Abstract
Adults with bipolar disorder (BD) and major depressive disorder (MDD) have higher circulating levels of proinflammatory cytokines than healthy controls. However, it is not known whether pediatric-onset patients with BD or MDD show increases in levels of inflammation or activation of nuclear factor kappa B (NF-κB), a key transcription factor in inflammatory signaling. Circulating levels of inflammatory cytokines, as well as spontaneous and stimulated levels of activated NF-κB in total peripheral blood mononuclear cells, monocytes and lymphocytes were measured in adolescents with BD (n=18), MDD (n=13), or no psychiatric history (n=20). Participants had a range of mood symptoms at time of testing. Adolescents with BD had significantly higher spontaneous levels of NF-κB in peripheral blood mononuclear cells, monocyte and lymphocyte populations, and higher plasma levels of IL-1β than healthy controls. Following stimulation with recombinant human TNF-α, participants with BD and MDD both had greater increases in NF-κB in monocytes than controls. Further, greater stimulated increases of NF-κB in monocytes were associated with the current severity of depressive symptoms. The results are limited by the small sample and cross-sectional design. Interventions that target early immunological dysregulation should be examined in relation to long-term outcomes in youth with bipolar and depressive disorders.
Keywords: Inflammation, interleukin-1, peripheral blood mononuclear cells, monocytes, lymphocytes, NF-κB, inflammatory signaling
1. Introduction
Bipolar disorder (BD), one of the world’s ten most disabling conditions, exerts a considerable toll on the psychological and physical health of the sufferer (Kupfer, 2005). The search for biological markers has yielded candidate endophenotypes including abnormal intracellular signaling cascades, inadequate cortical control over limbic activity when processing emotions, sleep and circadian rhythm dysregulation, heightened reward sensitivity, and low levels of brain-derived neurotrophic factor (Geddes and Miklowitz, 2013). Given the high rates of medical illness comorbidity in BD, the potential role of altered inflammatory activity as an illness mechanism has begun to receive attention (Goldstein et al., 2015).
It is well-established that inflammatory cytokines induce behaviors associated with depression, including changes in sleep, anhedonia, and decreased activity. As compared to healthy controls, adults with major depressive disorder (MDD) show increases in circulating levels of proinflammatory cytokines (Miller et al., 2009). Increases in systemic markers of inflammation are also found in patients with BD, especially among those in acutely depressed or manic states (Modabbernia et al., 2013; Brietzke et al., 2009; O’Brien et al., 2006; Guloksuz et al., 2010). These studies have focused almost exclusively on circulating levels of proinflammatory cytokines (e.g., IL-6) in adults with established MDD or BD. Fewer studies have examined upstream inflammatory signaling mechanisms such as nuclear factor kappa B (NF-κB), despite evidence that activation of these transcription factors plays a key role in the regulation of the inflammatory cascade and in responses to psychological stress (Keri et al., 2014; Pace et al., 2006; Slavich and Irwin, 2014; Wieck et al., 2013). Compared to healthy volunteers, higher levels of NF-κB activity have been found in adults with BD during depressed (Spiliotaki et al., 2006) and euthymic states (e.g., Amoruso et al., 2015). Barbosa et al. (2013) found a 7.2-fold increase in phosphorylated p65 NF-κB protein levels in euthymic bipolar I patients compared to healthy volunteers. These findings have been limited to the study of mixed peripheral blood mononuclear cell populations, despite evidence that activation of NF-κB in monocyte populations is key in initiating the inflammatory response in vivo.
Little is known about changes in the inflammatory biology of pediatric-onset major depression or BD. Limited cross-sectional data suggest that children with MDD show elevations in proinflammatory cytokines such as IL-1 beta (IL-1β) and IL-6 as compared to healthy controls (Henje-Blom et al., 2012; Gabbay et al., 2009; Brambilla et al., 2004; Mitchell and Goldstein, 2014). No study has examined whether levels of the anti-inflammatory cytokine IL-10 are correspondingly lower in pediatric MDD or BD. Preliminary findings suggest increases in pro-inflammatory gene expression in adolescent offspring of adults with BD (Padmos et al., 2008) and increases in a systemic marker of inflammation, C-reactive protein (CRP) in adolescents with a bipolar spectrum disorder (i.e., bipolar disorder I,II, or not elsewhere classified) (Goldstein et al., 2011). In a within-group analysis of 123 bipolar adolescents and young adults (mean age 20.4), there was a positive association between high-sensitivity CRP levels (hs-CRP) and earlier age at illness onset, as well as the severity of depressive symptoms over 6 months (Goldstein et al., 2015). Importantly, mean levels of hs-CRP in this young sample were above the established threshold for increased risk for cardiovascular disease among adults.
The present study examined systemic and cellular markers of inflammation in adolescents who had lifetime BD spectrum disorders or MDD (currently in remission or with subsyndromal symptoms) compared to healthy controls. Our primary hypothesis was that adolescents with BD and MDD would show greater circulating concentrations of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) and/or lower levels of IL-10, as compared to healthy volunteers. Secondarily, given the key role of the NF-κB transcription control pathway in regulating cellular expression of proinflammatory genes and increases in inflammatory cytokines, we hypothesized that among adolescents with BD or MDD, levels of NF-κB activation would be higher in peripheral blood mononuclear cells, including monocyte and lymphocyte subpopulations, than among adolescents with no psychiatric history. We measured levels of NF-κB signaling in spontaneous (unstimulated) states (i.e., activation in cells in the peripheral blood) to provide an index of in vivo activation of NF-κB. Additionally, we evaluated the ability of peripheral blood mononuclear cells and lymphocyte and monocyte populations to respond to a TNF-α challenge, because this proinflammatory cytokine induces a rapid (i.e., within 15 minutes) activation of the NF-κB signaling pathway, regardless of cell type, as would occur in an inflammatory response. NF-κB signaling was expressed as the change in intensity between unstimulated and stimulated cells. Finally, we examined the current severity of depressive and manic symptoms, body mass index, and medication regimens as additional independent variables or covariates for these group comparisons.
2. Material and Methods
2.1 Participants
All subjects were between age 12 and 18 yrs. Patient participants had to meet lifetime DSM-IV-TR (American Psychiatric Association, 2000) criteria for bipolar disorder (BD), type I (n = 7), type II (n = 5), or not otherwise specified (n = 6); or major depressive disorder (MDD, n = 13). Bipolar disorder not otherwise specified was operationalized as per the Course and Outcome of Bipolar Youth criteria (Birmaher et al., 2009): (1) a distinct period of abnormally elevated, expansive, or irritable mood plus at least 2 DSM-IV-TR symptoms of mania (3, if irritable mood only) that caused a clear change in functioning, (2) mood and associated symptoms were present for > 1 day, and (3) there have been at least 10 lifetime days in which the child met these mood, symptom, and functional change criteria. Youths who meet BD, not otherwise specified criteria are at substantially increased risk of converting to bipolar I or II disorder within 4 years (Birmaher et al., 2009).
Study candidates were recruited from consecutive admissions to a registry of patients who had completed an intake evaluation at the UCLA Child and Adolescent Mood Disorders clinic. Control subjects (n = 20) had no lifetime history of mental health diagnoses. Seven healthy controls were recruited directly for this study through web advertising and flyers posted at the UCLA Medical Center. Data from 13 additional controls came from two prior UCLA studies of inflammatory functioning in healthy adolescents that used nearly identical screening, sample collection and assay procedures (i.e., multiplex cytokine assays, flow cytometry) as those in this study (Muscatell et al., 2015; Moieni et al., 2015). Potential participants were excluded if they had a current illness (cold, flu, infection) and/or were taking antibiotics, had preexisting inflammatory disease, met DSM-IV-TR criteria for current (prior month) alcohol or substance abuse or dependence disorder, or had pervasive developmental disorder. Participants (and if under 18, a parent) provided written informed consent for the study after receiving a full explanation of the procedures. The study was approved by the UCLA Medical Institutional Review Board.
2.2. Assessment
All adolescents with suspected mood disorders and the 7 healthy volunteers recruited directly for the study were interviewed by a research staff member using the Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997; Chambers et al., 1985). The mood disorder modules of the KSADS-PL were replaced by the KSADS Depression and Mania Rating Scales (Chambers et al., 1985; Axelson et al., 2003) to enable finer-grained distinctions between manic or hypomanic states varying in severity, duration and functional impairment. The interview covered symptoms experienced during the worst week of the past month (current ratings) and the worst 1–2 weeks lifetime (past ratings). Parents were interviewed separately and KSADS item consensus judgments – leading to axis I diagnoses - were made using all sources of information. The 13 healthy controls from the two prior UCLA studies had no psychiatric history as reported on the Structured Clinical Interview for DSM-IV Axis I disorders (First et al., 1995) interview.
The first study visit lasted 2–3 hours and included dimensional ratings of current (prior week) manic/hypomanic symptoms using the Young Mania Rating Scale (YMRS; Young et al., 1978) and current (prior 2 weeks) depression symptoms using the Children’s Depression Rating Scale-Revised (CDRS-R; Poznanski and Mokros, 1995). These clinical interview-based rating scales were not administered to control subjects who, by design and by diagnostic interview, had no history of a mood disorder or other axis I disorders (Table 1). Interrater reliability for the YMRS averaged .98; for CDRS-R ratings, .93; for KSADS mania items, .90; and for KSADS depression items, .96 (intraclass rs).
Table 1.
Baseline Demographic and Clinical Characteristics of Study Population
| Variable | Bipolar Disorder (n = 18) | Major Depressive Disorder (n=13) | Healthy Control (n = 20) | P value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 16.0 | 2.1 | 14.8 | 1.7 | 16.6 | 2.2 | .05 |
| Education, years completed | 9.2 | 2.2 | 8.5 | 2.3 | 10.6 | 2.3 | .03 |
| Body Mass Index, kg/m2 | 23.0 | 4.1 | 22.4 | 4.4 | 22.6 | 3.8 | .92 |
| YMRS | 11.4 | 8.4 | 10.2 | 5.4 | -- | -- | .64 |
| CDRS-R | 40.9 | 14.2 | 44.2 | 18.1 | -- | -- | .58 |
| Number (%) | |||||||
| Female | 10 | 55.6 | 8 | 61.5 | 16 | 80.0 | .25 |
| Hispanic | 2 | 11.1 | 3 | 23.1 | 6 | 30.0 | .36 |
| Asian American | 4 | 22.2 | 2 | 15.4 | 3 | 15.0 | -- |
| African American | 2 | 11.1 | 0 | 0.0 | 3 | 15.0 | -- |
| Native American | 1 | 5.6 | 0 | 0.0 | 1 | 5.0 | -- |
| Mixed Race | 0 | 0.0 | 0 | 0.0 | 4 | 20.0 | -- |
| ADHD | 4 | 22.2 | 4 | 30.8 | -- | -- | .59 |
| Anxiety disorders | 2 | 11.1 | 29 | 38.5 | -- | -- | .07 |
| Mood stabilizer | 11 | 61.1 | 2 | 15.4 | -- | -- | .01 |
| Antipsychotic | 11 | 61.1 | 2 | 15.4 | -- | -- | .01 |
| Antidepressant | 5 | 27.8 | 10 | 76.9 | -- | -- | .01 |
| Stimulant | 4 | 22.2 | 4 | 30.8 | -- | -- | .59 |
Notes: YMRS = Young Mania Rating Scale; CDRS-R = Children’s Depression Rating Scale, Revised; ADHD = attention deficit hyperactivity disorder. P-values are based on F or χ2 statistics comparing means or frequencies across groups. The YMRS and CDRS-R were not administered to the healthy control participants. Comorbid disorders that were present in fewer than 4 participants are not listed.
Height and weight were obtained for all subjects, and Body Mass Index (BMI) was calculated (kg/m2). Blood samples (non-fasting) were obtained between 9:00 a.m. and 1:30 p.m. There were no effects of time of day of blood sampling on levels of cytokines (pg/ml) or NF-κB mean fluorescence intensity levels (for all comparisons, p > .10). Thus, these values were pooled in the primary group comparisons.
2.3. Blood sampling and immunological measures
Whole blood samples were collected in EDTA tubes. After collection, the samples were centrifuged at 4°C, plasma was harvested into multiple aliquots, and then stored in a −70°C freezer until assayed. Plasma concentrations of IL-1β, IL-6, IL-8, TNF-α, and IL-10 were measured in duplicate. Using a Bio-Plex 200 (Luminex) Instrument, Bio-Plex software v4.1, and a 5-parameter logistic curve fit, plasma levels of each cytokine were quantified by means of high sensitivity bead-based multiplex immunoassays (Performance High Sensitivity Human Cytokine, R& D Systems, Minneapolis, MN). This multiplex assay has been shown to have excellent intra- and inter-assay reproducibility in a temporal stability study of circulating cytokine levels (Epstein et al., 2013) and strong correlations (r> .94) across a wide range of concentrations with high sensitivity ELISA kits from the same manufacturer (Breen et al., 2014). All multiplex assays were performed on plasma samples diluted 2-fold according to the manufacturer’s protocol, and all calculated concentrations ≥0.1 pg/mL generated by the BioPlex Manager software were included in data analyses. A recent comparison of high-sensitivity multiplex cytokine assays indicated that assay sensitivity is effectively limited to concentrations above 0.1 pg/mL (Epstein et al., 2013). For this reason, we considered all extrapolated concentrations of <0.1 pg/mL as below the lower limit of detection. All observations deemed undetectable were assigned a value equal to one-half of the plate-specific lower limit and retained in the analysis.
Levels of activated NF-κB in peripheral blood mononuclear cells as well as in monocyte and lymphocyte subpopulations, were evaluated using flow cytometry (Compton et al., 2015). Briefly, heparinized blood samples were collected. Peripheral blood mononuclear cells were purified by Ficoll density centrifugation, and resuspended in phosphate buffered saline at 1 x 106 cells/mL. Aliquots of 1 x 106 peripheral blood mononuclear cells were either left unstimulated (spontaneous) or stimulated with 10 ng of recombinant human TNF-α (R&D Systems), and incubated for 15 minutes at 37°C. TNF-α was used because it activates the NF-κB signaling pathway, mimicking an inflammatory response in vivo. Lipopolysaccharide or ionomycin/PMA were not used, as these are highly artificial mitogens targeted to specific cell types. In particular, we were interested in responsiveness of the total lymphocyte population, so a T cell-specific mitogen was not appropriate. Moreover, lipopolysaccharide does not directly activate the NF-κB pathway, but acts indirectly by inducing cytokines such as TNF-α and IL-1β.
Each cell aliquot was then fixed in a final concentration of 2 percent paraformaldehyde and frozen at −80°C. For flow cytometric analyses, peripheral blood mononuclear cells were thawed, washed, and treated with 90 percent methanol to permeabilize the nuclear membrane. Peripheral blood cells were washed again, then stained with phycoerythrin-labeled monoclonal antibody specific for the phosphorylated (activated) serine 529 (pS529) in the transactivation domain of human NF-κB p65 (BD Biosciences). Stained peripheral blood mononuclear cells were analyzed by single color flow cytometry using CellQuest software (BD), gating on total cells, lymphocytes only, or monocytes only, based on forward versus side scatter. The amount of unstimulated NF-κB signaling was expressed as the mean fluorescent intensity (MFI) of the population of cells being analyzed; NF-κB signaling following in vitro stimulation with TNF-α was expressed as the change in MFI between unstimulated and stimulated cells (stimulated MFI – unstimulated MFI). All samples were analyzed using the same flow cytometer instrument and settings. Laboratory assistants were unaware of the participants’ control vs. patient status.
We present data using MFI analysis rather than flow cytometric plots for several reasons. Flow cytometric dot plots describe the proportion of cells positive for NF-κB, and hence provide a categorical indication of whether a cell was activated or not. Hence, dot plots show percentages of positive and negative cells, but this method does not reveal necessary information about the level of activation within an individual cell. Importantly, the number of positive cells may be similar in different individuals, yet levels of activated NF-kB within those cells could be different. MFI analysis provides an assessment of differences in the intensity of NF-κB signaling, particularly in unstimulated cells. In this study, we were interested in the levels of activation of NF-κB at the cellular level using peripheral blood mononuclear cell, lymphocyte and monocyte populations. We believe that the MFI analysis is appropriate for use in adolescent populations who are likely to have low levels of NF-κB activation, because MFI would provide a continuous measure of intensity of signaling, especially in the unstimulated cells that are most representative of the in vivo condition. Similarly, MFI analysis can be used to evaluate within person differences (stimulated minus unstimulated) in levels of NF-κB activation with a finer grain of analysis than is afforded by dot plots that classify cells as positive or negative.
2.4. Statistical analysis
The BD, MDD, and health volunteer groups were first compared on demographic and illness history variables at study intake. Upon visual inspection, the distributions of the cytokine and NF-κB intensity levels were found to be positively skewed. Thus, natural log transformations were undertaken to normalize the distributions prior to statistical analyses. Plasma concentrations of IL-1β or IL-6 that were below 0.1 pg/mL were assigned a value of 0.05 (one-half the lower limit of detection) prior to log transformation; this assignment was undertaken for IL-1β with 12 subjects (5 patients and 7 controls) and for IL-6 with 2 subjects (1 patient, 1 control). In one case, a subject’s IL-1β concentration (1.9 pg/mL) was 5 SDs above the sample mean and 3.5 SDs above the next highest concentration. As recommended by Dixon (1960) and others, this value was replaced by the next highest value in the distribution (0.8 pg/mL).
The primary hypotheses – that the BD and MDD groups would show higher levels of proinflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α), lower levels of the anti-inflammatory cytokine IL-10 and higher MFIs for NF-κB cell populations (peripheral blood mononuclear cells, monocytes, and lymphocytes in the spontaneous and stimulated states) than the healthy controls - were tested with univariate analyses of variance (ANOVAs), with planned comparisons of (1) bipolar (n = 18) vs. healthy control subjects (n = 20), and (2) MDD (n = 13) vs. control subjects. BMI was included as a covariate in these ANOVA models given evidence that BMI correlates with inflammation (O’Connor et al., 2009).
The majority of subjects with BD or MDD had become ill only recently. Thus, we limited our examination of illness variables to the severity of current symptoms and current medication regimens. Within the mood disorder subsample (n = 31), we examined whether immunological variables were related to YMRS or CDRS-R ratings of the worst week in the prior month. A series of dummy variables (present/absent) summarized the youths’ pharmacological treatment (mood stabilizers, antipsychotics, antidepressants, or stimulants). In one-way ANOVAs, we examined whether there were differences in inflammatory cytokines or NF-κB activity among participants who were on or off each class of medication.
All statistical comparisons used a two-tailed p-value of 0.05. Because the study addressed novel a priori hypotheses that relied on a limited number of dependent variables, we did not adjust p-values for multiple comparisons.
3. Results
3.1. Sample Composition
Of 150 candidates screened by telephone or in person, 60 refused and 52 were deemed ineligible. Of the 52 ineligible candidates, 34 were outside the 12–18 year age range, and 10 had autism spectrum disorders, psychosis, or chronic medical illnesses. Six candidates for the control group were excluded because they had siblings who were being treated for depression or BD. Two reported being afraid of needles. The remaining 38 participants met the study’s inclusionary criteria:18 had BD, 13 had MDD, and 7 had no psychiatric history. As explained earlier, data were included from 13 additional controls from prior studies who had been recruited and tested using methods very similar to the 7 youths without psychiatric history. There were no differences among the BD, MDD or healthy control (n = 20) groups in gender, race/ethnicity, or BMI (Table 1). The MDD participants were, however, an average of 2.0 years younger than the controls (F[2, 48]) = 3.21, p<0.05).
3.2. Cytokines and NF-κB activity across diagnostic groups
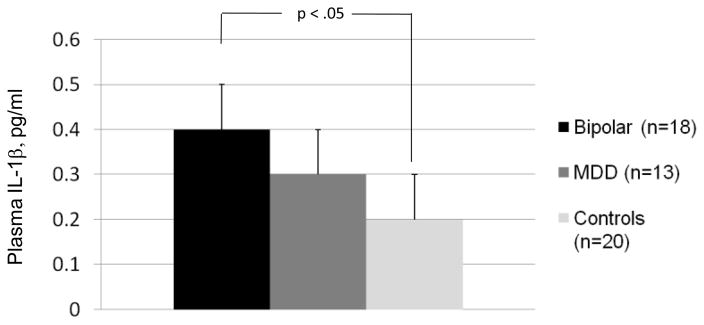
The means and standard errors for the cytokine variables are presented in table 2. For ease of interpretation, we report non-transformed values adjusted for covariates. With BMI covaried, plasma levels of IL-1β were found to be significantly higher in youth with BD than in controls (F[1,33] = 4.93, p = 0.03; unadjusted mean difference 0.17, 95% CI: .03 – .32; Figure 1). Exclusion of the 12 subjects with IL-1β plasma concentrations below 0.1 pg/mL did not change the size of this difference (F[1,24] = 4.54, p = 0.044; mean diff. 0.18, 95% CI, .01 – .35) nor did excluding the participant with the extreme IL-1β level (p = 0.049). The BD group did not differ from the healthy controls on IL-6, IL-8, TNF-α, or IL-10. Youth with MDD did not differ from bipolar participants or healthy controls on any of the cytokines.
Table 2.
Circulating Pro- and Anti-inflammatory Cytokines in Bipolar, Depressed, and Healthy Adolescents
| Cytokine | Bipolar Disorder (n = 18) | Major Depressive Disorder (n = 13) | Healthy Control (n = 20) | F-Value (Bipolar vs. Control) | P-value (Bipolar vs. Control) |
|---|---|---|---|---|---|
| Plasma Cytokine Concentrations (pg/ml), Mean (SE)
| |||||
| IL-1β | 0.4 (0.1) | 0.3 (0.1) | 0.2 (0.1) | 4.93 | .03 |
| IL-6 | 0.8 (0.2) | 1.3 (0.3) | 1.3 (0.2) | 2.90 | .10 |
| IL-8 | 3.5 (0.3) | 2.9 (0.4) | 2.7 (0.3) | 3.09 | .09 |
| IL-10 | 0.7 (0.1) | 0.5 (0.1) | 0.5 (0.1) | 3.26 | .08 |
| TNF-α | 5.9 (0.4) | 5.8 (0.5) | 5.1 (0.4) | 2.43 | .13 |
Note: All values were natural log-transformed prior to conducting group comparisons. For ease of interpretation, the non-transformed mean plasma cytokine concentrations, adjusted for BMI, are shown in the table. F- and p-values refer to the primary comparison of bipolar disorder vs. healthy control participants. The participants with major depressive disorder did not differ from the participants with bipolar disorder or the control participants in any comparison.
Fig. 1.
Interleukin -1β (IL-1β) Levels in Adolescents with Bipolar Disorder, Major Depressive Disorder (MDD), or No Psychiatric History (Controls). Note. Mean IL-1β levels + standard errors, adjusted for Body Mass Index, are pictured. Plasma levels of IL-1β were significantly higher in youth with BD than in controls (p = 0.03).
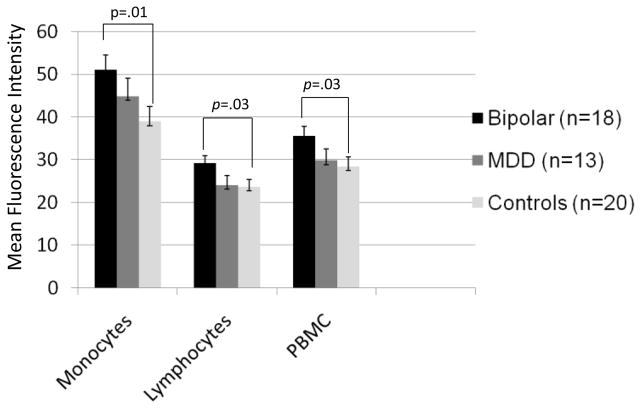
Spontaneous (unstimulated) levels of activated NF-κB in total peripheral blood mononuclear cells were higher in bipolar youth than healthy controls (F[1,33] = 4.94, p= 0.03; mean diff. 7.07, 95% CI: 0.67–13.48; Fig. 2). Further examination revealed that youth with BD had higher levels of spontaneous NF-κB in monocytes (F[1,33]=7.24, p = 0.01; mean diff. 12.04, 95% CI, 3.11 – 20.97) and lymphocytes (F[1,33]=4.98, p = 0.03; mean diff. = 5.48, 95% CI, 0.14 – 10.83) than controls (see online supplement, Table S1). In the sample as a whole, levels of NF-κB activation in monocytes were higher than levels of NF-κB activation in lymphocytes (t[50] = 19.41, p < .0001). Levels of IL-1β and unstimulated levels of activated NF-κB were uncorrelated (all p>0.10).
Fig. 2.
Spontaneous (Unstimulated) Nuclear Factor-Kappa B (NF-κB) Levels in Adolescents with Bipolar Disorder, Major Depressive Disorder (MDD), or No Psychiatric History (Controls). Notes. Values are mean (+ standard error) fluorescence intensity scores expressing amount of NF-κB signaling in monocyte, lymphocyte and total peripheral blood mononuclear cell (PBMC) populations. Spontaneous levels of NF-κB in monocytes, lymphocytes, and total peripheral blood mononuclear cells were higher in bipolar youth compared to healthy controls but did not differ from levels observed in adolescents with MDD.
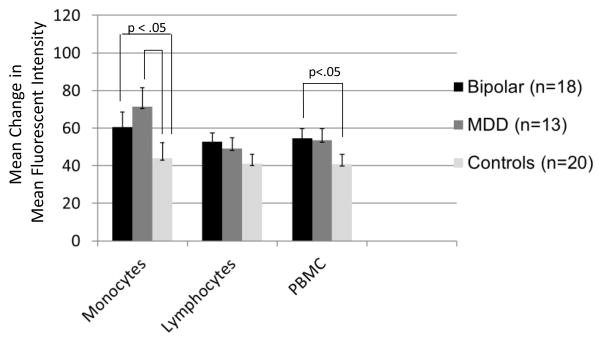
Higher levels of stimulated NF-κB in peripheral blood mononuclear cells were observed in youth with BD compared to control subjects (F([1,33])=4.30, p= 0.046; mean diff. 13.70, 95% CI, −1.18 to 28.58). Participants with BD (F([1,33])=4.50, p = 0.042; mean diff. 16.67, 95% CI, −7.02 to 40.36) and participants with MDD (F[1,27] = 6.31, p= 0.018; mean diff. 28.02, 95% CI, .61–55.44) had higher levels of stimulated monocytes than control subjects (Fig. 3). Youth with MDD did not differ from youth with BD on NF-κB monocytes in either the stimulated or unstimulated states.
Fig. 3.
Increases in Nuclear Factor - Kappa B (NF-κB) Activation following TNF-alpha Stimulation in Adolescents with Bipolar Disorder, Major Depressive Disorder (MDD), or No Psychiatric History (Controls). Notes. MDD = Major Depressive Disorder; PBMC= Peripheral Blood Mononuclear Cells. Values are mean (+ standard error) changes in fluorescence intensity scores (stimulated MFI - unstimulated MFI) in monocyte, lymphocyte and total peripheral blood mononuclear cell populations. The bipolar and MDD groups both had greater increases in stimulated monocyte levels than controls (p<0.05). The bipolar group also had greater increases in stimulated peripheral blood mononuclear cells than controls (p<0.05). The two mood groups did not significantly differ in increases in NF-κB activation following stimulation.
Secondary analyses were undertaken to compare participants who had diagnoses of bipolar disorder I, II, or not otherwise specified. There were no differences between cytokine levels or NF-κB MFI scores between these three subgroups, although cell sizes were too small to permit a fully powered comparison.
3.3. Effects of current mood symptoms and medications
Of the 31 participants with mood disorders, 13 (41.9%) had scores of 40 or above on the CDRS-R, indicating significant depressive symptoms; and 13 (41.9%) had scores of 12 or above on the YMRS, indicating significant hypomanic/manic symptoms. Overall, 15 of 31 patients (48.4%) were clinically remitted (CDRS-R < 40 and YMRS < 12) on both measures.
Levels of cytokines and NF-κB in the spontaneous and stimulated states were examined in relation to current CDRS-R and YMRS scores. Higher levels of stimulated NF-κB activity in peripheral blood mononuclear cells were associated with higher CDRS-R scores (r(31)=.42, p=0.02). This relationship between levels of NF-κB activation and CDRS-R scores held for stimulated monocytes (r(31) = .54, p= 0.002) but not for stimulated lymphocytes (r(31)=.29, p= 0.11). The association between stimulated monocytes and CDRS-R scores was significant in the bipolar subgroup (r(18)=.48, p= 0.046) and the MDD subgroup (r(13)=.59, p= 0.032). There were no associations between spontaneous levels of NF-κB activation and CDRS-R or YMRS scores, or between cytokines and concurrent mood scores in the two diagnostic groups.
As expected, participants with BD were more likely than participants with MDD to be taking mood stabilizers (i.e., lithium, divalproex, or lamotrigine) or second generation antipsychotics (χ2(1)=6.48, p= 0.01), whereas participants with MDD were more likely to be taking antidepressants than participants with BD (χ2(1)=7.30, p= 0.007; Table 1). The two mood groups were equally likely to be taking psychostimulants. There were no differences in levels of cytokines or NF-κB activation (either spontaneous or stimulated) between participants who were vs. were not taking mood stabilizers, antipsychotics, antidepressants, or stimulants. Inclusion of concurrent medications as covariates did not alter the associations between diagnostic group and IL-1β or NF-κB variables.
4. Discussion
Early onset of bipolar disorder (i.e., under age 18) is a consistent predictor of a poor course of illness over time, including an increased frequency of suicide attempts, more severe residual symptoms, higher rates of recurrence, and treatment resistance (Post et al., 2010). The present study examined whether adolescents with BD differed from adolescents with MDD or no psychiatric history on measures of inflammation. Examining a young sample reduces the role of illness burden or extensive histories of antipsychotic usage on inflammatory variables. Despite the limited sample size, a pattern of increased activation in spontaneous levels of NF-κB in total peripheral blood mononuclear cells, as well as within the monocyte and lymphocyte subpopulations, distinguished youth with BD from a group of healthy volunteers of the same age. These results are similar to those of Barbosa et al. (2013), who observed hyperactivation of the NF-κB and mitogen-activated protein kinase pathways in euthymic adults with BD compared to healthy controls.
NF-κB is a transcription factor that regulates proinflammatory cytokine gene expression and is implicated in a variety of cancer, autoimmune, and inflammatory disease processes (DiDonato et al., 2012). NF-κB is activated by acute stress (Bierhaus et al., 2003), as well as sleep loss (Irwin et al., 2008) and may have a role in mediating cellular responses to stressful life events, the latter of which have been strongly implicated in the relapse/remission course of MDD and BD (Slavich and Irwin, 2014; Johnson et al., 2008). The consequences of increased NF-κB inflammatory signaling may include increased expression of proinflammatory cytokines and attenuated neuroendocrine responses to stress (Wieck et al., 2013). NF-κB hyperactivation in BD may also be protective against cell loss, helping to compensate for the neurotoxic effects of repeated illness episodes (Barbosa et al., 2013).
Only one proinflammatory cytokine, IL-1β, observed at low circulating concentrations, was higher in youth with BD compared to healthy controls. Neither IL-6, IL-8, or TNF-α distinguished youth with BD or MDD from healthy volunteers, nor were there group differences in IL-10, which is considered an anti-inflammatory cytokine. One other study (Goldstein et al., 2011; Goldstein et al., 2015) observed high levels of inflammatory cytokines (notably, hs-CRP) in adolescents with BD, although without reference to a comparison group.
Prior studies have found that depressive symptoms – notably sleep disturbance or deprivation - are associated with increased cytokine levels and activation of NF-κB in adults with MDD (Motivala et al., 2005) and healthy controls (Irwin et al., 2008). Thus, it is surprising that the MDD participants did not differ from the BD participants or the healthy controls on these measures. Although the youths with MDD showed numerical elevations on several inflammatory variables, only one – stimulated levels of NF-κB monocytes – distinguished participants with MDD from controls. Independently of diagnosis, we observed that levels of activated NF-kB in stimulated peripheral blood mononuclear cells(and specifically, in stimulated monocytes) were associated with the severity of depression in the two mood disorder groups. In contrast, unstimulated NF-κB values and circulating cytokine levels were fully independent of concurrent clinical state. Examining inflammatory activity in BD and MDD patients across illness phases – and with consideration of depression as a severity dimension as well as a diagnostic category - will be necessary to determine whether increased inflammatory activity distinguishes youth with mood disorders from healthy youth, independent of clinical state.
As has been true in studies of adults with BD (e.g., do Prado et al., 2013), we found no associations between taking mood stabilizers, antipsychotics, antidepressants or psychostimulants and either levels of proinflammatory cytokines or NF-κB activation in pediatric-onset BD or MDD. Few studies have examined whether cytokine levels are abnormal in patients with BD or MDD when medications are controlled. One cross-sectional study (Henje Blom et al., 2012) found that levels of IL-6 in adolescent females with MDD were higher than among adolescent females without depression, but only among those not taking selective-serotonin reuptake inhibitors. Kim et al. (2004) found that medication-free adult patients with mania could be distinguished from healthy volunteers on elevated levels of IFN-y and IL-4 and lower levels of TGF-β1 at the time of a hospital admission; TGF-β1 levels increased significantly from admission to a reassessment after 8 weeks of mood stabilizer treatment. Among 10 treatment-naïve bipolar patients, three months of treatment with lithium were associated with normalization of inflammatory markers (Boufidou et al., 2004). Examining inflammatory cytokines or upstream inflammatory signaling mechanisms among pediatric patients with BD or MDD before and after pharmacological agents are added or discontinued may clarify the degree to which medications account for variations in inflammatory states.
Our results must be considered within the limitations of a cross-sectional study. A single assessment of peripheral blood is not adequate for determining the longer-term nature of immune system dysfunction. Relatedly, our sample was comprised of adolescents across the full spectrum of BD (I, II, and NOS) who may have had different illness trajectories before the study. Heterogeneous group composition has been the rule more than the exception in studies of pediatric bipolar disorder (Van Meter et al., 2011). Youth with subthreshold BD can present quite differently from those with bipolar I disorder, despite their increased risk of developing the full bipolar syndrome over time (Birmaher et al., 2009).
This study did not control variables that are important in determining health status in children and adolescents, such as family history of mood or immunological disorders, substance misuse, interpersonal stress, adherence with pharmacological treatments, and sleep irregularity (Mitchell and Goldstein, 2014; DelBello et al., 2007). Sleep disruption may be a mediating variable in the pathways between inflammatory dysregulation and mood instability, and treatments that help train adolescents to keep regular sleep/wake hours or maximize sleep efficiency may help regulate immune functioning (Irwin et al., 2015). Likewise, a greater frequency of negative interpersonal interactions with family members or peers is associated with higher levels of inflammation in healthy adolescents (Fuligni et al., 2009). The roles of sleep deprivation and adverse family interactions in the cycling of bipolar and other mood disorders are becoming increasingly clear. Indeed, most psychosocial interventions for BD emphasize sleep/wake regularity and stress management skills as central to long-term mood stability (Geddes and Miklowitz, 2013).
It is unknown whether early dysregulation in the immune system contributes to the later onset of chronic and life-threatening physical illnesses that often co-occur with BD, such as cardiovascular disease (Goldstein et al., 2015). Nonetheless, treatment options that would not ordinarily be considered in BD and that may target abnormalities in immunological functioning, such as nonsteroidal anti-inflammatory agents, cytokine antagonists, or insomnia treatments, should be examined as elements of maintenance care (Mitchell and Goldstein, 2014; Harvey, 2011). Examining inflammatory markers, including NF-κB, as mediators of the clinical effects of pharmacological or psychosocial interventions has the potential to refine identification of those at greatest risk and the targeted specificity of treatments, consistent with the broad research agenda of precision medicine (Pine and Leibenluft, 2015).
Supplementary Material
Highlights.
Few studies have examined immunological functioning in youths with bipolar disorder.
Bipolar, depressed, and healthy adolescents were compared on inflammatory measures.
Bipolar adolescents had higher levels of NF-κB and IL-1β than controls.
Immune dysregulation may be a target for early intervention in bipolar disorder.
Acknowledgments
Funding
This research was supported by National Institute of Mental Health grants R01MH093676 and R33MH097007 (DJM) and grants from the UCLA Clinical and Translational Sciences Institute, the Carl and Roberta Deutsch Foundation, the Kayne Family Foundation (DJM), and the UCLA Cousins Center for Psychoneuroimmunology (MRI). The funders had no role in the design or conduct of the study, in data collection, analysis or interpretation, or in writing the report.
The authors thank Marissa Caudill, Marc Weintraub, and Brittany Matkevich of the UCLA Semel Institute for their assistance with data collection, and Lisa Goehler of the University of Virginia for comments on an initial draft of this manuscript.
Footnotes
Clinical Trial registration information: Early Intervention for Youth at Risk for Bipolar Disorder, https://clinicaltrials.gov/ct2/show/NCT01483391.
Conflict of Interest
Dr. Miklowitz has received research funding from the National Institute of Mental Health, the UCLA Cousins Center for Psychoneuroimmunology, the Deutsch, Kayne, and Knapp Family Foundations, Danny Alberts Foundation, Attias Family Foundation, and the American Foundation for Suicide Prevention; and book royalties from Guilford Press and John Wiley and Sons. Dr. Irwin is supported by the NIMH, the UCLA Cousins Center for Psychoneuroimmunology; and the UCLA Claude D. Pepper Older Americans Independence Center. Dr. Breen is supported by the UCLA Cousins Center for Psychoneuroimmunology and the UCLA Claude D. Pepper Older Americans Independence Center. Dr. Eisenberger is supported by the NIMH, the National Institute on Drug Abuse, and the National Alliance for Research on Schizophrenia and Depression. The other authors report no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Press; 2000. (Text Revision) (DSM-IV-TR) [Google Scholar]
- Amoruso A, Bardelli C, Cattaneo CI, Fresu LG, Manzetti E, Brunelleschi S. Neurokinin (NK)-1 receptor expression in monocytes from bipolar disorder patients: a pilot study. Journal of Affective Disorders. 2015;178:188–192. doi: 10.1016/j.jad.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2003;13:463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- Barbosa IG, Nogueira CR, Rocha NP, Queiroz AL, Vago JP, Tavares LP, Assis F, Fagundes CT, Huguet RB, Bauer ME, Texiera AL, de Sousa LP. Altered intracellular signaling cascades in peripheral blood mononuclear cells from BD patients. Journal of Psychiatric Research. 2013;47:1949–1954. doi: 10.1016/j.jpsychires.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, Houck P, Ha W, Iyengar S, Kim E, Yen S, Hower H, Esposito-Smythers C, Goldstein T, Ryan N, Keller M. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. American Journal of Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boufidou F, Nikkolaou C, Alevizios B, Liappas IA, Christodoulou GN. Cytokine production in bipolar affective disorder patients under lithium treatment. Journal of Affective Disorders. 2004;82:309–313. doi: 10.1016/j.jad.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Monteleone P, Maj M. Interleukin-1 beta and tumor necrosis factor-alpha in children with major depressive disorder or dysthymia. Journal of Affective Disorders. 2004;78:273–277. doi: 10.1016/S0165-0327(02)00315-4. [DOI] [PubMed] [Google Scholar]
- Breen E, Perez C, Olmstead R, Eisenberger N, Irwin M. Comparison of multiplex immunoassays and ELISAs for the determination of circulating levels of inflammatory cytokines (Abstract) Brain Behavior and Immunity. 2014;40(supplement):e39. [Google Scholar]
- Brietzke E, Stertz L, Fernandes BS, Kauer-Sant’anna M, Mascarenhas M, Escosteguy VA, Chies JA, Kapczinski F. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. Journal of Affective Disorders. 2009;116:214–217. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semi-structured interview: test-retest reliability. Archives of General Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Compton P, Griffis C, Breen EC, Torrington M, Sadakane R, Tefera E, Irwin MR. Opioid treatment of experimental pain activates nuclear factor-κB. Journal of Opioid Management. 2015;11:115–125. doi: 10.5055/jom.2015.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelBello MP, Hanseman D, Adler CM, Fleck DE, Strakowski SM. Twelve month outcome of adolescents with bipolar disorder following first-hospitalization for a manic or mixed episode. American Journal of Psychiatry. 2007;164:582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunological Reviews. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Simplified estimation from censored normal samples. Annals of Mathematical Statistics. 1960;31:385–391. [Google Scholar]
- Do Prado CH, Rizzo LB, Wieck A, Lopes RP, Teixera AL, Grassi-Oliveira R, Bauer ME. Reduced regulatory T cells are associated with higher levels of Th1/TH17 cytokines and activated MAPK in type 1 bipolar disorder. Psychoneuroendocrinology. 2013;38:667–676. doi: 10.1016/j.psyneuen.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Epstein MM, Breen EC, Magpantay L, Detels R, Lepone L, Penugonda S, Bream JH, Jacobson LP, Martínez-Maza O, Birmann BM. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiology, Biomarkers and Prevention. 2013;22:2009–2015. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I, Version 2.0, Final Version) New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Fuligni AJ, Telzer EH, Bower J, Cole SW, Kiang L, Irwin MR. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosomatic Medicine. 2009;71:329–333. doi: 10.1097/PSY.0b013e3181921b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Hirsch GS, Hottinger-Blanc PM, Gonzalez CJ. Immune system dysregulation in adolescent major depressive disorder. Journal of Affective Disorders. 2009;115:177–182. doi: 10.1016/j.jad.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381:1672–1682. doi: 10.1016/S0140-6736(13)60857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, Stoney CM, Wasiak H, McCrindle BW. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:965–86. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Collinger K, Lotrich F, Marsland A, Gill MK, Axelson DA, Birmaher B. Preliminary findings regarding proinflammatory markers and Brain-Derived Neurotrophic Factor among adolescents with bipolar spectrum disorders. Journal of Child and Adolescent Psychopharmacology. 2011;21:479–484. doi: 10.1089/cap.2011.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Lotrich F, Axelson D, Gill MK, Hower H, Goldstein TR, Fan J, Yen S, Diler R, Dickstein D, Strober M, Iyengar S, Ryan ND, Keller MB, Birmaher B. Inflammatory markers among adolescents and young adults with bipolar spectrum disorders. Journal of Clinical Psychiatry. 2015;76(11):1556–63. doi: 10.4088/JCP.14m09395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guloksuz S, Cetin EA, Cetin T, Deniz G, ETO, Nutt DJ. Cytokine levels in euthymic bipolar patients. Journal of Affective Disorders. 2010;126:458–462. doi: 10.1016/j.jad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian functioning: critical mechanisms in the mood disorders? Annual Review of Clinical Psychology. 2011;7:297–319. doi: 10.1146/annurev-clinpsy-032210-104550. [DOI] [PubMed] [Google Scholar]
- Henje Blom E, Lekander M, Ingvar M, Åsberg M, Mobarrez F, Serlachius E. Pro-inflammatory cytokines are elevated in adolescent females with emotional disorders not treated with SSRIs. Journal of Affective Disorders. 2012;136:716–723. doi: 10.1016/j.jad.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological Psychiatry. 2015 Jun 1; doi: 10.1016/j.biopsych.2015.05.014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biological Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Cuellar A, Ruggero C, Perlman C, Goodnick P, White R, Miller I. Life events as predictors of mania and depression in bipolar I disorder. Journal of Abnormal Psychology. 2008;117:268–277. doi: 10.1037/0021-843X.117.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for school-age children - Present and Lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keri S, Szabo C, Kelemen O. Blood biomarkers of depression track clinical changes during cognitive-behavioral therapy. Journal of Affective Disorders. 2014;164:118–22. doi: 10.1016/j.jad.2014.04.030. [DOI] [PubMed] [Google Scholar]
- Kim YK, Myint AM, Lee BH, Han CS, Lee SW, Leonard BE, Steinbusch HW. T-helper types 1, 2, and 3 cytokine interactions in symptomatic manic patients. Psychiatry Research. 2004;129:267–272. doi: 10.1016/j.psychres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ. The increasing medical burden in bipolar disorder. Journal of the American Medical Association. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RHB, Goldstein BI. Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:274–296. doi: 10.1016/j.jaac.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biological Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Moieni M, Jevtic I, Irwin MR, Breen E, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: Implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosomatic Medicine. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, Irwin MR, Eisenberger NI. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain, Behavior and Immunity. 2015;43:46–53. doi: 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. Journal of Affective Disorders. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behavior and Immunity. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AHHCM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Padmos RC, Hillegers MHJ, Knijff EM, Vonk R, Bouvy A, Staal FJ, de Ridder D, Kupka RW, Nolen WA, Drexhage HA. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Archives of General Psychiatry. 2008;65:395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- Pine DS, Leibenluft E. Biomarkers with a mechanistic focus. JAMA Psychiatry. 2015;72:633–634. doi: 10.1001/jamapsychiatry.2015.0498. [DOI] [PubMed] [Google Scholar]
- Post RM, Leverich GS, Kupka RW, Keck PEJ, McElroy SL, Altshuler LL, Frye MA, Luckenbaugh DA, Rowe M, Grunze H, Suppes T, Nolen WA. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. Journal of Clinical Psychiatry. 2010;71:864–872. doi: 10.4088/JCP.08m04994yel. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. Children’s Depression Rating Scale, Revised (CDRS-R) Manual. Western Psychological Services; Los Angeles, CA: 1995. [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological Bulletin. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotaki M, Salpeas V, Malitas P, Alevizos V, Moutsatsou P. Altered glucocorticoid receptor signaling cascade in lymphocytes of bipolar disorder patients. Psychoneuroendocrinology. 2006;31(6):748–760. doi: 10.1016/j.psyneuen.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Van Meter AR, Moreira AL, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. Journal of Clinical Psychiatry. 2011;72:1250–1256. doi: 10.4088/JCP.10m06290. [DOI] [PubMed] [Google Scholar]
- Wieck A, Grassi-Oliveira R, do Prado CH, Rizzo LB, Schommer de Oliveira A, Kommers-Molina J, Viola TW, Teixera AL, Bauer ME. Differential neuroendocrine and immune responses to acute psychosocial stress in women with type 1 bipolar disorder. Brain, Behavior and Immunity. 2013;34:47–55. doi: 10.1016/j.bbi.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity, and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.