Abstract
Over one-third of patients with chronic lung disease undergoing lung transplantation have pre-existing antibodies against lung-restricted self-antigens, collagen type V (ColV) and k-alpha1 tubulin (KAT). These antibodies can also develop de novo following lung transplantation and mediate allograft rejection. However, the mechanisms leading to lung-restricted autoimmunity remain unknown. Since these self-antigens are normally sequestered, tissue injury is required to expose them to the immune system. We previously showed that respiratory viruses can induce apoptosis in CD4+CD25+Foxp3+ T cells (Tregs), the key mediators of self-tolerance. Therefore, we hypothesized that lung-tissue injury can lead to lung-restricted immunity if it occurs in a setting when Tregs are impaired. We found that human lung recipients who suffer respiratory viral infections experienced a decrease in peripheral Tregs. Pre-existing lung allograft injury from donor-directed antibodies or gastroesophageal reflux led to new ColV and KAT antibodies following respiratory viral infection. Similarly, murine parainfluenza (Sendai) respiratory viral infection caused a decrease in Tregs. Intratracheal instillation of anti-MHC class-I antibodies, but not isotype control, followed by murine Sendai virus (SdV) infection led to development of antibodies against ColV and KAT, but not collagen II (ColII), a cartilaginous protein. This was associated with expansion of IFN-γ producing CD4+ T cells specific to ColV and KAT, but not ColII. Intratracheal anti-MHC class-I antibodies or hydrochloric acid in Foxp3-DTR mice induced ColV and KAT, but not ColII, immunity, only if Tregs were depleted using diphtheria toxin. We conclude that tissue injury combined with loss of Tregs can lead to lung-tissue restricted immunity.
Introduction
Patients with chronic lung disease can develop immunity against lung-restricted antigens (1). In fact, over one-third of patients with end-stage lung disease who are undergoing lung transplantation have pre-existing non-human leukocyte antibodies (nHLAbs) against lung-restricted self-antigens collagen type V (ColV) and k-alpha1 tubulin V (KAT) (2–5). These antibodies strongly predispose to primary graft dysfunction (5, 6) as well as chronic lung rejection (5, 7), the predominant causes for early and late lung allograft failure, respectively (8). Immunity against lung-tissue restricted self-antigens can also develop de novo following lung transplantation. Over 70% of lung recipients have evidence of newly formed ColV and KAT antibodies within three years of transplantation (9). However, the mechanisms that lead to immunity against lung-restricted self-antigens remain undefined.
Antibodies against donor specific human leukocyte antigens (DSA) strongly predispose to the development of de novo nHLAbs. In a recent study, 96% of lung recipients with DSA developed nHLAbs (9). DSA likely lead to an inflammatory milieu in the lungs (10) that is favorable for immunity against lung tissue (11–13). Such an inflammatory milieu can also be induced by acid aspiration due to gastroesophageal reflux disease (14), a strong risk factor for lung allograft rejection (15–17). Inflammation and immune responses are suppressed by regulatory T cells (18–20). Naturally occurring CD4+CD25+Foxp3+ T cells (Tregs), one of the well-characterized regulatory T cells, can suppress the production of inflammatory cytokines and proliferation of cytotoxic T cells as well as antigen presenting cells (21–24). The loss of Tregs is associated with lung allograft rejection in both murine and human transplantation, suggesting that maintenance of Treg function is important for downregulating immune responses against allo- and self-antigens (21–26). In a murine model of orthotopic tracheal transplantation, we previously reported that respiratory viruses can upregulate FasL on infected airway epithelial cells, triggering the loss of Tregs (27). Therefore, we hypothesized that both ongoing injury to the lung graft by DSA or gastroesophageal reflux and a concomitant loss of Tregs, triggered for example by respiratory viruses, are necessary for the development of de novo lung-restricted immunity.
Materials and Methods
Human subjects
Blood specimens were collected from patients undergoing lung transplantation and normal volunteers after obtaining informed consent in accordance with a protocol approved by the Institutional Review Board. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation, and stored at −135°C. Plasma was stored at −70°C. Respiratory viral infection was defined as a positive microbial culture for respiratory syncytial virus (RSV), parainfluenza, influenza, or adenovirus from bronchoalveolar lavage, bronchial wash, tracheal aspirate, sputum culture, or nasopharyngeal swab specimens, as previously described (28).
Reagents and mice
Mouse anti-human CD4 (clone RPA-T4), mouse anti-human CD25 (clone M-A251), mouse anti-human Foxp3 (clone 259D/C7), rat anti-mouse CD4 (clone GK1.5), rat anti-mouse CD25 (clone 3C7), rat anti-mouse foxp3 (clone MF23), and isotype control antibodies including C1.18 were purchased from BD Biosciences (Pharmingen, San Diego, CA). Mouse FasL was analyzed in bronchoalveolar fluid using a standardized ELISA kit (R&D Systems, Minneapolis, MN). Six to eight week old C57Bl/6 (H-2kb), Fas mutant B6.MRL-Faslpr/J, and B6.129 (Cg)-Foxp3tm3(DTR/GFP)Ayr (Foxp3-DTR) mice were obtained (Jackson Laboratories, Bar Harbor, ME). All animal studies were performed with sterile precautions and approved by the institutional animal studies committee.
Animal Experiments
Antibody treatment: We administered anti-MHC class I Abs locally into the lung in order to avoid systemic effects. Murine mAb to H2Kb (IgG2a, Clone AF6-88.5 BD Biosciences), H2Db (IgG2a, Abcam, Clone B22-249.R1) or isotype control (C1.18, BioXcell Inc.), with no detectable endotoxin as measured by Limulus amebocyte lysate assay, were given intratracheally on days 1, 2, 3, and 6 and weekly thereafter (200 µg/administration). Sendai Viral Infection: Sendai Virus (SdV), FUSHIMI strain VR-105, (American Type Culture Collection, Manassas, VA) was stored at −70°C. Titration experiments were performed and a dose of 5,000 egg infectious dose (EID50)/animal of SdV (5K) was selected as previously described (27, 29, 30). Mice were anesthesized and 5K SdV diluted with 30 µl of PBS was slowly dropped into their nasal canal. Acid aspiration: To induce lung injury, 50µl of hydrochloric acid (0.1N) was administered into the trachea daily for seven days as previously described (31). Diphtheria toxin injection: Diphtheria toxin was administered to deplete the Foxp3 cells in the DTR mice as previously described (32). Briefly, 1 µg of diphtheria toxin or control phosphate-buffered saline were injected intraperitoneally on days 0 and 1.
Enzyme Linked Immunosorbent Assay (ELISA) for antibodies
Development of antibodies to k-alpha tubulin, collagen type V, and collagen type II (control) was determined by ELISA as previously described (10, 33). In brief, 1µg/mL of collagen type V, and collagen type II suspended in phosphate-buffered saline were coated onto an ELISA plate (Collagen type II and V were obtained from Chemicon/ Millipore, Billerica, MA, and recombinant k-alpha tubulin was expressed in our laboratory) and incubated overnight at 4°C. Diluted patient and normal sera were then added to these plates. Detection was done using anti-human IgG- horseradish peroxidase (1:10,000), developed using tetramethylbenzidine substrate and read at 450 nm. Antibody concentration was calculated using a standard curve from known concentrations of respective antibodies (Santa Cruz Biotechnology, CA).
Enzyme-linked immunospot (ELISPOT) for cellular immune response
Cellular immune responses to k-alpha tubulin, collagen type V, and collagen type II were enumerated using ELISPOT (10, 34). All antigens were endotoxin free. Briefly, multiscreen 96-well filtration plates were coated overnight at 4°C with 5.0µg/ml of capture human cytokine-specific mAb (BD Biosciences). The plates were then blocked with 1% BSA for 2h. Subsequently, 3×105 C57BL/6 splenocytes were cultured in triplicate in the presence of irradiated (3000 rads) syngeneic stimulators at a 1:1 ratio. The irradiated splenocytes were pulsed with the respective antigen overnight. After 48 h, the plates were washed and 2.0µg/ml of biotinylated human cytokine-specific mAb (BD Biosciences) was added to the wells. After an overnight incubation at 4°C, the plates were developed using horseradish peroxidase-labeled streptavidin (BD Biosciences), and 3-amino-9-ethylcarbazole substrate reagent (BD Biosciences). The spots were analyzed in an ImmunoSpot Series I analyzer (Cellular Technology, OH).
Histology
Tissue sections were stained with hematoxylin-eosin and trichrome and analyzed blindly. Images were obtained on a Nikon Eclipse microscope (Nikon, Melville, NY), and morphometric analysis performed using Nikon Elements software (Nikon, Melville, NY).
Statistical analysis
Morphometric analysis of tissue sections was performed using NIS-Elements Software (Nikon, Melville, NY). Analysis was performed on 5 different areas of the lungs and at least 5 fields were analyzed for each. Data were analyzed in SPSS v.19 (IBM Inc., Chicago, IL) and GraphPad Prism 5.0 (LaJolla, CA) and represented as mean±SD with at least 5 mice in each cohort. Chi-Square or Student T-tests were used as appropriate. Results were considered significant if p value was <0.05.
Results
Respiratory viral infections are associated with loss of Tregs and result in de novo lung-restricted immunity in the setting of concomitant lung injury
Lung transplant recipients with microbiologically proven respiratory viral infections (n=20) demonstrated a decrease in Tregs in the peripheral circulation (Pre-infection: 5.4±0.7% vs Post-infection 3.6±1.1%, p=0.006, Figure 1A). The decrease in Tregs was transient since at three months, the levels of Tregs in the peripheral blood had returned to their baseline (5.2±0.8%, Figure 1). However, at three months following the respiratory viral infection, 12 of these recipients developed de novo nHLAbs against lung-restricted antigens, ColV (Normal controls: 133±23.2 ng/ml, Pre-infection 152.0±41.8 ng/ml, Post-infection: 535.3±158.9 ng/ml, p<0.0001, Figure 1B-I) and KAT (Normal controls: 101.7±22.6 ng/ml, Pre-infection 97.2±18.4 ng/ml, Post-infection: 327.4±117.6 ng/ml, p<0.0001. Figure 1B-I). There were no significant differences between the clinical and demographic variables including age, gender, underlying disease process, course of transplant, acute rejection, or primary graft dysfunction between patients that developed nHLAbs and those that did not. Of the 12 recipients that developed lung-restricted nHLAbs, eight had antibodies against donor HLA class I antigens (DSA) prior to the viral infection and four suffered from gastro-esophageal reflux, as determined by 24-hour pH monitoring. Patients infected with respiratory viruses without DSA or GERD (n=8) did not develop antibodies against ColV (Normal controls: 133±23.2 ng/ml, Pre-infection 118.0±17.8 ng/ml, Post-infection: 120.0±19.3 ng/ml, p=0.9, Figure 1B-II) or KAT (Normal controls: 101.7±22.6 ng/ml, Pre-infection 105.0±16.9 ng/ml, Post-infection: 102.4±15.2 ng/ml, p=0.8. Figure 1B-II).
Figure 1. Decrease in Tregs following respiratory viral infection and development of lung-restricted immunity.
A) The levels of peripheral Tregs were analyzed using flow-cytometry prior to any respiratory infection (PRE-INFECTION), at 2-weeks following infection (INFECTION), and 3-months following the infection (POST-INFECTION). Each symbol represents individual human subjects. The decrease in Tregs at 2-weeks following infection was statistically significant (p=0.006). B-I) Of the 20 lung recipients with respiratory viral infection, 12 developed de novo nHLAbs against ColV and KAT at 3-months following infection. Of these 8 had donor specific antibodies (DSA) and 4 had gastroesophageal reflux (GERD). The serum titer of each antibody has been shown comparing pre-infection and post-infection levels. Additionally, antibody titer in 10 normal subjects has been shown. B-II) Eight lung recipients with respiratory infection but without DSA or GERD did not develop nHLAbs. Antibody titers pre-infection and 3-months post-infection have been demonstrated.
Sendai virus infection leads to loss of regulatory T cells
As previously observed (29, 30, 35), mice infected with SdV had a weight loss with a nadir between days 7–9 which resolved by 2 weeks in all groups, indicating clearance of the virus (Figure 2A). Additionally, at 4 weeks there was no SdV RNA detected in any group using polymerase chain reaction. We have previously shown that respiratory viruses upregulate FasL on airway epithelial cells and this might be responsible for apoptosis of Tregs (27). Consistent with the previous reports, SdV infection led to a significant increase in soluble FasL detected in bronchoalveolar lavage (Pre-infection: 53±7 ng/ml vs Post-infection 632.5±57.8 ng/ml, p<0.0001, Figure 2B). At day 7 following SdV infection, there was a decrease in Tregs in the peripheral blood of the mice compared to pre-infection levels (3.2±0.3% or 67±17 cells/µl blood vs 5.9±0.4% or 109±19 cells/µl blood, p=0.006) that resolved by two weeks (6.0±0.4% 115±28 cells/µl blood, Figure 2C). Lung analysis revealed a similar decrease in the CD4+CD25+Foxp3+ regulatory T cells on day 7 (Pre-infection 8.2±3.2% [4.5±1.8×105 /lung] 1.2± vs Post-infection 4.4±2.1% [2.9±1.4×105 /lung], p=0.02). However, mice deficient in Fas(lpr−/−) did not reveal loss of Tregs following SdV infection (Pre-infection: 6.9±1.4% vs Post-infection Day 7: 6.6.9±2.4%, p=0.9).
Figure 2. Sendai virus infection and loss of Tregs.
A) Following infection by SdV, mice were weighed daily. The weight loss and recovery has been represented in mice receiving SdV alone (solid blue line), SdV with anti-H2Kb antibodies (broken green line), and SdV with anti-H2Db antibodies (broken red line). The standard error bars have been represented. B) Sendai virus infection lead to increase in Fas ligand in lungs. Here, bronchoalveolar lavage was collected and soluble Fas ligand was analyzed at 48 hours following SdV infection (n=10, p=0.0001). C) The levels of Tregs were analyzed using flow-cytometry prior to any respiratory infection, at 7-days following infection, and 14-days following the infection. The black bars represent Tregs expressed as a percentage of CD4+ T cells (left y-axis) and white bars show total Tregs per microliter of blood (right y-axis). The decrease in Tregs at 7-days following infection was statistically significant (p=0.006).
Murine respiratory viral infection leads to de novo lung-restricted immunity following lung injury by anti-MHC I antibodies
We further hypothesized that lung injury by anti-MHC class I antibodies followed by loss of Tregs induced by respiratory viral infections would induce lung tissue-restricted immunity. B6 wild type mice were administered anti-H2Kb, anti-H2Db or isotype control antibodies prior to infection with a sublethal dose of SdV. Serum levels of de novo antibodies against lung specific antigens were measured 45 and 60 days after viral infection. Anti-H2Kb and Anti-H2Db treated mice developed high levels of antibodies against ColV (Anti-H2Kb - Day 30: 905±56 ng/ml, Day 45: 978±167 ng/ml, Day 60: 992±124 ng/ml, Anti-H2Db - Day 30: 899±101 ng/ml, Day 45: 900±120 ng/ml, Day 60: 934±99 ng/ml, Figure 3A) and KAT (Anti-H2Kb - Day 30: 16±5 ng/ml, Day 45: 23±8 ng/ml, Day 60: 34±11 ng/ml, Anti-H2Db - Day 30: 33±7 ng/ml, Day 45: 29±7 ng/ml, Day 60: 39±6 ng/ml, Figure 3A) following SdV infection. In contrast, SdV or anti-MHC class I antibody treatment alone did not result in significant increase in antibodies against ColV (SdV -Day 30: 210±33 ng/ml, Day 45: 189±45 ng/ml, Day 60: 176±56 ng/ml, Anti-H2Kb - Day 30: 412±66 ng/ml, Day 45: 501±78 ng/ml, Day 60: 476±98 ng/ml) or KAT (SdV - Day 30: 6±3 ng/ml, Day 45: 5±2 ng/ml, Day 60: 5±3 ng/ml, Anti-H2Kb - Day 30: 7±2 ng/ml, Day 45: 6±3 ng/ml, Day 60: 5±2 ng/ml). Similarly, treatment with isotype control antibodies followed by SdV infection did not result in an increase in antibodies against ColV (Day 30: 243±45 ng/ml, Day 45: 213±43 ng/ml, Day 60: 198±76 ng/ml) or KAT (Day 30: 5±3 ng/ml, Day 45: 7±4 ng/ml, Day 60: 8±3 ng/ml, Figure 3A). None of the mice developed antibodies against type II collagen (ColII), a cartilaginous protein that is the major component of joint articular cartilage and not found in the lungs (levels <10 ng/ml in all mice, Figure 3A).
Figure 3. Development of de novo lung-restricted immunity with sendai virus infection and anti-MHC class I antibody treatment.
A) Mice received intratracheal administration of MHC class I [anti-H2Kb and anti-H2Db, n=5 each, black bar) or control C1.18 (n=5) antibodies. Following this, a sublethal dose of SdV was administered. As control, a groups of mice (n=5) received SdV or anti-H2Kb antibodies alone. Serum was collected from mice on day 30, 45, and 60 following SdV infection and analyzed for antibodies against lung-restricted antigens ColV and KAT as well as a non-lung antigen ColII. The increase in antibody titers against lung antigens in mice treated with anti-H2Kb antibodies and SdV was statistically significant (p<0.001). Each group consisted of 5 mice. Standard error bars are illustrated. B) Blood was collected from mice above on day 30, and 45 following SdV infection and analyzed for development of IFN-γ producing CD4+ T cells specific to lung-restricted antigens ColV and KAT as well as a non-lung antigen ColII using ELISPOT assay. The increase in IFN-γ producing CD4+ T cells specific to lung antigens in mice treated with anti-H2Kb antibodies and SdV was statistically significant (p<0.001). Each group consisted of 5 mice. Standard error bars are illustrated. C) On days 45 and 60, mice were sacrificed and lungs analyzed using histology. While mice treated with C1.18 and SdV, anti-H2Kb antibodies, or SdV alone revealed lung fibrosis, those that received anti-H2Kb antibodies and SdV developed progressive airway luminal obliteration.
We further tested for the development of CD4+ T cells against the lung-restricted antigens. Towards this, irradiated syngeneic splenocytes were pulsed overnight with ColV, KAT, or ColII and then co-cultured with splenocytes from the mice from the different experimental groups. As shown in Figure 3B, anti-H2Kb + SdV and anti-H2Db + SdV treated mice showed high frequency of IFN-γ producing CD4+ T cells against ColV (Anti-H2Kb - Day 30: 850±89 spm, Day 45: 625±76 spm, Anti-H2Db - Day 30: 910±101 spm, Day 45: 799±99 spm) and KAT (Anti-H2Kb - Day 30: 1189±101 spm, Day 45: 815±83 spm, Anti-H2Db - Day 30: 1099±143 spm, Day 45: 990±103 spm, Figure 3B). In contrast, SdV or anti-MHC class I antibody treatment alone did not result in significant increase in IFN-γ producing CD4+ T cells against ColV (SdV - Day 30: 89±25 spm, Day 45: 91±36 spm, Anti-H2Kb - Day 30: 245±33 spm, Day 45: 276±43 spm) or KAT (SdV - Day 30: 103±54 spm, Day 45: 189±33 spm, Anti-H2Kb - Day 30: 312±55 spm, Day 45: 299±87 spm, Figure 3B). Treatment with control antibodies followed by SdV infection did not result in IFN-γ producing CD4+ T cells against ColV (Day 30: 101±46 spm, Day 45: 112±53 spm) or KAT (Day 30: 201±66 spm, Day 45: 176±44 spm). None of the mice developed IFN-γ producing CD4+ T cells against the control antigen ColII (<15 spm in all mice, Figure 3B).
On day 45 and 60, we assessed the lung injury histologically. Mice treated with SdV alone or isotype control Abs with SdV had developed fibrosis around the airways. However, the airway lumen was preserved in each of these groups. In stark contrast, administration of MHC class I Abs in combination with SdV had resulted in progressive airway luminal obliteration in addition to the fibrosis surrounding the airways (Figure 3C).
Loss of Tregs is crucial for de novo lung-restricted immunity
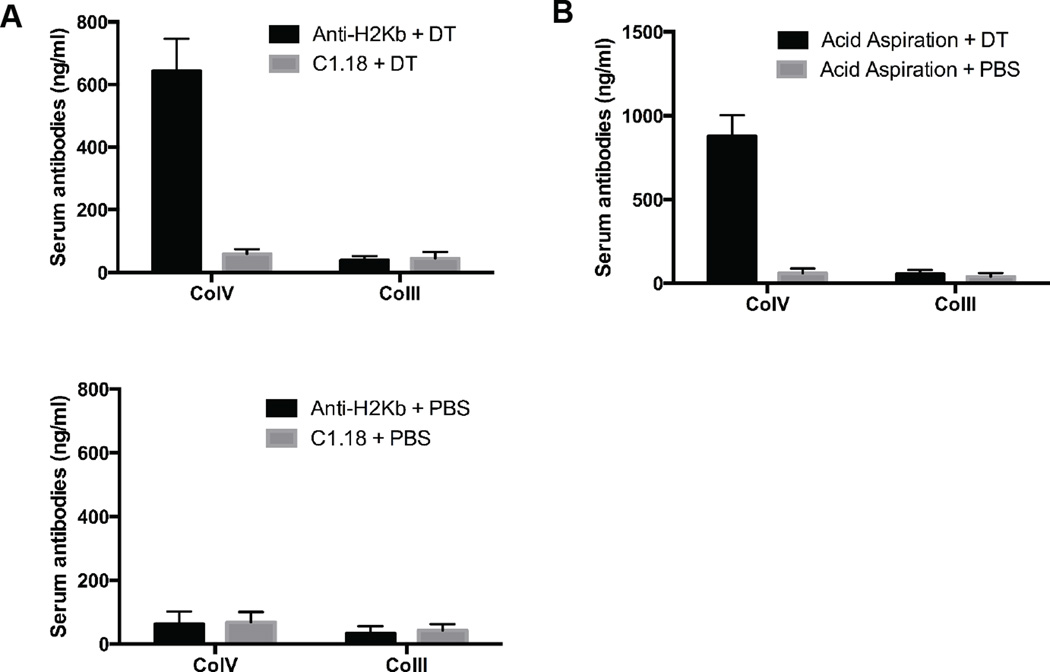
To further confirm that a loss of Tregs is necessary to induce de novo lung-restricted autoimmunity in the setting of pulmonary injury we took advantage of selective depletion of regulatory T cells in Foxp3-DTR mice (32). Foxp3-DTR animals were treated with anti-H2Kb or isotype control antibodies intratracheally. Subsequently, these mice received either diphtheria toxin (DT) or phosphate-buffered saline (PBS). At two weeks, development of ColV and ColII antibodies was determined using ELISA. As shown in Figure 4A, treatment with anti-MHC class I but not isotype control antibodies followed by administration of DT led to the development of ColV antibodies (643±103 ng/ml Vs 59±15 ng/ml, p<0.001). Mice injected with PBS, in lieu of DT, did not develop ColV antibodies (anti-H2Kb treatment: 63±39 ng/ml, C1.18 treatment: 68±33 ng/ml, Figure 4A). None of the mice developed antibodies against control antigen ColII (levels <50ng/ml in all, Figure 4A).
Figure 4. Development of lung-restricted antibodies in Foxp3-DTR mice following injury with MHC class I antibodies and acid aspiration.
A) Anti-H2Kb or control C1.18 antibodies were administered to Foxp3-DTR mice using intratracheal injection. Subsequently, these mice were treated with diphtheria toxin (DT) or phosphate-buffered saline (PBS). Two weeks after injection of DT or PBS, serum antibodies against ColV or ColII were analyzed. The increase in ColV antibodies in mice treated with anti-H2Kb antibodies and DT was statistically significant (p<0.001). Each group consisted of 5 mice. Standard error bars are illustrated. B) The Foxp3-DTR mice were administered 50µl of hydrochloric acid daily for seven days. Subsequently, these mice received diphtheria toxin (DT) or phosphate-buffered saline (PBS). Two weeks after injection of DT or PBS, serum antibodies against ColV or ColII were analyzed. The increase in ColV antibodies in mice receiving gastric acid and DT was statistically significant (p<0.001). Each group consisted of 5 mice. Standard error bars are illustrated.
Next we hypothesized that lung injury secondary to acid aspiration, an established risk factor for chronic lung allograft rejection, also predisposes to the development of nHLAbs upon depletion of Tregs (15). Similar to our observations after treatment with anti-H2Kb antibodies, intratracheal acid instillation followed by DT administration, led to the development of ColV antibodies (877±125 ng/ml) but not ColII antibodies (54±25 ng/ml, Figure 4B) in Foxp3-DTR mice. Acid injection followed by PBS did not induce either ColV or ColII antibodies (<50ng/ml for both, Figure 4B).
Discussion
Growing body of evidence supports the presence of lung-restricted immunity in patients with chronic lung disease (1–5). While the effects of lung-restricted antibodies in non-transplant patients remain unclear, they have been shown to significantly reduce allograft survival by causing both early (2–6) and chronic lung allograft rejection (36). The incidence of chronic lung allograft rejection has remained unchanged despite advances in contemporary immunosuppression (8) and remains the major cause of poor long-term lung allograft survival (37, 38). Prior studies indicated that DSA strongly predisposed to the development of chronic lung allograft rejection (39–41). Interestingly, nearly all patients with DSA also develop antibodies against lung-tissue restricted antigens (9). Treatment with antibody-directed therapy in patients with both DSA and de novo nHLAbs only reduces the incidence of chronic lung allograft rejection if the nHLAbs are cleared along with DSA. Clearance of DSA but not the nHLAbs does not prevent chronic rejection (9). We have recently validated the importance of antibodies against lung-restricted self-antigens in lung allograft rejection using a model of syngeneic murine lung transplant (42). Administration of ColV or KAT antibodies to recipients following syngeneic lung transplant led to rejection of the lung grafts and development of ColV and KAT specific IFN-γ and TH-17 cells. Additionally, pre-existing nHLAbs have been strongly implicated in the development of primary graft dysfunction immediately following transplantation as well as development of alloimmunity (2–6). Together, these data implicate an important role of lung-restricted immunity in the development of chronic lung allograft rejection.
Here we investigated the mechanisms that might lead to the development of lung-tissue restricted immunity. Lung allografts are unique amongst solid organs as they are exposed to the external milieu, making them susceptible to environmental toxins and pathogens. Additionally, gastro-esophageal reflux is common following lung transplantation and leads to tissue inflammation (15). Lung allografts are also the only solid organs that do not receive oxygenated blood following transplantation since the bronchial circulation is not routinely re-established (43, 44). As such, lung allografts are vulnerable to many forms of injury that can result in local inflammation (45–47). Such an inflammatory milieu can lead to the exposure of sequestered self-antigens that are otherwise concealed from the host’s immune system, predisposing to the development of tissue-restricted immunity (6, 48). This further becomes important since it is known that CD4+ T cells specific to tissue-restricted self-antigens are not deleted in the thymus but rather suppressed by Tregs (49). These Tregs play an important role in both self- and allo-tolerance (2, 25, 26, 50). Tregs can be found in many different tissues where they play an important role in suppressing inflammation and autoimmune responses (21–24). There is increasing evidence that alteration in cellular regulation of peripheral tolerance plays a crucial role in chronic allograft rejection (25, 26). The development of lung-restricted immunity in lung recipients (2–4) in the setting of tissue injury, therefore, suggests concomitant loss of self-tolerance maintained by Tregs.
We found that lung transplant recipients are predisposed to the development of de novo nHLAbs following respiratory viral infections. Furthermore, viral infections in humans were associated with decline in Tregs, a finding mimicked in our murine model. Respiratory viruses have been associated with a variety of autoimmune disorders in humans (12, 51). Using an orthotopic tracheal transplant model, we have previously shown that SdV can induce apoptosis in regional Tregs by upregulating FasL in airway epithelial cells (30). Data presented in this manuscript (Figure 2) further support the role of Fas-FasL interactions in apoptosis of Tregs following viral infections. Anz et al have also previously shown that immunostimulatory viral RNA can mediate Treg suppression through IL-6 production (52). They suggest that viruses induce Treg dysfunction by converting them into “effector” Th-17 cells. Hence, this might represent another pathway by which respiratory viruses lead to loss of T cell regulation.
Patients with DSA or gastro-esophageal reflux, both of which can cause lung injury, developed nHLAbs. Since Tregs eventually recover following respiratory infections, we postulate that the tissue injury needs to precede loss of Tregs for immunity to develop. In order to examine the role of tissue injury and loss of Tregs, we used wild type mice and injected them with MHC class I or isotype control antibodies. This was followed by SdV infection to induce loss of Tregs. Mice administered MHC class I antibodies developed both humoral and cellular immunity against lung-restricted self-antigens. In contrast, mice that received control antibodies or SdV infection alone did not develop immunity. It is well established that the thymus deletes autoreactive T cells against the dominant self-antigens including MHC class I and II antigens but not sequestered tissue-restricted self-antigens. Lungs, in particular, have been shown to have a subset of autoreactive T cells that can escape thymic deletion and are suppressed by peripheral Tregs (49). Therefore, a lung injury mechanism can uncover the lung-restricted self-antigens such as ColV and KAT and lead to the expansion of respective T cells, especially if Tregs are lost. It is noteworthy that administration of anti-H2Kb antibodies did not influence the natural course of SdV infection in the B6 mice by blocking CD8 T cell recognition of the immunodominant Kb-restricted epitope. Mice receiving anti-H2Kb treatment recovered within 2 weeks similar to those receiving SdV alone and SdV with anti-H2Db treatment. Additionally, we found that SdV RNA in bronchoalveolar lavage was cleared by 4 weeks in all groups. However, despite the fact that SdV infection was associated with decline in Tregs, it did not convincingly demonstrate that loss of Tregs was crucial for the development of immunity. Therefore, we used the Foxp3-DTR mice in which depletion of Tregs can be performed by systemic injection of DT. As shown in Figure 3, the loss of Tregs was insufficient to induce immunity against the lung unless accompanied by either MHC class I antibodies or acid aspiration. Loss of Tregs in these mice leads to systemic autoimmunity and death at three weeks. However, without injury to the lungs the mice did not show immunity to the lung-restricted self-antigens (data not shown).
How then can we explain the high incidence of de novo nHLAbs following human lung transplantation since respiratory viruses do not affect all lung transplant recipients (53, 54). Several of the immunosuppressive regimens that are in clinical use today are known to impair the function of Tregs. For example, it is known that Tregs are dependent on interleukin-2 (55) which is suppressed by calcineurin inhibitors such as tacrolimus and cyclosporine. Indeed, patients with chronic lung allograft rejection reveal significantly decreased Tregs (56). Therefore, it is possible that Tregs are affected by these drugs (57–61) which ultimately leads to the development of nHLAbs in the presence of ongoing injury mechanisms such as DSA or gastro-esophageal reflux. It is noteworthy that immunity against lung antigens has been demonstrated even in patients with other forms of lung disease such as asthma that do not require lung transplantation (1). Therefore, we postulate that similar mechanisms might be responsible for the development of lung-restricted immunity in patients with chronic lung disease. Smoking, for instance, in patients with emphysema, leads to ongoing lung tissue injury and inflammation. A loss or functional impairment of Tregs, for example by respiratory viruses, could predispose them to the development of lung-restricted immunity. Respiratory viral infections, indeed, are common in patients with underlying lung disease (62, 63).
We conclude that both tissue injury and loss of Tregs are essential for the development of lung-restricted immunity. Hence, we propose a “two-hit” hypothesis for the development of tissue-restricted immunity in lung recipients. Further investigation is necessary to determine strategies to ameliorate the tissue injury in lung allografts and prevent loss of Tregs. While our studies have shown that a complete elimination of Foxp3 T cells is sufficient to induce antibodies against lung antigens in previously injured lungs, future studies need to elucidate whether regulatory circuits other than Tregs contribute to the development of autoimmunity in lung transplant recipients.
Acknowledgments
Grant Support: SC is supported by T32 DK077662, Transplant Scientist Training Program. AB is supported by NIH HL125940 and American Lung Association Biomedical Grant. Studies in TM lab is supported by HL092514 and HL056643.
We thank Ms. Elena Susan for the formatting and submission of this manuscript.
Footnotes
There is no conflict of interest relevant to this manuscript by any of the authors.
References
- 1.Liu M, Subramanian V, Christie C, Castro M, Mohanakumar T. Immune responses to self-antigens in asthma patients: clinical and immunopathological implications. Hum Immunol. 2012;73(5):511–516. doi: 10.1016/j.humimm.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+CD25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human transplantation. American Journal of Transplantation. 2006;6(8):1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 3.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90(4):1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, Mickler E, Smith GN, Petrache I, Brand DB, Burlingham WJ, Gopalakrishnan B, Greenspan DS, Christie JD, Wilkes DS. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181(8):5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burlingham W, Wilkes DS, Sullivan JA. Why is the patient out of breath? Collagen V(alpha1) and K-alpha1-tubulin take center stage in lung transplantation. Am J Transplant. 2014;14(10):2201–2203. doi: 10.1111/ajt.12910. [DOI] [PubMed] [Google Scholar]
- 8.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17(12):1255–1263. [PubMed] [Google Scholar]
- 9.Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to K-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12(8):2164–2171. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148(1):32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludewig B, Zinkernagel RM, Hengartner H. Transgenic animal models for virus-induced autoimmune diseases. Exp Physiol. 2000;85(6):653–659. [PubMed] [Google Scholar]
- 13.Munoz LE, Janko C, Schulze C, Schorn C, Sarter K, Schett G, Herrmann M. Autoimmunity and chronic inflammation - two clearance-related steps in the etiopathogenesis of SLE. Autoimmun Rev. 2010;10(1):38–42. doi: 10.1016/j.autrev.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, Lee JS, Pianosi PT, Ryu JH. Aspiration-related pulmonary syndromes. Chest. 2015;147(3):815–823. doi: 10.1378/chest.14-1049. [DOI] [PubMed] [Google Scholar]
- 15.Bobadilla JL, Jankowska-Gan E, Xu Q, Haynes LD, Munoz del Rio A, Meyer K, Greenspan DS, De Oliveira N, Burlingham WJ, Maloney JD. Reflux-induced collagen type v sensitization: potential mediator of bronchiolitis obliterans syndrome. Chest. 2010;138(2):363–370. doi: 10.1378/chest.09-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartwig MG, Davis RD. Gastroesophageal reflux disease-induced aspiration injury following lung transplantation. Curr Opin Organ Transplant. 2012;17(5):474–478. doi: 10.1097/MOT.0b013e328357f84f. [DOI] [PubMed] [Google Scholar]
- 17.Hoppo T, Jarido V, Pennathur A, Morrell M, Crespo M, Shigemura N, Bermudez C, Hunter JG, Toyoda Y, Pilewski J, Luketich JD, Jobe BA. Antireflux surgery preserves lung function in patients with gastroesophageal reflux disease and end-stage lung disease before and after lung transplantation. Arch Surg. 2011;146(9):1041–1047. doi: 10.1001/archsurg.2011.216. [DOI] [PubMed] [Google Scholar]
- 18.Adeegbe D, Matsutani T, Yang J, Altman NH, Malek TR. CD4(+) CD25(+) Foxp3(+) T regulatory cells with limited TCR diversity in control of autoimmunity. J Immunol. 2010;184(1):56–66. doi: 10.4049/jimmunol.0902379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorsey NJ, Chapoval SP, Smith EP, Skupsky J, Scott DW, Keegan AD. STAT6 controls the number of regulatory T cells in vivo, thereby regulating allergic lung inflammation. J Immunol. 2013;191(4):1517–1528. doi: 10.4049/jimmunol.1300486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210(4):775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6(4):338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 23.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shevach EM. Biological functions of regulatory T cells. Adv Immunol. 2011;112:137–176. doi: 10.1016/B978-0-12-387827-4.00004-8. [DOI] [PubMed] [Google Scholar]
- 25.Tosiek MJ, Gruber AD, Bader SR, Mauel S, Hoymann HG, Prettin S, Tschernig T, Buer J, Gereke M, Bruder D. CD4+CD25+Foxp3+ regulatory T cells are dispensable for controlling CD8+ T cell-mediated lung inflammation. J Immunol. 2011;186(11):6106–6118. doi: 10.4049/jimmunol.1000632. [DOI] [PubMed] [Google Scholar]
- 26.Zhou W, Zhou X, Gaowa S, Meng Q, Zhan Z, Liu J, Li J, Fan H, Liu Z. The Critical Role of Induced CD4+ FoxP3+ Regulatory Cells in Suppression of Interleukin-17 Production and Attenuation of Mouse Orthotopic Lung Allograft Rejection. Transplantation. 2015;99(7):1356–1364. doi: 10.1097/TP.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 27.Bharat A, Kuo E, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Respiratory virus-induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg. 2010;90(5):1637–1644. doi: 10.1016/j.athoracsur.2010.06.048. discussion 1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, Mohanakumar T, Trulock EP, Walter MJ. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170(2):181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 29.Kuo E, Bharat A, Shih J, Street T, Norris J, Liu W, Parks W, Walter M, Patterson GA, Mohanakumar T. Role of airway epithelial injury in murine orthotopic tracheal allograft rejection. Ann Thorac Surg. 2006;82(4):1226–1233. doi: 10.1016/j.athoracsur.2006.03.122. [DOI] [PubMed] [Google Scholar]
- 30.Kuo E, Bharat A, Goers T, Chapman W, Yan L, Street T, Lu W, Walter M, Patterson A, Mohanakumar T. Respiratory viral infection in obliterative airway disease after orthotopic tracheal transplantation. Ann Thorac Surg. 2006;82(3):1043–1050. doi: 10.1016/j.athoracsur.2006.03.120. [DOI] [PubMed] [Google Scholar]
- 31.Maniatis NA, Sfika A, Nikitopoulou I, Vassiliou AG, Magkou C, Armaganidis A, Roussos C, Kollias G, Orfanos SE, Kotanidou A. Acid-induced acute lung injury in mice is associated with P44/42 and c-Jun N-terminal kinase activation and requires the function of tumor necrosis factor alpha receptor I. Shock. 2012;38(4):381–386. doi: 10.1097/SHK.0b013e3182690ea2. [DOI] [PubMed] [Google Scholar]
- 32.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 33.Tiriveedhi V, Gautam B, Sarma NJ, Askar M, Budev M, Aloush A, Hachem R, Trulock E, Myers B, Patterson AG, Mohanakumar T. Pre-transplant antibodies to Kalpha1 tubulin and collagen-V in lung transplantation: clinical correlations. J Heart Lung Transplant. 2013;32(8):807–814. doi: 10.1016/j.healun.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, Mohanakumar T. Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int J Cancer. 2002;102(5):499–506. doi: 10.1002/ijc.10736. [DOI] [PubMed] [Google Scholar]
- 35.Wetzel JL, Fensterl V, Sen GC. Sendai virus pathogenesis in mice is prevented by Ifit2 and exacerbated by interferon. J Virol. 2014;88(23):13593–13601. doi: 10.1128/JVI.02201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, Steward N, Aloush A, Hachem R, Trulock E, Meyers B, Patterson GA, Mohanakumar T. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30(6):624–631. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hachem RR, Khalifah AP, Chakinala MM, Yusen RD, Aloush AA, Mohanakumar T, Patterson GA, Trulock EP, Walter MJ. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005;80(10):1406–1413. doi: 10.1097/01.tp.0000181161.60638.fa. [DOI] [PubMed] [Google Scholar]
- 38.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, Tazelaar HD. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 39.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, Meyers B, Schuessler R, Trulock EP, Patterson GA, Mohanakumar T. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 40.Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86(1):189–195. doi: 10.1016/j.athoracsur.2008.03.073. discussion 196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyder LD, Wang Z, Chen DF, Reinsmoen NL, Finlen-Copeland CA, Davis WA, Zaas DW, Palmer SM. Implications for human leukocyte antigen antibodies after lung transplantation: a 10-year experience in 441 patients. Chest. 2013;144(1):226–233. doi: 10.1378/chest.12-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian V, Ramachandran S, Banan B, Bharat A, Wang X, Benshoff N, Kreisel D, Gelman AE, Mohanakumar T. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant. 2014;14(10):2359–2366. doi: 10.1111/ajt.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicolls MR, Zamora MR. Bronchial blood supply after lung transplantation without bronchial artery revascularization. Curr Opin Organ Transplant. 2010;15(5):563–567. doi: 10.1097/MOT.0b013e32833deca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pettersson GB, Yun JJ, Norgaard MA. Bronchial artery revascularization in lung transplantation: techniques, experience, and outcomes. Curr Opin Organ Transplant. 2010;15(5):572–577. doi: 10.1097/MOT.0b013e32833e16fc. [DOI] [PubMed] [Google Scholar]
- 45.Bharat A, Mohanakumar T. Autoimmunity and lung transplantation. Front Biosci (Elite Ed) 2012;4:2378–2388. doi: 10.2741/e549. [DOI] [PubMed] [Google Scholar]
- 46.Weber DJ, Wilkes DS. The role of autoimmunity in obliterative bronchiolitis after lung transplantation. Am J Physiol Lung Cell Mol Physiol. 2013;304(5):L307–L311. doi: 10.1152/ajplung.00378.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shilling RA, Wilkes DS. Immunobiology of chronic lung allograft dysfunction: new insights from the bench and beyond. Am J Transplant. 2009;9(8):1714–1718. doi: 10.1111/j.1600-6143.2009.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atassi MZ, Casali P. Molecular mechanisms of autoimmunity. Autoimmunity. 2008;41(2):123–132. doi: 10.1080/08916930801929021. [DOI] [PubMed] [Google Scholar]
- 49.Legoux FP, Lim JB, Cauley AW, Dikiy S, Ertelt J, Mariani TJ, Sparwasser T, Way SS, Moon JJ. CD4(+) T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion. Immunity. 2015;43(5):896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizobuchi T, Yasufuku K, Zheng Y, Haque MA, Heidler KM, Woods K, Smith GN, Jr, Cummings OW, Fujisawa T, Blum JS, Wilkes DS. Differential expression of Smad7 transcripts identifies the CD4+CD45RChigh regulatory T cells that mediate type V collagen-induced tolerance to lung allografts. J Immunol. 2003;171(3):1140–1147. doi: 10.4049/jimmunol.171.3.1140. [DOI] [PubMed] [Google Scholar]
- 51.Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. 2009;155(1):1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anz D, Koelzer VH, Moder S, Thaler R, Schwerd T, Lahl K, Sparwasser T, Besch R, Poeck H, Hornung V, Hartmann G, Rothenfusser S, Bourquin C, Endres S. Immunostimulatory RNA blocks suppression by regulatory T cells. J Immunol. 184(2):939–946. doi: 10.4049/jimmunol.0901245. [DOI] [PubMed] [Google Scholar]
- 53.Shah PD, McDyer JF. Viral infections in lung transplant recipients. Semin Respir Crit Care Med. 2010;31(2):243–254. doi: 10.1055/s-0030-1249120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uhlin M, Mattsson J, Maeurer M. Update on viral infections in lung transplantation. Curr Opin Pulm Med. 2012;18(3):264–270. doi: 10.1097/MCP.0b013e3283521066. [DOI] [PubMed] [Google Scholar]
- 55.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 56.Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177(8):5631–5638. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- 57.Huss R. In vitro determination of self-reactivity in the early postcyclosporine period. Transpl Immunol. 1993;1(3):228–234. doi: 10.1016/0966-3274(93)90051-9. [DOI] [PubMed] [Google Scholar]
- 58.Sakaguchi S, Sakaguchi N. Thymus and autoimmunity. Transplantation of the thymus from cyclosporin A-treated mice causes organ-specific autoimmune disease in athymic nude mice. J Exp Med. 1988;167(4):1479–1485. doi: 10.1084/jem.167.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakaguchi S, Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J Immunol. 1989;142(2):471–480. [PubMed] [Google Scholar]
- 60.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167(4):1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 61.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 62.Papakonstantinou E, Karakiulakis G, Batzios S, Savic S, Roth M, Tamm M, Stolz D. Acute exacerbations of COPD are associated with significant activation of matrix metalloproteinase 9 irrespectively of airway obstruction, emphysema and infection. Respir Res. 2015;16:78. doi: 10.1186/s12931-015-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer M, Jaspers I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. Am J Physiol Lung Cell Mol Physiol. 2015;308(12):L1189–L1201. doi: 10.1152/ajplung.00028.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]