Abstract
Influenza infection typically initiates at respiratory mucosal surfaces. Induction of immune responses at the sites where pathogens initiate replication is crucial for the prevention of infection. We studied the adjuvanticity of GPI-anchored CCL28 co-incorporated with influenza HA-antigens in chimeric virus-like particles (cVLPs), in boosting strong protective immune responses through an intranasal (i.n.) route in mice. We compared the immune responses to that from influenza VLPs without CCL28, or physically mixed with soluble CCL28 at systemic and various mucosal compartments. The cVLPs containing GPI-CCL28 showed in-vitro chemotactic activity towards spleen and lung cells expressing CCR3/CCR10 chemokine receptors. The cVLPs induced antigen specific endpoint titers and avidity indices of IgG in sera and IgA in tracheal, lung, and intestinal secretions, significantly higher (4–6 fold) than other formulations. Significantly higher (3–5 fold) hemagglutination inhibition titers and high serum neutralization against H3N2 viruses were also detected with CCL28-containing VLPs compared to other groups. The CCL28-containing VLPs showed complete and 80% protection, when vaccinated animals were challenged with A/Aichi/2/1968/H3N2 (homologous) and A/Philippines/2/1982/H3N2 (heterologous) viruses, respectively. Thus, GPI-anchored CCL28 in influenza VLPs act as a strong immunostimulator at both systemic and mucosal sites, boosting significant cross-protection in animals against heterologous viruses across a large distance.
Keywords: Antibody avidity, hemagglutination, influenza, mucosal adjuvant, neutralization, virus-like particles (VLPs)
Graphical abstract
Introduction
With recurrent worldwide epidemics, influenza presents a major health problem and affects hundreds of millions of people every year (1). In the United States alone, infections with influenza virus result in approximately 20,000 deaths and 100,000 hospitalizations each year (2). Vaccination is considered to be the most effective and economic strategy in preventing influenza virus infections (3). Despite having various vaccines in the market for decades, several limitations still exist such as relatively long production times, limited production capacity, moderate efficacy in certain populations, lack of generation of mucosal immunity, and limited cross-reactivity (4, 5). These are important issues that need to be addressed. We must focus mainly on cross-protection by inducing either a broadly cross-protecting systemic or mucosal immunity at the site of infection. The above goals could be achieved by utilizing a mucosal immunostimulator/adjuvant to generate mucosal immunity at viral entry sites which may effectively stop the initiation of a systemic infection (6–8).
Mucosal immunity may provide protection by mechanisms such as preventing the attachment, replication, and growth of the etiological agent at mucosal surfaces, and clearance of the pathogen with the development of innate and adaptive immune responses (8, 9). Compared to systemic immunization, mucosal immunization evokes local mucosal immune responses by targeting specific mucosal sites to produce IgA and neutralize viruses, thus avoiding the manifestation and dissemination of an infection (10, 11). Unfortunately, only a few approved human mucosal vaccines exist in the market and some have failed to stimulate strong IgA immune responses (12, 13). Therefore, there is great need for new vaccines and vaccination technologies that can effectively induce both systemic and mucosal immunity. The development of mucosal vaccines will also benefit from the development of safe and effective mucosal adjuvants and delivery systems. A mucosal adjuvant that can induce a protective immune response at various mucosal areas would be an important advance for development of the next generation of mucosal vaccines (14, 15).
Chemokines, as their name implies, are a group of small essential chemo-attractant peptides that regulate the immune system, both during homeostatic and inflammatory conditions (16). They are known to serve as regulators for the migration and activation of lymphocytes resulting in the enhancement of Th1 and Th2 immune responses (17). For example, in addition to chemotaxis, chemokines modulate both lymphocyte development, and priming and effector functions, as well as playing a critical role in immune surveillance (18). Thus, the modulation of immune responses through chemokines is an interesting concept for vaccine designs. The chemokine CCL28, also known as mucosa-associated epithelial chemokine (MEC), is expressed at high levels in the epithelium of various mucosal linings and is involved in the trafficking of antibody-secreting cells (ACSs) into gastrointestinal and respiratory mucosal tissues (19). This chemo-attractive effect is achieved by the binding of CCL28 to the CCR3 and CCR10 chemokine receptors (19, 20). Simultaneously, studies have revealed that CCL28 is involved in the more robust recruitment of relevant immune cells involved in antigen recognition, immune priming, and pathogen clearance (21–23). In order to increase the effectiveness of a vaccine with enhanced neutralization breadth and potency, we have investigated the effects of CCL28 as a mucosal immunostimulator/adjuvant. VLPs lack a viral genome, and retain the structure of a virus particle, and they can be engineered to have other membrane proteins on their surfaces. VLP-displayed antigens are efficiently taken up by antigen presenting cells (APCs) and induce potent immune responses (24). In the present study, a vaccine composition which contains influenza hemagglutinin (HA) and glycosylphosphatidylinositol (GPI)-anchored CCL28 co-incorporated into the same cVLP, was investigated as a strategy for influenza vaccine development. A unique advantage of our approach is the delivery of antigen and an immune stimulator to the same immune cells, recruiting IgA-secreting B cells to migrate or reside in respiratory mucosal tissues. Thus, GPI-anchored CCL28 represents a promising candidate to act as a novel mucosal adjuvant/immunostimulator in influenza vaccine development. We have previously investigated the importance of various immunostimulators such as GMCSF, GIFT-4 (25), and flagellin (26, 27) into VLPs, and found that these adjuvants significantly promoted antigen-specific humoral and cellular immune responses, conferring cross-protection against lethal viral challenges. The above findings, along with the importance of mucosal immunity during influenza infection, prompted us to design the current study. Thus, in the present study, we investigated the potential of the adjuvanticity of GPI-anchored CCL28 with influenza VLPs at systemic and mucosal compartments and compared immune responses to those obtained with influenza VLPs without CCL28, as well as VLPs co-delivered with soluble CCL28 (sCCL28).
Materials and Methods
Cell lines and viruses
Spodoptera frugiperda insect cells (SF9, ATCC: CRL-1711), and Madin Darby Canine Kidney cells (MDCK, ATCC: PTA-6500) were maintained as described previously (26). Mouse adapted A/Aichi/2/1968 (homologous), and A/Philippines/2/1982 (heterologous) H3N2 viruses were prepared as described previously (27). The LD50 (Lethal dose inducing 50% mortality) and TCID50 (Tissue culture infectious dose infecting 50% cells) of these strains were determined by infection of mice with serial viral dilutions and calculated by the Reed and Muench method (28).
Construction and expression of GPI-anchored CCL28
The membrane-bound form of CCL28 was engineered by fusing the signal peptide from honeybee mellitin to facilitate GPI-CCL28 expression in insect cells (29) and a sequence from murine CD59 glycolipid-anchoring to provide membrane-anchoring (30). The coding sequences of the signal peptide from the honeybee melittin and the murine CD59 GPI-anchor were fused to the 5′- and 3′- ends of the murine CCL28 coding sequence in frame to obtain the full-length gene encoding a GPI-anchored CCL28 by overlapping PCR (31–33). The resulting GPI-CCL28 encoding gene was then cloned into transfer vector pFastBac-1 plasmid (Invitrogen, Carlsbad, CA, USA). The accuracy of the full-length encoding sequence of GPI-CCL28 was confirmed by DNA sequence analysis. A recombinant baculovirus (rBV) expressing GPI-CCL28 was generated by using the Bac-to-Bac insect cell protein expression system (Invitrogen, Carlsbad, CA, USA). To confirm whether GPI-anchored CCL28 was membrane-oriented translocated and expressed on cell surfaces, SF9 cells (ATCC, Manassas, VA 20110 USA) were infected with rBVs expressing GPI-CCL28 at optimum multiplicity of infection (MOI). Two days later, cells were harvested and stained with FITC labeled goat anti-mouse CCL28 antibodies (BioLegend, San Diego, CA, USA). An unstained, and antibody isotype control were used as negative controls. Fluorescent intensity was recorded and analyzed by FACS with a BD FACSCanto II flow cytometer (BD Biosciences, San Diego, CA, USA).
Production of influenza VLPs
M1 VLPs used as a negative control were produced by infection of SF9 cells with rBV expressing M1, while Influenza VLPs (HA/M1 VLPs) were produced by co-infection of insect cells with rBVs expressing HA and M1 of Aichi virus as described previously (34). The cVLPs containing GPI-anchored CCL28 (HA/M1/GPI-CCL28 VLPs) were produced by co-infection of SF9 cells with rBVs expressing HA, M1, and GPI-CCL28 at the MOIs of 2:1:1 (35). At 60 h post-infection, VLPs were concentrated from cell culture supernatant by porous fiber filtration using the Quixstand benchtop system (GE Healthcare, Uppsala, Sweden) and purified using sucrose density gradient centrifugation (31). The VLP protein concentration was determined by Bio-Rad protein assay (Bio-Rad, California, LA, USA). The HA, M1, and CCL28 protein profiles in VLPs were analyzed by western blot using antibodies against the respective antigens. We confirmed the quality and purity of VLPs preparations by Transmission electron microscopy (TEM). A quantitative ELISA was also done as described previously with minor modifications, to determine the CCL28 and HA contents in VLPs, using recombinant CCL28 (ProSpec, Brunswick, NJ, USA) and HA (Sino Biological Inc., North Wales, PA, USA) as the calibration standards (36).
In-vitro chemotactic activity of GPI-anchored CCL28 in cVLPs
To determine whether GPI-CCL28-containing VLPs still retain their functional activity, we tested the potency of cVLPs exhibiting in-vitro chemotactic activity (37). The assay was conducted using Transwell plates with 5–8 µm pore inserts (Corning Costar, Cambridge, MA, USA). In brief, mouse spleen and lung tissues were harvested and single cells suspensions were prepared. Cells were suspended at 1 × 107 cells per ml in RPMI 1640 media containing 1 mg per ml bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) and 20 mol/ per m3 HEPES (pH 7.4), and applied to upper wells (100 µl per well). All VLPs with or without GPI-CCL28 (1 × 10−4 mol per m3) were applied to lower wells (600 µl per well) in complete RPMI 1640 media (38, 39). After 4 h at 37 °C, inserts were removed and cells were pelleted by centrifugation at 250 × g for 5 min, resuspended in the FACS buffer, and stained with APC labeled anti-mouse CCR3 and FITC labeled anti-mouse CCR10 antibodies. Cells were analyzed by FACS with a BD FACSCanto II flow cytometer (BD Biosciences, San Diego, CA, USA). Each chemotaxis experiment was performed in duplicate wells with minimum of three times, and a representative experiment is shown in the results.
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH). All animal studies were approved by the Emory University Institutional Animal Care and Use Committee (IACUC) (Approval Number: DAR-2003060-041318GA). The 6–8 week healthy Balb/c female mice were purchased from the Jackson Laboratory and housed in the animal facility at Emory University. Immunization and bleeding were performed under mild anesthesia that was induced and maintained with ketamine hydrochloride and xylazine, and all efforts were made to minimize pain.
Groups for immunization
Groups of 5–6 mice were used for immunization experiments. Animals were immunized through i.n. route with the VLPs, containing 1 µg of HA and 0.5 µg of GPI-anchored or sCCL28. Phosphate-buffered saline (PBS) and M1 VLPs were used as negative control groups. The immunization groups were:
PBS
M1 VLPs
HA/M1 VLPs
HA/M1 VLPs + sCCL28
cVLPs (HA/M1/GPI-CCL28).
For immunization, VLPs were resuspended in 20 µl PBS and instilled into nares of lightly anesthetized animals. For the sCCL28 group, 0.5 µg of recombinant CCL28 (ProSpec, Brunswick, NJ, USA) was physically mixed with HA/M1 VLPs prior to immunization. The doses of VLPs contained 1 µg HA with or without 0.5 µg of CCL28 (membrane-bound or soluble) per animal. Mice were immunized at day 0, and a booster was given on day 14.
Sample collection
Blood samples were collected by targeting submandibular veins of anesthetized animals and allowed to clot for 10 min at 37 °C. Sera were collected from the clotted blood by centrifugation at 1500 × g for 10 min at 4° C. At day 35, after collection of blood samples, animals were sacrificed followed by the collection of tracheal, lung, and intestinal lavage samples by repeated flushing of the respective cavities with 1 ml of ice cold lavage medium (0.9%, w/v, NaCl; 0.05%, v/v, Tween 20; 0.1%, w/v, NaN3; and 1 mol per dm3 PMSF). Sera and mucosal washes were stored at − 80 °C till further use (40).
Serum and mucosal ELISA endpoint titers
The influenza-specific endpoint titers of IgG, IgA, and IgG subtypes were determined by enzyme-linked immunosorbent assay (ELISA) (41). Briefly, 96-well flat bottom microtiter plates (Nunc-Immuno Plate Maxisorp; Nunc Life Technologies, Basel, Switzerland) were coated with 200 ng per well of inactivated Aichi virus in a carbonate coating buffer (pH 9.2) overnight at 4 °C. After blocking with 3% BSA (Sigma-Aldrich, St. Louis, MO, USA), the serially diluted serum and mucosal samples were added and incubated overnight at 4 °C. The detection color was developed using horseradish peroxidase (HRP)-labeled goat anti-mouse IgG, IgG1, IgG2a, IgG2b, and IgA antibodies (Southern Biotech, Birmingham, AL, USA) at RT for 1 h. After extensive washing, the substrate tetramethylbenzidine (TMB; Invitrogen, Carlsbad, CA, USA) was added and the reaction was stopped with 2N H2SO4. The optical density at 450 nm (OD450) was read with an ELISA reader (BioTek, Winooski, VT, USA). The highest dilution which gave an OD450, two fold higher than that of the naive group without dilution, was designated as the antibody endpoint titer.
ELISPOT assay
For estimating virus-specific IgG and IgA ASCs, Multiscreen 96-well filtration plates (Millipore, Bedford, MA, USA) were coated with inactivated Aichi virus at 2 µg per ml in PBS and incubated overnight at 4 °C. Coated plates were washed with 0.05% Tween 20 in PBS (PBST) before 200 µl of RPMI 1640 with 10% FCS was added to each well for 2 h at 37 °C to block non-specific binding. Freshly prepared cells from spleen (SP), lung (LG), mediastinal lymph nodes (LN), and Peyer’s patches (PP) at a concentration of 1 × 106 cells per ml, were suspended in 100 µl of complete RPMI medium and added to each well. After overnight incubation at 37 °C, plates were overlaid with 50 µl (2 µg per ml) of HRP-conjugated anti-mouse IgG/IgA antibody (Southern Biotech, Birmingham, AL, USA) and incubated for 1 h (1:1000 in PBST). After extensive washing, 3-3'-diaminobenzidine tetrahydrochloride (DAB; Research Genetics Inc., Huntsville, AL, USA) was added to develop spots in the plates (42). The plates were rinsed with water and air dried before counting using an ImmunoSpot ELISPOT reader (Cellular Technology, Shaker Heights, OH, USA).
Antibody avidity ELISA
Antibody avidity was determined by an ELISA method described previously (43) with minor modifications. Briefly, 96-well flat bottom microtiter plates (Nunc-Immuno Plate Maxisorp; Nunc Life Technologies, Basel, Switzerland) were coated with 200 ng per well of inactivated Aichi or Philippines viruses in a carbonate coating buffer (pH 9.2) overnight at 4 °C. Diluted serum samples were incubated for 1.5 h at 37 °C in duplicates. A sodium thiocyanate (NaSCN) solution (1.5 mol per dm3) was used to dissociate low-avidity antigen-antibody binding and added to one half, and PBS was added to the other half of each plate. After incubation for 15 min at RT, plates were incubated with HRP-conjugated antibodies (Southern Biotech, Birmingham, AL, USA) at RT for 1 h. After extensive washing, the substrate TMB (Invitrogen, Carlsbad, CA, USA) was added and the reaction was stopped with 2N H2SO4. The OD450 was read with an ELISA reader (BioTek, Winooski, VT, USA). The avidity index was calculated as follows: Avidity Index = (Titer with NaSCN) / (Titer without NaSCN) × 100 (42).
HAI assay
The HAI assay was performed as recommended by the World Health Organization (WHO) and the Center for Disease Control and Prevention (CDC) (44). In brief, sera and mucosal washes were treated with receptor destroying enzyme (RDE, Denka Seiken; Tokyo, Japan) overnight at 37 °C followed by RDE inactivation by incubating samples at 57 °C for 30 min. Treated samples were serially diluted and incubated with four HA units of Aichi virus for 1 h at RT. An equal volume of 0.5% washed chicken erythrocytes (Lampire Biological Laboratories; Pipersville, PA, USA) was added and agglutination was observed after 30 min.
Serum microneutralization assay
The serum neutralizing antibody titers were determined by microneutralization assay as described previously (45) with some modifications. Briefly, MDCK cells (ATCC, Manassas, VA 20110 USA) were seeded at 5 × 104 cells per well in 96-well flat bottom tissue culture treated plates (Corning Costar, Cambridge, MA, USA) and incubated overnight at 37 °C to form a monolayer. Serially diluted heat inactivated (56 °C for 45 min) sera samples were mixed separately with 100×TCID50 of Aichi or Philippines viruses and incubated at RT for 1 h. Then, the mixture was added to MDCK cell monolayers in triplicate wells. As control wells, uninfected cells (negative control) and cells infected with viruses (positive control) were used. After overnight incubation at 37 °C, cells were fixed with acetone and the expression of viral nucleoprotein (NP) was detected by ELISA. Furthermore, mouse monoclonal biotinylated anti-NP (Millipore, Temecula, CA, USA) and HRP-streptavidin conjugated anti-mouse IgG antibodies (Southern Biotech, Birmingham, AL, USA) were used. The highest serum dilution that generated a specific signal was considered to be the neutralization titer.
Challenge studies
Protective efficacies were determined by challenge with live mouse adapted A/Aichi/2/1968 (homologous), and A/Philippines/2/1982 (heterologous) H3N2 viruses. Three months after the last immunization, mouse groups were challenged with the two different H3N2 viruses to determine the protection level. For virus administration, mice were lightly anesthetized, and 10×LD50 of virus in 20 µl of PBS was administered into the mouse nostrils (46). Vaccinated and controlled groups were monitored up to 14 days for body weight changes, fever, hunched posture, illness features, and mortality.
Statistical analysis
The data for chemotactic activity, antigen-specific IgG/IgA levels, IgG subclasses, ASCs, antibody avidity indices, HAI, and serum neutralization titers were analyzed by paired student t-test and compared by parametric one-way ANOVA analysis of variance by ranks. Statistical analysis of FACS data was done using SPPS version 12.0.1 for Windows and by paired student t-test or one-way ANOVA test. n=5 mice per group and the results were expressed as mean ± standard deviation (SD). Survival differences were evaluated by Logrank Mantel-Cox test. Levels of significance (p value) were compared between the PBS, and influenza VLPs groups with cVLP group. Tests were performed using GraphPad Prism 6 software (San Diego, California). p values of less than 0.05 (p<0.05) were considered to be statistically significant. *p<0.05; **p<0.01, ***p<0.001, ****p<0.0001.
Results
GPI-CCL28 encoding gene construction and surface expression
The sequence encoding the membrane-bound form of CCL28 was constructed by fusing the signal peptide from honeybee mellitin and murine CD59 glycolipid-anchoring signal-coding sequences at the 5′- and 3′- ends of the murine CCL28 encoding sequence in the same open reading frame (ORF) (Fig 1A). The gene constructs were expressed in insect cells using the Bac-to-Bac expression system. FACS analysis further demonstrated that the expressed GPI-CCL28 was transported onto and retained at the surfaces of rBV-infected SF9 insect cells, as detected by the enhancement of fluorescent intensity in a dose dependent manner when compared with uninfected cells (Fig 1B). The results demonstrated that CCL28 can be expressed through rBV-driven protein expression in insect cells and can be expressed on cell surfaces by fusion to a GPI-signaling sequence. Protein expression was demonstrated by western blot of rBV-infected cell lysates. A characteristic band with a molecular weight of 20–25 KD was detected using anti-CCL28 antibody, which is consistent with the mass estimated from its amino acid composition (47).
Fig 1.
Characterization of GPI-CCL28 and VLPs. (A) Schematic representation of the GPI-CCL28 encoding gene. The coding sequences of the signal peptide from honeybee melittin were fused to the 5′- and murine CD59 GPI anchor to the 3′- ends of the murine CCL28 coding gene in frame to obtain the full-length encoding gene of a GPI-anchored CCL28 by overlapping PCR. (B) Expression of GPI-CCL28 in insect cells. FACS analysis was done to demonstrate the surface expression of GPI-CCL28 in rBV-infected SF9 insect cells. Blue, isotype antibody control; Red, Unstained cells; Orange, GPI-CCL28 expressing cells (MOI=2) + FITC anti-CCL28 antibodies (C) Western blot of M1, HA/M1, and HA/M1/GPI-CCL28 VLPs. Western blots were developed with the respective antibodies and results showed high expression of proteins with their expected sizes. (D) TEM analysis of VLPs. TEM data showed all VLPs were between 80–120 nm in size. (E)
In-vitro chemotactic activity of GPI-CCL28 containing VLPs towards spleen and lung cells. Spleen and lung cells (1 × 106) were placed in the upper chamber of Transwell plate (5–8 µm). In the lower chamber, VLPs were applied with complete RPMI media at 1 × 10−4 mol per m3 concentration of CCL28. After incubation for 4 h, cells migrated from the upper to the lower chamber were pelleted, and stained with APC labelled anti-mouse CCR3, and FITC labelled anti-mouse CCR10 antibodies. Figures show the chemotaxis indices of the CCR3+, and CCR10+ cells in spleen and lung towards VLPs with and without CCL28. The chemotactic index equals the number (%) of cells migrated to VLPs with or without CCL28 (at specific agonist concentration) divided by the number (%) to the cells migrated to medium alone. Each chemotaxis experiment was performed in duplicate wells with minimum of three times, and the results were expressed as the mean ± SD. [ ] M1 VLPs; [
] M1 VLPs; [ ] HA/M1 VLPs; [
] HA/M1 VLPs; [ ] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01.
] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01.
Characterization and quantification of VLPs
To produce high quality VLPs for immunization studies, optimal MOIs of rBVs for infection of SF9 cells were determined for different VLP production (data not shown). The cVLPs containing GPI-anchored CCL28 were produced by co-infection of SF9 cells with rBVs expressing HA, M1, and GPI-CCL28 at MOIs of 2:1:1, while HA/M1 or M1 VLPs were produced as described previously for comparison (48). Western blot data detected similar levels of M1, HA, and CCL28 proteins on the VLPs (Fig 1C). Together, these data represented that CCL28 can be incorporated into VLPs, or co-incorporated into cVLPs, through GPI-anchoring. We further determined the levels of GPI-CCL28 incorporation in cVLPs by quantitative ELISA, using purified recombinant CCL28 proteins as a standard. The amounts of CCL28 in cVLPs were similar between two independent preparations, at 20 ng per µg and 30 ng per µg, respectively (data not shown) (47). TEM analysis was also done to check the morphology and size of prepared VLPs. TEM data showed that the prepared VLPs were spherical in morphology and sized in between 80–120 nm (Fig 1D) (48).
VLPs containing GPI-CCL28 show in-vitro chemotactic activity towards CCR3/CCR10 expressing cells
Chemokines direct navigation of leukocytes during the initiation of adaptive immune responses in various tissues. As a member of the CC chemokine family, CCL28 binds to CCR3 and CCR10 chemokine receptors, particularly recruiting IgA-ASCs to the mucosal sites, and was found to be an effective vaccine adjuvant when co-delivered with several different antigens (20, 49). Using an in-vitro chemotactic assay, we demonstrated that CCL28-containing VLPs attracted CCR3 and CCR10 expressing cells; which migrated from the upper to the lower chambers using either pooled lung or spleen cells. An increase was observed in the chemotaxis indices with VLPs containing membrane-bound CCL28 compared to VLPs without CCL28 (Fig 1E). The singinificantly (p<0.05, p<0.01) enhanced chemotaxis indices support the conclusion that the migration of CCR3/CCR10 expressing cells was due to the membrane-bound CCL28 in cVLPs. These results indicated that GPI-CCL28 incorporated into VLPs retained their biological property of recruiting specific types of lymphocytes.
Intranasal immunization with CCL28-containing influenza VLPs enhanced strong systemic and mucosal immune responses
An advantage of mucosal immunization over other routes is the ability to induce both systemic and mucosal antigen-specific immune responses at distant effector sites (50). We immunized mice i.n. with and without CCL28-containing influenza VLPs and found that the presence of CCL28 in VLPs acts as a strong immunostimulator at both systemic and mucosal sites when compared to influenza VLPs without CCL28, or influenza VLPs mixed with sCCL28. Interestingly, the mean endpoint titers of IgG antibodies in immune sera of the cVLPs group were enhanced compared to the other formulations tested. While both PBS and M1 VLPs groups are negative controls, the cVLPs group showed significantly (p<0.001) higher immune responses than influenza VLPs group, demonstrating the adjuvant efficacy of GPI-CCL28. As a trend, cVLPs group also showed stronger immune responses than HA/M1 VLPs with sCCL28 group, although the difference is not stasignificant (p>0.05). The IgG antibody titers were ranged from 51200 to 102400 with cVLP group and found significantly (p<0.001) higher (2–4 fold) than HA/M1 VLPs (12800 – 25600) (Fig 2A). The IgG endpoint titers with soluble CCL28 group was observed between 25600 to 51200, which was also higher than HA/M1 VLP group. However, moderate to low serum IgA titers were detected in cVLPs groups with mean endpoint titers between 1600–3200. However, a robust increase was observed in the mean IgA endpoint titers at various mucosal sites after i.n. immunization with CCL28-containing VLPs. The mean endpoint titers for IgA were detected up to 12800 in tracheal and intestinal washes, significantly (p<0.01) higher (32 fold) than the VLPs with antigen alone group (200–400) (Fig 2B, 2C, 2D). However, the mucosal antibody responses in lung lavages were not induced significantly and reached only 6400 with cVLPs formulations. We also investigated total antigen-specific ASCs using ELISPOT at SP, LG, LN, and PP. The data showed significantly (p<0.01) higher levels of IgG (35–55 spots), (Fig 3A) and IgA-secreting cells (30–45 spots) (p<0.001) (Fig 3B) in cVLPs at various mucosal sites. In comparison, negligible ASC levels were observed in control groups. The ELISPOT data well correlated with the mean antibody endpoint titers in serum and different mucosal lavages. Thus, these data demonstrated a significant enhancement in the immune responses; not only at inductive, but at distal mucosal sites as well.
Fig 2.
Antibody endpoint titers in sera and mucosal lavages. Groups of mice were immunized with PBS, M1, HA/M1, HA/M1/sCCL28 (soluble), or HA/M1/GPI-CCL28 VLPs through i.n. route at day 0 and a booster dose was given with the same formulations at day 14. Sera and mucosal samples including tracheal, lung, and intestinal washes were collected at day 35, and antibody (IgG and IgA) endpoint titers determined. Figure shows the endpoint titers in (A) Sera, (B) Tracheal, (C) Lung, and (D) Intestinal washes. Assays were performed as described in Materials and Methods. Results were expressed as the mean ± SD (n=5). [ ] PBS; [
] PBS; [ ] M1 VLPs;[
] M1 VLPs;[ ] HA/M1 VLPs; [
] HA/M1 VLPs; [ ] HA/M1 VLPs + sCCL28 (soluble); [
] HA/M1 VLPs + sCCL28 (soluble); [ ] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Fig 3.
ELISPOT for estimating antigen-specific IgG and IgA-ASCs in different tissues. Cells from SP, LG, LN, and PP were collected and added to plates (1 × 106 cells per well) coated with the inactivated Aichi virus (2 µg per ml). Assays were performed as described in Materials and Methods. Results were expressed as the mean ± SD (n=5). Figure shows (A) IgG, and (B) IgA-ASCs counted in various tissues. [ ] PBS; [
] PBS; [ ] M1 VLPs; [
] M1 VLPs; [ ] HA/M1 VLPs; [
] HA/M1 VLPs; [ ] HA/M1 VLPs + sCCL28 (soluble); [
] HA/M1 VLPs + sCCL28 (soluble); [ ] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001.
] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001.
Chimeric VLPs strongly enhance Th1 type of immune responses in the system
IgG subtype switching and binding affinity evolution are important features of enhanced immunity (51). During evaluation of the IgG subtype patterns, a low IgG1 with high IgG2a/2b isotype responses were revealed with a significant (p<0.001) increase in responses by the inclusion of GPI-CCL28 into VLPs when compared with influenza VLPs alone. The IgG isotypic profile of serum IgG showed IgG2a/IgG2b as predominant isotypes with moderate to low levels of IgG1 levels. The serum IgG1 endpoint titers were ranged between 1600–3200 (Fig 4A) with the mean IgG2a/2b titers of approximately 25600 (Fig 4B, 4C) in CCL28 cVLPs. The mean IgG1 and IgG2a/2b endpoint titers were observed up to a maximum of 6400 and 12800, respectively in HA VLPs formulations. Thus, we found that IgG2a/2b levels were higher than IgG1 levels, with a ratio between 8 and 10, indicating that CCL28-containing VLPs preferentially enhance Th1 type of immune responses.
Fig 4.
IgG subtype endpoint titers in immune sera. IgG subtypes were determined using HRP-labeled goat anti-mouse IgG1, IgG2a, and IgG2b antibodies. Figure shows (A) IgG1, (B) IgG2a, and (C) IgG2b subtypes in sera. Assays were performed as described in Materials and Methods. Results were expressed as the mean ± SD (n=5). [ ] PBS; [
] PBS; [ ] M1 VLPs; [
] M1 VLPs; [ ] HA/M1 VLPs; [
] HA/M1 VLPs; [ ] HA/M1 VLPs + sCCL28 (soluble); [
] HA/M1 VLPs + sCCL28 (soluble); [ ] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001.
] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001.
Induction of antibodies with high avidity indices by Influenza VLPs containing GPI-CCL28
Antibody mediated protection against various infectious diseases have shown correlations between the antibody avidity and protective efficacy (52, 53). Therefore, antibody avidity analysis is an effective way to evaluate antibody quality for providing protection. An ELISA avidity assay was used to determine the antibody avidity index as measured by the relative stability of the antigen-antibody complexes to a 1.5 M NaSCN wash. The cVLP groups induced antibody responses with significantly (p<0.001) higher avidity than other formulations, against two different H3N2 viruses. The group of animals immunized with cVLPs produced antibodies with avidity indices substantially higher between 70–80% (p<0.001), and 40–45% (p<0.01) with Aichi, and Philippines viruses, respectively (Fig 5A, 5B). Notably, HA VLPs showed antibodies with some low avidity indices of 30–35% against Aichi, and 15–20% against Philippines viruses.
Fig 5.
Avidity indices of immune serum against selected H3N2 viruses. Antibody avidity assays were conducted with immune sera collected at day 35 from the immunized animals. Avidity indices of immune serum against (A) Aichi virus, (B) Philippines virus. Results were expressed as the mean ± SD (n=5). [ ] PBS; [
] PBS; [ ] M1 VLPs; [
] M1 VLPs; [ ] HA/M1 VLPs; [
] HA/M1 VLPs; [ ] HA/M1 VLPs + sCCL28 (soluble); [
] HA/M1 VLPs + sCCL28 (soluble); [ ] HA/M1/GPI-CCL28 VLPs. **p<0.01; ***p<0.001.
] HA/M1/GPI-CCL28 VLPs. **p<0.01; ***p<0.001.
GPI-CCL28-containing VLPs induced antibody responses conferring enhanced HAI titers
HAI titers are correlated to the protective efficacy of influenza antibody responses. The assay detects protective antibodies against influenza virus which act through preventing the attachment of virus to targeted host cells. For influenza vaccines, the induction of antibodies with HAI capacity directly correlates with their protective efficacy (27). To further compare the induced antibody responses, we determined the HAI activity of sera and various mucosal lavages against A/Aichi/2/1968 virus. A mean HAI titer in sera was reached up to 32 in the HA VLPs group, which was further induced significantly up to 115, by the inclusion of CCL28 (Fig 6A). In addition, the mean HAI titers in different mucosal washes were also observed to be higher (19.2 to 35) in animals immunized with cVLPs than HA VLPs alone (Fig 6B, 6C, 6D). The mean HAI titers were significantly (p<0.001) higher (3–5 fold) in the antibodies not only in sera, but also in mucosal washes using the vaccine formulations containing HA/M1/GPI-CCL28 VLPs than other groups. These results further demonstrated the strong adjuvant effect of GPI-CCL28, incorporated together with antigen in the same VLPs.
Fig 6.
HAI titers in sera and mucosal washes. The assay was performed according to protocols of the WHO global influenza program and the Center for Disease Control and Prevention (CDC). Figure shows the HAI titers in (A) Sera, (B) Tracheal, (C) Lung, and (D) Intestinal washes. Results were expressed as the mean ± SD (n=5). [ ] PBS; [
] PBS; [ ] M1 VLPs; [
] M1 VLPs; [ ] HA/M1 VLPs; [
] HA/M1 VLPs; [ ] HA/M1 VLPs + sCCL28 (soluble); [
] HA/M1 VLPs + sCCL28 (soluble); [ ] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001.
] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001.
GPI-CCL28 in VLPs enhance neutralizing antibody responses to Aichi and Philippines viruses
The microneutralization assay measures virus-specific neutralizing antibodies in sera and determines whether the vaccinated animals have antibodies that can neutralize the infectivity of a given virus strains (54). The serum microroneutralization test revealed that vaccination with influenza VLPs with GPI-CCL28 significantly (p<0.001) enhanced the serum neutralizing antibody titers against two H3N2 viral strains when compared with HA VLPs. HA VLPs mixed with sCCL28 also enhanced the serum neutralization titers but to a lesser degree compared with HA VLPs. The M1 and HA VLPs do not increase significant serum neutralization titers compared to the respective VLPs with CCL28 formulations. The mean serum neutralization for cVLP formulation reached up to 35000 and 12800 against Aichi and Philippines viruses, respectively. Moderate to low mean neutralization titers were found with VLPs containing HA antigen alone or mixed with sCCL28 (Fig 7A, 7B).
Fig 7.
Serum microneutralization assay with Aichi and Philippines viruses. Assay was conducted with immune sera, collected at day 35 from the mice immunized with various formulations. Figure shows the serum neutralization titers against (A) Aichi, and (B) Philippines viruses. Assays were performed as described in Materials and Methods. n=5 mice per group; Results were expressed as the mean ± SD (n=5). [ ] PBS; [
] PBS; [ ] M1 VLPs; [
] M1 VLPs; [ ] HA/M1 VLPs; [
] HA/M1 VLPs; [ ] HA/M1 VLPs + sCCL28 (soluble); [
] HA/M1 VLPs + sCCL28 (soluble); [ ] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001.
] HA/M1/GPI-CCL28 VLPs. *p<0.05; **p<0.01; ***p<0.001.
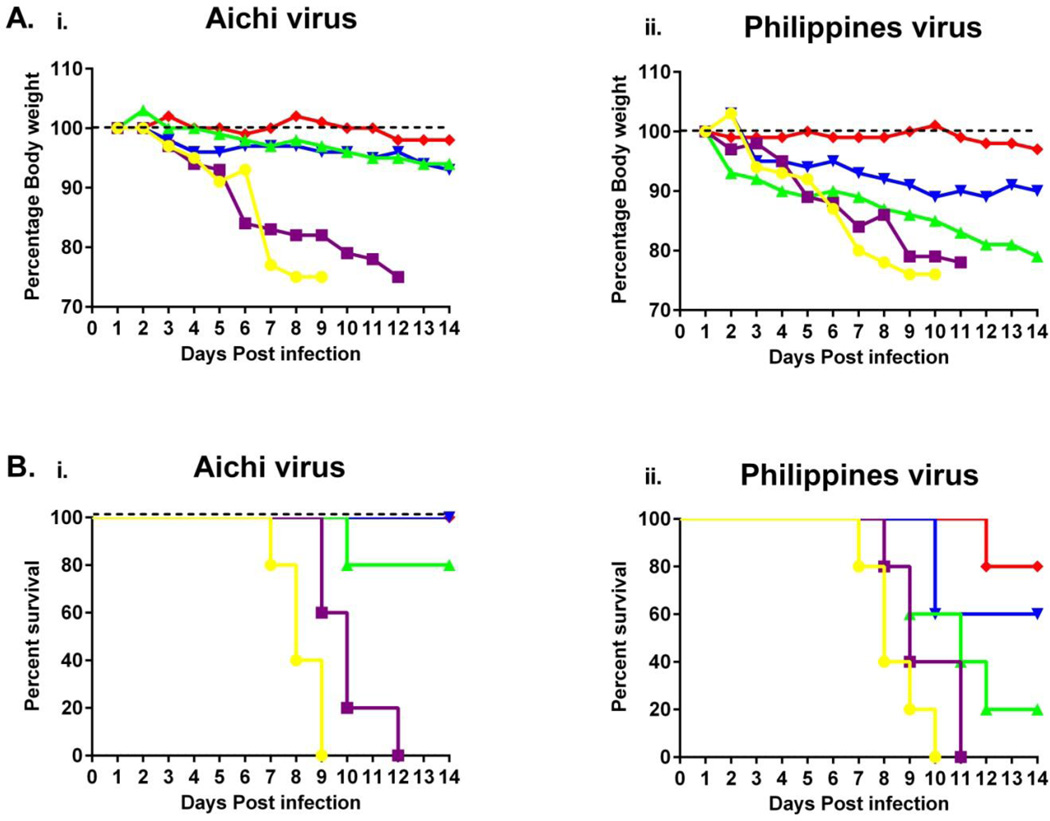
Influenza VLPs containing GPI-CCL28 demonstrated significant cross-protection against heterologous H3N2 virus
Protective efficacy was determined by challenging vaccinated animals with mouse adapted A/Aichi/2/1968 (homologous) or A/Philippines/2/1982 (heterologous) H3N2 viruses (46). Three months after the last immunization, mouse groups were challenged with 10×LD50 of two different H3N2 viruses to confirm the protection level by i.n. inoculation with 20 µl of the viruses. Following Aichi viral challenges, animals in the GPI-CCL28 VLP vaccinated group survived the challenge and remained healthy throughout the challenge period. However, weight loss, disease symptoms, and illness were observed in other groups. We observed body weight loss in other groups when compared to the GPI-anchored CCL28 VLPs (Fig 8A). With the homologous virus challenge (Aichi), the results demonstrated that HA VLPs conferred 80% survival in the experimental settings, which was improved to complete significant (p<0.001) protection (100%) with both the cVLP and VLPs with sCCL28 compared to influenza VLPs. Interestingly, we found significant (p<0.01) cross-protection against Philippines, a heterologous H3N2 virus, by immunization with cVLPs than HA VLPs. Philippines lethal viral challenge studies revealed that the protection level was reduced when compared to the homologous virus but cVLPs still were able to maintained survival up to 80%. Partial (60%) and 20% protection were observed with the mixed sCCL28 group and HA VLPs groups (Fig 8B). The challenges studies revealed that the protection level with cVLPs against Aichi and Philippines viruses was higher but not significant when compared with the HA VLPs containg sCCL28. The results indicate that the GPI-CCL28 containing VLPs provided significant cross-protection to a heterologous virus. Thus, GPI-CCL28 co-incorporated into VLPs boosted effective cross-protective immune responses leading to significant protection in animals across distantly related strain. These results pose a special importance when circulating viruses were found to be drifted from the vaccine strains.
Fig 8.
Protective efficacy of vaccination. Three-months post-vaccination; animals were challenged with 10×LD50 of mouse adapted A/Aichi/2/1968 or A/Philippines/2/1982 H3N2 viruses. Vaccinated and control groups were monitored up to 14 days for body weight changes, fever, hunched posture, illness features, and mortality. (A) Body weight changes in (i) Aichi, and (ii) Philippines viruses. Weight loss exceeding 25% was used as the experimental endpoint, at which mice were euthanized according to IACUC guidelines. Mean displayed; representative of one experiment. (B) Survival rates in (i) Aichi, and (ii) Philippines viruses. The survival differences were evaluated by Logrank Mantel-Cox test (n=5). [ ] PBS; [
] PBS; [ ] M1 VLPs; [
] M1 VLPs; [ ] HA/M1 VLPs; [
] HA/M1 VLPs; [ ] HA/M1 VLPs + sCCL28 (soluble); [
] HA/M1 VLPs + sCCL28 (soluble); [ ] HA/M1/GPI-CCL28 VLPs. **p<0.01; ***p<0.001.
] HA/M1/GPI-CCL28 VLPs. **p<0.01; ***p<0.001.
Discussion
Influenza, caused by the influenza virus, with intermittent epidemics, represents a major health threat to mankind. Pandemic and seasonal influenza viruses are constant public health problems causing significant morbidity and mortality worldwide (55). Influenza virus infection provides a model in which the relative contributions of the cellular, humoral, and mucosal immune systems to antiviral immunity can be addressed (55, 56). Although antibodies are thought to arbitrate protection, T cells mediate the clearance of the virus during infection (57). The most defensive and effective interventions against infectious diseases are vaccines. The available conventional inactivated influenza vaccines induce IgG antibodies in systemic circulation, but do not induce mucosal IgA antibodies (57). Current vaccine formulations rely on enhancing IgG responses to environmental insults that have bypassed mucosal immunity (58). Available vaccines have been shown to enhance serum HAI titers and protective IgG responses (59), but often do not display similar augmentation of mucosal IgA responses. Because IgA responses are linked to broad, cross-neutralizing activity at mucosal sites (60, 61), it would be advantageous to induce enhanced antigen-specific mucosal immunity as the next generation of influenza vaccines. In the present study, we investigated GPI-anchored CCL28 as a mucosal immunomodulator with influenza VLPs as vaccine antigens. We examined the immune-enhancing activity of GPI-CCL28 co-incorporated with HA VLPs when immunized through i.n. route in mice. We found that the GPI-CCL28 containing influenza VLPs vaccine approach bolsters IgA mucosal immune responses and confers significant cross-protection to a distantly related H3N2 virus. This novel vaccine approach may provide exciting new pathways for acquiring broad and effective immunity to pathogens requiring a mucosal site for infection.
The mucosal linings contribute nearly 80% of all the lymphocytes that accumulate in the mucosa-associated lymphoid tissue (MALT), providing specialized innate and adaptive defense against potential pathogens (62, 63). As influenza virus enters through mucosal surfaces, the induction of antibody responses in these sites is critical for the prevention of influenza virus infection. Previous studies have investigated the role of IgA and IgG in the protection against influenza virus infection, and found that IgA plays a dominant role in protection of the upper respiratory tract while IgG prevents lethal infection from influenza virus in the lower respiratory tract (10, 11). Several studies have provided evidence that IgA antibodies have better neutralization breadth and potency than IgG antibodies against various infectious agents (12). It has also been documented that polymeric IgA, transferred passively at nasal mucosa, confers nearly complete protection and clearance of viruses from the upper respiratory tract, whereas much larger amounts of influenza-specific IgG antibodies must be administered to provide the equivalent protection as IgA (13). Also, there are many encouraging studies suggesting the importance of trasudation of IgG antibodies to mucosal compartments (64, 65). For mucosal protection, IgA antibodies are the main protective antibodies while IgG, which transudate to mucosal compartments, have also shown a significant role (66). IgA antibodies are produced in the upper respiratory tract, and the passive transport of IgG antibodies from the systemic circulation onto the mucosal surfaces can also enhance protection from virus infection (12, 13).
Unfortunately, many mucosal vaccine candidates fail to elicit strong antibody immune responses (67). Thus, there is a great need for mucosal adjuvants as they can break the tolerance and lead to enhanced immune responses. The CC chemokine or CCL28, which we used as mucosal immunostimulator in the current study, interacts with CCR3/CCR10 chemokine receptors involved in both migration and recruitment of various cells at different mucosal inductive surfaces (68, 69). These interactions orchestrate the movement of cells which leads to the initiation of antigen-specific adaptive immunity. However, it has also been documented that CCL28 chemokines are involved in the migration and recruitment of IgA-ASCs at different mucosal sites using CCR3/CCR10 chemokine receptors (19, 20). The CCL28-CCR3/CCR10 circuit is considered to be a unifying system that plays a major role in the homing of plasmablasts and plasma cells at mucosal sites (21). Thus, CCL28 and CCR3/CCR10 receptors perform a critical function in coordinating the inter-dependent innate and adaptive immune responses (22, 23). As a result, it has become increasingly clear that their immunoattractant functions are necessary to translate innate immunity to adaptive immune responses. In agreement with above studies, our data indicate that GPI-CCL28 has potential to be used as a mucosal adjuvant with a broad range of applications.
In the present study, we successfully incorporated membrane-anchored CCL28 with HA antigens into VLPs containing influenza viral antigens, and determined their physical and biological functions. The preparation process is straightforward and efficient; and the resulting VLPs were relatively uniform, robust, and biologically active. We administered HA VLPs with or without GPI-CCL28 through i.n. route and evaluated protective antibody responses at systemic and various mucosal compartments. It was reported that i.n. immunization provides a practical means of vaccination for the induction of both systemic and mucosal immunity at distal sites (70). Our results demonstrated a robust increase in the antibody responses with high avidities, not only at systemic but also at mucosal sites such as tracheal, lung, and intestinal cavities. The immune responses showed significant cross-protection with high HAI and serum neutralization titers against distantly related Philippines H3N2 virus.
It is likely that the i.n. administered HA VLPs containing GPI-CCL28 are taken up by APCs in the nasal associated lymphoid tissues (NALT) and that co-incorporated CCL28 attracts the CCR3/CCR10 expressing cells at the inductive site and may provide signals for an efficient priming of resident naïve lymphocytes. Once primed, T and B cells in NALT can migrate via the lymph nodes to distant mucosal effector sites, and can mount distant immune responses. Thus, GPI-CCL28 acts as an adjuvant and may trigger the innate immune system resulting in enhanced specific humoral responses against the co-administered HA antigens present in the VLPs. It is noteworthy that these results suggested significant differences in the immune responses generated by the membrane-anchored CCL28 compared with the soluble form. A possible explanation could be that the particulate forms were easily taken up by APCs and induced immunity (71); but still more work is required in order to prove this concept. Simultaneously, our results are in agreement with the findings of other groups that immunization at one mucosal surface i.e. NALT, results in the generation of antigen-specific antibodies at distant mucosal sites such as tracheal, lung, and intestinal cavities (72).
Our results demonstrate that GPI-CCL28 indeed results in the enhancement of systemic and mucosal immunity to HA-containing VLPs, and these increases in the immune responses are associated with high neutralization breath and potency against different H3N2 viruses. Interestingly, despite having low IgG titers in the mucosal lavages, significantly high mean HAI titers and high neutralization potency were observed in these mucosal secretions. It demonstrates that there is direct correlation between IgA titers at mucosal compartments and neutralizing protective immunity.
In our study, we found that cVLPs showed significant cross protection in vaccinated animals against the heterologous H3N2 virus compared with those vaccinated with the influenza VLPs groups without CCL28. These results may suggest the protective role of broadly neutralizing IgG/IgA antibodies with high avidity/titers (73) and the effect of CCL28 in reshaping the immune direction to the conserved epitopes, conferring broad cross protection (74). The germinal center (GC) reactions may be involved with GPI-CCL28 in promoting heterologous protection. The long lasting GC reactions may encourage B cells to go through somatic hypermutation, a process by which they mutate their antibody encoding DNA and thus generate a diverse set of clones (75, 76). Thus, the phenomenon induces the bnAbs, which will be involved in cross pretection. Still, there is more work to do in order to obtain a better understanding behind the possible mechanism involved with GPI-anchored CCL28 in promoting heterologous protection. The above study shows that we may achieve the objective of up-regulating potentially beneficial mucosal immune responses with significant protection in animals against heterologous virus across a large distance by co-incorporation of GPI-CCL28 into VLPs.
Conclusion
The results of this study demonstrate that GPI-anchored CCL28 VLPs are effective vaccines when administered via intranasal route. Moreover, the most interesting feature of GPI-CCL28 VLPs is their ability to stimulate the comparatively balanced antigen-specific systemic and mucosal immune responses, making them a potentially effective and promising vaccine for mucosal derived infections. The present results warrant the further analysis of the efficacy of GPI-CCL28 influenza VLPs, which may provide a useful way for developing a platform for the next generation of mucosal influenza vaccines.
Supplementary Material
Acknowledgments
We thank Dahnide Taylor for her help in ordering of the materials needed for the study and, Lihua Shu and Scott Schaffer for their support in various experiments. We are thankful to the Robert P. Apkarian Integrated Electron Microscopy Core of Emory University for supporting the Electron Microscopy studies.
Funding:
This study was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH under the grants R01AI10104 and R01AI116835 to BZW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Contribution:
BZW designed and managed the project. TM conceived and executed the study, collected and analyzed data, and prepared the manuscript. JRK, and ZB assisted in the experiments related to this study while SW generated the CCL28 gene construct. RWC and BZW revised the manuscript.
Conflicts of Interests:
There are no known conflicts of interests.
References
- 1.Bridges CB, Winquist AG, Fukuda K, Cox NJ, Singleton JA, Strikas RA, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2000;49(RR-3):1–38. quiz CE1–7. [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Ehreth J. The value of vaccination: a global perspective. Vaccine. 2003;21(27–30):4105–4117. doi: 10.1016/s0264-410x(03)00377-3. [DOI] [PubMed] [Google Scholar]
- 4.Kamara L, Milstien JB, Patyna M, Lydon P, Levin A, Brenzel L. Strategies for financial sustainability of immunization programs: a review of the strategies from 50 national immunization program financial sustainability plans. Vaccine. 2008;26(51):6717–6726. doi: 10.1016/j.vaccine.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21(16):1776–1779. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 6.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature medicine. 2005;11(4 Suppl):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 7.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nature reviews Immunology. 2006;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 8.Rose MA, Zielen S, Baumann U. Mucosal immunity and nasal influenza vaccination. Expert review of vaccines. 2012;11(5):595–607. doi: 10.1586/erv.12.31. [DOI] [PubMed] [Google Scholar]
- 9.Magnusson KE, Stjernstrom I. Mucosal barrier mechanisms. Interplay between secretory IgA (SIgA), IgG and mucins on the surface properties and association of salmonellae with intestine and granulocytes. Immunology. 1982;45(2):239–248. [PMC free article] [PubMed] [Google Scholar]
- 10.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. Journal of immunology. 2004;173(3):1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 11.Asahi-Ozaki Y, Yoshikawa T, Iwakura Y, Suzuki Y, Tamura S, Kurata T, et al. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. Journal of medical virology. 2004;74(2):328–335. doi: 10.1002/jmv.20173. [DOI] [PubMed] [Google Scholar]
- 12.McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS biology. 2012;10(9):e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal immunology. 2008;1(1):11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 14.van den Brand JM, Kreijtz JH, Bodewes R, Stittelaar KJ, van Amerongen G, Kuiken T, et al. Efficacy of vaccination with different combinations of MF59-adjuvanted and nonadjuvanted seasonal and pandemic influenza vaccines against pandemic H1N1 (2009) influenza virus infection in ferrets. Journal of virology. 2011;85(6):2851–2858. doi: 10.1128/JVI.01939-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. Journal of virology. 2008;82(3):1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerwenka A, Morgan TM, Harmsen AG, Dutton RW. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. The Journal of experimental medicine. 1999;189(2):423–434. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MB. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. Journal of immunology. 2003;171(12):6838–6845. doi: 10.4049/jimmunol.171.12.6838. [DOI] [PubMed] [Google Scholar]
- 18.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. Journal of leukocyte biology. 2004;76(4):886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 19.Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, et al. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. The Journal of clinical investigation. 2003;111(7):1001–1010. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. The Journal of experimental medicine. 2004;200(6):805–809. doi: 10.1084/jem.20041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha HR, Ko HJ, Kim ED, Chang SY, Seo SU, Cuburu N, et al. Mucosa-associated epithelial chemokine/CCL28 expression in the uterus attracts CCR10+ IgA plasma cells following mucosal vaccination via estrogen control. Journal of immunology. 2011;187(6):3044–3052. doi: 10.4049/jimmunol.1100402. [DOI] [PubMed] [Google Scholar]
- 22.Hieshima K, Kawasaki Y, Hanamoto H, Nakayama T, Nagakubo D, Kanamaru A, et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. Journal of immunology. 2004;173(6):3668–3675. doi: 10.4049/jimmunol.173.6.3668. [DOI] [PubMed] [Google Scholar]
- 23.Tregoning JS, Buffa V, Oszmiana A, Klein K, Walters AA, Shattock RJ. A "prime-pull" vaccine strategy has a modest effect on local and systemic antibody responses to HIV gp140 in mice. PloS one. 2013;8(11):e80559. doi: 10.1371/journal.pone.0080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral immunology. 2005;18(1):244–251. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- 25.Feng H, Zhang H, Deng J, Wang L, He Y, Wang S, et al. Incorporation of a GPI-anchored engineered cytokine as a molecular adjuvant enhances the immunogenicity of HIV VLPs. Scientific reports. 2015;5:11856. doi: 10.1038/srep11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang BZ, Quan FS, Kang SM, Bozja J, Skountzou I, Compans RW. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. Journal of virology. 2008;82(23):11813–11823. doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang BZ, Xu R, Quan FS, Kang SM, Wang L, Compans RW. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PloS one. 2010;5(11):e13972. doi: 10.1371/journal.pone.0013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed LJMH. A simple method of estimating fifty percent endpoints. American Journal of Epidemiology. 1938;27(3):493–497. [Google Scholar]
- 29.Tessier DC, Thomas DY, Khouri HE, Laliberte F, Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991;98(2):177–183. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HF, Yu J, Chen S, Morgan BP, Abagyan R, Tomlinson S. Identification of the individual residues that determine human CD59 species selective activity. The Journal of biological chemistry. 1999;274(16):10969–10974. doi: 10.1074/jbc.274.16.10969. [DOI] [PubMed] [Google Scholar]
- 31.Nimal S, Heath AW, Thomas MS. Enhancement of immune responses to an HIV gp120 DNA vaccine by fusion to TNF alpha cDNA. Vaccine. 2006;24(16):3298–3308. doi: 10.1016/j.vaccine.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Skountzou I, Quan FS, Gangadhara S, Ye L, Vzorov A, Selvaraj P, et al. Incorporation of glycosylphosphatidylinositol-anchored granulocyte- macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. Journal of virology. 2007;81(3):1083–1094. doi: 10.1128/JVI.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poloso NJ, Nagarajan S, Mejia-Oneta JM, Selvaraj P. GPI-anchoring of GM-CSF results in active membrane-bound and partially shed cytokine. Molecular immunology. 2002;38(11):803–816. doi: 10.1016/s0161-5890(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 34.Wang BZ, Liu W, Kang SM, Alam M, Huang C, Ye L, et al. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. Journal of virology. 2007;81(20):10869–10878. doi: 10.1128/JVI.00542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassilieva EV, Wang BZ, Vzorov AN, Wang L, Wang YC, Bozja J, et al. Enhanced mucosal immune responses to HIV virus-like particles containing a membrane-anchored adjuvant. mBio. 2011;2(1):e00328-10. doi: 10.1128/mBio.00328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishanian P, Taylor JM, Korns E, Detels R, Saah A, Fahey JL. Significance of quantitative enzyme-linked immunosorbent assay (ELISA) results in evaluation of three ELISAs and Western blot tests for detection of antibodies to human immunodeficiency virus in a high-risk population. Journal of clinical microbiology. 1987;25(2):395–400. doi: 10.1128/jcm.25.2.395-400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. Journal of immunology. 2003;170(7):3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 38.Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7(2):291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- 39.Rossi DL, Hurst SD, Xu Y, Wang W, Menon S, Coffman RL, et al. Lungkine, a novel CXC chemokine, specifically expressed by lung bronchoepithelial cells. Journal of immunology. 1999;162(9):5490–5497. [PubMed] [Google Scholar]
- 40.Velasquez LS, Shira S, Berta AN, Kilbourne J, Medi BM, Tizard I, et al. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011;29(32):5221–5231. doi: 10.1016/j.vaccine.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Loon AM, van der Veen J. Enzyme-linked immunosorbent assay for quantitation of toxoplasma antibodies in human sera. Journal of clinical pathology. 1980;33(7):635–639. doi: 10.1136/jcp.33.7.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo L, Lu X, Kang SM, Chen C, Compans RW, Yao Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Virology. 2003;313(2):502–513. doi: 10.1016/s0042-6822(03)00372-6. [DOI] [PubMed] [Google Scholar]
- 43.Anttila M, Eskola J, Ahman H, Kayhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. The Journal of infectious diseases. 1998;177(6):1614–1621. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 44.Koutsonanos DG, Esser ES, McMaster SR, Kalluri P, Lee JW, Prausnitz MR, et al. Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine. 2015;33(37):4675–4682. doi: 10.1016/j.vaccine.2015.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PloS one. 2009;4(3):e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Wang YC, Feng H, Ahmed T, Compans RW, Wang BZ. Virus-like particles containing the tetrameric ectodomain of influenza matrix protein 2 and flagellin induce heterosubtypic protection in mice. BioMed research international. 2013;2013:686549. doi: 10.1155/2013/686549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pallister KB, Mason S, Nygaard TK, Liu B, Griffith S, Jones J, et al. Bovine CCL28 Mediates Chemotaxis via CCR10 and Demonstrates Direct Antimicrobial Activity against Mastitis Causing Bacteria. PloS one. 2015;10(9):e0138084. doi: 10.1371/journal.pone.0138084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pease LF, 3rd, Lipin DI, Tsai DH, Zachariah MR, Lua LH, Tarlov MJ, et al. Quantitative characterization of virus-like particles by asymmetrical flow field flow fractionation, electrospray differential mobility analysis, and transmission electron microscopy. Biotechnology and bioengineering. 2009;102(3):845–855. doi: 10.1002/bit.22085. [DOI] [PubMed] [Google Scholar]
- 49.Sundstrom P, Lundin SB, Nilsson LA, Quiding-Jarbrink M. Human IgA-secreting cells induced by intestinal, but not systemic, immunization respond to CCL25 (TECK) and CCL28 (MEC) European journal of immunology. 2008;38(12):3327–3338. doi: 10.1002/eji.200838506. [DOI] [PubMed] [Google Scholar]
- 50.Mohan T, Mitra D, Rao DN. Nasal delivery of PLG microparticle encapsulated defensin peptides adjuvanted gp41 antigen confers strong and long-lasting immunoprotective response against HIV-1. Immunologic research. 2014;58(1):139–153. doi: 10.1007/s12026-013-8428-5. [DOI] [PubMed] [Google Scholar]
- 51.Sousa AO, Henry S, Maroja FM, Lee FK, Brum L, Singh M, et al. IgG subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clinical and experimental immunology. 1998;111(1):48–55. doi: 10.1046/j.1365-2249.1998.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Derdeyn CA, Decker JM, Sfakianos JN, Zhang Z, O'Brien WA, Ratner L, et al. Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp120 interactions with the coreceptor. Journal of virology. 2001;75(18):8605–8614. doi: 10.1128/JVI.75.18.8605-8614.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai L, Vodros D, Kozlowski PA, Montefiori DC, Wilson RL, Akerstrom VL, et al. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369(1):153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein M, Schoppel K, Amvrossiadis N, Mach M. Strain-specific neutralization of human cytomegalovirus isolates by human sera. Journal of virology. 1999;73(2):878–886. doi: 10.1128/jvi.73.2.878-886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2008;57(RR-7):1–60. [PubMed] [Google Scholar]
- 56.Dougan SK, Ashour J, Karssemeijer RA, Popp MW, Avalos AM, Barisa M, et al. Antigen-specific B-cell receptor sensitizes B cells to infection by influenza virus. Nature. 2013;503(7476):406–409. doi: 10.1038/nature12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim TS, Sun J, Braciale TJ. T cell responses during influenza infection: getting and keeping control. Trends in immunology. 2011;32(5):225–231. doi: 10.1016/j.it.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moldoveanu Z, Clements ML, Prince SJ, Murphy BR, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13(11):1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 59.Brandtzaeg P, Pabst R. Let's go mucosal: communication on slippery ground. Trends in immunology. 2004;25(11):570–577. doi: 10.1016/j.it.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 60.van Riet E, Ainai A, Suzuki T, Hasegawa H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine. 2012;30(40):5893–5900. doi: 10.1016/j.vaccine.2012.04.109. [DOI] [PubMed] [Google Scholar]
- 61.Ichinohe T, Tamura S, Kawaguchi A, Ninomiya A, Imai M, Itamura S, et al. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. The Journal of infectious diseases. 2007;196(9):1313–1320. doi: 10.1086/521304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greenbaum E, Furst A, Kiderman A, Stewart B, Levy R, Schlesinger M, et al. Mucosal [SIgA] and serum [IgG] immunologic responses in the community after a single intra-nasal immunization with a new inactivated trivalent influenza vaccine. Vaccine. 2002;20(7–8):1232–1239. doi: 10.1016/s0264-410x(01)00396-6. [DOI] [PubMed] [Google Scholar]
- 63.Kutzler MA, Kraynyak KA, Nagle SJ, Parkinson RM, Zharikova D, Chattergoon M, et al. Plasmids encoding the mucosal chemokines CCL27 and CCL28 are effective adjuvants in eliciting antigen-specific immunity in vivo. Gene therapy. 2010;17(1):72–82. doi: 10.1038/gt.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bakaletz LO, Holmes KA. Evidence for transudation of specific antibody into the middle ears of parenterally immunized chinchillas after an upper respiratory tract infection with adenovirus. Clin Diagn Lab Immunol. 1997;4(2):223–225. doi: 10.1128/cdli.4.2.223-225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson DL. Synthesis and transudation of antibody during acute inflammation in the mammary gland. Inflammation. 1984;8(3):241–249. doi: 10.1007/BF00916414. [DOI] [PubMed] [Google Scholar]
- 66.Robert-Guroff M. IgG surfaces as an important component in mucosal protection. Nature medicine. 2000;6(2):129–130. doi: 10.1038/72206. [DOI] [PubMed] [Google Scholar]
- 67.Eng NF, Garlapati S, Gerdts V, Babiuk LA, Mutwiri GK. PCEP enhances IgA mucosal immune responses in mice following different immunization routes with influenza virus antigens. Journal of immune based therapies and vaccines. 2010;8:4. doi: 10.1186/1476-8518-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2) The Journal of biological chemistry. 2000;275(29):22313–22323. doi: 10.1074/jbc.M001461200. [DOI] [PubMed] [Google Scholar]
- 69.Hu K, Luo S, Tong L, Huang X, Jin W, Huang W, et al. CCL19 and CCL28 augment mucosal and systemic immune responses to HIV-1 gp140 by mobilizing responsive immunocytes into secondary lymph nodes and mucosal tissue. Journal of immunology. 2013;191(4):1935–1947. doi: 10.4049/jimmunol.1300120. [DOI] [PubMed] [Google Scholar]
- 70.Mohan T, Sharma C, Bhat AA, Rao DN. Modulation of HIV peptide antigen specific cellular immune response by synthetic alpha- and beta-defensin peptides. Vaccine. 2013;31(13):1707–1716. doi: 10.1016/j.vaccine.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 71.Mann JF, Shakir E, Carter KC, Mullen AB, Alexander J, Ferro VA. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009;27(27):3643–3649. doi: 10.1016/j.vaccine.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 72.Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science : JAALAS. 2011;50(5):600–613. [PMC free article] [PubMed] [Google Scholar]
- 73.Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E, et al. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine. 2008;26(12):1525–1529. doi: 10.1016/j.vaccine.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 74.Krammer F, Pica N, Hai R, Margine I, Palese P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. Journal of virology. 2013;87(12):6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 76.Randall KL, Lambe T, Johnson AL, Treanor B, Kucharska E, Domaschenz H, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10(12):1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.