Highlights
-
•
Prenatal brain development shapes later cognitive capacities and disease risks.
-
•
Sulcal patterns are considered postnatal proxies for early developmental events.
-
•
We tested the hypothesis that ACC sulcal pattern is a stable trait during postnatal life.
-
•
The ACC sulcal pattern was shown to be stable between childhood and adulthood.
-
•
Our findings support the interest of qualitative features of the cortex anatomy.
Keywords: ACC, Fetal life, Sulcal pattern, Neurodevelopment, MRI, Executive control
Abstract
Prenatal processes are likely critical for the differences in cognitive ability and disease risk that unfold in postnatal life. Prenatally established cortical folding patterns are increasingly studied as an adult proxy for earlier development events – under the as yet untested assumption that an individual's folding pattern is developmentally fixed. Here, we provide the first empirical test of this stability assumption using 263 longitudinally-acquired structural MRI brain scans from 75 typically developing individuals spanning ages 7 to 32 years. We focus on the anterior cingulate cortex (ACC) – an intensely studied cortical region that presents two qualitatively distinct and reliably classifiable sulcal patterns with links to postnatal behavior. We show – without exception–that individual ACC sulcal patterns are fixed from childhood to adulthood, at the same time that quantitative anatomical ACC metrics are undergoing profound developmental change. Our findings buttress use of folding typology as a postnatally-stable marker for linking variations in early brain development to later neurocognitive outcomes in ex utero life.
1. Introduction
Analysis of brain anatomy from in vivo structural magnetic resonance imaging (sMRI) has become a major tool in biological psychiatry and psychology. Disorders like schizophrenia and autism spectrum disorder, as well as traits like general cognitive abilities, have been linked to variation in diverse neuroanatomical features in postnatal life (Giedd and Rapoport, 2010, Kanai and Rees, 2011). However, there is a growing awareness that prenatal processes are likely to be critical for the differences in cognitive ability (Raznahan et al., 2012, Shenkin et al., 2004, Walhovd et al., 2012) and disease risk (Schlotz and Phillips, 2009) that unfold in postnatal life. Recognition of this has driven the search for neuroimaging features in late postnatal life that could serve as a proxy for earlier developmental events. A leading candidate in this effort has been typologies of cortical folding (Mangin et al., 2010, Sun et al., 2009).
Unlike quantitative features of the cortical sheet, such as thickness, surface area (Giedd and Rapoport, 2010) and curvature/gyrification (Armstrong et al., 1995, Li et al., 2014, Raznahan et al., 2011, White et al., 2010) – which can take decades to attain the levels observed in adulthood–the qualitative pattern formed by the characteristic set of primary, secondary and tertiary folds, or sulci, seen in human adulthood is already evident at birth (Chi et al., 1977, Mangin et al., 2010, Zilles et al., 2013). Inter-individual variation in this qualitative sulcal pattern–a classic example being the presence of a single or double parallel type of the ACC (Ono et al., 1990), determined between 10 and 15 weeks of fetal life (Chi et al., 1977) and present in 76 and 24% of adults respectively – has therefore been used as a marker for prenatal differences. Thus, ACC folding pattern has been linked to a host of cognitive domains including executive control (Cachia et al., 2014, Fornito et al., 2004), reality monitoring (Buda et al., 2011), temperament (Whittle et al., 2009) and social cognition (Fujiwara et al., 2007). The inference of these studies has been that observed qualitative differences reflect a constraint imposed by early neurodevelopmental processes on the subsequent abilities. However, a critical and as yet untested developmental assumption – upon which existing and future studies of sulcal pattern typology as an early developmental marker necessarily rest – is that the folding typologies are stable across postnatal brain development.
Here, we provide the first empirical test of this stability assumption using 263 magnetic resonance imaging (MRI) brain scans, taken from 75 healthy people longitudinally followed from 7 to 32 years. We focus on the ACC because it is a cortical region that presents two qualitatively distinct sulcal patterns (Ono et al., 1990) that can be easily and reliably classified with structural MRI from childhood (Cachia et al., 2014) to adulthood (Paus et al., 1996, Yucel et al., 2001). We evaluate the longitudinal stability of the ACC sulcal pattern from late childhood to adulthood, a developmental window characterized by significant structural remodeling of the brain as shown by the drastic ACC cortical thickness, surface area and curvature changes with time during this period (Hogstrom et al., 2013, Shaw et al., 2008). We complement our analysis of the longitudinal sulcal pattern stability by also investigating the longitudinal changes in cortical thickness.
2. Material and methods
2.1. Participants
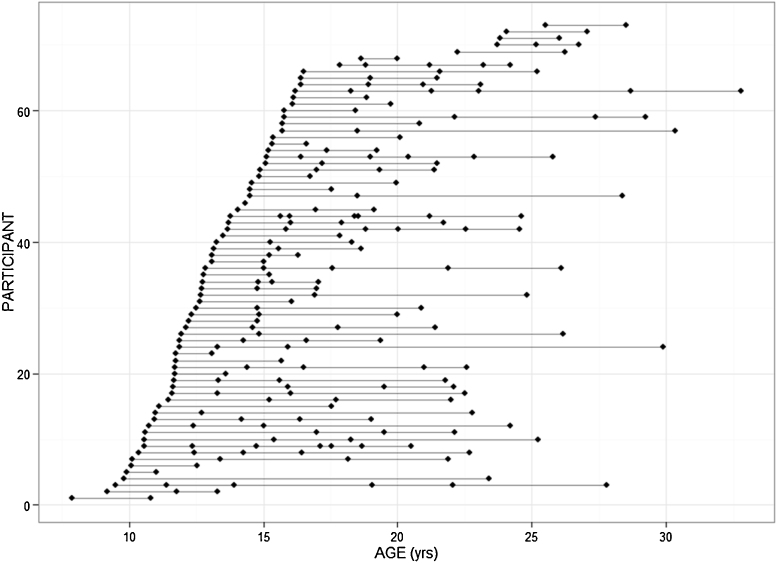
Seventy five healthy participants (age range: 7.88–32.8 years old; age at first scan: 7.88–25.5 years old; age latest scan: 10.38–32.8 years old), including 26 females and 49 males, were selected from a larger prospective study (Giedd et al., 1999) on brain development at the National Institute of Mental Health (NIMH) based on the presence of at least two longitudinally acquired brain scans per subject (Fig. 1). Participants were free of lifetime medical or psychiatric disorders, which were determined through clinical examination and standardized interview. Psychiatric illness in a first degree relative was also exclusionary. Further details on this sample has been described previously (Giedd et al., 1999). The research protocol was approved by the NIMH institutional review board. Written informed consent was obtained from parents and controls older than 18 years, and written informed assent was obtained from minors.
Fig. 1.
Participant age at scan. Each dot represents a participant scan. Each line represents a participant with repeated scans.
2.2. MRI acquisition
All structural magnetic resonance imaging (sMRI) brain scans were T-1 weighted images with contiguous 1.5 mm axial slices, obtained on the same 1.5-T General Electric (Milwaukee, WI) Signa scanner using a 3D spoiled gradient recalled echo sequence with the following parameters: Echo time, 5 ms; Repetition time, 24 ms; flip angle 45 (DEG); acquisition matrix, 256 × 192; number of excitations, 1; and field of view, 24 cm. Head placement was standardized as previously described (Castellanos et al., 2001).
2.3. Sulcal segmentation
Sulcal segmentation was performed with BrainVISA 4.2 software using the Morphologist toolbox (http://brainvisa.info). An automated pre-processing step skull-stripped T1 MRIs and segmented the brain tissues. No spatial normalization was applied to MRIs to overcome potential bias that may result from the sulcus shape deformations induced by the warping process. The cortical folds were automatically segmented throughout the cortex from the skeleton of the gray matter/cerebrospinal fluid mask, with the cortical folds corresponding to the crevasse bottoms of the ‘landscape’, the altitude of which is defined by its intensity on the MRIs. This definition provides a stable and robust sulcal surface definition that is not affected by variations in cortical thickness or gray matter/white matter contrast (Mangin et al., 2004). For each participant, images at each processing step were visually checked. No segmentation error was detected.
2.4. ACC classification
Analyses of ACC typology stability were carries out using the first and last scan available for each subject (i.e. total number of scans = 150). The sulcal pattern of the dorsal part of the ACC was visually assessed using three-dimensional, mesh-based reconstruction of cortical folds (Cachia et al., 2014) to measure the occurrence and extent of local folds (Fornito et al., 2004, Huster et al., 2007, Leonard et al., 2009, Yucel et al., 2001). This three-dimensional approach was used to overcome methodological issues inherent to the analysis of the three-dimensional sulcal pattern of the ACC from two-dimensional sagittal slices. The ACC sulcal pattern was assessed based on the absence or presence of a paracingulate sulcus (PCS), a variable secondary sulcus. The PCS was defined as the sulcus located dorsal to the cingulate sulcus with a course clearly parallel to the cingulate sulcus (Paus et al., 1996, Yucel et al., 2001). To reduce the ambiguity from the confluence of the PCS and the cingulate sulcus with the superior rostral sulcus, we determined the anterior limit of the PCS as the point at which the sulcus extends posteriorly from an imaginary vertical line running perpendicular to the line passing through the anterior and posterior commissures (AC–PC) and parallel to the anterior commissure; the PCS was anteriorly limited by an imaginary vertical line passing through the anterior commissure (Yucel et al., 2001). The PCS was considered absent if there were no clearly developed horizontal sulcus elements parallel to the cingulate sulcus and extending at least 20 mm (interruptions or gaps in the PCS course was not taken into account for the length measure). In order to control age-related differences on brain size, all the length measurements were performed in MNI space after a linear spatial registration using FSL software (www.fmrib.ox.ac.uk/fsl).
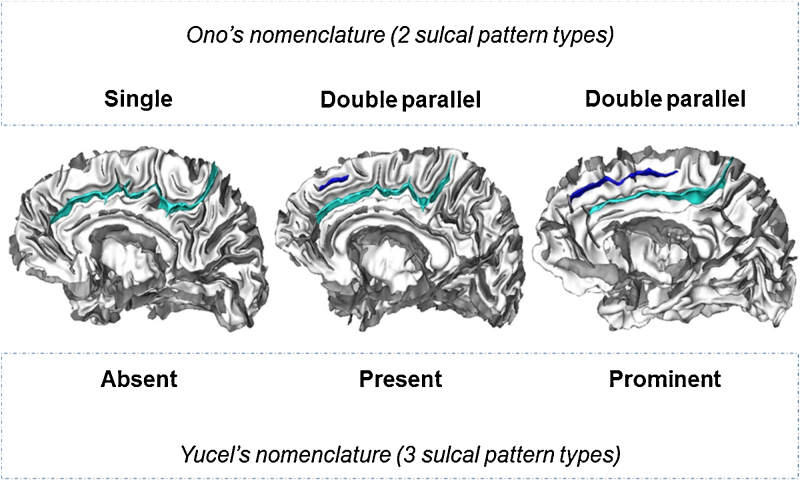
The individual ACC sulcal patterns were then classified with two commonly used nomenclatures (Fig. 2), Ono's nomenclature (e.g. Cachia et al., 2014, Fornito et al., 2006) and Yucel's nomenclature (e.g. Buda et al., 2011, Fornito et al., 2004, Fujiwara et al., 2007, Whittle et al., 2009). Ono's nomenclature (Ono et al., 1990) provides a classification of ACC sulcal pattern in two types: a ‘single’ type for ACC without PCS and a ‘double parallel’ type for ACC with a PCS. Yucel's nomenclature (Yucel et al., 2001), following Paus work on the cingulate cortex (Paus et al., 1996), provides a classification of ACC sulcal pattern in three types, using a finer distinction between ‘present’ and ‘prominent’ PCS based on PCS length (PCS greater than 40 mm were labelled as ‘prominent’).
Fig. 2.
The different sulcal pattern types of the anterior cingulate cortex (ACC) using Ono's nomenclature (two sulcal pattern types) and Yucel's nomenclature (three sulcal pattern types). Left panel: subject with only a cingulate sulcus (light blue), labeled as ‘single’ type in Ono's nomenclature or ‘absent’ type in Yucel's nomenclature. Middle panel: subject with a cingulate sulcus and a small (<40 mm) additional paracingulate sulcus (in blue), labeled as ‘double parallel’ type in Ono's nomenclature or ‘present’ type in Yucel's nomenclature. Right panel: subjects with a cingulate sulcus and a long (>40 mm) additional paracingulate sulcus (in blue), labeled as ‘double parallel’ type in Ono's nomenclature or ‘prominent’ type in Yucel's nomenclature.
The classification of baseline and follow-up MRI scans were examined separately and in random order. ACC sulcal pattern was done blinded to possible confounding information, including the participant's age as well as the label of the ACC sulcal pattern in the contralateral hemisphere and in the other time point. Interrater reliability (Cohen's weighted kappa) was assessed by using a second rater (O.G.) to evaluate 50 randomly chosen hemispheres.
Because quantitative features of cortex anatomy change during the development, including the PCS length (Clark et al., 2010), we anticipated possible longitudinal change in ACC sulcal pattern labelled with Yucel's nomenclature and expected longitudinal stability in ACC sulcal pattern labelled with Ono's nomenclature.
2.4.1. Cortical thickness (CT) analysis
Analyses of CT change were carried out using all available scans from all participants (i.e. total number of scans = 263). Native sMRI scans were submitted to the well-validated (Im et al., 2008, Shaw et al., 2008) CIVET pipeline (Ad-Dab’bagh et al., 2006) for automated modeling of gray/white and pial cortical surfaces in each scan. Briefly, this process begins with linear transformation, correction of non-uniformity artifacts, and segmentation of each image into white matter, gray matter and CSF (Zijdenbos et al., 2002). Next, each image is fitted with two deformable mesh models to extract the white/gray and pial surfaces. These surface representations are then used to calculate CT at approximately 80,000 points (vertices) across the cortex (MacDonald et al., 2000), and aligned with each other using a 2-D surface-based registration algorithm to allow comparison of equivalent vertices across different scans (Lyttelton et al., 2007).
We examined the linear effect of age on CT, controlling for sex, at each vertex using mixed-effects models. Significant vertex-wise main effects of age were visualized after applying a False Discovery Rate correction for multiple comparisons with q (the expected proportion of falsely rejected null hypotheses) set at 5%.
3. Results
3.1. ACC sulcal pattern
The longitudinal stability of the ACC sulcal pattern was assessed from the comparison of each individual hemisphere at baseline (mean ± sd age: 13.7 ± 3.5 years; range: 7.88–25.5 years old) and for the latest scan (20.8 ± 4.9 years; range: 10.38–32.8 years old).
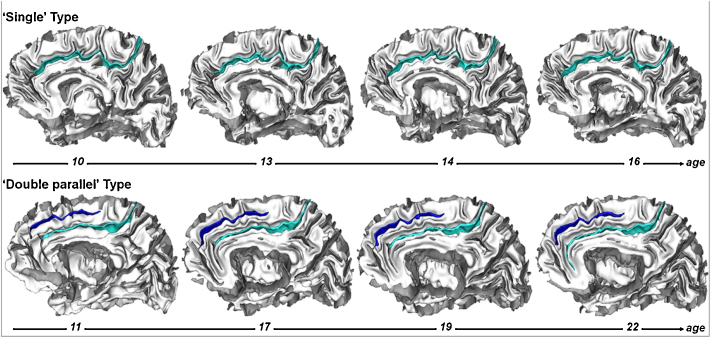
Analysis of the individual ACC sulcal patterns using Ono's nomenclature, with two qualitatively different types, revealed that there were 24 ‘single’ types and 51 ‘double parallel’ types in the left hemisphere and 41 ‘single’ types and 34 ‘double parallel’ types in the right hemisphere at the baseline. Individual qualitative sulcal patterns of ACC were found to remain - without exception - the same between the baseline and the follow-up scans (Fig. 3). The longitudinal stability of the ACC qualitative sulcal pattern was therefore of 100% in the left and right hemispheres. Interrater reliability was κ = 1.0
Fig. 3.
The sulcal pattern of the anterior cingulate cortex (ACC) remains stable from late childhood to adulthood. Longitudinal stability of the ACC sulcal pattern in a subject with a ‘single type’ ACC, with only the cingulate sulcus (light blue), and in a subject with a ‘double parallel type ACC’, with a cingulate sulcus and an additional paracingulate sulcus (in blue).
Analysis of the individual ACC sulcal patterns using Yucel's nomenclature (absent, present and prominent), mixing qualitative (PCS absence/presence) and quantitative (PCS length) features, revealed that there were 24 ‘absent’, 23 ‘present’ and 28 ‘prominent’ types in the left hemisphere and 41 ‘absent’, 19 ‘present’ and 15 ‘prominent’ types in the right hemisphere at the baseline. Individual ACC sulcal patterns in the left and right hemisphere were found to slightly change between the baseline and the follow-up scans. There were 24 ‘absent’, 25 ‘present’ and 26 ‘prominent’ types in the left hemisphere and 41 ‘absent’, 19 ‘present’ and 15 ‘prominent’ types in the right hemisphere at the follow-up. These longitudinal changes were not related to qualitative changes (i.e. switch between the presence or the absence of a PCS) but were only due to quantitative changes (i.e. present or prominent PCS). In the left hemisphere, 4 ACC sulcal patterns initially labeled ‘prominent’ were labeled ‘present’ at the follow-up and conversely 2 ACC sulcal patterns initially labeled ‘present’ were labeled ‘prominent’ at the follow-up; in the right hemisphere, 3 ACC sulcal patterns initially labeled ‘prominent’ were labeled ‘present’ at the follow-up and conversely 3 ACC sulcal patterns initially labeled ‘present’ were labeled ‘prominent’ at the follow-up. The longitudinal stability of the ACC sulcal pattern using Yucel's nomenclature that involves a quantitative criteria (PCS length) was therefore of 80% in the left and right hemispheres. Interrater reliability was κ = 0.72.
3.2. ACC cortical thickness
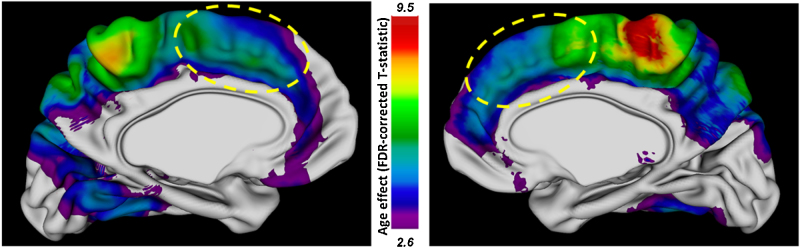
We also assessed the longitudinal change of the ACC cortical thickness (CT) in the same sample. Deformable mesh models were used to extract the white/gray and pial surfaces and calculate the CT at approximately 80,000 points (vertices) across the cortex. Whole brain analysis revealed a significant main effect of age on CT, surviving adjustment for multiple comparisons, from late childhood to adulthood, in several cortical areas. Noteworthy, there was a significant longitudinal change in CT in several medial regions, including the dorsal parts of ACC (Fig. 4).
Fig. 4.
The cortical thickness in the anterior cingulate cortex (ACC) changes with age from late childhood to adulthood. Map of the linear age effect (FDR-corrected T-statistic, q ≤ 0.05) on the cortical thickness. The dorsal part of ACC is indicated with a yellow dashed line.
4. Discussion
Our large scale longitudinal study provides the first evidence that the qualitative sulcal pattern of ACC is stable from late childhood to adulthood, a developmental window of quantitative changes in ACC structure (cortical thickness and PCS length). Our finding supports use of sulcal patterning, based on qualitative but not quantitative features, as a relevant marker to study long-term effects of early cerebral constraints on cognitive abilities.
Cortical folding is a hallmark of many, but not all, mammalian brains (Welker, 1988). The degree of folding increases with brain size across mammals, but at different scales between orders and families (Zilles et al., 2013). The mature sulcal pattern results from pre- and peri-natal processes that shape the cortex from a smooth lissencephalic structure to a highly convoluted surface (Chi et al., 1977, Haukvik et al., 2012). The precise mechanism underlying cortical folding is still unknown but several factors likely contribute to prenatal processes that influence the shape of the folded cerebral cortex, including cortical growth (Chenn and Walsh, 2002, Haydar et al., 1999, Kuida et al., 1996, Toro and Burnod, 2005), apoptosis or programmed cell death (Haydar et al., 1999), differential expansion of superior and inferior cortical layers (Kriegstein et al., 2006, Richmann et al., 1975), differential tangential expansion (Ronan et al., 2013) and/or structural connectivity through axonal tension forces (Dehay et al., 1996, Hilgetag and Barbas, 2006, Van Essen, 1997). Recent progress in characterizing basal progenitor cells and the genes that regulate their proliferation has contributed to our understanding of genetic basis of cortical folding (Sun and Hevner, 2014, for review). These different early processes lead to a compact layout that optimizes the transmission of neuronal signals between brain regions (Klyachko and Stevens, 2003) and thus the efficacy of brain network functioning.
This association between cortical folding and network functioning may mediate relationships between early sulcal patterns and later functional development (Mangin et al., 2010, for review). For example, we recently showed in a longitudinal study in preschoolers (Borst et al., 2014) that the ACC sulcal pattern, but not the cortical thickness or the surface area of the ACC, at age 5 predicts the efficiency in cognitive control not only at age 5 but also four years later (i.e., at age 9). Of note, the efficiency of cognitive control is only partly explained by these early cerebral constraints given that it can be improved by training and practice during childhood (Diamond, 2013 for a review)–improvements sustained by neuroplasticity mechanisms. An open issue is thus to determine to what extent the receptivity to cognitive training and practice depends on early neurodevelopmental constraints such as the ACC sulcal pattern.
The stability assumption of the sulcal pattern was tested in this study from the analysis of ACC morphology from late childhood to adulthood. This cortical region and this developmental period optimize the possibility to detect possible developmental changes in sulcal pattern types. Indeed, ACC presents qualitatively distinct sulcal patterns that can be reliably classified with structural MRI from childhood to adulthood (Cachia et al., 2014, Paus et al., 1996, Yucel et al., 2001), which limits false positive findings (i.e. detecting changes in sulcal pattern because of classification issues instead of actual morphological changes). While our data provide the first direct evidence that ACC folding type is a developmentally stable trait in humans, further longitudinal studies will be required to directly establish that this trait is also stable outside the age-range considered in our analyses. However, available data suggest that cortical shape within the anterior cingulate region is most-likely stable during the first years after birth, as evidenced by recent longitudinal studies reporting that several markers of cortical shape of stable within the ACC region during the first year of postnatal life, including cortical surface curvature (Li et al., 2014), surface area (Li et al., 2013) and local gyrification (Mutlu et al., 2013). Nevertheless, an important goal for future longitudinal studies will be testing if our findings regarding the developmental stability of folding typology generalize to other segments of the life span, and other regions of the cortical sheet. Testing for generalization to the entire cortex will raise the issues of the definition of the qualitative sulcal patterns to be used for the assessment of each cortical area. This will requires the establishment of a dictionary of human brain folding patterns (Sun et al., 2009), for instance based on the ‘sulcal root’ (Regis et al., 2005), namely indivisible and stable sulcal units corresponding to the first folding locations during antenatal life and that can be recovered after birth from the analysis of the local cortex curvature (Cachia et al., 2003) or depth (Im et al., 2010).
5. Conclusion
By providing direct evidence that sulcal typology remains fixed en route to adulthood, we provide firmer ground for researchers to harness the powerful opportunity of using adult folding patterns to retrospectively stratify cohorts based on categorical differences in their earlier brain development. This approach provides a means to assess how early life influences cognitive abilities in later life, which in turn may illuminate mechanisms of fundamental importance to basic developmental cognitive neuroscience.
References
- Ad-Dab’bagh Y., Einarson D., Lyttelton O., Muehlboeck S., Mok K., Ivanov O., Vincent R., Lepage C., Lerch J., Fombonne E., Evans A. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research; Proceedings of the 12th annual meeting of the organization for human brain mapping, Florence, Italy; 2006. p. 2266. [Google Scholar]
- Armstrong E., Schleicher A., Omran H., Curtis M., Zilles K. The ontogeny of human gyrification. Cereb. Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Borst G., Cachia A., Vidal J., Simon G., Fischer C., Pineau A., Poirel N., Mangin J.F., Houde O. Folding of the anterior cingulate cortex partially explains inhibitory control during childhood: a longitudinal study. Dev. Cogn. Neurosci. 2014;9:126–135. doi: 10.1016/j.dcn.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda M., Fornito A., Bergstrom Z.M., Simons J.S. A specific brain structural basis for individual differences in reality monitoring. J. Neurosci. 2011;31:14308–14313. doi: 10.1523/JNEUROSCI.3595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachia A., Borst G., Vidal J., Fischer C., Pineau A., Mangin J.F., Houde O. The shape of the ACC contributes to cognitive control efficiency in preschoolers. J. Cogn. Neurosci. 2014;26:96–106. doi: 10.1162/jocn_a_00459. [DOI] [PubMed] [Google Scholar]
- Cachia A., Mangin J.F., Riviere D., Kherif F., Boddaert N., Andrade A., Papadopoulos-Orfanos D., Poline J.B., Bloch I., Zilbovicius M., Sonigo P., Brunelle F., Regis J. A primal sketch of the cortex mean curvature: a morphogenesis based approach to study the variability of the folding patterns. IEEE Trans Med. Imaging. 2003;22:754–765. doi: 10.1109/TMI.2003.814781. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Giedd J.N., Berquin P.C., Walter J.M., Sharp W., Tran T., Vaituzis A.C., Blumenthal J.D., Nelson J., Bastain T.M., Zijdenbos A., Evans A.C., Rapoport J.L. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- Chenn A., Walsh C.A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chi J.G., Dooling E.C., Gilles F.H. Gyral development of the human brain. Ann. Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- Clark G.M., Mackay C.E., Davidson M.E., Iversen S.D., Collinson S.L., James A.C., Roberts N., Crow T.J. Paracingulate sulcus asymmetry; sex difference, correlation with semantic fluency and change over time in adolescent onset psychosis. Psychiatry Res. 2010;184:10–15. doi: 10.1016/j.pscychresns.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Dehay C., Giroud P., Berland M., Killackey H., Kennedy H. Contribution of thalamic input to the specification of cytoarchitectonic cortical fields in the primate: effects of bilateral enucleation in the fetal monkey on the boundaries, dimensions, and gyrification of striate and extrastriate cortex. J. Comp. Neurol. 1996;367:70–89. doi: 10.1002/(SICI)1096-9861(19960325)367:1<70::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Ann. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Yucel M., Wood S., Stuart G.W., Buchanan J.A., Proffitt T., Anderson V., Velakoulis D., Pantelis C. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb. Cortex. 2004;14:424–431. doi: 10.1093/cercor/bhh004. [DOI] [PubMed] [Google Scholar]
- Fornito A., Yucel M., Wood S.J., Proffitt T., McGorry P.D., Velakoulis D., Pantelis C. Morphology of the paracingulate sulcus and executive cognition in schizophrenia. Schizophr. Res. 2006;88:192–197. doi: 10.1016/j.schres.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Hirao K., Namiki C., Yamada M., Shimizu M., Fukuyama H., Hayashi T., Murai T. Anterior cingulate pathology and social cognition in schizophrenia: a study of gray matter, white matter and sulcal morphometry. Neuroimage. 2007;36:1236–1245. doi: 10.1016/j.neuroimage.2007.03.068. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukvik U.K., Schaer M., Nesvag R., McNeil T., Hartberg C.B., Jonsson E.G., Eliez S., Agartz I. Cortical folding in Broca's area relates to obstetric complications in schizophrenia patients and healthy controls. Psychol. Med. 2012;42:1329–1337. doi: 10.1017/S0033291711002315. [DOI] [PubMed] [Google Scholar]
- Haydar T.F., Kuan C.Y., Flavell R.A., Rakic P. The role of cell death in regulating the size and shape of the mammalian forebrain. Cereb. Cortex. 1999;9:621–626. doi: 10.1093/cercor/9.6.621. [DOI] [PubMed] [Google Scholar]
- Hilgetag C.C., Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput. Biol. 2006;2:e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogstrom L.J., Westlye L.T., Walhovd K.B., Fjell A.M. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb. Cortex. 2013;23:2521–2530. doi: 10.1093/cercor/bhs231. [DOI] [PubMed] [Google Scholar]
- Huster R.J., Westerhausen R., Kreuder F., Schweiger E., Wittling W. Morphologic asymmetry of the human anterior cingulate cortex. Neuroimage. 2007;34:888–895. doi: 10.1016/j.neuroimage.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Im K., Jo H.J., Mangin J.F., Evans A.C., Kim S.I., Lee J.M. Spatial distribution of deep sulcal landmarks and hemispherical asymmetry on the cortical surface. Cereb. Cortex. 2010;20:602–611. doi: 10.1093/cercor/bhp127. [DOI] [PubMed] [Google Scholar]
- Im K., Lee J.M., Lyttelton O., Kim S.H., Evans A.C., Kim S.I. Brain size and cortical structure in the adult human brain. Cereb. Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Kanai R., Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Klyachko V.A., Stevens C.F. Connectivity optimization and the positioning of cortical areas. Proc. Natl. Acad. Sci. USA. 2003;100:7937–7941. doi: 10.1073/pnas.0932745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A., Noctor S., Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Kuida K., Zheng T.S., Na S., Kuan C., Yang D., Karasuyama H., Rakic P., Flavell R.A. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Leonard C.M., Towler S., Welcome S., Chiarello C. Paracingulate asymmetry in anterior and midcingulate cortex: sex differences and the effect of measurement technique. Brain Struct. Funct. 2009;213:553–569. doi: 10.1007/s00429-009-0210-z. [DOI] [PubMed] [Google Scholar]
- Li G., Nie J., Wang L., Shi F., Lin W., Gilmore J.H., Shen D. Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age. Cereb. Cortex. 2013;23:2724–2733. doi: 10.1093/cercor/bhs265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Wang L., Shi F., Lyall A.E., Lin W., Gilmore J.H., Shen D. Mapping longitudinal development of local cortical gyrification in infants from birth to 2 years of age. J. Neurosci. 2014;34:4228–4238. doi: 10.1523/JNEUROSCI.3976-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton O., Boucher M., Robbins S., Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- MacDonald D., Kabani N., Avis D., Evans A.C. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Mangin J.F., Jouvent E., Cachia A. In-vivo measurement of cortical morphology: means and meanings. Curr. Opin. Neurol. 2010;23:359–367. doi: 10.1097/WCO.0b013e32833a0afc. [DOI] [PubMed] [Google Scholar]
- Mangin J.F., Riviere D., Cachia A., Duchesnay E., Cointepas Y., Papadopoulos-Orfanos D., Scifo P., Ochiai T., Brunelle F., Regis J. A framework to study the cortical folding patterns. Neuroimage. 2004;23(Suppl 1):S129–S138. doi: 10.1016/j.neuroimage.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Mutlu A.K., Schneider M., Debbane M., Badoud D., Eliez S., Schaer M. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage. 2013;82:200–207. doi: 10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- Ono M., Kubik S., Abarnathey C.D. Georg Thieme; New York: 1990. Atlas of the Cerebral Sulci. [Google Scholar]
- Paus T., Tomaiuolo F., Otaky N., MacDonald D., Petrides M., Atlas J., Morris R., Evans A.C. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb. Cortex. 1996;6:207–214. doi: 10.1093/cercor/6.2.207. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Greenstein D., Lee N.R., Clasen L.S., Giedd J.N. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc. Natl. Acad. Sci. USA. 2012;109:11366–11371. doi: 10.1073/pnas.1203350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regis J., Mangin J.F., Ochiai T., Frouin V., Riviere D., Cachia A., Tamura M., Samson Y. Sulcal root” generic model: a hypothesis to overcome the variability of the human cortex folding patterns. Neurol. Med. Chir. (Tokyo) 2005;45:1–17. doi: 10.2176/nmc.45.1. [DOI] [PubMed] [Google Scholar]
- Richmann D., Stewart R., Hutchinson J., Caviness V. Mechanical model of brain convolutional development. Science. 1975;189:18–21. doi: 10.1126/science.1135626. [DOI] [PubMed] [Google Scholar]
- Ronan L., Voets N., Rua C., Alexander-Bloch A., Hough M., Mackay C., Crow T.J., James A., Giedd J.N., Fletcher P.C. Differential tangential expansion as a mechanism for cortical gyrification. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W., Phillips D.I. Fetal origins of mental health: evidence and mechanisms. Brain Behav. Immun. 2009;23:905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkin S.D., Starr J.M., Deary I.J. Birth weight and cognitive ability in childhood: a systematic review. Psychol. Bull. 2004;130:989–1013. doi: 10.1037/0033-2909.130.6.989. [DOI] [PubMed] [Google Scholar]
- Sun T., Hevner R.F. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat. Rev. Neurosci. 2014;15:217–232. doi: 10.1038/nrn3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.Y., Perrot M., Tucholka A., Riviere D., Mangin J.F. Constructing a dictionary of human brain folding patterns. Med. Image Comput. Comput. Assist. Interv. 2009;12:117–124. doi: 10.1007/978-3-642-04271-3_15. [DOI] [PubMed] [Google Scholar]
- Toro R., Burnod Y. A morphogenetic model for the development of cortical convolutions. Cereb. Cortex. 2005;15:1900–1913. doi: 10.1093/cercor/bhi068. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Brown T.T., Kuperman J.M., Chung Y., Hagler D.J., Jr., Roddey J.C., Erhart M., McCabe C., Akshoomoff N., Amaral D.G., Bloss C.S., Libiger O., Schork N.J., Darst B.F., Casey B.J., Chang L., Ernst T.M., Frazier J., Gruen J.R., Kaufmann W.E., Murray S.S., van Zijl P., Mostofsky S., Dale A.M. Long-term influence of normal variation in neonatal characteristics on human brain development. Proc. Natl. Acad. Sci. USA. 2012;109:20089–20094. doi: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker W. Why does cerebral cortex fissure and fold? Cereb. Cortex. 1988;8B:3–135. [Google Scholar]
- White T., Su S., Schmidt M., Kao C.Y., Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Allen N.B., Fornito A., Lubman D.I., Simmons J.G., Pantelis C., Yucel M. Variations in cortical folding patterns are related to individual differences in temperament. Psychiatry Res. 2009;172:68–74. doi: 10.1016/j.pscychresns.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Yucel M., Stuart G.W., Maruff P., Velakoulis D., Crowe S.F., Savage G., Pantelis C. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb. Cortex. 2001;11:17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- Zijdenbos A.P., Forghani R., Evans A.C. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: Application to multiple sclerosis. Ieee Trans. Med. Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- Zilles K., Palomero-Gallagher N., Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36:275–284. doi: 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]