Abstract
Cancer immunotherapies are increasingly effective in the clinic, especially immune checkpoint blockade delivered to patients who have T cell-infiltrated tumors. Agonistic CD40 mAb promotes stromal degradation, and in combination with chemotherapy, drives T cell infiltration and de novo responses against tumors, rendering resistant tumors susceptible to current immunotherapies. Partnering αCD40 with different treatments is an attractive approach for the next phase of cancer immunotherapies, with a number of clinical trials using αCD40 combinations ongoing, but the optimal therapeutic regimens with αCD40 are not well understood. Pancreatic ductal adenocarcinoma (PDA) is classically resistant to immunotherapy and lacks baseline T cell infiltration. Here, we utilized a tumor cell line derived from a genetically engineered mouse model of PDA to investigate alterations in the sequence of αCD40 and chemotherapy as an approach to enhance pharmacological delivery of chemotherapy. Unexpectedly, despite our previous studies showing αCD40 treatment after chemotherapy is safe in both mice and patients with PDA, we report here that αCD40 administration less than three days in advance of chemotherapy is lethal in over half of treated C57Bl/6 mice. αCD40 treatment two or three days before chemotherapy resulted in significantly increased populations of both activated myeloid cells and macrophages, and lethal hepatotoxicity. Liver damage was fully abrogated when macrophage activation was blocked using αCSF-1R mAb. These studies highlight the dual nature of CD40 in activating both macrophages and T cell responses, and the need for preclinical investigation of optimal αCD40 treatment regimens for safe design of clinical trials.
Introduction
Immunotherapies such as αPD-1/L1 (programmed cell death-1/ligand-1) and αCTLA-4 have shown significant clinical efficacy in some patients with certain cancers, including those with metastatic disease (1–3). However, these therapies are most often successful in the subset of patients who have an ongoing immune response against the tumor, and are less effective against tumors lacking baseline T cell infiltration (4). Patients with poorly infiltrated tumors have a much lower prognosis, even for classically immunogenic cancers such as melanoma (5). Pancreatic ductal adenocarcinoma (PDA) is a canonical example of a poorly immunogenic tumor as it largely lacks strong neo-epitopes (6–8) and T cell infiltration, correlating with a dismal 5-year survival rate of less than 5%. Gemcitabine (Gem) is part of a standard-of-care for patients with PDA but serves to extend overall survival by only a few weeks to months (9). Patients with PDA are resistant to CTLA-4 or PD-1 antibody therapy (1, 10, 11). Thus, improved treatments that are effective in tumors lacking endogenous T cell infiltration are needed in the clinic.
Agonistic CD40 mAb functions analogous to CD40-ligand in vivo, activating and maturing APCs (12–14). In some highly immunogenic mouse models of cancers, αCD40 therapy results in T cell-mediated tumor regressions (15), but in other models of solid tumors, αCD40 alone is not sufficient to mediate anti-tumor T cell responses (16, 17). Indeed, using the genetically engineered KPC mouse model of PDA, in which oncogenic KrasG12D and mutant p53R172H are under the control of Cre recombinase specifically expressed in the pancreas (18), we have shown that αCD40 alone fails to prime T cell responses against PDA (16). KPC mice faithfully recapitulate key features of human disease, including a dearth of non-synonymous mutations (similar to other Kras-induced mouse models of cancer (19)) and minimal effector T cell infiltration (20). The lack of T cells in PDA tumors correlates with resistance to current immunotherapies, including αPD-1 and αCTLA-4 in mice (21), as is also observed in patients with PDA (1). However, agonistic αCD40 activates dendritic cells and is capable of driving T cell infiltration and T cell-dependent regression of established tumors when administered 48 hours after treatment with Gem (17, 22), and is sufficient to render PDA susceptible to αPD-1/αCTLA-4 treatment (21). Gem is hypothesized to augment αCD40 therapy by killing tumor cells and liberating tumor antigens that are then picked up and presented by antigen presenting cells (17). Thus, αCD40 is an immunotherapy capable of converting tumors devoid of T cells (and refractory to αPD-1/αCTLA-4) to a tumor that is sensitive to T cell-mediated destruction, potentially filling a void in the clinical toolbox for treating patients with cancer.
In addition to the ability of αCD40 to activate APCs and prime T cell responses, we have shown that αCD40 stimulation alters tumor stroma and activates macrophages to become tumoricidal (16). Therefore, αCD40 plays dual roles, both activating APCs to destroy tumor stroma as well as driving anti-tumor T cell responses. To develop an optimal adaptive T cell response, the hypothesis has been that Gem must precede αCD40, but given the stroma degradation observed with αCD40 alone, another option would be to deliver chemotherapy after αCD40 to potentially deliver more chemotherapy to the tumor microenvironment which is otherwise difficult to penetrate pharmacologically (23). If this is possible, the sequence of αCD40 administration relative to chemotherapy may be relevant for improved treatment design.
In this study, we investigate the efficacy of αCD40 treatment when provided 48 hours before, instead of after, standard-of-care chemotherapy for PDA. While αCD40 treatment alone or after Gem therapy resulted in reduction of tumor growth as expected (16, 22), we found that pretreatment with αCD40 followed by Gem was lethal in half of all treated mice, whether mice were tumor-bearing or not. Although αCD40 therapy recruited large numbers of myeloid cells in to the liver, blockade of the inflammatory macrophage population via the administration of αCSF-1/1R antibodies abrogated the toxicity associated with αCD40 pretreatment. As αCD40 is being actively investigated in the clinical setting in combination with a number of treatments, including chemotherapy, these studies have implications for the design of clinical trials using αCD40.
Materials and Methods
Mice, tumor cell lines, and in vivo growth
The mouse pancreatic cancer cell line 4662 (CD40-negative) was generated from a C57BL/6 KrasLSL-G12D/+,Trp53LSL-R172H/+, Pdx1-Cre (KPC) mouse (18) as previously described (24). The genetic background of the C57BL/6 KPC mice was confirmed using the DartMouse™ Speed Congenic Core Facility at the Geisel School of Medicine at Dartmouth College (Hanover, NH). DartMouse uses the Illumina, Inc. (San Diego, CA) GoldenGate Genotyping Assay to interrogate 1449 SNPs spread throughout the genome. The raw SNP data were analyzed using DartMouse’s SNaP-Map™ and Map-Synth™ software, allowing the determination for each mouse of the genetic background at each SNP location. Wild-type C57BL/6 were purchased from Jackson Laboratory (Bar Harbor, ME). Animal protocols were reviewed and approved by the Institute of Animal Care and Use Committee at the University of Pennsylvania. PDA cells were used in experiments after 3–5 passages in vitro; C57Bl/6 mice received 2.5×105 PDA cells subcutaneously only if viability was >94%. Cell lines were tested by using the Infectious Microbe PCR Amplification Test (IMPACT) and authenticated by the Research Animal Diagnostic Laboratory (RADIL) at the University of Missouri. Tumors were measured thrice weekly by calipers, and the volume was calculated by (LxW2)/2, where L is the longest diameter and W is the perpendicular diameter. Mice were euthanized when tumor volume reached 1000m3.
Drug preparation
Gemcitabine (Gem; Hospira, Lake Forest, IL) pharmaceutical grade suspension at 38mg/mL 2’-deoxy-2’,2’-difluorocytidine was purchased through the Hospital of the University of Pennsylvania Pharmacy. Gem was diluted to 12mg/mL in PBS and administered at 120mg/kg via intraperitoneal (i.p.) injection as we have previously reported (16). Nab-paclitaxel (nP; Abraxane, Celgene, Summit, NJ) pharmaceutical grade powder was purchased through the Hospital of the University of Pennsylvania Pharmacy and resuspended at 12mg/mL in PBS. Mice received 120mg/kg of nP i.p. as previously reported (25).
Monoclonal antibodies
Mice received 100 or 300 µg of agonist CD40 rat anti-mouse IgG2a monoclonal antibody (mAb; clone FGK45), or the isotype control IgG2a mAb (clone 2A3) as previously reported (16) on day 0 (tumor-free mice), or specified day after 4662 injection. Indicated mice received 1mg of anti-CSF-1R mAb (clone AFS98) i.p. on day 6 after 4662 injection, and 0.5mg every 3 days afterwards. Some mice also received 1mg of anti-CSF-1 (clone 5A1) i.p. starting on day 6 and repeated every 3 days (26). All mAbs were purchased from BioXCell (Lebanon, NH) and were endotoxin-free.
Preparation of livers, spleen, and lungs for flow cytometry
Livers, spleen, and lungs were harvested from mice on indicated day. Livers and lungs were minced, and incubated for one hour in 1mg/mL collagenase IV in DMEM at 37°C, with the addition of 50U/mL of DNAse (Roche) for lung tissues. Livers, spleen, and lungs were then mechanically dissociated and passed through a 70 µM cell strainer, incubated in ACK lysis buffer (BioWhittaker, Allendale, NJ), and used for flow cytometric analysis.
Flow Cytometry
Cell surface molecules were analyzed by incubating single cell suspensions of tissues with primary fluorochrome-labeled antibodies at 4°C for 30 minutes in PBS with 0.5% BSA and 2mM EDTA. Antibodies used in flow analysis were ordered from BD Biosciences (Sparks, MD), eBioscience (San Diego, CA) or Biolegend (San Diego, CA), and included FITC labeled CD40 (clone HM40-3), CD45 (PE, PerCP, Alexa-Fluor 700, and QDot 605, clone 30-F11), CD115 (CSF-1R, PE, clone AFS98), CD31 (Brilliant Violet 450, clone 390), PerCP labeled BD Viaprobe, CD11b (PerCP Cy5.5 and APC, clone M1/70), F4/80 (PerCP, APC Cy7, and PE Cy7, clone BM8), Gr-1 (APC Cy7, clone RB6–8C5), CD11c (Brilliant Violet V450 or APC, clone N418), Ly6G (APC Cy7, clone 1A8), Ly6C (Brilliant Violet 570, clone HK1.4), and Live/Dead Aqua (Life Technologies, Carlsbad, CA). Flow cytometric analysis was performed on a FACSCanto (BD Biosciences, Sparks, MD). Collected data were analyzed using FlowJo software (Treestar, Ashland, OR).
Immunohistochemistry
Livers, spleen, and lungs were harvested from mice two or four days after receiving αCD40 injection as indicated, and snap frozen in OCT, or fixed in zinc formalin and paraffin embedded. Tissue sections were cut at 5um, stained with H&E, and quantified by counting all lesions visible per liver lobe at 4X magnification. Images were taken using a Nikon DS-Fi2 digital camera at 4X and 20X magnification on a Nikon Eclipse 50i and assessed at the Comparative Pathology Core at the University of Pennsylvania School of Veterinary Medicine.
AST and ALT analysis
Whole blood was collected from mice sacrificed 12 hours after αCD40 injection, and samples were tested for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) by the Clinical Pathology Lab at the Ryan Veterinary Hospital at the University of Pennsylvania.
Statistical Analyses
Significance of overall survival was determined using Kaplan-Meier survival curve with Log-rank analysis. All other comparisons were performed using one- or two-way ANOVA with Tukey’s HSD post-test, or Mann-Whitney T test, as indicated. All statistical analyses were performed with Graphpad Prism 6 (GraphPad, La Jolla, CA). Standard deviation (SD) or Standard Error of the Mean (SEM) as indicated by error bars. P < 0.05 was considered statistically significant, * indicates P <0.05, ** P <0.01, *** P < 0.001, and *** P < 0.0001, ns denotes not significant.
Results
CD40 agonist antibody is lethal when administered before chemotherapy
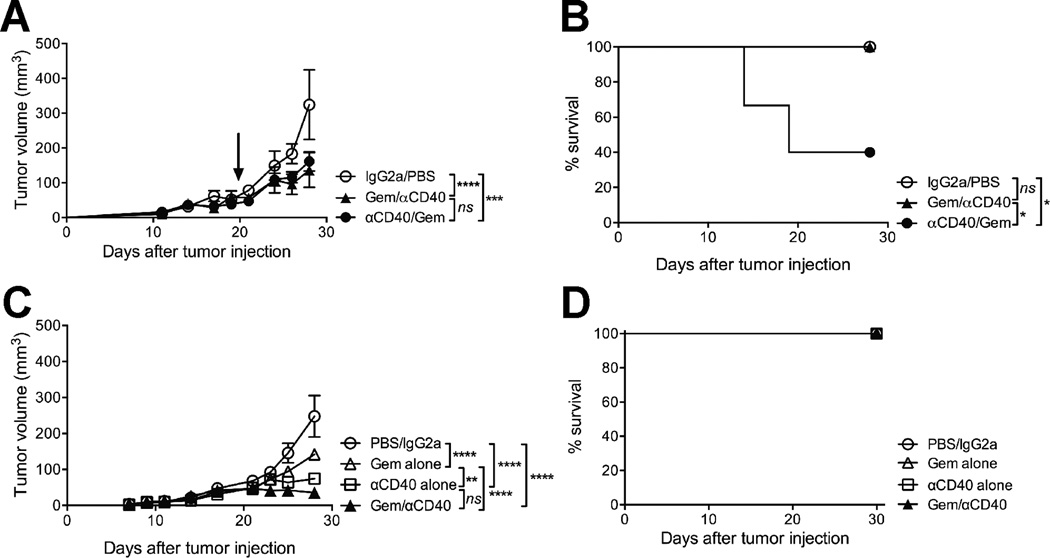
We harvested a spontaneous PDA tumor from a C57BL/6 KPC mouse and generated a cell line (4662) that upon implantation shares features with spontaneous PDA tumors, including desmoplastic stroma, extracellular matrix deposition, mutant Kras expression, and loss of p53 (24). The 4662 PDA cell line grew progressively when 2.5×105 cells were implanted subcutaneously in C57BL/6 syngeneic hosts, and reached a diameter of 3–5mm by 12 days after implantation. Treatment of mice bearing established 4662 tumors with Gem on day 12, followed by agonistic CD40 antibody on day 14 (Gem/αCD40) significantly reduced tumor growth rates compared to mice receiving PBS and isotype control (Fig. 1A). Tumor growth rates were also significantly reduced in mice that received the treatment regimen in reverse (αCD40 on day 12, followed by Gem on day 14; αCD40/Gem) (Fig. 1A); however, 4/10 of mice treated with αCD40 followed by Gem died within 5 days of starting therapy (Fig. 1B). In comparison, 0/10 mice died with Gem/αCD40 treatment or vehicle control treatment (Fig. 1B). A similar reduction in tumor growth was observed with αCD40 alone (Fig. 1C), as we have previously reported (16), but there was no lethal toxicity with αCD40 alone, Gem alone, or Gem followed by CD40 (Fig. 1D). After treatment with either αCD40 or Gem alone, 0/12 mice died across two independent experiments, and we have previously reported the safety of these drugs as monotherapies (16). But across 10 independent experiments, 46/82 (56%) mice treated with αCD40 followed by Gem died, compared to 0/22 mice after Gem/αCD40 in four experiments, and 0/14 mice after IgG2a/PBS in three experiments
Figure 1. Agonistic CD40 antibody is lethal when administered before chemotherapy.
Mice were injected with 4662 PDA tumor cell line, and received either Gem on day 12 and αCD40 on day 14 (Gem/αCD40), or αCD40 on day 12 and Gem on day 14 (αCD40/Gem), or vehicle controls (IgG2a on day 12, PBS on day 14). (A) Tumor growth curves of mice treated as indicated, representative of two independent experiments, n=5–10 mice/group. Arrow indicates time point at which 4/10 mice died in αCD40/Gem treatment group, following this time point only tumor growth on the surviving 6/10 mice is shown. (B) Survival curve of mice treated as indicated, from two combined experiments, n=5–10 mice/group. (C) Tumor growth curves of mice treated as indicated including Gem alone or αCD40 alone on day 12, representative of 2 independent experiments, n=6 mice per group. (D) Survival curve of mice from (C). Statistical analysis by two-way ANOVA with Tukey’s HSD post-test (A, C) or by Kaplan-Meier (B, D). Each symbol represents a group of mice, error bars indicate SEM, ns indicates not significant, * indicates P < 0.05, *** P < 0.001, **** P < 0.0001.
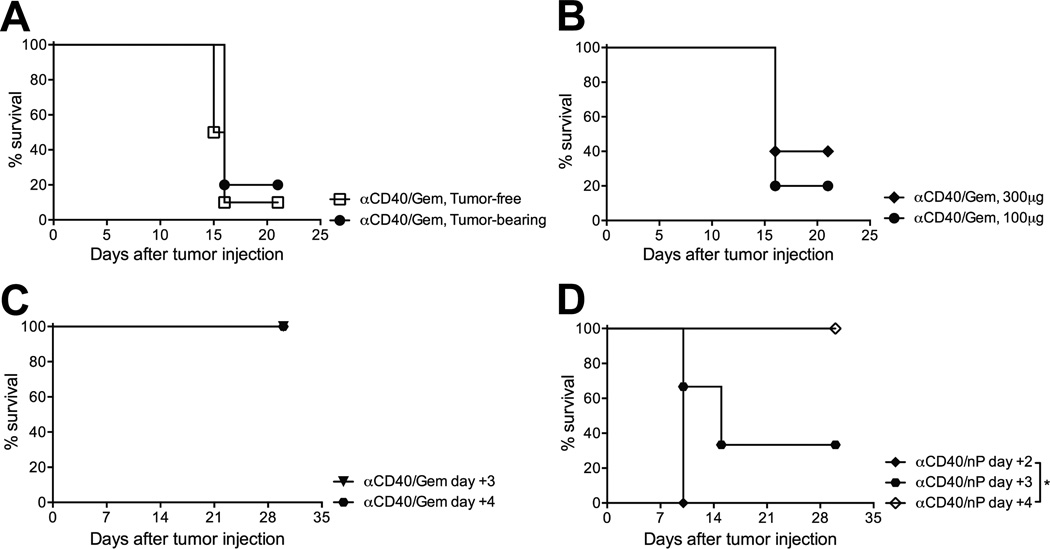
To determine if the presence of a growing tumor was required for lethal sensitivity to αCD40/Gem therapy, tumor-free C57BL/6 mice were treated with αCD40/Gem and survival rates were similar to those observed in αCD40/Gem mice bearing established tumors (Fig. 2A) and across 6 experiments, 11/34 (32.2%) of tumor-free mice died after αCD40/Gem treatment. To investigate the potential for dose-mediated lethality after αCD40/Gem treatment, we increased the amount of αCD40 administered to mice from 100µg to 300µg but found that survival rates were similar in mice regardless of the amount of αCD40 received before Gem administration (Fig. 2B). Across 15 experiments, with both tumor-bearing and tumor-free mice, 56/124 (45.2%) of mice treated with 300µg of αCD40 followed by Gem died, compared to 57/98 (58.1%) of mice treated with 100µg of αCD40 followed by Gem (data not shown). We did not test lower doses of αCD40 in these experiments because we have previously observed that lower doses fail to demonstrate a pharmacodynamic effect.
Figure 2. Chemotherapy is toxic when administered two days after αCD40 therapy, regardless of tumor-bearing status of the host.
(A) Mice were injected with 4662 PDA tumor cells (tumor-bearing) or left uninjected (tumor-free) on day 0, and received αCD40 on day 11 and Gem on day 13. Survival curve representative of 4 experiments, 5–10 mice/group. (B) Mice were treated as described in Fig. 1, except that tumor-bearing mice received either 100 or 300 µg of αCD40 on day 11 as indicated, followed by Gem on day 13. Survival representative of 2 experiments, n=5 mice/group. (C) Mice were treated as described in Fig. 1, except that Gem was administered 3 days or 4 days after αCD40, as indicated. Survival curve representative of 2 experiments, n=3–5 mice/group. (D) Mice were treated as described in Fig. 1, except that mice received nab-paclitaxel (nP) instead of Gem on 2, 3, or 4 days, as indicated, after αCD40 treatment, n=3 mice/group. Each symbol represents a group of mice, statistical analyses by Kaplan-Meier (* indicates P < 0.05).
Given that both mice and patients can be successfully treated with Gem 5 days after αCD40 as part of a combination therapeutic schema (16), we next investigated the time frame in which Gem administration after αCD40 treatment is lethal. While the administration of Gem 2 days after αCD40 is toxic in 56% of treated mice (Fig. 1), Gem administration 3 or 4 days after αCD40 was not lethal to mice (Fig. 2C). Furthermore, when substituting nab-paclitaxel (Abraxane; nP), a different chemotherapy but also FDA approved for PDA, the combination was also toxic when nab-paclitaxel was administered within 2 days after αCD40, and remained lethal even on day 3 (Fig. 2D). Hence, there is a temporal window after αCD40 administration when treatment with chemotherapy significantly reduces the survival of treated mice.
Agonistic CD40 stimulation combined with chemotherapy drives hepatotoxicity
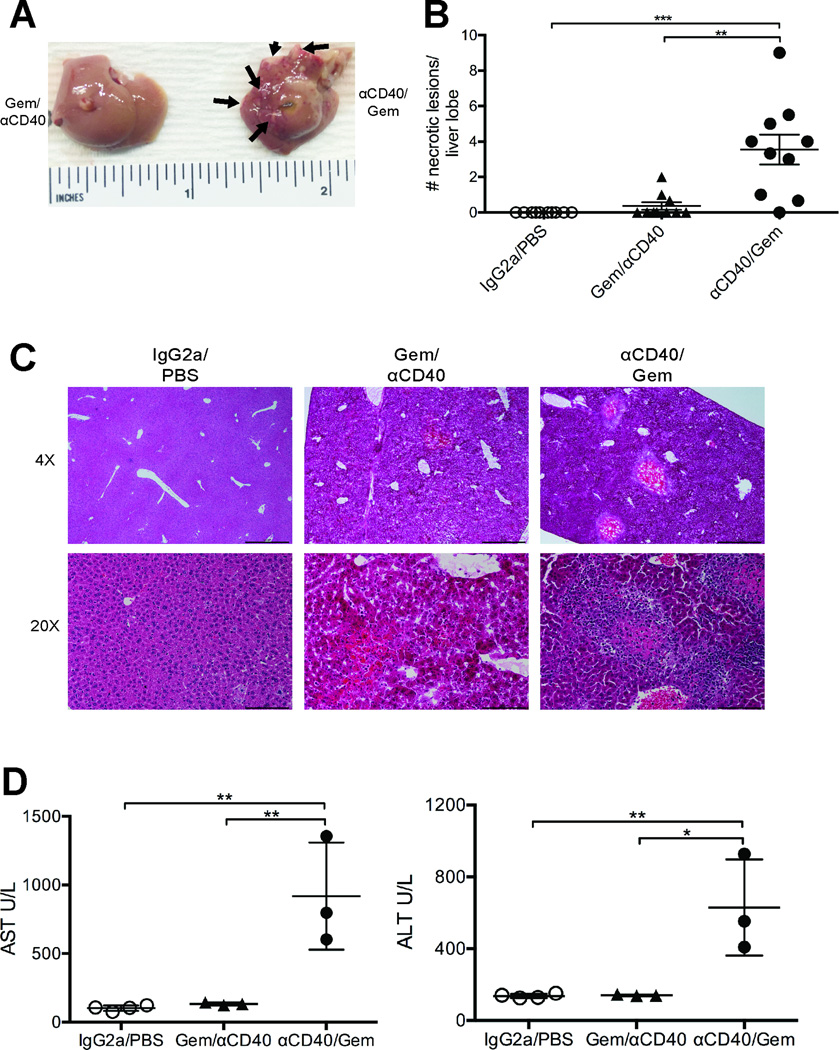
Given that a subset of mice were dying after 2 days after completing αCD40/Gem therapy, cohorts of mice were sacrificed 48 hours after the completion of treatment to investigate causality. Pathologic lesions were grossly observed in liver parenchyma and on the capsule of αCD40/Gem-treated mice that were not present in the livers of Gem/αCD40 treated mice (Fig. 3A, lesions indicated by arrow). No other pathological findings were noted on gross inspection. Using H&E staining, the number of lesions observed per lobe of liver was significantly higher in mice treated with αCD40/Gem (mean 2.9 ± 0.6) as compared to mice with Gem/αCD40 (mean 0.2 ± 0.2, p < 0.01) (Fig. 3B). Microscopically, livers from both αCD40/Gem and Gem/αCD40 treated mice exhibited coagulative hepatocellular necrosis compared to IgG2a/PBS control treated mice, primarily surrounding veins (both central and portal) and frequently associated with occlusive to partially occlusive fibrin thrombi (Fig. 3C). These findings are consistent with infarcts, with the likely cause of death in αCD40/Gem treated mice being multiple hepatic infarcts. However, moderate to severe multifocal granulatomous and neutrophilic infiltrates were observed in all mice treated with αCD40/Gem (14/14), whereas only 2/5 mice treated with Gem/αCD40 were found to have minimal to mild granulatomous and neutrophilic infiltrates. Assessment of both the spleen and lung at the same time point revealed no macroscopic lesions at the time of sacrifice, and upon histopathological examination, no evidence of necrosis within the tissues, although 3/5 mice treated with αCD40/Gem were observed to have minimal multifocal lymphohistiocytic perivascular infiltrates in the lung. Additionally, 3/20 mice treated with αCD40 +/− Gem presented with capillary fibrin thrombi in the lung, associated with the systemic hypercoagulable state observed in the liver tissue.
Figure 3. αCD40 followed by chemotherapy causes hepatotoxicity.
Mice were treated as described in Fig. 1, and mice were euthanized 48 hours after the end of treatment (day 16). (A) Representative livers from indicated treatment groups with macroscopic lesions indicated by arrows. (B) Quantification of lesions observed at 4X per lobe of liver for indicated groups, from two combined experiments with n=4–5 mice/group. (C) Representative liver H&E sections shown from indicated treatment group. (D) Mice were treated as described in Fig. 1, and mice were euthanized 12 hours after the end of treatment (day 14.5). Serum was collected from whole blood, and analyzed for AST and ALT as indicated. Each symbol represents a single mouse, error bars indicated SEM (B) and SD (D). Statistical analysis by one-way ANOVA with Tukey’s HSD post-test, * indicates P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
To quantify further the magnitude of hepatotoxicity after αCD40/Gem therapy, serum levels of alanine and aspartate aminotransferases (ALT and AST, respectively) were determined 12 hours after the completion of treatment in all cohorts of mice. Both ALT and AST levels were significantly increased (628.7 ± 267.4 U/L and 918.7 ± 391 U/L, respectively) in mice receiving αCD40/Gem compared to Gem/αCD40 (140 ± 4.4 U/L and 133.7 ± 10.2 U/L) or vehicle controls (136 ± 11.9 U/L and 102.3 ± 19.9 U/L) (Fig. 3D), revealing that the administration of αCD40 followed by Gem results in rapid and significant liver damage in mice.
CD40 stimulation increases activated myeloid populations in the liver
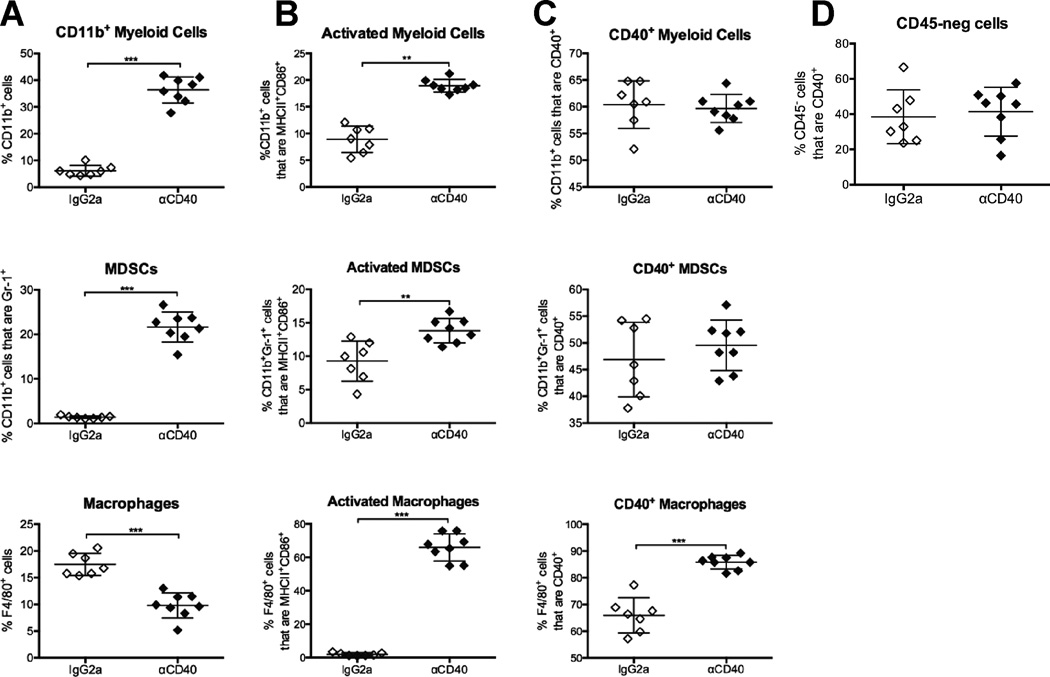
Given the hepatotoxicity caused by αCD40/Gem, and that αCD40 stimulates APCs, including dendritic cells and macrophages (16), we hypothesized that αCD40 was activating APCs within the liver compartment. Indeed, 24 hours after αCD40, the overall proportion of total CD11b+ myeloid cells in the liver was significantly increased to 36.4%, as was the frequency of CD11b+ Gr1+ neutrophils/myeloid derived suppressor cells (MDSCs) (21.6%), compared to IgG2a/PBS vehicle control treatment (from 6.2% myeloid cells and 1.4% MDSCs, respectively) (Fig. 4A, top and middle). Conversely, the total frequency of F4/80+ macrophages was significantly decreased in the livers of mice treated with αCD40 to 9.8% versus 17.5% in control treated mice (Fig. 4A, bottom).
Figure 4. αCD40 therapy increases the frequency of activated macrophages in the livers of treated mice.
Mice were treated with αCD40 and sacrificed one day after treatment. Livers were harvested and analyzed by flow cytometry with regard to the indicated cell populations among live, CD45+ cells (A–C) or live, CD45-neg cells (D). Each symbol represents an individual mouse, horizontal bars indicated mean, and error bars indicate SD, data representative of two independent experiments with n=5–8mice/group. Statistical analysis by Mann-Whitney unpaired T test, * indicates P < 0.05, ** P < 0.01 and *** P < 0.001.
The proportion of activated CD86+ MHCII+ CD11b+ cells was significantly increased in the livers of αCD40 treated mice (mean 18.9%, versus 8.9% in control treated mice), as were the frequencies of activated MDSCs, although to a lesser extent (mean 13.8% versus 9.3% in control treated mice) (Fig. 4B, top and middle). Although the frequency of the total macrophage population was reduced, the frequency of activated macrophages, as measured by the proportion of CD86+ MHCII+ F4/80+ cells, was significantly increased after αCD40 (mean 65.9% versus 1.4% in control treated mice) (Fig. B, bottom). Additionally, even though nearly 70% of F4/80+ macrophages were CD40+, stimulation with αCD40 further increased CD40+ expression (mean 85.5% versus 65.9% in control treated mice, MFI 2795±321 in αCD40 treated mice versus 1345±258 in control treated mice). In comparison, activated myeloid cells and MDSCs exhibited no significant alterations in the proportion of CD40+ cells after αCD40 treatment (Fig. 4C). Furthermore, the expression of CD40 on CD45-neg cells, including hepatocytes and vascular endothelial cells known to express CD40 (27–29), was not increased after αCD40 therapy (Fig. 4D). The changes in the hepatic myeloid subsets were observed only within the relative frequencies of cells, as the absolute cell numbers per gram of liver were not significantly different across groups (Supp. Fig. 1), potentially due to alterations in the frequencies of other (non-myeloid) leukocyte subsets. Furthermore, treatment with αCD40 resulted in splenomegaly regardless of the timing of Gem administration (Supp. Fig. 2A and 2B), associated with moderate to severe white pulp hyperplasia as a result of systemic immune activation (Supp. Fig. 2C). The total splenic myeloid cell population per gram of tissue was significantly reduced after αCD40 therapy, as well as both monocytic (Mo-) and granulocytic (G-) MDSCs in mice treated with αCD40/Gem (Supp. Fig. 2D and 2E). A slight increase in the proportion of CD40+ Mo-MDSCs was observed in the lungs of αCD40/Gem treated mice compared to vehicle control treated mice, but no other obvious alterations in myeloid cell subsets were observed (Supp. Fig. 3). Thus, αCD40 therapy primarily alters the hepatic myeloid cell compartment, and significantly increases the frequency of activated, mature macrophages within the liver after treatment.
Blockade of macrophage activation abrogates lethality of CD40/chemotherapy combination
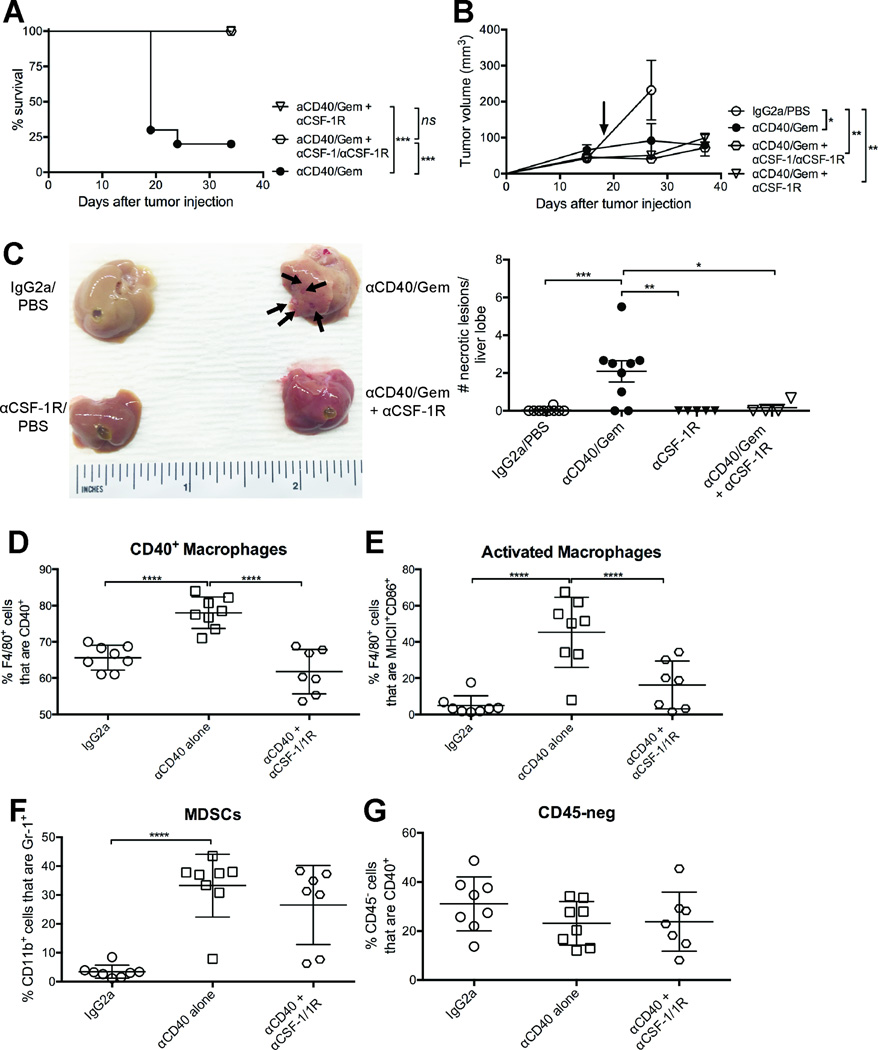
To ascertain a role of activated macrophages in mediating liver damage, CSF-1 and CSF-1R mAbs were administered to mice bearing established tumors starting on day 7. After treatment with αCD40 on day 13, followed by Gem on day 15, 10/10 mice survived (Fig. 5A). Using CSF-1R mAb alone also protected mice from αCD40/Gem lethality (9/9 survived), while only 2/10 survived without αCSF-1 and/or αCSF-1R (Fig. 5A). Furthermore, the addition of αCSF-1/1R in combination with αCD40/Gem maintained the reduced tumor growth rate observed in mice that survived αCD40/Gem therapy (Fig. 5B, and Fig. 1A). Thus, blockade of CSF-1/1R nullifies the lethality mediated by αCD40/Gem treatment.
Figure 5. Blockade of macrophage activation abrogates hepatotoxicity of αCD40/Gem treatment.
Mice were treated as described in Figure 1, except some mice also received αCSF-1 and/or αCSF-1R starting on day 6 and repeated every three days for the duration of the experiment. (A–B) Survival curve (A) or tumor growth kinetics (B) of mice treated with αCD40/Gem or αCD40/Gem with αCSF-1 and/or αCSF-1R. Data representative of three independent experiments with n=4–10 mice/group, and from two combined experiments (right). Each symbol represents a group of mice, error bars indicate mean ± SEM, and arrow indicates time point when 7/19 mice died in αCD40/Gem treated group. (C) Mice were treated as in (A), except that mice were sacrificed on day 16. (Left) Representative livers from indicated treatment groups with macroscopic lesions indicated by arrows. (Right) Quantification of lesions observed at 4X per lobe of liver for indicated groups, from two combined experiments with n=4–5 mice/group. (D–G) Mice were euthanized on day 12, and livers were analyzed by flow cytometry with regard to the indicated cell populations among live, CD45+ cells (D–F) or live, CD45-neg cells (G). Each symbol represents a single mouse, n=7–8mice/group, data representative of two independent experiments, horizontal line indicates mean ± SD. Statistical analyses by Kaplan-Meier (A,), two-way ANOVA (B), or one-way ANOVA with Tukey’s HSD post-test (C–G), * indicates P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001.
To determine if the hepatotoxicity observed after αCD40/Gem was alleviated with the addition of anti-CSF-1R, mice were sacrificed two days after Gem administration. No macroscopic lesions were observed in mice treated with αCD40/Gem and anti-CSF-1R, and the frequency of necrotic lesions in the liver was significantly reduced compared to mice receiving αCD40/Gem (Fig. 5C). Upon histological examination, 5/5 livers from mice treated with αCD40/Gem exhibited severe multifocal to coalescing coagulative to lytic hepatocellular necrosis, while only 1/4 livers from mice treated with αCD40/Gem and anti-CSF-1R exhibited moderate multifocal acute coagulative hepatocellular necrosis (with 2/4 mild, and 1/4 minimal). Furthermore, the livers from αCD40/Gem treated mice had widely dispersed hepatocyte necrosis, which was individualized and had indistinct margins, compared to livers from αCD40/Gem + anti-CSF-1R treated mice, for which the coagulative necrosis was observed in confluent areas with clear distinction between normal and necrotic hepatocytes. No necrotic foci were noted in the spleen of mice treated with αCD40/Gem +/− anti-CSF-1R, despite an increase in spleen weight with anti-CSF-1R alone (Supp. Fig. 4A). Thus, blocking CSF-1R abrogates many of the hepatotoxic histopathological effects observed with αCD40/Gem treatment.
To determine the cell populations targeted by αCSF-1R mAbs – and mediating the hepatotoxicity of αCD40/Gem – flow cytometry was performed on livers 24 hours after αCD40 treatment. A significant reduction in the frequencies of activated macrophages was observed in the livers of mice treated with CD40 and CSF-1/1R mAb (Fig. 5D and 5E). The proportions of both CD40+ F4/80+ macrophages and CD86+ MHCII+ F4/80+ macrophages were increased with αCD40, and reduced to frequencies similar to IgG2a treated mice with the addition of αCSF-1/1R mAbs (Fig. 5D and 5E, respectively). Despite previous studies reporting a major role of MDSCs in mediating CD40 heptatotoxicity in the absence of chemotherapy (30), the proportion of MDSCs in the livers of αCD40 treated mice receiving αCSF-1/1R were not altered (Fig. 5F), in agreement with increased CSF-1R expression on macrophages, but not MDSC subsets, in the livers of αCD40/Gem treated mice (Supp. Fig. 4B). Nevertheless, the frequency of F4/80+ CD11b+ Gr1+ cells was significantly reduced after treatment with αCD40/Gem +/− anti-CSF-1R (Supp. Fig. 4C). The frequency and total number of both Mo- and G-MDSC subsets per gram of liver in mice treated with αCD40/Gem were not significantly altered with the addition of anti-CSF-1R (Supp. Fig. 4D and 4E). Furthermore, the proportion of CD40+ CD45-neg cells, including hepatocytes, was also not significantly altered after αCD40/Gem and anti-CSF-1R treatment (Fig. 5G). Instead, these data reveal that activated macrophages mediate liver damage after αCD40/Gem administration, and inhibiting macrophage activation via CSF-1R blockade abrogated liver toxicity and significantly inhibited tumor growth and enhanced overall survival.
Discussion
The success of immunotherapies in tumors replete with T cell infiltration is in stark contrast to the failure of the same treatments in T cell-poor tumors such as PDA. αCD40 is capable of converting immunotherapy-resistant tumors susceptible to T cell infiltration and destruction, but the requirement of concomitant therapies, such as chemotherapy, is poorly understood. Here, in a mouse model of PDA, we show that αCD40 therapy can inhibit tumor growth but causes significant and lethal hepatotoxicity when administered 48 hours in advance of (instead of after) Gem, regardless of the tumor-bearing status of the mouse. αCD40 treatment significantly increased the proportion of activated macrophages within the liver, and blockade of macrophage activation using αCSF-1/1R mAbs abrogated the lethality of αCD40/Gem treatment without reducing the anti-tumor efficacy of the combination treatment. The data presented here highlight at once the potency of αCD40 therapy to activate both innate and adaptive immunity but also important pharmacological limitations with regard to sequencing with chemotherapy. These studies have implications for the design of upcoming clinical trials investigating αCD40-chemotherapy combinations in cancer.
CD40 antibody has been administered to patients in a number of clinical trials with minimal adverse events (31–34), but the murine studies herein reveal a previously unrecognized temporal window after αCD40 administration during which chemotherapy should not be administered. We have found that separating αCD40 and subsequent Gem administration by 5 days is safe in both mouse models and in patients with PDA (16). However, administration of Gem or nab-paclitaxel only 2 or 3 days after αCD40 resulted in significant and lethal hepatotoxicity, and was likely the cause of treatment related deaths in mice after repeated doses of Gem and αCD40 in overlapping treatment schedules (17). This finding is despite the fact that Gem therapy rarely results liver damage in patients with PDA (35), non-small cell lung cancer (36), or other tumor types (37), and that αCD40 therapy as a single agent has been reported to drive only mild, transient, and non-lethal hepatotoxicity in mice (30, 38) and humans (34). However, αCD40 combined with IL-2 has also been reported to result in lethal hepatotoxicity in tumor-bearing mice (39, 40), indicating that the potency of αCD40 needs to be considered in all combinatorial treatments, not only those with chemotherapy. These studies highlight the need for understanding the context and timing of combination therapies for the safe use of αCD40. Our data support the need for careful sequential administration of αCD40 after, not before, chemotherapy, in the absence of macrophage modulation. This finding is important because the stroma depletion caused by αCD40 alone (16) might have otherwise been considered a useful first maneuver to improve the delivery of increased concentrations of chemotherapy to highly desmoplastic tumors, thereby overcoming chemotherapy resistance in PDA.
Mechanistic studies to determine the cells mediating hepatotoxicity after αCD40/Gem revealed a role for activated macrophages, similar to what has been shown in hepatotoxicity observed after αCD40/IL-2 in aged or obese mice (39, 40). While MDSCs were increased in the liver after αCD40, the frequency of MDSCs was unaltered after αCSF-1/1R therapy, even though hepatotoxocity was resolved. We also found no significant alterations after αCSF-1/1R therapy within the CD45-neg cellular compartment, which includes hepatocytes and vascular endothelial cells, despite previous reports that CD40-ligand stimulation of hepatocytes promotes the upregulation of CD40 by the hepatocytes and exacerbation of fulminant hepatitis in a FasL dependent manner (28, 29). The high frequency of occlusive hepatic infarcts in αCD40/Gem treated mice is consistent with the significant role for CD40 in two mouse models of thrombosis (41) in which macrophages are strongly activated, similar to αCD40 treatment. In αCD40/Gem treated mice, liver damage is CSF-1R dependent, and can be alleviated via blockade of macrophage activation using αCSF-1/1R mAbs.
The fact that blunted macrophage activation did not alter the anti-tumor efficacy in αCD40/Gem treated mice is in contrast to our previous work identifying a major role for αCD40-induced tumoricidal macrophages in mediating tumor regressions of spontaneous PDA tumors in KPC mice (16). Although macrophages mediated αCD40-induced anti-tumor immunity as a single agent, in the absence of Gem, T cell immunity did not develop with this treatment (16). However, T cell immunity against PDA is generated when αCD40 is administered subsequent to Gem (22), and is further potentiated by the addition of nab-paclitaxel, as well as αPD-1/αCTLA-4 (21). Thus, the approach in which αCD40 therapy is used can significantly alter the dominant mechanism of anti-tumor immune activation, switching between innate and adaptive immune-mediated tumor regressions depending on the presence or absence of chemotherapy. Although reversing the treatment schedule by providing αCD40 before Gem successfully increased the activity of innate immune cells during treatment, the activated macrophages mediated liver damage. Blocking macrophage activation via αCSF-1/1R mAbs prevented lethal hepatotoxicity without further enhancement (nor reduction) of anti-tumor immunity. Indeed, in the absence of αCD40 treatment, αCSF-1/1R mAbs have been shown to contribute to the reprogramming of macrophages in the PDA tumor microenvironment rendering the tumor sensitive to immune checkpoint blockade therapies (26). Thus, αCD40/Gem with αCSF-1/1R and αPD-1/αCTLA-4 may be a highly effective therapy that can be investigated in future studies.
The important opportunity for using immunotherapies for patients with cancer is clear, and identifying treatments that can generate effective T cell responses in cancers currently resistant to newly approved immune interventions is of high priority. Our studies here build upon the identification of αCD40 as a potent activator of anti-tumor immunity in both preclinical and clinical studies. However, our finding that αCD40 can be hepatotoxic and lethal when administered in advance of chemotherapy reveals the need to continue investigating αCD40 combinations in preclinical studies. These data provide key information to design safe αCD40 clinical trials, maintaining the ability to harness the potential of αCD40 in cancer therapies while minimizing potential toxicity.
Supplementary Material
Acknowledgments
We thank Drs. Elizabeth Buza and Amy Durham from the Abramson Cancer Center Comparative Pathology Core at the University of Pennsylvania School of Veterinary Medicine for their assistance with our samples.
Grant support
Supported by the American Cancer Society 125403-PF-14-135-01-LIB (to KTB) and NIH R01-CA-169123 and Pancreatic Cancer Action Network-American Association for Cancer Research (to RHV).
References
- 1.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, investigators K- Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sausen M, Phallen J, Adleff V, Jones S, Leary RJ, Barrett MT, Anagnostou V, Parpart-Li S, Murphy D, Kay Li Q, Hruban CA, Scharpf R, White JR, O'Dwyer PJ, Allen PJ, Eshleman JR, Thompson CB, Klimstra DS, Linehan DC, Maitra A, Hruban RH, Diaz LA, Jr, Von Hoff DD, Johansen JS, Drebin JA, Velculescu VE. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat. Commun. 2015;6:7686. doi: 10.1038/ncomms8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van 't Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome I, Consortium IBC, Consortium IM-S, PedBrain I, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 10.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Jr, Donehower RC, Jaffee EM, Laheru DA. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 13.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 14.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 15.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky HI. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat. Med. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 16.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 18.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Westcott PM, Halliwill KD, To MD, Rashid M, Rust AG, Keane TM, Delrosario R, Jen KY, Gurley KE, Kemp CJ, Fredlund E, Quigley DA, Adams DJ, Balmain A. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature. 2015;517:489–492. doi: 10.1038/nature13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 21.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol. Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, Vonderheide RH. Exclusion of T cells From pancreatic carcinomas in mice is regulated by Ly6C(low) F4/80(+) extratumoral macrophages. Gastroenterology. 2015;149:201–210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo A, Wang LC, Scholler J, Monslow J, Avery D, Newick K, O'Brien S, Evans RA, Bajor DJ, Clendenin C, Durham AC, Buza EL, Vonderheide RH, June CH, Albelda SM, Pure E. Tumor-Promoting desmoplasia Is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, Tuveson DA. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260–269. doi: 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA, Aruffo A. Expression of functional CD40 by vascular endothelial cells. J. Exp. Med. 1995;182:33–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas-mediated hepatocyte death during allograft rejection. J. Exp. Med. 1999;189:441–446. doi: 10.1084/jem.189.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F, Ajuebor MN, Beck PL, Le T, Hogaboam CM, Swain MG. CD154-CD40 interactions drive hepatocyte apoptosis in murine fulminant hepatitis. Hepatology. 2005;42:372–380. doi: 10.1002/hep.20802. [DOI] [PubMed] [Google Scholar]
- 30.Medina-Echeverz J, Ma C, Duffy AG, Eggert T, Hawk N, Kleiner DE, Korangy F, Greten TF. Systemic agonistic anti-CD40 treatment of tumor-bearing mice modulates hepatic myeloid-suppressive cells and causes immune-mediated liver lamage. Cancer Immunol. Res. 2015;3:557–566. doi: 10.1158/2326-6066.CIR-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 33.Bajor DL, Xu X, Torigian DA, Mick R, Garcia LR, Richman LP, Desmarais C, Nathanson KL, Schuchter LM, Kalos M, Vonderheide RH. Immune activation and a 9-year ongoing complete remission following CD40 antibody therapy and metastasectomy in a patient with metastatic melanoma. Cancer Immunol. Res. 2014;2:1051–1058. doi: 10.1158/2326-6066.CIR-14-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O'Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, Blatter J. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br. J. Cancer. 1996:101–105. doi: 10.1038/bjc.1996.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson H, Lund B, Bach F, Thatcher N, Walling J, Hansen HH. Single-agent activity of weekly gemcitabine in advanced non-small-cell lung cancer: a phase II study. J. Clin. Oncol. 1994;12:1821–1826. doi: 10.1200/JCO.1994.12.9.1821. [DOI] [PubMed] [Google Scholar]
- 37.Green MR. Gemcitabine safety overview. Semin. Oncol. 1996;23:32–35. [PubMed] [Google Scholar]
- 38.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin. Cancer Res. 2011;17:2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 39.Bouchlaka MN, Sckisel GD, Chen M, Mirsoian A, Zamora AE, Maverakis E, Wilkins DE, Alderson KL, Hsiao HH, Weiss JM, Monjazeb AM, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Redelman D, Taub DD, Murphy WJ. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J. Exp. Med. 2013;210:2223–2237. doi: 10.1084/jem.20131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirsoian A, Bouchlaka MN, Sckisel GD, Chen M, Pai CC, Maverakis E, Spencer RG, Fishbein KW, Siddiqui S, Monjazeb AM, Martin B, Maudsley S, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Taub DD, Murphy WJ. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J. Exp. Med. 2014;211:2373–2383. doi: 10.1084/jem.20140116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavins FN, Li G, Russell J, Perretti M, Granger DN. Microvascular thrombosis and CD40/CD40L signaling. J. Thromb. Haemost. 2011;9:574–581. doi: 10.1111/j.1538-7836.2010.04176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.