Abstract
Objective
Elevated rates of epilepsy and motor impairments including cerebral palsy are seen in children who were born prematurely. Maternal antenatal magnesium supplementation has been associated with decreased rates of cerebral palsy in infants born prematurely. Our objective was to determine whether the neonatal serum magnesium level between 24 and 48 hours following birth is associated with better long-term neurodevelopmental outcomes (epilepsy, motor impairment) in premature infants.
Methods
We performed a retrospective cohort analysis in infants born <37 weeks gestation over a 10-year period. Prenatal, perinatal, and postnatal clinical and demographic information was collected. Crude and adjusted odds ratios were estimated under generalized linear models with generalized estimating equations to examine the association of the neonatal serum magnesium level between 24 and 48 hours following birth with the risk of epilepsy and/or motor impairment (spasticity; hypotonia; cerebral palsy).
Results
The final cohort included 5,461 infants born <37 weeks gestation from 2002–2011. The adjusted relative risk ratio (RR) for the combined outcomes of epilepsy and/or motor impairment, controlling for gestational age, current age, maternal magnesium supplementation, maternal steroid administration, five minute Apgar score, neonatal infection, need for vasopressor use, and birth weight; and with serum magnesium level as the main independent variable, was 0.85 (p 0.24). Stratified analyses by gestational age <32 weeks or >32 weeks were not significantly associated with adverse neurodevelopmental outcome (respectively RR 0.79 and 1.2, p 0.12 and 0.49). A multivariate analysis for the risk of motor impairment alone had a RR of 0.94 (p 0.72).
Conclusion
This study shows that the neonatal magnesium level between 24 and 48 hours of life in premature infants is not significantly associated with the risk for developing epilepsy or motor impairment.
Keywords: magnesium, prematurity, neurological, neuroprotection, neonate
INTRODUCTION
Premature birth is a significant public health problem affecting 11.4% of births each year in the U.S.(Osterman et al., 2015) Children who were born prematurely are at increased risk for developing a range of adverse neurodevelopment outcomes including cerebral palsy, epilepsy, autism spectrum disorder, intellectual disability and school difficulty, and behavioral and neuropsychiatric problems including attention-deficit hyperactivity disorder.(Bass et al., 2004; Saigal and Doyle, 2008; Van Lieshout et al., 2015; Vohr et al., 2000)
The pathophysiology leading to the neurodevelopmental problems of ex-premature infants and children are complex.(Ferriero, 2004; Inder and Volpe, 2000) There is increasing evidence that both developing neurons and oligodendrocytes are at risk in the premature brain,(Thomas et al., 2005; Volpe, 2009) with potential to cause gross structural damage or more subtle disruptions in measures of connectivity.(Deng et al., 2008; Li et al., 2015; Murray et al., 2015)
There are few proven therapeutic options for preventing the adverse neurodevelopmental outcomes of premature birth, although antenatal maternal steroid administration, antenatal magnesium sulfate, N-acetylcysteine, erythropoietin, melatonin, and stem cell transplants are under active investigation.(Chang, 2015; Leuchter et al., 2014; Sotiriadis et al., 2015) In particular, antenatal magnesium sulfate has been found to reduce rates of subsequent cerebral palsy in multiple studies and in meta-analyses though a large randomized trial did not find association between antenatal magnesium sulfate administration and rates of cerebral palsy or abnormal motor function.(Doyle et al., 2009; Rouse et al., 2008; Doyle et al., 2014; Chollat et al., 2014) Maternal antenatal magnesium sulfate was also found to decreased abnormal echogenicities on head ultrasounds in infants born at less than 32 weeks gestation. (Hirtz et al., 2015) Experimental evidence from tissue culture and animal models ranging from mouse to zebrafish support potential mechanisms of magnesium to exert neuroprotective effects.(Cetinkaya et al., 2011; Goni-de-Cerio et al., 2012; Stevenson et al., 2012; Suzuki-Kakisaka et al., 2013) However, concerns have been raised that magnesium sulfate can have adverse effects;(Basu et al., 2012; Mitani et al., 2011; Mittendorf et al., 2002) and therefore an improved understanding of dose and timing of administration and levels are important.(McPherson et al., 2014; Sherwin et al., 2014; Shimada et al., 2013) Further, it is not clear if the neuroprotective effects are limited to prenatal or perinatal exposure; or whether postnatal levels and roles in the postnatal brain are important. If magnesium continued to play neuroprotective effects in premature infants after delivery, then serum levels of magnesium in these infants would potentially correlate with their neurodevelopmental outcomes. For example, a small pilot study from our group suggested that higher postnatal serum magnesium levels in premature infants was associated with decreased risks for motor impairment.(Doll et al., 2014)
Our objective was to test the hypothesis that the neonatal serum magnesium level following birth was associated with better long-term neurodevelopmental outcomes in premature infants. The outcomes of interest were for epilepsy, for motor impairment including cerebral palsy, spasticity, or hypotonia; or for a composite including both epilepsy and motor impairment. To test our hypothesis we performed a retrospective analysis in which we evaluated serum magnesium levels in premature infants between 24 and 48 hours after birth, and related the levels to subsequent diagnoses of epilepsy and/or motor impairment in a cohort of 5461 infants.
METHODS
This study was approved by Institutional Review Boards of the University of Utah and Intermountain Healthcare (IH). Data was anonymously collected and analyzed with no identifying information, and a waiver of informed consent was obtained. IH is a vertically integrated not-for-profit health care system in the Intermountain West encompassing 23 hospitals including the sole children’s hospital. Antenatal, perinatal, and follow-up data were obtained from the Enterprise Data Warehouse (EDW) maintained by IH.
Data extraction and analysis were performed retrospectively in premature infants born at an IH hospital between January 1, 2002 and December 31, 2011. Follow-up for all outcomes was through December 31, 2014. Inclusion criteria were a gestational age less than 37 weeks and linkage to mother’s records. Exclusion criteria were infants with known or likely genetic conditions or chromosomal abnormalities; infants with bacterial or viral meningitis during their initial post-birth hospitalization; infants with congenital hydrocephalus; infants with epileptic encephalopathies; infants with a chromosomal abnormality or genetic syndrome; infants with a congenital brain malformation; infants with congenital heart disease; and infants who developed meningitis, encephalitis, stroke, or traumatic head injury.
Using unique identifiers assigned to each of the cohort infants, we queried the EDW for the study and follow-up periods, using ICD-9-CM codes to identify outcomes. Data collected from the EDW included name; date of birth; gender; ethnicity; birth weight; gestational age; presence of multiple gestation; administration of corticosteroids prior to delivery; administration of magnesium sulfate prior to delivery; length of hospitalization; neonatal total serum magnesium levels drawn between ages 24 and 48 hours of life; days requiring mechanical ventilation; and the presence of seizures.
A total of 6874 potential patients were identified. We excluded subjects that did not have a serum magnesium level obtained between 24–48 hours post birth (n=1370) and subjects missing gestational age or birth weight (n=43); leaving 5461 infants for the final cohort (Figure 1).
Figure 1.
Patient enrollment. Potential patients were identified from a computer-based ICD-9-CM code search of the IH EDW for the study time period.
The main exposure variable was defined as the serum magnesium level (mg/dL) obtained between 24–48 hours post birth. In subjects where multiple serum magnesium levels were drawn in this timeframe, only the first observation was used. Covariates included gestational age at delivery, current age (years), maternal magnesium supplementation, maternal steroids, five-minute Apgar score, neonatal infection, need for vasopressors, and birth weight. Maternal magnesium supplementation was included as a covariate because we wished to evaluate the relationship between the outcomes and variation in neonatal magnesium levels that was not accounted for by maternal supplementation.
The two primary outcome variables were: 1) motor impairment, defined as the presence of at least one of the following: use of a wheelchair, baclofen prescription, or botox injection, (all identified using charge codes); dorsal rhizotomy (03.1); heel cord release (83.11; 83.85); hypotonia (342.x); or spasticity or cerebral palsy (333.71; 343.x); and 2) epilepsy, defined by ICD-9 codes 345.x. For the diagnosis of epilepsy, diagnoses of seizures at any time during the NICU hospitalization were excluded (779.0), as was a diagnosis of febrile seizures.
The relationships of the neonatal magnesium level with the outcomes were represented using discrete proportional hazards regression models to relate the age of occurrence (categorized into age intervals of 1 to 2 years) of the outcomes to the neonatal magnesium exposure variables; with adjustment for gestational age at delivery, maternal magnesium supplementation, maternal steroids, five-minute Apgar score, neonatal infection, need for vasopressors, and birth weight as covariates. Because the probability of diagnosis during any 1 to 2 year age interval was small, we fit the proportional hazard regression models by applying generalized estimating equations (GEE) with logarithmic link functions to a data set with separate binary indicator variables for a positive diagnosis defined for each 1–2 year age interval for each child. Robust empirical standard errors were computed under a working independence covariance model to account for correlated outcomes between siblings when computing p-values and confidence intervals. We assumed that the underlying disease condition (motor impairment or epilepsy) is defined as present or absent at birth, and that age of diagnosis is unrelated to neonatal magnesium and the covariates among those with the disease conditions, the relative risks from the GEE analyses can be interpreted as closely approximating the relative risks relating the underlying disease conditions to neonatal magnesium. Separate GEE analyses were performed with neonatal magnesium levels expressed as a continuous variable, in which case relative risks of the outcomes were expressed relative to 1 mg/dL increases in neonatal magnesium, and as tertiles, in which case the relative risks compared the upper two tertiles to the lowest tertile. Thus relative risks smaller than 1 suggest protective effects of higher serum levels of neonatal magnesium.
All analyses were carried out in SAS statistical software version 9.4 (SAS Institute Inc., Cary, North Carolina). We used descriptive univariate statistics to characterize demographics, risk factors, and potential confounders, as well as the prevalence of motor impairment and epilepsy. Serum magnesium was analyzed both as a continuous variable and after categorization into tertiles. Statistical models were performed for the full cohort, as well as also stratified separately by gestational age (>32 vs ≤32 weeks); as well as by the presence or absence of maternal magnesium sulfate supplementation.
RESULTS
We collected serum magnesium levels on a cohort of 5461 premature infants between 24–48 hours after birth (Figure 1 shows selection of the cohort). Serum magnesium levels within the first 24 hours were not used, as a sharp decrease in magnesium concentrations were observed within the first 24 hours post-birth, and thus reflected whether maternal magnesium supplementation had occurred. After 48 hours of life most infants did not have magnesium levels checked, and those infants that did have levels checked were sporadically timed with respect to postconceptual age as well as compared to other infants.
The cohort of premature infants was 57% male, and the majority were Caucasian (81%), reflecting Utah population demographics (Table 1). 26% of the cohort received antenatal magnesium, and 31% received steroids. The median gestational age was 33 weeks; and median birth weight was 1973g.
Table 1.
Demographic characteristics of the study group (n= 5,461 premature infants); and outcomes. IQR, interquartile range; S.D., standard deviation.
| Characteristic | n (%) |
|---|---|
| Gender (male) | 3097 (57%) |
| Ethnicity | |
| Caucasian | 4401 (81%) |
| Hispanic | 590 (11%) |
| Pacific-Islander | 48 (0.88%) |
| African-American | 32 (0.59%) |
| Native American | 18 (0.33%) |
| Asian | 67 (1.2%) |
| Unknown/Other | 298 (5.5%) |
| Multiple gestation | 1469 (27%) |
| Deaths | 145 (2.7%) |
| Antenatal magnesium | 1430 (26%) |
| Antenatal steroids | 1680 (31%) |
| Gestational age (weeks) | |
| Median (S.D.; IQR) | 33.3 (3.3; 30.7–35.0) |
| Birth weight (g) | |
| Median (S.D.; IQR) | 1973 (695; 1459–2422) |
| Apgar Score at 5 minutes | |
| Median (S.D.; IQR) | 8 (1.4; 8–9) |
| Neonatal infection | 2044 (37%) |
| Vasopressor requirement | 482 (8.8%) |
| Intraventricular hemorrhage | 410 (7.5%) |
| Outcomes | |
| Combined Epilepsy and Motor Impairment | 98 (1.8%) |
| Epilepsy | 70 (1.3%) |
| Motor Impairment | 84 (1.5%) |
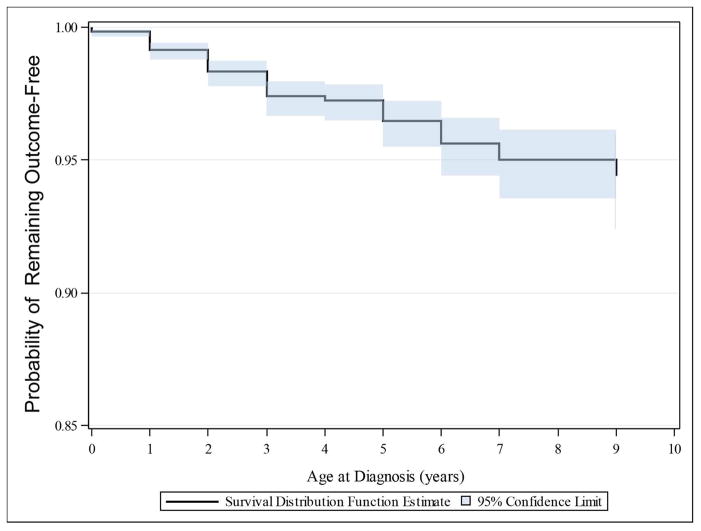
We examined outcomes in the cohort by ICD-9 codes, procedure codes, and billing codes, for the outcomes of motor impairment (cerebral palsy, hypotonia, or spasticity), and/or epilepsy. Outcomes were followed for no less than three additional years after the final birth. Electronic records were examined from all in-patient and out-patient encounters, including with the only pediatric neurology physicians in Utah. A total of 1.8% of the cohort was diagnosed with motor impairment and/or epilepsy (Table 1; Figure 2). There were 70 (1.3%) infants that had epilepsy, and 84 (1.5%) with motor impairment.
Figure 2.
Kaplan-Meier curve demonstrating risk of remaining free from developing combined outcome of motor impairment or epilepsy in cohort of 5461 premature infants.
To test whether the serum magnesium level in the premature infants correlated with their long-term neurodevelopmental outcomes, we compared the serum magnesium level drawn between 24 and 48 hours of life, and the neurodevelopmental outcomes. To control for other variables that might affect outcomes, we performed a multivariate logistic regression analysis in which we also compared for prenatal maternal magnesium administration; prenatal maternal steroid administration; the five minute Apgar score; the presence of neonatal infection; whether the infant required vasopressors during hospitalization; the gestational age; and the birth weight.
Table 2 summarizes the results of the multivariable analysis relating risk of the epilepsy/motor composite to neonatal magnesium with covariate adjustment. Each 1mg/dL increase in the serum magnesium level was associated with a relative risk for the composite outcome of 0.85 (95% confidence limits 0.66 – 1.1), which was not statistically significant (p = 0.24). The covariate adjusted association of the epilepsy/motor composite with neonatal magnesium also did not approach statistical significant when neonatal magnesium was expressed in tertiles with the first tertile as the referent ((2nd quartile, RR 0.83 (0.51–1.3), p 0.43; 3rd quartile, RR 0.80 (0.48 – 1.3), p 0.39) (Table 3). As displayed in Table 4, there was also no statistically significant relationship between the epilepsy/motor composite and neonatal magnesium in the subgroup of infants born to mothers who had not magnesium supplementation (RR 0.68, 95% CI 0.32 – 1.5 per 1 mg/dL increase, p = 0.32) or in the subgroup with maternal magnesium supplementation (RR 0.88, 95% CI 0.66 – 1.2 per 1 mg/dL increase, p = 0.39) after covariate adjustment.
Table 2.
Multivariable model relating the epilepsy/motor composite outcome to neontatal magnesium and covariates. The first row in the table provides the relative risk of the epilepsy/motor composite outcome associated with a 1 mg/dL increase in serum magnesium.
| Parameter | Relative Risk (95% C.I.) | P value |
|---|---|---|
| Serum Mg (mg/dL, continuous) | 0.85 (0.66, 1.1) | 0.24 |
|
| ||
| Maternal Mg administration (yes) | 1.2 (0.75, 1.8) | 0.50 |
| Maternal Steroid administration (yes) | 0.62 (0.41, 0.94) | 0.02 |
| Apgar Score, 5′ (continuous) | 0.87 (0.79, 0.95) | <0.01 |
| Neonatal infection (yes) | 1.6 (1.1, 2.4) | 0.03 |
| Vasopressor Requirement (yes) | 1.1 (0.68, 1.7) | 0.77 |
| Gestational Age (days, continuous) | 0.99 (0.98, 1.0) | 0.16 |
| Birth weight (standardized, continuous) | 0.63 (0.44, 0.89) | <0.01 |
Table 3.
Multivariable model relating the epilepsy/motor composite outcome to neonatal magnesium concentrations organized into tertiles, and to covariates. The first two rows in the table provides the relative risk of the epilepsy/motor composite associated with the second and third neonatal magnesium tertiles compared to the lowest/first magnesium tertile. RR, relative risk ratio; LCL and UCL, 95% lower and upper confidence levels.
| Parameter | RR | LCL | UCL | p value |
|---|---|---|---|---|
| Serum Mg (mg/dL, 1st Tertile is referent) | ||||
| 2nd Tertile | 0.83 | 0.51 | 1.3 | 0.43 |
| 3rd Tertile | 0.80 | 0.48 | 1.3 | 0.39 |
| Maternal Mg administration (yes) | 1.1 | 0.68 | 1.7 | 0.75 |
| Maternal Steroid administration (yes) | 0.87 | 0.80 | 0.95 | 0.0025 |
| Apgar Score, 5′ (continuous) | 1.6 | 1.0 | 2.4 | 0.032 |
| Neonatal infection (yes) | 1.1 | 0.67 | 1.7 | 0.80 |
| Vasopressor Requirement (yes) | 0.99 | 0.98 | 1.0 | 0.14 |
| Gestational Age (days, continuous) | 0.63 | 0.44 | 0.89 | 0.0088 |
| Birth weight (standardized, continuous) | 0.60 | 0.40 | 0.91 | 0.016 |
Table 4.
Multivariable model of serum Mg concentration stratified for absence or presence of maternal magnesium administration; and combined risk for epilepsy/motor impairment. RR, relative risk ratio; LCL and UCL, 95% lower and upper confidence levels.
| No Maternal Mg | |||||
|---|---|---|---|---|---|
| Parameter | Level | RR | LCL | UCL | p value |
| Serum Mg | 0.68 | 0.32 | 1.5 | 0.32 | |
| Age | |||||
| 1 | 3.1 | 1.6 | 6.0 | 0.0009 | |
| 2 | 1.9 | 0.82 | 4.5 | 0.13 | |
| 3 | 1.9 | 0.73 | 5.1 | 0.19 | |
| 4 | 2.6 | 0.87 | 7.8 | 0.088 | |
| Maternal Steroids | 0.63 | 0.34 | 1.2 | 0.13 | |
| APGAR_5_ | 0.84 | 0.74 | 0.95 | 0.0053 | |
| Neonatal infection | 1.3 | 0.75 | 2.2 | 0.34 | |
| VASOPRES | 1.1 | 0.59 | 2.0 | 0.80 | |
| GA_TOTAL | 0.99 | 0.97 | 1.0 | 0.19 | |
| BIRTH_WT | 0.67 | 0.45 | 1.0 | 0.049 | |
| Yes Maternal Mg | |||||
|---|---|---|---|---|---|
| Parameter | Level | RR | LCL | UCL | pvalue |
| Serum Mg | 0.88 | 0.66 | 1.17 | 0.3859 | |
| Maternal Steroids | 0.60 | 0.33 | 1.06 | 0.0800 | |
| APGAR_5 min | 0.92 | 0.81 | 1.03 | 0.1441 | |
| Neonatal Infection | 2.11 | 1.15 | 3.88 | 0.0158 | |
| VASOPRESSOR | 1.09 | 0.52 | 2.26 | 0.8230 | |
| GA_TOTAL | 1.00 | 0.98 | 1.02 | 0.7210 | |
| BIRTH_WT | 0.51 | 0.24 | 1.07 | 0.0744 | |
With advancing gestational age the risk for adverse neurodevelopmental outcomes decreases, so we compared the association between the neurodevelopmental outcome and magnesium levels separately in infants older or younger than 32 weeks gestation (Table 5). Again, there was no statistically significant association between the magnesium level and the risk for adverse neurodevelopmental outcomes within either subgroup (< 32 weeks RR 0.79, CI [0.59 – 1.1] per 1 mg/dL increase, p = 0.12; > 32 weeks RR 1.2, CI [0.74 – 1.9] per 1 mg/dL increase, p = 0.49).
Table 5.
Multivariable model of serum Mg concentration stratified by gestational age > or <32 weeks; and combined risk for epilepsy/motor impairment. RR, relative risk ratio; LCL and UCL, 95% lower and upper confidence levels.
| <=32 Weeks gestation | |||||
|---|---|---|---|---|---|
| Parameter | Level | RR | LCL | UCL | p value |
| Serum Mg | 0.79 | 0.59 | 1.1 | 0.12 | |
| Maternal Mg | 1.2 | 0.72 | 2.1 | 0.45 | |
| Maternal steroids | 0.74 | 0.47 | 1.2 | 0.19 | |
| APGAR 5 min | 0.89 | 0.81 | 0.99 | 0.025 | |
| Neonatal infection | 1.5 | 0.85 | 2.5 | 0.17 | |
| VASOPRESSOR | 1.1 | 0.64 | 1.8 | 0.82 | |
| BIRTH_WT | 0.37 | 0.23 | 0.57 | <0.0001 | |
| >32 Weeks gestation | |||||
|---|---|---|---|---|---|
| Parameter | Level | RR | LCL | UCL | p value |
| Serum Mg | 1.2 | 0.74 | 1. 9 | 0.49 | |
| Maternal Mg | 0.81 | 0.33 | 2.0 | 0.64 | |
| Maternal Steroids | 0.36 | 0.11 | 1.2 | 0.087 | |
| APGAR_5 | 0.83 | 0.68 | 1.0 | 0.046 | |
| Neonatal Infection | 1.7 | 0.87 | 3.1 | 0.12 | |
| VASOPRESSOR | 1.2 | 0.45 | 3.3 | 0.69 | |
| BIRTH_WT | 0.74 | 0.50 | 1.1 | 0.14 | |
Because most literature has examined the association between antenatal magnesium and the risk for cerebral palsy, and not a combined risk as we had, we also evaluated association between magnesium level and the risk for cerebral palsy in a multivariate analysis (Table 6). There was no significant association between the serum magnesium level and the outcome of cerebral palsy (RR 0.89, CI [0.63 – 1.3] p = 0.49).
Table 6.
Multivariable model of serum Mg concentration and risk for cerebral palsy. RR, relative risk ratio; LCL and UCL, 95% lower and upper confidence levels.
| parameter | RR | LCL | UCL | p value |
|---|---|---|---|---|
| Serum Mg (ng/dL, continuous) | 0.89 | 0.63 | 1.3 | 0.49 |
| Maternal Mg administration (yes) | 1.1 | 0.59 | 1.9 | 0.85 |
| Maternal Steroid administration (yes) | 0.52 | 0.30 | 0.89 | 0.017 |
| Apgar Score, 5′ (continuous) | 0.85 | 0.77 | 0.95 | 0.0039 |
| Neonatal infection (yes) | 1.7 | 0.98 | 2.8 | 0.060 |
| Vasopressor Requirement (yes) | 1.2 | 0.70 | 2.1 | 0.49 |
| Gestational Age (days, continuous) | 0.99 | 0.97 | 1.0 | 0.17 |
| Birth weight (standardized, continuous) | 0.53 | 0.36 | 0.78 | 0.0013 |
DISCUSSION
Our study examined the correlation between serum neonatal magnesium levels and neurodevelopmental outcomes including epilepsy and motor impairment including cerebral palsy in premature infants. While we observed trends towards lower rates of adverse neurodevelopmental outcomes, these were not statistically significant. The absence of statistical significance remained when motor impairment was examined separately from the outcome of epilepsy; and also when outcomes were compared for infants born before or after 32 weeks gestation.
Our objective was to determine whether postnatal serum magnesium levels in premature infants correlates with their likelihood of developing adverse neurodevelopmental outcomes. In particular, our long-term goal is to determine if magnesium might play a neuroprotective role in premature infants beyond the immediate peripartum period. That is, perhaps continued magnesium supplementation in premature infants might improve outcomes. Previous work from our group had shown in a pilot study that higher magnesium levels in premature infants were correlated with lower risk for motor impairment.(Doll et al., 2014) However, because of the limited data available retrospectively, in this larger cohort analysis we limited use of magnesium levels from only 24 to 48 hours after birth. This was done because magnesium levels in the first 24 hours of life directly correlated with whether the mother had received magnesium; and magnesium levels after 48 hours of life were few and sporadic, making comparisons impossible.
Strengths of the study included the large cohort size; the ability to comprehensively track outcomes through the EDW since most healthcare in Utah is provided by Intermountain Healthcare and since there is only one children’s hospital; and the extended follow-up time period. Weaknesses of this work include its retrospective analysis; and that outcomes were indirectly assessed using ICD-9 codes. Although the IH EDW does contain both in- and out-patient data, a child with motor impairment followed by their primary care provider would not have been captured in our analysis. Magnesium levels are affected by both endogenous and iatrogenic factors, which were not addressed in our work. We observed a low rate of impairments (only 1.8% combined for epilepsy and motor impairment), which made it difficult to determine if magnesium was having an effect. Reasons for this low rate of impairments are not obvious, but could include that the neonates spanned a range of ages from the very premature to near term; or that the families were from less disadvantaged socioeconomic backgrounds. While it is possible that we did not identify all patients with neurological impairment, we think this is a less likely explanation because of the strengths mentioned above.
Previous studies have shown that prenatal magnesium administration is associated with a decreased risk of developing cerebral palsy,(Doyle et al., 2009; Rouse et al., 2008) and our own pilot study showed in a small cohort that higher serum magnesium levels in premature infants was associated with a lower risk for cerebral palsy.(Doll et al., 2014) However, there are not any studies examining the association between the maternal serum magnesium level or cord magnesium level and the neurodevelopmental outcome of the infant. Further, based on our experience with this current analysis, it will be difficult to perform a retrospective analysis to determine whether premature infant magnesium levels, other than the time period we examined, correlate with outcomes. This is because of the scattered and non-standardized timing of draws for magnesium levels obtained in most premature infants. This limitation will make it difficult to retrospectively test whether there is a broader window of potential neuroprotection using magnesium in premature infants.
Because of the limitations with currently available data from human studies, mechanisms of magnesium neuroprotection need to be more fully examined using animal models. Mechanisms by which magnesium has been shown to be neuroprotective include blocking calcium influx through the N-methyl-D-aspartate (NMDA) receptor channel, reduction of inflammatory cytokine production, limiting blood pressure fluctuations, and preventing activation of the hypoxia inducible factor 1α (HIF1α) pathway which disrupts connectivity (27, 31–33).(Stevenson et al., 2012) Other future studies to be considered would be to track a cohort prospectively; and to determine if magnesium has more neuroprotective effects in certain groups of infants, for example, those with intraventricular hemorrhage. The significant extent of prematurity, affecting more than 12.9 million infants worldwide every year, and the neurodevelopmental morbidity associated with premature birth, make further research into this area of continued importance.
Acknowledgments
SOURCES OF FINANCIAL SUPPORT
JLB was funded in part by NIH grant DP2 MH100008 and by a Research Grant from the March of Dimes Foundation. TB and TG were supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105.
Abbreviations
- CNS
central nervous system
- EDW
enterprise data warehouse
- IH
Intermountain Healthcare
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, Schonwald A, Wilker RE, Stehle S, Kinane TB. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- Basu SK, Chickajajur V, Lopez V, Bhutada A, Pagala M, Rastogi S. Immediate clinical outcomes in preterm neonates receiving antenatal magnesium for neuroprotection. Journal of perinatal medicine. 2012;40:185–189. doi: 10.1515/JPM.2011.094. [DOI] [PubMed] [Google Scholar]
- Cetinkaya M, Alkan T, Ozyener F, Kafa IM, Kurt MA, Koksal N. Possible neuroprotective effects of magnesium sulfate and melatonin as both pre- and post-treatment in a neonatal hypoxic-ischemic rat model. Neonatology. 2011;99:302–310. doi: 10.1159/000320643. [DOI] [PubMed] [Google Scholar]
- Chang E. Preterm birth and the role of neuroprotection. Bmj. 2015;350:g6661. doi: 10.1136/bmj.g6661. [DOI] [PubMed] [Google Scholar]
- Deng W, Pleasure J, Pleasure D. Progress in periventricular leukomalacia. Archives of neurology. 2008;65:1291–1295. doi: 10.1001/archneur.65.10.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollat C, Enser M, Houivet E, Provost D, Benichou J, Marpeau L, Marret S. School-age outcomes followinga randomized controlled trial of magnesium sulfate for neuroprotection of preterm infants. The journal of pediatrics. 2014;165:398–400. doi: 10.1016/j.jpeds.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Doll E, Wilkes J, Cook LJ, Korgenski EK, Faix RG, Yoder BA, Srivastava R, Sherwin CM, Spigarelli MG, Clark EA, et al. Neonatal magnesium levels correlate with motor outcomes in premature infants: a long-term retrospective cohort study. Frontiers in pediatrics. 2014;2:120. doi: 10.3389/fped.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. The Cochrane database of systematic reviews. 2009:CD004661. doi: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Anderson PJ, Haslem R, Lee KJ, Crowther C. School-age Outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. Jama. 2014;312:1105–1113. doi: 10.1001/jama.2014.11189. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. The New England journal of medicine. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Goni-de-Cerio F, Alvarez A, Lara-Celador I, Alvarez FJ, Alonso-Alconada D, Hilario E. Magnesium sulfate treatment decreases the initial brain damage alterations produced after perinatal asphyxia in fetal lambs. Journal of neuroscience research. 2012;90:1932–1940. doi: 10.1002/jnr.23091. [DOI] [PubMed] [Google Scholar]
- Inder TE, Volpe JJ. Mechanisms of perinatal brain injury. Seminars in neonatology : SN. 2000;5:3–16. doi: 10.1053/siny.1999.0112. [DOI] [PubMed] [Google Scholar]
- Hirtz DG, Weiner SJ, Bulas D, DiPietro M, Seibert J, Rouse DJ, Mercer BM, Varner MW, Reddy Um, Iams JD, et al. Antenatal Magnesium and cerebral palsy in preterm infants. The journal of pediatrics. 2015;167:834–839. doi: 10.1016/j.jpeds.2015.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter RH, Gui L, Poncet A, Hagmann C, Lodygensky GA, Martin E, Koller B, Darque A, Bucher HU, Huppi PS. Association between early administration of high-dose erythropoietin in preterm infants and brain MRI abnormality at term-equivalent age. Jama. 2014;312:817–824. doi: 10.1001/jama.2014.9645. [DOI] [PubMed] [Google Scholar]
- Li K, Sun Z, Han Y, Gao L, Yuan L, Zeng D. Fractional anisotropy alterations in individuals born preterm: a diffusion tensor imaging meta-analysis. Developmental medicine and child neurology. 2015;57:328–338. doi: 10.1111/dmcn.12618. [DOI] [PubMed] [Google Scholar]
- McPherson JA, Rouse DJ, Grobman WA, Palatnik A, Stamilio DM. Association of duration of neuroprotective magnesium sulfate infusion with neonatal and maternal outcomes. Obstetrics and gynecology. 2014;124:749–755. doi: 10.1097/AOG.0000000000000467. [DOI] [PubMed] [Google Scholar]
- Mitani M, Matsuda Y, Shimada E Perinatal Research Network Group in J. Short- and long-term outcomes in babies born after antenatal magnesium treatment. The journal of obstetrics and gynaecology research. 2011;37:1609–1614. doi: 10.1111/j.1447-0756.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, Tomich PG. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. American journal of obstetrics and gynecology. 2002;186:1111–1118. doi: 10.1067/mob.2002.123544. [DOI] [PubMed] [Google Scholar]
- Murray AL, Thompson DK, Pascoe L, Leemans A, Inder TE, Doyle LW, Anderson JF, Anderson PJ. White matter abnormalities and impaired attention abilities in children born very preterm. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman MJ, Kochanek KD, MacDorman MF, Strobino DM, Guyer B. Annual summary of vital statistics: 2012–2013. Pediatrics. 2015;135:1115–1125. doi: 10.1542/peds.2015-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, Iams JD, Wapner RJ, Sorokin Y, Alexander JM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. The New England journal of medicine. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Sherwin CM, Balch A, Campbell SC, Fredrickson J, Clark EA, Varner M, Stockmann C, Korgenski EK, Bonkowsky JL, Spigarelli MG. Maternal magnesium sulphate exposure predicts neonatal magnesium blood concentrations. Basic & clinical pharmacology & toxicology. 2014;114:318–322. doi: 10.1111/bcpt.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada E, Ogawa M, Matsuda Y, Mitani M, Matsui H. Umbilical artery pH may be a possible confounder for neonatal adverse outcomes in preterm infants exposed to antenatal magnesium. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26:270–274. doi: 10.3109/14767058.2012.733749. [DOI] [PubMed] [Google Scholar]
- Sotiriadis A, Tsiami A, Papatheodorou S, Baschat AA, Sarafidis K, Makrydimas G. Neurodevelopmental Outcome After a Single Course of Antenatal Steroids in Children Born Preterm: A Systematic Review and Meta-analysis. Obstetrics and gynecology. 2015;125:1385–1396. doi: 10.1097/AOG.0000000000000748. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Trinh T, Kogelschatz C, Fujimoto E, Lush ME, Piotrowski T, Brimley CJ, Bonkowsky JL. Hypoxia disruption of vertebrate CNS pathfinding through ephrinB2 Is rescued by magnesium. PLoS genetics. 2012;8:e1002638. doi: 10.1371/journal.pgen.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki-Kakisaka H, Sugimoto J, Tetarbe M, Romani AM, Ramirez Kitchen CM, Bernstein HB. Magnesium sulfate increases intracellular magnesium reducing inflammatory cytokine release in neonates. American journal of reproductive immunology. 2013;70:213–220. doi: 10.1111/aji.12118. [DOI] [PubMed] [Google Scholar]
- Thomas B, Eyssen M, Peeters R, Molenaers G, Van Hecke P, De Cock P, Sunaert S. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain : a journal of neurology. 2005;128:2562–2577. doi: 10.1093/brain/awh600. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, Boyle MH, Saigal S, Morrison K, Schmidt LA. Mental health of extremely low birth weight survivors in their 30s. Pediatrics. 2015;135:452–459. doi: 10.1542/peds.2014-3143. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, Simon NP, Wilson DC, Broyles S, Bauer CR, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]