Abstract
Background
Intimal hyperplasia (IH) remains a major cause of poor patient outcomes after surgical revascularization to treat atherosclerosis. A multitude of drugs have been shown to prevent the development of IH. Moreover, endovascular drug delivery following angioplasty and stenting has been achieved with a marked diminution in the incidence of restenosis. Despite advances in endovascular drug delivery, there is currently no clinically available method of periadventitial drug delivery suitable for open vascular reconstructions. Herein we provide an overview of the recent literature regarding innovative polymer platforms for periadventitial drug delivery in preclinical models of IH as well as insights about barriers to clinical translation.
Methods
A comprehensive PubMed search confined to the past 15 years was performed for studies of periadventitial drug delivery. Additional searches were performed for relevant clinical trials, patents, meeting abstracts, and awards of NIH funding.
Results
Most of the research involving direct periadventitial delivery without a drug carrier was published prior to 2000. Over the past 15 years there have been a surge of reports utilizing periadventitial drug-releasing polymer platforms, most commonly bioresorbable hydrogels and wraps. These methods proved to be effective for the inhibition of IH in various animal models (e.g. balloon angioplasty, wire injury, and vein graft), but very few have advanced to clinical trials. There are a number of barriers that may account for this lack of translation. Promising new approaches including the use of nanoparticles will be described.
Conclusions
No periadventitial drug delivery system has reached clinical application. For periadventitial delivery, polymer hydrogels, wraps, and nanoparticles exhibit overlapping and complementary properties. The ideal periadventitial delivery platform would allow for sustained drug release yet exert minimal mechanical and inflammatory stresses to the vessel wall. A clinically applicable strategy for periadventital drug delivery would benefit thousands of patients undergoing open vascular reconstruction each year.
Keywords: Open surgery, periadventitial drug delivery, intimal hyperplasia, hydrogel, wrap, nanoparticles
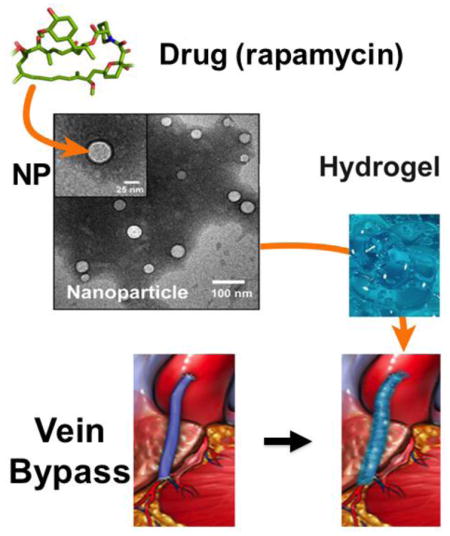
Graphic abstract
Periadventitial Drug Delivery — schematic depicting a periadventitial drug delivery platform of nanoparticles carried in a hydrogel. Based on Shi et al, PLoS One, 9 (2014) e89227.
Each year over a million patients in the US are treated with vascular surgical procedures for flow-limiting atherosclerosis or for hemodialysis access[1]. Although initially successful, a large proportion of these reconstructions eventually fail due to intimal hyperplasia (IH)[2]. IH can result from injury that occurs at the time of arterial reconstruction, for example, manipulation of a vein being prepared for bypass[3]. Alterations of hemodynamics can provide a more persistent stimulus for IH. An example of this is the exposure of a vein graft to arterial pressures and subsequent arteriolization of the vein[4]. The development of recurrent disease leads to the narrowing of the new conduit with the eventual development of stenosis or occlusion. Recently, significant progress has been made in the development of endovascular drug eluting stents (DESs) and balloons (DEBs), which have significantly reduced the incidence of restenosis [5, 6]. However, limitations of these technologies are quickly becoming evident. For example, drugs (rapamycin analogs or paclitaxel) released directly into the luminal vessel wall can impair reendothelialization, thereby increasing the risk of thrombosis[7]. Most importantly, drug delivery methods designed for stents or balloons are not applicable for the patients undergoing open vascular surgery. To date, there are no clinically available methods for drug delivery to prevent IH in patients undergoing open vascular reconstruction.
With a host of new biocompatible drug-carrying materials developed over the past decade, there is a refreshed interest in periadventitial drug delivery. It is somewhat surprising that the technology for endovascular drug delivery is well developed whereas a periadventitial approach for open vascular reconstructions, which is at least theoretically less challenging, has not yet reached clinical use. Endovascular delivery requires attachment of a drug to a balloon or stent and remote application. Release kinetics can be altered by the hemodynamics of blood flow. Alternatively, for open surgery, the drug carrier can be conveniently applied to the periadventitial surface of an artery or vein at the time of reconstruction and is unaffected by adverse flow or hemodynamics. Moreover, since the drug does not directly enter the circulation, there is improved bioavailability to the vessel wall while minimizing systemic toxicity. Since the drug is applied to the adventitia, away from the endothelial layer, there is diminished impairment of endothelial healing. The ideal periadventitial drug delivery system for effective prevention of IH would be one that allows for sustained and steady release of drug considering that the stimuli for IH following arterial reconstruction is often persistent. The ideal strategy would also produce minimal periadventitial inflammation as inflammation of the adventitia can stimulate myofibroblast migration into the subintimal space thereby enhancing IH or producing unfavorable constrictive remodeling[8]. Lastly, the ideal drug carrier would be one that is not bulky and has minimal effect on the hemodynamics of the treated arterial wall. Herewith we review the current options for periadventitial delivery and the associated benefits and disadvantages with regard to potential clinical use. Our focus is primarily on the literature published over the past 15 years and approaches that have at minimum been applied in preclinical models.
Direct Application
Until the beginning of this century, the most commonly used periadventitial delivery technique was the direct application of an anti-restenotic agent without a carrier. Using this approach, a number of therapeutic agents including small molecules, DNA, viral vectors, proteins and antibodies have been tested for their efficacy in preventing IH[9]. These agents have been directly applied to the arterial wall in either a powder form or in solution. Alternatively, a drug in-solution can be administered into the vessel wall of an ex-vivo vein (prior to grafting) using a pressure-mediated delivery system[10]. These studies show that direct application of anti-restenotic agents is somewhat effective in reducing IH in short-term animal models. While the greatest advantage of this approach is its simplicity, there are obvious limitations. Without a carrier, the therapeutic agent quickly diffuses into the capillary bed and into surrounding tissues making it necessary to use high concentrations of drug[11]. As such, there is potential toxicity to adjacent tissues and there is little ability to provide uniform drug administration. For these reasons, direct periadventitial application of drug has been replaced with carriers or platforms that allow more controlled and directed drug release.
Hydrogels
Hydrogels have become the most widely utilized platform for the periadventitial delivery of drugs to prevent IH in animal models. Examples of synthetic hydrogels used for periadventitial delivery include block copolymers of polyethylene glycol (PEG) such as PEG-polypropylene glycol (PPG)-PEG (e.g., Pluronic gel), poly(lactic-co-glycolic acid) (PLGA)-PEG-PLGA (e.g., ReGel gel) as well as gels derived from Sodium Alginate[12–15]. There are a number of reports where hydrogels have been used to deliver small molecules, DNA plasmids, siRNAs, viruses, as well as proteins/peptides into the perivascular space[16–18] Much of the enthusiasm surrounding this approach centers around its ease of use and its customizability.
The properties of hydrogels can be readily adjusted by controlling the polymer concentration, structure and molecular weight[19]. Many of the widely studied hydrogels used for biomedical applications are thermoreversible gels including Pluronic gel and ReGel [20, 21]. Thermoreversible gels typically remain in a liquid form at relatively low temperatures, convenient for mixing with various therapeutic agents; however, they can rapidly form gels at body temperature creating a drug reservoir localized at the treatment site. A prominent feature of thermoreversible hydrogels is their ability to mold into any shape, in this case forming an attached layer surrounding the vessel. This formability is particularly advantageous for periadventitial drug delivery since the artery is circumferential and the anatomy around bifurcations and anastomoses is even more complex. In addition, also showing excellent formability, a self-assembling nanofiber gel capable of nitric oxide release effectively reduced IH after periadevtitial application in a rat carotid injury model [22].
Although hydrogels are the most commonly used approach for perivascular drug delivery, they have inherent limitations. First, many studies including our own, reveal that hydrogels often produce an initial burst of drug release immediately after the drug-laden hydrogel is applied to the arterial wall[13, 23]. Although there are some advantages of the early release of a bolus of drug for the prevention of IH, this early loss of large quantities of drug leaves less available for long-term delivery to the arterial wall. After an initial burst, drug is then released quite slowly from the hydrogel until the hydrogel itself dissolves[24]. With this dissolution, there is often the rapid release of a second large bolus of drug causing similar issues. The timing of the second bolus is related to the kinetics of dissolution of the hydrogel[25]. For example, Pluronic gel F127 dissolves over 3 days and as such the second bolus is almost immediate. Alternatively, a hydrogel made in our laboratory, comprised of PLGA-PEG-PLGA block copolymer (referred to as Triblock gel), dissolves at 6 weeks so the second large bolus occurs around 6 weeks after application of the hydrogel (Kent et al, unpublished data). Thus drug release from hydrogels is typically bimodal, with a large bolus at the time of initial application and a second large bolus when the gel dissolves. Although a bimodal bolus of drug may be effective in preventing IH, theoretically a steady and sustainable release of drug would be more desirable.
The duration of drug delivery required to prevent IH has not been well established and may vary with the type of reconstruction. For example with angioplasty, there is an acute injury to the arterial wall and drug may be required for only a short period during which the injury resolves and the artery heals. That said, it is not clear how short is short, and even with acute arterial injury healing is not immediate and drug may be required for 6 weeks or more following injury. To this end, recently published data using a rat carotid angioplasty model suggest that delivery of rapamycin through Pluronic gel produces only temporary inhibition of IH at two weeks with recurrence of disease by four weeks[13]. Alternatively following a vein bypass, exposure of the vein which is accustomed to a low pressure system, now to arterial pressures (arterialization), produces a stimulus for IH that continues throughout the life span of the vein graft. Therefore, prevention of IH following bypass grafting with an autogenous vein may require prolonged release of the drug. Thus use of a hydrogel such as Pluronic gel for drug delivery, which dissolves over three days, may be an ineffective approach to IH.
There are additional disadvantages of hydrogels. Hydrogels may not be homogenous. Moreover, the quantity of drug that can be loaded into a hydrogel is limited compared to other deliver methods[19]. In addition, it is difficult to control hydrogel elasticity and degradability[26]. Hydrogels can also produce an inflammatory response due to decomposition. Reid et al. demonstrated that implantation of a hydrogel into adipose tissue in rats increased macrophage and neutrophil infiltration[27]. Zhu et al echoed these findings with significant increases in T cell and macrophage infiltration after intramuscular injection of the PNIPAM hydrogel[28].
It is worth noting that considerable efforts have been devoted to circumvent the foregoing limitations of hydrogels. A PLGA-PEG-PLGA triblock hydrogel exhibits improved properties over Pluronic gel. This hydrogel has a life-span of 60 days with continuous release of rapamycin throughout[29]. By adding a second layer that covers the hydrogel and directionally “caps” drug diffusion, Sanders et al. was able to significantly reduce drug loss to the surrounding tissues[30]. In order to prevent the initial drug loss due to burst release, Chun et al covalently crosslinked paclitaxel to the hydrogel resulting in markedly prolonged drug release[31]. This technique has not been attempted with periadventitial drug delivery for the prevention of IH. Moreover, natural hydrogels have also been used for periadventitial drug delivery, examples being hyaluronic acid (HA), collagen and others[32, 33]. Although these materials elicit a minimal inflammatory response, they have a relatively short life span because of their susceptibility to biodegradation. As new materials are being discovered, more opportunities will emerge for producing hydrogels with improved properties to achieve the goal of steady prolonged periadventitial release with minimal mechanical and inflammatory stress to the vessel wall.
Wraps
Another important platform for periadventitial drug delivery is polymer wraps. Wraps share a few favorable characteristics with hydrogels. For example, they can be easily produced. Following evaporation of an organic solvent used to dissolve the drug/polymer mixture, a drug-loaded polymer film (or wrap) can be formed. The thickness of the film and drug loading capacity are readily adjustable by controlling the amount of the various polymer(s)[34]. A number of FDA-approved biocompatible and biodegradable polymers including aliphatic polyesters such as polyglycolic acid (PGA), poly(lactic-co-glycolic acid) (PLGA), poly(lactic acid) (PLLA), and poly(ε-caprolactone) (PCL) can be used to create polymer wraps. Such polymers have been used for a variety of clinical applications particularly in orthopedics[35]. Moreover, a number of different versions, such as polymer cuffs, meshes, films, or sheaths, collectively termed “wraps” for purposes of this review, have been used for periadventitial drug delivery in animal models of intimal hyperplasia.
Like hydrogels, polymer wraps possess distinct properties desirable for periadventitial drug delivery. 1) Polymer wraps provide the mechanical strength and rigidity to allow for relatively easy deployment and immobilization around a blood vessel. 2) The hydrophobic environment inside the polymer matrix confers a relatively high loading capacity for hydrophobic drugs while protecting the drug from early hydrolysis. For example, in a PCL film of 0.5 cm2 size and 50 μm thickness, at least 100 μg of rapamycin can be loaded[34]. 3) Polymer wraps can persist for longer periods, leading to more prolonged drug release than with hydrogels. For example, a PCL wrap provides a steady rapamycin release for at least 2 months[34]. 4) An initial loss of the drug due to burst release is less of a problem with polymer wraps than with hydrogels, likely due to the polymer wraps having a more condensed structure or greater hydrophobicity, or both. 5) In addition, polymers with different drug release properties can be conveniently mixed to produce co-polymers attaining desirable “customized” release profiles[34]
A number of polymer wraps have been tested for their effectness in preventing neointimal hyperplasia in various animal models. Pires et al reported that paclitaxel- and rapamycin-eluting PCL cuffs placed around the mouse femoral artery reduced IH by ~75% at 21 days, compared to drug-free control[36]. Using a rabbit vein graft model, Skalsky et al found that a sirolimus-releasing polyester mesh wrapped around the graft reduced the intima-media thickness by 60% and 30% after 3 and 6 weeks, respectively [37]. Yu et al showed that a periadventitial PCL sheath steadily releasing rapamycin, greatly reduced (by 85%) IH compared to a drug-free sheath two weeks after balloon angioplasty of the rat common carotid artery[34]. Most recently, Gregory et al used a citrate-based polyester membrane for periadventitial delivery of all trans retinoic acid to balloon injured rat carotid arteries and observed a 50% reduction of restenosis two weeks after surgery[38].
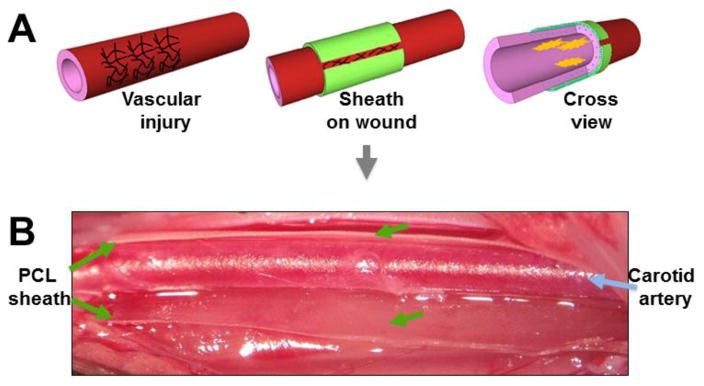
While various drug-loaded polymer wraps have been shown to be effective in mitigating IH, it is important to note that these wraps have also exhibited various limitations. A common problem is the mechanical stress imposed on the vessel due to the rigidity of the wrap. For example, while the PCL cuff serves as a drug-releasing device, the cuff itself (without drug) can induce IH in the mouse artery[36]. In studies by Yu et al, PLGA and PLLA wraps were soft when initially applied around the vessel wall, but became stiff and rigid in vivo and produced thrombosis of arteries surrounded by the hardened structure[34]. To circumvent this problem, when using a PCL sheath the authors created a modification such that the shealth was not placed in a fully circumferential manner thus leaving the wrap open at the top (Figure 1). Since this provided flexibility allowing normal hemodynamics, thrombosis was prevented. Alternatively, to alleviate the issue of arterial constriction produced by more traditional wraps, Serrano et al employed elastomers, which provided enhanced elasticity[39].
Figure 1. Non-constrictive periadventitial sheath.
Diagram (A) and picture (B) showing a PCL sheath wrapped around a balloon-injured segment of rat carotid artery with an open slot, which generates flexibility and reduces constriction. (Reproduced from Yu et al (JCR) with permission).
Although periadventitial wraps, much like hydorgels, deliver drug to the vessel wall, they inevitably release drug into the surrounding tissues with the potential of local toxicity. Sanders et al elegantly addressed this issue with a bilayer polymer wrap employing a drug-free non-porous outer barrier laminated onto a drug-loaded porous inner layer which is in direct contact with the vessel wall [30]. Using Sunitinib as the drug in a pig model of arteriovenous (AV) hemodialysis graft access, they found this bilayer PLGA wrap was able to provide unidirectional drug delivery to the vessel with minimal drug loss to extravascular tissues. While multilayer drug delivery systems are conceptually ideal to produce directed drug delivery, they are bulky and complex to produce. These issues in turn can affect arterial hemodynamics and degradation products can promote inflammation leading to constrictive vessel remodeling. With this in mind, some investigators have explored naturally occuring materials such as hyaluronic acid and collagens as drug carriers[40, 41]. A recent example is the evaluation of a sirolimus-eluting collagen wrap in humans. Patients undergoing AVG placement received the collagen membrane (Coll-R) around the site of the venous and PTFE anastamosis. Unfortunately, the lack of appropriate controls prohibited any definitive conclusions regarding the efficacy of the collagen wrap. Although associated with minimal inflammation, natural materials are generally not able to provide the same amount of mechanical strength (for wrapping) or durability compared to synthetic polymers. These disadvantages are also shared by hydrogels as previously discussed. Interestingly, progress has been made in the development of “hybrids” that reconcile the properties of synthetic polymer wraps and hydrogels formed by natural materials. For example, a prototypical design is a bilayer wrap recently produced by Sanders et al, in which the PCL outer layer provides durable mechanical support while the natural HA hydrogel infused into the porous inner layer produces prolonged drug release in vitro [30]. To date, the in vivo efficacy of this hybrid wrap for inhibiting neointima remains to be assessed. Nevertheless, continued research is required for the invention of next-generation periadventitial wraps that are 1) durable, 2) bioresorbable, 3) non-inflammatory, 4) not disruptive to the vessel, and 5) can directionally release drug in a controlled and sustained manner. Ultimately, a wrap with all of these characteristics would be highly effective in preventing IH.
Nanoparticles
The latest development in periadventitial drug delivery is the use of nanoparticles (NP) as a drug-releasing platform, which echoes the recent surge of research in nanomedicine. Compared to traditional platforms, such as hydrogels and polymer wraps, NPs possess a number of properties favorable for periadventitial delivery. (1) Their small size (typically 10–200 nm) allows for efficient infiltration of NPs into the arterial wall with drug release or endocytosis of cells[42]. (2) Due to their minute mass, their degradation products are less likely to produce an inflammatory response as compared to bulky hydrogels or wraps[43–45]. In addition, NPs are less likely impose mechanical stress on the vessel wall. (3) NPs are highly customizable and can be readily tailored for controlled and sustained drug release[46]. (4) NPs can be produced with hydrophobic cores to harbor a hydrophobic drug, while a hydrophilic outer surface renders the drug-loaded NPs highly soluble[47]. (5) NPs can be fluorescently labeled, facilitating in vitro and in vivo imaging and localization[47]. (6) For targeting a specific tissue or cell population, the surface of the NP can be conjugated with targeting ligands[48, 49]. As such targeted delivery using NPs could provide high local drug concentrations to pathogenic cells with minimal collateral damage to normal cells, a unique strength of NPs that cannot be achieved with traditional platforms (e.g. hydrogels or wraps) [50].
NPs have been widely applied in various disease models particularly in cancer[49]. While considerable attention has been given to the endovascular delivery of NPs via systemic injection[51], research on NPs for periadventitial drug delivery is just emerging In a study of periadventitial application, Rajathurai et al utilized rapamycin-loaded microspheres in Pluronic gel to treat IH in a pig vein graft model[52]. While low doses of rapamycin did not inhibit IH, high doses were associated with toxicity as manifested by graft rupture and the paradoxical acceleration of vein graft stenosis. Nonetheless, they demonstrated a 63% reduction in neointimal growth after 4 weeks of treatment with a rapamycin dose of 60 ug/cm2. Although microspheres (or microbeads) share similarities with NPs, they are of a larger size and possess different release kinetics. The first study using NPs for periadventitial delivery was reported by Li et al in 2010[53]. These investigators used a lysine-based NP incorporating the siRNA of NOX2 (an enzyme generating reactive oxygen species) to inhibit IH after balloon angioplasty. Two weeks after periadventitial application of these NPs, IH was reduced by 83% compared to control NPs without NOX2 siRNA. Efficient siRNA delivery was demonstrated by penetration of fluorescent-labeled NPs into the artery wall and an 85% knockdown of NOX2. In another study Gasper et al used a unique approach to deliver NPs formed by conjugating albumin and rapamycin (termed Nab-rapamycin), by injecting these particles into the adventitia via an intraluminally inserted microinfusion balloon catheter with needles that transgress the arterial wall[54]. Although this periadventitial delivery method is intrusive to the vessel, the treatment proved safe and reduced IH following balloon angioplasty. More recently, Shi et al used periadventitial NPs to deliver rapamycin for inhibition of restenosis in a rat carotid balloon injury model[13]. Almost all the drug carriers including hydrogels, wraps, microspheres and NPs are associated with an initial burst release at the time of application. Thus a prominent question arises, i.e. to produce sustained inhibition of IH, is the initial burst release sufficient or is continued release over some period of time required? To address this question, Shi et al compared the inhibitory effect (on IH) of rapamycin mixed in Pluronic gel, which dissolves over 3 days thus only providing early burst release, and rapamycin loaded in PLGA NPs (suspended in Pluronic gel), which produces release of rapamycin over two weeks. These investigators found that rapamycin loaded in Pluronic gel inhibited smooth muscle cell proliferation (Ki-67 staining) and IH at 2 weeks after periadventitial application, but with recurrence of both by 4 weeks. Alternatively, rapamycin-loaded NPs produced durable inhibition of smooth muscle cell proliferation and IH at two as well as four weeks. These studies strongly support the notion that prolonged drug delivery facilitated by NPs promotes lasting inhibition of IH.
Depending on the required functionality, an array of NPs, e.g., solid polymer nanoparticles, polymer micelles, and solid lipid nanoparticles have been produced and tested in a variety of in vitro and in vivo experimental systems [55, 56]. The “payload” that can be delivered ranges from small molecules, proteins/peptides, to nucleic acid (e.g., siRNAs and DNA). Recently, unimolecular micelle NPs, formed by individual/single multi-arm star amphiphilic block copolymer molecules, have gained more attention due to their excellent in vitro and in vivo stability, and chemical versatility including the ability of conjugating various types of targeting ligands [47, 57–59]. NPs conjugated with a specific type of targeting ligand can recognize its cognate receptor highly expressed on the surface of a specific population of cells allowing for preferential cellular uptake of targeted NPs. In a recent study, Chan et al synthesized paclitaxel-containing NPs that target Collagen IV. After systemical delivery following balloon injury[60], they observed greater efficacy of the targeted NPs versus non-targeted NPs 14 days after application. However, there have been no reports of periadventitial application using NPs targeting specific pathogenic smooth muscle cell populations or collagen in the vessel wall, underscoring a need for research in this area.
Though a highly promising tool for periadventitial drug delivery, NPs are not without drawbacks. Because of their small size and high solubility, periadventitially applied NPs may quickly diffuse into the surrounding tissues and the capillary bed, or be cleared by immune cells such as macrophages[61]. These issues could be resolved by specific tailoring, e.g. crosslinking NPs to the adventitia, or via combined use of NPs together with traditional platforms such as hydrogels[13]. While NPs embedded in a hydrogel can be effectively sequestered around the adventitia, evidence also indicates that coating with PEG and polylactic acid (PLA) copolymers can reduce the loss of NP to the immune system [62–64]. It is clear that the combination of hydrogels and NPs offers another layer of manipulation to generate optimal drug release kinetics.
Clinical Trials
A variety of polymer platforms have been used for periadventitial drug delivery and many of these have proven efficacious for inhibition of IH in preclinical models. However, none of these have advanced to clinical application. In fact, only a few clinical trials have been carried out to evaluate periadventitial drug delivery. The trials that have been performed are generally for patients receiving hemodialysis access. A rapamycin-impregnated polymer mesh was recently tested for safety and efficacy for hemodialysis access. Unfortunately this clinical trial (NCT01033357) was terminated due to an increase in the rate of graft infection in the experimental group. Another clinical trial (NCT00895479) which involved the periadventitial application of a VEGF-D-expressing adenovirus injected into a reservoir formed by collagen collars entered Phase III but was terminated for ‘strategic reasons’. In addition, the V-Health Phase I/II trials by Conte et al tested periadventitially applied endothelial cells in a gelfoam matrix in an effort to reduce neointimal hyperplasia formation in patients undergoing placement of dialysis access [65]. Unfortunately, the investigators were unable to demonstrate a significant difference in primary or secondary patency by using this approach. Most recently, a phase I/II trial of periadventitial application of recombinant human pancreatic elastase I dissolved in phosphate buffered saline administered to patients undergoing dialysis access was completed (NCT01001351)[66, 67]. The overall 30-day outcomes were not significantly different for drug-treated patients. The subgroup of patients undergoing placement of a radiocephalic fistula and treated with elastase did demonstrate a statistically significant increase in primary patency at 3-years. Encouraged by these results, Proteon Therapeutics has begun to enroll this group of patients in a Phase III trial (NCT02110901) (NCT02414841). The PREVENT trial was a major investigation of the use of an oligonucleotide decoy of the transcription factor E2F (edifoligide) applied intraluminally to vein grafts at the time of arterial bypass. It is important to note that in this trial, the transcription factor was not delivered periadventially but through a pressurized intaluminal technique [68–70]. Unfortunately, the Phase III/IV trials indicated that the decoy was not more effective than placebo in preventing vein graft failure. In summary, to date there have been no successful clinical trials proving benefit to periadvential drug delivery in the prevention of intimal hyperplasia. This is surprising considering the great success achieved to date in preclinical studies. This realization underscores the need for further investigation into strategies to translate these various approaches to drug delivery into the clinical arena.
Conclusion
Over the past 15 years, in contrast to a rapid advancement in endovascular drug delivery technologies including drug-eluting stents and dru-eluting balloons, there remains a conspicuous lack of a clinically available approach to perivascular drug delivery for open vascular reconstructive surgery. As open surgeries including coronary artery bypass, peripheral bypass, as well as renal hemodialysis access remain common interventions, there is no doubt that a clinically applicable periadventitial drug delivery system is an urgent medical need. During the past decade, encouraging progress has been made in evaluating a variety of polymer materials and formats for periadventitial delivery, some of which have shown substantial efficacy for inhibiting IH in various preclinical models. Of particular note, nanoparticles open a new frontier for this endeavor. However, human clinical trials to translate these preclinical outcomes have severely lagged. Continued research to identify innovative periadventitial delivery methods to prevent recurrent vascular disease followed by properly designed clinical trials will ultimately benefit thousands of patients undergoing vascular reconstruction each year.
Table 1.
Comparison of periadventitial drug delivery methods
| Methods | Advantages | Disadvantages |
|---|---|---|
| Direct Application |
|
|
| Hydrogels |
|
|
| Wraps |
|
|
| Nanoparticles |
|
|
Acknowledgments
This work was supported by a National Heart Lung and Blood Institute R01 grant (HL-068673, to KCK), a T-32 training (for MAC) grant (HL-110853, to KCK), an American Heart Association Grant-In-Aid fund (14GRNT20380854, to KCK), and a State of Wisconsin Partnership Program New Investigator Award (ID2832, to L-WG). In addition, this work was also supported by University of Wisconsin ICTR-Clinical and Type 1 Translational Research Pilot Program (UL1TR000427, to K.C.K.). This funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jim J, Owens PL, Sanchez LA, Rubin BG. Population-based analysis of inpatient vascular procedures and predicting future workload and implications for training. J Vasc Surg. 2012;55:1394–1399. doi: 10.1016/j.jvs.2011.11.061. discussion 1399–1400. [DOI] [PubMed] [Google Scholar]
- 2.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 3.Samano N, Geijer H, Liden M, Fremes S, Bodin L, Souza D. The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J Thorac Cardiovasc Surg. 2015;150:880–888. doi: 10.1016/j.jtcvs.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Kwei S, Stavrakis G, Takahas M, Taylor G, Folkman MJ, Gimbrone MA, García-Cardeña G. Early Adaptive Responses of the Vascular Wall during Venous Arterialization in Mice. Am J Pathol. 2004;164:81–89. doi: 10.1016/S0002-9440(10)63099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Snyder SA, O’Leary EE, Tepe G, Scheinert D, Zeller T. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J Am Coll Cardiol. 2013;61:2417–2427. doi: 10.1016/j.jacc.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 8.Zalewski A, Shi Y. Vascular myofibroblasts. Lessons from coronary repair and remodeling. Arterioscler Thromb Vasc Biol. 1997;17:417–422. doi: 10.1161/01.atv.17.3.417. [DOI] [PubMed] [Google Scholar]
- 9.Wiedemann D, Kocher A, Bonaros N, Semsroth S, Laufer G, Grimm M, Schachner T. Perivascular administration of drugs and genes as a means of reducing vein graft failure. Curr Opin Pharmacol. 2012;12:203–216. doi: 10.1016/j.coph.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Kibbe MR, Tzeng E, Gleixner SL, Watkins SC, Kovesdi I, Lizonova A, Makaroun MS, Billiar TR, Rhee RY. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J Vasc Surg. 2001;34:156–165. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 11.Lovich MA, Brown L, Edelman ER. Drug clearance and arterial uptake after local perivascular delivery to the rat carotid artery. J Am Coll Cardiol. 1997;29:1645–1650. doi: 10.1016/s0735-1097(97)00123-x. [DOI] [PubMed] [Google Scholar]
- 12.McLennan G, Johnson MS, Stookey KR, Zhang Z, Fife WK. Kinetics of release of heparin from alginate hydrogel. J Vasc Interv Radiol. 2000;11:1087–1094. doi: 10.1016/s1051-0443(07)61344-x. [DOI] [PubMed] [Google Scholar]
- 13.Shi X, Chen G, Guo LW, Si Y, Zhu M, Pilla S, Liu B, Gong S, Kent KC. Periadventitial application of rapamycin-loaded nanoparticles produces sustained inhibition of vascular restenosis. PLoS One. 2014;9:e89227. doi: 10.1371/journal.pone.0089227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forte A, Grossi M, Turczynska KM, Svedberg K, Rinaldi B, Donniacuo M, Holm A, Baldetorp B, Vicchio M, De Feo M, Sante P, Galderisi U, Berrino L, Rossi F, Hellstrand P, Nilsson BO, Cipollaro M. Local inhibition of ornithine decarboxylase reduces vascular stenosis in a murine model of carotid injury. Int J Cardiol. 2013;168:3370–3380. doi: 10.1016/j.ijcard.2013.04.153. [DOI] [PubMed] [Google Scholar]
- 15.Owen SC, Li H, Sanders WG, Cheung AK, Terry CM. Correlation of tissue drug concentrations with in vivo magnetic resonance images of polymer drug depot around arteriovenous graft. J Control Release. 2010;146:23–30. doi: 10.1016/j.jconrel.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seedial SM, Ghosh S, Saunders RS, Suwanabol PA, Shi X, Liu B, Kent KC. Local Drug Delivery to Prevent Restenosis. J Vasc Surg. 2013;57:1403–1414. doi: 10.1016/j.jvs.2012.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baek S, March KL. Gene therapy for restenosis: getting nearer the heart of the matter. Circ Res. 1998;82:295–305. doi: 10.1161/01.res.82.3.295. [DOI] [PubMed] [Google Scholar]
- 18.Ahanchi SS, Varu VN, Tsihlis ND, Martinez J, Pearce CG, Kapadia MR, Jiang Q, Saavedra JE, Keefer LK, Hrabie JA, Kibbe MR. Heightened efficacy of nitric oxide-based therapies in type II diabetes mellitus and metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008:H2388–2398. doi: 10.1152/ajpheart.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoare TK, Daniel Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49:1993–2007. [Google Scholar]
- 20.Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharm Sci. 2006;9:339–358. [PubMed] [Google Scholar]
- 21.Zentner GM, Rathi R, Shih C, McRea JC, Seo MH, Oh H, Rhee BG, Mestecky J, Moldoveanu Z, Morgan M, Weitman S. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release. 2001;72:203–215. doi: 10.1016/s0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]
- 22.Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Eng JW, Murar J, Martinez J, Popowich DA, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Hulvat JF, Stupp SI, Kibbe MR. Nitric Oxide and Nanotechnology: A Novel Approach to Inhibit Neointimal Hyperplasia. J Vasc Surg. 2008;47:173–182. doi: 10.1016/j.jvs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 24.dos Santos ACM, Akkari ACS, Ferreira IRS, Maruyama CR, Pascoli M, Guilherme VA, de Paula E, Fraceto LF, de Lima R, da Melo SP, de Araujo DR. Poloxamer-based binary hydrogels for delivering tramadol hydrochloride: sol-gel transition studies, dissolution-release kinetics, in vitro toxicity, and pharmacological evaluation. Int J Nanomedicine. 2015:2391–2401. doi: 10.2147/IJN.S72337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong B, Bae YH, Kim SW. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J Control Release. 2000;63:155–163. doi: 10.1016/s0168-3659(99)00194-7. [DOI] [PubMed] [Google Scholar]
- 26.Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release. 2011;154:258–266. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid B, Gibson M, Singh A, Taube J, Furlong C, Murcia M, Elisseeff J. PEG hydrogel degradation and the role of the surrounding tissue environment. J Tissue Eng Regen Med. 2015;9:315–318. doi: 10.1002/term.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu R, Wu G, Liu X, Shi D, Cao B, Gu R, Xiao J, Liao H. PNIPAM hydrogel induces skeletal muscle inflammation response. 2015 [Google Scholar]
- 29.Zhu W, Masaki T, Cheung AK, Kern SE. In-vitro Release of Rapamycin from a Thermosensitive Polymer for the Inhibition of Vascular Smooth Muscle Cell Proliferation. J Bioequiv Availab. 2009;1:3–12. doi: 10.4172/jbb.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders WG, Hogrebe PC, Grainger DW, Cheung AK, Terry CM. A biodegradable perivascular wrap for controlled, local and directed drug delivery. J Control Release. 2012;161:81–89. doi: 10.1016/j.jconrel.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chun C, Lee SM, Kim SY, Yang HK, Song SC. Thermosensitive poly(organophosphazene)-paclitaxel conjugate gels for antitumor applications. Biomaterials. 2009;30:2349–2360. doi: 10.1016/j.biomaterials.2008.12.083. [DOI] [PubMed] [Google Scholar]
- 32.Kjøniksen AL, Calejo MT, Zhu K, Nyström B, Sande SA S.o.P.U.o.O.D.o.P.O. Norway, Ø.U.C.F.o.E.H. Norway, S.o.P.U.o.O.D.o.P.O. Norway, T.U.o.T.D.o.E.a.C.E.T. Finland, U.o.O.D.o.C.O. Norway, U.o.O.D.o.C.O. Norway, S.o.P.U.o.O.D.o.P.O. Norway. Stabilization of pluronic gels in the presence of different polysaccharides. Journal of Applied Polymer Science. 2015;131 [Google Scholar]
- 33.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Takayama T, Goel SA, Shi X, Zhou Y, Kent KC, Murphy WL, Guo LW. A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia. J Control Release. 2014;191:47–53. doi: 10.1016/j.jconrel.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciccone WJ, 2nd, Motz C, Bentley C, Tasto JP. Bioabsorbable implants in orthopaedics: new developments and clinical applications. J Am Acad Orthop Surg. 2001;9:280–288. doi: 10.5435/00124635-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Pires NM, van der Hoeven BL, de Vries MR, Havekes LM, van Vlijmen BJ, Hennink WE, Quax PH, Jukema JW. Local perivascular delivery of anti-restenotic agents from a drug-eluting poly(epsilon-caprolactone) stent cuff. Biomaterials. 2005;26:5386–5394. doi: 10.1016/j.biomaterials.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 37.Skalsky I, Szarszoi O, Filova E, Parizek M, Lytvynets A, Maluskova J, Lodererova A, Brynda E, Lisa V, Burdikova Z, Capek M, Pirk J, Bacakova L. A perivascular system releasing sirolimus prevented intimal hyperplasia in a rabbit model in a medium-term study. Int J Pharm. 2012;427:311–319. doi: 10.1016/j.ijpharm.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Gregory EK, Webb AR, Vercammen JM, Flynn ME, Ameer GA, Kibbe MR. Periadventitial at RA citrate-based polyester membranes reduce neointimal hyperplasia and restenosis after carotid injury in rats. Am J Physiol Heart Circ Physiol. 2014;307:H1419–1429. doi: 10.1152/ajpheart.00914.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serrano MC, Vavra AK, Jen M, Hogg ME, Murar J, Martinez J, Keefer LK, Ameer GA, Kibbe MR. Poly(diol-co-citrate)s as novel elastomeric perivascular wraps for the reduction of neointimal hyperplasia. Macromol Biosci. 2011;11:700–709. doi: 10.1002/mabi.201000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bahcivan M, Yucel S, Kefeli M, Gol MK, Can B, Keceligil HT. Inhibition of vein graft intimal hyperplasia by periadventitial application of hyaluronic acid-carboxymethyl cellulose: an experimental study. Scand Cardiovasc J. 2008;42:161–165. doi: 10.1080/14017430701747108. [DOI] [PubMed] [Google Scholar]
- 41.Paulson WD, Kipshidze N, Kipiani K, Beridze N, DeVita MV, Shenoy S, Iyer SS. Safety and efficacy of local periadventitial delivery of sirolimus for improving hemodialysis graft patency: first human experience with a sirolimus-eluting collagen membrane (Coll-R) Nephrol Dial Transplant. 2012;27:1219–1224. doi: 10.1093/ndt/gfr667. [DOI] [PubMed] [Google Scholar]
- 42.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8:147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Tian J, Wong KK, Ho CM, Lok CN, Yu WY, Che CM, Chiu JF, Tam PK. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem. 2007;2:129–136. doi: 10.1002/cmdc.200600171. [DOI] [PubMed] [Google Scholar]
- 44.Bhol KC, Schechter PJ. Effects of nanocrystalline silver (NPI 32101) in a rat model of ulcerative colitis. Dig Dis Sci. 2007;52:2732–2742. doi: 10.1007/s10620-006-9738-4. [DOI] [PubMed] [Google Scholar]
- 45.Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJ, Verharen HW, Briede JJ, van Loveren H, de Jong WH. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011;32:9810–9817. doi: 10.1016/j.biomaterials.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 47.Chen G, Wang L, Cordie T, Vokoun C, Eliceiri KW, Gong S. Multi-functional self-fluorescent unimolecular micelles for tumor-targeted drug delivery and bioimaging. Biomaterials. 2015;47:41–50. doi: 10.1016/j.biomaterials.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan CE, Wasserman MA, Kibbe MR. Targeted Nanotherapies for the Treatment of Surgical Diseases. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Hussain S, Pluckthun A, Allen TM, Zangemeister-Wittke U. Antitumor activity of an epithelial cell adhesion molecule targeted nanovesicular drug delivery system. Mol Cancer Ther. 2007;6:3019–3027. doi: 10.1158/1535-7163.MCT-07-0615. [DOI] [PubMed] [Google Scholar]
- 51.Yin RX, Yang DZ, Wu JZ. Nanoparticle Drug- and Gene-eluting Stents for the Prevention and Treatment of Coronary Restenosis. Theranostics. 2014:175–200. doi: 10.7150/thno.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajathurai T, Rizvi SI, Lin H, Angelini GD, Newby AC, Murphy GJ. Periadventitial rapamycin-eluting microbeads promote vein graft disease in long-term pig vein-into-artery interposition grafts. Circ Cardiovasc Interv. 2010;3:157–165. doi: 10.1161/CIRCINTERVENTIONS.109.864660. [DOI] [PubMed] [Google Scholar]
- 53.Li JM, Newburger PE, Gounis MJ, Dargon P, Zhang X, Messina LM. Local arterial nanoparticle delivery of siRNA for NOX2 knockdown to prevent restenosis in an atherosclerotic rat model. Gene Ther. 2010;17:1279–1287. doi: 10.1038/gt.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gasper WJ, Jimenez CA, Walker J, Conte MS, Seward K, Owens CD. Adventitial nab-rapamycin injection reduces porcine femoral artery luminal stenosis induced by balloon angioplasty via inhibition of medial proliferation and adventitial inflammation. Circ Cardiovasc Interv. 2013;6:701–709. doi: 10.1161/CIRCINTERVENTIONS.113.000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48:416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Reviews Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 57.Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn H40, poly(L-lactide) and poly(ethylene glycol) for tumor-targeted drug delivery. Biomaterials. 2009;30:3009–3019. doi: 10.1016/j.biomaterials.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Guo J, Hong H, Chen G, Shi S, Zheng Q, Zhang Y, Theuer CP, Barnhart TE, Cai W, Gong S. Image-guided and tumor-targeted drug delivery with radiolabeled unimolecular micelles. Biomaterials. 2013;34:8323–8332. doi: 10.1016/j.biomaterials.2013.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu W, Siddiqui IA, Nihal M, Pilla S, Rosenthal K, Mukhtar H, Gong S. Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials. 2013;34:5244–5253. doi: 10.1016/j.biomaterials.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan JM, Rhee JW, Drum CL, Bronson RT, Golomb G, Langer R, Farokhzad OC. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid-polymeric nanoparticles. Proc Natl Acad Sci USA. 2011;108:19347–19352. doi: 10.1073/pnas.1115945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 62.Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Deliv Rev. 1995;16:215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peracchia MT, Fattal E, Desmaele D, Besnard M, Noel JP, Gomis JM, Appel M, d’Angelo J, Couvreur P. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release. 1999;60:121–128. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 64.He G, Ma LL, Pan J, Venkatraman S. ABA and BAB type triblock copolymers of PEG and PLA: a comparative study of drug release properties and “stealth” particle characteristics. Int J Pharm. 2007;334:48–55. doi: 10.1016/j.ijpharm.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 65.Conte MS, Nugent HM, Gaccione P, Guleria I, Roy-Chaudhury P, Lawson JH. Multicenter phase I/II trial of the safety of allogeneic endothelial cell implants after the creation of arteriovenous access for hemodialysis use: the V-HEALTH study. J Vasc Surg. 2009;50:1359–1368.e1351. doi: 10.1016/j.jvs.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 66.Dwivedi AJ, Roy-Chaudhury P, Peden EK, Browne BJ, Ladenheim ED, Scavo VA, Gustafson PN, Wong MD, Magill M, Lindow F, Blair AT, Jaff MR, Franano FN, Burke SK. Application of human type I pancreatic elastase (PRT-201) to the venous anastomosis of arteriovenous grafts in patients with chronic kidney disease. J Vasc Access. 2014;15:376–384. doi: 10.5301/jva.5000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hye RJ, Peden EK, O’Connor TP, Browne BJ, Dixon BS, Schanzer AS, Jensik SC, Dember LM, Jaff MR, Burke SK. Human type I pancreatic elastase treatment of arteriovenous fistulas in patients with chronic kidney disease. J Vasc Surg. 2014;60:454–461.e451. doi: 10.1016/j.jvs.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 68.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. Jama. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 69.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 70.Mann MJ, Whittemore AD, Donaldson MC, Belkin M, Conte MS, Polak JF, Orav EJ, Ehsan A, Dell’Acqua G, Dzau VJ. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354:1493–1498. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]