Abstract
Objective
To evaluate the impact of Roux-en-Y gastric bypass (RYGB) on quality of life in obese diabetic patients compared to standard medical therapy for type 2 diabetes mellitus.
Methods
We prospectively studied two matched obese populations with type 2 diabetes. Thirty patients underwent laparoscopic RYGB and 31 received standard medical therapy combined with a diabetes support and education program (DSE), consisting of educational sessions on diet and exercise. Groups were matched by age, gender, weight, glucostatic parameters, and use of glucose-lowering medications (oral agents and insulin therapy). Health-related quality of life (HRQOL) was assessed using the normalized SF-36 questionnaire and data were collected at baseline and at 12-month follow-up.
Results
Diabetic patients who underwent RYGB experienced a statistically significant increase in their overall HRQOL. However, the role-physical and mental health domains increased but did not reach statistical significance. Diabetic patients in the medical therapy and DSE group did not show any significant increase in HRQOL. The between-group differences for the HRQOL changes from baseline were significant, other than for role-physical and mental health domains. Percentage changes in glucostatic parameters, discontinuation of glucose-lowering medications, and T2DM remission were not found to predict the percentage change in SF-36 scores at 12 months after RYGB.
Conclusions
For the first time, with a prospective matched control study, we demonstrate a significant improvement in HRQOL in obese diabetic patients who underwent RYGB, but not in those who were offered standard medical therapy and DSE.
Keywords: Diabetes, Bariatric Syurgery, Gastric Bypass, Quality of Life
Introduction
The alarming rise in the global prevalence of obesity is made a more pressing public health challenge by the concurrent rise in the prevalence of type 2 diabetes. Worldwide, the prevalence of overweight or obesity is projected to increase from an estimated 33% in 2005 to 57.8% by 2030 if the current trends persist [1]. Similarly, the worldwide prevalence of type 2 diabetes mellitus (T2DM) in the adult population is projected to increase from an estimated 8.3% in 2010 to 9.9% by 2030 [2]. The reported percentage of obese U.S. adults with T2DM ranges from 25 to more than 30% [3, 4].
Bariatric surgery is a highly effective long-term treatment for patients with obesity and type 2 diabetes, providing durable weight loss and improving obesity-related comorbidities, quality of life, and survival [5-7]. More recently, laparoscopic Roux-en-Y gastric bypass (RYGB) has been shown to be superior to medical therapy alone for the treatment of T2DM [2, 8].
Quality of life is a broad multi-dimensional concept that includes subjective evaluations of both positive and negative aspects of life [9]. Health is arguably one of the most important domains of overall quality of life, and the concept of health-related quality of life (HRQOL) focuses on those aspects of quality of life that reflect the evaluation and reaction of the subject to health or illness. Clinical interventions, therefore, often strive to improve not only medical outcomes but health-related quality of life (HRQOL) as well. Regrettably, no consensus exists on the importance of HRQOL as an independent outcome measure for bariatric and metabolic surgery along with weight loss and comorbidity resolution [10]. The Medical Outcomes Study Short Form (SF-36) is a well-known HRQOL measure that has been studied and validated in a variety of populations by Ware and colleagues [11]. The second version of the SF-36 questionnaire provides normative data that allow interpretation of whether or not an observed score is typical compared to the distribution of scores for other individuals.
Patients with T2DM have been shown to have a worse health-related quality of life (HRQOL) than the general population, [12] and diabetes with obesity were significantly more detrimental to measures of health outcomes than either condition alone or in combination with other comorbidities [13]. Furthermore, the presence of T2DM has been shown to have a negative impact on improvement in HRQOL after Roux-en-Y Gastric Bypass [14].
Several studies have reported improved HRQOL after bariatric surgery [14-18], but the impact of RYGB on HRQOL in obese patients with T2DM has been less defined. The impact of RYGB in such a population compared to a matched control group who underwent standard medical therapy and a Diabetes Support and Education program (DSE) has yet to be studied. Furthermore, to date, a study evaluating the potential impact of the post-operatively improved glucose homeostasis on the changes in HRQOL experienced by obese diabetic patients undergoing RYGB has yet to be published. In this investigation we prospectively studied two matched obese populations with T2DM. The aim of our study was to evaluate the impact of gastric bypass on quality of life in obese diabetic patients compared to standard medical therapy. We hypothesize that obese diabetic patients who undergo RYGB will have a significant improvement in HRQOL when prospectively compared to a matched control group who undergo standard medical therapy and DSE. A secondary aim of this study is to evaluate the likelihood that the improvement in glucose homeostasis, suspension or cessation of glucose-lowering medications, and remission of T2DM correlate with the expected improvement in HRQOL seen in this patient population following RYGB.
Methods
In a prospective cohort study, we evaluated sixty-one patients diagnosed with T2DM and morbid obesity (BMI ≥ 35 kg/m2). Clinical diagnosis of T2DM was made when any one of the following was present: current regular use of oral hypoglycemic medication, current regular use of insulin, documented diabetes by ADA criteria [19].
Based on patient selection and/or insurance approval for RYGB, 30 patients underwent laparoscopic RYGB and 31 patients received 1-year of DSE, as previously described [20]. This program was run on a per-year basis and consisted of three group educational and support sessions during the year. The educational sessions were designed to include one seminar on dietary and nutrition counseling and one on exercise. The support session allowed for the discussion in a group format of the problems associated with living with T2DM. A major aim was to encourage weight loss. The DSE was delivered by a certified diabetic educator and nutritionist. During the study, all patients received comprehensive medical management of their T2DM and of other CVD risk factors from by their primary health care provider. The goals of medical therapy were to achieve HbA1c <7%, blood pressure less than 130/80 mm Hg, LDL cholesterol less than 100mg/dL, and triglycerides less than 200 mg/dL. The treating provider made adjustments in the therapeutic agents as deemed necessary to achieve these goals.
Groups were matched by weight, age, gender, waist-to-hip ratio, glucostatic parameters and use of glucose-lowering medications.
Data were collected prospectively at baseline and at 12-month follow up in both groups. All participants signed written informed consent prior to enrollment, and all aspects of the study were approved by the Duke University Institutional Review Board.
The second version of the SF-36 questionnaire, consisting of 36 questions, was used to map eight domains, the physical component scale (PCS) and the mental component scale (MCS) for the impact of disease on health status. The 8 scales of the questionnaire include: general health, physical functioning, bodily pain, role-physical (role limitations due to physical problems), mental health, social functioning, vitality, and role-emotional (role limitations due to emotional problems). The first four domains correlate with the PCS and summarize the overall individual physical function. The remaining four domains correlate with the MCS and summarize the overall individual mental function. The second version of the SF-36 questionnaire provides normative data, and we used as a reference the norms for the standard scales in the 1998 general U.S. population. Using normative data allows comparison of the SF-36 domain or summary scores observed in our obese diabetic population with those that would be expected in the general U.S. population. Scores observed in our diabetic obese population could then be understood as a departure from expected or typical scores found in the general U.S. population. Scoring above the average general U.S. population would indicate a better HRQOL.
In all obese patients with T2DM, a set number of pre-intervention variables were examined for their effect on each SF-36 domain and summary scores. Furthermore, the effects of percentage changes in these variables were examined for their influence on the percentage change in SF-36 domain and summary scores at 12 months after RYGB. These variables included weight, BMI, waist-to-hip ratio, age, sex, fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), insulin, insulin sensitivity (Si), insulin resistance (IR), oral glucose-lowering medications, insulin therapy, and T2DM remission at 12 months. Si was estimated using the mathematical modeling methods of Bergman [21] (MINMOD Millennium, ver. 6.02), with a 3-hour frequently sampled intravenous glucose tolerance test (IVGTT). IR was estimated by HOMA according to the method described by Matthews et al. [22]. Insulin therapy and oral glucose-lowering medications, including metformin and/or GLP-1 agonists, were assessed as being present or absent. T2DM remission, also assessed as being present or absent, was defined as the patient being off glucose-lowering medications and no longer meeting the American Diabetes Association criteria for T2DM (HbA1c ≥ 6.5% (48 mmol/mol) or FPG ≥ 126mg/dL) [19].
Statistical analysis
The Shapiro–Wilk test was performed to determine the type of distribution for all tested variables. For continuous variables, arithmetic means and standard deviations were calculated, while for categorical variables, the frequency (percentage) was determined.
Paired t-test and independent t-test were used respectively to compare pre- and post-intervention normally distributed quantitative variables and to determine differences between the two groups. Related-samples Wilcoxon Signed Rank test and independent-samples Mann-Whitney U test were used respectively to compare pre- and post-intervention non-normally distributed quantitative variables and to determine differences between the two groups. McNemar Test without Yates' correction and Chi-square Test were used respectively to compare pre- and post-intervention categorical variables and to determine differences between the two groups.
Linear regression analysis with forward and backward modeling was used to examine for independent variables influencing the SF-36 scores in an obese diabetic population. Linear regression analysis was also used to examine for percentage changes in independent factors influencing the percentage change in SF-36 scores in a diabetic obese population at 12 months after RYGB. Beta coefficient and combined r2 have been reported.
The SPSS statistical software program (version 21.0; SPSS, Chicago, IL) was used for all analyses. All tests were 2-tailed. The results are expressed as mean ± SD and statistical significance was considered for P values of less than 0.05.
Results
The cohort population consisted of sixty-one subjects of which 30 underwent RYGB and 31 underwent standard medical therapy and DSE. Patients in the two groups were matched by age (RYGB: 48.8 ± 7.5 years versus DSE: 47.4 ± 8.4 years, P=0.463), gender (RYGB: 66.7 % female versus DSE: 67.7% female, P=0.929), weight (RYGB: 120.1 ± 17.0 kg versus DSE: 114.3 ± 17.1 kg, P=0.186), waist-to-hip ratio (RYGB: 0.98 ± 0.08 versus DSE: 0.97 ± 0.09, P=0.580), IR (RYGB: 8.1 ± 12.2 versus DSE: 6.1 ± 6.3, P=0.444), Si (RYGB: 1.5 ± 1.2 mU/L-1*min-1 versus DSE: 1.4 ± 1.2 mU/L-1*min-1, P=0.753), FPG (RYGB: 155.6 ± 47.2 mg/dL versus DSE: 149.1 ± 50.6 mg/dL, P=0.607), HbA1c (RYGB: 7.53 ± 1.3 % (59 ± 14.2 mmol/mol) versus DSE: 7.51 ± 1.3 % (59 ± 14.2 mmol/mol), P=0.943), Insulin (RYGB: 21.9 ± 29.4 μU/mL versus DSE: 16.9 ± 14.6 μU/mL, P=0.401), oral anti-diabetic medications (RYGB: 27% versus DSE: 29%, P=0.837) and insulin therapy (RYGB: 73% versus DSE: 71%, P=0.837). These data have been previously published [20].
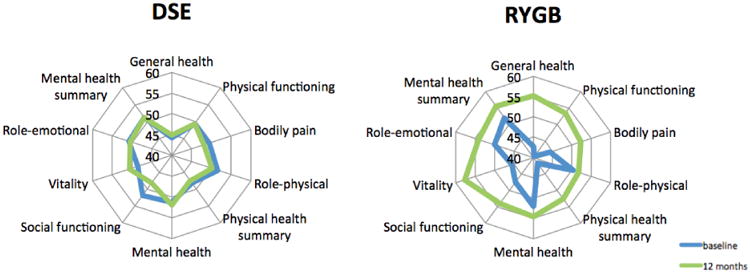
All patients completed the SF-36 questionnaire at enrollment and at 12 months after intervention. Table 1 shows SF-36 mean (± SD) scores for the eight domains and the two component summary scores for both groups at baseline and at 12 months. Obese diabetic patients have lower HRQOL than the general US population for several SF-36 domains scores other than role-physical, mental health and role emotional (Table 1). PCS in the obese diabetic population is much lower compared to the general U.S. population, whereas the MCS is slightly higher than the one experienced in the general U.S. population.
Table 1.
HRQOL assessed using the normalized SF-36 questionnaire. Data are presented with mean ± SD.
| All | DSE | RYGB | |||||
|---|---|---|---|---|---|---|---|
| baseline | baseline | 12 months | Δ % at 12m | baseline | 12 months | Δ % at 12m | |
| General health | 43.50±7.88 | 44.30±7.76 | 44.861±7.69b | 1.77±15.80b | 42.67+8.06 | 55.07±6.43a,b | 60.56±55.84b |
| Physical functioning | 44.94±10.23 | 49.42±8.21b | 49.351±6.55b | 1.601±21.58b | 40.32+10.16b | 53.43±5.30a,b | 126.48±293.90b |
| Bodily pain | 46.92±10.94 | 49.45±9.99 | 48.14+11.78 | -1.12±20.38b | 44.311±11.43 | 52.36±9.67a | 41.701±47.57b |
| Role-physical | S1.00±8.82 | 51.661±8.83 | 50.16±8.95 | -1.56±14.39 | 50.331±8.91 | 51.82±8.51 | 40.281±116.09 |
| Physical component summary | 45.08±9.49 | 48.251±7.49b | 47.46±7.13b | 0.76±16.43b | 41.811±10.33b | 52.51±6.65a,b | 41.561±53.83b |
| Mental health | 51.62±8,91 | 51.261±9.16 | 51.90±8.96 | 3.061±17.86 | 51.991±8.78 | 54.56±7.39 | 6.221±19.77 |
| Social functioning | 49.741±8.85 | 51.871±7.83 | 48.211±10.02a,b | -6.231±16.05b | 47.541±9.41 | 53.931±5.70a,b | 45.291±130.40b |
| Vitality | 47.101±10.38 | 48.621±10.60 | 50.671±8.74b | 7.361±24.48b | 45.531±10.08 | 57.801±8.35a,b | 95.971±122.30b |
| Role-emotional | 50.461±8.74 | 50.891±10.09 | 50.421±9.27 | 1.641±20.98b | 50,021±7.23 | 54.211±4.30a | 29.321±63.50b |
| Mental component summary | 51.681±8.72 | 51.251±8.73 | 51.171±8.3b | 1.231±11.01b | 51.991±8.78 | 55.621±6.22a,b | 7.661±18.29b |
P<0.05 follow-up vs baseline within group.
P<0.05 between groups
As shown in Figure 1, diabetic patients who underwent RYGB experienced a significant increase in their HRQOL, except in the role-physical and mental health domains, which increased but did not reach statistical significance. Control diabetic subjects who underwent 12 months of standard medical therapy and DSE did not show any significant increase in any HRQOL scores. In fact, these patients had worsened scores for physical functioning, bodily pain, role-physical, PCS, social functioning, role-emotional and MCS post-intervention. In this group, the worsening in social functioning at 12 months was statistical significant. The between-group differences for the percentage changes from baseline in all the HRQOL were significant, except in role physical and mental health at 12 months.
Figure 1. HRQOL assessed using the normalized SF-36 questionnaire.
There was one post-operative superficial wound infection in the RYGB group. There was no mortality. There were no other surgical complications, including anastomotic leak, stricture, obstruction, or hernia in this cohort. At 12 months the percentage change in weight was − 28.0 ± 8.3 % in the RYGB group and 0.2 ± 4.36 % in the DSE group (respectively, P<0.001 and P=0.831). At 12 months 60% in the RYGB group experienced T2DM remission (P<0.001), while in the DSE group no remission was observed. Within the RYGB group, 73% of the patients were taking oral glucose-lowering medications pre-operatively and 27% required insulin, while at 12 months after the surgery the prevalence of oral agents decreased to 20% (P<0.001) and no patients required insulin therapy (P<0.001). Within the DSE group, 71% were on oral glucose-lowering medications at enrollment and 29% required insulin. At 12 months after DSE one patient discontinued his insulin therapy and started oral glucose-lowering agents. The between-group differences for the changes from baseline in the use of both oral glucose-lowering agents and insulin therapy were significant (P<0.001).
Predictors of HRQOL improvement in obese diabetic patients at 12 months after RYGB
Patient demographics at enrollment (age and sex), percentage changes in anthropometric measurements (weight, BMI and waist-to-hip ratio), glucostatic parameters (FPG, HbA1c, insulin, IR and Si) and glucose-lowering medications (oral agents and insulin therapy) were run against the percentage changes in SF-36 domains and component summaries scores in a univariate analysis model. Percentage change in BMI (Beta =-.482, p=0.008) is a significant predictor of percentage change in vitality 12 months after RYGB. Greater improvement in BMI was associated with a greater increase in vitality after RYGB. Percent change in glucostatic parameters or other studied variables were not found to affect the percent change in SF-36 scores 12 months post-RYGB.
Baseline predictors of HRQOL in obese diabetic patients
Patient demographics (age and sex), anthropometric measurements (weight, BMI and waist-to-hip ratio), glucostatic parameters (FPG, A1c, insulin, IR and Si) and glucose-lowering medications (oral agents and insulin therapy) were run against SF-36 domains and component summaries scores in a univariate analysis model. Age (Beta =-.301, p=0.014) and BMI (Beta =-.331, p=0.007) are significant predictors of physical functioning in obese diabetic subjects: higher BMI and increased age are predictors of lower physical functioning scores (combined r2=.19, p=0.002). Age (Beta =-.272, p=0.034) is also a significant predictor of bodily pain in obese diabetic subjects: older subjects experienced worse bodily pain scores. Gender is a significant predictor of general health and PCS in obese diabetic subjects (respectively, Beta =.273, p=0.033 and Beta =.272, p=0.036): males experienced higher general health and PCS scores (respectively, r2=.0.74, p=0.033 and r2=.102, p=0.036). Gender (Beta =.343, p=0.007) is also a significant predictor of vitality in obese diabetic subjects: males experienced higher vitality scores. Among the analyzed variables, no predictors were found for all the other SF-36 domains (role-physical, social functioning, role-emotional and mental health) and MCS in our obese diabetic population.
Discussion
Our study demonstrates the superiority of RYGB in improving HRQOL, as measured by the SF-36 questionnaire, in obese patients with T2 DM over standard medical therapy combined with a diabetes support and education program. However, the associated percentage changes in glucostatic parameters, discontinuation of glucose-lowering medications (oral agents and insulin therapy) and T2DM remission do not appear to influence the percentage change in SF-36 scores at 12 months after RYGB.
Several studies have shown an improved HRQOL after bariatric surgery, starting as early as 3 months and leveling off thereafter in the long term [14-18, 23]. However, in obese diabetic patients the impact of RYGB in improving HRQOL has never been prospectively compared to the effects on HRQOL of standard medical therapy and DSE in a matched control group who did not undergo bariatric surgery. For the first time, to our knowledge, with a prospective study, we show that obese diabetic patients reported worse HRQOL than the general U.S. population. Additionally, HRQOL significantly improves and exceeds the general U.S. population average after RYGB, but not after standard medical therapy and DSE. After RYGB, improvement in mental health and role-physical domains was not statistically significant in our study. However, scores in both domains improved at 12 months. Furthermore, in line with published data [17, 23], our data show that obese diabetic patients report a mental health related quality of life less impaired than other SF-36 scores and comparable to mean values of the general US population, and this might partly explain the less significant improvement following surgery. Randomized clinical trials with standard medical interventions for diabetes fail to show improvements in HRQOL despite improvements in primary clinical outcomes [24, 25]. Indeed, Hill-Briggs et al. [26] suggested that since functional health assessed by SF-36 appears to exhibit a pattern of deterioration over time independent of intervention, stability in SF-36 scores may indicate a positive effect of medical intervention on HRQOL. This is consistent with the statistically significant worsening scores for physical functioning, bodily pain, role-physical, PCS, social functioning, role-emotional and MCS observed in our study. This further heightens the value of surgical therapy, as this intervention resulted in an improvement in HRQOL, whereas the best that might be expected from standard medical therapy is stabilization in HRQOL scores.
At any rate, these findings challenge standard medical therapy alone for T2DM in morbidly obese patients because while dietary change and medical intervention may help delay disease progression and the onset of diabetes complications [27], they might themselves be associated with a decreased quality of life. Recently, in patients with diabetes, Bjorner et al. [28] showed that 1-point lower selected SF-36 scores was associated with an excess risk of up to 9% for mortality and hospitalization and 12% for inability to work. That surgical therapy for obesity-related diabetes is associated with an improvement in quality of life along with comorbidity resolution is, therefore, is an extraordinarily important finding.
No published study to date has evaluated the impact of changes in glucostatic parameters, glucose-lowering medications, and DM remission on the changes in HRQOL (longitudinal) of obese diabetic patients after RYGB. A few studies have analyzed the effect of change in diabetes status on change in HRQOL in patients who underwent bariatric surgery [12, 17]. Weiner et al. [12] studied 524 patients who underwent gastric banding, RYGB, and BPD (Scopinaro) and reported the HRQOL up to 12 months using four questionnaires including the Short Form 12. Patients were grouped into non-diabetic, diabetic with remission, and diabetic with improvement based on the bariatric quality of life questionnaire, which was developed and validated by the authors only in its German version within their study. Following bariatric surgery, HRQOL improved more in diabetic patients with remission and/or improvement compared to the non-diabetic group. A noted weakness of that study is neither HbA1c nor FPG were collected, and the diabetes remission after surgery was assessed only through the questionnaire. In another study, Julia et al. [17] evaluated 71 patients who underwent RYGB and reported the HRQOL up to 12 months using the SF-36 questionnaire. Change in PCS was mostly associated with changes in either BMI or comorbidity status except for diabetes, dyslipidemia and sleep apnea. They concluded that improvement in diabetes was not significantly related to change in PCS and speculated that this might be explained by the lack of power due to the limited number of patients with T2DM at baseline (n=24), with 41.7% (n=10) experiencing diabetes remission at 12 months.
There is conflicting data regarding the expected association between extent of weight loss and the improvement of HRQOL after bariatric surgery. [14, 15, 17, 23, 30] A few of these studies used the SF-36 questionnaire. Sarwer et al. [23] showed that 92 weeks after gastric bypass, greater weight loss was associated with significant improvements on the general health and vitality domains of the SF-36. Julie et al. [17], reporting data only for the SF-36 summaries scores, showed that PCS was mainly associated with longitudinal modification of BMI 12 months after RYGB. In this study we show that a larger decrease in BMI is a predictor only of higher increase in vitality at 12 months after RYGB. The extent of weight loss, as measured by the percentage of both BMI and weight changes at 1 year, failed to predict other SF-36 score changes at 1 year after RYGB. We caution here that SF-36 has been shown to be less sensitive to weight loss than other obesity-specific questionnaires, and this should be taken into account when looking for relationships between weight loss and HRQOL addressed with the SF-36 questionnaire. Kolotkin et al alluded to a potential value of using more than one measure in such a trial. [31]
In our study, improvement in glucose homeostasis, T2DM remission, and discontinuation of glucose-lowering medications did not correlate with the improvement in SF-36 domain and summary scores seen in patients after RYGB. This finding is somewhat puzzling. It is important to note that other studies assessing the HRQOL of diabetic subjects using a questionnaire such as the SF-36, usually did not detect a relationship between diabetes markers and HRQOL [12, 29]. We suspect that assessing the HRQOL by a diabetes-specific questionnaire rather than a general one might have revealed some relationship between the improved glycemic control and the HRQOL after RYGB. A diabetes-specific questionnaire might have properly assessed diabetes-specific symptoms and experiences and thereby uncover a positive impact of a better glycemic control on health issues that are specific to diabetic patients.
Conceptualization of HRQOL in diabetes must include patient experiences of associated symptoms, complications, and even treatment of the disease. We surmise that patient expectations from the surgical procedure itself and an awareness of having made a decision to pursue a surgical therapy that is known to be associated with long-term amelioration of comorbidities might play an important role in overall health perception after RYGB. Furthermore, obese diabetic patients exploring bariatric surgery are well aware of the unparalleled impact of RYGB on diabetes, and the expectation that a long-term disability might be remitted could also influence quality of life perception post-operatively. The complications of T2DM have been shown to impact the HRQOL of diabetic patients [32]. Our prospective study was not designed to assess the complications of T2DM their rate of remission after RYGB. However, we suspect that the expected reduced number and severity of T2DM related complications after RYGB could also impact quality of life perception and should be investigated by further studies.
Existing literature [12] and our data confirm that age, gender and BMI can be predictors of the degree of HRQOL in obese diabetic patients. Focusing also on diabetes relevant variables, we did not find any relationship between parameters assessing glycemic control (HbA1c, FPG, insulin, IR and Si) or T2DM treatment regimen and HRQOL in T2DM obese patients. Klein et al. [33] using the SF-36 questionnaire found that physical function, physical role and general health scores were associated with HbA1c levels only in the sub-populations of subjects with younger onset of diabetes. Weiner et al. [12], supported by studies using disease-specific questionnaires and showing significant associations between HbA1c and HRQOL in some sub-populations, suggested that “there may be a curvilinear relationship between HbA1c level and health-related quality of life, perhaps as a result of decrements in quality of life associated with more complex treatment regimens”. There is also evidence that increasing treatment intensity in T2DM patients from diet and exercise alone, to oral medications, and to insulin, is associated with worsening quality of life [29, 34-36]. Jacobson et al. [29] pointed out that for T2DM patients “insulin treatment was associated with lower levels of satisfaction with diabetes and greater impact of diabetes on quality of life”. Our data support neither the notion that a relationship exists between glycemic control and HRQOL, nor that T2DM patients treated with glucose-lowering medications experienced lower quality of life than those not so treated. Additionally, it is important to emphasize that our study design did not stratify our obese diabetic population in terms of HbA1c levels. Furthermore, as previously pointed out, one limitation of our study is not having used a diabetes-specific HRQOL questionnaire along with the SF-36.
Another limitation of our study is that we did not collect data regarding the duration of diabetes and use of insulin therapy before the enrollment. Rubin et al. [34] pointed out that the duration of diabetes has not been consistently associated with HRQOL. Because of the absence of these data we could not address their impact on both the HRQOL in the diabetic obese patients and on the changes of HRQOL after RYGB.
In conclusion, obese patients with T2DM have poor HRQOL as measured by the SF-36 health survey. With a prospective matched control study, we demonstrate a significant improvement in HRQOL in obese diabetic patients who underwent RYGB, but not in those who were offered standard medical therapy and DSE. However, the improvement in glucose homeostasis, reduced use of glucose-lowering medications, and remission of diabetes were not predictors of the observed improved HRQOL at 12 months after RYGB.
Table 2. Changes in Glucose metabolism Parameters.
| Parameter | DSE (n=31) | RYGB (n=30) | P1 | P2 | |||
|---|---|---|---|---|---|---|---|
| Baseline | 12-month | Baseline | 12-month | DSE | RYGB | ||
| HbA1c (%) | 7.5±0.2 | 7.9±0.3 | 7.5±0.2 | 6.3±0.2 | NS | <0.001 | <0.001 |
| Fasting glucose (mg/dL) | 149.1±9.2 | 155.5±8.4 | 155.6±8.6 | 113.5±8.7 | NS | <0.001 | <0.001 |
| Fasting Insulin (μU/mL) | 16.9±2.6 | 18.1±3.1 | 21.9±5.4 | 3.6±5.1 | NS | <0.001 | 0.002 |
| Insulin Sensitivity (mU/L-1·min-1) | 1.4±0.2 | 1.0±0.2 | 1.5±0.2 | 4.0±0.5 | NS | <0.001 | 0.001 |
P1=statistical significance within group, P2= statistical significance between groups
Acknowledgments
Research supported by National Institute of Diabetes and Digestive and Kidney Diseases grant no.K23 DK075907 from the National Institutes of Health, Bethesda, MD to Dr Alfonso Torquati.
Footnotes
Disclosures: Drs. Philip Omotosho, Alessandro Mor, Prapimporn Chattranukulchai Shantavasinkul, Leonor Corsino, and Alfonso Torquati have no conflicts of interest or financial ties to disclose.
References
- 1.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 2.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. The New England journal of medicine. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 3.Hofso D, Jenssen T, Hager H, Roislien J, Hjelmesaeth J. Fasting plasma glucose in the screening for type 2 diabetes in morbidly obese subjects. Obesity surgery. 2010;20:302–307. doi: 10.1007/s11695-009-0022-5. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. The American journal of medicine. 2009;122:248–256 e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009:CD003641. doi: 10.1002/14651858.CD003641.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. The New England journal of medicine. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. Social science & medicine (1982) 1998;46:1569–1585. doi: 10.1016/s0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 10.Kral JG, Sjostrom LV, Sullivan MB. Assessment of quality of life before and after surgery for severe obesity. The American journal of clinical nutrition. 1992;55:611s–614s. doi: 10.1093/ajcn/50.5.1195. [DOI] [PubMed] [Google Scholar]
- 11.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2001;10:405–413. doi: 10.1023/a:1012588218728. discussion 415-420. [DOI] [PubMed] [Google Scholar]
- 12.Weiner S, Neugehauer EA. Quality of life of diabetic patients with medical or surgical treatment. Nutricion hospitalaria. 2013;28(Suppl 2):66–77. doi: 10.3305/nh.2013.28.sup2.6716. [DOI] [PubMed] [Google Scholar]
- 13.Oldridge NB, Stump TE, Nothwehr FK, Clark DO. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. Journal of clinical epidemiology. 2001;54:928–934. doi: 10.1016/s0895-4356(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 14.Torquati A, Lutfi RE, Richards WO. Predictors of early quality-of-life improvement after laparoscopic gastric bypass surgery. American journal of surgery. 2007;193:471–475. doi: 10.1016/j.amjsurg.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 15.Dixon JB, Dixon ME, O'Brien PE. Quality of life after lap-band placement: influence of time, weight loss, and comorbidities. Obesity research. 2001;9:713–721. doi: 10.1038/oby.2001.96. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)--an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1998;22:113–126. doi: 10.1038/sj.ijo.0800553. [DOI] [PubMed] [Google Scholar]
- 17.Julia C, Ciangura C, Capuron L, Bouillot JL, Basdevant A, Poitou C, Oppert JM. Quality of life after Roux-en-Y gastric bypass and changes in body mass index and obesity-related comorbidities. Diabetes & metabolism. 2013;39:148–154. doi: 10.1016/j.diabet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Vincent HK, Ben-David K, Conrad BP, Lamb KM, Seay AN, Vincent KR. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2012;8:346–354. doi: 10.1016/j.soard.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Standards of medical care in diabetes--2013. Diabetes care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoo CM, Chen J, Pamuklar Z, Torquati A. Effects of Roux-en-Y Gastric Bypass or Diabetes Support and Education on Insulin Sensitivity and Insulin Secretion in Morbidly Obese Patients With Type 2 Diabetes. Annals of surgery. 2013 doi: 10.1097/SLA.0b013e318294d19c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocrine reviews. 1985;6:45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Sarwer DB, Wadden TA, Moore RH, Eisenberg MH, Raper SE, Williams NN. Changes in quality of life and body image after gastric bypass surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2010;6:608–614. doi: 10.1016/j.soard.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill-Briggs F, Gary TL, Baptiste-Roberts K, Brancati FL. Thirty-Six-Item Short-Form Outcomes Following a Randomized Controlled Trial in Type 2 Diabetes. Diabetes Care. 2005;28:443–444. doi: 10.2337/diacare.28.2.443. [DOI] [PubMed] [Google Scholar]
- 25.Lobo CM, Frijling BD, Hulscher ME, Bernsen RM, Grol RP, Prins A, van der Wouden JC. Effect of a comprehensive intervention program targeting general practice staff on quality of life in patients at high cardiovascular risk: a randomized controlled trial. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2004;13:73–80. doi: 10.1023/B:QURE.0000015285.08673.42. [DOI] [PubMed] [Google Scholar]
- 26.Hill-Briggs F, Gary TL, Baptiste-Roberts K, Brancati FL. Thirty-six-item short-form outcomes following a randomized controlled trial in type 2 diabetes. Diabetes care. 2005;28:443–444. doi: 10.2337/diacare.28.2.443. [DOI] [PubMed] [Google Scholar]
- 27.Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes care. 2004;27:2067–2073. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- 28.Bjorner JB, Lyng Wolden M, Gundgaard J, Miller KA. Benchmarks for interpretation of score differences on the SF-36 health survey for patients with diabetes. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2013;16:993–1000. doi: 10.1016/j.jval.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes care. 1994;17:267–274. doi: 10.2337/diacare.17.4.267. [DOI] [PubMed] [Google Scholar]
- 30.Chang CY, Huang CK, Chang YY, Tai CM, Lin JT, Wang JD. Prospective study of health-related quality of life after Roux-en-Y bypass surgery for morbid obesity. The British journal of surgery. 2010;97:1541–1546. doi: 10.1002/bjs.7179. [DOI] [PubMed] [Google Scholar]
- 31.Kolotkin RL, Norquist JM, Crosby RD, Suryawanshi S, Teixeira PJ, Heymsfield SB, Erondu N, Nguyen AM. One-year health-related quality of life outcomes in weight loss trial participants: comparison of three measures. Health and quality of life outcomes. 2009;7:53. doi: 10.1186/1477-7525-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donald M, Dower J, Coll JR, Baker P, Mukandi B, Doi SA. Mental health issues decrease diabetes-specific quality of life independent of glycaemic control and complications: findings from Australia's living with diabetes cohort study. Health and quality of life outcomes. 2013;11:170. doi: 10.1186/1477-7525-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein BE, Klein R, Moss SE. Self-rated health and diabetes of long duration. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes care. 1998;21:236–240. doi: 10.2337/diacare.21.2.236. [DOI] [PubMed] [Google Scholar]
- 34.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes/metabolism research and reviews. 1999;15:205–218. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 35.Petterson T, Lee P, Hollis S, Young B, Newton P, Dornan T. Well-being and treatment satisfaction in older people with diabetes. Diabetes care. 1998;21:930–935. doi: 10.2337/diacare.21.6.930. [DOI] [PubMed] [Google Scholar]
- 36.Glasgow RE, Ruggiero L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes care. 1997;20:562–567. doi: 10.2337/diacare.20.4.562. [DOI] [PubMed] [Google Scholar]


