Abstract
Cognitive training is an emergent approach to improve cognitive functions in various neurodevelopmental and neurodegenerative diseases. However, current training programs can be relatively lengthy, making adherence potentially difficult for patients with cognitive difficulties. Previous studies suggest that providing individuals with real-time feedback about the level of brain activity (neurofeedback) can potentially help them learn to control the activation of specific brain regions. In the present study, we developed a novel task-based neurofeedback training paradigm that benefits from the effects of neurofeedback in parallel with computerized training. We focused on executive function training given its core involvement in various developmental and neurodegenerative diseases. Near-infrared spectroscopy (NIRS) was employed for providing neurofeedback by measuring changes in oxygenated hemoglobin in the prefrontal cortex. Of the twenty healthy adult participants, ten received real neurofeedback (NFB) on prefrontal activity during cognitive training, and ten were presented with sham feedback (SHAM). Compared with SHAM, the NFB group showed significantly improved executive function performance including measures of working memory after four sessions of training (100 minutes total). The NFB group also showed significantly reduced training-related brain activity in the executive function network including right middle frontal and inferior frontal regions compared with SHAM. Our data suggest that providing neurofeedback along with cognitive training can enhance executive function after a relatively short period of training. Similar designs could potentially be used for patient populations with known neuropathology, potentially helping them to boost/recover the activity in the affected brain regions.
Keywords: functional plasticity, neurofeedback, cognitive stimulation, prefrontal cortex, NIRS
1. Introduction
Cognitive training is an emergent approach that has been adopted in recent years as a potential intervention for a number of developmental and neurodegenerative disorders (Fisher et al. 2010; Belleville et al. 2011; Kray et al. 2011; Ranganath et al. 2011; Hampstead et al. 2012; Vinogradov et al. 2012; Chacko et al. 2014). Cognitive training theoretically promotes several neuroplastic mechanisms in the brain (Zatorre et al. 2012) and has been shown to improve cognitive functioning in various healthy and patient populations (Ball et al. 2002; Olesen et al. 2004; McNab et al. 2009; Jaeggi et al. 2011; Kesler et al. 2011; Anguera et al. 2013; Kesler et al. 2013; Wolinsky et al. 2013). While the mechanisms underlying these plasticity-related changes in the brain are still unclear, it is speculated that cognitive training enhances cognitive reserve (i.e. brain’s ability to perform cognitive tasks) (Vance 2012; Barulli and Stern 2013).
The main goal of cognitive training is to boost or restore cognitive skills and brain function by employing a set of adaptive, practice-based paradigms. In order for the training programs to be effective, individuals are usually asked to perform a distributed set of cognitive exercises. The training time can thus be lengthy (up to 6 months), making adherence potentially difficult for some patients, especially those with more severe cognitive difficulties and/or comorbid behavioral and/or psychological conditions.
Previous studies have shown that providing individuals with real-time feedback about the level of brain activity or rhythm (neurofeedback) can potentially help them learn to control the activation of specific brain regions (deCharms et al. 2005; Hampstead et al. 2012; Scharnowski et al. 2012; Mihara et al. 2013; Ruiz et al. 2013; Kober et al. 2014) [see (Sulzer et al. 2013) for a review]. These studies have successfully employed electroencephalography (EEG), functional magnetic resonance imaging (fMRI), and functional near-infrared spectroscopy (NIRS) for providing neurofeedback. For example, using stimulus-free fMRI and EEG neurofeedback paradigms, patients with Parkinson’s disease and ADHD successfully learned to improve the activity in regions related to motor control and attention, respectively (Levesque et al. 2006; Subramanian et al. 2011). Similar reports have shown that providing neurofeedback using NIRS can enhance the cortical activation related to motor imagery in healthy participants and post-stroke patients (Mihara et al. 2013; Kober et al. 2014) [see (Birbaumer et al. 2009) for a review]. This body of evidence implies that providing neurofeedback in parallel with computerized training (i.e. task-based neurofeedback training) might be helpful for targeted enhancement of a specific brain network and developing effective cognitive training programs.
In the present study, we developed a novel cognitive training program that included task-based neurofeedback. Specifically, we provided participants with behavioral feedback regarding their performance on the task as well as their brain activity in the targeted brain network, simultaneously. Near-infrared spectroscopy (NIRS) was employed for providing neurofeedback by measuring changes in oxygenated hemoglobin in the prefrontal cortex (Irani et al. 2007; Ferrari and Quaresima 2012). Providing neurofeedback along with task performance may have several advantages. First, participants can test different strategies to determine how they can best perform the task by efficient use of neural resources associated with the targeted cognitive domain. Therefore, the training provides a practice for efficient use of cognitive resources. Second, the training is potentially more engaging and reinforcing than cognitive training without neurofeedback since participants are actively monitoring their brain activity to improve their strategies. This monitoring potentially improves the training effect by integrating an additional level of working memory and attention into the training exercise. For example, behavioral weight loss studies suggest that feedback and self-monitoring results in greater intervention gains (Burke et al. 2011). Feedback and self-monitoring enhances self-regulatory skills in relation to the performance of the target behavior which in turn results in change in behavior (Burke et al. 2011). Finally, the training design accounts for individual differences in brain networks. Specifically, the training can be customized for patients depending on the type of pathology involved; the feedback can be programmed specifically for up- or down-regulating the activity in a specific brain region as appropriate.
We focused on training executive function given its core involvement in various developmental and neurodegenerative diseases including attention deficit hyperactivity disorder (ADHD), mild cognitive impairment (MCI) and major depressive disorder (MDD) (Corbett et al. 2009; Minzenberg et al. 2009; O'Brien et al. 2010; Marshall et al. 2011; Snyder 2013). A delayed verbal working memory task also known as the Sternberg task (Sternberg 1969), was used for neurofeedback training. Regulating brain activity while doing a cognitive task might interfere with task performance. Therefore, we designed the training paradigm such that the feedback was presented intermittently and updated at the end of each trial in order to limit the interfering effect that feedback might have on task performance. Previous studies have shown the involvement of prefrontal and inferior parietal regions in the Sternberg task (Veltman et al. 2003; Woodward et al. 2006). However, our NIRS system does not have the capability of measuring the whole brain. Therefore, we focused on the prefrontal cortex, given the significant role of this region in executive functioning (Tsujimoto et al. 2004; Sato et al. 2013).
Twenty healthy adult participants underwent four sessions of neurofeedback training (100 min total) and their performance on executive function tests were measured before and after completion of training. Ten participants received real feedback (NFB) on brain activity in the prefrontal cortex and ten of them were presented with sham feedback (SHAM). Previous neuroimaging studies have consistently shown increased prefrontal activity associated with increasing cognitive load (Rympa et al. 1999; Veltman et al. 2003). In line with these observations, a number of working memory training studies suggest that mastering a working memory task through practice results in decreased prefrontal activity that might reflect employing a more efficient information processing (Schneiders et al., 2011; Sayala et al., 2006; Jansma et al., 2001). Therefore we instructed the subjects to recruit a metacognitive strategy that results in reduction in neurofeedback signal without compromising their performance. We expected that the NFB group would show significantly higher improvement in executive function scores and reduced neurofeedback signal compared with SHAM.
2. Materials and Methods
2.1. Participants
20 healthy adults (10 female, age 19 – 33 years old) participated in the study. Ten participants (5 female, mean (SD) age = 24.1 (3.9)) were in the experimental group and received real feedback on their brain activity (NFB). The other ten participants (5 female, mean (SD) age = 25.1 (3.9)) were in the control group and received sham feedback (SHAM). There was a gap in recruiting the two groups because of resource limitations. Therefore the groups were not randomized. However, the NFB and SHAM were group-matched for age and gender. Participants were excluded for any history of medical (e.g. diabetes, cancer, etc.), neurologic (e.g. stroke, brain injury, etc.) or psychiatric conditions (Depression, neurogenetic disorders, attention deficit hyperactivity disorder, etc.) that are known to affect cognition. Individuals who were currently participating in other cognitive training studies or activities were also excluded. Participants were recruited via mailing lists and flyers. The study was approved by the Stanford University Institutional Review Board. Written informed consent was obtained from participants.
2.2. NIRS data acquisition
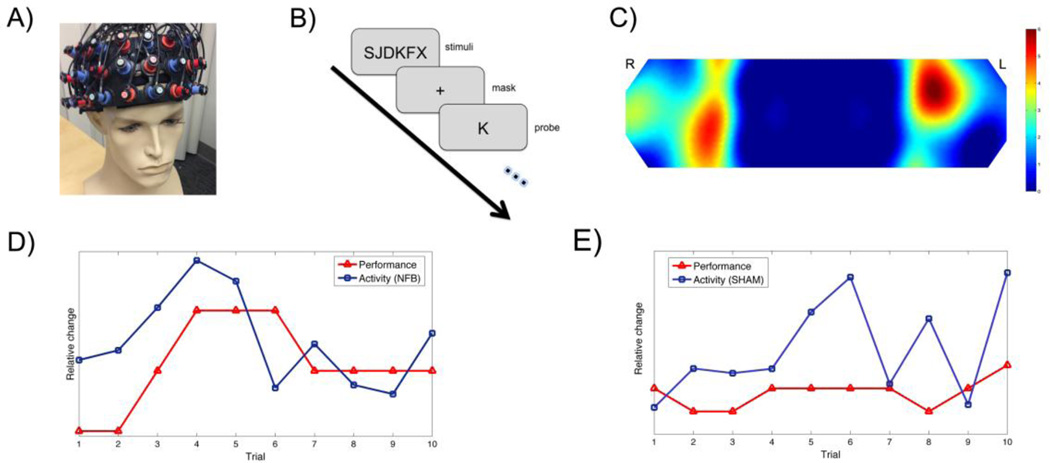
An ETG-4000 (Hitachi Medical Co., Japan) system was used for NIRS measurements. This continuous-wave system employs two different wavelengths (695 and 830 nm) for detecting relative changes in concentration of oxygenated (OHb) and deoxygenated hemoglobin (RHb) in the blood. We used a 3 × 11 optode probe set consisting of 17 light emitters and 16 photodetectors resulting in a total of 52 channels. The 52-channel probe set was placed over the prefrontal regions with its lowest-row center optode at the electrode position (FPz) according to the international 10–20 system (Figure 1A) (Okamoto et al. 2004; Hosseini et al. 2011). The sampling frequency was set at 10 Hz.
Figure 1. Neurofeedback setting.
A) The 52-channel NIRS probe set covering the prefrontal regions. B) The training task paradigm. C) Distribution of targeted regions across subjects. The colorbar indicates the overlap among subjects (hot color indicates more overlap across subjects). The unit is the number of subjects. The distribution of the selected target regions is consistent with previous data showing activity in the bilateral prefrontal cortex during Sternberg task performance. The line plot presentation of feedback of brain activity and task performance in the last ten trials for (D) NFB and (E) sham feedback for the SHAM groups. The horizontal axis represents the last ten trials of training while the vertical axis indicates the relative changes in performance and brain activity compared to baseline (arbitrary unit). The blue line (circle markers) shows changes in activity and the red line (rectangle markers) shows changes in performance compared to baseline.
2.3. Neurofeedback procedure
A delayed verbal working memory task also known as the Sternberg task (Sternberg 1969), was used for neurofeedback training (Figure 1B). In each trial of the task, a set of letters was presented to the subject for 2 s (encoding phase). After a jittered delay period (6 to 8 s, average 7 s) representing the retention phase, a single inquiry letter was presented on the screen and the subject had to respond within 2 s (inquiry phase) if the inquiry letter was included in the original stimuli set or not. A jittered fixation period (6 to 8 s, average 7 s) separated the probe phase from the subsequent trial. Each training session consisted of 80 trials (around 25 min total). Subjects underwent a total of four training sessions across two weeks.
Since our NIRS system does not have the capability of measuring the whole brain, we focused on the prefrontal cortex, given the significant role of this region in executive functioning (Tsujimoto et al. 2004; Sato et al. 2013). The experiment started with a calibration period to locate regions in the dorsolateral prefrontal cortex (target regions) associated with working memory load. The calibration period consisted of twelve trials of the working memory task and lasted approximately four minutes. The target regions were selected automatically through general linear modeling at the end of the calibration period. A NIRS channel was selected as the target if its t-value was 1 SD higher than the mean. It should be noted that the specific targeted regions were different among subjects because of individual neurocognitive differences in task performance. The between-subject overlap in targeted regions is presented in Figure 1C. The distribution of the selected target regions is consistent with previous data showing activity in the bilateral prefrontal cortex during Sternberg task performance (Rympa et al. 1999; Veltman et al. 2003).
In subsequent trials, subjects were presented with feedback regarding activity in the targeted channels as well as their behavioral performance (i.e. accuracy) on the task. The neurofeedback was designed to represent brain activity in the target channels during encoding (2 s) and retention (average 7 s) phases (Coyle et al. 2007). Therefore, the OHb measurements over a 9 s window were used for neurofeedback calculation, accounting for hemodynamic delay. Specifically, the recorded OHb signals were first band-pass filtered with cutoff frequencies of 0.01 and 0.5 Hz (Cui et al. 2011). Then, the average OHb signal over the targeted channels (over 9 s window) was calculated and its relative change compared with the OHb signal in the calibration period was presented to the subject. For the behavioral performance feedback, the relative change in accuracy compared with the average accuracy in the calibration period was presented.
The feedback was visually presented as a line plot showing the history of changes in neural activity and behavioral performance during the previous ten trials (Figure 1D). A decrease/increase in the slope of the line represents decrease/increase in average activity in the targeted channels. The feedback was intermittent (on a trial-by-trial basis) and updated 2 s after each inquiry phase during the inter-trial interval to allow for the hemodynamic delay and to limit the interfering effect that feedback might have on task performance. Subjects were instructed to focus on task performance during each trial. In the inter-trial interval, the feedback was updated and the subjects could analyze the feedback in order to modify their cognitive strategy. Subjects were instructed to recruit a metacognitive strategy that results in reduction in neurofeedback signal without compromising their performance.
The same procedure and instructions were provided to the SHAM group except that the presented neurofeedback was not actual and derived from the NFB participants’ feedback. Specifically, the neurofeedback from each individual in the NFB group was scrambled and pseudorandomly assigned to one of the SHAM participants. However, the behavioral performance feedback was real for the SHAM group.
2.4. Outcome Measures
The effect of neurofeedback training on executive function was measured using a set of cognitive tests. Subjects performed these tests at baseline and after the end of training. Average accuracy in a classic verbal n-back test (Jonides et al. 1997) was used as the primary outcome measure to test subjects’ improvement in working memory performance. Secondary outcome measures included the Letter-Number Sequencing Test from the Wechsler Adult Intelligence Scale (WAIS) 4th Edition, a measure of working memory span (Wechsler 2008), the Trail Making Test (standard and alternate forms), a measure of task switching (Lezak et al. 2004), the Color-Word Interference test from the Delis-Kaplan Executive Function System (DKEFS), a measure of response inhibition and cognitive flexibility (Homack et al. 2005) and the Switching task from our in-house mobile cognitive assessment battery (MCAB) (Kesler and Blayney 2014), which measures task switching, response bias and cognitive flexibility.
2.5. Statistical analysis
2.5.1. Behavioral data analysis
To evaluate changes in executive function performance between the two groups, a linear mixed model was used treating group as fixed effect and individuals as random effect. The n-back accuracy (primary outcome) before and after training was entered as the dependent variable in the model (alpha = 0.05). We performed this analysis with and without age and gender as covariates. Similar models were built for evaluating changes in the secondary outcome measures between groups. It should be noted that the analyses of secondary outcomes were considered exploratory and therefore we did not correct for multiple comparisons.
To test if providing discordant feedback would affect the performance of the SHAM group during the training, we compared the performance of SHAM group during the calibration trials (i.e. the first twelve trials with no feedback) and the feedback training trials (i.e. the rest of the trials with sham feedback). A one-sample t-test was used to compare the average performance across sessions. In addition, we compared the behavioral practice effects of Sternberg task between SHAM and NFB. A linear mixed model was employed with the performance in the Sternberg task in each session as the dependent variable.
2.5.3. Neurofeedback analysis
To compare the training-related brain activity between NFB and SHAM groups, a general linear model was first used to quantify the beta estimates of activity (OHb) of each channel within an individual session. This resulted in one beta estimate corresponding to each training session at the individual level. To identify the regions that showed significant differences in prefrontal cortex activity between groups across training sessions, the extracted beta estimates were input to a linear mixed model. This analysis tests if neurofeedback training had a significant effect on brain function.
In addition, a one-sample t-test was performed to evaluate the changes in neurofeedback signal within the NFB group across training sessions. Specifically, the average slope of change in neurofeedback signal across sessions was entered into this analysis. This analysis measures if NFB group could significantly reduce the neurofeedback signal during training. Exploratory Spearman’s correlation analyses (alpha = 0.05) were also performed to identify association between the amount of change in neurofeedback signal and participants’ performance in executive function tests within the NFB group.
3. Results
3.1. Behavioral data
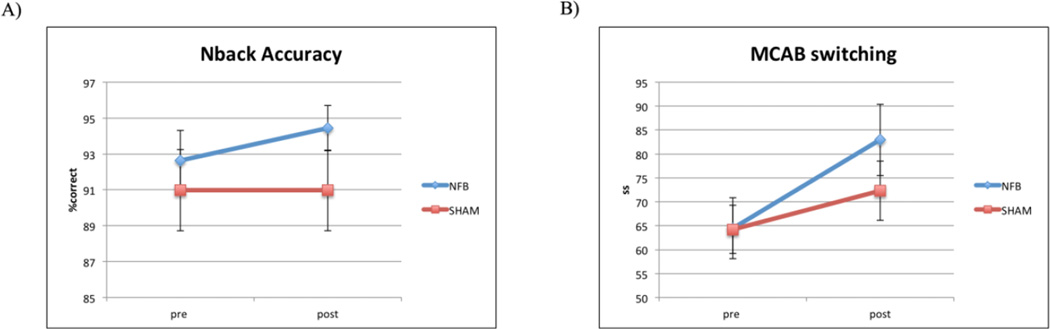
Performance of NFB and SHAM groups in cognitive tests and training are given in Table 1. A significant group by time interaction was observed for the primary outcome measure (n-back accuracy) (F1,19.7 = 5.42, p = 0.031, Cohen’s d = 0.69), with the NFB group improving more than SHAM group after training (Figure 2A). Similar effect was observed without correcting for age and gender (significant group by time interaction, p = 0.049). Among secondary outcome measures, a significant group by time interaction was only observed for the MCAB switching score (F1,20 = 5.44, p = 0.03, Cohen’s d = 0.79), with NFB improving more than SHAM after training (Figure 2B). It should be noted that this effect did not survive the correction for multiple comparison.
Table 1.
Performance in cognitive tests and training
| Test | NFB | SHAM | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| N-back accuracy (%) | 92.6 (5.3) | 94.4 (4.0) | 91.0 (7.2) | 91.0 (7.1) |
| N-back RT (s) | 0.65 (0.1) | 0.58 (0.1) | 0.54 (0.07) | 0.56 (0.1) |
| LNS (ss) | 10.6 (1.1) | 13.3 (3.7) | 10.5 (2.8) | 12.1 (3.8) |
| TMT (completion time) | 32.8 (14.9) | 31.4 (19.3) | 32.0 (15.2) | 31.9 (15.6) |
| CW Switch (ss) | 11.8 (1.6) | 12.7 (1.6) | 11.4 (2.6) | 12.3 (2.11) |
| CW Inhibit (ss) | 11.8 (4.0) | 13.3 (1.8) | 11.7 (1.9) | 12.6 (1.7) |
| MCAB Switching (rs) | 64.5 (20.1) | 83.0 (23.6) | 64.2 (16.0) | 72.3 (19.6) |
| Sternberg accuracy* (%) | 89.3 (6.5) | 89.2(5.6) | 77.4 (14.6) | 77.2 (16.7) |
RT: Response Time; LNS: Letter-Number Sequencing; TMT: Trail Making Test; CW: Color-Word Interference Test; %: percent accuracy; s: seconds; ss: scaled score; rs: raw score. Higher score indicates higher performance except for trail-making test.
The performance in the Sternberg task is given for the first (pre) and last (pre) session of the training.
Figure 2. Changes in outcome measures after neurofeedback training.
A) Primary outcome measure. The NFB group showed significant improvement in n-back accuracy compared with SHAM. B) NFB improvement in secondary outcome measures. NFB showed significant improvement in MCAB switching score compared with SHAM.
We did not find any significant difference in subjects’ performance in the calibration trials (no feedback) and feedback trials within the SHAM (p = 0.61) and NFB (p = 0.62) groups. While the average performance of NFB in Sternberg task was significantly higher compared with SHAM, the group by time interaction was not significant (p = 0.26). In other words, there was no significant difference in practice effect of Sternberg tasks between groups.
3.2. Neurofeedback data
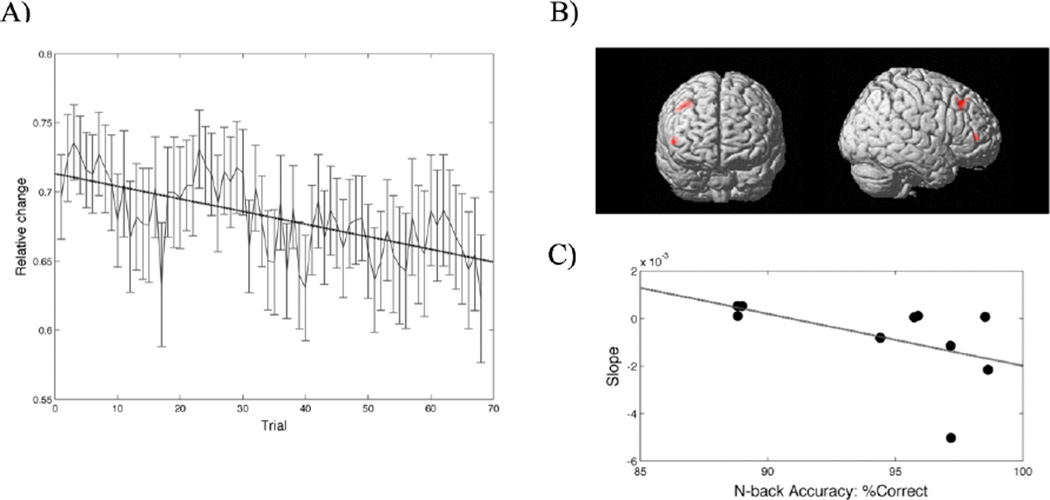
The reduction in neurofeedback signal in the NFB was only marginally significant in the second session (p = 0.055, Cohen’s d = 0.56; p > 0.14 for other sessions). The average reduction in neurofeedback signal over sessions was also marginal (p = 0.094, Cohen’s d = 0.45) (Figure 3A). These results suggest that NFB reduced the presented feedback signal but the observed decrease was marginally significant. Comparison of beta estimates of activity between groups showed significantly decreased activity in the NFB in the right inferior frontal and middle frontal gyrus compared with SHAM (Figure 3B).
Figure 3. Neurofeedback signal.
A) The average change in neurofeedback signal across neurofeedback trials within the NFB group. The dotted line represents the linear fit and shows a decrease in neurofeedback signal (p = .094, Cohen’s d = 0.45). B) Between-group differences in brain activity in response to training: Significant training-related decrease in activity was observed in the right inferior and middle frontal gyrus in NFB compared to SHAM. C) A significant negative correlation was observed between the change in neurofeedback signal and post-training n-back performance within the NFB group.
3.3. Correlation analysis
Reduction in neurofeedback signal was significantly correlated with post-training n-back accuracy in the NFB group (Spearman’s rho = 0.71, p = 0.023) (Figure 3C).
4. Discussion
In this novel study, we aimed to determine if providing neurofeedback in parallel with computerized training is helpful for targeted enhancement of a specific brain network and developing effective cognitive training programs. We engaged participants in a training program during which we provided them with the behavioral performance feedback typical of cognitive training paradigms but additionally gave them feedback about their brain activity. Participants in the neurofeedback group (NFB) showed significantly improved working memory performance compared with participants who received sham feedback (SHAM). These results suggest that task-based neurofeedback training design has the potential for developing effective cognitive training paradigms.
Compared with SHAM group, the NFB group significantly reduced the activity in the right middle and inferior frontal regions consistent with the provided training instructions. It suggests that providing neurofeedback helped the NFB participants significantly reduce brain activity in the targeted network within the prefrontal regions while preserving their performance. Several functional MRI studies have reported decreased activity in the right prefrontal regions after working memory cognitive training. For example, Schneiders and colleagues (Schneiders et al. 2011) reported a significant decrease in activity in the right superior middle frontal gyrus after two weeks (8 hours total) of visual and auditory working memory training. Interestingly, they reported an additional decrease in activity in the right middle frontal gyrus specific to visual working memory training that resulted in greater training gain as opposed to auditory working memory training and no training. There was no training-related increase in activity observed during this study. The authors suggested that training leads to greater efficiency of storage, access, and updating of stimuli mediated by the right middle frontal gyrus.
Decrease in activity of the right middle frontal gyrus was also reported after practice of object and spatial delayed working memory tasks and was linked to increased efficiency of maintaining task-relevant information and improved ability to filter-out task-irrelevant information (Sayala et al. 2006). Conversely, training in a verbal Sternberg task resulted in decreased activity in a network of brain regions including the bilateral dorsolateral prefrontal cortex (Jansma et al. 2001). These regions were part of the neural substrates of working memory performance before practice. The authors attributed the dominant practice-related decrease in activity, along with improved performance, to more efficient information processing within the neural network underlying working memory performance.
There are several mechanisms underlying a decrease in neural activity (Kelly et al. 2006; Poldrack 2014). For example, participants may have used various metacognitive strategies to regulate their neurofeedback signal which could have resulted in experienced-based neural reorganization. We did not inquire regarding participants’ subjective experiences such as if they used metacognitive strategies and if so, what strategies they used. We also did not have complementary neuroimaging data available such as gray matter volumes or white matter pathway measurements. Therefore, further research is necessary to determine how neurofeedback affects the neurocircuitry underlying executive function performance. It is noteworthy that the decreased brain activity observed in our study cannot be entirely attributed to priming and/or practice effects (Klingberg 2010). Specifically, while the experimental settings were the same for both the NFB and SHAM groups (except the feedback information), the behavioral practice effects in Sternberg task were not different between groups. This data further justifies that the observed reduction in brain activity in prefrontal cortex in NFB compared with SHAM is not affected by between group differences in learning the Stenrberg task.
Although it was a marginal effect, we demonstrated that participants in the NFB group were able to down-regulate their prefrontal cortex activity during training. Previous studies have also shown that stimulus-based neurofeedback is successful for helping subjects regulate a target brain region. Participants have been trained to regulate activity in the auditory cortex while listening to auditory stimuli (Haller et al. 2013), to control pain in response to noxious thermal stimuli (deCharms et al. 2005), to up- and down-regulate the emotional network in the presence of threat-related stimuli (Veit et al. 2012), and to down-regulate the activity in amygdala while presented with negative emotional faces (Bruhl et al. 2014). To our knowledge, this is the first study to apply task-based neurofeedback cognitive training on executive function.
There is no consensus whether the instruction about the neurofeedback signal should be implicit or explicit (Sulzer et al. 2013), or whether subject should try to up-regulate or down-regulate the activity in a target region (Ruiz et al. 2014). Our results correspond with our training instruction to down-regulate. Instructions should likely depend on the target population’s specific neuropathology. For example, in patients with mild cognitive impairment (MCI), training mainly results in increased activity within putatively compensatory networks (Hosseini et al. 2014). These “compensatory” networks are commonly employed in task performance by healthy older adults but are negatively affected by MCI. Therefore, MCI participants might be instructed to up-regulate the activity of these networks using task-based neurofeedback training.
We did not have a cognitive-training only group to measure the pure effect of neurofeedback on top of the effect of cognitive training. Specifically, one might argue that providing SHAM group with discordant feedback might have detrimental effect on their performance. In order to investigate this effect, we compared the performance of SHAM group during the calibration trials (with no feedback) and the feedback training trials. We did not find any significant difference in performance for trials with no feedback and those with sham or real feedback in SHAM and NFB groups. Further, the group by time interaction in the Sternberg task performance was not significant. These data indicate that there was no significant difference in practice effects of Sternberg task between groups and corroborate the idea that sham feedback did not suppress the performance in the Sternberg task.
Cognitive training has been criticized for lacking transfer to non-trained cognitive domains (Park and Bischof 2013). Our neurofeedback cognitive training was associated with an improvement in working memory, as expected, but also an increase in task switching performance. The time-based resource sharing model of working memory suggests that there are shared resources underlying task switching and working memory processes and that task-switching induces a cost on working memory processes (Liefooghe et al. 2008). Conversely, neuroimaging studies have shown an overlap between neurocircuitry underlying working memory and task switching processes (Dove et al. 2000; Wager et al. 2004). Specifically, overlapping regions within the lateral prefrontal cortex respond to both working memory and task switching paradigms (Dove et al. 2000). We speculate that the present neurofeedback training tap neural resources shared between working memory and task switching processes. It should be noted that this analysis was exploratory and the transfer effect needs to be tested more rigorously in future studies.
The results of the current study demonstrate the feasibility of task-based neurofeedback training for improving higher cognitive functions. However, a few considerations are noteworthy. Although NIRS can be used in more natural settings compared with fMRI (Irani et al. 2007), the training still needs to be done in person, a disadvantage compared to computerized training programs that can be done at home. However, previous fMRI neurofeedback studies have shown that subjects can successfully apply the acquired regulatory strategy learned during neurofeedback training in the absence of feedback (deCharms et al. 2004; Haller et al. 2013; Ruiz et al. 2013). Therefore, trained subjects could potentially practice cognitive training tasks at home using the learned metacognitive strategy. Nonetheless, new generation NIRS systems are more portable/wearable and affordable (Atsumori et al. 2007; Sagara et al. 2009; Lareau et al. 2011) and have the potential to be used with personal computers in the near future (Sagara et al. 2009). We did not follow-up the participants to investigate the long-term effect of training. Cognitive training studies have shown the stability of training effects after several months (Willis et al. 2006; Park and Bischof 2013). Future studies need to test the long-term effect of neurofeedback training. In addition, our study was a feasibility study where the sample size was small. However, the effect size data are quite promising showing a medium to large effects. Finally, a recent cognitive training study demonstrated changes in white matter indices in the limbic structure of healthy individuals following just 2 hours of cognitive training (Sagi et al. 2012). Our neurofeedback training program was slightly shorter in duration and also takes into account individual differences in brain networks and can therefore be customized for patients depending on the type of pathology involved; a feature that is unique to neurofeedback training.
5. Conclusions
This study reports a preliminary investigation regarding the effectiveness of task-based neurofeedback for executive function training using NIRS. The results suggest that providing neurofeedback can significantly enhance executive function after a short period of training. While our current study focused on young, healthy adults, a similar design could potentially be used for patient populations with known pathology, potentially helping them to boost/recover the activity in the affected brain regions. Additionally, the proposed design could potentially be helpful for improving the efficiency of cognitive training paradigms making it more feasible for certain patient populations.
Highlights.
-
-
Significantly improved EF test scores in the NFB group compared with SHAM
-
-
Reduced post-training activity in the prefrontal regions in NFB compared with SHAM
-
-
Targeted enhancement of executive functions using task-based neurofeedback training
Acknowledgments
This work was supported by funding from the National Institutes of Health (1DP2OD004445, 1R01NR014195, and 1R01CA172145 to S.R.K.), Lucile Packard Foundation for Children's Health (LPFCH), Spectrum Child Health, Pilot Early Career Award (to SMH), and Brain & Behavior Foundation, NARSAD Young Investigator Award (to SMH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Kong E, Larraburo Y, Rolle C, Johnston E, Gazzaley A. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumori H, Kiguchi M, Obata A, Sato H, Katura T, Utsugi K, Funane T, Maki A. Development of a multi-channel, portable optical topography system. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2007;2007:3362–3364. doi: 10.1109/IEMBS.2007.4353051. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL Advanced Cognitive Training for I, Vital Elderly Study G. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in cognitive sciences. 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S, Clement F, Mellah S, Gilbert B, Fontaine F, Gauthier S. Training-related brain plasticity in subjects at risk of developing Alzheimer's disease. Brain : a journal of neurology. 2011;134:1623–1634. doi: 10.1093/brain/awr037. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Ramos Murguialday A, Weber C, Montoya P. Neurofeedback and brain-computer interface clinical applications. International review of neurobiology. 2009;86:107–117. doi: 10.1016/S0074-7742(09)86008-X. [DOI] [PubMed] [Google Scholar]
- Bruhl AB, Scherpiet S, Sulzer J, Stampfli P, Seifritz E, Herwig U. Real-time neurofeedback using functional MRI could improve down-regulation of amygdala activity during emotional stimulation: a proof-of-concept study. Brain topography. 2014;27:138–148. doi: 10.1007/s10548-013-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. Journal of the American Dietetic Association. 2011;111:92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko A, Bedard AC, Marks DJ, Feirsen N, Uderman JZ, Chimiklis A, Rajwan E, Cornwell M, Anderson L, Zwilling A, Ramon M. A randomized clinical trial of Cogmed Working Memory Training in school-age children with ADHD: a replication in a diverse sample using a control condition. Journal of child psychology and psychiatry, and allied disciplines. 2014;55:247–255. doi: 10.1111/jcpp.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry research. 2009;166:210–222. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle SM, Ward TE, Markham CM. Brain-computer interface using a simplified functional near-infrared spectroscopy system. Journal of neural engineering. 2007;4:219–226. doi: 10.1088/1741-2560/4/3/007. [DOI] [PubMed] [Google Scholar]
- Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage. 2011;54:2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JD. Learned regulation of spatially localized brain activation using real-time fMRI. NeuroImage. 2004;21:436–443. doi: 10.1016/j.neuroimage.2003.08.041. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain research Cognitive brain research. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage. 2012;63:921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophrenia bulletin. 2010;36:869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Kopel R, Jhooti P, Haas T, Scharnowski F, Lovblad KO, Scheffler K, Van De Ville D. Dynamic reconfiguration of human brain functional networks through neurofeedback. NeuroImage. 2013;81:243–252. doi: 10.1016/j.neuroimage.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Giddens M, Sathian K. Mnemonic strategy training partially restores hippocampal activity in patients with mild cognitive impairment. Hippocampus. 2012;22:1652–1658. doi: 10.1002/hipo.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homack S, Lee D, Riccio CA. Test review: Delis-Kaplan executive function system. Journal of clinical and experimental neuropsychology. 2005;27:599–609. doi: 10.1080/13803390490918444. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Kramer JH, Kesler SR. Neural correlates of cognitive intervention in persons at risk of developing Alzheimer's disease. Frontiers in aging neuroscience. 2014;6:231. doi: 10.3389/fnagi.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Mano Y, Rostami M, Takahashi M, Sugiura M, Kawashima R. Decoding what one likes or dislikes from single-trial fNIRS measurements. Neuroreport. 2011;22:269–273. doi: 10.1097/WNR.0b013e3283451f8f. [DOI] [PubMed] [Google Scholar]
- Irani F, Platek SM, Bunce S, Ruocco AC, Chute D. Functional near infrared spectroscopy (fNIRS): an emerging neuroimaging technology with important applications for the study of brain disorders. The Clinical neuropsychologist. 2007;21:9–37. doi: 10.1080/13854040600910018. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10081–10086. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Slagter HA, Kahn RS. Functional anatomical correlates of controlled and automatic processing. Journal of cognitive neuroscience. 2001;13:730–743. doi: 10.1162/08989290152541403. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal Working Memory Load Affects Regional Brain Activation as Measured by PET. Journal of cognitive neuroscience. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kelly C, Foxe JJ, Garavan H. Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Archives of physical medicine and rehabilitation. 2006;87:S20–S29. doi: 10.1016/j.apmr.2006.08.333. [DOI] [PubMed] [Google Scholar]
- Kesler S, Blayney DW. Mobile cognitive assessment battery (MCAB) for assessment of cancer-related cognitive changes. Journal of Clinical Oncology. 2014;32S:A9571. [Google Scholar]
- Kesler S, Hadi Hosseini SM, Heckler C, Janelsins M, Palesh O, Mustian K, Morrow G. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clinical breast cancer. 2013;13:299–306. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Lacayo NJ, Jo B. A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain injury : [BI] 2011;25:101–112. doi: 10.3109/02699052.2010.536194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends in cognitive sciences. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Kober SE, Wood G, Kurzmann J, Friedrich EV, Stangl M, Wippel T, Valjamae A, Neuper C. Near-infrared spectroscopy based neurofeedback training increases specific motor imagery related cortical activation compared to sham feedback. Biological psychology. 2014;95:21–30. doi: 10.1016/j.biopsycho.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Kray J, Karbach J, Haenig S, Freitag C. Can task-switching training enhance executive control functioning in children with attention deficit/-hyperactivity disorder? Frontiers in human neuroscience. 2011;5:180. doi: 10.3389/fnhum.2011.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lareau E, Lesage F, Pouliot P, Nguyen D, Le Lan J, Sawan M. Multichannel wearable system dedicated for simultaneous electroencephalographynear-infrared spectroscopy real-time data acquisitions. Journal of biomedical optics. 2011;16:096014. doi: 10.1117/1.3625575. [DOI] [PubMed] [Google Scholar]
- Levesque J, Beauregard M, Mensour B. Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Neuroscience letters. 2006;394:216–221. doi: 10.1016/j.neulet.2005.10.100. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th. New York: Oxford University Press; 2004. [Google Scholar]
- Liefooghe B, Barrouillet P, Vandierendonck A, Camos V. Working memory costs of task switching. Journal of experimental psychology Learning, memory, and cognition. 2008;34:478–494. doi: 10.1037/0278-7393.34.3.478. [DOI] [PubMed] [Google Scholar]
- Marshall GA, Rentz DM, Frey MT, Locascio JJ, Johnson KA, Sperling RA Alzheimer's Disease Neuroimaging I. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7:300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Mihara M, Hattori N, Hatakenaka M, Yagura H, Kawano T, Hino T, Miyai I. Near-infrared spectroscopy-mediated neurofeedback enhances efficacy of motor imagery-based training in poststroke victims: a pilot study. Stroke; a journal of cerebral circulation. 2013;44:1091–1098. doi: 10.1161/STROKEAHA.111.674507. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of general psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JW, Dowell LR, Mostofsky SH, Denckla MB, Mahone EM. Neuropsychological profile of executive function in girls with attention-deficit/hyperactivity disorder. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2010;25:656–670. doi: 10.1093/arclin/acq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Oda I, Isobe S, Suzuki T, Kohyama K, Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature neuroscience. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Park DC, Bischof GN. The aging mind: neuroplasticity in response to cognitive training. Dialogues in clinical neuroscience. 2013;15:109–119. doi: 10.31887/DCNS.2013.15.1/dpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Is "efficiency" a useful concept in cognitive neuroscience? Developmental cognitive neuroscience. Developmental cognitive neuroscience. 2014 doi: 10.1016/j.dcn.2014.06.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Flegal KE, Kelly LL. Can cognitive training improve episodic memory? Neuron. 2011;72:688–691. doi: 10.1016/j.neuron.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S, Buyukturkoglu K, Rana M, Birbaumer N, Sitaram R. Real-time fMRI brain computer interfaces: Self-regulation of single brain regions to networks. Biological psychology. 2014;95:4–20. doi: 10.1016/j.biopsycho.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Ruiz S, Lee S, Soekadar SR, Caria A, Veit R, Kircher T, Birbaumer N, Sitaram R. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Human brain mapping. 2013;34:200–212. doi: 10.1002/hbm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rympa B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JDE. Load-dependent roles of frontal brain regions in the maintenance of working memory. NeuroImage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Sagara K, Kido K, Ozawa K. Portable single-channel NIRS-based BMI system for motor disabilities' communication tools. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2009;2009:602–605. doi: 10.1109/IEMBS.2009.5333071. [DOI] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Sato H, Yahata N, Funane T, Takizawa R, Katura T, Atsumori H, Nishimura Y, Kinoshita A, Kiguchi M, Koizumi H, Fukuda M, Kasai K. A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. NeuroImage. 2013;83:158–173. doi: 10.1016/j.neuroimage.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Sayala S, Sala JB, Courtney SM. Increased neural efficiency with repeated performance of a working memory task is information-type dependent. Cerebral cortex. 2006;16:609–617. doi: 10.1093/cercor/bhj007. [DOI] [PubMed] [Google Scholar]
- Scharnowski F, Hutton C, Josephs O, Weiskopf N, Rees G. Improving visual perception through neurofeedback. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17830–17841. doi: 10.1523/JNEUROSCI.6334-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiders JA, Opitz B, Krick CM, Mecklinger A. Separating intra-modal and across-modal training effects in visual working memory: an fMRI investigation. Cerebral cortex. 2011;21:2555–2564. doi: 10.1093/cercor/bhr037. [DOI] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. Discovery of Processing Stages - Extensions of Donders Method. Acta Psychol. 1969;30:276-+. [Google Scholar]
- Subramanian L, Hindle JV, Johnston S, Roberts MV, Husain M, Goebel R, Linden D. Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinson's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:16309–16317. doi: 10.1523/JNEUROSCI.3498-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer J, Haller S, Scharnowski F, Weiskopf N, Birbaumer N, Blefari ML, Bruehl AB, Cohen LG, DeCharms RC, Gassert R, Goebel R, Herwig U, LaConte S, Linden D, Luft A, Seifritz E, Sitaram R. Real-time fMRI neurofeedback: progress and challenges. NeuroImage. 2013;76:386–399. doi: 10.1016/j.neuroimage.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S, Yamamoto T, Kawaguchi H, Koizumi H, Sawaguchi T. Prefrontal cortical activation associated with working memory in adults and preschool children: an event-related optical topography study. Cerebral cortex. 2004;14:703–712. doi: 10.1093/cercor/bhh030. [DOI] [PubMed] [Google Scholar]
- Vance DE. Potential factors that may promote successful cognitive aging. Nursing: Research and Reviews. 2012;2:27–32. [Google Scholar]
- Veit R, Singh V, Sitaram R, Caria A, Rauss K, Birbaumer N. Using real-time fMRI to learn voluntary regulation of the anterior insula in the presence of threat-related stimuli. Social cognitive and affective neuroscience. 2012;7:623–634. doi: 10.1093/scan/nsr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. NeuroImage. 2003;18:247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale Fourth Edition. San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E, Group AS. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA : the journal of the American Medical Association. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PloS one. 2013;8:e61624. doi: 10.1371/journal.pone.0061624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan ET. Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience. 2006;139:317–325. doi: 10.1016/j.neuroscience.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature neuroscience. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]