Abstract
OBJECT
This report describes the stereotactic technique, hospitalization, and 90-day perioperative safety of bilateral deep brain stimulation (DBS) of the fornix in patients who underwent DBS for the treatment of mild, probable Alzheimer’s disease (AD).
METHODS
The ADvance Trial is a multicenter, 12-month, double-blind, randomized, controlled feasibility study being conducted to evaluate the safety, efficacy, and tolerability of DBS of the fornix in patients with mild, probable AD. Intra-operative and perioperative data were collected prospectively. All patients underwent postoperative MRI. Stereotactic analyses were performed in a blinded fashion by a single surgeon. Adverse events (AEs) were reported to an independent clinical events committee and adjudicated to determine the relationship between the AE and the study procedure.
RESULTS
Between June 6, 2012, and April 28, 2014, a total of 42 patients with mild, probable AD were treated with bilateral fornix DBS (mean age 68.2 ± 7.8 years; range 48.0–79.7 years; 23 men and 19 women). The mean planned target coordinates were x = 5.2 ± 1.0 mm (range 3.0–7.9 mm), y = 9.6 ± 0.9 mm (range 8.0–11.6 mm), z = −7.5 ± 1.2 mm (range −5.4 to −10.0 mm), and the mean postoperative stereotactic radial error on MRI was 1.5 ± 1.0 mm (range 0.2–4.0 mm). The mean length of hospitalization was 1.4 ± 0.8 days. Twenty-six (61.9%) patients experienced 64 AEs related to the study procedure, of which 7 were serious AEs experienced by 5 patients (11.9%). Four (9.5%) patients required return to surgery: 2 patients for explantation due to infection, 1 patient for lead repositioning, and 1 patient for chronic subdural hematoma. No patients experienced neurological deficits as a result of the study, and no deaths were reported.
CONCLUSIONS
Accurate targeting of DBS to the fornix without direct injury to it is feasible across surgeons and treatment centers. At 90 days after surgery, bilateral fornix DBS was well tolerated by patients with mild, probable AD.
Keywords: Alzheimer’s disease, deep brain stimulation, dementia, fornix, memory, functional neurosurgery
Alzheimer’s disease (AD) poses a significant threat to public health, and current treatment options have limited efficacy. New AD treatments are urgently needed. To this end, deep brain stimulation (DBS) of the fornix (DBS-f) was previously evaluated in a small pilot study.12 In the United States, DBS is approved by the US FDA as a therapy for Parkinson’s disease (PD) and essential tremor. The use of DBS has also been studied as a treatment for other neurological disorders, such as primary dystonia, obsessive-compulsive disorder, epilepsy, Tourette’s syndrome, pain, treatment-resistant depression, bipolar disorder, anorexia nervosa, addiction, traumatic brain injury, and obesity.
The ADvance Trial is a multicenter, 12-month, double-blind, randomized, controlled feasibility study designed to evaluate the safety, efficacy, and tolerability of DBS-f in patients with mild, probable AD. Patients are randomized to receive stimulation-on or stimulation-off in a double-blind manner for a period of 1 year. The primary end point of the ADvance Trial is to evaluate the safety of DBS-f, and the secondary end point evaluates the preliminary efficacy of therapy. This report describes the 90-day perioperative surgical experience of patients undergoing DBS-f, including surgical technique, stereotactic accuracy, hospital course, and adverse events (AEs) occurring within 90 days of surgery. This is the largest study reported to date on DBS performed for AD.
METHODS
Study Design and Objectives
The study design, methods, and informed consent were approved by the institutional review board overseeing each of the respective 7 participating sites. The study was registered with the clinicaltrials.gov database (registration no. NCT01608061).
All patients were enrolled and treated according to the ADvance study protocol. The inclusion criteria required that patients be between the ages of 45 and 85 years (inclusive) and have an Alzheimer’s Disease Assessment Scale cognitive subscale (ADAS-cog-11)16 score between 12 and 24 (inclusive).
Surgical Technique and Patient Monitoring
Patients underwent surgical implantation of DBS electrodes (model 3387; Medtronic, Inc.), which were inserted bilaterally through 2 bur holes, followed by the implantation of a dual-channel internal pulse generator (IPG) into the infraclavicular area (Activa PC, Medtronic, Inc.). These were connected via a lead extension (model 37085, Medtronic, Inc.). After surgery, stereotactic MRI was performed, and patients were admitted to the hospital. Patients were seen by the treating neurosurgeon at 2 weeks and again 6 to 12 weeks after the operation. In a separate setting, 2 weeks after the operation, monopolar review was carried out, followed by activation versus no activation of the device in a randomized, double-blind fashion, per the study design. All treating physicians and patients were blinded to the activation status of the IPG. Close clinical follow-up was maintained, as per the study protocol, beyond the perioperative neurosurgical clinic visits.
Stereotactic Analysis
Stereotactic analysis of all postoperative MRI scans was performed in a blinded fashion by a single rater. Both preoperative and postoperative MRI scan sets were imported into a stereotactic surgical planning software package (FrameLink, Medtronic, Inc.), computationally fused, and reformatted to produce images orthogonal to the anterior commissure–posterior commissure line and midsagittal plane.20 Data collected included the position of the deepest contact, the coronal and sagittal angles of the trajectory, the distance of the bur hole from the midline and target, the coordinates of the active contact and the distance of that contact from the fornix, lead and fornix coordinates at z = 0, lead and mammillary body coordinates at the level of the mammillary bodies, ventricular width, third ventricular width, skull width,4 and the sagittal angle of the column of the fornix. In addition, there were 54 leads for which the coordinates for the preoperative stereotactic plan were documented; for these leads, the vector and radial errors relative to the final lead placement (Fig. 1) were collected. Pearson’s correlation coefficients were used to measure the extent of the linear association between 2 variables.
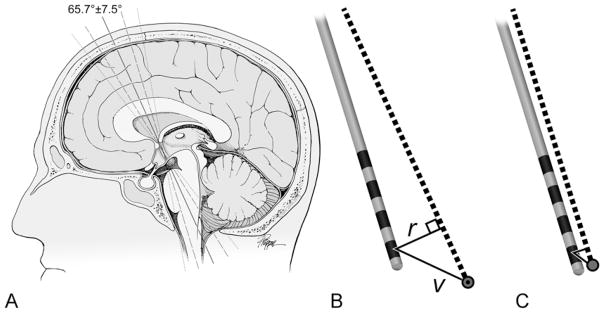
FIG. 1.
Stereotactic accuracy. A: The angles encountered for the descending columns of the fornix (the solid line indicates the mean angle, 65.7°; the dashed line indicates the standard deviation, ± 7.5°; the dotted line indicates the range, 52.2–79.6°). B: Calculation of the radial (r) and vector (v) errors. The intended trajectory (dotted line) and target (circle) are shown, with the distance from the distal-most contact to the intended target measured. C: To-scale drawing showing the mean radial and vector errors to the distal-most contact of the Medtronic 3387 (Medtronic, Inc.) DBS lead. The bands indicate the 4 contacts on the lead. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Patient Outcomes
AEs were recorded at each institution and presented to an independent clinical events committee for adjudication. Each AE was adjudicated for relatedness to the study, surgical procedure, IPG, leads, and programming. AEs were also categorized as general medical, psychiatric, surgical, or programming in nature. In addition, adjudications were made regarding whether an event was a serious AE (SAE) or an unanticipated adverse device effect. Typically, events requiring extended hospitalization, hospital readmission, or reoperation were considered to be SAEs.
RESULTS
Patients
Between June 6, 2012, and April 28, 2014, a total of 42 patients were enrolled who underwent surgery by 7 neurosurgeons at 6 participating hospitals. The mean age of these patients was 68.2 ± 7.8 years (Table 1), and the mean duration since the initial diagnosis of AD was 2.3 ± 1.7 years. The mean ADAS-cog-11 score was 16.9 ± 2.9.
TABLE 1.
Summary of patient demographics and incidence of adjudicated AEs within 90 days of surgery*
| Factor | Trial Site | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Toronto | BAI | Brown | UF | JH | BSHRI | Penn | ||
| No. of patients | 12 | 9 | 6 | 5 | 4 | 4 | 2 | 42 |
| Sex (no. of patients) | ||||||||
| Female | 5 | 4 | 2 | 3 | 2 | 1 | 2 | 19 |
| Male | 7 | 5 | 4 | 3 | 2 | 3 | 0 | 23 |
| Age (yrs) | 68.7 ± 6.8 (52.9–77.1) | 71.1 ± 3.0 (66.7–74.9) | 75.3 ± 3.2 (71.6–79.7) | 62.7 ± 6.7 (57.1–72.9) | 60.3 ± 10.2 (48.0–72.7) | 64.8 ± 12.1 (51.1–78.0) | 66.1 ± 10.6 (58.6–73.6) | 68.2 ± 7.8 (48.0–79.7) |
| Duration of surgery (hrs) | 4.6 ± 1.7 | 2.7 ± 0.6 | 4.5 ± 0.3 | 6.4 ± 1.6 | 4.5 ± 0.7 | 2.6 ± 0.5 | 3.3 ± 0.4 | 4.1 ± 1.6 |
| Duration of hospitalization (days) | 1.3 ± 0.6 | 1.0 ± 0.0 | 1.2 ± 0.4 | 1.2 ± 0.4 | 3.3 ± 0.5 | 1.0 ± 0.0 | 2.0 ± 0.0 | 1.4 ± 0.8 |
| Interval from diagnosis (yrs) | 2.6 ± 1.2 (0.7–4.4) | 0.9 ± 0.8 (0.0–2.8) | 4.1 ± 1.5 (1.5–5.9) | 2.6 ± 2.1 (0.4–5.6) | 2.6 ± 2.5 (0.3–5.9) | 1.6 ± 1.5 (0.4–3.8) | 2.1 ± 2.0 (0.6–3.5) | 2.3 ± 1.7 (0.0–5.9) |
| No. of AEs | 8 | 17 | 20 | 2 | 6 | 2 | 9 | 64 |
| No. of SAEs | 2 | 1 | 1 | 1 | 2 | 0 | 0 | 7 |
BAI = Banner Alzheimer’s Institute; Brown = Brown University; BSHRI = Banner Sun Health Research Institute; JH = Johns Hopkins Bayview; Penn = Hospital of the University of Pennsylvania, Penn Memory Clinic; Toronto = Toronto Western Hospital; UF = University of Florida–Gainesville.
Data are presented as the mean ± SD (range), unless otherwise noted.
Operative Data
Stereotactic Targeting
Stereotactic planning was performed on a surgical navigation station. Direct targeting methods were used, based on visualization of the descending columns of the fornix on MRI. A transventricular trajectory was necessary, and planning took into account the orientation of the fornix, location of the optic tracts, and avoidance of the sulci and blood vessels. The electrodes exited the ventricles above the midcommissural plane and passed posterior to the anterior commissure, from which point the intended trajectory ran parallel and approximately 2 mm anterior to the columns of the fornix, traversing the hypothalamus and ending posteromedial to the optic tracts (Fig. 2).
FIG. 2.
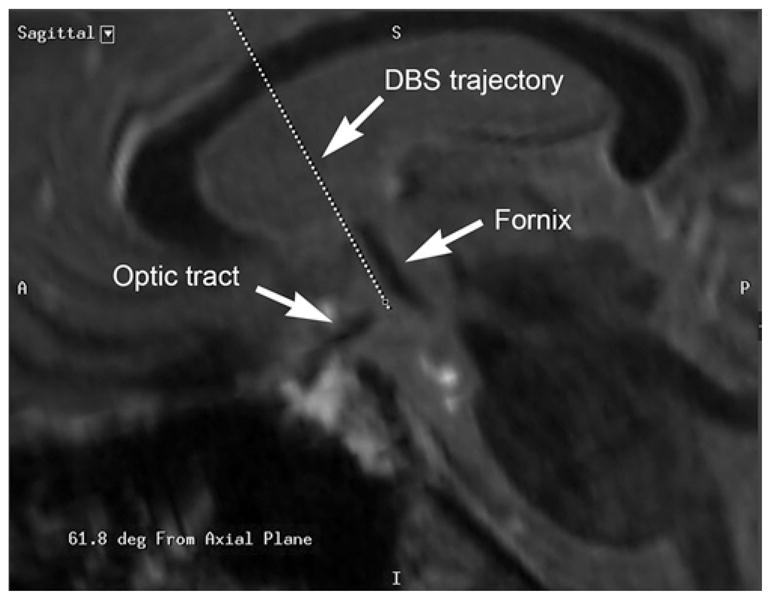
Example of the stereotactic plan for targeting the fornix. The trajectory (dotted line) passes approximately 2 mm anterior to the anterior border of the fornix. A = anterior; DBS = deep brain stimulation; deg = degrees; I = inferior; P = posterior; S = superior.
The mean coordinates of the planned target relative to the midcommissural point were x = 5.2 ± 1.0 mm (range 3.0–7.9 mm), y = 9.6 ± 0.9 mm (range 8.0–11.6 mm), and z = −7.5 ± 1.2 mm (range −5.4 to −10.0 mm). (For the purpose of clarity, the left- and right-sided electrodes are combined for this analysis, and the x coordinates are listed as positive values. However, in the Cartesian coordinate system used for stereotactic planning and analysis, the points on the left side of midline are assigned a negative value and the points on the right are assigned a positive value.) The mean coronal angle was 12.5 ± 4.6° (range 1.7°–26.5°) and the mean sagittal angle was 67.9 ± 8.5° (range 41.7°–82.0°). The mean distance from the midline of the bur hole was 21.5 mm, and distance to the target from the outer skull was 83.7 mm.
Operative Technique and Duration
The DBS electrodes were placed using standard frame-based stereotactic methods (Leksell [Elekta AB], n = 37; CRW [Integra Radionics, Inc.], n = 5), followed by the placement of the IPG under general anesthesia. Because of the proximity of the ventricle ventral to the contacts, guide cannulas were placed to the target and subsequently raised to expose the electrodes for intraoperative test stimulation. Testing was performed in a monopolar or bipolar configuration at a frequency of 130 Hz and a pulse width of 60 μsec, with the voltage increased slowly up to 7 V or until side effects occurred. The observed AEs included autonomic phenomena and cognitive effects. All patients experienced autonomic AEs, which consisted of increased blood pressure and heart rate in response to stimulation. Experiential phenomena, similar to those previously reported,12 were observed in 3 patients. Intraoperative imaging consisted ofanteroposteriorandlateralfluoroscopy. One lead was repositioned intraoperatively, due to deflection of the cannula into the third ventricle. The mean total operative time, including lead and battery placement, was 4.1 ± 1.6 hours (range 2.1–8.5 hours).
Postoperative Imaging and Stereotactic Accuracy
The mean coordinates of the targeted contact (i.e., contact 0) on postoperative MRI was x = 4.4 ± 1.1, y = 9.8 ± 1.8, and z = −7.2 ± 1.5. Relative to the stereotactic plan, the mean vector error was 2.2 ± 1.2 mm (range 0.4–6.3 mm) and the mean radial error was 1.5 ± 1.0 mm (range 0.2–4.0 mm). There was no statistically significant difference in error between the left- and right-sided leads (vector error, p = 0.98; radial error, p = 0.89).
At the midcommissural plane (i.e., z = 0), the mean coordinates of the electrodes were x = 5.8 mm and y = 12.4 mm, and the mean coordinates of the fornix were x = 4.9 mm and y = 10.5 mm. The mean distance from the center of the lead to the center of the fornix at the midcommissural plane was therefore 2.7 ± 1.0 mm. The tip of the electrode was typically near the plane of the mammillary bodies. The mean coordinates for the mammillary bodies were x = 2.8, y = 3.5, and z = −7.2; at the plane of the mammillary bodies, the coordinates of the lead were x = 4.5, y = 9.8, and z = −7.2. Therefore, the mean distance between the electrode and the mammillary bodies was 6.8 ± 1.5 mm.
Anatomical Considerations
The mean intercommissural distance was 25.9 ± 1.7 mm (range 22.2–29.4 mm). The mean third ventricular width was 8.5 ± 2.5 mm (range 3.8 –13.6 mm), and the bicaudate ratio was 0.19 ± 0.03 (range 0.13 –0.26). There was no significant correlation between stereotactic error (radial or vector) versus atrophy (third ventricular width or bicaudate ratio) (r2 ≤ 0.0124 for all 4 comparisons). The mean angle of the fornix in the sagittal plane was 65.7° ± 7.5° (range 52.2°–79.6°; Fig. 1A). The difference between the mean sagittal angles of the fornix and of the lead was 6.8 ± 5.5° (range 0°–25.5°).
Postoperative Course
Hospitalization
All patients were admitted to the hospital for at least 1 night. In this cohort, the mean interval from the end of surgery to discharge from the hospital was 1.4 ± 0.8 days. One patient underwent prolonged hospitalization due to headaches on postoperative Day 1, followed by nausea and vomiting on postoperative Day 2. All patients were discharged home under the care of their families.
Active Contacts
Programming was set after monopolar review and documentation of thresholds and description of AEs. On the basis of this and the surgeon’s input regarding the closest contact to the fornix on postoperative imaging, a clinician who was not blind to the programming would designate an active contact. This was Contact 2 (i.e., the third contact from the tip) in 79.2% of leads, Contact 1 in 11.0% of leads, and Contact 3 in 9.8% of leads. The mean coordinates relative to the midcommissural point of the active contact were x = 5.6 ± 1.2 mm, y = 12 ± 1.6 mm, and z = −1.5 ± 1.4 mm. These were on average 1.8 ± 0.9 mm (range 0–3.8) away from the fornix.
AEs Within 90 Days
Before adjudication, 32 (76.2%) patients were reported to have 131 AEs within 90 days of surgery. After adjudication, 26 (61.9%) patients were found to have 64 AEs likely related to the implant procedure (Table 2). Nonrelated events typically included worsening or fluctuation of pre-existing problems (such as arthritis pain or baseline mood disorder), early reoperation for unrelated disease (e.g., cataract surgery or dental procedures), new diagnoses not likely related to AD (e.g., basal cell carcinoma), unrelated traumatic injury (including orthopedic fractures and lacerations), infectious illness unlikely to be nosocomial (e.g., influenza), unexpected laboratory findings that could not be mechanistically linked to the procedure (e.g., hypo- or hyperglycemia), or the expected normal sequelae of DBS-f surgery (e.g., sensation of some battery movement or experiential phenomena).
TABLE 2.
Description of 64 adjudicated AEs occurring in 26 patients in the ADvance trial
| AE | No. of Patients |
|---|---|
| Dermatological | |
| Bruising, left forehead and cheek | 1 |
| Contact dermatitis | 1 |
| Rash | 1 |
| Fatigue | |
| Fatigue | 5 |
| Increased fatigue | 1 |
| Gastrointestinal | |
| Nausea | 2 |
| Nausea resulting in prolonged hospitalization* | 1 |
| Nausea and vomiting | 1 |
| Vomiting | 2 |
| Genitourinary | |
| Urinary retention | 3 |
| Headache or other pain | |
| Headache | 11 |
| Headache, nausea, and vomiting* | 1 |
| Left mastoid pain | 1 |
| Left neck discomfort | 1 |
| Neck/shoulder pain | 1 |
| Major surgical site | |
| Bilateral subacute & chronic subdural hematoma* | 1 |
| IPG infection | 3 |
| Left electrode lead misplacement* | 1 |
| Mental status change | |
| Confusion | 2 |
| Delirium | 2 |
| Depressed mood | 1 |
| Minor surgical site | |
| Fluid collection | 1 |
| Neck discomfort due to pulling of extensions | 1 |
| Redness of the left frontal incision w/o drainage or swelling | 1 |
| Right periorbital edema | 2 |
| Small pustule at right chest near surgical scar | 1 |
| Surgical site pain in right chest | 1 |
| Swelling at suture behind right ear w/ pain | 1 |
| Swelling of left eyelid | 1 |
| Bilateral eyelid swelling | 1 |
| Extraaxial collection | 1 |
| Inflammation at IPG site | 1 |
| Left cerebral convexity subdural hematoma | 1 |
| Pink skin behind right ear | 1 |
| Pinkish discharge | 1 |
| Possible infection at surgical site | 1 |
| Rash on right chest around incision | 1 |
| Small right intracerebral hemorrhage | 1 |
| Surgical pain | 1 |
| Swelling & redness | 1 |
| Trauma | |
| Fall | 1 |
SAE.
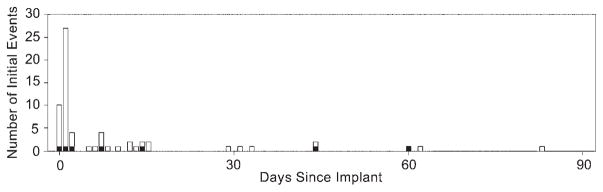
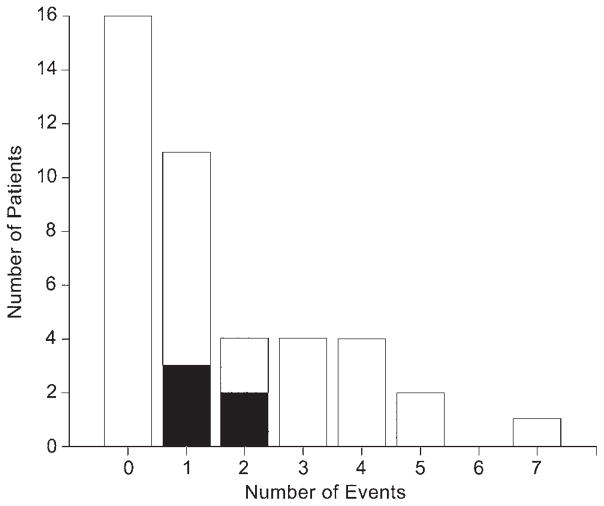
Of the 64 AEs related to the procedure, 57 (89.1%) occurred within 30 days of implant (Fig. 3). Sixteen of 42 patients (38.1%) had no AEs reported. Eleven of 42 patients (26.2%) experienced 1 AE, and the remaining 15 (35.7%) experienced more than 1 AE (Fig. 4).
FIG. 3.
Chronological occurrence of adjudicated AEs in the ADvance Trial. Black indicates serious AEs.
FIG. 4.
Number of patients experiencing multiple AEs. Black indicates serious AEs.
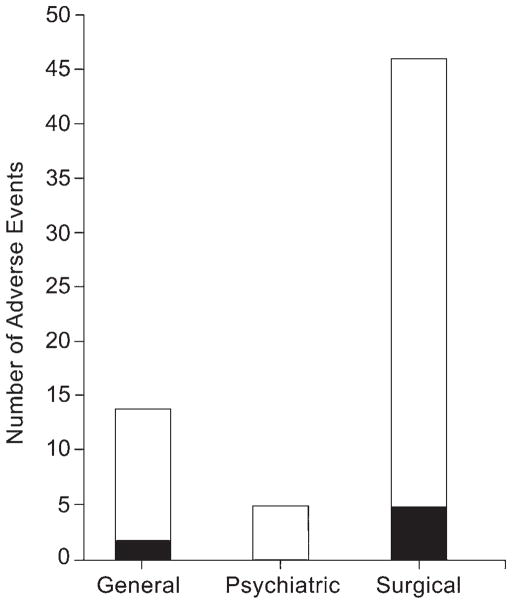
Most AEs (46 of 64; 71.9%) were directly related to the operative procedure itself (Fig. 5). General AEs (14 of 64; 21.9%) included potentially nosocomial infections (such as early postoperative urinary tract infections), contact dermatitis (likely due to surgical adhesives), headache, nausea, and other medical problems. Psychiatric events (such as postoperative delirium or confusion) accounted for 4 of the 64 AEs (6.3%).
FIG. 5.
Classification and number of AEs.Black indicates serious AEs.
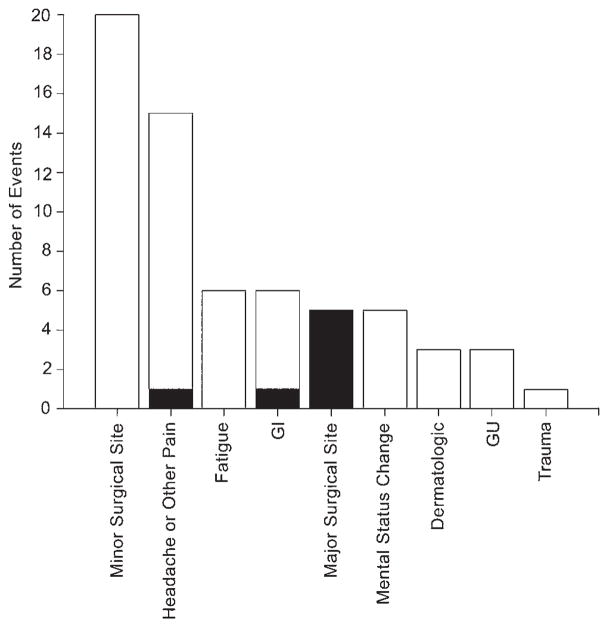
The specific categories most frequently associated with AEs consisted of findings related to the surgical sites (Fig. 6): 20 of 64 (31.3%) AEs were determined to be “minor” and related to the surgical site (including transient erythema or swelling; discomfort due to implanted hardware; and, in 1 case, a small, nonoperative, incidentally identified intracerebral hematoma); 5 of 64 (7.8%) AEs determined to be “serious” were related to the surgical site (see below). The next most common AE (15 of 64; 23.4%) was headache and/or other pain, which is frequently associated with neurosurgery in general.
FIG. 6.
Rates of occurrence of specific AEs. Black indicates serious adverse events. GI = gastrointestinal; GU = genitourinary.
Five patients (11.9%) experienced 7 SAEs (Table 2; Fig. 6). Four (9.5%) patients required a return to surgery: 2 of 3 patients who had IPG infections required explantation (the third patient was treated with oral antibiotics), 1 patient required repositioning of a misplaced electrode lead, and 1 patient was treated for chronic subdural hematoma. The remaining 2 SAEs consisted of 2 episodes of headache and/or nausea and vomiting in a single patient who required 2 extra nights of hospitalization. The mean interval from initial surgery to the 7 serious AEs was 18.3 days (range 0–60 days). No programming-related AEs or unanticipated adverse device effects were reported. There were no reported neurological deficits, and no instances of mortality in the study population.
Readmissions
Hardware Infection
One patient presented with signs of infection around the IPG site starting at postoperative Day 7, and the device was immediately explanted. Two days later, after further observation and the return of the laboratory results, the remaining components of the system (extensions and leads) were also explanted. This patient then successfully had an IPG reimplanted 2 months later. Another patient presented with signs of infection at the IPG site at 14 days postoperatively and underwent washout without explantation. This patient subsequently presented with a chest abscess at 87 days after the initial implant, at which point complete explantation was performed.
Hematoma
One patient presented 60 days postoperatively, after a ground-level fall that caused a clavicle fracture, and was found to have bilateral chronic subdural hematomas with mass effect. Along the parasagittal frontal lobe, which was the entry point for the DBS lead, about 1 cm of cerebrospinal fluid was present between the skull and the gyrus, and the patient had marked pneumocephalus postoperatively. The hematomas were believed to be a consequence of the pneumocephalus, and were surgically drained via bilateral bur holes. A CT scan of the head (obtained as part of the 6-month PET study included in the full ADvance Trial) was obtained 4.4 months after the hematoma evacuation and demonstrated the complete resolution of the hematoma.
Lead Repositioning
One lead was determined to be inappropriately positioned relative to the fornix on postoperative imaging, and the patient was readmitted 7 days later for uncomplicated lead repositioning.
DISCUSSION
The purpose of the ADvance Trial is to evaluate the feasibility of using DBS-f to treat patients with AD. In this multicenter, double-blind, randomized, controlled study, the descending limbs of the fornix are targeted for electrical stimulation in patients with mild, probable AD.
The rationale for this therapeutic approach is 2-fold: 1) there is an absence of promising pharmacological options for patients with AD,10 but 2) there are prior pilot data evaluating DBS-f.12 The pilot study that preceded the ADvance Trial suggested a possible slowing of cognitive decline in some patients, as well as increased glucose metabolism in the parietal and temporal lobes that persisted after 1 year of DBS-f, in contrast to the decreased metabolism that is typically observed in AD patients.19 More recently, it has been reported that 2 patients from the pilot study demonstrated an unprecedented reversal of the progressive hippocampal atrophy that is characteristic of AD.17
More than 140,000 patients have been implanted with DBS systems worldwide for other indications (http://professional.medtronic.com/pt/neuro/dbs-md/prod/index.htm). DBS-f consists of the use of an FDA-approved medical device in an off-label manner. The surgical technique for DBS-f in AD patients is nearly identical to that used for other applications of DBS. Unique to DBS-f are the brain locations where the electrodes are placed (i.e., the target), the purpose of electrical stimulation for intraoperative testing (i.e., autonomic side effects and possible experiential phenomena), and the patient population (i.e., AD patients). The safety profile of DBS surgery when used for other indications has been well characterized and can be used for comparison with the experience reported here.
Stereotactic Targeting
Some technical considerations are specific to DBS-f. First, stereotactic targeting for this procedure is performed via direct visualization of the fornix. Placing a cannula such that it ends 10 to 20 mm above target, as is often done with DBS in order to leave the electrodes exposed for intraoperative testing, would leave the end of the cannula within the ventricle, potentially allowing the bare electrode to deflect off the wall of the ventricle and thus miss the target. Therefore, in this study, the cannulas were typically placed fully on the target.
Second, whereas stereotactic targeting typically has the goal of placing the electrode into the targeted structure, such an approach with the fornix would run the risk of injuring the fornix and worsening cognitive impairment. Therefore, careful attention must be paid during planning to avoid the fornix, yet nevertheless the fornix must be within sufficiently close proximity to the electrodes to be within the field produced by the electrical stimulation. Microlesion effect, which is seen with DBS of the ventral intermediate nucleus, globus pallidus pars interna, or subthalamic nucleus, is to be avoided in DBS-f. In our experience, there was no radiographic evidence of fornix damage and there were no acute postoperative cognitive changes suggestive of injury to the fornix.
Third, the ventral-medial to dorsal-lateral trajectory of the descending columns of the fornix is different from the ventral-lateral to dorsal-medial trajectory of DBS electrodes. While the trajectories on sagittal imaging appear parallel, they are actually skewed and this demands a more nuanced approach to targeting in order to optimize the proximity of the 10.5-mm span of the DBS contacts to the fornix. Furthermore, as noted, the surgical plan requires a transventricular approach, which is further addressed below.
Intraoperative Test Stimulation
DBS surgery offers the opportunity for intraoperative test stimulation. For example, with essential tremor, a lead can be repositioned if tremor arrest is not produced or if the side effects are intolerable during test stimulation. Consistently throughout this trial, activation of the distal contacts resulted in autonomic AEs such as tachycardia and hypertension. This served as one form of validation that the electrodes were intraparenchymal (as opposed to intraventricular) and within the hypothalamus. This maneuver is not without risk and could conceivably increase the risk of hemorrhage or affect a patient’s cardiac status; however, these events were not observed in our cohort.
Safety Considerations With DBS-f
When compared with the safety profile of DBS for PD, the safety profile of DBS-f in the ADvance Trial was noteworthy in part because of 3 factors: 1) surgery was performed on patients with dementia; 2) simultaneous bilateral electrodes were placed; and 3) a transventricular trajectory was used. These 3 factors may increase the risk of DBS-f compared with DBS of other targets, and they have been regarded as risk factors in the setting of DBS for PD.
Regarding the first factor, patients with PD who are considering DBS typically undergo screening by a neuropsychologist, and patients who demonstrate significant cognitive decline are typically not considered good surgical candidates.11,13,15 Mild cognitive impairment is frequently diagnosed during this preoperative workup, and it becomes an area to address when counseling patients and families on the risks and cognitive side effects of DBS.
Significant cognitive impairment is typically regarded as a relative contraindication to DBS surgery. Nevertheless, in our cohort of patients with early-stage, probable AD, there were relatively few reports of mental status change in the postoperative period. Only 4 instances of confusion or delirium were reported, and these were transient. This surprisingly low incidence may have been related to the early stage of the disease at which patients underwent implantation, or perhaps to factors related to this specific target and trajectory. Alternatively, delirium may have been underreported because active delirium screening was not done. Longer term assessment of potential impairment is underway.
Another potentially relevant feature of our patient population is the degree of cerebral atrophy, which is typically greater in patients with AD than in other patients undergoing DBS. Cerebral atrophy could impair accurate stereotactic targeting because a smaller ratio of cerebrum to cranium could allow for greater “brain shift” once bur holes are made to allow cerebrospinal fluid to escape and air to enter. However, stereotactic accuracy in this study was comparable with that reported for other patient populations.5
In contrast to the placement of simultaneous bilateral electrodes in our study, many centers stage the placement of electrodes into the left and right cerebral hemispheres. Staging may be recommended due to concerns about safety,8 brain shift that may result in stereotactic error,14 or cognitive AEs, with the latter being particularly relevant in the setting of elderly patients or patients with mild cognitive impairment. No difference in accuracy between the right and left leads was noted.
A transventricular trajectory was used in our patients; however, there has been a growing consensus that the ventricles should be avoided in DBS surgery for reasons related to accuracy as well as safety,23 the latter because a transventricular approach entails the additional penetration of 2 ependymal surfaces. Ben-Haim et al.2 reported that traversing the ventricle contributed significantly to the overall hemorrhage rate in 246 microelectrode-guided lead implantations, while Kramer et al.9 recently reported a comparison of 46 patients with a transventricular trajectory to 19 patients with no transventricular trajectory that identified no significant difference in the incidence of intraventricular hemorrhage or the number of microelectrode trajectory attempts. In addition to the fornix, other DBS targets, such as the pedunculopontine nucleus and the anterior nucleus of the thalamus, require a transventricular approach, and electrode placements have been performed safely without intraventricular hemorrhage.7,21,24 There were no intraventricular hematomas in the present cohort of 42 patients and 84 ventricular penetrations. On the basis of our experience, we believe that the risk attributed to ventricular penetration with other DBS procedures may be overstated in the medical literature.
Overall, these specific factors did not appear to contribute to the overall morbidity of patients undergoing DBS-f. This may be due to strict screening for healthy patients, as is typical in such trials, or it may suggest a low or nonexistent incremental risk associated with simultaneous bilateral electrode placement, transventricular trajectories, or the performance of DBS-f in patients who are mildly cognitively impaired.
AEs
The complication rates associated with DBS surgery include symptomatic hemorrhage (1.1%–1.4%), wound infection (1.7%–8%), and hardware failure (1.5%–36%).3,6,18 Less common complications include seizure, delirium/ psychosis, venous air embolism, and ischemic stroke. Serious AEs occurred in 11.9% (5 of 42) of the patients in this study, which is consistent with rates reported in other DBS trials.8,22
Because AD is characterized by marked, progressive brain atrophy, the patient population in this study would be expected to have more atrophy than others who might undergo DBS surgery. Cerebral atrophy is generally considered a risk factor for DBS surgery, and the degree of brain atrophy is typically reviewed as part of the preoperative workup. The mean bicaudate ratio reported in this study, 0.19 ± 0.03, is slightly larger than the ratio of 0.16 ± 0.02 reported by Brickman et al. 4 for a cohort of 84 patients with probable AD. (A higher bicaudate ratio suggests a greater degree of cerebral atrophy; in the study by Aylward et al.,1 the mean bicaudate ratio for normal controls was 0.09 ± 0.02.) A greater volume of extraaxial space could potentially contribute to a greater risk of procedure-related subdural hematoma, whether due to the stretching of bridging veins across this space or less resilience of the atrophic brain to reexpand in the event of postoperative pneumocephalus. Certain other risks would not be expected to differ in this procedure compared with other DBS procedures. For example, the risk of infection seems unlikely to be modified in this setting. Nevertheless, the AD patient population is potentially different from the PD, essential tremor, or dystonia populations in ways that are currently unappreciated yet relevant to infection risk.
Limitations and Future Directions
Discussing the potential therapeutic benefit of DBS-f for patients with AD is beyond the scope of the present report. In the context of the ADvance Trial, these data will remain unknown until the last patient completes the 1-year, double-blind, stimulation-versus-sham phase of the study. The primary purpose of this report is to describe the surgical and technical aspects of DBS-f, as well as the safety profile in this specific cohort of patients with mild probable AD.
In light of the relatively small size of this study, no conclusive risk-related statements can be made at this time. Nonetheless, the surgical experience reported here can inform future studies that aim to assess DBS for the treatment of AD. These data do suggest that transventricular surgery can be performed safely in this cohort of patients with dementia without causing surgical damage to the fornix.
There may be other risks specific to the application of electrical stimulation to this region of the brain, such as alterations that affect cognition or perception. However, because the study remains in a blinded phase, the attribution of any functional derangement to stimulation cannot yet be made; such an assessment cannot be made until it is known which patients were assigned to the “on-stimulation” state.
The mechanism by which DBS-f may enhance cognition is unknown. For other conditions, the effect of electrical stimulation is not to reverse the primary disease process or modify the natural history, but rather to modulate neural circuits in such a way as to functionally compensate for the deficits resulting from that pathology. In addition to clinical outcomes, data being collected for the ADvance Trial include structural and metabolic imaging that may contribute to our mechanistic understanding of the disease.
CONCLUSIONS
In the ADvance Trial, patients with mild, probable AD underwent bilateral DBS-f electrode placement via a transventricular approach, followed by the placement of an IPG. The perioperative course was similar to that seen in DBS trials for PD, including the incidence of complications. Up to postoperative Day 90, there was no evidence of permanent neurological morbidity and no instances of mortality. This experience suggests that bilateral DBS-f can be performed safely and is well tolerated in this population. The safety and efficacy of 1-year electrical stimulation in this patient cohort remain to be evaluated.
Acknowledgments
The ADvance Trial is supported by grant from the National Institutes of Health’s National Institute on Aging (R01AG042165) and a vendor grant received from Functional Neuromodulation, Ltd., the sponsor of the ADvance Trial. The Neuroscience Publications office of Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona, assisted with the editing and preparation of this manuscript.
ABBREVIATIONS
- ADAS-cog-11
Alzheimer’s Disease Assessment Scale cognitive subscale
- AD
Alzheimer’s disease
- AE
adverse event
- DBS
deep brain stimulation
- DBS-f
deep brain stimulation of the fornix
- IPG
internal pulse generator
- PD
Parkinson’s disease
- SAE
serious adverse event
APPENDIX
The ADvance Trial team includes Todd Langevin, Lisa Fosdick, Kristen Drake, Donald E. Reymers, Robyn Moxon, Dan O’Connell, Vince Owens, Cara Pendergrass, Susan Klees, Steven D. Targum, and the 7 participating clinical trial sites listed below.
Chair’s Office at Johns Hopkins University and University of Toronto: Constantine G. Lyketsos, MD, MHS, Co-Principal Investigator, Elizabeth Plank Althouse Professor and Chair of Psychiatry at Johns Hopkins Bayview; Andres Lozano, MD, PhD, FRCSC, FACS, Co-Principal Investigator, Professor, and Chair of Neurosurgery, Tasker Chair of Functional Neurosurgery; Gwenn Smith, PhD, Imaging Core Director, Professor of Psychiatry and Behavioral Sciences, Johns Hopkins University; Cynthia Munro, PhD, Neuropsychologist, Associate Professor of Psychiatry and Behavioral Sciences, Johns Hopkins University; Esther Oh, MD, Medical Monitor, Assistant Professor of Geriatric Medicine, Johns Hopkins University; Jeannie Sheppard Leoutsakos, PhD, Data Core Leader, Assistant Professor of Psychiatry and Behavioral Sciences, Johns Hopkins University.
Clinical Trial Sites
Johns Hopkins University School of Medicine, Baltimore, MD: Paul Rosenberg, MD, Associate Professor, Associate Director, Memory and Alzheimer’s Treatment Center; William S. Anderson, MD, PhD, Associate Professor of Neurosurgery
University of Toronto/Toronto Western Hospital: Andres Lozano, MD, PhD, FRCSC, FACS, Professor of Neurosurgery, Tasker Chair of Functional Neurosurgery; David Tang-Wai, MDCM, Assistant Professor of Neurology.
Banner Alzheimer’s Institute, Phoenix: Anna Burke, MD, Geriatric Psychiatrist, Dementia Specialist; Francisco Ponce, MD, Associate Professor of Neurosurgery, Director, Barrow Center for Neuromodulation, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center.
Banner Sun Health Research Institute, Sun City: Marwan Sabbagh, MD, Director, Banner Sun Health Research Institute; Francisco Ponce, MD, Associate Professor of Neurosurgery, Director, Barrow Center for Neuromodulation, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center.
Brown University, Rhode Island Hospital, Butler Hospital: Stephen Salloway, MD, MS, Professor of Neurology, Director of Neurology and Memory and Aging Program; Rees Cosgrove, MD, PhD, Chair of Neurosurgery; Wael Asaad, MD/PhD, Assistant Professor of Neurosurgery.
University of Florida–Gainesville: Michael S. Okun, MD, Professor of Neurology, Co-Director, Center for Movement Disorders and Neurorestoration; Kelly Foote, MD, Professor of Neurosurgery, Co-Director, Center for Movement Disorders and Neurorestoration.
University of Pennsylvania: David Wolk, MD, Associate Professor of Neurology, Assistant Director, Penn Memory Center; Gordon Baltuch, MD, PhD, Professor of Neurosurgery, Director Center for Functional and Neurorestorative Neurosurgery.
Footnotes
Disclosures
The authors state the following. Dr. Ponce is a consultant for Medtronic, Inc., and has had travel expenses reimbursed from Functional Neuromodulation, Ltd. Dr. Asaad has had travel expenses reimbursed from Medtronic, Inc., and Functional Neuromodulation, Ltd. Dr. Foote has received research support, fellowship support, and an honorarium for moderating a deep brain stimulation practitioners’ meeting from Medtronic, Inc. He has received research support and consultant fees from NeuroPace, Inc. He has received research support from St. Jude Medical, Inc., Boston Scientific Corp., National Institutes of Health, National Institute on Aging, and Functional Neuromodulation, Ltd. Dr. Anderson has received funding support from the National Institutes of Health (1R01AG042165-01A1 PAR-11-100). Dr. Beasley has served as a consultant on neuronavigation for Functional Neuromodulation, Ltd. and Medtronic, Inc. Dr. Smith is supported by the National Institutes of Health/National Institute on Aging/National Institute of Mental Health (AG038893, AG041633, MH 086881, AG042165, AG037298). Dr. Lyketsos has received grant support from the National Institute of Mental Health, the National Institute on Aging, The Associated: Jewish Community Federation of Baltimore, Weinberg Foundation, Forest Laboratories, Inc., GlaxoSmithKline, Eisai Co., Ltd., Pfizer, Inc., AstraZeneca, Eli Lilly and Co., Ortho-McNeil-Janssen Pharmaceuticals, Inc., Bristol-Myers Squibb Co., Novartis AG, National Football League, Elan Pharmaceuticals, and Functional Neuromodulation, Ltd. He has received payment as consultant or advisor from AstraZeneca, GlaxoSmithKline, Eisai Co., Ltd., Novartis AG, Forest Laboratories, Inc., Supernus Pharmaceuticals, Inc., Adlyfe, Inc., Takeda Pharmaceutical Co., Ltd., Wyeth Pharmaceuticals, H. Lundbeck A/S, Merz Pharma, Eli Lilly and Co., Pfizer, Inc., Genentech, Elan Pharmaceuticals, NFL Players Association, NFL Benefits Office, Avanir Pharmaceuticals, Inc., Zinfandel Pharmaceuticals, Inc., Bristol-Myers Squibb Co., AbbVie, Inc., Janssen Pharmaceuticals, Inc., Orion Corp., Otsuka Pharmaceutical Co., Ltd., Servier, and Astellas Pharma, Inc. He has received honoraria or travel support from Pfizer, Inc., Forest Laboratories, Inc., GlaxoSmithKline, Health Monitor. Dr. Lozano is a co-founder of Functional Neuromodulation, Ltd., holds intellectual property in the field of deep brain stimulation, and works as a consultant for Medtronic, Boston Scientific, and St. Jude. Dr. Reymers is a consultant for Medtronic, Inc., and has a financial relationship with Functional Neuromodulation, Ltd.
Author Contributions
Conception and design: Ponce, Asaad, Lyketsos, Lozano. Acquisition of data: Ponce, Asaad, Beasley. Analysis and interpretation of data: Ponce, Asaad, Oh, Smith, Lyketsos, Lozano. Drafting the article: Ponce, Asaad, Lyketsos, Lozano. Critically revising the article: all authors. Reviewed submitted version of manuscript: Ponce, Asaad. Statistical analysis: Ponce, Asaad. Administrative/ technical/material support: Beasley, Reymers. Study supervision: Targum, Smith, Lyketsos, Lozano.
References
- 1.Aylward EH, Schwartz J, Machlin S, Pearlson G. Bicaudate ratio as a measure of caudate volume on MR images. AJNR Am J Neuroradiol. 1991;12:1217–1222. [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Haim S, Asaad WF, Gale JT, Eskandar EN. Risk factors for hemorrhage during microelectrode-guided deep brain stimulation and the introduction of an improved microelectrode design. Neurosurgery. 2009;64:754–763. doi: 10.1227/01.NEU.0000339173.77240.34. [DOI] [PubMed] [Google Scholar]
- 3.Binder DK, Rau G, Starr PA. Hemorrhagic complications of microelectrode-guided deep brain stimulation. Stereotact Funct Neurosurg. 2003;80:28–31. doi: 10.1159/000075156. [DOI] [PubMed] [Google Scholar]
- 4.Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch Neurol. 2008;65:1202–1208. doi: 10.1001/archneur.65.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burchiel KJ, McCartney S, Lee A, Raslan AM. Accuracy of deep brain stimulation electrode placement using intraoperative computed tomography without microelectrode recording. J Neurosurg. 2013;119:301–306. doi: 10.3171/2013.4.JNS122324. [DOI] [PubMed] [Google Scholar]
- 6.Fenoy AJ, Simpson RK., Jr Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120:132–139. doi: 10.3171/2013.10.JNS131225. [DOI] [PubMed] [Google Scholar]
- 7.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 8.Gunduz A, Morita H, Rossi PJ, Allen WL, Alterman RL, Bronte-Stewart H, et al. Proceedings of the Second Annual Deep Brain Stimulation Think Tank: What’s in the Pipeline. Int J Neurosci. 2015;125:475–485. doi: 10.3109/00207454.2014.999268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer DR, Halpern CH, Danish SF, Jaggi JL, Baltuch GH. The effect of intraventricular trajectory on brain shift in deep brain stimulation. Stereotact Funct Neurosurg. 2012;90:20–24. doi: 10.1159/000332056. [DOI] [PubMed] [Google Scholar]
- 10.Lanctôt KL, Rajaram RD, Herrmann N. Therapy for Alzheimer’s disease: how effective are current treatments? Ther Adv Neurol Disorder. 2009;2:163–180. doi: 10.1177/1756285609102724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang AE, Houeto JL, Krack P, Kubu C, Lyons KE, Moro E, et al. Deep brain stimulation: preoperative issues. Mov Disord. 2006;21(Suppl 14):S171–S196. doi: 10.1002/mds.20955. [DOI] [PubMed] [Google Scholar]
- 12.Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 13.Massano J, Garrett C. Deep brain stimulation and cognitive decline in Parkinson’s disease: a clinical review. Front Neurol. 2012;3:66. doi: 10.3389/fneur.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyagi Y, Shima F, Sasaki T. Brain shift: an error factor during implantation of deep brain stimulation electrodes. J Neurosurg. 2007;107:989–997. doi: 10.3171/JNS-07/11/0989. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez RL, Fernandez HH, Haq I, Okun MS. Pearls in patient selection for deep brain stimulation. Neurologist. 2007;13:253–260. doi: 10.1097/NRL.0b013e318095a4d5. [DOI] [PubMed] [Google Scholar]
- 16.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 17.Sankar T, Chakravarty M, Bescos A, Lara M, Obuchi T, Laxton A, et al. Deep Brain Stimulation influences brain structure in Alzheimer’s Disease. Brain Stimulat. 2015;8:645–654. doi: 10.1016/j.brs.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery. 2008;62:360–367. doi: 10.1227/01.neu.0000316002.03765.33. [DOI] [PubMed] [Google Scholar]
- 19.Smith GS, Laxton AW, Tang-Wai DF, McAndrews MP, Diaconescu AO, Workman CI, et al. Increased cerebral metabolism after 1 year of deep brain stimulation in Alzheimer disease. Arch Neurol. 2012;69:1141–1148. doi: 10.1001/archneurol.2012.590. [DOI] [PubMed] [Google Scholar]
- 20.Starr PA, Turner RS, Rau G, Lindsey N, Heath S, Volz M, et al. Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: techniques, electrode locations, and outcomes. J Neurosurg. 2006;104:488–501. doi: 10.3171/jns.2006.104.4.488. [DOI] [PubMed] [Google Scholar]
- 21.Velasco AL, Velasco F, Jiménez F, Velasco M, Castro G, Carrillo-Ruiz JD, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47:1203–1212. doi: 10.1111/j.1528-1167.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 22.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zrinzo L, van Hulzen ALJ, Gorgulho AA, Limousin P, Staal MJ, De Salles AAF, et al. Avoiding the ventricle: a simple step to improve accuracy of anatomical targeting during deep brain stimulation. J Neurosurg. 2009;110:1283–1290. doi: 10.3171/2008.12.JNS08885. [DOI] [PubMed] [Google Scholar]
- 24.Zrinzo L, Zrinzo LV, Tisch S, Limousin PD, Yousry TA, Afshar F, et al. Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. 2008;131:1588–1598. doi: 10.1093/brain/awn075. [DOI] [PubMed] [Google Scholar]