Abstract
Background & Aims
Colonic diverticula are the most common finding from colonoscopy examinations. Little is known about the distribution of colonic diverticula, which are responsible for symptomatic and costly diverticular disease. We aimed to assess the number, location, and characteristics of colonic diverticula in a large US screening population.
Methods
We analyzed data from a prospective study of 624 patients (mean age, 54 years) undergoing screening colonoscopy at the University of North Carolina Hospital from 2013 through 2015. The examination included a detailed assessment of colonic diverticula. To assess the association between participant characteristics and diverticula, we used logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Of our population, 260 patients (42%) had one or more diverticula (mean number 14; range, 1–158). Participants with diverticula were more likely to be older, male, and have a higher body mass index than those without diverticula. The distribution of diverticula differed significantly by race. Among Whites, 75% of diverticula were in the sigmoid colon, 11% in the descending splenic flexure, 6% in the transverse colon, and 8% were in the ascending colon or hepatic flexure; in Blacks 64% of diverticula were in the sigmoid colon, 8% in the descending colon or splenic flexure, 7% in the transverse colon, and 20% in the ascending colon or hepatic flexure (P=.0008). The proportion of patients with diverticula increased with age: 35% were 50 years or younger, 40% were 51–60 years, and 58% were older than 60 years. The proportion of patients with more than 10 diverticula increased with age: 8% were 50 years or younger, 15% were 51–60 years, and 30% were older than 60 years.
Conclusions
Older individuals not only have a higher prevalence of diverticula than younger individuals, but also a greater density, indicating that this is a progressive disease. Blacks have a greater percentage of their diverticula in the proximal colon and fewer in the distal colon compared with Whites. Understanding the distribution and determinants of diverticula is the first step in preventing diverticulosis and its complications.
Keywords: BMI, diverticular disease, obesity, race
Colonic diverticula are the most common finding on colonoscopy in the United States (U.S.).1 Almost all of these lesions are pseudodiverticula that result when colonic mucosa and submucosa herniate through the muscularis propria where the vasa recta penetrate.2 Colonic diverticula can be complicated by a spectrum of morbid and mortal diseases including diverticulitis, diverticular bleeding, and free rupture.3 Diverticular pathology is associated with a 5-fold increased risk of developing chronic gastrointestinal symptoms or a mood disorder.4
Despite this burden of disease, little is known about the distribution and characteristics of colonic diverticula in Western populations. The prevalence of diverticulosis has been estimated using the Clinical Outcomes Research Initiative National Endoscopy Database.1 Among persons at routine risk only, the prevalence of diverticulosis was 33% in individuals 50–59 years old and 71% in those over the age of 80 years. The fidelity of detecting and reporting diverticula on colonoscopy is unknown, raising questions about the validity of these estimates. There has been no prospective colonoscopy-based study in a Western population that included a standardized detailed examination for diverticula and associated characteristics.
The widespread utilization of screening colonoscopy offers an unprecedented opportunity to learn more about colonic diverticula. The aim of this study was to describe the distribution and characteristics of colonic diverticula enumerating them by number, size, depth, and location in the colon in a large US screening population. We also aimed to assess whether participant characteristics were associated with a greater burden, size and depth of colonic diverticula.
METHODS
The purpose of this study was to assess risk factors associated with colonic diverticula (NIH R01DK094738). In brief, the study recruited outpatients between 2013–2015 undergoing a first-time screening colonoscopy at the University of North Carolina Hospital in Chapel Hill, North Carolina. Eligible participants were 30 years and older, had a satisfactory preparation for colonoscopy, and a complete exam to the cecum. Exclusion criteria included any prior colonoscopy, a history of previous colon resection, or a prior diagnosis of polyposis, colitis, colon cancer, diverticulosis or diverticular disease. Informed consent was obtained from all participants. The University of North Carolina School of Medicine Institutional Review Board approved this study.
Prior to the colonoscopy, each participant completed a detailed telephone interview on diet and physical activity. Race was self-reported. The day of the colonoscopy, a research assistant measured the participant’s height and weight. During the procedure, the gastroenterologist counted the number of diverticula in each segment of the colon (cecum, ascending, hepatic flexure, transverse, splenic flexure, descending, and sigmoid colon) and reported the findings to a research assistant. The research assistant was present during the entire examination to record the information on a standard data collection form. Diverticula were assessed on either insertion or withdrawal at the discretion of the gastroenterologist. The size of diverticula in each segment was estimated as small only, large only, or both small and large, where large was defined as greater than 7 mm. The depth of diverticula in each segment was estimated as shallow only, deep only, or both shallow and deep. Deep was defined as a depth greater than width. Each segment was assessed for the presence or absence of muscular hypertrophy and inflammation. Muscular hypertrophy was defined as the presence of large bands between the diverticula. Inflammation was any sign of redness or ulceration near a diverticulum. An instruction sheet including photographic examples (Supplemental Figure 1) was distributed to the endoscopists and posted in the physician workroom.
Statistical Analysis
Means and standard deviations were calculated for continuous variables, medians for skewed distributions of continuous variables, and proportions for categorical data. We determined the distribution of diverticula both at the individual level and at the group level. We summarized, at the individual level, the within-person distribution of diverticula by colonic location, as the proportion of that individual’s diverticula found in each segment. We used repeated measures logistic regression to assess the association between race and distribution of diverticula by location. We used the same method to assess the association between sex and distribution of diverticula. We used Spearman’s correlation to assess for an association between body mass index and distribution of diverticula. We also created a box and whisker plot to demonstrate, at the group level, the distribution of diverticula in each location. We generated bar graphs to display the characteristics of diverticula. To assess the association between participant characteristics and multiple (>10), large and deep diverticula, we estimated odds ratios and 95% confidence intervals using logistic regression, adjusting for age, body mass index, sex and race. All tests of significance were two-tailed and p-values <0.05 were considered significant. The analysis was performed using SAS 9.4 (SAS, Cary, North Carolina).
RESULTS
Our analysis included 624 participants. Of these, 260 (42%) had one or more diverticula on colonoscopy. Among those with diverticula, the mean number was 14 (range 1 to 158). Participants with diverticula were more likely to be older, male, and have a higher body mass index and waist circumference than those without diverticula (Table 1).
Table 1.
Characteristics of study participants.
| Diverticula n = 260 |
No Diverticula n =364 |
|
|---|---|---|
| Age, year, mean ± SD | 56 ± 7 | 53 ± 6 |
| Age, years, % | ||
| ≤50 | 18 | 23 |
| 51–60 | 59 | 64 |
| >60 | 23 | 13 |
| Sex, % | ||
| Male | 48 | 41 |
| Female | 52 | 59 |
| Race, % | ||
| White | 77 | 75 |
| Non-white | 20 | 24 |
| BMI, kg/m2, mean ± SD | 30 ± 7 | 28 ± 7 |
| Waist circumference, cm, mean ± SD | 98 ± 17 | 93 ± 16 |
Abbreviations: SD, standard deviation; BMI, body mass index
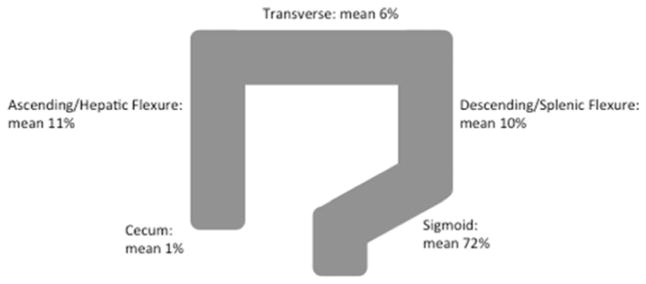
Most diverticula were located in the sigmoid colon (Figure 1) although the distribution differed significantly by race. Among Whites, 75% of diverticula were in the sigmoid colon, 11% in the descending colon or splenic flexure, 6% in the transverse colon and 8% were in the ascending colon or hepatic flexure; in Blacks, 64% of diverticula were in the sigmoid colon, 8% in the descending colon or splenic flexure, 7% in the transverse colon and 20% in the ascending colon or hepatic flexure (p=0.0008). There was no difference in the mean age or proportion male between the two races. The overall prevalence of diverticula was similar in Whites and Blacks (42% and 40% respectively). There was no difference in the average number of diverticula between Whites and Blacks. Sex and body mass index were not associated with the presence of proximal compared with distal diverticula.
Figure 1.
Within-individual distributions of colonic diverticula among participants having diverticula
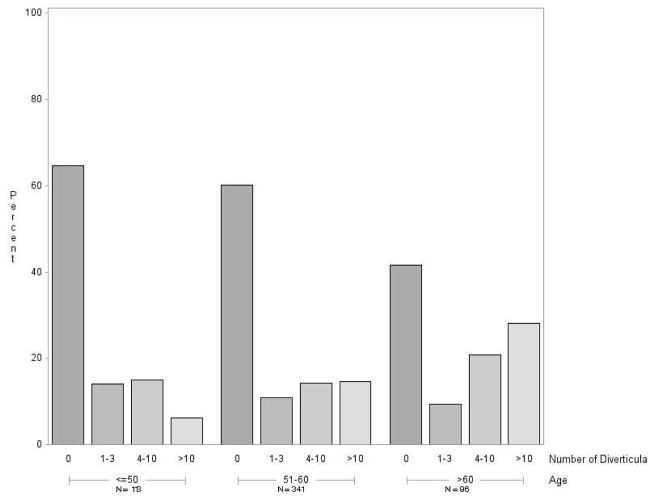
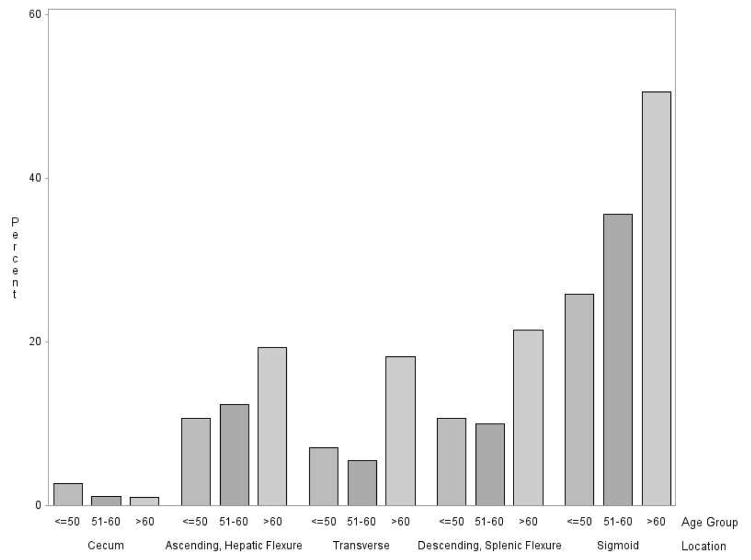
The prevalence of diverticulosis increased with age (Figure 2). Prevalence rose from 35% among participants 50 years of age or younger, to 40% among 51 to 60 year-olds, to 58% among those older than 60. The number of diverticula also increased with age. The proportion having more than 10 diverticula was 8% for those age ≤50, 15% for those age 51–60, and 30% for those age >60 (Figure 2). With the exception of the cecum, the prevalence of diverticula generally increased with age across all locations in the colon (Figure 3).
Figure 2.
Distribution of number of diverticula by age group
Figure 3.
Age distribution of patients with one or more diverticula in each colonic location.
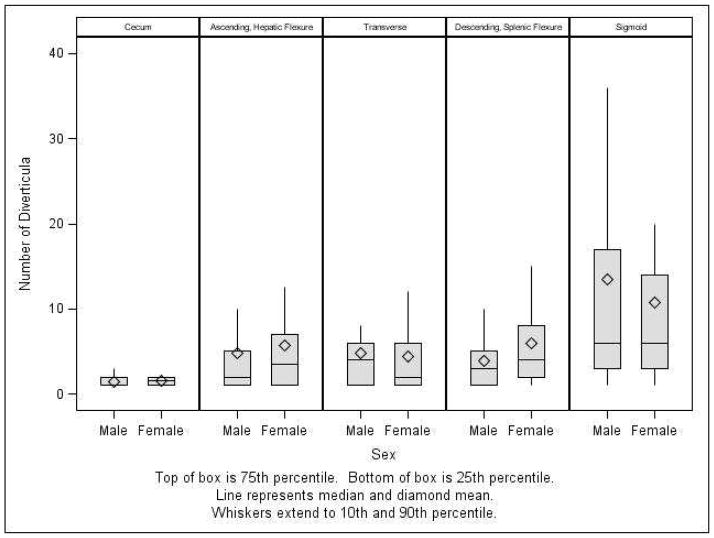
Across the group of participants with diverticula, the density or number of diverticula in the sigmoid colon (mean 12; median 6) was twice that of other locations, compared with the other locations which were all similar: ascending/hepatic flexure (mean 5; median 2), transverse (mean 5; median 2) and descending colon/splenic flexure (mean 5; median 3) (Figure 4). The sigmoid colon also had the widest range in number of diverticula (range 0 – 140). There were no significant differences in the location-specific number of diverticula between the sexes (Figure 4).
Figure 4.
Distribution of all diverticula across study group, by location and sex
The proportion of participants having one or more diverticula characterized as large or deep increased with age (Supplemental Figure 2). Among those with diverticula, few participants (3%) had peridiverticular muscular hypertrophy. When present, muscular hypertrophy was limited to the descending and sigmoid colon. Similarly, few participants (2%) had peridiverticular inflammation. Most had this finding only in the sigmoid colon. There was no overlap among those participants with peridiverticular muscular hypertrophy and those with inflammation.
Older age was associated with an increased likelihood of having multiple (>10), large or deep diverticula on colonoscopy (Table 2). Compared with participants ≤50 years old, participants 51–60 years old (adjusted OR 2.4, 95% CI 1.1–5.0) and > 60 years old (adjusted OR 5.6, 95% CI 2.5–12.6) were more likely to have a greater burden (>10) of diverticula. Participants who were overweight (adjusted OR 2.0, 95% CI 1.0–3.8) or obese (adjusted OR 3.1, 95% CI 1.7–5.8) also had an increased odds of having >10 diverticula. Nonwhite race compared with White race was not associated with having multiple diverticula (>10) but was associated with having at least one large (adjusted OR 2.1, 95% CI 1.0–4.1) or deep diverticulum (adjusted OR 2.5, 95% CI 1.2–4.9).
Table 2.
Participant characteristics and associations with multiple, large, or deep diverticula.
| >10 diverticula aOR (95% CI) |
Large diverticula aOR (95% CI) |
Deep diverticula aOR (95% CI) |
|
|---|---|---|---|
| Age | |||
| ≤50 years | 1.0 | 1.0 | 1.0 |
| 51–60 | 2.4 (1.1–5.0) | 2.6 (1.2–5.5) | 2.8 (1.3–6.2) |
| >60 | 5.6 (2.5–12.6) | 3.8 (1.6–9.0) | 4.3 (1.8–10.5) |
| Sex | |||
| Male | 1.0 | 1.0 | 1.0 |
| Female | 0.79 (0.50–1.3) | 0.8 (0.48–1.4) | 0.9 (0.50–1.5) |
| Race | |||
| White | 1.0 | 1.0 | 1.0 |
| Nonwhite | 0.75 (0.43–1.31) | 2.1 (1.0–4.1) | 2.5 (1.2–4.9) |
| BMI | |||
| Normal 18.5–25 | 1.0 | 1.0 | 1.0 |
| Overweight (25–30) | 2.0 (1.0–3.8) | 1.6 (0.80–3.2) | 1.4 (0.68–2.7) |
| Obese (>30) | 3.1 (1.7–5.8) | 1.6 (0.80–3.1) | 1.3 (0.68–2.7) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; BMI, body mass index
DISCUSSION
In the United States, many individuals over age thirty have sigmoid diverticula with the prevalence and density increasing with age suggesting this is a progressive disease. Although the sigmoid colon is disproportionately affected by diverticula compared with other locations, the distribution differs by race. Blacks have significantly more of their diverticula in the proximal colon and fewer in the distal colon compared with Whites. Peridiverticular muscular hypertrophy and inflammation is rare. Being overweight or obese is a risk factor for multiple colonic diverticula.
The results of this study are important for several reasons. It has been generally assumed that diverticulosis was a feature of old age. While we found a clear association with older age, we found one or more diverticula in 35% of subjects under the age of 50. Whether the proportion of younger individuals with diverticula is increasing over time is unknown because there have been no prior studies with careful enumeration of diverticula. Although we currently do not understand the cause of diverticulosis, nutrition or other factors linked with body mass index may be involved as we found a clear association with body mass index. We also found that a notable proportion of subjects had diverticula in the ascending and transverse colon. The more proximal location argues against high pressure, lumen-occluding contractions as the primary cause for diverticular outpouchings.5 We also found considerable variation in number – one individual had 140 diverticula. One could speculate that there could be genetic defects in collagen that might be responsible.
The prevalence of diverticulosis was higher in our age-stratified population than that reported from Clinical Outcomes Research Initiative National Endoscopy Database (CORI-NED).1 Specifically, the prevalence of diverticulosis in our population of participants 51 to 60 years old was 40% compared with 33% in those 50–59 years old in CORI-NED. This discrepancy with CORI-NED is likely to due to under detection and under reporting during routine clinical colonoscopies. Our prevalence estimates were similar to our prior colonoscopy based estimates,6 a recent CT colonography study that included Caucasian patients having an examination for colon cancer screening or a clinical indication,7 and a study from the United Kingdom that utilized barium enema for non-emergent gastrointestinal symptoms.7, 8
Curiously, there is substantial geographic variation in the prevalence and distribution of colonic diverticula. The prevalence of diverticulosis has been estimated prospectively in several colonoscopy-based studies in Asia. Colonic diverticula were less prevalent in these populations compared with our population. The prevalence of diverticulosis was 12% in Korea (mean age 51)9 and 14% in Taiwan (mean age 53).10 The prevalence of diverticulosis was 25% in Japan (mean age 58).11 Proximal diverticula were more common in these populations although the distal colon was not spared. In Korea - 15% had distal diverticula while half of the Japanese and Taiwanese population had distal diverticula. Increasing age was a risk factor for colonic diverticula across all of these populations, which is evidence that diverticula are acquired in these populations and not congenital. The prevalence and distribution of colonic diverticula was also prospectively assessed in a study from the Middle East and was more similar with our population in the United States. The prevalence of diverticulosis was 33% (mean age 61) and most had distal diverticula.12
While we had too few participants of Asian race to look for variation in the prevalence and distribution of diverticula, we were able to compare these statistics between Whites and Blacks. There was no difference in the prevalence or overall burden of diverticula among Whites and Blacks. However, the distribution of diverticula differed significantly by race, with Blacks having a greater percentage of diverticula in the proximal colon compared with Whites. Similarly, a study of 1000 barium enemas in patients with non-emergent gastrointestinal symptoms found that Black Africans were more likely to have diverticulosis that involved the proximal colon than Whites.8 This finding supports a possible heritable contribution to the development of diverticulosis but may also be due to differences in health behaviors or environmental factors. Moreover, this disparity in distribution may have clinical implications. In a prospective study of patients with severe hematochezia, among those with a definitive diagnosis of diverticula hemorrhage, the source of bleeding was more likely to be diverticula in the proximal colon compared with the distal colon.13 And in a recent analysis using data from the Nationwide Inpatient Sample, hospitalizations for diverticular bleeding were significantly more common in Blacks compared with Whites.14 The greater burden of proximal diverticula in Blacks compared with Whites may contribute to this significant difference.
In Western populations, acute diverticulitis classically develops in the descending and/or sigmoid colon and only rarely occurs in the proximal colon.15 In a retrospective cohort of patients in the United States with acute diverticulitis, 72% had disease in the sigmoid colon, 33% in the descending colon, 3% in the transverse colon and 5% in the ascending colon. The distribution of disease is similar to the distribution of diverticula in our population. This similarity suggests that diverticulitis is most common in the descending and/or sigmoid colon because diverticula are much more common in these locations. Race was not reported in this study.
There is growing evidence that obesity is associated with an increased risk of diverticulosis. In another colonoscopy-based study, we found that overweight or obese participants were 65% more likely to have diverticula compared to those with a normal body mass index.16 Several other colonoscopy-based studies have also confirmed this association.17–19 Similarly, abdominal obesity as measured by computed tomography is a risk factor for colonoscopy-proven diverticula.20 The mechanism by which obesity is associated with colonic diverticula is unknown. Given the complex relationship between metabolism, the immune system and gut micobiota,21 we hypothesize that obesity and associated inflammation and alterations in the microbiome may play a role in the development of colonic diverticula.
Peridiverticular muscular hypertrophy and inflammation was rare in our population. Another colonoscopy-based study also found peridiverticular inflammation is rare and that patients were asymptomatic in follow up. 22 The significance of this finding in the asymptomatic population is unknown. Others have also assessed for peridiverticular inflammation but only in symptomatic populations.23
This work has notable strengths. This was a prospective study specifically designed to quantify and characterize diverticula. Every colonoscopy was performed by a gastroenterologist. A research assistant was present during the entire colonoscopy to question the gastroenterologist about diverticula in each colon segment and record the results on a data collection form. Participants were asymptomatic and undergoing a first time colonoscopy for screening. Our work has limitations. Diverticula may have been missed, although our prevalence estimates were similar to two recent studies to characterize colonic diverticula. The first study assessed diverticula by barium enemas8 and the second utilized CT colonography.7 Because our population was limited to individuals undergoing a first-time screening colonoscopy, we have limited data on diverticula in the elderly with only 10 individuals in our population were over the age of 70.
The burden of diverticular disease in the US is considerable.24, 25. We have shown that older individuals had a higher prevalence and density of diverticula than younger individuals suggesting this is a progressive disease. We have also found that Blacks have a greater percentage of their diverticula in the proximal colon and fewer in the distal colon compared with Whites. The present study is the first step in understanding the distribution and determinants of diverticulosis with the eventual hope of prevention.
Supplementary Material
Supplemental Figure 1. Study instructions to endoscopists
Supplemental Figure 2. Percentage by Age With Any Large or Deep Diverticula
Acknowledgments
Funding: This research was supported, in part, by grants from the National Institutes of Health (P30 DK034987, R01DK09473, T32DK07634 and by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant KL2TR001109.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures: None
Author Contributions: Design and conduct of study (AFP, TOK, CFM, RSS); collection (AFP, TOK, CFM, RSS), management (CFM, RSS), analysis (AFP, TOK, CFM, SE, TR, JAP, RSS), and interpretation of data; and preparation and final approval of manuscript (AFP, TOK, CFM, SE, TR, JAP, RSS).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009;136(3):741–54. doi: 10.1053/j.gastro.2009.01.015. Epub 2009/01/27. [DOI] [PubMed] [Google Scholar]
- 2.Brian West A. The pathology of diverticulosis: classical concepts and mucosal changes in diverticula. J Clin Gastroenterol. 2006;40(Suppl 3):S126–31. doi: 10.1097/01.mcg.0000225508.90417.07. Epub 2006/08/04. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs DO. Clinical practice. Diverticulitis. The New England journal of medicine. 2007;357(20):2057–66. doi: 10.1056/NEJMcp073228. [DOI] [PubMed] [Google Scholar]
- 4.Cohen E, Fuller G, Bolus R, et al. Increased risk for irritable bowel syndrome after acute diverticulitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(12):1614–9. doi: 10.1016/j.cgh.2013.03.007. Epub 2013/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Painter NS, Truelove SC, Ardran GM, et al. Segmentation and the Localization of Intraluminal Pressures in the Human Colon, with Special Reference to the Pathogenesis of Colonic Diverticula. Gastroenterology. 1965;49:169–77. Epub 1965/08/01. [PubMed] [Google Scholar]
- 6.Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142(2):266–72. e1. doi: 10.1053/j.gastro.2011.10.035. Epub 2011/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Cecco CN, Ciolina M, Annibale B, et al. Prevalence and distribution of colonic diverticula assessed with CT colonography (CTC) European radiology. 2015 doi: 10.1007/s00330-015-3866-1. Epub 2015/06/25. [DOI] [PubMed] [Google Scholar]
- 8.Golder M, Ster IC, Babu P, et al. Demographic determinants of risk, colon distribution and density scores of diverticular disease. World journal of gastroenterology. 2011;17(8):1009–17. doi: 10.3748/wjg.v17.i8.1009. Epub 2011/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song JH, Kim YS, Lee JH, et al. Clinical characteristics of colonic diverticulosis in Korea: a prospective study. The Korean journal of internal medicine. 2010;25(2):140–6. doi: 10.3904/kjim.2010.25.2.140. Epub 2010/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang FW, Chuang HY, Tu MS, et al. Prevalence and risk factors of asymptomatic colorectal diverticulosis in Taiwan. BMC gastroenterology. 2015;15:40. doi: 10.1186/s12876-015-0267-5. Epub 2015/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata N, Niikura R, Shimbo T, et al. Alcohol and smoking affect risk of uncomplicated colonic diverticulosis in Japan. PloS one. 2013;8(12):e81137. doi: 10.1371/journal.pone.0081137. Epub 2013/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharara AI, El-Halabi MM, Mansour NM, et al. Alcohol consumption is a risk factor for colonic diverticulosis. Journal of clinical gastroenterology. 2013;47(5):420–5. doi: 10.1097/MCG.0b013e31826be847. Epub 2012/11/21. [DOI] [PubMed] [Google Scholar]
- 13.Jensen DM. The ins and outs of diverticular bleeding. Gastrointestinal endoscopy. 2012;75(2):388–91. doi: 10.1016/j.gie.2011.09.004. Epub 2012/01/18. [DOI] [PubMed] [Google Scholar]
- 14.Wheat CL, Strate LL. Trends in Hospitalization for Diverticulitis and Diverticular Bleeding in the United States From 2000 to 2010. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14(1):96–103. e1. doi: 10.1016/j.cgh.2015.03.030. Epub 2015/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall JF, Roberts PL, Ricciardi R, et al. Long-term follow-up after an initial episode of diverticulitis: what are the predictors of recurrence? Diseases of the colon and rectum. 2011;54(3):283–8. doi: 10.1007/DCR.0b013e3182028576. Epub 2011/02/10. [DOI] [PubMed] [Google Scholar]
- 16.Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(12):1622–7. doi: 10.1016/j.cgh.2013.06.033. Epub 2013/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braunschmid T, Stift A, Mittlbock M, et al. Constipation is not associated with diverticular disease - Analysis of 976 patients. International journal of surgery. 2015;19:42–5. doi: 10.1016/j.ijsu.2015.04.045. Epub 2015/05/20. [DOI] [PubMed] [Google Scholar]
- 18.Kopylov U, Ben-Horin S, Lahat A, et al. Obesity, metabolic syndrome and the risk of development of colonic diverticulosis. Digestion. 2012;86(3):201–5. doi: 10.1159/000339881. Epub 2012/08/22. [DOI] [PubMed] [Google Scholar]
- 19.Comstock SS, Lewis MM, Pathak DR, et al. Cross-sectional analysis of obesity and serum analytes in males identifies sRAGE as a novel biomarker inversely associated with diverticulosis. PloS one. 2014;9(4):e95232. doi: 10.1371/journal.pone.0095232. Epub 2014/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata N, Sakamoto K, Arai T, et al. Visceral Abdominal Obesity Measured by Computed Tomography is Associated With Increased Risk of Colonic Diverticulosis. Journal of clinical gastroenterology. 2015;49(10):816–22. doi: 10.1097/MCG.0000000000000267. Epub 2014/11/21. [DOI] [PubMed] [Google Scholar]
- 21.Cavalcante-Silva LH, Galvao JG, da Silva JS, et al. Obesity-Driven Gut Microbiota Inflammatory Pathways to Metabolic Syndrome. Frontiers in physiology. 2015;6:341. doi: 10.3389/fphys.2015.00341. Epub 2015/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghorai S, Ulbright TM, Rex DK. Endoscopic findings of diverticular inflammation in colonoscopy patients without clinical acute diverticulitis: prevalence and endoscopic spectrum. The American journal of gastroenterology. 2003;98(4):802–6. doi: 10.1111/j.1572-0241.2003.07383.x. Epub 2003/05/10. [DOI] [PubMed] [Google Scholar]
- 23.Tursi A, Elisei W, Giorgetti GM, et al. Inflammatory manifestations at colonoscopy in patients with colonic diverticular disease. Alimentary pharmacology & therapeutics. 2011;33(3):358–65. doi: 10.1111/j.1365-2036.2010.04530.x. Epub 2010/12/08. [DOI] [PubMed] [Google Scholar]
- 24.Peery AF, Crockett SD, Barritt AS, et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.08.045. Epub 2015/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharucha AE, Parthasarathy G, Ditah I, et al. Temporal Trends in the Incidence and Natural History of Diverticulitis: A Population-Based Study. The American journal of gastroenterology. 2015 doi: 10.1038/ajg.2015.302. Epub 2015/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Study instructions to endoscopists
Supplemental Figure 2. Percentage by Age With Any Large or Deep Diverticula