Abstract
While previous work has suggested that nicotine may transiently improve attention deficits in schizophrenia, the neuronal mechanisms are poorly understood. This study is the first to examine the effects of nicotine on connectivity within the ventral attention network (VAN) during a selective attention task in schizophrenia. Using a crossover design, 17 nonsmoking patients with schizophrenia and 20 age/gender-matched nonsmoking healthy controls performed a go/no-go task with environmental noise distractors during application of a 7 mg nicotine or placebo patch. Psychophysiological interaction analysis was performed to analyze task-associated changes in connectivity between a ventral parietal cortex (VPC) seed and the inferior frontal gyrus (IFG), key components of the human VAN. Effects of nicotine on resting state VAN connectivity were also examined. A significant diagnosis X drug interaction was observed on task-associated connectivity between the VPC seed and the left IFG (F(1,35) = 8.03, p < 0.01). This effect was driven by decreased connectivity after placebo in patients and greater connectivity after nicotine. Resting state connectivity analysis showed a significant main effect of diagnosis between the seed and right IFG (F = 4.25, p = 0.023) due to increased connectivity in patients during placebo, but no drug X diagnosis interactions or main effects of drug. This study is the first to demonstrate that 1) the VAN is disconnected in schizophrenia during selective attention, and 2) nicotine may normalize this pathological state.
Keywords: Attention, Connectivity, fMRI, Nicotine, Schizophrenia, Ventral Attention Network
1. Introduction
Attention deficits, particularly in the presence of distracting stimuli, are among the most striking features of schizophrenia. As documented by McGhie and Chapman (McGhie and Chapman, 1961) and later Venables (Venables, 1964), patients are often overwhelmed by noisy environmental stimuli (such as a clock ticking) while trying to perform cognitive tasks.
The deleterious effects of sensory “overload” and distractibility in schizophrenia have led researchers to examine their neurobiological underpinnings using functional neuroimaging. A plausible mechanism by which this phenomenon may occur is dysfunction in the neuronal circuitry underlying attention. Two primary attention networks are known to exist in the human brain. “Top-down,” goal-directed attention is the primary function of a dorsal attention network consisting of the intraparietal sulcus of the dorsal parietal cortex and frontal eye fields (Corbetta and Shulman, 2002; Vossel et al., 2014). “Bottom-up,” stimulus-driven attention (e.g. reorienting to stimuli when they appear in unexpected locations) is the primary function of a ventral attention network (VAN) consisting of the ventral parietal cortex (VPC) and inferior frontal gyrus (IFG) (Corbetta and Shulman, 2002; Vossel et al., 2014). Although dysfunction of both networks has been reported in schizophrenia, abnormal activity of the VAN is most frequently reported during selective attention tasks involving distracting stimuli (Keedy et al., 2015; Kiehl and Liddle, 2001; Laurens et al., 2005; Roiser et al., 2013; Smucny et al., 2015; Smucny et al., 2013b; Tregellas et al., 2012).
If the VAN is dysfunctional during selective attention in schizophrenia, pharmacologically targeting the network may have clinical utility. One promising class of drugs that may target the VAN to improve attention in the illness is nicotinic agonists. Nicotine has been shown to improve attention deficits in schizophrenia, including in nonsmokers (Barr et al., 2008; Harris et al., 2004). Nicotine and α7 nicotinic agonists improve sensory gating, an electrophysiological phenomenon that may predict distractibility, in patients (Adler et al., 1993; Adler et al., 1992; Olincy et al., 2006; Smucny et al., 2013a; Zhang et al., 2012). Furthermore, a recent study by our laboratory has demonstrated that nicotine reduces hyperactivity of the VPC during an auditory selective attention task in the disease (Smucny et al., 2015).
Although our previous work suggests that nicotine may target VPC activity during attention tasks, it is unclear if nicotine affects connectivity between the VPC and the second primary hub of the VAN, the IFG. In the present study, we examined the effect of nicotine vs. placebo treatment on connectivity between these regions in nonsmoking patients and healthy control subjects during an auditory selective attention task. Because we found that nicotine affects task-associated VPC activity in our previous study in patients (Smucny et al., 2015), we used an identical VPC region of interest as a seed region in the present connectivity study. As disrupted VAN connectivity has been observed in other disorders with known attention deficits such as autism and attention-deficit hyperactivity disorder (Fitzgerald et al., 2015; McCarthy et al., 2013), we hypothesized decreased connectivity between this seed and the IFG (i.e. decreased VAN connectivity) in schizophrenia during placebo conditions (relative to controls). We also hypothesized that nicotine would increase connectivity between these areas in patients, reflecting restoration of within-network connectivity. Finally, we also examined task-independent effects of nicotine on VAN connectivity during the resting state.
2. Material and methods
2.1. Subjects
Demographic and clinical information for participants was assessed by interview and is shown in Table 1. 37 subjects participated in this study — 17 stable outpatients who had a primary diagnosis of schizophrenia and 20 healthy comparison subjects. As the analysis was conducted using the same task and dataset from our previous study examining VPC activity (Smucny et al., 2015), it included all subjects who had been included in that study. Patients were recruited using a database at the University of Colorado Schizophrenia Research Center and had generally participated in previous studies at the Center. Symptoms were measured by the Brief Psychiatric Rating Scale (BPRS; 27 point) and the Scale for the Assessment of Negative Symptoms (SANS; 4 domains including Affective Flattening, Alogia, Avolition, and Anhedonia). Median time between assessment and study participation was 7 months, over which time symptoms are expected to be relatively consistent in stable outpatients (Fennig et al., 1996). As stated in Table 1 and listed in Supplementary Table 1, 16 patients were being treated with atypical antipsychotics at the time of the study, and 1 patient with a typical antipsychotic (haloperidol). Patients were medication stable (> 3 mo. with no change in medication). No significant group differences in age, gender, handedness, or ratio of never smokers/former smokers (> 3 months from last cigarette) were observed. No subjects were taking smoking cessation medication (e.g. varenicline) at the time of the study. Patients were recruited by referral from a University of Colorado psychiatrist. Patients were excluded for a diagnosis of neurological illness, head trauma, current smoking (< 3 months from last cigarette) or substance abuse, poor (inability to hear 60 dB SPL 1000 and 1500 Hz tones in either ear) or unbalanced (> 10 dB threshold difference between each ear) hearing, failure to pass a physical examination, and magnetic resonance imaging (MRI) exclusion criteria (claustrophobia, weight > 250 lbs, metal in the body). Control subjects were excluded for all of the above as well as a diagnosis of Axis I mental illness or first-degree family history of Axis I mental illness. All subjects were required to pass a nicotine tolerance test, in which the nicotine dose used for the experiment (7 mg) was administered > 3 d prior to the first fMRI scan. Criteria for passing the tolerance test were 1) less than a 20% change in heart rate or blood pressure (BP) for up to 90 minutes (m) post patch-application, 2) no side effects other than mild/minor nausea, headache, lightheadness, buzz, clouded thinking, anxiety, or mouth tingling. All participants provided written informed consent in accordance with the principles of the Declaration of Helsinki and could withdraw from the study at any time. The Colorado Multiple Institutional Review Board approved the study.
Table 1.
Demographic and Clinical Data of Participants.
| Controls | Schizophrenia | Test Statistic (p) | |
|---|---|---|---|
| Age | 38.4 (12) | 44 (12) | t = 1.68 (0.10) |
| Gender (M/F) | 11/9 | 12/5 | χ2 = 0.95 (0.33) |
| Smoking (Never/Former Smokers) | 15/5 | 10/7 | χ2 = 0.01 (0.92) |
| Handedness (R/L) | 18/2 | 13/4 | χ2 = 1.24 (0.27) |
| Average Total BPRS | 36.6 (7.7) | n/a | |
| Average Total SANS | 4.59 (3.4) | n/a | |
| Meds: Typ/ATyp | 1/16 | n/a |
Parentheses contain the standard deviation. Abbreviations: BPRS = Brief Psychiatric Rating Scale, SANS = Scale for the Assessment of Negative Symptoms, Typ = # Treated with Typical Antipsychotic Medications, ATyp = # Treated with Atypical Antipsychotic Medications.
2.2. Study Design
This was a single-blind, pseudo-randomized, placebo-controlled crossover study. On each of two study visits, subjects were administered a 7 mg nicotine patch (Nicoderm) or a placebo patch (made in-house) 70 m prior to MRI scanning. The order of study visits (placebo or nicotine) was counterbalanced across subjects. Subjects wore patches throughout scanning. Total time of patch application was approximately 130 m (70 m before scanning, 60 m during scanning). The attention task was performed approximately 10 m after the subject was placed in the scanner (~80 m after patch application); the delay was due to localizer, high-order shimming and anatomical scans that preceded the functional scan. The 80 m latent period was used such that the attention task occurred during a time window corresponding to the peak plasma concentration of nicotine (Dempsey et al., 2013). Based on previous work, the expected nicotine concentration during this period is expected to be approximately 4 ng/ml (Dempsey et al., 2013). The placebo patch was tactilely similar to the nicotine patch and was affixed to the skin (upper arm) in the same manner as the nicotine patch. Subjects were asked to refrain from examining either patch during or after application as the placebo and drug patches were not visually identical. Furthermore, clothing covered patches such that they could not be readily observed after affixation. Patches were removed immediately after scanning. Visits were scheduled > 3 d apart. Heart rate and BP were monitored immediately prior to patch application, 30 and 60 m after patch application, and up to 60 m after patch removal. Physiological effects of nicotine were analyzed using a mixed-effects model analysis of variance (ANOVA) in SPSS22, with time (pretreatment vs. posttreatment) and drug (placebo vs. nicotine) as within-subjects factors and diagnosis (control vs. patient) as a between-subjects factor.
2.3. Task Description
Subjects performed an auditory version of the Sustained Attention to Response Task (SART) (Seli et al., 2012). For the SART, single-digit numbers were aurally presented one at a time, and the subject was asked to respond (button press) (Lumina Response Pad, Cedrus Corp.) after each auditory stimulus (70 dB, presented in either the right or left ear), except for the number ‘3,’ in which case the subject was asked to withhold from responding. Subjects used their dominant hand for motor responses. The ear (right or left) in which the numbers were presented was pseudo-randomized between subjects. Stimulus duration was 250 ms and inter-stimulus interval was 900 ms. Subjects performed two variations of the SART, the Ordered SART and the Random SART. In the Ordered SART, the numbers were presented in order; in the Random SART, the numbers were presented pseudo-randomly. Due to the predictability of Ordered SART, subjects may be able to correctly respond or withhold responding to the presence of any auditory stimulus while minimally engaging attention-associated neuronal systems. The unpredictability of Random SART, however, requires subjects to focus on specific stimulus features before making the appropriate response, increasing attentional demands (Smucny et al., 2013b). The current SART variation (Ordered or Random) was highlighted and visually presented through MR-compatible goggles (Resonance Technologies, Inc.) throughout the experiment. The identifier cue was presented 2.3 s before the first set of stimuli, as well 2.3 s before each time the condition switched from Ordered to Random (or vice-versa). The subject was asked to respond as quickly and accurately as possible to help induce attentiveness.
The SART was presented as a block design, with four pseudo-randomly dispersed conditions: Ordered-Silent (ordered numbers with no noise distraction), Ordered-Noisy (ordered numbers with noise distraction), Random-Silent (random numbers with no noise distraction), and Random-Noisy (random numbers with noise distraction). 72 blocks of 12.65 s each were administered, with 18 blocks per condition. Each block consisted of 9–11 trials. Baseline data was collected from six 37.95 s fixation periods interspersed at regular intervals throughout the experiment. Total task duration was 18 m.
Recorded performance measures on the SART were 1) errors of commission, or incorrect button presses on ‘3’, 2) errors of omission, or failure to button press on the numbers 1, 2, and 4–9, and 3) reaction time. Percent correct responses were calculated as 100 – (percent errors commission + percent errors of omission). As a combination of all these measures provides a more accurate assessment of performance than each individual measure (Seli et al., 2013), they were combined into a single measure, “efficiency,” based on a previous SART study in schizophrenia (Chan et al., 2009). Specifically, efficiency was defined as arcsin (√ (Percent Correct Responses / Reaction Time for Correct Responses)). Efficiency data were analyzed by mixed-effects ANOVA in SPSS22 with drug (placebo vs. nicotine), SART difficulty (Ordered vs. Random) and distraction level (Silent vs. Noisy) as within-subjects factors and diagnosis (Control vs. Patient) as a between-subjects factor.
2.4. Auditory Stimuli
For the attention task (see “Task Description”), synthetic audio recordings for the numbers 1–9 were downloaded from www.modeltalker.com. Number stimuli were adjusted to have the same onset with Adobe Audition.
For task-overlaid noise distraction, environmental, “urban” noise stimuli were mixed as described previously (Tregellas et al., 2009). The subjective experience of the sound mixture was that of standing in a busy crowd of people, in which multiple conversations were occurring, with a low level of indistinguishable background music and other sounds. Urban noise distraction was presented at 80 dB in the ear opposite the task-relevant stimuli with MR-compatible headphones (Resonance Technologies, Inc.).
2.5. fMRI Scanning Parameters: SART
Functional scans were collected using a clustered volume approach as described previously (Smucny et al., 2013b, c). Use of the clustered volume approach allowed stimuli to be presented while minimizing scanner noise. This technique has been shown to substantially improve signal detection in fMRI experiments using auditory stimuli, despite reducing the overall number of scans collected per experimental condition (Edmister et al., 1999). Indeed, a previous connectivity analysis was able to extract robust, readily identifiable intrinsic networks during a listening task using clustered volume acquisition (including frontal-parietal networks) (Langers and van Dijk, 2011). We have previously used clustered volume acquisition in a number of auditory tasks in schizophrenia, including the SART (Smucny et al., 2014a; Smucny et al., 2013b, c; Tregellas et al., 2007; Tregellas et al., 2009; Tregellas et al., 2012).
Studies were performed with a 3T GE Signa MR system using a standard quadrature head coil. Functional images were acquired with a gradient-echo T2* Blood Oxygenation Level Dependent (BOLD) contrast technique, with TR = 12650 ms (as a clustered volume acquisition of 2000 ms, plus an additional 10650 ms silence interval), TE = 30 ms, FOV = 220 mm2, 642 matrix, 38 slices, 3.5 mm thick, 0.5 mm gap. Additionally, one inversion recovery echo planar image (IR-EPI; TI = 505 ms) volume was acquired to improve spatial normalization (see “fMRI Preprocessing”).
2.6. fMRI Scanning Parameters: Resting State
Resting state functional images were acquired with the following parameters: scan time 10 m, TR = 2000 ms, TE = 26 ms, FOV = 220 mm2, 642 matrix, 27 slices, 2.6 mm thick, 1.4 mm gap. The first four volumes of the 300-volume scan were excluded from analysis. Subjects were instructed to rest with eyes closed and to “not think about anything in particular.”
2.7. fMRI Preprocessing (SART and Resting State)
Data were preprocessed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London). Data from each subject were realigned to the first volume, normalized to the Montreal Neurological Institute template using the IR-EPI as an intermediate to improve coregistration between images, and smoothed with an 8 mm FWHM Gaussian kernel. The images were motion-corrected using rigid-body motion parameters. No significant effect of diagnosis, drug treatment, or drug X diagnosis interaction was observed for overall movement. White matter and csf signal confounds were removed. Mean overall gray matter signal was not included as a confound as doing so shifted the whole brain connectivity distribution towards predominantly negative values. The data were detrended and a 0.01 to 0.1 Hz bandpass filter applied to remove low-frequency drifts and physiological high-frequency noise, consistent with previous research using connectivity analysis of sparse acquisition fMRI data (Yakunina et al., 2015).
2.8. Connectivity Analysis: Seed and Target ROI Definitions
As we have previously reported task-associated effects of nicotine on BOLD signal in schizophrenia using the anatomically defined ROI of the left VPC/supermarginal gyrus in Wake Forest University Pickatlas (Maldjian et al., 2003; Smucny et al., 2015), we used an identical ROI as a seed in the present analysis. Connectivity was then analyzed between this seed and 6 mm radius spherical target ROIs centered at the coordinates (x,y,z = −45, 36, −6) and (x,y,z = 45, 36, −6), respectively. These ROIs have been previously identified in the literature as the brain regions most closely linked to stimulus driven attention reorienting and the ventral attention network (Daselaar et al., 2013). Connectivity was analyzed between the left VPC seed and both the left and right IFG because previous work has shown significant interhemispheric intrinsic connectivity of the VAN (Kucyi et al., 2012). Exploratory analyses were also performed on task-associated connectivity between the left VPC seed and the whole brain (voxelwise). Significance threshold was set at voxelwise p < 0.01, clusterwise p < 0.05 FDR-corrected for multiple comparisons.
2.9. Connectivity Analysis: Implementation
Psychophysiological interaction (PPI) describes functional connectivity between brain regions contingent on a psychological context (Friston et al., 1997; Gitelman et al., 2003). Here, we examined PPI of the VAN using the Conn v.15 toolbox (http://www.nitrc.org/projects/conn). A generalized psychophysiological interaction (gPPI) routine was implemented. Briefly, gPPI allows for an analysis of task-associated connectivity without the two-condition constraint necessary for traditional PPI analysis by controlling for the main effects of any number of conditions across the scanning session in a single model (e.g. Ordered-Silent, Ordered-Noisy, Random-Silent, and Random-Noisy in this study) (McLaren et al., 2012). “Task-associated” connectivity can therefore be analyzed independent of task-associated effects on BOLD response. Identical to our previous study (Smucny et al., 2015), task-associated connectivity (Δconnectivity) was defined using the contrast ((Random-Noisy > Random-Silent) > (Ordered-Noisy >Ordered-Silent)). Connectivity during fixation was used as a baseline and subtracted from each condition as implemented in a previous gPPI analysis (McDaniel et al., 2013). Using this contrast, Δconnectivity parameter estimates (beta weights) between the VPC seed and left/right IFG target ROIs were generated for each individual in a first-level analysis. Because Δconnectivity is defined as a comparison between conditions, it should not be considered a “pure” estimate of connectivity (e.g. a negative beta weight should not be interpreted as a negative correlation between the seed and target ROI). Confounding task-associated BOLD response was modeled with a double-gamma hemodynamic response function without temporal derivatives.
First level Δconnectivity parameter estimates were analyzed via second level ANOVA with drug (placebo vs. nicotine) as a within-subjects factor and diagnosis (control vs. patient) as a between-subjects factor. A separate ANOVA was performed between the seed ROI (left VPC) and each target ROI (left and right IFG). Significant interaction effects were followed up by analysis of simple main effects to describe the direction of the interactions. For the whole brain analysis, task-associated connectivity between the seed and the whole brain (voxelwise) was analyzed using the interaction contrast ((Patient Drug > Patient Placebo) > (Control Drug > Control Placebo)).
2.10. Task-Independent Effects of Nicotine on VAN Connectivity
Task-independent connectivity between the VPC seed and IFG target ROIs was analyzed using data from 10 m (duration) resting state sessions that immediately followed the SART task after both placebo and nicotine administration. Resting state data from one control subject could not be analyzed due to a scan ending prematurely. ANOVA analysis was performed in the same manner as the gPPI analysis.
2.11. Correlation Analyses
Exploratory correlation analyses were performed with significance threshold set to p < 0.05. Correlations were examined between neuronal effects, behavior, and clinical measures.
3. Results
3.1. Physiological Effects of Nicotine
Physiological effects of placebo vs. nicotine treatment have been published elsewhere (Smucny et al., 2015). Briefly, no significant time X drug X diagnosis interactions were observed on systolic BP, diastolic BP, or heart rate. Across all subjects, no significant time (pretreatment vs. 60 m post-treatment) X drug interactions were observed for systolic BP, diastolic BP, or heart rate.
3.2. Behavioral Data
The primary behavioral measure of interest in this study was performance efficiency, a single metric that combines accuracy and reaction time (see Methods). Efficiency data and base behavioral measures (errors of commission, omission, and reaction time) for each SART condition have been published elsewhere (Smucny et al., 2015). Briefly, using this measure, significant main effects of difficulty (Ordered vs. Random; F(1,35) = 46.4, p < 0.001) and distraction level (Silent vs. Noisy; F(1,35) = 17.2, p < 0.001) were observed. This result is indicative of decreased efficiency during the Random condition and Noisy condition relative to the Ordered and Silent conditions, respectively. No significant interactions were observed between SART condition and diagnosis (Control vs. Patient) or drug (Placebo vs. Nicotine). Furthermore, no significant effects of nicotine were observed for either controls or patients vs. placebo.
3.3. gPPI Analysis
gPPI (see Methods) was performed to analyze the effects of nicotine (vs. placebo) on task-associated change in connectivity (Δconnectivity) between an anatomically defined VPC seed and the left and right IFG. Identical to our previous analysis (Smucny et al., 2015), “task” was defined using the contrast ((Random-Noisy > Random-Silent) > (Ordered-Noisy > Ordered-Silent)). Single-subject Δconnectivity values were then analyzed by ANOVA using drug (nicotine vs. placebo) as a within-subjects factor and diagnosis (patient vs. control) as a between-subjects factor.
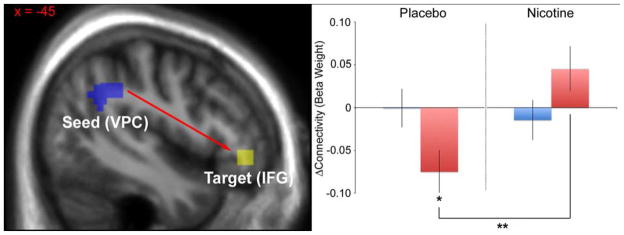
Average Δconnectivity values (beta weights) for each group (control-placebo, control-nicotine, patient-placebo, patient-nicotine) during the task are presented in Table 2a and Figure 1. A significant drug X diagnosis interaction was observed on Δconnectivity between the left VPC seed and the left IFG (F(1,35) = 8.03, p < 0.01). The main effect of drug was also significant (F(1,35) = 5.07, p = 0.031). Post-hoc analyses determined the interaction effect was driven by 1) reduced Δconnectivity in patients (relative to controls) during placebo (Δβ = −0.074, p = 0.035) and 2) increased Δconnectivity in patients during nicotine (relative to placebo) (Δβ = 0.12, p < 0.01). Nicotine did not significantly affect Δconnectivity in control subjects.
Table 2a.
Δconnectivity (beta weights) between the VPC seed and left and right IFG. Parentheses contain the standard error.
| Controls | Schizophrenia Patients | |||
|---|---|---|---|---|
| Target ROI | Placebo | Nicotine | Placebo | Nicotine |
| Left IFG | 0 (0.023) | −0.015 (0.023) | −0.075 (0.025) | 0.045 (0.026) |
| Right IFG | 0.055 (0.020) | 0.028 (0.035) | −0.033 (0.028) | 0.038 (0.043) |
Figure 1.
Effect of nicotine on task-associated connectivity between the VPC and left IFG. Left: Diagram showing location of the seed ROI (left VPC) and the left target ROI (IFG). Right: Charts illustrating the direction and magnitude of the significant interaction effect. Beta weights represent relative connectivity between the left VPC seed and the left IFG ROI. *p < 0.05, controls vs. patients during placebo. **p < 0.05, placebo vs. nicotine in patients. Error bars represent the standard error.
No significant drug X diagnosis interaction, main effect of drug, or main effect of diagnosis was observed on Δconnectivity between the left VPC seed and the right IFG ROI.
Analysis of task-associated connectivity between the seed and the whole brain (voxelwise) revealed additional significant drug X diagnosis interaction effects in bilateral inferior frontal and occipital extrastriate/visual association cortices (Supplementary Table 2).
3.4. Resting State Connectivity Analysis
Resting state connectivity values (beta weights) for each group are presented in Table 2b. No significant drug X diagnosis interactions or main effects of drug were observed for connectivity between the seed and either the left or right IFG. A main effect of diagnosis was observed for connectivity between the VPC and right IFG (F(1,34) = 6.80, p = 0.013). This effect was driven by increased connectivity in patients (vs. controls) during placebo (Δβ = 0.10, p < 0.01).
Table 2b.
Resting connectivity (beta weights) between the VPC seed and left and right IFG. Parentheses contain the standard error.
| Controls | Schizophrenia Patients | |||
|---|---|---|---|---|
| Target ROI | Placebo | Nicotine | Placebo | Nicotine |
| Left IFG | 0.23 (0.043) | 0.21 (0.036) | 0.29 (0.033) | 0.25 (0.050) |
| Right IFG | 0.11 (0.023) | 0.14 (0.026) | 0.21 (0.025) | 0.22 (0.042) |
3.5. Correlation Analysis
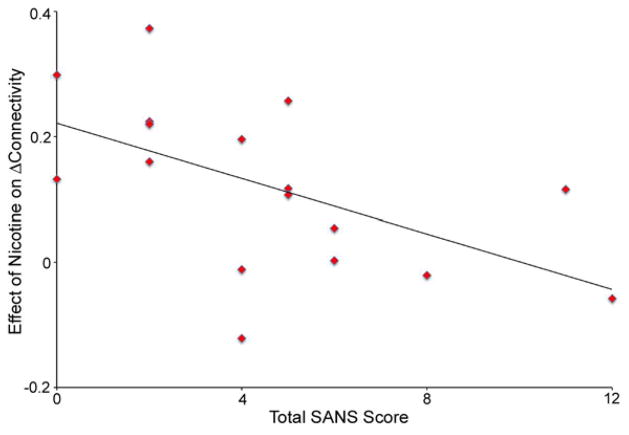
A significant negative correlation was observed between total SANS score and the effect of nicotine on Δconnectivity between the VPC and left IFG (r = −0.56, p = 0.21, Figure 2), suggesting that patients with the least severe negative symptoms were the most responsive to nicotine. The effect was driven by significant negative correlations with SANS Avolition (r = −0.60, p = 0.011) and SANS Asociality (r = −0.60, p = 0.011) subscales. No significant correlations were observed between behavioral measures and task-associated or resting VAN connectivity.
Figure 2.
Negative correlation between total SANS score and the effect of nicotine on Δconnectivity in patients.
We have previously reported a significant drug X diagnosis interaction effect on left VPC response, driven by relative VPC hyperactivity during placebo in patients and normalization after nicotine (Smucny et al., 2015). Exploratory correlation analyses revealed no significant associations between left VPC response and left VPC to left IFG connectivity, suggesting that these two phenotypes are not directly related to one another.
4. Discussion
In agreement with our hypothesis, significant drug X diagnosis interactions were observed on task-associated VAN connectivity between a VPC seed and left IFG target, driven by 1) reduced Δconnectivity in schizophrenia patients (relative to healthy controls) during placebo administration, and 2) increased Δconnectivity in patients during nicotine. Exploratory whole brain analysis also revealed significant interaction effects between the VPC seed and bilateral IFG as well as accessory visual cortex. Patients who showed the greatest improvement in performance after nicotine also showed the greatest increase in Δconnectivity. No significant interaction effects or main effects of drug were observed on resting state connectivity, despite the observation that patients showed increased connectivity during placebo. To our knowledge, these results are the first to suggest that functional abnormalities of the VAN during selective attention may be pharmacologically targeted by acute nicotine administration in schizophrenia.
Functional abnormalities within the VAN are consistent with previous observations in schizophrenia on a variety of attention tasks, including visual oddball (Wynn et al., 2015), auditory oddball (Kiehl and Liddle, 2001; Laurens et al., 2005; Tregellas et al., 2012) and visual targets combined with auditory distractors (Smucny et al., 2013b). These results also expand upon our previous findings showing abnormalities in VPC response during this task in patients by suggesting that functional abnormalities may extend throughout the VAN (Smucny et al., 2015). Taken together, these results suggest that “bottom-up” attentional processing systems are abnormal in schizophrenia, consistent with the view that early stimulus processing deficits may contribute to higher level cognitive dysfunction in the illness (Javitt, 2009).
Interestingly, the directionality of connectivity effects observed in this study was in the opposite direction of activity effects observed in our previous report that examined VPC response during this task (Smucny et al., 2015). Specifically, our previous work revealed increased activity of the VPC in patients during placebo, whereas in the present study decreased connectivity was observed. Both phenotypes were then reversed by nicotine. One interpretation of these findings is that reduced connectivity in patients is a compensatory response to abnormally high VPC response during task. Or, similarly, greater VPC activity could occur as a result of reduced connectivity. The observed lack of significant association between connectivity and response, however, is incongruent with these explanations. Another possibility is that the VAN disconnectivity and VPS activity are less directly related, such that VAN disconnectivity is effectively a separate mechanism by which patients are more distracted in noisy environments, and a second target which nicotine may ameliorate functional attention deficits in schizophrenia. Schizophrenia is frequently referred to as disease of “disconnectivity,” particularly of long-distance connections and networks (Karbasforoushan and Woodward, 2012; Uhlhaas, 2013). The observed results may therefore be another manifestation of this phenotype.
The nicotinic modulation of VAN connectivity observed in this study is consistent with previous studies showing that functional abnormalities in schizophrenia may be “corrected” by administration of nicotinic agonists (Freedman, 2014; Smucny et al., 2015; Smucny and Tregellas, 2013; Tanabe et al., 2006; Tregellas et al., 2010; Tregellas et al., 2011; Tregellas et al., 2005). One striking feature of schizophrenia is the high rate of smoking in the illness (70% or greater (Winterer, 2010)). Furthermore, smoking schizophrenia patients smoke more cigarettes per day and inhale more nicotine per cigarette than healthy smokers (Olincy et al., 1997). High rates of smoking in schizophrenia have been hypothesized to be a form of “self-medication” to correct a deficit in endogenous nicotinic signaling contributing to cognitive dysfunction (Winterer, 2010). Consistent with this view, patients show reduced expression of nicotinic receptors in several brain areas, including the parietal cortex (D’Souza et al., 2012). Future studies may examine how loss of these receptors affects attention task-associated VAN connectivity in schizophrenia and other populations. Interestingly, genetic polymorphisms in genes for nicotinic receptors (e.g. CHRNA4) have been shown to affect parietal response during attention tasks (Giessing et al., 2012).
The effectiveness of nicotine at improving VAN connectivity in patients was negatively correlated with negative symptom severity, suggesting that patients with the most severe negative symptoms were the least neuronally responsive to nicotine. Although preliminary, this result suggests that it may be possible to predict nicotine’s effectiveness at normalizing loss of network connectivity. The ability to predict treatment efficacy is a topic of great interest in psychiatry. Previous studies have reported significant interactions between baseline symptom severity and antipsychotic efficacy in schizophrenia (Furukawa et al., 2015) and antidepressant efficacy in depression (Fournier et al., 2010). Previous work has also demonstrated that first episode patients with higher levels of baseline function benefit more from cognitive behavior therapy (Allott et al., 2011). Our lab has demonstrated that responsiveness to an α7 nicotinic agonist may depend upon the allele expressed near the α7 promoter, possibly due to allelic-driven variation in α7 receptor expression level (Tregellas et al., 2011). Future studies may more closely examine the ability to predict the neuronal response to nicotine during attention tasks in schizophrenia through a combination of clinical and genetic factors.
In addition to the IFG, seed to voxel (i.e. whole brain) analysis revealed significant drug X diagnosis interaction effects on connectivity between the VPC and the visual association cortex. Attention-dependent modulation of connectivity has previously been observed between the parietal cortex and extrastriate visual cortex during a visual task (Buchel and Friston, 1997). Given that the attention task used in the present study was primarily auditory, the significance of the nicotinic modulation of VPC to visual connectivity in the present study is unclear. One possibility is that it may be related to the visual component of the task, in that the task difficulty (“Ordered” or “Random”) was graphically displayed as an instructional aide throughout.
The effects of nicotine on VAN connectivity in the present study were task-specific, as no drug effects on resting state connectivity were observed despite the finding that patients showed increased connectivity (relative to controls) during placebo. The effects of pharmacologic manipulation on resting state connectivity in neuropsychiatric disease is a topic of recently increased interest, due in part to its potential applications in drug development (Smucny and Tregellas, 2013; Smucny et al., 2014b; Wylie et al., 2016). Comparatively few studies, however, have used task-associated connectivity in ascertain the neuronal effects of potential treatment interventions. These results suggest that task-based connectivity should also be considered when developing fMRI-based protocols for evaluating the neuronal effects of investigational compounds.
4.1. Limitations
A potential limitation of this study was the single-blind design. The experiment was carried out in this manner as the nicotine and placebo patches were not visually identical and therefore it was impractical to blind the experimenter to the treatment. For this reason, subjects were instructed to refrain from examining the patches during the study. Furthermore, nicotine can have physiological effects that may reduce the effectiveness of the blind (Benowitz, 1998). It should be noted, however, that 1) nicotine did not have any significant effects on blood pressure or heart rate during scanning in this study, and 2) subjects most likely to have noticeably adverse reactions to nicotine were excluded by prescreening (see Methods). Although it was somewhat surprising to not observe significant physiological effects of the drug in this study, 1) previous work has found only small physiological effects of 7 mg transdermal nicotine (vs. placebo) in nonsmokers up to 120 min post-treatment (Wignall and de Wit, 2011) and 2) exclusion criteria included screening for subjects who showed large physiological effects of nicotine during screening. The latent period (subjects scanned 80 m post-patch application) was chosen as it was expected to capture the peak plasma absorption of nicotine (Dempsey et al., 2013). It remains possible, however, that later time points may show more profound physiological as well as neuronal effects.
An additional limitation of this study was that no significant correlations were observed between the behavioral and neuronal effects of nicotine. This negative finding is not altogether unexpected in that several previous studies have reported neuronal effects of nicotinic agonists during cognitive tasks but no corresponding change in behavior (reviewed by Newhouse et al., 2011). Future studies using larger sample sizes are necessary to more thoroughly examine the relationships between effects of nicotine on network connectivity and behavior during attention tasks.
4.2. Conclusion
The ability of nicotinic agents to pharmacologically target the neuronal mechanisms that underlie cognitive dysfunction in schizophrenia remains a priority for psychiatry research. Along with our previous study examining nicotinic effects on VPC activity, this study potentially identifies task-associated VAN abnormalities as a potential nicotinic target in schizophrenia. Future imaging studies may investigate the ability of nicotine and nicotinic agonists to target VAN abnormalities in other schizophrenia and schizophrenia-associated populations, such as smokers, first-degree relatives, and at-risk individuals.
Supplementary Material
Highlights.
We analyzed effects of nicotine on attention network connectivity in schizophrenia
Nicotine increased ventral attention network connectivity during an attention task
Nicotine did not affect ventral attention network connectivity at rest
Acknowledgments
The authors thank Debra Singel and Lindsey S. Eichman for assistance with data acquisition, and Eugene Kronberg for helpful comments.
Role of the Funding Source
This work was supported by the VA Biomedical Laboratory and Clinical Science Research and Development Service grant CX000459 (grant to Dr. Tregellas), the Brain & Behavior Research Foundation (grant to Dr. Tregellas), NIH grants MH-089095, DK-103691, MH-102224 (grants to Dr. Tregellas), and NIH fellowship MH-102879 (grant to Mr. Smucny).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Allott K, Alvarez-Jimenez M, Killackey EJ, Bendall S, McGorry PD, Jackson HJ. Patient predictors of symptom and functional outcome following cognitive behaviour therapy or befriending in first-episode psychosis. Schizophr Res. 2011;132:125–130. doi: 10.1016/j.schres.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine Safety and Toxicity. Oxford University Press; New York, NY: 1998. [Google Scholar]
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb Cortex. 1997;7:768–778. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Chan RC, Wang Y, Cheung EF, Cui J, Deng Y, Yuan Y, Ma Z, Yu X, Li Z, Gong Q. Sustained attention deficit along the psychosis proneness continuum: a study on the Sustained Attention to Response Task (SART) Cogn Behav Neurol. 2009;22:180–185. doi: 10.1097/WNN.0b013e3181b7ef84. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, Pittman B, Ranganathan M, Cosgrove K, Staley J. Lower beta2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry. 2012;169:326–334. doi: 10.1176/appi.ajp.2011.11020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Huijbers W, Eklund K, Moscovitch M, Cabeza R. Resting-state functional connectivity of ventral parietal regions associated with attention reorienting and episodic recollection. Front Hum Neurosci. 2013;7:38. doi: 10.3389/fnhum.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DA, St Helen G, Jacob P, 3rd, Tyndale RF, Benowitz NL. Genetic and pharmacokinetic determinants of response to transdermal nicotine in white, black, and Asian nonsmokers. Clin Pharmacol Ther. 2013;94:687–694. doi: 10.1038/clpt.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisitions. Hum Brain Mapp. 1999;7:89–97. doi: 10.1002/(SICI)1097-0193(1999)7:2<89::AID-HBM2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennig S, Bromet EJ, Galambos N, Putnam K. Diagnosis and six-month stability of negative symptoms in psychotic disorders. Eur Arch Psychiatry Clin Neurosci. 1996;246:63–70. doi: 10.1007/BF02274895. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Johnson K, Kehoe E, Bokde AL, Garavan H, Gallagher L, McGrath J. Disrupted functional connectivity in dorsal and ventral attention networks during attention orienting in autism spectrum disorders. Autism Res. 2015;8:136–152. doi: 10.1002/aur.1430. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med. 2014;65:245–261. doi: 10.1146/annurev-med-092112-142937. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Furukawa TA, Levine SZ, Tanaka S, Goldberg Y, Samara M, Davis JM, Cipriani A, Leucht S. Initial severity of schizophrenia and efficacy of antipsychotics: participant-level meta-analysis of 6 placebo-controlled studies. JAMA Psychiatry. 2015;72:14–21. doi: 10.1001/jamapsychiatry.2014.2127. [DOI] [PubMed] [Google Scholar]
- Giessing C, Neber T, Thiel CM. Genetic variation in nicotinic receptors affects brain networks involved in reorienting attention. Neuroimage. 2012;59:831–839. doi: 10.1016/j.neuroimage.2011.07.061. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Curr Top Med Chem. 2012;12:2404–2414. doi: 10.2174/156802612805289863. [DOI] [PubMed] [Google Scholar]
- Keedy SK, Reilly JL, Bishop JR, Weiden PJ, Sweeney JA. Impact of antipsychotic treatment on attention and motor learning systems in first-episode schizophrenia. Schizophr Bull. 2015;41:355–365. doi: 10.1093/schbul/sbu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF. An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizophr Res. 2001;48:159–171. doi: 10.1016/s0920-9964(00)00117-1. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol. 2012;108:3382–3392. doi: 10.1152/jn.00674.2012. [DOI] [PubMed] [Google Scholar]
- Langers DR, van Dijk P. Robustness of intrinsic connectivity networks in the human brain to the presence of acoustic scanner noise. Neuroimage. 2011;55:1617–1632. doi: 10.1016/j.neuroimage.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Kiehl KA, Ngan ET, Liddle PF. Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schizophr Res. 2005;75:159–171. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCarthy H, Skokauskas N, Mulligan A, Donohoe G, Mullins D, Kelly J, Johnson K, Fagan A, Gill M, Meaney J, Frodl T. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70:1329–1337. doi: 10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Lamontagne P, Beck SM, Scullin MK, Braver TS. Dissociable neural routes to successful prospective memory. Psychol Sci. 2013;24:1791–1800. doi: 10.1177/0956797613481233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Potter AS, Dumas JA, Thiel CM. Functional brain imaging of nicotinic effects on higher cognitive processes. Biochem Pharmacol. 2011;82:943–951. doi: 10.1016/j.bcp.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Wigton R, Kilner JM, Mendez MA, Hon N, Friston KJ, Joyce EM. Dysconnectivity in the frontoparietal attention network in schizophrenia. Front Psychiatry. 2013;4:176. doi: 10.3389/fpsyt.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P, Cheyne JA, Barton KR, Smilek D. Consistency of sustained attention across modalities: comparing visual and auditory versions of the SART. Can J Exp Psychol. 2012;66:44–50. doi: 10.1037/a0025111. [DOI] [PubMed] [Google Scholar]
- Seli P, Jonker TR, Solman GJ, Cheyne JA, Smilek D. A methodological note on evaluating performance in a sustained-attention-to-response task. Behav Res Methods. 2013;45:355–363. doi: 10.3758/s13428-012-0266-1. [DOI] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Eichman LC, Lyons E, Tregellas JR. Early sensory processing deficits predict sensitivity to distraction in schizophrenia. Schizophr Res. 2013a;147:196–200. doi: 10.1016/j.schres.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Eichman LS, Tregellas JR. Neuronal effects of nicotine during auditory selective attention. Psychopharmacology (Berl) 2014a doi: 10.1007/s00213-014-3832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Rojas DC, Tregellas JR. Neuronal effects of nicotine during auditory selective attention in schizophrenia. Hum Brain Mapp. 2015 doi: 10.1002/hbm.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Rojas DC, Eichman LC, Tregellas JR. Neural effects of auditory distraction on visual attention in schizophrenia. PLoS One. 2013b;8:e60606. doi: 10.1371/journal.pone.0060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Rojas DC, Eichman LC, Tregellas JR. Neuronal effects of auditory distraction on visual attention. Brain Cogn. 2013c;81:263–270. doi: 10.1016/j.bandc.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Tregellas J. Nicotinic modulation of intrinsic brain networks in schizophrenia. Biochem Pharmacol. 2013;86:1163–1172. doi: 10.1016/j.bcp.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Wylie KP, Tregellas JR. Functional magnetic resonance imaging of intrinsic brain networks for translational drug discovery. Trends Pharmacol Sci. 2014b;35:397–403. doi: 10.1016/j.tips.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Martin LF, Freedman R. Effects of nicotine on hippocampal and cingulate activity during smooth pursuit eye movement in schizophrenia. Biol Psychiatry. 2006;59:754–761. doi: 10.1016/j.biopsych.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, Du YP, Freedman R. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Ellis J, Shatti S, Du YP, Rojas DC. Increased hippocampal, thalamic, and prefrontal hemodynamic response to an urban noise stimulus in schizophrenia. Am J Psychiatry. 2009;166:354–360. doi: 10.1176/appi.ajp.2008.08030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Olincy A, Johnson L, Tanabe J, Shatti S, Martin LF, Singel D, Du YP, Soti F, Kem WR, Freedman R. Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology. 2010;35:938–942. doi: 10.1038/npp.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Eichman L, Rojas DC. The effect of distracting noise on the neuronal mechanisms of attention in schizophrenia. Schizophr Res. 2012;142:230–236. doi: 10.1016/j.schres.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe J, Rojas DC, Shatti S, Olincy A, Johnson L, Martin LF, Soti F, Kem WR, Leonard S, Freedman R. Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biol Psychiatry. 2011;69:7–11. doi: 10.1016/j.biopsych.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Martin LF, Freedman R. FMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. Am J Psychiatry. 2005;162:391–393. doi: 10.1176/appi.ajp.162.2.391. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr Opin Neurobiol. 2013;23:283–290. doi: 10.1016/j.conb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Venables PH. Input Dysfunction in Schizophrenia. Prog Exp Pers Res. 1964;72:1–47. [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall ND, de Wit H. Effects of nicotine on attention and inhibitory control in healthy nonsmokers. Exp Clin Psychopharmacol. 2011;19:183–191. doi: 10.1037/a0023292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatry. 2010;23:112–119. doi: 10.1097/YCO.0b013e3283366643. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Smucny J, Legget KT, Tregellas JR. Targeting Functional Biomarkers in Schizophrenia with Neuroimaging. Curr Pharm Des. 2016 doi: 10.2174/1381612822666160127113912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JK, Jimenez AM, Roach BJ, Korb A, Lee J, Horan WP, Ford JM, Green MF. Impaired target detection in schizophrenia and the ventral attentional network: Findings from a joint event-related potential-functional MRI analysis. Neuroimage Clin. 2015;9:95–102. doi: 10.1016/j.nicl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunina N, Kim TS, Tae WS, Kim SS, Nam EC. Applicability of the Sparse Temporal Acquisition Technique in Resting-State Brain Network Analysis. AJNR Am J Neuroradiol. 2015 doi: 10.3174/ajnr.A4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Liu L, Liu S, Hong X, Chen da C, Xiu MH, Yang FD, Zhang Z, Zhang X, Kosten TA, Kosten TR. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry. 2012;169:974–981. doi: 10.1176/appi.ajp.2012.11081289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.