Abstract
BACKGROUND
Cerebral palsy is the most common cause of motor dysfunction in children worldwide and is often accompanied by multiple comorbidities. Although cerebral palsy has been studied extensively in high-resource settings, there are few published studies on cerebral palsy etiology, outcomes and comorbidities in low-resource settings.
METHODS
Children with cerebral palsy were prospectively enrolled from inpatient and outpatient settings at a referral center in Gaborone, Botswana, in a cross-sectional study conducted from 2013 to 2014. Cerebral palsy etiology, outcomes, and comorbidities were determined through caregiver interviews, review of medical records, and direct physical examination.
RESULTS
Sixty-eight children with cerebral palsy were enrolled. Subjects were 41% male, with a median age of 4 years (interquartile range = 2 to 7). The most common etiologies for cerebral palsy in our cohort were intrapartum hypoxic events (18%), postnatal infections (15%), prematurity (15%), focal ischemic strokes (10%), and prenatal infections (10%). Severe motor impairment was common, with the most severe category present in 41%. The predominant comorbidities were cognitive impairment (84%), epilepsy (77%), and visual impairment (46%).
CONCLUSIONS
Cerebral palsy in Botswana has different etiologies and is associated with poorer outcomes and higher prevalence of comorbidities than what has been reported in high-resource settings. Further studies are necessary to determine optimal preventative and treatment strategies in this population.
Keywords: International Child Health, neurologic disorders, cerebral palsy, Africa
Introduction
Cerebral palsy is the most common cause of motor impairment in children worldwide1–3 and is associated with significant morbidity and mortality.4–6 A variety of prenatal, perinatal, and postnatal insults can lead to the development of cerebral palsy, including hypoxic events, congenital brain malformations, and infections.3,7,8 Cerebral palsy is associated with a variety of comorbidities such as visual impairment, epilepsy, and cognitive impairment.9 In many children with cerebral palsy, associated comorbidities are the major drivers of outcome and quality of life.10
Cerebral palsy etiology and outcomes have been well characterized in high-resource settings, which has contributed to risk-reduction interventions and facilitated appropriate allocation of resources. However, there have been few high-quality studies conducted in low-resource settings. A recent systematic review of the literature on cerebral palsy in Africa11 noted few African studies of cerebral palsy etiology and no high-quality studies on outcomes and comorbidities. The few studies that have been performed in Sub-Saharan Africa have identified birth asphyxia and neonatal infections as the most common etiologies, suggesting that cerebral palsy etiology may be significantly different in Africa compared with the United States and Europe. A recent conference on cerebral palsy conducted jointly by the International Child Neurology Association and the African Child Neurology Association identified research on cerebral palsy etiology and outcomes as a key research priority to improve care for children in the region.11
The objective of this study was to systematically evaluate cerebral palsy etiology, outcomes, and comorbidities in a prospective cross-sectional study of children with cerebral palsy recruited from a referral center in Gaborone, Botswana. We hypothesized that intrapartum hypoxic events would be a major contributor to cerebral palsy in Botswana and that children with cerebral palsy in Botswana would have poorer outcomes and a higher frequency of comorbidities than historical controls in high-resource settings. We also sought to determine if outcomes and comorbidities would vary by etiology or by severity of illness.
Methods
Study design and setting
We conducted a prospective cross-sectional study of all children with cerebral palsy at Princess Marina Hospital. Princess Marina Hospital, the largest hospital in the country, is a 525-bed tertiary referral center located in Gaborone, Botswana’s capital city. Enrollment in the study was conducted from June–September in 2013 to June–September 2014. This sampling strategy was used to maximize the number of subjects enrolled. We attempted to capture all subjects with cerebral palsy coming into contact with the health care system during the period of data collection. Patients were recruited from all settings at which children with cerebral palsy receive care at Princess Marina Hospital including general pediatric clinic, pediatric neurology clinic, physical therapy, occupational therapy, and inpatient wards. Ethical approval was obtained from institutional review boards at University of Botswana, Botswana Ministry of Health, and Princess Marina Hospital.
Inclusion criteria
Subjects met all of the following inclusion criteria: (1) children ages two to 18 years with a clinical diagnosis of cerebral palsy (confirmed by study author D.R.B., a board-certified pediatric neurologist), (2) motor weakness defined as a score of less than or equal to four of five on the Medical Research Council Scale for Muscle Strength in at least one limb associated with activity limitation, (3) onset of signs before the age of 12 months, and (4) presumed central origin of weakness based on neurological examination.
Exclusion criteria
Presence of one or more of the following: (1) human immunodeficiency virus (HIV) infection, (2) obstructive hydrocephalus, (3) history of malignancy, (4) evidence of developmental regression, (5) diagnosis of a known genetic syndrome, (6) primary neuromuscular disorder, or (7) major extracerebral birth defects. HIV status was determined by review of records. All children enrolled in the study had documented negative HIV tests after the age of six months.
Recruitment and case ascertainment
Potential recruitment sites were surveyed daily by study personnel for potential subjects. All potential subjects were approached and screened by one of the study authors and invited to participate in the study. Parents or relevant caregivers provided verbal and written informed consent in either Setswana or English as preferred by the participant and when possible subjects were asked for verbal assent. A diagnosis of cerebral palsy was confirmed through caregiver interviews, evaluation of medical records, examination of the child, and review of all relevant studies and imaging (by study author D.R.B.). Activity limitation was determined through caretaker interview.
Data sources and variable definitions
Data were obtained through caregiver interviews, chart review of inpatient and outpatient records, standardized physical examinations, cognitive testing, and review of imaging. Interviews were conducted in English or Setswana based on caregiver preference. Interviews in Setswana were conducted by a study team member fluent in Setswana or with the assistance of an interpreter fluent in Setswana. All data were collected on paper case report forms and verified (by study author D.R.B.) before entry into a password protected anonymized database.
Variables and data sources are reported in Tables 1 and 2. Cerebral palsy was defined according to the consensus definition by Bax et al.1 operationalized through the inclusion/exclusion criteria above. Years of maternal education included primary and secondary schooling as well as years of postsecondary education. Outcomes determined included Gross Motor Function Classification System (GMFCS) score,12 Pediatric Cerebral Performance Category score,13 and Pediatric Overall Performance Category score.13 Scores for each outcome were determined according to published criteria and were assigned by study author D.R.B. after review of all available information. GMFCS score was additionally classified as either “ambulatory” (GMFCS I-III) or “nonambulatory” (GMFCS IV-V).
TABLE 1.
Demographics of Study Cohort
| Subject Characteristics | Total n = 68 |
|---|---|
| Age in years, median (IQR) | 4 (2–6.5) |
| Male sex | 28 (41%) |
| Birth weight in kg, median (IQR) | 3.0 (2.6–3.2) |
| Living situation | |
| Large city | 48 (71%) |
| Small city/town | 12 (18%) |
| Small village/rural area | 8 (12%) |
| Electricity in home | 48 (71%) |
| Running water in home | 49 (72%) |
| Maternal characteristics | |
| Maternal age at delivery, median (IQR) | 29 (24–32) |
| Maternal HIV infection | 17 (25%) |
| Maternal years of education, median (IQR) | 12 (10–14) |
| Received prenatal care during pregnancy | 61 (90%) |
| Characteristics of the delivery | |
| Delivered in hospital | 65 (96%) |
| Preterm delivery (<34 wk) | 10 (15%) |
| Multiple gestation | 2 (3%) |
| Complications during delivery | 31 (46%) |
| Caesarean section | 18 (27%) |
| Admitted to NICU | 44 (65%) |
| Days in NICU, median (IQR) | 7 (0–14) |
Abbreviations:
HIV = human immunodeficiency virus
IQR = interquartile range
NICU = neonatal intensive care unit
Values are n (%), or median (IQR) where noted.
TABLE 2.
Variables and Data Sources
| Data Source* | Variable |
|---|---|
| Caregiver interview | Age, sex, location of residence, years of maternal education, complications during delivery, history of intrapartum hypoxic event, history of epilepsy, developmental history, history of orthopedic complications. |
| Chart review | Age, gestational age at birth, birth weight, Apgar scores, complications during delivery, history of intrapartum hypoxic event, nutritional status, history of neonatal infection, history of hyperbilirubinemia, history of epilepsy, developmental history, history of orthopedic complications. |
| Physical examination | Cerebral palsy type, hearing impairment, vision impairment, contractures, other orthopedic complications. |
| Investigator determination | Gross Motor Function Classification System score, cerebral performance category, overall performance category, etiology. |
Some variables were obtained from multiple data sources. When there was a discrepancy between data sources, this was resolved by determination of the investigators.
The World Health Organization Ten Question Screen was used to screen for additional comorbidities; children testing positive on screening then had these comorbidities confirmed by standardized evaluations. Computed tomography and magnetic resonance imaging reports and images were obtained and reviewed by study author D.R.B. Ascertained comorbidities included epilepsy, cognitive impairment, contractures, orthopedic problems (including scoliosis, club foot, and pathologic fractures), hearing impairment, and visual impairment. Hearing impairment was assessed using three-level voice test (for children able to participate) or audiometry. Hearing impairment was considered to be present when a child failed to respond to mid-level spoken voice or had a 70 dB or greater hearing loss on audiometry in either ear. Visual impairment was assessed by physical examination and was considered to be present when vision was 20/100 or worse in both eyes in children able to participate in vision testing. In children too young or cognitively impaired to formally test vision, visual impairment was considered to be present in children with response to other stimuli but no response to light or threat in at least one eye. Cognitive impairment was determined through caregiver interview regarding cognitive and language milestones. A standardized developmental evaluation was completed for all children. For children ages six years and older, cognitive testing was performed using the International Cognitive Assessment, a short cognitive test developed for use in low-resource settings and concurrently validated during this study. Cognitive impairment was considered to be present when children performed below a cutoff on developmental assessment or cognitive testing representing two standard deviations below the mean for age.
Determination of etiology
We used a hierarchical mutually exclusive categorization system to determine etiology based on the World Health Organization verbal autopsy method.14 Definitions were first tested for applicability and appropriate performance characteristics during a pilot phase of the study. Because we were interested in examining outcomes and comorbidities by etiology, each case was categorized as secondary only to one etiology. Where there was evidence of multiple possible etiologies, etiology was categorized based on the earliest timing of injury (e.g., if there was evidence of both congenital malformation and neonatal infection, the etiology was assigned as congenital malformation). The specific etiology for each case was determined through caregiver interview, analysis of relevant imaging, laboratory results, and review of the obstetric history and the child’s peripartum and postpartum history.
Etiologies were categorized into the following hierarchy based on presumed timing of insult from earliest to latest: (1) congenital brain malformations,15 (2) prenatal (toxoplasmosis other rubella cytomegalovirus herpes—type) infections,14,16,17 (3) prematurity,14,16,17 (4) focal ischemic stroke,15 (5) intrapartum hypoxic event,18 (6) postnatal infections,14,16,17 (7) kernicterus,19 (8) head trauma,14,16,17 and (9) unknown. Criteria for each etiology are described in Table 3.
TABLE 3.
Criteria Used to Determine the Etiology of Cerebral Palsy
| Etiology | Criteria/Definition |
|---|---|
| Congenital brain malformation | Brain imaging (CT or MRI) consistent with congenital brain malformation. |
| Prenatal infection | (1) Characteristic neuroimaging (e.g., calcifications in the basal ganglia) and (2) elevated TORCH titers or positive PCR for cytomegalovirus. |
| Prematurity | Gestational age less than or equal to 34 wk and characteristic neuroimaging. |
| Focal ischemic stroke | Brain imaging (CT or MRI) consistent with focal ischemic stroke. |
| Intrapartum hypoxic event | 5-min Apgar score less than or equal to five and clinical history of birth asphyxia in the patient’s medical record or reported by the patient’s caregiver and clinical history of birth complications either recorded in the patient’s chart or described by the caregiver and imaging consistent with global hypoxic event. |
| Postnatal infection | Clinical history of meningitis or encephalitis and one or more of the following: (1) documentation of positive bacterial culture from cerebrospinal fluid, (2) documentation of herpes simplex virus from serum or cerebrospinal fluid, or (3) CSF pleocytosis with >10 WBCs/HPF. |
| Kernicterus | Total serum bilirubin documented during neonatal period >20 mg/dL (342 μmol/L) and either (1) movement disorder involving choreoathetosis or dystonia or (2) imaging consistent with bilirubin deposition in the globus pallidus or subthalamic nuclei. |
| Head trauma | Clinical history of head trauma described by the caregiver or recorded in the medical record and brain imaging consistent with traumatic brain injury. |
| Other | Other specified etiology determined by the investigator and not meeting any of the criteria specified above. |
| Unknown | Cerebral palsy not fitting criteria for any of the above definitions and not better explained by an alternative etiology. |
Abbreviations:
CSF = cerebrospinal fluid
CT = computed tomography
MRI = magnetic resonance imaging
PCR = polymerase chain reaction
TORCH = toxoplasmosis other rubella cytomegalovirus herpes
WBCs/HPF = white blood cells per high-powered field
Statistical analysis
Statistical analyses were performed using Stata 12.1 (StataCorp LP, College Station, TX). Standard descriptive statistics are reported including medians and interquartile ranges for non-normally distributed continuous variables and percentages for categorical variables. Comparisons between categorical variables were performed using a chi-square test. Comparisons between continuous variables were performed using the Kruskal—Wallis test. Correlations between ranked outcome variables were performed using Spearman correlation coefficient. Tests for trend across ordered groups were performed using the nptrend test in Stata, a nonparametric test which is an extension of the Wilcoxon rank sum test. Significance level was set at P = 0.05. Missing data were handled using pairwise deletion. The sample size of 68 subjects was calculated based on simulations of possible numbers of patients within groups and had 80% power to detect a 20% difference between dichotomously characterized groups assuming variables of interest occurred in at least 30% of subjects.
Results
Demographics
A total of 68 subjects with cerebral palsy were enrolled in the study. All subjects who were approached agreed to participate in the study. All children had previously undergone imaging with computed tomography or magnetic resonance imaging. Fifty-four subjects (79%) were recruited from outpatient settings, whereas 14 subjects (21%) were recruited from inpatient wards. Patients recruited from inpatient wards were admitted for seizures (six subjects), pneumonia (three subjects), gastroenteritis (two subjects), diagnostic evaluation of cerebral palsy (two subjects), and motor vehicle accident (one subject.) All subjects were of African ethnicity. Subjects were 41% male with a median age of 4 years (interquartile range = 2 to 6.5). Most subjects (48 subjects, 71% of total) were living in Gaborone; 12 (18%) were categorized as living in a small city or town in the surrounding area, and eight (12%) were categorized as living in a small village or rural area. Additional demographic data and descriptive statistics are presented in Table 1.
Etiology
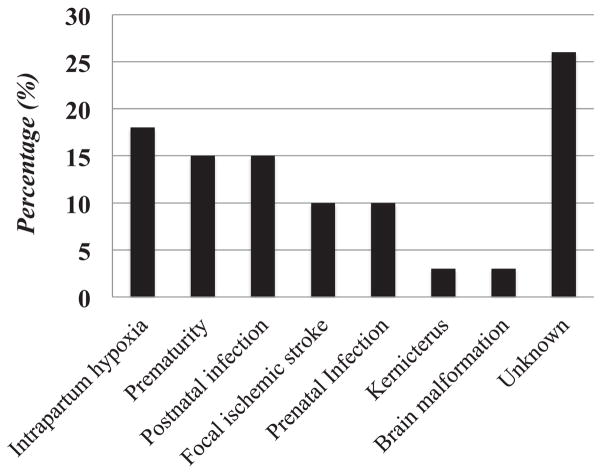
The most common etiologies are reported in Figure 1 and included intrapartum hypoxic events (12 subjects, 18%), prematurity (10 subjects, 15%), and postnatal infections (10 subjects, 15%), followed by focal ischemic stroke (seven subjects, 10%) and prenatal infections (seven subjects, 10%). Two subjects (3%) had cerebral palsy secondary to kernicterus, and two subjects (3%) had congenital brain malformations. We did not identify any subjects with cerebral palsy secondary to head trauma. The etiology in 18 subjects (26%) could not be determined. Among the ten subjects with cerebral palsy secondary to postnatal infection, seven had meningitis in the first month of life and three developed meningitis between the age of one and six months.
FIGURE 1.
Etiology of cerebral palsy in Botswana.
Cerebral palsy type
Spastic cerebral palsy was the most common type (56 subjects, 82%), including 31 subjects (46% of total) with spastic quadriplegia, 16 subjects (24%) with spastic hemiplegia, three subjects (4%) with spastic diplegia, and six subjects (9%) with mixed spastic/dyskinetic cerebral palsy. Among the remaining 12 subjects with nonspastic cerebral palsy, five subjects (7%) had hypotonic cerebral palsy, two subjects (6%) had dyskinetic cerebral palsy, two subjects (3%) had ataxic cerebral palsy, and three subjects (3%) had other cerebral palsy.
Outcomes
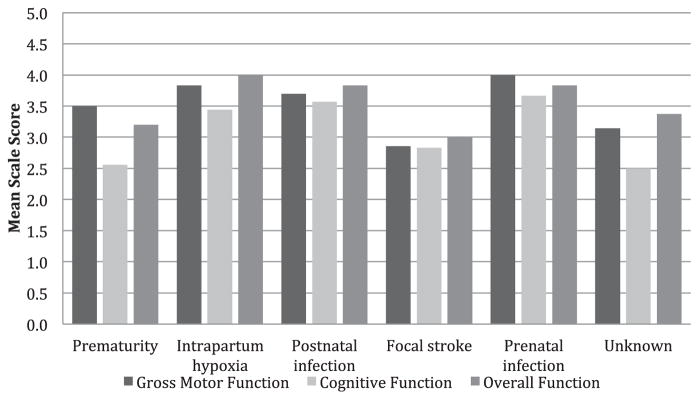
Severe motor impairment was common, with the most severe category (GMFCS V) being present in 28 subjects (41%). Another 11 subjects (16%) were categorized as GMFCS IV. The total number of nonambulatory subjects (GMFCS IV and V) was 39 (58%). Cerebral performance score was highly correlated with GMFCS score (Spearman correlation coefficient = 0.59, P < 0.001) as was overall performance score (Spearman correlation coefficient = 0.83, P < 0.001, Figure 2). Outcomes on GMFCS, cerebral performance score, and overall performance score did not vary by etiology (Kruskal—Wallis test for GMFCS, H = 6.6, 7 d.f., P = 0.47, other outcomes all with P > 0.5), but small numbers of subjects in each group limited power for this analysis. Outcomes also did not vary by inpatient versus outpatient status (P = 0.7).
FIGURE 2.
Cerebral Palsy Outcomes, by Etiology. There were no significant differences in outcomes between etiologies. Gross Motor Function was rated using the Gross Motor Function Classification System, a scale from 1 (least severe) to 5 (most severe). Cognitive Function was rated using the Pediatric Cerebral Performance Category Scale, a scale from 1 (normal) to 6 (brain death). Overall function was rated using the Pediatric Overall Performance Scale, a scale from 1 (normal) to 6 (brain death).
Comorbidities
The most common comorbidities of cerebral palsy in our study are described in Table 4 and were cognitive impairment in 56 subjects (82%, 95% confidence interval [CI] = 73%–91%), epilepsy in 52 subjects (76%, CI = 62%–88%), and visual impairment in 31 subjects (46%, CI = 33%–58%). In addition, 29 subjects had malnutrition (43%), 11 subjects (16%) had hearing impairment, 18 (26%) had contractures, and 28% had other orthopedic complications. Cognitive impairment, malnutrition, and visual impairment were all significantly more common in subjects with higher GMFCS scores (Table 4). Comorbidities did not vary by etiology or by inpatient status.
TABLE 4.
Proportion of Subjects With Each Comorbidity by GMFCS Score
| GMFCS Score | Cognitive Impairment | Epilepsy | Visual Impairment | Malnutrition | Contractures | Hearing Impairment |
|---|---|---|---|---|---|---|
| 1 (n = 6) | 83% | 83% | 0% | 0% | 17% | 0% |
| 2 (n = 14) | 57% | 60% | 20% | 25% | 14% | 15% |
| 3 (n = 8) | 71% | 86% | 20% | 0% | 40% | 0% |
| 4 (n = 11) | 100% | 63% | 33% | 22% | 36% | 10% |
| 5 (n = 28) | 100% | 92% | 59% | 73% | 36% | 26% |
| P value* | 0.02 | 0.12 | 0.02 | <0.001 | 0.07 | 0.80 |
Abbreviation:
GMFCS = Gross Motor Function Classification System.
Using test for trend across group defined by GMFCS score. P values <0.05 are in bold and indicate a significant increase in the comorbidity with increasing GMFCS score.
Discussion
Key results
Our study aimed to determine the etiology, clinical outcomes, and comorbidities of children with cerebral palsy in Botswana. We identified 68 children with a diagnosis of cerebral palsy at Princess Marina Hospital during our recruitment period in 2013 and 2014. The most common causes of cerebral palsy in our study included hypoxic events, with 28% of cases due to either global hypoxic events or focal ischemic strokes, with prenatal or postnatal infections accounting for another 25% of cases. In addition, we found that severe cerebral palsy (GMFCS 4 or 5) was common, accounting for 57% of all children with cerebral palsy. The most common comorbidities were cognitive impairment, epilepsy, visual impairment, and malnutrition. Comorbidities were more common in children with more severe cerebral palsy but did not vary by etiology.
Interpretation
The relative contribution of intrapartum hypoxic events to cerebral palsy is much higher in our study than what has been reported from high-resource settings but similar to what has been reported in other African studies. For example, data from the South Australia Cerebral Palsy Registry published in 2006 found that only two of 46 term neonates with cerebral palsy had a history of an acute intrapartum hypoxic event occurring during labor and delivery.20 Several recent review articles from high-resource settings have concluded that intrapartum hypoxic events are uncommon causes of cerebral palsy, accounting for at most 8% to 10% of all cases.21 In contrast, most studies of cerebral palsy etiologies in Africa have also found relatively high percentages of birth asphyxia as the primary etiology of cerebral palsy, accounting for 35% to 58% of cases of cerebral palsy.22–25 These studies had relatively loose definitions of birth asphyxia, preventing a direct comparison to our study. Future studies of cerebral palsy in Africa would benefit from a multicenter approach using standardized definitions to better allow comparisons between centers.
We found that 15% of cerebral palsy cases were attributable to prematurity. The percentage of cases attributable to premature delivery is low compared with epidemiologic data obtained in developed countries, where it has been reported that 78% of cases of cerebral palsy occur due to preterm birth.20 The relatively low percentage of cerebral palsy cases attributable to preterm birth is at least partially explained by poor survival rates of early preterm and very low birth weight infants. Among neonates born with a birth weight between 500 g and 900 g in Botswana during the period of this study, the mortality rate was 80% or higher (unpublished data).
The proportion of patients with a confirmed etiology of postnatal infection (15%) is also high compared with the proportions reported in developed countries. In a retrospective Australian study of cerebral palsy cases born between 1970 and 1999, only 2.4% (75 of 3162) were caused by postnatal infection.26 However, our results are similar to what has been reported from other African studies.23 Given that many postnatal infections are potentially preventable, this is a target for future interventions.
The proportion of children distributed among each GMFCS category in Botswana is remarkably different from the distribution observed in most studies from high-resource settings. The most frequently assigned GMFCS category in the United States is category I, the mildest category of disability in children with cerebral palsy where the affected individuals are able to perform all age-appropriate motor functions. In a retrospective record review of 8-year-old children with cerebral palsy in the United States, it was found that 38% were assigned GMFCS category I, with only 17% assigned the most severe category, GMFCS V.27 In contrast, the most frequently assigned GMFCS category in Botswana was category V (41%). This stark inversion of the motor and cerebral outcomes of cerebral palsy in Botswana vs the United States can be potentially explained by a number of factors, including different etiologies, differences in management of infants in the intensive care unit, and differences in rehabilitative therapies.28 Lack of access to therapy services, orthotics, and assistive technology are all potential contributing factors to poor outcomes that should be targeted.
Limitation, bias, and generalizability
Because our sampling strategy attempted to capture all children with cerebral palsy coming into contact with the health care system, our sample is likely to be representative of children with cerebral palsy receiving health care services in Botswana. Our study demographics are similar to what has been reported in prior studies of children in Gaborone.29 However, compared with the overall population in Botswana, our study population was more urban, had higher levels of education, and had higher levels of literacy.
Because this study recruited children from inpatient and outpatient sites at a tertiary care center, it is likely that there is some selection bias. More severely affected children may be over-represented because children with milder cerebral palsy may not be brought in for care to a tertiary care center. We attempted to examine this by looking at rates of outcomes and comorbidities by inpatient status and found no difference between these groups. Thus, need for hospitalization is unlikely to have introduced bias into the study. Nevertheless, future studies of cerebral palsy in Africa should also perform community-based sampling to capture a more representative sample. Generalizability of our study to other African settings is likely to be moderate because labor and delivery practices and postnatal care in hospitals in Botswana are similar to practices in other neighboring countries.11 However, Botswana is a middle-income country with universal health care, likely resulting in relatively better maternal and child health than are found in most countries in sub-Saharan Africa. Caregivers in our study were highly educated, and most mothers in our study had prenatal care during pregnancy and gave birth in a hospital setting. Also notable was that our population was very urban. Future studies should also include rural sites to identify differences in prevalence and severity between urban and rural centers.
Conclusion
This study confirms the importance of potentially preventable etiologies as major contributors to cerebral palsy in Botswana. In the future, multicenter international studies with recruitment from both urban and rural areas using standardized definitions are necessary to make meaningful comparisons between countries and to provide additional insight into optimal treatment and preventative strategies for cerebral palsy. Low technology community-based early intervention programs need to be developed and scaled up for use in Botswana and similar African settings specifically targeting the most common comorbidities. The large etiologic contribution of both intrapartum hypoxic events and postnatal infections to cerebral palsy suggests interventions in birth practices and neonatal care could make a significant impact in decreasing rates of cerebral palsy in Botswana.
Acknowledgments
Funding Source: This study was supported by institutional funding from the University of Botswana and Children’s Hospital of Philadelphia, and was made possible through core services and support from the Penn Center for AIDS Research (CFAR), an NIH-funded program (P30 AI 045008).
Footnotes
Contributors Statement: D.R.B. conceptualized and designed the study, collected data, performed data analysis, drafted and revised the article, and approved the final article as submitted. B.M. conceptualized and designed the study, collected data, assisted in drafting and revising the article, and approved the final article as submitted. A.P.S. and L.M. assisted in conceptualizing and designing the study, advised on the process of data collection, assisted in drafting and revising the article, and approved the final article as submitted. E.K., J.B., and E.B. assisted in conceptualizing and designing the study, collected data, assisted with data analysis, assisted in drafting and revising the article, and approved the final article as submitted. K.W. assisted with data analysis, assisted in drafting and revising the article, and approved the final article as submitted.
Financial Disclosure: All authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: Dr. D.R.B. has served as an expert witness in legal cases involving cerebral palsy. The other authors have no conflicts of interest to disclose.
Clinical Trial Registration: Not applicable.
References
- 1.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–576. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 2.Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil. 2006;28:183–191. doi: 10.1080/09638280500158422. [DOI] [PubMed] [Google Scholar]
- 3.Pakula AT, Van Naarden Braun K, Yeargin-Allsopp M. Cerebral palsy: classification and epidemiology. Phys Med Rehabil Clin N Am. 2009;20:425–452. doi: 10.1016/j.pmr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Chen KL, Tseng MH, Shieh JY, Lu L, Huang CY. Determinants of quality of life in children with cerebral palsy: a comprehensive biopsychosocial approach. Res Dev Disabil. 2014;35:520–528. doi: 10.1016/j.ridd.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Maher CA, Olds T, Williams MT, Lane AE. Self-reported quality of life in adolescents with cerebral palsy. Phys Occup Ther Pediatr. 2008;28:41–57. doi: 10.1300/j006v28n01_04. [DOI] [PubMed] [Google Scholar]
- 6.Raina P, O’Donnell M, Rosenbaum P, et al. The health and well-being of caregivers of children with cerebral palsy. Pediatrics. 2005;115:e626–e636. doi: 10.1542/peds.2004-1689. [DOI] [PubMed] [Google Scholar]
- 7.Torfs CP, van den Berg B, Oechsli FW, Cummins S. Prenatal and perinatal factors in the etiology of cerebral palsy. J Pediatr. 1990;116:615–619. doi: 10.1016/s0022-3476(05)81615-4. [DOI] [PubMed] [Google Scholar]
- 8.Meberg A, Broch H. Etiology of cerebral palsy. J Perinat Med. 2004;32:434–439. doi: 10.1515/JPM.2004.143. [DOI] [PubMed] [Google Scholar]
- 9.Colver A, Fairhurst C, Pharoah PO. Cerebral palsy. Lancet. 2014;383:1240–1249. doi: 10.1016/S0140-6736(13)61835-8. [DOI] [PubMed] [Google Scholar]
- 10.Shevell MI, Dagenais L, Hall N REPACQ Consortium. Comorbidities in cerebral palsy and their relationship to neurologic subtype and GMFCS level. Neurology. 2009;72:2090–2096. doi: 10.1212/WNL.0b013e3181aa537b. [DOI] [PubMed] [Google Scholar]
- 11.Donald KA, Samia P, Kakooza-Mwesige A, Bearden D. Pediatric cerebral palsy in Africa: a systematic review. Semin Pediatr Neurol. 2014;21:30–35. doi: 10.1016/j.spen.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 13.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 14.Verbal autopsy standards: ascertaining and attributing causes of death. Geneva: World Health Organization; [Accessed November 2, 2015]. Available at, http://www.who.int/healthinfo/statistics/verbalautopsystandards/en/on11/2/15. [Google Scholar]
- 15.Ashwal S, Russman BS, Blasco PA, et al. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2004;62:851–863. doi: 10.1212/01.wnl.0000117981.35364.1b. [DOI] [PubMed] [Google Scholar]
- 16.Anker M, Black R, Coldham C, Kalter H, Quigley M, Ross D. A standard verbal autopsy method for investigating causes of death in infants and children. Geneva: World Health Organization; 1999. WHO/CDS/CSR/ISR/99.4. [Google Scholar]
- 17.Bang AT, Bang RA. Diagnosis of causes of childhood deaths in developing countries by verbal autopsy: suggested criteria. The SEARCH team. Bull World Health Organ. 1992;70:499–507. [PMC free article] [PubMed] [Google Scholar]
- 18.Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901. doi: 10.1097/01.AOG.0000445580.65983.d2. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro SM. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND) J Perinatol. 2005;25:54–59. doi: 10.1038/sj.jp.7211157. [DOI] [PubMed] [Google Scholar]
- 20.Strijbis EM, Oudman I, van Essen P, MacLennan AH. Cerebral palsy and the application of the international criteria for acute intrapartum hypoxia. Obstet Gynecol. 2006;107:1357–1365. doi: 10.1097/01.AOG.0000220544.21316.80. [DOI] [PubMed] [Google Scholar]
- 21.McIntyre S, Taitz D, Keogh J, Goldsmith S, Badawi N, Blair E. A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol. 2013;55:499–508. doi: 10.1111/dmcn.12017. [DOI] [PubMed] [Google Scholar]
- 22.El Tallawy HN, Farghaly WM, Rageh TA, et al. Epidemiology of major neurological disorders project in Al Kharga district, New Valley, Egypt. Neuroepidemiology. 2010;35:291–297. doi: 10.1159/000320240. [DOI] [PubMed] [Google Scholar]
- 23.Ogunlesi T, Ogundeyi M, Ogunfowora O, Olowu A. Socio-clinical issues in cerebral palsy in Sagamu, Nigeria. S Afr J Child Health. 2008;3:120–124. [Google Scholar]
- 24.Karumuna JM, Mgone CS. Cerebral palsy in Dar Es Salaam. Cent Afr J Med. 1990;36:8–10. [PubMed] [Google Scholar]
- 25.Belonwu RO, Gwarzo GD, Adeleke SI. Cerebral palsy in Kano, Nigeria—a review. Niger J Med. 2009;18:186–189. doi: 10.4314/njm.v18i2.45062. [DOI] [PubMed] [Google Scholar]
- 26.Reid SM, Lanigan A, Reddihough DS. Post-neonatally acquired cerebral palsy in Victoria, Australia, 1970–1999. J Paediatr Child Health. 2006;42:606–611. doi: 10.1111/j.1440-1754.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 27.Kirby RS, Wingate MS, Van Naarden Braun K, et al. Prevalence and functioning of children with cerebral palsy in four areas of the United States in 2006: a report from the Autism and Developmental Disabilities Monitoring Network. Res Dev Disabil. 2011;32:462–469. doi: 10.1016/j.ridd.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 28.Donald KA, Kakooza AM, Robinson DW, et al. Pediatric cerebral palsy in Africa: where are we? J Child Neurol. 2015;30:963–971. doi: 10.1177/0883073814549245. [DOI] [PubMed] [Google Scholar]
- 29.Bearden D, Steenhoff AP, Dlugos DJ, et al. Early antiretroviral therapy is protective against epilepsy in children with human immunodeficiency virus infection in Botswana. J Acquir Immune Defic Syndr. 2015;69:193–199. doi: 10.1097/QAI.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]