Abstract
Obesity and metabolic diseases are linked to chronic stress and low socio-economic status. The mechanistic link between stress and obesity has not been clarified, partly due to the inherent complexity exemplified by the bidirectional effect of stress on eating and body weight. Recent studies focusing on adaptive-thermogenesis and brown adipose tissue (BAT) function support a dichotomous relationship to explain the impact of stress on obesity: stress promotes obesity in the presence of hyperphagia and unchanged BAT function; stress results in weight-loss/obesity-resistance in the presence of hypophagia, or when hyperphagia is associated with BAT recruitment and enhanced thermogenesis. Mechanistically dissecting the bidirectional effects of stress on metabolic outcomes might open new avenues for innovative pharmacotherapies for the treatment of obesity-associated diseases.
Keywords: β-adrenergic receptors, hypothalamus-pituitary-adrenocortical axis, social stress, sympathetic nervous system, purinergic
The dichotomous consequences of stress on energy balance
In the last few decades, obesity-associated metabolic diseases have become a pandemic, seemingly due to the widespread consumption of Western-type diet combined with sedentary lifestyle and increased psycho-emotional stress [1]. However, industrialized societies also suffer from stress-associated eating disorders that can negatively impact energy balance, such as anorexia nervosa, characterized by self-starvation and excessive weight loss, and bulimia, characterized by periods of excessive overeating followed by purging [2].
We refer to stress here as a coordinated set of physiological responses to unpredictable and uncontrollable conditions where an environmental/internal demand exceeds the natural regulatory range and adaptive capacity of an organism [3] (Box 1). It is key to emphasize that individuals can vary in their response to stress, considerably. This intrinsic feature of the stress responses has been well studied in the context of central nervous system (CNS) disorders [4]. Similarly, metabolic responses vary in both human and animal models, with some subjects exhibiting decreased while others increased food intake and body weight when exposed to stress. The stress-eating paradox has been well documented in studies on college students reporting changes in food intake following a “stressful” experience [5–7]. The same dichotomous outcome has been confirmed in more general populations. Eating behavior has been linked to psychosocial stress related to work, personal relationships, life constraints, and finances [8]. Stress-associated hypophagia results in weight loss or resistance to obesity. However, stress-associated hyperphagia is not always followed by concomitant increases in body weight and risk for obesity, thus pointing to multiple mechanisms that control energy expenditure being simultaneously activated under certain conditions [9,10]. A recent study extended this knowledge to demonstrate that stress and a history of major depression alter metabolic responses to an experimental meal, ranging from lower energy expenditure to altered lipid metabolism and endocrine output [11]. Overall, although the effect of stress on energy balance has been well documented, a mechanistic explanation is still lacking.
BOX 1. STRESS, “STRESS HORMONES” AND STRESS-RELATED METABOLIC ORGANS.
A major difficulty in the study of stress physiology is the varying definition which has been proposed and used operationally by scientists [3,88,89]. A revised terminology has been presented to restrict the use of “stress” to conditions characterized by unpredictability and uncontrollability as well as to limit the definition of stimuli eliciting a true stress-response to conditions where an environmental/internal demand exceeds the natural regulatory range and adaptive capacity of an organism [3]. Consistently, physiological reactions that are a prerequisite of any behavior and that occur in conditions of acute challenge to homeostasis, arousal or, adaptation to predictable changes in environmental conditions within the regulatory range of a species, should not be called “stress” [3]. Accordingly, animal models consisting of an acute challenge to homeostasis or arousal as well as acclimation to environmental temperatures within the regulatory range of a species should not be considered stressors. It has now been established that stress-induced pathological effects are more likely to develop when unpredictable and uncontrollable chronic stressors of social nature (social stress) induce physiological and behavioral adjustments leading to a continuous “wear and tear” on underlying physiology. There are three major neuroendocrine systems mediating most components of the stress response [e.g.12,89]. The first is the hypothalamic–pituitary–adrenocortical (HPA) axis, which stimulates the adrenal cortex to release glucocorticoids such as cortisol in humans or corticosterone in rodents into the blood. The HPA-axis is a closed neuroendocrine axis regulated by inhibitory feedback loops that is also regulated by upstream nuclei including the hippocampus, the bed nucleolus of the stria terminalis and, as recently demonstrated, a small group of cells in the olfactory cortex [88,100]. The second system is the sympatho-adrenomedullary axis composed by premotor neuron-derived nerves triggering the release of epinephrine and norepinephrine from the chromaffin cells in adrenal medulla into the blood. Furthermore, sympathetic nerve innervating essentially every organ in the body, secrete norepinephrine and, in an organ specific manner, also ATP, NPY and other neuropeptides [39,88]. Finally, several peptides recently identified to modulate energy homeostasis such as ghrelin, orexin-A, FGF-21, TLQP-21, etc are also implicated in the stress response [64–66].
Classical studies investigated the metabolic role of stress-associated hormones on liver, skeletal muscle and WAT. However, to understand the role of stress on obesity and energy homeostasis the activation of metabolic pathways in these key metabolic organs needs to be considered in light of the CNS circuits regulating food intake and adaptive thermogenesis and its master regulator, the BAT.
Our limited understanding of the molecular mechanisms involved in the stress-induced modulation of energy balance is at least in part due to a common view that oversimplifies the stress response by focusing mainly on the activation of the hypothalamus-pituitary-adrenal (HPA)-axis (Box 1). Glucocorticoids affect food intake and metabolism by favoring a positive energy balance [12]. However, other “stress-hormones” (Box 1), such as the catecholamines epinephrine (E) and norepinephrine (NE) secreted by the sympathetic nervous system (SNS) and sympatho-adreno-medullary axis (SAM), affect metabolism in a direction which is usually opposite to glucocorticoids. Catecholamines are mostly responsible for lipolysis in adipocytes, via activation of β-adrenergic receptors (β-AR) that in brown adipose tissue (BAT) is coupled with increased thermogenesis [13,14]. Furthermore, HPA-axis derived hormones can exert opposite effects on BAT: the adrenocorticotropic hormone (ACTH) enhances, while corticosterone (cortisol in humans) suppresses thermogenesis, uncoupling protein 1 (UCP1) expression and BAT activation [15,16].
This review aims to reconcile the opposite metabolic outcomes of stress, proposing a key role for stress-induced changes in adaptive thermogenesis and BAT functions, in modulating the impact of stress-induced hyperphagia or hypophagia.
Molecular mechanisms of stress-induced positive and negative energy balance
Central mechanisms of stress-induced positive energy balance
Laboratory rodents can respond with hyperphagic behavior to several psychological and physical stress models. For example, chronic mild stress and chronic social stress (see Glossary) have been linked to sustained hyperphagia and binge eating like disorders [17–19]. During chronic stress and the corresponding hyperactivation of the HPA axis, glucocorticoids and insulin increase craving for calorie rich meals [20]. The preference for palatable food ingestion has been proposed to reduce the negative effects of stress via downregulation of glucocorticoids, ACTH, and corticotropin releasing hormone (CRH) in the amygdala and stimulation of the anterior nucleus accumbens (NAc) shell to outweigh the contribution of the stress-stimulated posterior, defensive part of NAc [12,20].
The main orexigenic pathway is controlled by agouti related peptide (AgRP) and neuropeptide Y (NPY) expressing neurons located in the arcuate nucleus (ARC) of the hypothalamus [21]. ARC-AgRP neurons drive food intake via direct and indirect inhibition of second-order satiety neurons located in the paraventricular nucleus of the hypothalamus (PVH), anterior bed nucleus of the stria terminalis (aBNST), lateral hypothalamus (LH) and lateral parabrachial nucleus (LPBN) [22,23]. Chronic subordination stress has been associated with increased hypothalamic expression of AgRP and NPY mRNA [24]. Low doses of glucocorticoids can stimulate food intake by facilitating the orexigenic activity of NPY and drive the hypothalamic expression of AgRP [25]. However, the involvement of this circuit has not been functionally dissected yet in relation to chronic stress-associated metabolic functions.
Conversely, other neuropeptides have received more attention. Orexin is a neuropeptide that regulates arousal, wakefulness, and appetite, and orexin-expressing neurons are thought to be involved in the stress response through direct and indirect effects on hypothalamic CRH neurons and the brainstem, respectively. Orexin-A has been classically linked to the induction of hyperphagia and increased thermogenesis [26,27]. It has been proposed that this peptide elevates the set-point of the core body temperature thereby shaping the feeding response [28]. The orexigenic effect of Orexin-A is diminished when the body temperature is already increased, such as during postprandial thermogenesis, to facilitate postprandial anorexia [29]. This might explain the opposite effects of cold and restraint stress on food intake, both of which are reported to activate orexin neurons in rats, but that tend to respectively enhance and reduce/have no effect on eating [29]. The thermogenic demand elicited by cold exposure is indeed constantly high, while restraint stress only induces a transient rise in BAT temperature [30].
Several recent studies also point to a key role for the gut-derived hormone ghrelin on the central responses to stress, mood and food intake largely via modulation of ARC-AgRP/NPY neurons [31]. A recent study demonstrated that ghrelin modulation of PVH-CRF can be dissociated from ARC-NPY→PVH signaling while is mediated by growth hormone secretagogue receptors (GHSR) expressed on GABAergic terminal in the PVH [32]. Acyl-ghrelin plasma levels are lower in obesity and diabetes and elevated after several types of stress [31,33]. In rodent models of chronic social stress the plasma concentration of acyl-ghrelin is increased [24,34,35] while total ghrelin can be decreased [19]. Chronic subordination stress induces weight gain and hyperphagia that can be blocked by genetic or pharmacological blockage of the ghrelin receptor GHSR [24,35]. Consistently, third cerebral ventricle infusion of acyl-ghrelin promotes food intake and results in the inhibition of on BAT activity and temperature [36], all in all promoting positive energy balance.
Peripheral mechanisms of stress-induced positive energy balance
Unremitting stress may result in chronic hyperactivation of the HPA-axis: sustained glucocorticoid production stimulates gluconeogenesis and inhibits glycolysis resulting in hyperglycemia and increased insulin secretion. The combination of hypercortisolemia and hyperinsulinemia is thought to promote visceral obesity [12,20,37]. Elevated glucocorticoid levels modulate pathways involved in lipolysis and triglyceride synthesis in a tissue-specific manner. In the liver, they have been linked to the development of hypertriglyceridemia and hepatic steatosis due to reduced lipolysis and lipogenesis [37]. Glucocorticoids exert an adipose depot-specific effect that can vary from lipogenesis, adipogenesis, and lipolysis, as exemplified in the Cushing’s syndrome where abdominal obesity develops while subcutaneous fat becomes reduced [38]. Glucocorticoids also act on myocytes to oppose the actions of insulin, resulting in enhanced protein degradation, decreased protein synthesis, and suppressed insulin-stimulated glucose uptake [37].
A pivotal role in stress-induced enhanced fat mass has also been attributed to NPY release from sympathetic nerves [39]. Mice exposed to intermittent cold stress and fed a hypercaloric diet show increased fat mass and circulating glucocorticoids as well as increased NPY, Y2 receptors (Y2R) and dipetidyl peptidase IV (DPP4) mRNA in perigonadal WAT. Conversely, germline Y2R knockout mice exhibit reduced adiposity when exposed to stress. It has thus been hypothesized that intermittent cold stress could activate the NPY/Y2R pathway that promotes adipogenesis by blunting the NE/βAR signaling in WAT in a glucocorticoid dependent manner [39].
Central mechanisms of stress-induced negative energy balance
Reduced food intake is common to many stress models, mostly when stress appears to be life threatening or traumatic. Coherently, physical stressors such as heat stress, to which mammals can adapt only to a very limited extent before the upper temperature survival limit is reached [14,40], consistently reduce food consumption in rodents [41]. Repeated resident/intruder or unstable social settings stress can lead to a persistent hypophagic response and a corresponding and sustained body weight loss [42]. Finally, in some [43] but not all studies [34,35] using the chronic social defeat stress, mice become hypophagic.
Surprisingly, despite the accumulating experimental evidence on stress-elicited hypophagia, the central regulatory pathways remained so far poorly characterized. The main central anorexigenic pathway encompasses the inhibition of ARC-AgRP neurons [44], and the activation of the melanocortin pathway downstream of the ARC proopiomelanocortin (POMC) and cocaine and amphetamine-regulated transcript (CART) expressing neurons [44,45]. Leptin inhibits food intake and enhances adaptive thermogenesis at least in part via ARC-POMC neurons activation [46–48], thus suggesting that it might be involved in stress-induced hypophagia and thermogenesis. However, a context-dependent effect is suggested by the demonstration that leptin counteracts HPA-axis activation and opposes restraint stress-induced hypophagia and behavioral effects [49,50].
On the other hand, it is well established that CRH and its analogs urocortins 1–3 released from PVH neurons (which are modulated by ARC-POMC and ARC-AgRP neurons [22]), mediate restraint-stress induced hypophagia and weight loss with a mechanism requiring CRH receptor 1 activation [51,52], while controversial findings have been reported on the role of CRH receptor 2 activation [52,53].
Recent studies also identified a role for glucagon-like peptide (GLP)-1 and calcitonin gene-related peptide (CGRP)-expressing neurons in stress-mediated catabolic effects.
CGRP-expressing neurons in the PBN are tonically inhibited by GABAergic input from ARC-AgRP neurons [54]. The activation of the amygdala-projecting PBN neurons using genetic, optogenetic, and pharmacogenetic tools suppresses appetite while their inhibition increases food intake, in spite of the use of anorexigenic compounds such as lithium chloride or amylin that inhibit eating. Based on these data and given the anatomical connections between AgRP and CGRP neurons, it has been proposed that AgRP neurons inhibit CGRP neurons involved in anorectic circuits under normal conditions, while, where stress prevails, the CGRP circuit becomes disinhibited and feeding ceases [55].
Other neurons that are involved in stress-induced metabolic effects are the hindbrain GLP-1 expressing neurons, which are known to play a crucial role in regulating the HPA-axis [56]. Chronic mild stress and chronic restraint stress may reduce expression of the pre-pro-glucagon mRNA (which encodes for glucagon and GLP-1) in the nucleus tractus solitarius (NTS). In rats under chronic mild stress, chronic central administration of GLP-1 induces HPA-axis hyperactivity, decreases basal glucose levels, and body weight gain. Since food intake remains unchanged, this outcome could depend on GLP-1 acting on energy expenditure [57]. In line with this, GLP-1 receptor agonism can induce BAT activation, via increased sympathetic outflow, resulting in enhanced triglyceride and glucose clearance, both in lean and obese mice [58].
Peripheral mechanisms of stress-induced negative energy balance
Catecholamines are major pro-lipolytic and thermogenic factors in mammals via activation of βARs in white and brown adipocytes [59]. NE derived from sympathetic neurons increases triglyceride hydrolysis in white and brown adipocytes and activates the expression of mitochondrial UCP1 in brown adipocytes, thus enhancing thermogenesis. Rodent obesity has been associated with decreased expression of βARs [60,61], and treatment with selective βAR agonists may reduce diet-induced obesity (DIO) [62]. In response to chronic social defeat stress, mice lose weight but when allowed to recover they regain weight, due to hyperphagia, although fat mass and leptin remain lower than control levels [35]. Consistent with a catabolic role of βAR activation, in a rat model of metabolic syndrome, chronic restraint stress-induced weight loss can be ameliorated by the βAR antagonist propranolol [63].
In addition to catecholamines, other mediators that are released under certain stress conditions such as thyroid hormones, natriuretic peptides, fibroblast growth factor-21 (FGF-21), and TLQP-21 (not acronymic) have been shown to increase lipolysis, to oppose the anabolic action of glucocorticoids or to increase thermogenesis, overall exerting a negative effect on energy balance [64–66]. However, their mechanistic role in stress so far remains poorly characterized.
Adaptive thermogenesis and BAT function at the core of the stress-induced regulation of obesity
A major factor hindering the understanding of the dichotomous metabolic responses to stress reviewed above is the intrinsic complexity of the physiologic regulation of metabolism [67]. In this context, CNS plays a key role by exerting efferent control on adipose depot functions. The autonomic innervation of the WAT is fundamental in the regulation of adipogenesis and lipolysis [48], and under certain conditions thermogenesis [68]. Sympathectomy causes increased accumulation of lipids in WAT [48], suggesting that excessive fat deposition could be due to malfunctioning of the SNS. The subcutaneous (sc)WAT depots are also characterized by a remarkable plasticity and can undergo browning/beigeing, i.e. the activation of an inducible thermogenic program, under different environmental and pathological conditions such as cold or cancer cachexia [69,70].
The SNS is also an essential regulator of BAT and adaptive thermogenesis [13,14]. CNS networks, which respond reflexively to thermal afferent signals from skin thermoreceptors, regulate BAT by activating the postganglionic sympathetic neurons [71]. NE released from sympathetic nerves signals through βARs to induce tissue differentiation and UCP1-induced thermogenesis [14]. BAT recruitment is maximal in conditions of cold acclimation and is minimal in conditions of low demand for adaptive thermogenesis such as thermoneutrality [14,40]. Importantly, acclimation to thermoneutrality over a protracted period of time predisposes mice to hypercaloric diet-induced cardio-metabolic diseases [72]. Similarly to rodents housed at thermoneutrality, adult humans experience only a modest basal BAT activation due to a high body weight/surface area and predominant exposure to thermoneutrality, resulting in low sympathetic tone and NE/βAR signaling [14,73]. The adrenergic stimulation of BAT can be recapitulated in humans pharmacologically with βAR agonists [74]; furthermore, a brown genetic program in human adipose tissue can be induced with a severe adrenergic stress or exposure to cold [75]. BAT activity/amount is inversely associated with obesity and diabetes in humans [73], thus suggesting that targeting BAT differentiation and activation could be a viable therapeutic approach to obesity [76,77].
The first observations of stress-induced BAT activation date back to the 1940’s [14]. Later data demonstrated that exposing rats to chronic restraint stress increased their cold tolerance due to BAT recruitment, sustained UCP1 expression and activity, and thermogenic potential in correspondence to body weight and fat loss [78,79]. Recent studies provide a critical mechanistic demonstration that acute social defeat stress activates a neuronal pathway largely overlapping the one stimulated by cold to induce BAT thermogenesis and hyperthermia. Social defeat, like cold exposure, activates dorsomedial hypothalamic (DMH) neurons leading to stimulation of sympathetic premotor neurons in the rostral medullary raphe nuclei and culminating in βAR-dependent BAT activation and thermogenesis [80,81]. It is important to note that DMH is also a crucial node regulating stress-induced cardiovascular and neuroendocrine functions [71]. Importantly, the thermogenic efficacy of stress has recently been replicated in humans where an acute psychological challenge in thermoneutral conditions has been associated to increased supraclavicular BAT temperature [82]. Altogether, these evidences constitute a strong case for the inclusion of psycho-emotional stress as a naturalistic stimulus for BAT function, together with cold and diet induced thermogenesis in rodent models and humans.
In line with these data, it has been demonstrated that under physiological conditions promoting low adaptive thermogenesis and BAT recruitment/activation such as in wild-type mice housed at thermoneutrality or in mice genetically deficient in β1,β2,β3–ARs [83], chronic subordination stress leads to a selective interscapular BAT browning and sympathetic hyper-innervation [84]. These changes are associated with resilience to DIO in the presence of hyperphagia. Interestingly, this model elicits a browning of the BAT but not of visceral WAT or, scWAT that harbors the beige/brite adipocytes [70], overall suggesting that enhanced thermogenesis and resistance to obesity are mediated prevalently by BAT browning. These results are consistent with subordination stress eliciting a selective activation of sympathetic neurons innervating the BAT but not other fat pads [84].
Contrarily to mice acclimated to thermoneutrality, in wild-type mice acclimated to room temperature, therefore characterized by sustained BAT recruitment/activation, exposure to chronic subordination stress increases vulnerability to obesity and insulin resistance [85] in presence of an overall normal BAT morphology [84]. Subordinate mice housed at room temperature also show a molecular signature in the BAT characteristic of the myogenic lineage and de-differentiated brown adipocytes [84]. Consistent with a role of AgRP neurons in mediating stress-induced positive energy balance in mice housed at room temperature (see Central mechanisms of stress-induced positive energy balance), recent data demonstrated that opto- and chemo-genetic activation of ARC-AgRP neurons inhibit sympathetic activity to BAT, increase myogenic markers in the BAT and impair insulin sensitivity [86].
The dichotomous effect of stress on obesity: an energy balance perspective
The main components of energy balance are food intake, nutrients absorption, and energy expenditure. Energy expenditure is classically subdivided into three main components, basal thermogenesis, physical activity-related thermogenesis, and adaptive thermogenesis [13]. We propose that there are two main factors promoting stress-induced vulnerability vs. resilience to obesity: i) changes in food intake and ii) recruitment/activation of BAT and its impact on nutrient clearance and adaptive thermogenesis (Figure 1). The other two components of energy expenditure, i.e. physical activity and basal metabolism, are unlikely to explain the vulnerability vs. resilience to obesity. Firstly, stress in both laboratory models or in naturalistic settings consistently elicits a depression-like state characterized by diminished locomotor activity [3,4,84], thus failing to explain the dichotomous effect on energy balance [18, 34,35,84]. Secondly, the basal metabolic rate is relatively stable in adult subjects over relatively short periods of time [13,40]. Finally, it must be noted that stress can also influence nutrient transit and absorption and elicit gastrointestinal disorders [87], but the role of altered gastrointestinal functions on changes in energy balance remains unclear.
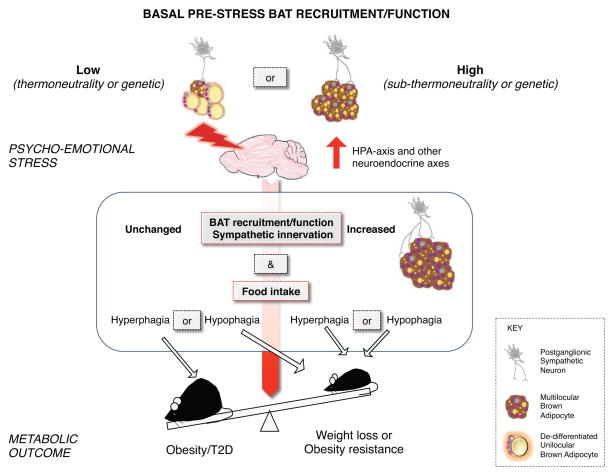
Figure 1. Proposed model to explain the dichotomous effect of stress on energy balance.
We propose that the metabolic outcome (exemplified by an obese/diabetic and a lean mouse) of chronic psycho-emotional stress is mediated by changes in food intake and recruitment/activation of the brown adipose tissue (BAT) and their impact on nutrient clearance and thermogenesis. Stress promotes obesity in the presence of increased caloric intake and unchanged BAT recruitment and function. Stress results in weight loss or resistance to obesity in the presence of hypophagia or hyperphagia, the latter paralleled by increased thermogenesis and BAT recruitment/function.
Based on the experimental evidences reviewed above we propose that an energy balance perspective explains the dichotomous impact of stress on obesity. Stress promotes obesity in the presence of hyperphagia only if BAT recruitment and function remain unchanged, when compared to basal pre-stress conditions. Most laboratory rodents are studied under conditions of moderate cold challenge (standard housing at temperatures is ~20–22°C), leading to a relatively high basal pre-stress BAT function. Conversely, in humans thermoneutrality is the norm, thus subjects are often characterized by minimal BAT recruitment and function. In rodent models as well as humans, the lack of net change in BAT recruitment/function will fail to compensate an excessive caloric intake resulting in increased stored nutrients, obesity, and metabolic disorders. Conversely, stress results in weight loss or resistance to obesity in two conditions, the first when stress induces hypophagia. However, the same metabolic outcome can occur when stress-induced hyperphagia will fail to compensate for an excessive thermogenesis and BAT recruitment/function compared to basal pre-stress conditions, thus limiting DIO and metabolic disorders.
This energy balance view also predicts that genetic predispositions (or pathological conditions) imposing low basal pre-stress adaptive thermogenesis requirement and resulting in minimal BAT recruitment would be permissive for stress-induced BAT recruitment, thus favoring obesity resistance even in the presence of hyperphagia (Figure 1). Conversely, genetic or pathological conditions associated with sustained basal pre-stress adaptive thermogenesis requirement and resulting in high BAT recruitment would facilitate obesity in the presence of stress-induced hyperphagia (Figure 1).
One characteristic likely to be associated with BAT recruitment and function is the intensity of stress-induced activation of sympathetic nerves innervating the BAT and neuroendocrine axes modulating brown adipocytes functions (Figure 2). Different stressors trigger the HPA-axis, SNS/SAM or other neuroendocrine axes with varied intensity [3,88,89] and stimuli of different intensity differentially regulate nerve activity leading to the preferential release of neurotransmitters and/or neuropeptides [90]. Acute social defeat activates the sympathetic nerves innervating the BAT with a magnitude comparable to acute cold exposure [85]. A direct experimental comparison of how different stress models activate the BAT sympathetic nerves is not available yet. However, a hypothesis can be introduced based on indirect evidences. Models characterized by low to mild challenge such as exposure to open field, result in BAT-independent thermogenesis ineffectual for modulating energy balance [91]. Similarly, an intermittent version of the chronic mild stress model exerted minimal and transient effects on energy balance [92]. Exposing mice to intermitted cold resulted in increased adiposity in the absence of reported increase in thermogenesis or BAT recruitment/activation [38]. Interestingly, mouse models that appear very similar such as the chronic social defeat and chronic subordination stress models induce overlapping neuro-endocrine and behavioral effects but have divergent effects on energy balance [18,19,24,34,35,43]. This striking difference could be attributed the stability (chronic subordination stress) vs. the instability (chronic social defeat stress) of the housing environment and the social rank experienced by the subordinate animal in the two models and result in a differential activation of BAT sympathetic nerves. Therefore, the chronic subordination stress model might represent a milder stimulus for subordinate mice as supported by the less dramatic but still significant elevation in corticosterone levels [93] and related to threatening but not traumatizing stressors, known to be linked to hyperphagia and positive energy balance in humans [9,10]. On the other hand, in the chronic social defeat stress model, the larger elevation in corticosterone [34,35], could be associated to other traumatizing stimuli involving immediate actual danger and often associated with a catabolic outcome [94]. Moving rightward along the intensity scale (Figure 2) are the phenotypes induced by restraint stress. Restraint stress elicits a milder HPA-axis activation compared to social stress that eventually undergoes habituation [3,95], whereas it acutely increases BAT activation and induces BAT recruitment [78,79]. Furthermore, dominant mice in the chronic psychosocial stress model become as hyperphagic as subordinate mice but are resilient to DIO, while manifesting a sustained increase in energy expenditure and thermogenesis as well as increased sympathetic innervation to the adipose organ [18,96].
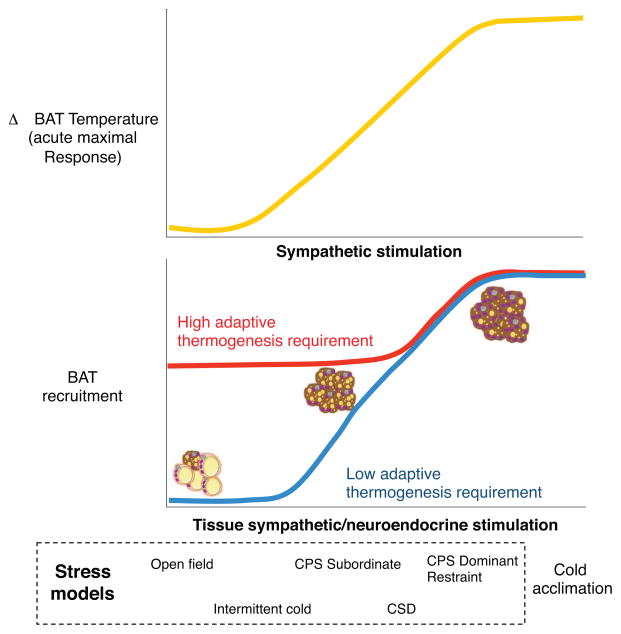
Figure 2. Model of the impact of stress-induced sympathetic and neuroendocrine function on BAT activation and recruitment.
Stress acutely enhances BAT activation and thermogenesis (top graph) and chronically induces BAT recruitment (lower panel) as a function of the intensity of the sympathetic and neuroendocrine activation. Experimental evidences support a model in which the basal pre-stress adaptive thermogenesis requirements modulate the capacity of stress stimuli to elicit BAT recruitment. Specifically, in subjects characterized by high thermogenesis requirement, only stress leading to maximal sympathetic/neuroendocrine activation will enhance BAT recruitment and thermogenic capacity. Conversely, in subjects characterized by low thermogenesis requirement even stress of mild intensity will induce a sympathetic/neuroendocrine activation effectual for BAT recruitment. CPS= Chronic Psychosocial Stress; CSD= Chronic Social Defeat.
Finally, it must be noted that the extreme on the intensity scale is represented by cold acclimation which, despite not being a stressor per se (Box 1), is the prototypical stimulus for BAT recruitment and activation (Figure 2) as well as browning/beigeing [14].
In a translational perspective, most of the human studies report ~5–8% of people with identifiable BAT even at thermoneutrality [73]. Mild mental arithmetic challenge elicits acute BAT activation in healthy lean humans [97], supporting the hypothesis that subjects manifesting detectable BAT at thermoneutrality could be characterized by increased sensitivity to stress experienced during the PET or MRI scans. Furthermore, it is well established that cold acutely activates and chronically recruits the BAT in humans [76,77]. The conserved nature of the stress-response and adaptive thermogenesis function in mammals suggests that the bidirectional relationship between stress and energy balance applies to human as well.
Concluding Remarks and Future Perspectives
The metabolic effects of chronic stress in rodents are heterogeneous and reminiscent of human data. While progress has been made on the identification of the mediators of stress-induced metabolic effects using classical neuroendocrine approaches, the mechanistic understanding of how stress affects energy balance is still at an early stage. Accumulated evidences now support a major role for sympathetically-derived factors in brown adipocyte function. Classically, NE-mediated βAR activation has been associated with BAT recruitment and activation [14]. Peripheral NPY has been proposed as a key neuromodulator blunting the effect of adrenergic signaling on adipocyte functions [39]. Conversely, and of particular relevance, recent studies identified a sympathetic/brown adipocyte purinergic (ATP secretion) system in rodent and human BAT that is downregulated in condition of low adaptive thermogenesis, is upregulated by cold or chronic subordination stress and mediates browning [84,98,99]. These recent studies, along with the demonstration that βAR are dispensable for BAT recruitment as well as tonic, but not acute, thermogenesis [84], point to a new mechanism of stress-associated BAT regulation. Additional preclinical and clinical studies are required to clarify the mechanistic details, but these studies suggest that neuromodulation and sympathetic co-transmission in the adipose tissue should be considered as novel druggable targets for human obesity, thus creating the opportunity for novel combinatorial pharmacotherapies to treat obesity by enhancing BAT functions at thermoneutrality.
Trends.
Chronic stress and socio-economic status have been associated with metabolic diseases.
-
A beneficial metabolic potential of brown adipose tissue (BAT) has been identified in humans. Recent data suggest that psychological stress is a natural stimulus for BAT function, together with classically recognized cold- and diet-induced thermogenesis.
Recent data also demonstrate that the dichotomous metabolic outcome of social stress can be related to differential levels of basal BAT activity and stress-induced recruitment and activation of BAT.
We propose a bidirectional relationship between stress and energy balance whereby stress promotes obesity in the presence of hyperphagia and unchanged BAT function, but results in weight-loss/obesity-resistance in the presence of hypophagia or when hyperphagia is associated with enhanced BAT recruitment/thermogenesis.
Acknowledgments
We would like to thank past and current members of the laboratory as well as colleagues and collaborators for help generating the experimental data discussed in the review and helpful discussions. We apologize to those authors whose papers could not be cited owing to space constraints. AB is supported by NIH/NIDDK R01DK102496, NIH/NIA R01AG043972.
GLOSSARY
- Chronic mild stress
sequential exposure of experimental animals to a variety of mild stressors (e.g. overnight illumination; periods of food and/or water deprivation; cage tilt; change of cage mate)
- Chronic social stress
two male mice are paired and allowed to aggressively interact for a short period of time daily; they are thereafter in sensory contact allowed by a perforated partition in the housing cage for up to 6 weeks. A dominant mouse and a subordinate mouse are identified by behavioral observations. Two main protocols have been developed. In the chronic social defeat (originally named sensory contact stress), the subordinate individual is moved to the cage of an unfamiliar aggressive mouse on a daily basis. In the chronic psychosocial stress (also defined as chronic subordination stress if only subordinate mice are investigated), dyads of dominant and subordinate mice are stable and live chronically in sensory contact and interact physically on a daily basis
- Intermittent cold stress
mice are placed in 0.5 cm ice-cold water for 1 h per day from two weeks to three months and returned to their home-cage afterward
- Open field
Rodents are exposed to an unfamiliar open field arena for a few minutes to a few hours. The test is classically used to assess locomotor activity and anxiety but has also been used as mild stressor
- Restraint or immobilization stress
experimental subjects are limited in their ability to move by either their limbs being tied to a surface or being placed in restrainers for different durations and with different schedules (once/twice day) over a number of days (consecutive or not)
- Resident/intruder stress (or social defeat)
based on manipulating rodent territorial disparity, by allowing brief confrontations between a resident aggressor and an intruder defeated experimental subject. After displaying defeat, the intruder can be protected from the potential injury of the resident’s attack and returned to his home-cage
- Sympathetic co-transmission
the secretion of more than one neurotransmitter or neuropeptide from sympathetic nerves activating different classes of receptors on target cells. The classical example is the release of norepinephrine, ATP and neuropeptide Y from sympathetic nerve terminals
- Thermoneutrality
operationally defined as the thermoneutral zone for metabolic rate, is the specie-specific range of environmental temperatures at which the requirement for adaptive or facultative thermogenesis to maintain core body temperature in homoeothermic vertebrates is negligible
- Unstable social settings stress
a heterogeneous category of models inclusive of procedures based on the alternate application of social defeat/overcrowding/isolation/group composition change/etc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.GBD 2013 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteford HA, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 3.Koolhaas JM, et al. Stress revisited: a critical evaluation of the stress concept. Neurosc Biobehav Rev. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS, et al. Recognizing Resilience: Learning from the Effects of Stress on the Brain. Neurobiol Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav. 1999;66(3):511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- 6.Serlachius A, et al. Stress and weight change in university students in the United Kingdom. Physiol Behav. 2007;92(4):548–553. doi: 10.1016/j.physbeh.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Epel E, et al. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sc. 2004;1032:208–210. doi: 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- 8.Block JP, et al. Psychosocial stress and change in weight among US adults. Am J Epidemiol. 2009;170(2):181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epel ER, et al. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26(1):37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 10.Rutters F, et al. Acute stress-related changes in eating in the absence of hunger. Obesity (Silver Spring) 2009;17(1):72–77. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- 11.Kiecolt-Glaser JK, et al. Daily stressors, past depression, and metabolic responses to high-fat meals: a novel path to obesity. Biol Psychiatry. 2015;77(7):653–660. doi: 10.1016/j.biopsych.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrin Met. 2010;21(3):159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404(6778):652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 14.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 15.Soumano K, et al. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol Cell Endocrinol. 2000;165:7–15. doi: 10.1016/s0303-7207(00)00276-8. [DOI] [PubMed] [Google Scholar]
- 16.van den Beukel JC, et al. Direct activating effects of adrenocorticotropic hormone (ACTH) on brown adipose tissue are attenuated by corticosterone. FASEB J. 2014;28(11):4857–4867. doi: 10.1096/fj.14-254839. [DOI] [PubMed] [Google Scholar]
- 17.Teegarden SL, Bale TL. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Phys Behav. 2008;93(4–5):713–723. doi: 10.1016/j.physbeh.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanghez V, et al. Psychosocial stress induces hyperphagia and exacerbates diet-induced insulin resistance and the manifestations of the Metabolic Syndrome. Psychoneuroendocrino. 2013;38(12):2933–2942. doi: 10.1016/j.psyneuen.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Razzoli M, et al. Chronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder. Front Nutr. 2015;1(30) doi: 10.3389/fnut.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallman MF, et al. Chronic stress and obesity: a new view of “comfort food”. P Natl Acad Sci USA. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krashes MJ, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507(7491):238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betley JN, et al. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garfield AS, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18(6):863–71. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson ZR, et al. Central Ghrelin Signaling Mediates the Metabolic Response of C57BL/6 Male Mice to Chronic Social Defeat Stress. Endocrinology. 2013;154(3):1080–1091. doi: 10.1210/en.2012-1834. [DOI] [PubMed] [Google Scholar]
- 25.Lu XY, et al. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143:3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Leighton CE, et al. Behavioral responses to orexin, orexin receptor gene expression, and spontaneous physical activity contribute to individual sensitivity to obesity. Am J Physiol Endocrinol Metab. 2012;303(7):E865–874. doi: 10.1152/ajpendo.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monda M, et al. Olanzapine blocks the sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Peptides. 2008;29:120–126. doi: 10.1016/j.peptides.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Messina G, et al. Orexin-A controls sympathetic activity and eating behavior. Front Psychol. 2014;5:997. doi: 10.3389/fpsyg.2014.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L, et al. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. NeuroReport. 2002;13:1351–1353. doi: 10.1097/00001756-200207190-00027. [DOI] [PubMed] [Google Scholar]
- 30.Ootsuka Y, et al. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress. 2008;11(2):125–133. doi: 10.1080/10253890701638303. [DOI] [PubMed] [Google Scholar]
- 31.Müller TD, et al. Ghrelin. Mol Metab. 2015;4(6):437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabral A, et al. Ghrelin activates hypophysiotropic corticotropin-releasing factor neurons independently of the arcuate nucleus. Psychoneuroendocrinology. 2016;67:27–39. doi: 10.1016/j.psyneuen.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouach V, et al. The acute ghrelin response to a psychological stress challenge does not predict the poststress urge to eat. Psychoneuroendocrino. 2007;32(6):693–702. doi: 10.1016/j.psyneuen.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Lutter M, et al. Orexin Signaling Mediates the Antidepressant-Like Effect of Calorie Restriction. J Neurosc. 2008;28(12):3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuang JC. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasuda T, et al. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci Lett. 2003;349(2):75–78. doi: 10.1016/s0304-3940(03)00789-4. [DOI] [PubMed] [Google Scholar]
- 37.de Guia RM, Herzig S. How Do Glucocorticoids Regulate Lipid Metabolism? Adv Exp Med Biol. 2015;872:127–144. doi: 10.1007/978-1-4939-2895-8_6. [DOI] [PubMed] [Google Scholar]
- 38.Geer EB, et al. MRI assessment of lean and adipose tissue distribution in female patients with Cushing’s disease. Clin Endocrinol (Oxf) 2010;73(4):469–475. doi: 10.1111/j.1365-2265.2010.03829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo LE, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 40.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214(Pt 2):242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 41.Morera P, et al. Chronic heat stress up-regulates leptin and adiponectin secretion and expression and improves leptin, adiponectin and insulin sensitivity in mice. J Mol Endocrinol. 2012;48:129–138. doi: 10.1530/JME-11-0054. [DOI] [PubMed] [Google Scholar]
- 42.Ilio W, et al. Effects of chronic social defeat stress on peripheral leptin and its hypothalamic actions. BMC Neurosci. 2014;15:72. doi: 10.1186/1471-2202-15-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar K, et al. Differential effects of chronic social stress and fluoxetine on meal patterns in mice. Appetite. 2013;64:81–88. doi: 10.1016/j.appet.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q, et al. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137(7):1225–34. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atasoy D, et al. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172–7. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dodd GT, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160(1–2):88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–91. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Zeng W, et al. Sympathetic Neuro-adipose Connections Mediate Leptin-Driven Lipolysis. Cell. 2015;163(1):84–94. doi: 10.1016/j.cell.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morton GJ, et al. Evidence against hypothalamic-pituitary-adrenal axis suppression in the antidiabetic action of leptin. J Clin Invest. 2015;125(12):4587–91. doi: 10.1172/JCI82723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haque Z, et al. Inhibition of immobilization stress-induced anorexia, behavioral deficits, and plasma corticosterone secretion by injected leptin in rats. Stress. 2013;16(3):353–62. doi: 10.3109/10253890.2012.736047. [DOI] [PubMed] [Google Scholar]
- 51.Jochman KA, et al. Corticotropin-releasing factor-1 receptors in the basolateral amygdala mediate stress-induced anorexia. Behav Neurosci. 2005;119(6):1448–58. doi: 10.1037/0735-7044.119.6.1448. [DOI] [PubMed] [Google Scholar]
- 52.Chotiwat C, et al. The effects of repeated restraint stress on energy balance and behavior of mice with selective deletion of CRF receptors. Stress. 2010;13(3):203–13. doi: 10.3109/10253890903207527. [DOI] [PubMed] [Google Scholar]
- 53.Bale TL, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24(4):410–4. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 54.Carter ME, et al. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503(7474):111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morton GJ, et al. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15(6):367–78. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinzig KP, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosc. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tauchi M, et al. Role of central glucagon-like peptide-1 in hypothalamo-pituitary-adrenocortical facilitation following chronic stress. Exp Neurol. 2008;210(2):458–466. doi: 10.1016/j.expneurol.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kooijman S, et al. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia. 2015;58(11):2637–2646. doi: 10.1007/s00125-015-3727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab. 2014;25(5):255–262. doi: 10.1016/j.tem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Atgié C, et al. Role of beta1- and beta3-adrenoceptors in the regulation of lipolysis and thermogenesis in rat brown adipocytes. Am J Physiol. 1997;273(4 Pt 1):C1136–1142. doi: 10.1152/ajpcell.1997.273.4.C1136. [DOI] [PubMed] [Google Scholar]
- 61.Girousse A, et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PloS Biol. 2013;11(2):e1001485. doi: 10.1371/journal.pbio.1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mottillo EP, et al. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J Lipid Res. 2014;55(11):2276–2286. doi: 10.1194/jlr.M050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuura N, et al. Restraint stress exacerbates cardiac and adipose tissue pathology via β-adrenergic signaling in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol. 2015;308(10):H1275–1286. doi: 10.1152/ajpheart.00906.2014. [DOI] [PubMed] [Google Scholar]
- 64.Rojas JM, et al. Glucose intolerance induced by blockade of central FGF receptors is linked to an acute stress response. Mol Metab. 2015;4(8):561–568. doi: 10.1016/j.molmet.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bordicchia M, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Possenti, et al. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem J. 2012;441(1):511–22. doi: 10.1042/BJ20111165. [DOI] [PubMed] [Google Scholar]
- 67.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav R. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 68.Kazak L, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163(3):643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–6. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 70.de Jong JM, et al. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab. 2015;308(12):E1085–105. doi: 10.1152/ajpendo.00023.2015. [DOI] [PubMed] [Google Scholar]
- 71.Morrison SF, et al. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19(5):741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian XY, et al. Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell Metab. 2016;23:165–178. doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broeders E, et al. Endogenous ways to stimulate brown adipose tissue in humans. Ann Med. 2015;47:123–132. doi: 10.3109/07853890.2013.874663. [DOI] [PubMed] [Google Scholar]
- 74.Cypess AM, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21(1):33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sidossis LS, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015;22(2):219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanssen MJ, et al. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes. 2015 Dec 30; doi: 10.2337/db15-1372. In press. [DOI] [PubMed] [Google Scholar]
- 77.Yoneshiro T, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuroshima A, et al. Cross adaption between stress and cold in rats. Pflugers Arch. 1984;402(4):402–8. doi: 10.1007/BF00583941. [DOI] [PubMed] [Google Scholar]
- 79.Gao B, et al. Repeated immobilization stress increases uncoupling protein 1 expression and activity in Wistar rats. Jpn J Physiol. 2003;53(3):205–213. doi: 10.2170/jjphysiol.53.205. [DOI] [PubMed] [Google Scholar]
- 80.Lkhagvasuren B, et al. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci. 2011;34(9):1442–1152. doi: 10.1111/j.1460-9568.2011.07863.x. [DOI] [PubMed] [Google Scholar]
- 81.Kataoka N, et al. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Met. 2014;20:346–358. doi: 10.1016/j.cmet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 82.Robinson LJ, et al. Brown adipose tissue activation as measured by infrared thermography by mild anticipatory psychological stress in lean healthy females. Exp Physiol. 2016 doi: 10.1113/EP085642. [DOI] [PubMed] [Google Scholar]
- 83.Bachman ES, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297(5582):843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 84.Razzoli M, et al. Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Mol Metab. 2016;5(1):19–33. doi: 10.1016/j.molmet.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanghez V, et al. Chronic subordination stress selectively downregulates the insulin signaling pathway in liver and skeletal muscle but not adipose tissue of male mice. STRESS. 2016 doi: 10.3109/10253890.2016.1151491. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steculorum SM, et al. AgRP Neurons Control Systemic Insulin Sensitivity via Myostatin Expression in Brown Adipose Tissue. Cell. 2016;165(1):125–38. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–12. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 88.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 10(6):397–409. doi: 10.1038/nrn2647. 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sapolsky RM, et al. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. End Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 90.Bulloch JM, Daly CJ. Autonomic nerves and perivascular fat: interactive mechanisms. Pharmacol Ther. 2014;143(1):61–73. doi: 10.1016/j.pharmthera.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 91.Daniel ML, et al. Stress-induced hyperthermia is not mediated by brown adipose tissue in mice. J Therm Biol. 2012;37(2):125–129. [Google Scholar]
- 92.Thompson AK, et al. Metabolic consequences of chronic intermittent mild stress exposure. Physiol Behav. 2015;150:24–30. doi: 10.1016/j.physbeh.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Razzoli M, et al. Chronic subordination stress phase advances adrenal and anterior pituitary clock gene rhythms. Am J Physiol Regul Integr Comp Physiol. 2014 Jul;307(2):R198–205. doi: 10.1152/ajpregu.00101.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Warren BL, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73(1):7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Girotti M, et al. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138(4):1067–81. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 96.Bartolomucci A, et al. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS One. 2009;4(1):e4331. doi: 10.1371/journal.pone.0004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robinson LJ, et al. Brown adipose tissue activation as measured by infrared thermography by mild anticipatory psychological stress in lean healthy females. Exp Physiol. 2016 doi: 10.1113/EP085642. [DOI] [PubMed] [Google Scholar]
- 98.Ussar S, et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Science Transl Med. 2014;6:247ra103. doi: 10.1126/scitranslmed.3008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gnad T, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 100.Kondoh K, et al. A specific area of olfactory cortex involved in stress hormone responses to predator odours. Nature. 2016;532:103–106. doi: 10.1038/nature17156. [DOI] [PMC free article] [PubMed] [Google Scholar]