Abstract
Monocytes and macrophages make up part of the innate immune system and provide one of the first defenses against variety of treats. Macrophages can also modulate the adaptive immune system. Efficient sensing and response to tissue environmental cues highlights the complexity and dynamic nature of macrophages and their plasticity. Macrophages may have divergent roles depending on their polarity and stimulus received. Accumulating evidence demonstrates the critical role played by macrophages in tumor initiation, development, and progression. In this review, we discuss the characteristics of tumor-associated macrophages (TAMs) and their role in pancreatic adenocarcinoma. In addition, we give an overview on recent advances related to the therapeutic implication associated with targeting TAMs in pancreas cancer.
Keywords: macrophages, tumor associated macrophages, pancreas cancer, pancreatic ductal adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive tumors and the fourth leading cause of cancer-associated death in the United States [72, 83]. PDAC develops by an adenoma to carcinoma sequence as a result of accumulating genetic alterations, which provide signals (e.g. TGFβ via SMAD4) that promote recruitment of immune cells [8, 80, 89]. Histologically, microscopic pancreatic intraepithelial neoplasms (PanIN) progress from intraepithelial to invasive pancreatic cancer and parallel the accumulating genetic alterations in the adenoma to carcinoma succession of PDAC [8, 33, 50]. Macrophages are one of the early infiltrating immune cells in PanIN lesions and continue to persist through the invasive cancer [12]. Moreover, recent studies showing macrophage involvement even at the early stages of acinar cell dedifferentiation to ductal cells or acinar-to-ductal metaplasia (ADM) underscores the critical role these cells play in pancreatic cancer initiation, progression, and metastasis. Tumor macrophage infiltration is associated with poor outcome. Analysis of human pancreatic cancer tissues showed that the number of infiltrating macrophages in tumor tissue from patients with metastases was significantly greater compared to patients with no metastasis [23].
The tumor environment is rich in immune cell infiltrates yet tumors are able to circumvent interference through mechanisms that inhibit immune cell mediated anti-tumor effects. Such modification of immune cell functions creates a favorable microenvironment that allows tumor progression (Figure 1). This review attempts to summarize the knowledge gained during the last few years on the role of macrophages in pancreatic cancer.
Figure 1. Tumor associated macrophages (TAM) in pancreatic cancer.
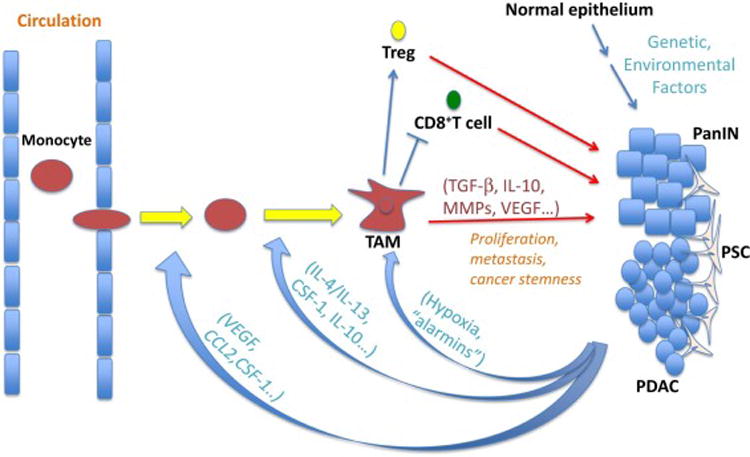
Macrophages play an important role in pancreas cancer development and progression. CCL2, chemokine (C-C motif) ligand 2; CSF-1, colony stimulating factor 1; IL, interleukin; MMPs, matrix metalloproteinases; PDAC, pancreatic ductal adenocarcinoma; PanIN, pancreatic intraepithelial neoplasia; TGFβ, transforming growth factor beta; Treg, regulatory T cell; VEGF, vascular endothelial growth factor.
Characteristics of tumor associated macrophages (TAMs)
Macrophages are efficient phagocytic cells of the innate immune system originating from bone marrow derived monocytes that constantly reconstitute most of the gastrointestinal tissues including the pancreas under homeostatic and inflammatory conditions [26, 27, 86]. In addition, local proliferation also contributes to maintain tissue resident macrophages. As a result of their plasticity, macrophages make up a heterogeneous population of immune cells with distinct functional and phenotypic characteristics [26]. More recently, classification of macrophages based on their functions, namely host defense, wound healing, and immune regulation, has been suggested [59]. More broadly and for simplicity, macrophages have been classified as classically activated (M1; commonly activated by IFN-γ and TLR ligands, express higher levels of IL-12, IL-23, TNFα, MHCII, IL-6, and inducible nitric oxide synthase or iNOS) or alternatively activated (M2; commonly activated by IL-4 and IL-13, express higher levels of IL-10 and TGFβ) [52, 53]. M1- and M2-polarized macrophages predominate in acute and chronic pancreatitis respectively [84, 86], with tumor-associated macrophages (TAMs) infiltrating solid tumors including PDAC thought to bear similarities to M2s and associated with poor prognosis [41, 89].
TAMs have been studied in different cancer types, play an immunosuppressive role, and are frequently associated with poor prognosis due to their abilities in promoting tumor growth, invasion, angiogenesis, and metastasis as discussed below [6, 90]. In addition to general macrophage markers (F4/80 in mouse, CD68 in human), scavenger receptors such as CD163, CD204, and CD206 have been used to identify TAMs in cancers [30]. Functional properties of TAMs include production of IL-10 and matrix metalloproteinases (MMPs), as well as M1-associated iNOS suggesting heterogeneous or mixed characteristics amongst tumor infiltrating macrophages [29, 82]. Recent study found that TAMs were predominantly M2 and associated with poor prognosis, whereas M1 predominated in the non-tumor inflammatory region surrounding the caner cells, highlighting the importance of spatial localization of TAMs [34].
The importance of TAMs in PDAC is emphasized by the results coming from several groups showing relationships between number and location of TAMs in surgically resected tumors and patient outcome. Of note, the 5 year survival rate after surgery performed with curative intent is only about 20% [18]. Thus, the correlation between disease recurrence and survival after surgery represents opportunities to determine factors associated with disease recurrence and survival. Examples include findings that metastasis to lymph nodes found in surgical specimens is highly associated with M2s in the primary tumor and poor outcome [41]; and that high level of M2s in tumors as mentioned above is associated with shorter survival times while a greater ratio of M1s to overall macrophage number is associated with a longer survival after surgery [34]. It is known that extra-pancreatic nerve plexus metastasis of PDAC is associated with a poor outcome. One study showed that there is an association between a high number of M2s in the extra-pancreatic nerve plexus and decreased disease free survival and overall survival in patients undergoing resection of PDAC [74]. These studies confirm the longstanding findings in PDAC that a poor prognosis is associated with neural invasion and lymph node metastasis, and show that these biomarkers of outcome are associated with greater numbers of M2 type of macrophages.
Tumor microenvironment/evasive mechanisms recruiting and promoting TAM
The tumor microenvironment includes proliferating tumor cells, tumor-associated stromal fibroblasts (pancreatic stellate cells or PSCs in the case of PDAC), immune cells, blood vessels, and other tissue cells. The tumor microenvironment is enriched by factors that recruit circulating monocytes and favor generation of TAMs resembling M2 (Figure 1). Such factors include colony-stimulating factor (CSF)-1, chemokines and cytokines such as IL-4, IL-13, TGFβ and IL-10 [3, 15, 52, 71, 73]. CSF-1 promotes myeloid progenitor differentiation into monocytes and macrophages; and regulates proliferation, function, survival and migration of macrophages [43]. CSF-1 has also been implicated in promoting generation of alternatively activated macrophages or M2 [54, 75]. In experimental PDAC models CSF-1/CSF-1R inhibition depleted CD206hi TAMs and altered residual TAMs to support anti-tumor responses [91]. Thus, tumor-derived cytokines and chemokines not only promote macrophage recruitment and survival but also TAM functional properties that enhance tumor growth. In addition, other tumor-derived factors such as vascular endothelial growth factor (VEGF) also promote TAM infiltration as discussed below [87]. Dineen et al. showed in an animal model of pancreatic cancer that TAMs express epidermal growth factor receptor (EGFR) 2 where as, macrophages from the animals without tumors did not express the receptor. They also found that VEGF recruits TAMs to the tumor microenvironment and that inhibition of VEGF prevented this effect [20].
CCL2/CCR2 axis is important for monocyte egress from the bone marrow and their recruitment into tissues under homeostasis and inflammation [42, 69, 70]. Of the chemoattractants identified to play a role in tumor monocyte/macrophage recruitment, the CCL2/CCR2 axis has been the best studied. Bone marrow monocyte mobilization and tumor infiltration was shown to depend on CCR2 in PDAC model, and increased bone marrow monocyte mobilization correlated with increased PDAC infiltration with CCR2+ macrophages and was associated with poor survival in patients with PDAC [66]. In addition, CCR2 inhibition led to a decrease in monocyte recruitment, tumor growth and metastasis in an orthotropic model of PDAC. Results from this study have led to the ongoing Phase Ib trial testing CCR2 blockade in combination with chemotherapy in patients with advanced PDAC (NCT01413022).
TAMs are found in high concentration in hypoxic and avascular areas of the tumor [44]. Presence of large areas of hypoxia has been correlated with poor prognosis and resistance to anti-tumor therapies [78]. In a mammary tumor model, TAMs were shown to up regulate VEGF expression in areas of hypoxia [44]. In addition, increased TAMs were present in poorly vascularized areas VEGF-positive as compared to VEGF-negative areas of the tumor and the authors proposed that VEGF might provide macrophage chemoattraction. Hypoxia up regulates VEGF and class-3 semaphorins (Sema3A, a ligand for neruopilin-1 or Nrp1) [10]. Inhibition of Nrp1 in experimental tumor models was shown to inhibit angiogenesis and tumor growth [32, 63]. A recent study using models of PDAC and breast cancer showed that Nrp1-dependent (via Sema3A) and Nrp1-independent VEGF receptor transactivation led to TAM localization and entrapment in hypoxic tumor niches [10]. In addition to the Nrp1 and pro-angiogenic factor VEGF, hypoxia induces macrophages to up regulate hypoxia-inducible factor (HIF)-regulated genes and MMPs that allow them not only to survive in harsh environment but also to favor revascularization of the ischemic sites of the tumor [61]. TAMs are an important source of VEGF especially in poorly vascularized areas [44]. In fact, TAMs in hypoxic tumor regions have been shown to represent a macrophage population (closely resembling M2, expressing HIF-1α) that promotes angiogenesis, suppresses anti-tumor immunity, and metastasis [21, 60].
Alarmins such as high mobility group box protein 1(HMGB1), S100A8, S100A9, serum amyloid A3 (SAA3), and fibronectin have been implicated to recruit myeloid cells to tumor areas [13, 14]. HMGB1 is released from necrotic cells and activated macrophages [81]. Recent study showed that HMGB1 was over expressed in tumor tissues of PDAC patients and associated with poor outcome [45]. However, more studies are needed to understand the role of alarmins in PDAC and their effect on TAM localization as well as function.
Tumor growth and immune evasion is maintained by immunosuppressive factors such as IL-10, TGFβ, and prostaglandin E2 (PGE2) released by TAMs that favor Treg recruitment and inhibit anti-tumor effector CD8+ T cell activities [62]. Several effects of IL-10 have been implicated in promoting TAM recruitment and TAM immunosuppressive properties: i) IL-10 enhances monocyte differentiation into macrophages and suppresses anti-tumor dendritic cell generation; ii) IL-10 suppresses myeloid cells derived IL-12 release, thus enhancing Th2 differentiation and production of high levels of IL-4/13 that further promote TAM generation. Additional tumor evasive mechanisms involve up regulation of CD47 or the “do not eat me signal” [35, 77]. CD47 interacts with macrophage regulatory protein-α and CD47 overexpressing cancer cells are protected from phagocytosis by TAMs [11].
Factors secreted by primary tumors have been shown to mediate formation of pre-metastatic niche in particular organs and lead to organotropism [1, 88]. Some of the secreted factors implicated include VEGF and placenta growth factor or PIGF [37, 38]. Exosomes play an important role as molecular carriers and provide mechanism for cross talk between cells; and more recently tumor derived exosomes have been shown to promote pre-metastatic niche formation [64]. Interestingly, a recent study showed that PDAC-released exosomes were enriched with the chemokine macrophage migration inhibitory factor (MIF) and induced recruitment of TAMs and formation of pre-metastatic niche in the liver [17]. These studies demonstrate the ability of the primary tumor not only to influence local TAM recruitment but also their recruitment to distant pre-metastatic sites.
Role of TAMs in tumor initiation and progression
Accumulating evidence shows that acinar cells give rise to PDAC, whereas duct and centro-acinar cells are resistant to oncogenic transformation suggesting that acinar cells must convert or dedifferentiate into duct-like cells to give rise to PDAC [9, 19, 39]. Recent study in experimental model of acute pancreatitis showed that macrophage-secreted factors TNFα and CCL5 (RANTES) induce ADM through activation of NFκB, and macrophage depletion blocked generation of pancreatic ADM [47]. Folias and coworkers showed that macrophages play an important role in acinar de-differentiation and pancreatic regeneration in experimental model of acute pancreatitis [22]. In the presence of oncogenic Kras, such regenerative process of ADM can progress into neoplastic transformation [31, 46, 58]. Macrophage infiltration is also noted early on around neoplastic ducts in PanIN lesion and persists around neoplastic epithelium in invasive carcinomas [12]. Thus, TNFα-producing macrophages (M1) likely play an important role in the initiation of pre-neoplastic lesions in the right settings [48].
TAMs have been shown to promote immunosuppressive environment by suppressing adaptive immunity, which provides tumors a survival and growth advantage [51, 55]. TAMs have been shown to secrete chemokines that allow regulatory T cell (Treg) recruitment and provide co-stimulatory signals that inhibit T cell proliferation [40]. Accordingly, increased Treg and decreased CD8+ T cell tumor infiltration is associated with PDAC progression and poor prognosis [34, 76]. Moreover, tumor derived-mucins were shown to bind mannose receptor CD206 on TAMs and enhance immunosuppressive properties of TAMs [2]. Notably, functionally distinct MHC IIhi and MHC IIlow TAMs were identified within normoxic and hypoxic mammary tumor microenvironment respectively, yet both TAMs were inefficient at activating T cells and suppressed T cell proliferation highlighting T cell suppressive characteristics of TAMs [60].
Angiogenesis is critical for tumor progression and metastasis. As discussed in the above, TAMs are important source of VEGF and can regulate angiogenesis. Hypoxic tumor microenvironments recruit TAMs that promote vascularization and suppress anti-tumor responses favoring tumor escape [10, 21, 60]. In addition to TAM-derived MMPs that degrade extracellular matrix proteins and promote metastasis [13, 28], a recent study showed that TAM-derived cathepsins promote new blood vessel formation and enhance tumor invasiveness [24]. Based on these findings, the authors proposed a model in which macrophage cathepsins cleave E-cadherin, contribute to ECM degradation, and decrease cell-to-cell contacts thereby creating ways for cancer cells to migrate.
Similar to angiogenesis, lymphangiogenesis is an essential step for tumor spread as evidenced by the clinical information presented earlier. Two pathways have been proposed for macrophages mediated lymphangiogenesis. These include secretion of pro-lymphangiogenic factors by the TAMs and trans-differentiation of TAMs into lymphatic vessel structures [56, 68]. In both human and experimental tumors, TAMs have been reported to express lymphatic vessel endothelial hyaluronan receptor, a major lymphatic vessel marker [67, 92]. In experimental models of prostate cancer and pancreatic insulinoma, such TAMs have been reported to directly integrate into peritumoral lymphatic vessels [67, 92]. Recent lineage tracing in dermal lymphatic vessel experiments support macrophages as regulator of lymphatic endothelial cell proliferation however do not support macrophages or myeloid cells as lymphatic endothelial progenitor cells [25]. Interestingly, a recent murine orthotropic lung carcinoma study showed that tumor derived TNFα activated TAMs to produce VEGF-C, and VEGF-C in turn induced lymphatic vessel tip cell formation, lymphatic endothelial cell proliferation, and migration [36]. This study demonstrates an inflammatory-mediated cross talk between tumor cells, TAMs, and lymphatic endothelial cells in promoting metastasis. Although it seems clear that TAMs influence lymphangiogenesis, it remains to be further determined whether they trans-differentiate into lymphatic endothelial cells in PDAC.
Epithelial-to-mesenchymal transition (EMT) is an important process via which primary tumors progress into metastatic state [5]. The tumor cells up regulate mesenchymal cell markers, lose intrinsic polarity, and lose cell-cell junctions by down regulating epithelial cell markers such as E-cadherin [16]. Macrophages contribute to metastasis by producing chemotactic and other factors as well as by promoting degradation of ECM barriers. More recently, in a co-culture system M2-polarized TAMs where shown to promote EMT phenotypic changes in pancreatic cancer cell lines Panc-1 and BxPC-3 [49]. This effect was mediated via macrophage TLR4 activation since knockdown of TLR4 or inhibition of TLR4/IL-10 signaling abolished the pancreatic cancer cells EMT. In addition to the role in EMT, TAMs have also been implicated to promote cancer stemness, a hallmark for cancer cells to self-renew, differentiate into tumor cells, migrate and regrow [57, 84]. Moreover, TAMs were shown to directly induce tumor-initiating cell properties in PDAC by enhancing STAT3 activation and targeting TAMs led to better chemotherapeutic responses [57].
Therapeutic Implication
As a result of macrophage involvement from tumor initiation to invasion and metastasis, there is a lot of interest in targeting TAMs. Many preclinical and experimental studies initially focused in depleting macrophages, but with more recent studies identifying functional and phenotypic heterogeneity among TAMs and differences in their micro environmental localization, therapeutic strategies that re-educate TAMs to those with anti-tumor responses have led to interesting results. A landmark study-using antibody based CD40 agonist showed beneficial effects in experimental models and patients with PDAC [85]. CD40 is expressed by many antigen-presenting cells (APCs) and the CD40 agonistic antibodies activate APCs and promote anti-tumor responses [79]. Commonly CD40 activation licenses dendritic cells in promoting cytotoxic T cell activity against tumors. The effect of agonistic CD40 antibody on PDAC highlighted important yet not previously well-appreciated generation of tumoricidal TAMs and activation of extratumoral macrophages that enhanced anti-tumor T cell responses [4, 85].
Histidine-rich glycoprotein (HRG), which is down regulated in tumors including in pancreas cancer as compared to normal tissues, was shown to support tumor progression and vascularization [7, 65]. Rolny and co-investigators showed a critical role for HRG in reprograming TAMs from pro-angiogenic and immunosuppressive M2 phenotype into TAMs with M1 marker expression and anti-tumor properties leading to inhibition of tumor growth and metastasis [65]. The investigators went on to show that HRG effect was mediated via a member of the VEGF family placenta growth factor (PIGF), where PIGF deletion in macrophages reproduced the anti-tumor and vascular normalization effects of HRG. Thus HRG offers a potential therapeutic target for reeducating TAMs and suppress tumor evasion.
As mentioned above CSF-1/CSF1R inhibition in experimental PDAC led not only to depletion of TAMs but also to re-education of residual TAMs that promoted antigen presentation and enhanced anti-tumor T cell response [91]. Similarly CCR2 inhibition decreased TAMs and tumor-initiating cells in pancreatic tumors, improved chemotherapeutic and anti-tumor T cell responses [57]. These experimental studies show that targeting TAMs in PDAC improves responses to T-cell checkpoint immunotherapy and likely to provide means to tackle therapeutic resistance mediated by the tumor.
Conclusion
Macrophages are a heterogeneous population with remarkable plasticity, as a result they become an important evasive target for tumors, where pro-inflammatory macrophages provide signals for tumor initiation and anti- and immunosuppressive-macrophages promote tumor growth and metastasis. Hence, macrophage polarization state and spatial localization within the tumor microenvironment influences tumor development and progression. Better understanding of these factors will have significant impact in macrophage directed therapies and means via which macrophages can be reprogramed to promote tumoricidal activities and suppress tumor escape.
Highlights.
Macrophages infiltrate pancreatic adenocarcinomas
Presence of tumor-associated macrophages (TAMs) correlates with poor prognosis
TAMs are involved in pancreas cancer development and progression
Targeting TAMs offers a novel therapy against pancreas cancer
Acknowledgments
This work was supported in part by the National Pancreas Foundation Grant, Department of Veterans Affairs, NIH DK092421, NIH P01CA163200 and, NIH P50 AA11999, and NIH K01AA019996.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Alderton GK. Metastasis. Exosomes drive premetastatic niche formation, Nature reviews. Cancer. 2012;12:447. doi: 10.1038/nrc3304. [DOI] [PubMed] [Google Scholar]
- 2.Allavena P, Chieppa M, Bianchi G, Solinas G, Fabbri M, Laskarin G, Mantovani A. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clinical & developmental immunology. 2010;2010:547179. doi: 10.1155/2010/547179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill F. Cancer and the chemokine network, Nature reviews. Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 4.Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, Vonderheide RH. Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages. Gastroenterology. 2015;149:201–210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biddle A, Mackenzie IC. Cancer stem cells and EMT in carcinoma. Cancer metastasis reviews. 2012 doi: 10.1007/s10555-012-9345-0. [DOI] [PubMed] [Google Scholar]
- 6.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 7.Blank M, Shoenfeld Y. Histidine-rich glycoprotein modulation of immune/autoimmune, vascular, and coagulation systems. Clinical reviews in allergy & immunology. 2008;34:307–312. doi: 10.1007/s12016-007-8058-6. [DOI] [PubMed] [Google Scholar]
- 8.Brat DJ, Lillemoe KD, Yeo CJ, Warfield PB, Hruban RH. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. The American journal of surgical pathology. 1998;22:163–169. doi: 10.1097/00000478-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Carriere C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4437–4442. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Cioffi M, Trabulo S, Hidalgo M, Costello E, Greenhalf W, Erkan M, Kleeff J, Sainz B, Jr, Heeschen C. Inhibition of CD47 Effectively Targets Pancreatic Cancer Stem Cells via Dual Mechanisms. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:2325–2337. doi: 10.1158/1078-0432.CCR-14-1399. [DOI] [PubMed] [Google Scholar]
- 12.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer research. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 13.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochimica et biophysica acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Coffelt SB, Scandurro AB. Tumors sound the alarmin(s) Cancer research. 2008;68:6482–6485. doi: 10.1158/0008-5472.CAN-08-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours, Nature reviews. Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 17.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature cell biology. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dal Molin M, Zhang M, de Wilde RF, Ottenhof NA, Rezaee N, Wolfgang CL, Blackford A, Vogelstein B, Kinzler KW, Papadopoulos N, Hruban RH, Maitra A, Wood LD. Very Long-term Survival Following Resection for Pancreatic Cancer Is Not Explained by Commonly Mutated Genes: Results of Whole-Exome Sequencing Analysis. Clin Cancer Res. 2015;21:1944–1950. doi: 10.1158/1078-0432.CCR-14-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De La OJ, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer research. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 21.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer research. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folias AE, Penaranda C, Su AL, Bluestone JA, Hebrok M. Aberrant innate immune activation following tissue injury impairs pancreatic regeneration. PloS one. 2014;9:e102125. doi: 10.1371/journal.pone.0102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardian K, Janczewska S, Olszewski WL, Durlik M. Analysis of pancreatic cancer microenvironment: role of macrophage infiltrates and growth factors expression. Journal of Cancer. 2012;3:285–291. doi: 10.7150/jca.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes & development. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, Harvey NL. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development. 2010;137:3899–3910. doi: 10.1242/dev.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunological reviews. 2014;262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity, Nature reviews. Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 28.Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 29.Helm O, Held-Feindt J, Grage-Griebenow E, Reiling N, Ungefroren H, Vogel I, Kruger U, Becker T, Ebsen M, Rocken C, Kabelitz D, Schafer H, Sebens S. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis, International journal of cancer. Journal international du cancer. 2014;135:843–861. doi: 10.1002/ijc.28736. [DOI] [PubMed] [Google Scholar]
- 30.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. Journal of translational medicine. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 32.Hong TM, Chen YL, Wu YY, Yuan A, Chao YC, Chung YC, Wu MH, Yang SC, Pan SH, Shih JY, Chan WK, Yang PC. Targeting neuropilin 1 as an antitumor strategy in lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4759–4768. doi: 10.1158/1078-0432.CCR-07-0001. [DOI] [PubMed] [Google Scholar]
- 33.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. The American journal of surgical pathology. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. British journal of cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H, Lim S, Nakamura M, Andersson P, Wang J, Sun Y, Dissing S, He X, Yang X, Cao Y. TNFR1 mediates TNF-alpha-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nature communications. 2014;5:4944. doi: 10.1038/ncomms5944. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer research. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JPt, Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, Sander M. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. The Journal of experimental medicine. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. The Journal of surgical research. 2011;167:e211–219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. The Journal of experimental medicine. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity, Nature reviews. Immunology. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 44.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. The Journal of pathology. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 45.Liang X, Li Q, Zhang Z, Jia C, Liu C, Yang Y, Cheng B. High Mobility Group Box 1 (HMGB1) is Associated with Progression and Poor Prognosis in Pancreatic Cancer. Journal of gastrointestinal & digestive system. 2014;4:1–7. [Google Scholar]
- 46.Liou GY, Doppler H, Necela B, Edenfield B, Zhang L, Dawson DW, Storz P. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer discovery. 2015;5:52–63. doi: 10.1158/2159-8290.CD-14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liou GY, Doppler H, Necela B, Krishna M, Crawford HC, Raimondo M, Storz P. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. The Journal of cell biology. 2013;202:563–577. doi: 10.1083/jcb.201301001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liou GY, Storz P. Inflammatory macrophages in pancreatic acinar cell metaplasia and initiation of pancreatic cancer. Oncoscience. 2015;2:247–251. doi: 10.18632/oncoscience.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Laboratory investigation; a journal of technical methods and pathology. 2013;93:844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 50.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer, Best practice & research. Clinical gastroenterology. 2006;20:211–226. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Mantovani A, Schioppa T, Biswas SK, Marchesi F, Allavena P, Sica A. Tumor-associated macrophages and dendritic cells as prototypic type II polarized myeloid populations. Tumori. 2003;89:459–468. doi: 10.1177/030089160308900501. [DOI] [PubMed] [Google Scholar]
- 52.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. Journal of immunology. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 55.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Frontiers in bioscience : a journal and virtual library. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 56.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. The Journal of clinical investigation. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer research. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma, Nature reviews. Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation, Nature reviews. Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer research. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 61.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. Journal of immunology. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 62.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends in immunology. 2015;36:229–239. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature medicine. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Akerud P, De Mol M, Salomaki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, Goedegebuure SP, Linehan DC. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, Demory A, Falkowska-Hansen B, Kurzen H, Ugurel S, Geginat G, Arnold B, Goerdt S. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. The Journal of pathology. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 68.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. The American journal of pathology. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature immunology. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 70.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation, Nature reviews. Immunology. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sica A, Saccani A, Mantovani A. Tumor-associated macrophages: a molecular perspective. International immunopharmacology. 2002;2:1045–1054. doi: 10.1016/s1567-5769(02)00064-4. [DOI] [PubMed] [Google Scholar]
- 72.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 73.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. Journal of leukocyte biology. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 74.Sugimoto M, Mitsunaga S, Yoshikawa K, Kato Y, Gotohda N, Takahashi S, Konishi M, Ikeda M, Kojima M, Ochiai A, Kaneko H. Prognostic impact of M2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas. Eur J Cancer. 2014;50:1900–1908. doi: 10.1016/j.ejca.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. Journal of immunology. 2011;187:3671–3682. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 76.Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, Ni B, Lu B, Wang H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PloS one. 2014;9:e91551. doi: 10.1371/journal.pone.0091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, Weissman IL. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Seminars in oncology. 2001;28:29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- 79.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wachsmann MB, Pop LM, Vitetta ES. Pancreatic ductal adenocarcinoma: a review of immunologic aspects. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2012;60:643–663. doi: 10.231/JIM.0b013e31824a4d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 82.Wang J, Ye Q, She QB. New insights into 4E-BP1-regulated translation in cancer progression and metastasis. Cancer cell & microenvironment. 2014;1 doi: 10.14800/ccm.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA: a cancer journal for clinicians. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu S, Chheda C, Ouhaddi Y, Benhaddou H, Bourhim M, Grippo PJ, Principe DR, Mascarinas E, DeCant B, Tsukamoto H, Pandol SJ, Edderkaoui M. Characterization of Mouse Models of Early Pancreatic Lesions Induced by Alcohol and Chronic Pancreatitis. Pancreas. 2015;44:882–887. doi: 10.1097/MPA.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu X, Han X, Liu L, Ji Y, Li J, Lou W. Clinical Analysis and Comprehensive Treatment of Nonfunctional Pancreatic Neuroendocrine Tumor With Liver Metastases: A Retrospective Single-Center Study. Pancreas. 2015;44:995–996. doi: 10.1097/MPA.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 86.Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, Habtezion A. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nature communications. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, Li M, Luo C, Yu S, Bian X, Feng H. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. Journal of neuroimmunology. 2011;232:75–82. doi: 10.1016/j.jneuroim.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Wang XF. A niche role for cancer exosomes in metastasis. Nature cell biology. 2015;17:709–711. doi: 10.1038/ncb3181. [DOI] [PubMed] [Google Scholar]
- 89.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230–1240. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 91.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer research. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PloS one. 2009;4:e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]


