin gene duplication and divergence in ray-finned fish. Mol Phylogenet Evol. 2012;62(3):986–1008. doi: 10.1016/j.ympev.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Sabbah S, Gray SM, Boss ES, Fraser JM, Zatha R, Hawryshyn CW. The underwater photic environment of Cape Maclear, Lake Malawi: comparison between rock- and sand-bottom habitats and implications for cichlid fish vision. J Exp Biol. 2011;214(Pt 3):487–500. doi: 10.1242/jeb.051284. [DOI] [PubMed] [Google Scholar]
- Sabbah S, Hui J, Hauser FE, Nelson WA, Hawryshyn CW. Ontogeny in the visual system of Nile tilapia. J Exp Biol. 2012;215(Pt 15):2684–2695. doi: 10.1242/jeb.069922. [DOI] [PubMed] [Google Scholar]
- Sabbah S, Laria RL, Gray SM, Hawryshyn CW. Functional diversity in the color vision of cichlid fishes. BMC Biol. 2010;8:133. doi: 10.1186/1741-7007-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol Biol. 2005;5(1):17. doi: 10.1186/1471-2148-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott RK, Refvik SP, Hauser FE, Lopez-Fernandez H, Chang BS. Divergent positive selection in rhodopsin from lake and riverine cichlid fishes. Mol Biol Evol. 2014;31(5):1149–1165. doi: 10.1093/molbev/msu064. [DOI] [PubMed] [Google Scholar]
- Schulte JE, O'Brien CS, Conte MA, O'Quin KE, Carleton KL. Interspecific Variation in Rx1 Expression Controls Opsin Expression and Causes Visual System Diversity in African Cichlid Fishes. Mol Biol Evol. 2014;31(9):2297–2308. doi: 10.1093/molbev/msu172. [DOI] [PubMed] [Google Scholar]
- Seehausen O. Lake Victoria rock cichlids. Germany: Verduijn Cichlids; 1996. [Google Scholar]
- Seehausen O. Hybridization and adaptive radiation. Trends Ecol Evol. 2004;19(4):198–207. doi: 10.1016/j.tree.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Seehausen O. Process and pattern in cichlid radiations - inferences for understanding unusually high rates of evolutionary diversification. New Phytol. 2015;207(2):304–312. doi: 10.1111/nph.13450. [DOI] [PubMed] [Google Scholar]
- Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HD, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, Imai H, Okada N. Speciation through sensory drive in cichlid fish. Nature. 2008;455(7213):620–626. doi: 10.1038/nature07285. [DOI] [PubMed] [Google Scholar]
- Seehausen O, van Alphen JJ. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex) Behavioral Ecology and Sociobiology. 1998;42:1–8. [Google Scholar]
- Seehausen O, van Alphen JJ, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277:1808–1811. [Google Scholar]
- Selz OM, Pierotti MER, Maan ME, SC, Seehausen O. Female preference for male color is necessary and sufficient for assortative mating in 2 cichlid sister species. Behavioral Ecology. 2014;25(3):612–626. [Google Scholar]
- Shand J, Davies WL, Thomas N, Balmer L, Cowing JA, Pointer M, Carvalho LS, Trezise AE, Collin SP, Beazley LD, Hunt DM. The influence of ontogeny and light environment on the expression of visual pigment opsins in the retina of the black bream, Acanthopagrus butcheri. J Exp Biol. 2008;211(Pt 9):1495–1503. doi: 10.1242/jeb.012047. [DOI] [PubMed] [Google Scholar]
- Siebeck UE, Marshall NJ. Ocular media transmission of coral reef fish--can coral reef fish see ultraviolet light? Vision Res. 2001;41(2):133–149. doi: 10.1016/s0042-6989(00)00240-6. [DOI] [PubMed] [Google Scholar]
- Sivak JG. Through the lens clearly: phylogeny and development: the Proctor lecture. Invest Ophthalmol Vis Sci. 2004;45(3):740–747. 739. doi: 10.1167/iovs.03-0466. [DOI] [PubMed] [Google Scholar]
- Smith AR, Carleton KL. Allelic variation in Malawi cichlid opsins: a tale of two genera. J Mol Evol. 2010;70(6):593–604. doi: 10.1007/s00239-010-9355-x. [DOI] [PubMed] [Google Scholar]
- Smith AR, D'Annunzio L, Smith AE, Sharma A, Hofmann CM, Marshall NJ, Carleton KL. Intraspecific cone opsin expression variation in the cichlids of Lake Malawi. Mol Ecol. 2011;20(2):299–310. doi: 10.1111/j.1365-294X.2010.04935.x. [DOI] [PubMed] [Google Scholar]
- Smith AR, Ma K, Soares D, Carleton KL. Relative LWS cone opsin expression determines optomotor thresholds in Malawi cichlid fish. Genes Brain Behav. 2012;11(2):185–192. doi: 10.1111/j.1601-183X.2011.00739.x. [DOI] [PubMed] [Google Scholar]
- Spady TC, Parry JW, Robinson PR, Hunt DM, Bowmaker JK, Carleton KL. Evolution of the cichlid visual palette through ontogenetic subfunctionalization of the opsin gene arrays. Mol Biol Evol. 2006;23(8):1538–1547. doi: 10.1093/molbev/msl014. [DOI] [PubMed] [Google Scholar]
- Spady TC, Seehausen O, Loew ER, Jordan RC, Kocher TD, Carleton KL. Adaptive molecular evolution in the opsin genes of rapidly speciating cichlid species. Mol Biol Evol. 2005;22(6):1412–1422. doi: 10.1093/molbev/msi137. [DOI] [PubMed] [Google Scholar]
- Stern DL, Orgogozo V. The loci of evolution: how predictable is genetic evolution? Evolution. 2008;62(9):2155–2177. doi: 10.1111/j.1558-5646.2008.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DL, Orgogozo V. Is genetic evolution predictable? Science. 2009;323(5915):746–751. doi: 10.1126/science.1158997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Terai Y, Imai H, Turner GF, Koblmuller S, Sturmbauer C, Shichida Y, Okada N. Parallelism of amino acid changes at the RH1 affecting spectral sensitivity among deep-water cichlids from Lakes Tanganyika and Malawi. Proc Natl Acad Sci U S A. 2005;102(15):5448–5453. doi: 10.1073/pnas.0405302102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Terai Y, Okada N. Natural selection of the rhodopsin gene during the adaptive radiation of East African Great Lakes cichlid fishes. Mol Biol Evol. 2002;19(10):1807–1811. doi: 10.1093/oxfordjournals.molbev.a004004. [DOI] [PubMed] [Google Scholar]
- Terai Y, Mayer WE, Klein J, Tichy H, Okada N. The effect of selection on a long wavelength-sensitive (LWS) opsin gene of Lake Victoria cichlid fishes. Proc Natl Acad Sci U S A. 2002;99(24):15501–15506. doi: 10.1073/pnas.232561099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai Y, Seehausen O, Sasaki T, Takahashi K, Mizoiri S, Sugawara T, Sato T, Watanabe M, Konijnendijk N, Mrosso HD, Tachida H, Imai H, Shichida Y, Okada N. Divergent selection on opsins drives incipient speciation in Lake Victoria cichlids. PLoS Biol. 2006;4(12):e433. doi: 10.1371/journal.pbio.0040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe A, Douglas RH. Spectral transmission and short-wave absorbing pigments in the fish lens - II. Effects of age. Vision Res. 1993;33(3):301–307. doi: 10.1016/0042-6989(93)90086-c. [DOI] [PubMed] [Google Scholar]
- Thorpe A, Douglas RH, Truscott RJW. Spectral transmission and short-wave absorbing pigments in the fish lens - I. Phylogenetic distribution and identity. Vision Res. 1993;33(3):289–300. doi: 10.1016/0042-6989(93)90085-b. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. On aims and methods of ethology. Zeitschrift fur Tierpsychologie. 1963;20:410–433. [Google Scholar]
- Torres-Dowdall J, Henning F, Elmer KR, Meyer A. Ecological and Lineage-Specific Factors Drive the Molecular Evolution of Rhodopsin in Cichlid Fishes. Mol Biol Evol. 2015;32(11):2876–2882. doi: 10.1093/molbev/msv159. [DOI] [PubMed] [Google Scholar]
- van der Meer HJ. Visual resolution during growth in a cichlid fish: a morphological and behavioural case study. Brain Behav Evol. 1995;45(1):25–33. doi: 10.1159/000113383. [DOI] [PubMed] [Google Scholar]
- van der Meer HJ, Bowmaker JK. Interspecific variation of photoreceptors in four co-existing haplochromine cichlid fishes. Brain Behav Evol. 1995;45(4):232–240. doi: 10.1159/000113552. [DOI] [PubMed] [Google Scholar]
- Wagner CE, Harmon LJ, Seehausen O. Cichlid species-area relationships are shaped by adaptive radiations that scale with area. Ecol Lett. 2014;17(5):583–592. doi: 10.1111/ele.12260. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Kroger RH. Adaptive plasticity during the development of colour vision. Prog Retin Eye Res. 2005;24(4):521–536. doi: 10.1016/j.preteyeres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Weadick CJ, Chang BS. Complex patterns of divergence among green-sensitive (RH2a) African cichlid opsins revealed by Clade model analyses. BMC Evol Biol. 2012;12:206. doi: 10.1186/1471-2148-12-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weadick CJ, Loew ER, Rodd FH, Chang BS. Visual pigment molecular evolution in the Trinidadian pike cichlid < (Crenicichla frenata): a less colorful world for neotropical cichlids? Mol Biol Evol. 2012;29(10):3045–3060. doi: 10.1093/molbev/mss115. [DOI] [PubMed] [Google Scholar]
- Won YJ, Sivasundar A, Wang Y, Hey J. On the origin of Lake Malawi cichlid species: a population genetic analysis of divergence. Proc Natl Acad Sci U S A. 2005;102(Suppl 1):6581–6586. doi: 10.1073/pnas.0502127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray GA. The evolutionary significance of cis-regulatory mutations. Nat Rev Genet. 2007;8(3):206–216. doi: 10.1038/nrg2063. [DOI] [PubMed] [Google Scholar]
- Yokoyama S. Evolution of dim-light and color vision pigments. Annu Rev Genomics Hum Genet. 2008;9:259–282. doi: 10.1146/annurev.genom.9.081307.164228. [DOI] [PubMed] [Google Scholar]
- Young RW. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Invest Ophthalmol Vis Sci. 1978;17(2):105–116. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.