Abstract
The field of psychiatry is approaching a major inflection point. The basic science behind cognition, emotion, behavior and social processes has been advancing rapidly in the past 20 years. However, clinical research supporting the classification system in psychiatry has not kept up with these scientific advances. In order to begin organizing the basic science of psychiatry in a comprehensive manner, we begin by selecting fragile X syndrome (FraX), a neurogenetic disease with cognitive-behavioral manifestations, to illustrate key concepts in an integrative, multi-dimensional model. Specifically, we will describe key genetic and molecular mechanisms (e.g. GABAergic dysfunction and mGluR5-associated long-term depression) relevant to the pathophysiology of FraX, as well as neural correlates of cognitive-behavioral symptoms. We will then describe what we have learned from FraX, which may be applicable to other psychiatric disorders. We conclude the article by discussing on-going and future opportunities in both diagnosing and treating psychiatric diseases in the future.
Keywords: Fragile X syndrome, RDoC, DSM5, multi-dimensional research, GABA, behavior
INTRODUCTION
A 23-year-old woman is referred to you for long-standing symptoms of anxiety and social avoidance, and recent onset of depression. In the process of conducting your history and mental status exam you discover that she lives at home, and has a history of learning and attentional problems with below-average grades throughout school. She also has a brother with significant intellectual disability and autism spectrum disorder (ASD) and a (maternal) grandfather with a progressive tremor-ataxia syndrome. Your recommendations to this patient include testing for fragile X syndrome by Southern blot analysis. This test shows that she has a methylated FMR1 mutation with 275 CGG repeats on one of her two X chromosomes.
What can we presently offer a patient with such a history? Diagnostically, she might meet current or past criteria for social or generalized anxiety disorder, attention deficit hyperactivity disorder (ADHD), specific learning disorder or intellectual disability, and a depressive disorder. Indeed, based on the Diagnostic and Statistical Manual (DSM), she might receive two or more “co-morbid” diagnoses. In the best of circumstances, we might offer this patient individual and/or group therapy, perhaps an anti-depressant, and work with the patient and her family to determine how to optimize her social supports and vocational potential given her cognitive and psychiatric disabilities. This approach is symptom-focused, and (hopefully) based on the clinician’s knowledge of evidence-based clinical trial results.
Alternatively, the clinician conducting the initial evaluation might be aware that the patient’s personal and family history as well as current symptoms are consistent with a diagnosis of fragile X syndrome (FraX), a relatively common genetic condition associated with mutations of the FMR1 gene on the X chromosome. Why is this important? As we describe below, the extraordinary accumulation of knowledge about FraX in the past 25 years has led directly to disease-specific trials of biologic agents designed to interrupt pathophysiological processes occurring downstream to the genetic mutation. Modulating these processes in a disease-focused manner thus brings about the promise of greater specificity and efficacy of biologic interventions, thereby increasing the likelihood that concomitant environmental approaches (e.g., vocational, cognitive, family-based) will be more effective in persons with FraX.
As highlighted in this paper, knowledge of specific risk factors, disease pathophysiology, aberrant brain circuitry and core symptoms provides an unprecedented opportunity to diagnose and treat individuals with FraX more effectively. With an ultimate goal of generalizing this model to other specific brain disorders that have psychiatric symptoms, we will critically analyze this multi-dimensional model of FraX, formulate the key lessons learned, and describe potential opportunities for advancing the field of psychiatry.
FRAGILE X SYNDROME – A COMPLEX NEUROPSYCHIATRIC DISEASE
Fragile X syndrome is the most common genetic cause of ASD (1) and inherited cause of intellectual disability (2). The general prevalence of males with a full FMR1 mutation is estimated as 1 in 4000, while the female prevalence is approximately 1 in 5000–8000 (3). Families of children with FraX experience substantial financial burden (4). The society at large is clearly impacted by this neuropsychiatric disease.
Genetics
The mutation responsible for FraX consists of large expansions of trinucleotide CGG repeats within the 5′ untranslated region of the FMR1 gene on the long arm of the X chromosome. Typically developing individuals have about 30 CGG repeats while those with the FMR1 pre-mutation have repeat lengths ranging between 55 and 200 copies. Individuals with the FMR1 full mutation (and hence the diagnosis of FraX) typically have more than 200 CGG repeats. This expansion leads to DNA hypermethylation within FMR1 (5), resulting in its transcriptional silencing, and therefore the absence or attenuation of the gene product, FMR1 protein (FMRP) (6). In addition to expansion of CGG repeats, point mutations of FMR1 have been identified as risk factors for development of FraX (7).
Physical and Cognitive-Behavioral Phenotypes
Individuals with FMR1 pre-mutation may have milder physical and cognitive symptoms. Some (particularly men) are at risk for developing fragile X-associated tremor/ataxia syndrome (FXTAS), which is characterized by problems with movement and potentially, cognition, as in the case of the grandfather of the index patient described in the beginning of this article. Patients with FMR1 full mutations can have mild dysmorphic features (long face with large mandible, large everted ears, high-arched palate (8)) as well as mild to severe cognitive deficits and behavioral abnormalities (1). These individuals particularly exhibit deficits in executive function (9), including attention, inhibition, working memory and impulse control. Children and adults with FraX often exhibit gaze aversion, increased social anxiety and avoidance (10). Furthermore, impairments in visuospatial processing are common. Collectively, these factors may contribute to profound difficulties in maintaining appropriate social interactions with others.
Because FraX is a condition due to mutations within a specific gene on the X chromosome, males with this disease tend to have more severe symptoms than their female counterparts. Among females with FraX, the range in severity of symptoms is large, thought to be mainly due to the genetic variation in the form of X-inactivation, a process by which one of the two copies of the X chromosome present in females is inactivated. While females with FMR1 full mutation demonstrate intellectual abilities ranging from average function to moderate disability, males with full mutation often suffer from severe to profound intellectual disability. Similarly, females with FraX tend to have less specific cognitive deficits than males with FraX (11). Most boys and about one-third of girls with FraX satisfy the DSM criteria for ADHD, with hyperactivity subtype more common in boys and inattentiveness more common in girls (12).
How can a single gene mutation (i.e. FMR1) lead to such complex deficits in cognitive-behavioral function in individuals with FraX? To answer this question, we shall attempt to fill in the gaps between genetics and behavior by describing what we know about the physiological (neural circuit) and molecular/cellular processes in FraX.
Neural Correlates of Cognitive-Behavioral Phenotypes
Our first step in attempting to explain the relationships between FMR1 mutations in FraX and the syndrome’s complex behavioral manifestations is to determine the structural, functional, and connectional abnormalities of the brain. We will organize the findings into the following three sections: (A) cognitive processes; (B) social processes; and (C) emotion regulation.
(A) Cognitive processes
As mentioned, individuals with FraX commonly have prominent deficits in executive function. The frontostriatal pathway is known to be central to this important function. A large body of literature indicates that the caudate (part of the striatum) is dramatically enlarged in individuals with FraX. This finding holds true in toddlers (13–15), children, adolescents (16, 17), and adults (18), suggesting that this abnormality starts as early as the first 1–2 years of life in individuals with FraX. Interestingly, this finding was identified not only when toddlers with FraX were compared with neurotypical controls but also when they were compared with their age and sex matched counterparts with idiopathic ASD (14, 15). Such findings indicate that caudate enlargement is a key brain phenotype in FraX.
Anatomical studies have also examined the frontal component of the frontostriatal system in FraX. In a longitudinal study reported by Bray et al, the growth trajectory of prefrontal cortical regions (superior, middle, orbito-frontal gyri) from late childhood to young adulthood (age range of all subjects: 9–22 years) showed larger overall volumes and more volume increase in FraX relative to typically developing individuals, particularly in male subjects (19). However, in a cross-sectional study in older adult males (30±9 years for FraX subjects, and 35±14 years for control subjects) reported by Hallahan et al, the left frontal lobe (manually traced) was smaller in individuals with FraX relative to healthy controls (however, only after controlling for IQ) (18). The reason for inconsistent findings between these two studies is not clear. However, the two studies had significant differences in age range, methodology (longitudinal vs. cross-sectional), sample size (n=68 vs. 17) and image analysis approaches, which might explain the discrepancies in their findings. Despite these differences, it is also possible that prefrontal cortical development in FraX has a biphasic trajectory such that an initial phase of higher volume increase is followed by a faster decrease in volume during adulthood as compared to neurotypical controls. This possibility can only be addressed with additional longitudinal studies. Abnormalities in the frontostriatal pathway were also demonstrated by diffusion tensor imaging (DTI) studies of white matter tracts comprising this system, which revealed lower fractional anisotropy (FA) in female adolescents with FraX (20) and increased density of fibers in the left ventral frontostriatal pathway in male toddlers with FraX (21). In addition to structural MRI and DTI studies, functional MRI studies further support the notion that the frontostriatal pathway is abnormal in FraX (22–24) (please see Table 1 for more details).
Table 1.
Relationships between cognitive-behavioral profiles and their potential neural correlates in Fragile X Syndrome. The biobehavioral dimensions are adopted from the Research Domain Criteria (RDoC).
| SUB-POPULATION | CONTROL GROUP(S) | COGNITIVE-BEHAVIORAL PHENOTYPES | PUTATIVE NEURAL CORRELATES (findings in the fragile X group, as compared to controls) | |
|---|---|---|---|---|
| COGNITIVE SYSTEMS | ||||
| Attention | M infants with full mutation | HC | Look duration and increased latency to disengage attention were correlated with severity of autistic behavior but not mental age (80) | |
| Young boys with full mutation | DD and HC | Attention deficit at higher levels of attention function/executive functioning (81) | ||
| M and F adolescents with full mutation | DD and HC | Insula cortex was smaller (82). | ||
| M and F adolescents and young adults with full mutation | DD | Converging structural and functional abnormalities in the left insular cortex (83). | ||
| F adolescents with full mutation | HC | Salience network is abnormal – ACC had reduced activation during a Go/NoGo task (23) | ||
| F adolescents with full mutation | HC | Dorsal attention network (consisting of SPL and DLPFC) may be abnormal – reduced activation in the SPL during visuospatial working memory tasks (27). | ||
| Perception | Young boys with full mutation | HC | Impairments in visuospatial processing (84) | |
| M adolescents and young adults with full mutation | DD and HC | Severe impairments in first- and second-order motion perception (26) | ||
| M adolescents and young adults with full mutation | DD | Abnormalities in the magnocellular/dorsal pathway from post-mortem samples (25). | ||
| Young M adults with full mutation | HC | Auditory information processing is critically impaired relative to visual information processing (85). | EEG recordings revealed exaggerated N1 and N2b amplitudes (85). | |
| F adults with premutation | IQ-matched HC | Lower sensitivities for biological and mechanical motion (86) | ||
| Working memory | Young boys with full mutation | IQ-matched HC | Deficits visuospatial and auditory working memory (84). | |
| M adults with full mutation | HC | Increased size of parietal lobe bilaterally (18). | ||
| M and F children and adolescents with full mutation | HC | Increased size of IPL (16). | ||
| M and F adolescents with full mutation | None | Deficits in auditory working memory (87) | ||
| F adolescents with full mutation | HC | Deficits in visuospatial working memory (27). | Reduced activation in the SPL and IPL during visuospatial working memory tasks; unable to modulate activation in the prefrontal and parietal cortex in response to an increasing working memory load. Correlation between FMRP levels and activation in the right inferior and bilateral middle frontal gyri and the bilateral supramarginal gyri (27). | |
| F adolescents and young adults with full mutation | HC | Decreased white matter connectivity in PCG (20). | ||
| Declarative memory | M and F elderly adults with premutation | HC | Poor declarative verbal memory (88) | Higher levels of FMR1 mRNA were associated with smaller N400s (in EEG) to incongruous words and larger positive amplitudes to congruous words (88). |
| Language | Boys and M adolescents with full mutation | HC | Impaired phonological memory (84) | |
| M and F children and adults with full mutation | DD and HC | Decreased neurocognitive performance, including verbal performance IQ (29) | Reduced size of posterior cerebellar vermis (29) | |
| M and F adolescents with full mutation | None | Impaired phonological and verbal working memory (87) | ||
| Girls and young women with full mutation | HC | Reduced volume of STG (89). | ||
| Cognitive control | Toddler boys with full mutation | DD and HC | Increased size of caudate (13, 14) | |
| Boys with full mutation | DD and HC | DTI studies showed aberrant white matter structure was localized in the left ventral frontostriatal pathway (21) | ||
| M adolescents with full mutation | DD and HC | Aberrant response inhibition (22) | Reduced activation in the right VLPFC and right caudate head, and increased contralateral (left) VLPFC activation (22). | |
| M adults with full mutation | HC | Reduced volume of frontal lobe and increased volume of caudate nucleus (18). | ||
| M and F children and adolescents with full mutation | HC | Increased volume of caudate nucleus (16) | ||
| F adolescents with full mutation | HC | Aberrant response inhibition (23). | Reduced deactivation in the ventromedial PFC (23), and reduced activation in the supplementary motor area, anterior cingulate and midcingulate cortex, basal ganglia, and hippocampus (23). | |
| F adolescents with full mutation | HC | Longer reaction times during the Stroop interference task, and adopted a strategy trading speed for accuracy (24). | More extensive activation in the anterior region of the PFC (24), and reduced activation in the left orbitofrontal gyrus (24). | |
| F adolescents with full mutation | HC | Lower FA values in white matter in frontostriatal pathways, as well as in parietal sensory-motor tracts (20). | ||
| SYSTEMS FOR SOCIAL PROCESSES¶ | ||||
| Social communication | ||||
| Reception of facial communication | Toddler boys with full mutation | DD and HC | Increased size of FG (13). | |
| M adolescents with full mutation | DD and HC | Decreased accuracy in gaze trials (32). | Less activation in prefrontal cortices and elevated left insula activation to direct eye gaze stimuli, as well as greater sensitization in the left amygdala with successive exposure to direct gaze (32). | |
| M and F adolescents with full mutation | DD | No significant differences in accuracy and reaction time, compared to DD group (37) | Abnormal habituation in the anterior cingulate, fusiform gyrus, as well as frontal cortex of young adults and adolescents with FraX in response to face/eye gaze (37). | |
| M and F adolescents with full mutation | ASD and HC | Decreased duration of eye fixation (38) | Decreased activation of FG (38) | |
| M and F adolescents with full mutation | HC | Decreased activation of medial and superior frontal cortex, during successful face encoding (90). | ||
| M and F adolescents with full mutation | DD and HC | Increased size of insula (82). | ||
| F adolescents with full mutation | HC | Deficit in recognizing neutral and sad, but not happy, faces (91) | Reduced activation in the ACC for neutral faces compared with scrambled faces; reduced activation in the caudate for sad faces compared with scrambled faces (91). FMRP levels positively correlated with activation in the dorsal ACC for neutral, happy, and sad faces when independently compared with scrambled faces (91). | |
| Production of facial communication | M adults with premutation | HC | Lack of startle potentiation while viewing fearful faces and reduction of skin conductance response when greeting an unfamiliar experimenter (92) | Diminished activation in amygdala and several brain areas that mediate social cognition while viewing fearful faces (92). |
| Production of non-facial communication | Children with full mutation | FraX with ASD | Difficulties with imitation (93) | |
| Perception and understanding of self | M and F adolescents with full mutation | DD and HC | Total, anterior and posterior insular volumes were found to be reduced (82). | |
| Perception and understanding of others | M children with full mutation | DD | Difficulties with theory of mind (94) | |
| F adolescents with full mutation | HC | Deficit in recognizing neutral and sad, but not happy, faces (91) | Reduced activation in the ACC for neutral faces compared with scrambled faces; reduced activation in the caudate for sad faces compared with scrambled faces (91). | |
| NEGATIVE VALENCE SYSTEMS* | ||||
| Acute threat (“Fear”) | Girls with full mutation; boys and girls with premutation | HC | Elevated baseline anxiety (33). | Attenuated amygdala activation in “Fearful-Scrambled” and “Fearful-Happy” contrasts; normal size of amygdala (33). Significant relationships between FMR1 gene expression, anxiety/social dysfunction scores, and reduced amygdala activation (33). |
| Potential threat (“Anxiety”) | Young boys with full mutation | ASD and HC | Abnormally small amygdala volumes (15). | |
| M and F children with full mutation | Unaffected siblings | Dysregulated HPA axis (41) | ||
| M and F children and adolescents with full mutation | HC | Abnormally small amygdala volumes (16) | ||
| M and F adolescents with full mutation | HC | Decreased activation of medial and superior frontal cortices during successful face encoding (90) | ||
| M and F adolescents with full mutation | DD and HC | Increased size of insula (82) | ||
| Sustained threat | M and F children with full mutation | Study 1: HC; Study 2: Williams syndrome | More preservative errors (95). | |
| POSITIVE VALENCE SYSTEMS# | ||||
| AROUSAL/MODULATORY SYSTEMS§ | ||||
| Arousal | M infants with full mutation | HC | Lower HR variability, shallower HR decelerations, and prolonged look durations (96) | |
| Adolescents with full mutation | DD and HC | Increased size of insula (82) | ||
| Young M adults with full mutation | HC | Reduced alpha and exaggerated theta power during the resting-state EEG (97) | ||
Abbreviations: ACC, anterior cingulate cortex; ASD, autism spectrum disorder; DD, developmental delayed controls; DTI, diffusion tensor imaging; F, female; FA, fractional anisotropy; FG, fusiform gyrus; FMRP, FraX mental retardation protein; fMRI, functional magnetic resonance imaging; HC, healthy controls; HPA, hypothalamus-pituitary-adrenal axis; HR, heart rate; IPL, inferior parietal lobe; M, male; MD, mean diffusivity; PCG, postcentral gyrus; PFC, prefrontal cortex; SPL, superior parietal lobe; STG, superior temporal gyrus; VBM, voxel-based morphometry; VLPFC, ventrolateral prefrontal cortex.
N/A: not available.
No known abnormalities in RDoC-defined “Affiliation and attachment”.
No known abnormalities in RDoC-defined “Loss” and “Frustrative, non-reward”.
No known abnormalities in RDoC-defined “Approach motivation”, “Initial responsiveness to reward”, “Sustained responsiveness to reward”, “Reward learning”, and “Habit”.
No known abnormalities in RDoC-defined “Biological rhythms” and “Sleep-wake”.
Cognitive functions may also be disturbed when sensory functions are abnormal. The magnocellular/dorsal pathway (the “where” stream) is mainly responsible for processing an object’s spatial location relative to the viewer. Aberrations in this pathway were found in males (16, 18, 25, 26) and females with FraX (18, 20, 27) with FraX (Table 1).
(B) Social processes
Communication is the centerpiece of social processes. Individuals with FraX often have stereotypic speech and aberrant language development (28). As well, the brain regions associated with language abilities also appear abnormal in this condition. The most notable examples include smaller superior temporal gyrus (16) and posterior cerebellar vermis (29) in persons with FraX.
In addition to overt language, non-verbal communication is another crucial component of social processes. Face and emotion recognition is associated with the “fusiform face area” [FFA; (30, 31)], the amygdala, and the superior temporal sulcus. In neurotypical humans, the perception of emotionally expressive faces results in greater activation in the FFA (32, 33) and bilateral amygdala (34–36). In humans with FraX, increased activity and abnormal habituation of the amygdala (32, 33) were observed when individuals viewed faces. It is possible that these results were a reflection of compromised capacity in processing facial information, which is potentially consistent with the small size of the amygdala in individuals with FraX (15, 16). In addition to the amygdala, abnormal habituation was also found in the anterior cingulate, fusiform gyrus, as well as frontal cortex of young adults and adolescents with FraX when responding to face/eye gaze (37). Finally, individuals with FraX have decreased duration of eye fixation, which is putatively associated with decreased activation of FFA (38).
(C) Emotion regulation
While the amygdala is important for social processes, this structure is also well known for its function in emotion regulation. Amygdala-prefrontal circuitry has been associated with a wide range of behavioral functions, such as fear conditioning, extinction, as well as anxiety-related conditions such as social anxiety (39). Toddlers with FraX were shown to have smaller amygdala as well as smaller dorsolateral PFC than those with ASD or control participants (15). The physiology of emotion dysregulation in FraX goes beyond the cerebrum and extends to the autonomic nervous system. Compared to their unaffected siblings, individuals with FraX have significantly higher heart rates, lower vagal tone, and lower heart rate variability (40), suggesting that both sympathetic and parasympathetic nervous systems are dysregulated in FraX. Further, when challenged by a social task, children with FraX show excess cortisol reactivity, suggesting dysregulation of the hypothalamus-pituitary-adrenal (HPA) axis (41).
Uncovering the neural correlates of behavioral abnormalities brings us a step closer to understanding the pathophysiology of the complex symptom profile in individuals with FraX. While MRI and EEG studies have uncovered associations between neuroanatomical structure and behavior, these studies do not provide opportunities to determine how neural circuits are controlled at the molecular and cellular scales. To provide avenues for targeted biological treatments in FraX, molecular and cellular processes modulating neural circuits need to be elucidated. Accordingly, in the next section we describe some of the salient features of molecular and cellular physiology in FraX.
Molecular and cellular biology
Individuals with FraX have absent or reduced levels of FMRP, a protein which has a prominent role in regulating the translation of a subset of mRNAs associated with synaptic plasticity, dendritic pruning, and axonal development (42). We will summarize some of the key molecular mechanisms affected by FMRP.
mGluR Theory of FraX
Neural circuits involving glutamatergic pathways are known to serve important functions in learning, memory and behavior. In particular, as illustrated in Figure 1, long-term depression (LTD) regulated by the group 5 metabotropic glutamate receptor (mGluR5) is a well-established form of synaptic plasticity (43). Activation of mGluR5 leads to cascades of signaling events driving the activation of protein synthesis involved in the internalization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). In the absence or substantial attenuation of FMRP, some of the proteins important for AMPAR trafficking become too abundant, thus increasing the internalization of AMPARs and resulting in exaggerated mGluR5-dependent LTD (44). AMPAR density at the synapse was found to correlate inversely with dendritic spine density (45). In the case of FraX, the internalization of AMPARs is increased and therefore the density of dendritic spines is expected to increase. Indeed, longer and thinner dendritic protrusions with increased density were found in the temporal and visual cortices of patients with FraX (46, 47). Similarly, this altered dendritic protrusion phenotype was also shown in the layer V pyramidal neurons of the visual cortices (48, 49) and the CA1 region of the hippocampus (50) in adult Fmr1 knock-out (KO) mice. When these animals were treated with mGluR antagonists, their dendritic morphologic abnormalities were normalized (50). Remarkably, the aberrant behaviors in these animals [abnormal pre-pulse inhibition of startle (50), decreased sociability (51)] were also reversed. However, in the first randomized, double-blind study of a mGluR5 antagonist, AFQ056, in adult patients with FraX, the compound failed to show improvement in the primary behavioral endpoint [Aberrant Behavior Checklist – Irritability Subscale (ABC-I)] (52). Additional trials of two mGluR5 antagonists did not demonstrate efficacy (53).
Figure 1.
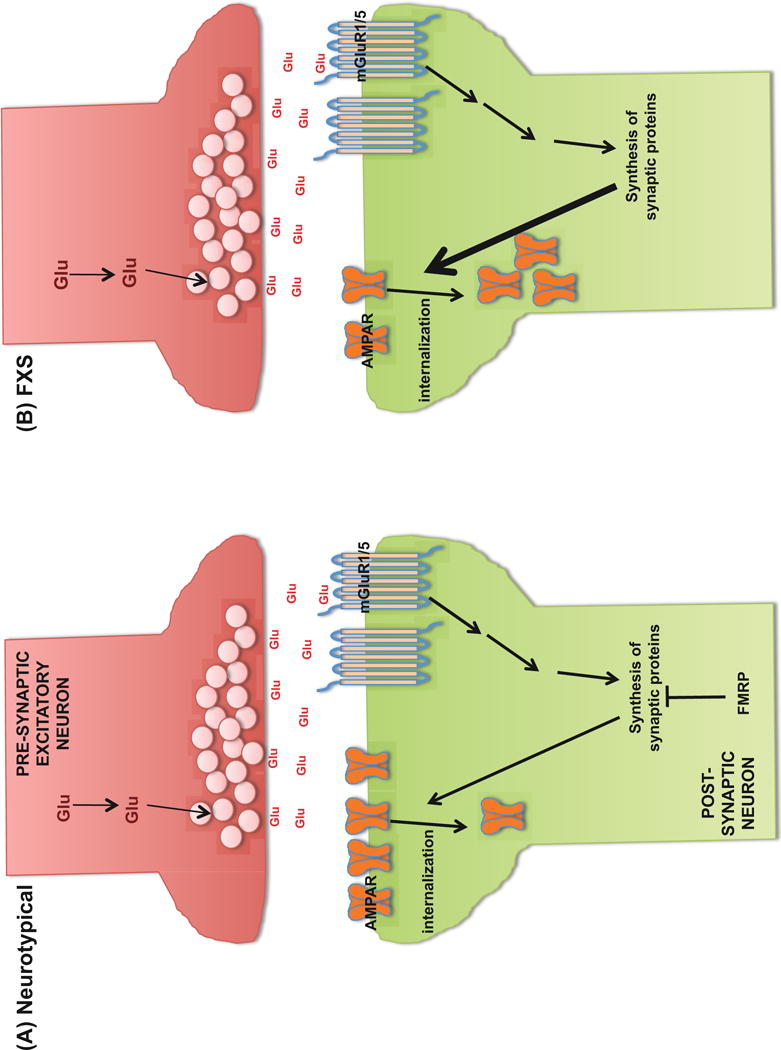
Internalization of AMPA receptors via mGluR5 stimulation in (A) neurotypical individuals and (B) individuals with fragile X syndrome.
GABAergic dysfunction in FraX
The GABAergic system is responsible for numerous vital brain functions. GABA is the most abundant inhibitory neurotransmitter in the brain. In addition to neurotransmission, GABAergic neurons were shown to regulate critical periods of brain development (54), excitatory-inhibitory shift (55), as well as neural synchrony (56). The developmental switch in GABA polarity was found to be delayed in Fmr1 KO mice (57). Many components of the GABAergic system are known to be dysfunctional in FraX (58). The mRNA for the δ subunit of the GABAAR is a known target of FMRP (59). Abnormal levels of various subunits of the GABAAR (60, 61), GABAAR’s scaffolding protein (61), enzymes involved in the metabolism of GABA (60–62), as well as cellular transport of GABA (60–62) were found in Fmr1 KO mice. Furthermore, these mice were found to have decreased inhibitory synapse number in the basolateral amygdala (62) and striatum (63), but increased inhibitory synapse density in the CA1 region of the hippocampus (64). These findings motivated researchers to design treatments targeting the abnormal GABA physiology in animal models of FraX. Chronic oral administration of arbaclofen, a GABAB receptor agonist, in juvenile mice, corrected aberrant increased spine density in the visual cortex of Fmr1 KO mice (65). This compound was also shown to modify behavioral (stereotypic behaviors, anxiety) and neurologic (motor coordination, audiogenic seizures) symptoms in these animals (65). Despite this elegant demonstration of pharmacologic effects in mice, the first randomized, double-blind, placebo-controlled study of arbaclofen failed to show efficacy in reducing ABC-I (primary endpoint of the study) in humans with FraX (66). However, post-hoc analysis suggested that arbaclofen reduced ABC-social avoidance in those with FraX (66).
Other molecular targets of FMRP
FMRP regulates the mRNAs of signal transduction molecules (e.g. Rgs5, CamKIIα), molecules for synthesis of various proteins (e.g. eukaryotic translation elongation factor 1A (eEF1A)), proteins involved in axonal development (e.g. Sema3F), and those associated with neuroplasticity (e.g. Arc (67) and hASH1 (68)). FMRP stalls the translation of mRNAs linked to synaptic function (69). The dysfunction of protein expression from these genes is expected to result in downstream effects in key neurological pathways. In addition, FMRP was shown to have a role in synaptic plasticity and signaling that involves retinoic acid (RA) (70). In normal mice, synaptic RA signaling was found to regulate inhibitory synaptic transmission in response to reduced synaptic excitation (71). Different from RA’s action at excitatory synapses, RA at inhibitory synapses was shown to cause a loss of GABAARs. Interestingly, in the absence of FMRP (as in Fmr1 KO mice), RA fails to regulate inhibitory synaptic strength, resulting in an imbalance between synaptic excitation and inhibition which may contribute to the pathogenesis of FraX (71). Emerging evidence supports that microRNAs may be involved in the translational regulation of major synaptic proteins in FraX (72). In hippocampal neurons of Fmr1 KO mice, microRNA 125b regulates the expression of one of the subunits of N-methyl D-aspartate (NMDA) receptor (72), which controls a type of long-term potentiation (LTP) important for memory and learning. Finally, converging evidence has shown that hyperexcitability of the primary somatosensory neocortex (S1) of Fmr1 KO mice was attributable to the reduction and dysfunction of dendritic h- and BKCa channels, pointing to the potential utility of BKCa channel openers for the treatment of sensory hypersensitivity, a common problem in individuals with FraX (73).
Collectively, reduction in FMRP leads to various downstream molecular effects. These effects are likely to be associated with abnormalities in cell morphologies (e.g. dense and thin dendritic spines) as well as functioning of local and global neural circuits. Some of the major challenges in FraX research are therefore related to organizing the existing knowledge, and establishing more definitive and causal relationships among various domains of biology.
LESSONS LEARNED FROM FRAGILE X SYNDROME
As illustrated above, an increasingly elaborate understanding of the pathophysiology and phenotype of FraX has been developed at multiple levels – genetic, molecular, cellular, neurocircuit/physiology, and behavior. What have we learned from this multi-dimensional research? How can we organize and integrate the complex multi-dimensional data regarding FraX? Can such a model be used to understand other psychiatric diseases? Below, we describe some of the lessons learned from FraX research, which will hopefully shed light on pathways for understanding the complex pathogenesis of other neurosychiatric diseases.
Lesson 1: One molecular abnormality can lead to various cognitive-behavioral symptoms
Many known neurogenetic diseases are associated with a single gene mutation. Yet, their behavioral manifestations are often complex, as in the case of FraX. Based on the high prevalence rates of several DSM diagnoses in FraX, most individuals with this well-defined neurogenetic disease fulfill the criteria for multiple psychiatric diagnoses. In the case of the young woman who has a single primary condition (described in the beginning of this article), she will likely meet criteria for at least 4 co-morbid diagnoses (ADHD, social anxiety disorder, mood disorder, specific learning disorder) within the DSM framework. This case vignette illustrates that a single genetic abnormality can lead to multiple DSM diagnoses. In a large-scale genome-wide analysis of single nucleotide polymorphisms (SNP) for 5 DSM disorders (ASD, ADHD, bipolar disorder, major depressive disorder, and schizophrenia), SNPs at four loci surpassed the cutoff for genome-wide significance (74). These results are consistent with the “one-to-many” relationships (i.e. common risk loci with shared effects on multiple DSM-defined psychiatric disorders) that we deduce from our experience with FraX.
Lesson 2: Multi-dimensional organization of biological information is a natural framework for FraX and other neuropsychiatric diseases
In this article, we have illustrated a multidimensional understanding of the pathophysiology of FraX. How can we capitalize on our knowledge of the neuroscience of FraX in treating individuals with this disease? In 2009, the National Institute of Mental Health (NIMH) launched the RDoC project to “develop, for research purposes, new ways of classifying mental disorders based on dimensions of observable behavior and neurobiological measures” (75). In Table 1, we organized selected behaviors observed in FraX and their putative neural correlates (i.e. circuitry/physiology) based on the RDoC domains. It is instructive to see that FraX, a monogenic disease, is represented in 4 out of 5 domains. The widespread effects of FMR1 deletion may be explained by the gene product (FMRP’s) function. As FMRP regulates the mRNA’s of many proteins including synaptic proteins, a monogenic disease actually acts like a polygenic disease. We anticipate that other biological risk factors associated with neurodevelopmental and neuropsychiatric disorders will behave in a similar manner. Therefore, as much as we anticipate that RDoC will provide a framework to capture the pathogenic and pathophysiologic bases in various neurobiological domains, we also anticipate that the framework will be utilized interactively. Components of each RDoC domain will not operate in isolation, but will likely interact with components of other domains. For example, as illustrated in Figure 2, we have depicted data-driven and hypothesis-driven relationships between components of several RDoC domains (cognitive processes, social processes, and negative valence systems) in the context of GABAergic dysfunction (elaborated earlier in the “Molecular and Cellular Biology” section) in FraX and ASD. This is an example of defining an integrative, multi-dimensional system using the RDoC framework. Overall, we predict that RDoC will evolve gradually to a more inter-connected and interactive matrix to represent more inter-related biological phenomena across RDoC domains.
Figure 2.
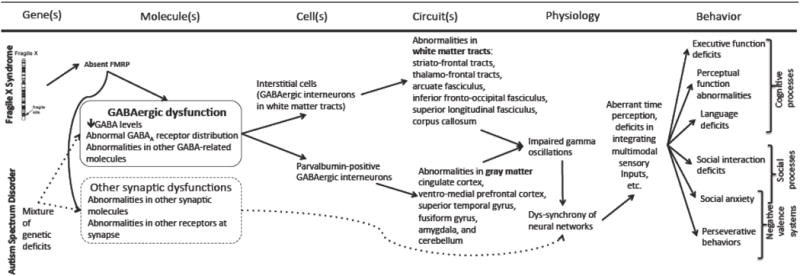
Conceptual framework for GABAergic dysfunction in fragile X syndrome and autism spectrum disorder.
Lesson 3: Neurodevelopmental trajectories are key to understanding neuropsychiatric diseases and developing the next generation of treatments
In addition to the biological domains constructed in RDoC, another key dimension of interest is time. FraX and many current DSM psychiatric disorders are known to be neurodevelopmental in nature. Therefore, capturing neurodevelopmental trajectories of neural circuits in future RDoC-defined neuropsychiatric diseases will provide us with opportunities to understand when and what to correct. Cortical networks in Fmr1 KO mice are hyperexcitable in a brain state-dependent manner during a critical period for experience-dependent plasticity (76). As noted earlier, imaging studies of toddlers with FraX showed that the neuroanatomy of these children was distinctly different from neurotypical controls, ASD, and idiopathic intellectual disability (13, 15). We are continuing to follow these children over time as they enter adolescence. In parallel, other groups have also conducted longitudinal neuroimaging studies in neuropsychiatric populations – attention-deficit/hyperactivity disorder (77), schizophrenia (78), autism (79). We anticipate that one of the next steps to advance our understanding of brain development is to supplement our knowledge of anatomical neurodevelopment by charting the developmental course of molecular targets relevant to specific RDoC domains. If abnormalities in structural and/or molecular brain biomarkers can be detected early in life (e.g. infancy), we may be able to prevent “at risk” brains from further developing abnormal neurocircuits by utilizing early, effective interventions.
Lesson 4: Focusing on specific molecular targets can be an attractive tactic but we need neural-based biomarkers to develop treatments for diseases in psychiatry
Targeting specific molecular mechanisms, such as the glutamatergic and GABAergic systems, appear to be good starting points for developing more specific biological treatments for FraX. However, as discussed, results from the initial human trials of AFQ056 (mGluR5 antagonist) and arbaclofen (GABAB agonist) were not positive – likely due to a variety of factors. One problem in studies conducted to date is the non-specific nature of behavioral endpoints. As we march toward the future of neuroscience-based psychiatric research, we will need to consider using more objective neural based recruitment criteria and endpoints to track treatment effects. Over time, the development of novel compounds will certainly be accomplished in a translational manner. To date, however, many of the results from preclinical models of compounds in FraX and other disorders haven’t translated to positive results in studies of humans with the disorder.
CONCLUSION
As outlined in this article, researchers have already begun developing molecular-based treatments for FraX. We are hopeful that further understanding of these treatments will be fine-tuned thereby improving the quality of lives of individuals with FraX. We have also shared what we have learned from FraX, which may be applicable to other neuropsychiatric diseases. In particular, we predict that the development of RDoC will create a platform for other molecular- and circuit-based strategies to be utilized in the discoveries of novel interventions for other neuropsychiatric diseases. A paradigm shift in psychiatry has begun.
Acknowledgments
Dr. Fung was supported by NIMH grant T32 MH019908 to Dr. Reiss. Some of the research described in this paper was also supported by NIMH grant MH064708 to Dr. Reiss.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Drs. Fung and Reiss report no biomedical financial interests or potential conflicts of interest.
References
- 1.Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: analyzing gene-brain-behavior relationships in child developmental psychopathologies. Dev Psychopathol. 2003;15:927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- 2.Freund LS, Reiss AL. Cognitive profiles associated with the fra(X) syndrome in males and females. American journal of medical genetics. 1991;38:542–547. doi: 10.1002/ajmg.1320380409. [DOI] [PubMed] [Google Scholar]
- 3.Hill MK, Archibald AD, Cohen J, Metcalfe SA. A systematic review of population screening for fragile X syndrome. Genet Med. 2010;12:396–410. doi: 10.1097/GIM.0b013e3181e38fb6. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang L, Grosse S, Raspa M, Bailey D. Employment impact and financial burden for families of children with fragile X syndrome: findings from the National Fragile X Survey. J Intellect Disabil Res. 2010;54:918–928. doi: 10.1111/j.1365-2788.2010.01320.x. [DOI] [PubMed] [Google Scholar]
- 5.Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, et al. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 6.Reiss AL, Freund LS, Baumgardner TL, Abrams MT, Denckla MB. Contribution of the FMR1 gene mutation to human intellectual dysfunction. Nat Genet. 1995;11:331–334. doi: 10.1038/ng1195-331. [DOI] [PubMed] [Google Scholar]
- 7.De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, et al. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993;3:31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- 8.Meryash DL, Cronk CE, Sachs B, Gerald PS. An anthropometric study of males with the fragile-X syndrome. Am J Med Genet. 1984;17:159–174. doi: 10.1002/ajmg.1320170110. [DOI] [PubMed] [Google Scholar]
- 9.Van der Molen MJ, Huizinga M, Huizenga HM, Ridderinkhof KR, Van der Molen MW, Hamel BJ, et al. Profiling Fragile X Syndrome in males: strengths and weaknesses in cognitive abilities. Res Dev Disabil. 2010;31:426–439. doi: 10.1016/j.ridd.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Feinstein C, Singh S. Social phenotypes in neurogenetic syndromes. Child and adolescent psychiatric clinics of North America. 2007;16:631–647. doi: 10.1016/j.chc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Rinehart NJ, Cornish KM, Tonge BJ. Gender differences in neurodevelopmental disorders: autism and fragile x syndrome. Curr Top Behav Neurosci. 2011;8:209–229. doi: 10.1007/7854_2010_96. [DOI] [PubMed] [Google Scholar]
- 12.Mazzocco MM, Baumgardner T, Freund LS, Reiss AL. Social functioning among girls with fragile X or Turner syndrome and their sisters. Journal of autism and developmental disorders. 1998;28:509–517. doi: 10.1023/a:1026000111467. [DOI] [PubMed] [Google Scholar]
- 13.Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9335–9339. doi: 10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazlett HC, Poe MD, Lightbody AA, Gerig G, Macfall JR, Ross AK, et al. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with fragile X syndrome and autism. J Neurodev Disord. 2009;1:81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeft F, Walter E, Lightbody AA, Hazlett HC, Chang C, Piven J, et al. Neuroanatomical differences in toddler boys with fragile x syndrome and idiopathic autism. Archives of general psychiatry. 2011;68:295–305. doi: 10.1001/archgenpsychiatry.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Annals of neurology. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AD, Leow AD, Lu A, Reiss AL, Hall S, Chiang MC, et al. 3D pattern of brain abnormalities in Fragile X syndrome visualized using tensor-based morphometry. Neuroimage. 2007;34:924–938. doi: 10.1016/j.neuroimage.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, et al. In vivo brain anatomy of adult males with Fragile X syndrome: an MRI study. Neuroimage. 2011;54:16–24. doi: 10.1016/j.neuroimage.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Bray S, Hirt M, Jo B, Hall SS, Lightbody AA, Walter E, et al. Aberrant Frontal Lobe Maturation in Adolescents with Fragile X Syndrome is Related to Delayed Cognitive Maturation. Biological psychiatry. 2011 doi: 10.1016/j.biopsych.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, et al. White matter tract alterations in fragile X syndrome: preliminary evidence from diffusion tensor imaging. American journal of medical genetics Part B, Neuropsychiatric genetics. 2003;118B:81–88. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- 21.Haas BW, Barnea-Goraly N, Lightbody AA, Patnaik SS, Hoeft F, Hazlett H, et al. Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Developmental medicine and child neurology. 2009;51:593–599. doi: 10.1111/j.1469-8749.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoeft F, Hernandez A, Parthasarathy S, Watson CL, Hall SS, Reiss AL. Frontostriatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Hum Brain Mapp. 2007;28:543–554. doi: 10.1002/hbm.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon V, Leroux J, White CD, Reiss AL. Frontostriatal deficits in fragile X syndrome: relation to FMR1 gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3615–3620. doi: 10.1073/pnas.0304544101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamm L, Menon V, Johnston CK, Hessl DR, Reiss AL. fMRI study of cognitive interference processing in females with fragile X syndrome. J Cogn Neurosci. 2002;14:160–171. doi: 10.1162/089892902317236812. [DOI] [PubMed] [Google Scholar]
- 25.Kogan CS, Boutet I, Cornish K, Zangenehpour S, Mullen KT, Holden JJ, et al. Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain: a journal of neurology. 2004;127:591–601. doi: 10.1093/brain/awh069. [DOI] [PubMed] [Google Scholar]
- 26.Kogan CS, Bertone A, Cornish K, Boutet I, Der Kaloustian VM, Andermann E, et al. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology. 2004;63:1634–1639. doi: 10.1212/01.wnl.0000142987.44035.3b. [DOI] [PubMed] [Google Scholar]
- 27.Kwon H, Menon V, Eliez S, Warsofsky IS, White CD, Dyer-Friedman J, et al. Functional neuroanatomy of visuospatial working memory in fragile X syndrome: relation to behavioral and molecular measures. The American journal of psychiatry. 2001;158:1040–1051. doi: 10.1176/appi.ajp.158.7.1040. [DOI] [PubMed] [Google Scholar]
- 28.Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, et al. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. J Intellect Disabil Res. 2006;50:532–545. doi: 10.1111/j.1365-2788.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 29.Mostofsky SH, Mazzocco MM, Aakalu G, Warsofsky IS, Denckla MB, Reiss AL. Decreased cerebellar posterior vermis size in fragile X syndrome: correlation with neurocognitive performance. Neurology. 1998;50:121–130. doi: 10.1212/wnl.50.1.121. [DOI] [PubMed] [Google Scholar]
- 30.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 31.Kanwisher N, Stanley D, Harris A. The fusiform face area is selective for faces not animals. Neuroreport. 1999;10:183–187. doi: 10.1097/00001756-199901180-00035. [DOI] [PubMed] [Google Scholar]
- 32.Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Archives of general psychiatry. 2008;65:1315–1323. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SY, Burris J, Bassal F, Koldewyn K, Chattarji S, Tassone F, et al. Fear-Specific Amygdala Function in Children and Adolescents on the Fragile X Spectrum: A Dosage Response of the FMR1 Gene. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 35.Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 36.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 37.Bruno JL, Garrett AS, Quintin EM, Mazaika PK, Reiss AL. Aberrant face and gaze habituation in fragile x syndrome. Am J Psychiatry. 2014;171:1099–1106. doi: 10.1176/appi.ajp.2014.13111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalton KM, Holsen L, Abbeduto L, Davidson RJ. Brain function and gaze fixation during facial-emotion processing in fragile X and autism. Autism research: official journal of the International Society for Autism Research. 2008;1:231–239. doi: 10.1002/aur.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile x syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hessl D, Glaser B, Dyer-Friedman J, Reiss AL. Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of child psychology and psychiatry, and allied disciplines. 2006;47:602–610. doi: 10.1111/j.1469-7610.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 42.Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- 43.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanley JG. AMPA receptor trafficking pathways and links to dendritic spine morphogenesis. Cell Adh Migr. 2008;2:276–282. doi: 10.4161/cam.2.4.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- 47.Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 48.Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKinney BC, Grossman AW, Elisseou NM, Greenough WT. Dendritic spine abnormalities in the occipital cortex of C57BL/6 Fmr1 knockout mice. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2005;136B:98–102. doi: 10.1002/ajmg.b.30183. [DOI] [PubMed] [Google Scholar]
- 50.Levenga J, de Vrij FM, Buijsen RA, Li T, Nieuwenhuizen IM, Pop A, et al. Subregion-specific dendritic spine abnormalities in the hippocampus of Fmr1 KO mice. Neurobiology of learning and memory. 2011;95:467–472. doi: 10.1016/j.nlm.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Gantois I, Pop AS, de Esch CE, Buijsen RA, Pooters T, Gomez-Mancilla B, et al. Chronic administration of AFQ056/Mavoglurant restores social behaviour in Fmr1 knockout mice. Behav Brain Res. 2013;239:72–79. doi: 10.1016/j.bbr.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 52.Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011;3:64ra61. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- 53.Scharf SH, Jaeschke G, Wettstein JG, Lindemann L. Metabotropic glutamate receptor 5 as drug target for Fragile X syndrome. Curr Opin Pharmacol. 2015;20:124–134. doi: 10.1016/j.coph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 56.Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- 57.He Q, Nomura T, Xu J, Contractor A. The developmental switch in GABA polarity is delayed in fragile X mice. J Neurosci. 2014;34:446–450. doi: 10.1523/JNEUROSCI.4447-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X Syndrome: The GABAergic System and Circuit Dysfunction. Dev Neurosci. 2011 doi: 10.1159/000329420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, et al. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 60.D’Hulst C, Heulens I, Brouwer JR, Willemsen R, De Geest N, Reeve SP, et al. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS) Brain research. 2009;1253:176–183. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- 61.Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology. 2010;59:167–171. doi: 10.1016/j.neuropharm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Centonze D, Rossi S, Mercaldo V, Napoli I, Ciotti MT, De Chiara V, et al. Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. Biol Psychiatry. 2008;63:963–973. doi: 10.1016/j.biopsych.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Dahlhaus R, El-Husseini A. Altered neuroligin expression is involved in social deficits in a mouse model of the fragile X syndrome. Behav Brain Res. 2010;208:96–105. doi: 10.1016/j.bbr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 65.Henderson C, Wijetunge L, Kinoshita MN, Shumway M, Hammond RS, Postma FR, et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABA(B) receptors with arbaclofen. Sci Transl Med. 2012;4:152ra128. doi: 10.1126/scitranslmed.3004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, et al. Effects of STX209 (Arbaclofen) on Neurobehavioral Function in Children and Adults with Fragile X Syndrome: A Randomized, Controlled, Phase 2 Trial. Sci Transl Med. 2012;4:152ra127. doi: 10.1126/scitranslmed.3004214. [DOI] [PubMed] [Google Scholar]
- 67.Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci U S A. 2001;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fahling M, Mrowka R, Steege A, Kirschner KM, Benko E, Forstera B, et al. Translational regulation of the human achaete-scute homologue-1 by fragile X mental retardation protein. J Biol Chem. 2009;284:4255–4266. doi: 10.1074/jbc.M807354200. [DOI] [PubMed] [Google Scholar]
- 69.Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarti F, Zhang Z, Schroeder J, Chen L. Rapid suppression of inhibitory synaptic transmission by retinoic acid. J Neurosci. 2013;33:11440–11450. doi: 10.1523/JNEUROSCI.1710-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat Neurosci. 2014;17:1701–1709. doi: 10.1038/nn.3864. [DOI] [PubMed] [Google Scholar]
- 74.Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013 doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 76.Goncalves JT, Anstey JE, Golshani P, Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci. 2013;16:903–909. doi: 10.1038/nn.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Archives of general psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Molecular psychiatry. 2012;17:1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. The American journal of psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roberts JE, Hatton DD, Long AC, Anello V, Colombo J. Visual attention and autistic behavior in infants with fragile X syndrome. Journal of autism and developmental disorders. 2012;42:937–946. doi: 10.1007/s10803-011-1316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munir F, Cornish KM, Wilding J. A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia. 2000;38:1261–1270. doi: 10.1016/s0028-3932(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 82.Cohen JD, Nichols T, Brignone L, Hall SS, Reiss AL. Insular volume reduction in fragile X syndrome. Int J Dev Neurosci. 2011;29:489–494. doi: 10.1016/j.ijdevneu.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hall SS, Jiang H, Reiss AL, Greicius MD. Identifying Large-Scale Brain Networks in Fragile X Syndrome. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baker S, Hooper S, Skinner M, Hatton D, Schaaf J, Ornstein P, et al. Working memory subsystems and task complexity in young boys with Fragile X syndrome. J Intellect Disabil Res. 2011;55:19–29. doi: 10.1111/j.1365-2788.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van der Molen MJ, Van der Molen MW, Ridderinkhof KR, Hamel BC, Curfs LM, Ramakers GJ. Auditory and visual cortical activity during selective attention in fragile X syndrome: a cascade of processing deficiencies. Clin Neurophysiol. 2012;123:720–729. doi: 10.1016/j.clinph.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 86.Keri S, Benedek G. The perception of biological and mechanical motion in female fragile X premutation carriers. Brain Cogn. 2010;72:197–201. doi: 10.1016/j.bandc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 87.Pierpont EI, Richmond EK, Abbeduto L, Kover ST, Brown WT. Contributions of phonological and verbal working memory to language development in adolescents with fragile X syndrome. J Neurodev Disord. 2011;3:335–347. doi: 10.1007/s11689-011-9095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olichney JM, Chan S, Wong LM, Schneider A, Seritan A, Niese A, et al. Abnormal N400 word repetition effects in fragile X-associated tremor/ataxia syndrome. Brain: a journal of neurology. 2010;133:1438–1450. doi: 10.1093/brain/awq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reiss AL, Lee J, Freund L. Neuroanatomy of fragile X syndrome: the temporal lobe. Neurology. 1994;44:1317–1324. doi: 10.1212/wnl.44.7.1317. [DOI] [PubMed] [Google Scholar]
- 90.Holsen LM, Dalton KM, Johnstone T, Davidson RJ. Prefrontal social cognition network dysfunction underlying face encoding and social anxiety in fragile X syndrome. Neuroimage. 2008;43:592–604. doi: 10.1016/j.neuroimage.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hagan CC, Hoeft F, Mackey A, Mobbs D, Reiss AL. Aberrant neural function during emotion attribution in female subjects with fragile X syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1443–1354. doi: 10.1097/CHI.0b013e3181886e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, et al. Amygdala dysfunction in men with the fragile X premutation. Brain: a journal of neurology. 2007;130:404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- 93.Macedoni-Luksic M, Greiss-Hess L, Rogers SJ, Gosar D, Lemons-Chitwood K, Hagerman R. Imitation in fragile X syndrome. Implications for autism. Autism: the international journal of research and practice. 2009;13:599–611. doi: 10.1177/1362361309337850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grant CM, Apperly I, Oliver C. Is theory of mind understanding impaired in males with fragile X syndrome? J Abnorm Child Psychol. 2007;35:17–28. doi: 10.1007/s10802-006-9077-0. [DOI] [PubMed] [Google Scholar]
- 95.Scerif G, Cornish K, Wilding J, Driver J, Karmiloff-Smith A. Visual search in typically developing toddlers and toddlers with Fragile X or Williams syndrome. Dev Sci. 2004;7:116–130. doi: 10.1111/j.1467-7687.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 96.Roberts JE, Boccia ML, Bailey DB, Jr, Hatton DD, Skinner M. Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Dev Psychobiol. 2001;39:107–123. doi: 10.1002/dev.1035. [DOI] [PubMed] [Google Scholar]
- 97.Van der Molen MJ, Van der Molen MW. Reduced alpha and exaggerated theta power during the resting-state EEG in fragile X syndrome. Biol Psychol. 2013;92:216–219. doi: 10.1016/j.biopsycho.2012.11.013. [DOI] [PubMed] [Google Scholar]


