Abstract
Objectives
Pancreatic Ductal AdenoCarcinoma (PDAC) has not experienced a meaningful mortality improvement over the past few decades. Successful screening is difficult to accomplish because most PDACs present late in their natural history, and current interventions have not provided significant benefit. Our goal was to identify determinants of survival for early PDAC to help inform future screening strategies.
Methods
Early pancreatic ductal adenocarcinomas from the NCI SEER database (2000–2010) were analyzed. We stratified by size and included carcinomas-in-situ (Tis). Overall cancer-specific survival were calculated. A Cox-Proportional Hazards model was developed and the significance of key covariates for survival prediction was evaluated.
Results
A Kaplan-Meier plot demonstrated significant differences in survival by size at diagnosis; these survival benefits persisted after adjustment for key covariates in the Cox-Proportional Hazard analysis. Additional, relatively weaker predictors of worse survival included: older age, male sex, black race, nodal involvement, tumor location within the head of the pancreas, and no surgery or radiotherapy.
Conclusions
For early PDAC, we found tumor size to be the strongest predictor of survival, even after adjustment for other patient characteristics. Our findings suggest that early PDAC detection can have clinical benefit, which has positive implications for future screening strategies.
Keywords: pancreatic cancer, SEER registry, survival, pancreas, adenocarcinoma, pancreatic neoplasms
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer mortality in the United States, with an estimated 48,960 diagnoses and 40,560 deaths expected in 2015 (1). Based on current epidemiologic trends, it is expected to be the second leading cause of cancer mortality by 2030 (2). While the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER) data suggest that mortality rates are slightly increasing overall at 0.4% each year from 2002–2011 (3), the prognosis of diagnosed patients remains extremely poor, with a 1-year relative survival rate of 28% and 5-year rate of 7% (1).
Cancer screening programs for breast, colorectal, cervical, and lung cancer have been shown to reduce cancer-specific mortality rates (4–13). In the case of pancreatic cancer, while studies have suggested that imaging-based screening of asymptomatic, average-risk-individuals is unlikely to be of benefit due to the low incidence of pancreatic cancer (14–17), the clinical impact of targeted screening for high-risk individuals remains unclear and an active area of research. While high-risk screening programs for pancreatic cancer exist, there is no consensus on starting and stopping ages and optimal screening modalities (17). Furthermore, the risk of overtreatment is heightened in pancreatic cancer treatment from the high morbidity and mortality risk of pancreatic surgery (14, 17, 18). Our goal was to examine determinants of survival differences in individuals with early pancreatic cancer to determine the potential role of targeted screening on pancreatic cancer mortality rates. We hypothesized that if survival differences exist based on factors such as tumor size, targeted screening of high-risk individuals has a potential role to play in the early detection of treatable pancreatic cancers.
MATERIALS AND METHODS
Cohort Inclusion/Exclusion Criteria
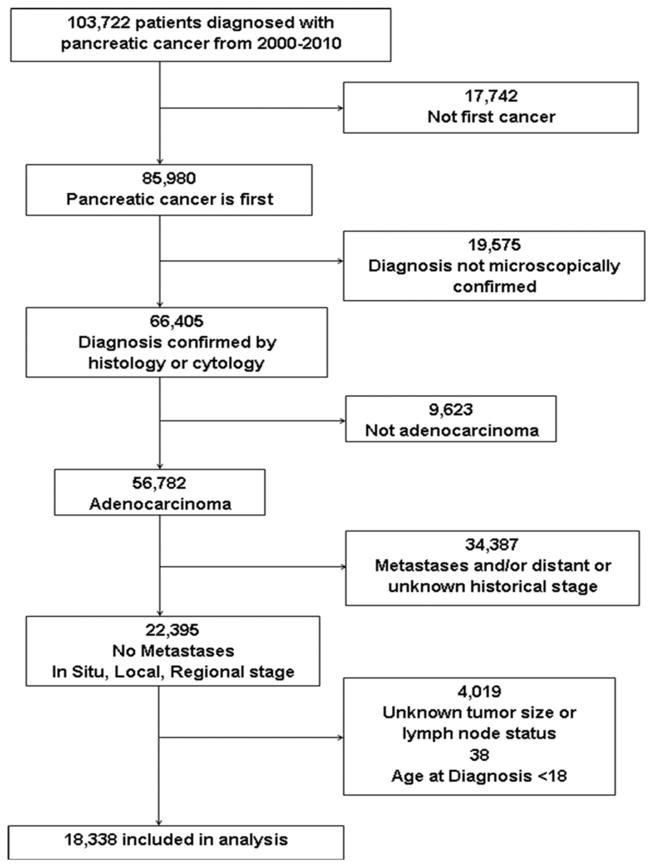
We used data from the November 2013 release of the Surveillance, Epidemiology, and End Results (SEER) database by the National Cancer Institute (19). SEER collects information on cancer incidence and survival for eighteen U.S. registries, which represent approximately 28% of the population. The database includes information on demographics, cancer site, histology, treatment, and survival. Pancreatic cancer patients were included if they were 18 years or older and had non-metastatic, microscopically confirmed (histology and cytology) adenocarcinoma diagnosed between January 1, 2000 and December 31, 2010. Histology was based on diagnosis codes from the International Classification of Diseases for Oncology (ICD-O-3). A cancer was classified as adenocarcinoma using the codes 8050, 8140–8147, 8160–8162, 8180–8221, 8250–8507, 8514, 8520–8551, 8560, 8570–8574, 8576, and 8940–8941. Patients with islet cell carcinomas, epidermoid carcinomas, sarcomas, neuroendocrine, and other carcinomas were excluded. Patients were also excluded if the pancreatic cancer was not their first primary cancer, had a diagnostic confirmation of tumor marker, direct visualization, imaging, or clinical diagnosis, had a distant or unknown historical stage, or had unknown tumor size or lymph node status. Detailed information on the numbers of patients included and excluded, consequent to each of the above criteria, are shown in Figure 1. The final analysis included a cohort of 18,338 patients.
Figure 1.
Flowchart of the inclusion and exclusion process used to create the final pancreatic cancer cohort from SEER.
Statistical Analysis
Our primary outcome was overall survival among early PDAC. The following independent variables were evaluated to identify associations with the dependent variable of survival: age at diagnosis; year of diagnosis; sex; race and ethnicity; tumor size; N stage; receipt of surgery or radiation; SEER region; and tumor location (pancreatic head or body/tail) in our analysis. The staging of early pancreatic cancers relies on tumor size and regional lymph node involvement. Patients were classified into four different tumor sizes (In Situ, <=1 cm, 1.1–2 cm, and > 2 cm), with tumor sizes less than 2 cm corresponding to stage category of T1 in the American Joint Committee on Cancer (AJCC) TNM staging system. Early PDAC cancers were defined as in situ cancers or those with tumor size less than 2 cm. Lymph node status was used to classify patients as N0 (no lymph node involvement) or N1 (spread to regional lymph nodes). Patients with a tumor site code of C25.0 were categorized as having cancer in the pancreatic head, patients with a tumor site code of C25.1 and C25.2 were categorized as having cancer located in the pancreatic body/tail, and the remaining site codes were categorized as “other.” Surgery was defined as having any pancreatic surgery code in the “surgery of primary site” variable field. Radiation was defined as having received any type of radiation for their cancer (beam, radioactive isotopes, implants, or unknown method/other). Survival was defined as time from date of diagnosis to date of death or December 31, 2011, whichever came first; patients who were identified as still alive on December 31, 2011 were censored in survival curves. Cancer-specific survival was based on the cause-specific death classification in the SEER registry.
Descriptive statistics were calculated for all demographic variables included in the analysis. Five-year survival was calculated for both overall and cancer-specific survival and stratified by tumor size. Kaplan-Meier estimates of survival were plotted stratified by tumor size and differences were analyzed using the log-rank test. Multivariable survival analysis of overall survival was performed using the Cox proportional hazard model. Statistical significance was defined as having a p-value <0.05 in a two-sided test. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc).
RESULTS
Cohort Description
A total of 18,338 pancreatic cancer patients were eligible for analysis based on our inclusion and exclusion criteria (Figure 1). The majority of the patients were white (N=13,293; 72.5%); the median age was 68. There were slightly more females than males (51.4% vs 48.6%). Most tumors were greater than 2 cm in size (N=15,625, 85.2%); 1.4% (N=264) of the patients had in situ cancer, 1.7% (N=311) had tumors less than 1 cm, and 11.7% (N=2,138) had tumors that were 1.1–2 cm. All diagnoses were pathologically confirmed, with the majority (N=14,126; 77%) confirmed with positive histology. The majority of patients (N=13,333; 72.7%) had pancreatic head cancer. There were 8,678 (47.3%) patients with some regional lymph node involvement (N1). Among patients with <=1 cm tumors, 28.3% were N1, vs. 53.9% of those with 1.1–2 cm tumors and 51.3% of those with >2 cm tumors. All in situ cancers were N0. A total of 9,473 (51.7%) patients received some form of pancreatic cancer surgery and 7,103 (38.7%) of the cohort received radiation. Detailed descriptive statistics are included in Table 1.
Table 1.
Hazard Ratios for Overall Survival Adjusted for Patient Characteristics
| Characteristic | N (%) | HR (95% Cl) | p-value |
|---|---|---|---|
| Unadjusted Model | |||
| Tumor Size | |||
| In Situ | 264 (1.4%) | 1.0 (ref) | |
| <=1 cm | 311 (1.7%) | 3.26 (2.49–4.28) | <0.0001 |
| 1.1–2 cm | 2,138 (11.7%) | 4.99 (3.94–6.33) | <0.0001 |
| >2 cm | 15,625 (85.2%) | 7.43 (5.88–9.83) | <0.0001 |
| Adjusted Model | |||
| Age | |||
| <50 | 1,358 (7.4%) | 1.0 (ref) | |
| 50–59 | 3,477 (19.0%) | 1.07 (0.99–1.15) | 0.09 |
| 60–69 | 5,351 (29.2%) | 1.22 (1.14–1.31) | <0.0001 |
| 70–79 | 5,484 (29.9%) | 1.42 (1.33–1.52) | <0.0001 |
| 80+ | 2,668 (14.6%) | 1.75 (1.62–1.88) | <0.0001 |
| Sex | |||
| Male | 8,920 (48.6%) | 1.0 (ref) | |
| Female | 9,418 (51.4%) | 0.93 (0.90–0.96) | <0.0001 |
| Race/Ethnicity | |||
| White | 13,293 (72.5%) | 1.0 (ref) | |
| Black | 1,947 (10.6%) | 1.10 (1.04–1.16) | 0.0004 |
| Asian | 1,246 (6.8%) | 1.01 (0.94–1.08) | 0.78 |
| Hispanic | 1,679 (9.2%) | 1.04 (0.98–1.10) | 0.2 |
| Unknown | 173 (0.9%) | not included* | -- |
| Tumor Size | |||
| In Situ | 264 (1.4%) | 1.0 (ref) | |
| <=1 cm | 311 (1.7%) | 3.09 (2.35–4.07) | <0.0001 |
| 1.1–2 cm | 2,138 (11.7%) | 4.35 (3.42–5.53) | <0.0001 |
| >2 cm | 15,625 (85.2%) | 5.69 (4.49–7.21) | <0.0001 |
| N Stage | |||
| N0 | 9,660 (52.7%) | 1.0 (ref) | |
| N1 | 8,678 (47.3%) | 1.31 (1.28–1.37) | <0.0001 |
| Pancreas Location | |||
| Head | 13,333 (72.7%) | 1.0 (ref) | |
| Tail/Body | 2,674 (14.6%) | 0.92 (0.88–0.96) | 0.0003 |
| Other/Unknown | 2,331 (12.7%) | 1.02 (0.97–1.07) | 0.38 |
| Surgery | |||
| No | 8,810 (48.0%) | 1.0 (ref) | |
| Yes | 9,473 (51.7%) | 0.38 (0.36–0.39) | <0.0001 |
| Unknown | 55 (0.3%) | not included* | -- |
| Radiation | |||
| No | 10,899 (59.4%) | 1.0 (ref) | |
| Yes | 7,103 (38.7%) | 0.71 (0.69–0.74) | <0.0001 |
| Unknown | 336 (1.8%) | not included* | -- |
| Year of Diagnosis | |||
| 2000–2001 | 2,511 (13.7%) | 1.0 (ref) | |
| 2002–2003 | 2,801 (15.3%) | 1.01 (0.95–1.06) | 0.77 |
| 2004–2005 | 3,074 (16.8%) | 0.94 (0.89–0.99) | 0.03 |
| 2006–2007 | 3,509 (19.1%) | 0.91 (0.86–0.96) | 0.0005 |
| 2008–2010 | 6,443 (35.1%) | 0.82 (0.78–0.87) | <0.0001 |
| SEER Region | |||
| Northeast | 3,244 (17.7%) | 1.0 (ref) | |
| South | 3,812 (20.8%) | 1.25 (1.19–1.32) | <0.0001 |
| Midwest | 2,001 (10.9%) | 1.14 (1.07–1.21) | <0.0001 |
| West/Hawaii | 9,281 (50.6%) | 1.03 (0.98–1.08) | 0.2 |
Abbreviations: N, sample size; HR, hazard ratio
Unknown race, surgery, and radiation categories were not included in the model due to small size
Survival Outcomes
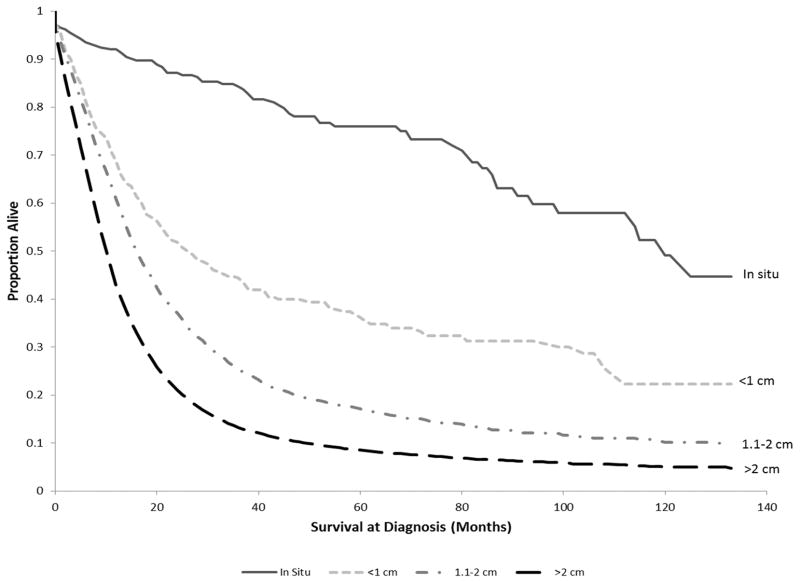
PDAC Survival outcomes were analyzed among the four tumor size subgroups. The five-year overall survival rate decreased as tumor size increased, from 76% for in situ patients to 9% for patients with tumors >2 cm (Table 2). Similar results were seen in the five-year cancer-specific survival rates, which decreased from 84% for in situ patients to 10% for patients with tumors >2 cm. The overall and cancer-specific five-year survival rate for the entire cohort was 11% and 13%, respectively. The Kaplan-Meier curve for the cohort stratified by tumor size is shown in Figure 2. The median survival was 120 months for in situ, 27 months for cancers <=1 cm, 17 months for cancers 1.1–2 cm, and 11 months for cancers greater than 2 cm. The overall median survival was 11 months. The difference in the Kaplan-Meier survival probabilities among the tumor size subgroups was statistically significant (p<0.0001).
Table 2.
5-Year Survival Rate According to Tumor Size for Overall and Cancer-Specific Survival
| Tumor Size | Overall Survival | Cancer Specific Survival |
|---|---|---|
| In Situ | 76% | 84% |
| <=1 cm | 36% | 40% |
| 1.1–2 cm | 17% | 20% |
| >2 cm | 11% | 13% |
Figure 2.
Kaplan-Meier survival curve of overall survival (in months) among pancreatic cancer patients in SEER diagnosed in 2000–2010, stratified by tumor size (labeled in the graph).
In an unadjusted Cox proportional hazards model, we found a statistically significant difference in overall survival between patients with smaller tumors and those with larger tumors, when compared to in situ cancers (HR 3.26, 95% CI 2.49–4.28; HR 4.99, 95% CI 3.94–6.33; HR 7.43, 95% CI 5.88–9.83, for tumors <1 cm, 1.1–2 cm, and >2 cm, respectively, p<0.0001).
In the adjusted multi-covariate model, when controlling for the specified demographic and clinical characteristics (independent variables), this difference remained significant (HR 3.09, 95% CI 2.35–4.07; HR 4.35, 95% CI 3.42–5.53; HR 5.69, 95% CI 4.49–7.21, for tumors sized <=1 cm, 1.1–2 cm, and >2 cm, respectively, p<0.0001). Patients with N1 cancers had worse survival when compared to those with N0 cancers (HR 1.32, 95% CI 1.28–1.37, p<0.0001). Patients who received treatment had significantly better survival outcomes than those who did not (HR 0.38, 95% CI 0.36–0.39 for surgery, p<0.0001; HR 0.71, 95% CI 0.69–0.74 for radiation, p<0.0001). Those who had body/tail pancreatic cancers had a better survival than those with pancreatic head cancer (HR 0.92, 95% CI 0.88–0.96, p=0.0003). The hazard ratios for all characteristics included in the adjusted model are listed in Table 1. When Tis cases were excluded, tumor size remained a strong predictor of survival (1.41, 95% CI 1.21–1.64 and 1.84, 95% CI 1.59–2.13, for 1.1–2 cm and >2 cm, respectively, when compared to <1 cm).
In a subgroup analysis of patients who were treated with surgery, tumor size remained a strong predictor of survival in the entire cohort (HR 2.67, 95% CI 1.96–3.65; HR 3.97, 95% CI 3.04–5.17; HR 5.37, 95% CI 4.14–6.96, for tumors sized <=1 cm, 1.1–2 cm, and >2 cm, respectively, p<0.0001) and when Tis cases were excluded (1.48, 95% CI 1.23–1.79 and 2.00, 95% CI 1.67–2.40, for 1.1–2 cm and >2 cm, respectively, when compared to <1 cm).
DISCUSSION
We analyzed NCI SEER cancer registry data to identify determinants of survival for early PDAC, with the goal of informing future strategies for targeted screening. We found that survival among patients with non-metastatic PDAC was strongly predicted by tumor size. Kaplan Meier survival curves demonstrated a clear correlation between survival and tumor size at detection including early carcinomas in situ (Tis or PanIN-3), an effect that was evident when either overall survival or cancer-specific survivals were analyzed. Moreover, when adjusting for multiple key covariates that could affect PDAC survival in a Cox-Proportional Hazard model, tumor size at diagnosis continued to be the strongest predictor of survival, including the subgroup of patients treated with surgery. Interestingly, nodal status, or lymph node involvement, was significantly associated with worsened survival (HR=1.31, p<0.0001); however, the risk ratio was substantially less than for size.
The importance of our findings pertains to the implications for future, emerging screening technologies. The close relationship between tumor size and survival observed for early PDAC suggests that even if the window of opportunity to detect very small – or in situ – PDAC is limited, if such a window is identifiable, survival benefits from screening may be gained.
Other studies have used SEER data to analyze pancreatic cancer survival. A 2013 study of 7,135 patients with pancreatic head cancers treated with pancreaticoduonenectomy by Franco et al (20) demonstrated poorer survival among patients with a larger tumor size compared to those with tumors <1 cm, using a Cox-Proportional Hazard model (HR 1.67, p<0.001; HR 1.18, p=0.15, for >2 cm and 1.1–2 cm, respectively). Their study also showed that N1 nodal status had a significantly worse survival when compared to N0 patients (HR 1.50, p<0.001). Given our study goals, our analytic approach differed. Most importantly, our analysis included carcinoma in situ cases. In addition, we did not impose limitations related to the specific cancer anatomical location within the pancreas or surgical status. Additionally, we used more recent SEER data (2000–2010). These differences resulted in an analysis that was broader in scope, and which included more than twice the number of cases.
A significant strength of our analysis is the use of NCI SEER data which are representative of the U.S. population and provided over 18,000 PAC patients to analyze. Current screening technologies including MRI or endoscopic ultrasound are not able accurately detect Tis or PanIN-3 tumors (21–22), thus the 264 patients with Tis in our analysis were cases that were most likely found incidentally (e.g. surgery for another indication). In addition, these higher survival rates of these cancers could be a result of lead-time bias, inherent in all screening programs, rather than the result of earlier treatment. However, this sub-group comprised a relatively small percentage of our cohort. To potentially control for this bias, we ran the adjusted Cox proportional hazards model with Tis cases excluded. The significant correlation of size to survival persisted, albeit with a somewhat attenuated effect.
Additionally, although we attempted to control for potential biases that could affect cancer survival by developing and analyzing a regression model, additional biases that we are not aware of may be present; this limitation is common to all similar, retrospective, observational studies. Furthermore, we were unable to include chemotherapy or recurrence as predictors due to the unavailability of this information in SEER. Nevertheless, it is unlikely that another methodological approach – such as a prospective clinical trial – could be feasibly designed and executed to address the principal hypotheses tested in our analysis.
Future investigations, likely based on large, longitudinal cohort studies, may be able to provide additional information regarding the natural history of PDAC. These would be helpful for confirming our results, and for providing additional, similar insights into the natural history of PDAC for the development of viable screening technologies. Although pancreatic carcinomas in situ, or PanIN-3, are not detectable by currently available imaging and screening modalities, emerging technologies including molecular imaging, and serum biomarkers such as circulating tumor cells (CTCs) (23–25), may allow for the detection of pancreatic neoplasia at significantly earlier stages within their natural history.
In conclusion, when conducting an analysis of >18,000 patients with early PDAC using the SEER cancer registry, we found that tumor size at diagnosis is the greatest predictor of survival, even after adjustments for other patient characteristics using a Cox-Proportional Hazard model. Our findings have positive implications for the viability and potential clinical benefit of future early detection technologies for PDAC.
Acknowledgments
Source of Funding: This work was supported by the National Cancer Institute (award number K07CA133097 to P.V. Pandharipande). Pandharipande received research funding from the Medical Imaging and Technology Alliance. Brugge received research funding from Lustgarten, Celgene, and RedPath, and is consultant to Boston Scientific.
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
References
- 1.American Cancer Society. Cancer Facts and Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.Rahib L, Smith B, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Surveillance Epidemiology and End Results. [Accessed March 4, 2015];SEER Stat Fact Sheet. Available at: http://seer.cancer.gov/statfacts/html/pancreas.html.
- 4.Nyström L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359:909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 5.Tabár L, Vitak B, Chen HH, et al. The Swedish two-county trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin N Am. 2000;38:625–651. doi: 10.1016/s0033-8389(05)70191-3. [DOI] [PubMed] [Google Scholar]
- 6.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkin WS, Edwards R, Kralj-Hans I, et al. UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 9.Mandel JS, Church TR, Ederer F, et al. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–437. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 10.Hakama M, Louhivuori K. A screening programme for cervical cancer that worked. Cancer Surv. 1988;7:403–416. [PubMed] [Google Scholar]
- 11.Hakama M, Miller AB, Day NE. IARC Scientific Publication. 76. Lyon: IARC Press; 1986. Screening for Cancers of the Uterine Cervix. [Google Scholar]
- 12.Sigurdsson K. The Icelandic and Nordic cervical screening programs: trends in incidence and mortality rates through 1995. Acta Obstet Gynecol Scand. 1999;78:478–485. [PubMed] [Google Scholar]
- 13.Lynge E. Screening for cancer of the cervix uteri. World J Surg. 1989;13:71–78. doi: 10.1007/BF01671157. [DOI] [PubMed] [Google Scholar]
- 14.Pandharipande PV, Heberle C, Dowling EC, et al. Targeted screening of individuals at high risk for pancreatic cancer: results of a simulation model. Radiology. 2015;275:177–187. doi: 10.1148/radiol.14141282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein AP, Lindström S, Mendelsohn JB, et al. An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. [Accessed March 6, 2015];PLoS One [serial online] 2013 8:e72311. doi: 10.1371/journal.pone.0072311. Available at: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0072311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Canto MI, Harinck F, Hruban RH, et al. International cancer of the pancreas screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–347. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surveillance Epidemiology and End Results Program (www.seer.cancer.gov) Research Data (1973–2011), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
- 20.Franco J, Hugec V, Lopes TL, et al. Survival among pancreaticoduodenectomy patients treated for pancreatic head cancer <1 or 2 cm. Ann Surg Oncol. 2013;20:357–361. doi: 10.1245/s10434-012-2621-y. [DOI] [PubMed] [Google Scholar]
- 21.Goggins M. Markers of pancreatic cancer: working toward early detection. Clin Cancer Res. 2011;17:635–637. doi: 10.1158/1078-0432.CCR-10-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Li Y, Cui L, et al. Monitoring pancreatic carcinogenesis by the molecular imaging of cathepsin E in vivo using confocal laser endomicroscopy. [Accessed March 9, 2015];PLoS One [serial online] 2014 9:e106566. doi: 10.1371/journal.pone.0106566. Available at: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0106566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhim AD, Thege FI, Santana SM, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146:647–651. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ting DT, Wittner BS, Ligorio M, et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;9:1905–1918. doi: 10.1016/j.celrep.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]