Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, for which a number of genetic, environmental, and lifestyle risk factors have been identified. A significant modifiable risk factor is obesity in mid-life. Interestingly, both obesity and AD exhibit sex differences and are regulated by sex steroid hormones. Accumulating evidence suggests interactions between obesity and sex in regulation of AD risk, although the pathways underlying this relationship are unclear. Inflammation and the E4 allele of apolipoprotein E have been identified as independent risk factors for AD and both interact with obesity and sex steroid hormones. We review the individual and cooperative effects of obesity and sex on development of AD and examine the potential contributions of apolipoprotein E, inflammation, and their interactions to this relationship.
Keywords: Alzheimer’s disease, β-amyloid, apolipoprotein E, estrogen, inflammation, obesity, sex differences, testosterone
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that is the leading cause of dementia. The neuropathological hallmarks of AD include neuron loss, accumulation of amyloid-β (Aβ) plaques and hyperphosphorylated tau in the form of neurofibrillary tangles and neuropil threads, and gliosis (Cherry et al., 2014; Glass et al., 2010; LaFerla, 2010; Morris et al., 2014). There is compelling evidence that abnormal Aβ accumulation (Mucke and Selkoe, 2012; Tanzi, 2012) or hyperphosphorylated tau (Iqbal et al., 2010) or both (Zempel and Mandelkow, 2014) are the primary driving force(s) in the pathogenesis as well as strong support for key contributions by activated microglia and astrocytes (Cherry et al., 2014; Glass et al., 2010). Regardless of the proximal cause(s) of the neural injury in the AD brain, successful therapeutic intervention will require understanding of the factors that culminate in development of pathology.
The risk of AD is affected by numerous factors. Aging is the single greatest risk factor for AD, with the prevalence doubling every five years after the age of 65 (Hebert et al., 2003). However, the age-related physiological changes that contribute to this effect are uncertain. In addition to aging, AD risk is regulated by genetic factors. A small percentage of AD cases result from autosomal dominant mutations in the Aβ precursor protein, presenilin-1, and presenilin-2. The key consequences of these mutations appear to be increased production of A β and/or a change in the ratio of Aβ species, both of which foster Aβ accumulation (LaFerla, 2010; Tanzi, 2012). The most prevalent genetic risk factor for AD is the E4 allele (apoE4) of the cholesterol transporter apolipoprotein E (Saunders et al., 1993; Strittmatter et al., 1993), which also appears to regulate Aβ accumulation. In addition to apoE, there are a number of single nucleotide polymorphisms in genes that are associated with relatively subtle increases in AD risk. Among these are several genes associated with innate immunity (Tanzi, 2012), pointing to a role of the immune system, and microglia in particular, in AD pathogenesis. As with most diseases, AD risk is also significantly affected by several environmental and lifestyle factors, including education (Ferrari et al., 2014; Sharp and Gatz, 2011), head injury (Breunig et al., 2013), air pollution (Calderón-Garcidueñas et al., 2012), and physical exercise (Brown et al., 2013; Tolppanen et al., 2015). In recent years, an especially interesting risk factor has been obesity (Emmerzaal et al., 2015), which may contribute to links between cardiovascular diseases and AD (Hayden et al., 2006).
As with many disorders, significant sex differences exist in AD risk and development, with women being disproportionately affected by AD. These sex differences are likely to be mediated both via actions of sex steroid hormones, as well as by differences in neurophysiological substrates between men and women. Moreover, several normal age-related changes significantly increase AD risk including (i) estrogen depletion associated with menopause, (ii) age-related decreases in testosterone in men, and (iii) increasing adiposity in men and women. Since both estrogen and testosterone regulate adiposity, there are likely interactions between sex steroid hormones, adiposity, and AD risk that may be expected to exhibit sex differences.
In this review, we consider the individual and interactive effects of these AD risk factors as well as possible mechanisms that may be underlying these relationships. We begin by examining obesity as a risk factor for AD and sex differences in AD development. We then examine how sex differences and obesity interact in the context of AD, before exploring mechanisms underlying this relationship. Though there are likely to be a number of important mechanisms, our review will focus on inflammation, apoE4, and their interaction in the context of sex differences, obesity, and AD.
1. Obesity/metabolic syndrome as risk factors for AD
A. Epidemiological studies
Accumulating evidence over the past several years has identified obesity and related conditions as significant risk factors for the development of AD. Body mass index (BMI) is a commonly used measure of obesity, and though some studies show an association between BMI and AD, with an up to 40% increased risk for obese individuals (Fitzpatrick et al., 2009; Gustafson et al., 2003), others have found no association (Qizilbash et al., 2015; Yoshitake et al., 1995) (reviewed in Profenno et al., 2010). However, central adiposity may be a more important factor and better predictor of AD risk than BMI (Gustafson et al., 2009; Luchsinger et al., 2012; Whitmer et al., 2008), as visceral fat has been shown to be particularly harmful (Bloor and Symonds, 2014). Central adiposity has been shown to be a risk factor for AD as well as for cognitive impairment (Feng et al., 2013; Gustafson et al., 2009; Luchsinger et al., 2012; Whitmer et al., 2008), and visceral fat deposits are associated with lower brain volumes at middle age (Debette et al., 2010). Importantly, it appears that obesity at midlife is a particularly strong risk factor for onset of AD in late life (Emmerzaal et al., 2015; Fitzpatrick et al., 2009; Meng et al., 2014; Profenno et al., 2010; Xu et al., 2011). Intriguingly, the association between obesity and AD risk diminishes with age. Weight loss and low BMI are actually associated with increased risk of AD in older adults, whereas a higher BMI may be protective at advanced ages (Besser et al., 2014; Emmerzaal et al., 2015; Fitzpatrick et al., 2009; Hughes et al., 2009; Profenno et al., 2010). In fact, one study found that overweight and obese older adults were protected against AD, mild cognitive impairment (MCI), and vascular dementia (Doruk et al., 2010). One interpretation of these findings is that obesity at midlife may serve as a triggering factor for AD neuropathology, the effects of which do not become apparent until onset of clinical dementia later in life.
Obesity is associated with increased risk for the development of metabolic syndrome and type 2 diabetes (T2D), both of which are also independent risk factors for AD (Biessels et al., 2006; Samaras and Sachdev, 2012; Strachan et al., 2011). In addition, both obesity and T2D are risk factors for MCI (Samaras and Sachdev, 2012), and obesity is also linked with cognitive impairments in the absence of dementia (Benito-León et al., 2013; Gustafson et al., 2003; Mazzoccoli et al., 2014). In particular, central adiposity is a risk factor for cognitive decline, as increased visceral adipose tissue is associated with decreased performance on verbal memory and attention tasks, and with lower hippocampal volume (Isaac et al., 2011). Additionally, obesity can impair cognition even in children and young adults (Khan et al., 2014; Reinert et al., 2013; Schwartz et al., 2013; Yau et al., 2012). Thus, there are likely to be two independent pathways; one by which obesity impairs cognition and another pathway by which it promotes AD pathogenesis, that in turn impairs cognition in late life. Interestingly, while the relationship between higher visceral adipose tissue and lower cognitive performance is true in individuals under 70 years of age, this association does not exist in those over age 70 (Yoon et al., 2012), again indicating a protective effect of increased weight at older ages.
Obesity and related metabolic syndromes increase the risk of vascular dementia to an even greater extent than the risk of AD (Hayden et al., 2006; Whitmer et al., 2007; Xu et al., 2011; Yoshitake et al., 1995). Many vascular components are associated with AD neuropathology, including blood brain barrier disruption (Bell, 2012; Bell and Zlokovic, 2009) and cerebral amyloid angiopathy, the accumulation of β-amyloid (Aβ) deposits in the cerebrovasculature (Hultman et al., 2013). In addition, the presence of modifiable vascular risk factors at midlife increases risk of all types of dementia, including AD, later in life (Exalto et al., 2014; Whitmer et al., 2007). As obesity is a major risk factor for cardiovascular disease as well as AD, obesity and vascular factors likely cooperatively contribute to AD pathogenesis.
B. Experimental studies
In agreement with epidemiological findings, experimental studies in animals have demonstrated that obesity and T2D are associated with promotion of AD. First, various animal models of obesity and diabetes exhibit brain changes consistent with early AD pathology (Jayaraman and Pike, 2014). A commonly used approach is the use of high fat diet (HFD) in rodents, which yields diet-induced obesity (DIO). Using this model, our lab and others have shown that DIO in transgenic mouse models of AD increases levels of Aβ (Barron et al., 2013; L. Ho et al., 2004; Julien et al., 2010; Kohjima et al., 2010), a key protein in the initiation and progression of AD (Selkoe, 2011). Tau pathology, the other neuropathological hallmark of AD, is also increased by DIO in a number of strains of transgenic mice (Julien et al., 2010; Leboucher et al., 2013; Mehla et al., 2014; Takalo et al., 2014).
As in the human literature, DIO is also associated with cognitive deficits in animal models independent of underlying AD-related pathology. That is, rodents show impairments on a number of cognitive tasks following HFD, without apparent changes in Aβ accumulation (Davidson et al., 2013; Granholm et al., 2008; Hsu et al., 2014; Kanoski and Davidson, 2011; Kanoski et al., 2010; Knight et al., 2014; Stranahan et al., 2008). In fact, even a short 9-day exposure to HFD can cause cognitive impairment in rats (Murray et al., 2009).
In addition to DIO, genetic and pharmacological manipulations that model diabetes also increase AD-related neuropathology. For example, the BBZDR/Wor rats, a strain genetically prone to T2D, have greater neuronal loss and Aβ pathology than do BB/Wor rats, which are genetically prone to Type 1 diabetes (Li et al., 2007) Further, treatment with streptozotocin (STZ), which kills pancreatic β-cells, is commonly used to induce type I diabetes in animal models. STZ has been found to increase Aβ in both mouse (Currais et al., 2012; Jolivalt et al., 2008; Wang et al., 2010) and rat (Yang et al., 2013) models, as well as increase tau phosphorylation in brain (Jolivalt et al., 2008; Kim et al., 2009; Planel et al., 2007). Transgenic mouse models of obesity include leptin deficient mice (ob/ob) and leptin receptor deficient mice (db/db). Even in the absence of HFD, these transgenic mice show cognitive impairments and Aβ pathology (Li et al., 2012; Ramos-Rodriguez et al., 2013), as well as increased tau phosphorylation (Li et al., 2012; Kim, et al., 2013; Ramos-Rodriguez et al., 2013). Moreover, endothelial cells cultured from db/db mice show increased susceptibility to the toxic effects of Aβ (Carvalho et al., 2014). Interestingly, the antidiabetic drug metformin has been shown to reduce AD-like pathology in db/db mice, though it did not improve cognition (Li et al., 2012).
Notably, crossing the genetically obese and diabetic ob/ob and NSY mice with an AD transgenic mouse increases cognitive impairment and diabetic outcomes (Takeda et al., 2010), and is associated with severe cerebrovascular pathology (Niedowicz et al., 2014) without affecting Aβ pathology (Niedowicz et al., 2014; Takeda et al., 2010). These studies suggest that adverse metabolic outcomes can be exaggerated in the presence of AD, but genetically induced metabolic outcomes do not necessarily increase AD pathology. Interestingly, complementary findings in an AD transgenic mouse with DIO also suggest that metabolic disturbance may not be the driving force in promotion of AD pathogenesis (Barron et al., 2013). In summary, a number of studies in animal models have demonstrated increased AD-like pathology in the presence of diet- and pharmacologically-induced as well as genetically-induced obesity and metabolic disturbances. Moreover, the literature suggests that dietary components may be important in regulating AD pathology, even in the absence of obesity and metabolic syndromes.
C. Dietary components affect AD risk
Studies in both human and animal models suggest that particular dietary constituents may be important in modulating AD risk. For example, trans and saturated fatty acids are associated with higher risk of AD and MCI (Barnard et al., 2014; M. C. Morris and Tangney, 2014). In rodents, a diet high in saturated fatty acids was found to be more detrimental than a high cholesterol diet (Takechi et al., 2013). Other rodent studies have shown that trans and saturated fatty acids lead to a particularly robust increase in Aβ (Grimm et al., 2012; Oksman et al., 2006). Conversely, diets with high omega 3 polyunsaturated fatty acids are associated with decreased Aβ levels (Hjorth et al., 2013; Julien et al., 2010; Lebbadi et al., 2011; Zerbi et al., 2014), and one study found that a diet low in fat and high in oleic acid was able to reduce Aβ levels and pathology in transgenic mice (Amtul et al., 2010). In addition, the high sucrose and fructose contents of Western diets are also associated with cognitive impairment in humans (Francis and Stevenson, 2011) and increased Aβ (Lakhan and Kirchgessner, 2013; Moreira, 2013; Orr et al., 2014), and tau pathology (Orr et al., 2014) in rodents. Even in the absence of HFD, 10% sucrose water increased Aβ in a mouse model of AD (Cao et al., 2007), and a high fructose diet impaired spatial memory in rats (Ross et al., 2009). Thus, diets high in saturated fatty acids, sucrose, and fructose may contribute to AD pathogenesis, whereas diets high in certain types of fatty acids may be protective.
2. Sex differences in AD risk
The prevalence of AD is greater in women than in men, which holds true even after controlling for the fact that women have an increased lifespan (Li and Singh, 2014). Moreover, apolipoprotein E ε4 allele (ApoE4), the strongest genetic risk factor for AD, has a greater effect in women than it does in men such that a single copy of ApoE4 increases risk about 4-fold in women without significantly affecting AD risk in men (Payami et al., 1994). Similarly, ApoE4 increases rates of conversion from cognitively normal to MCI and from MCI to AD significantly more in women than in men (Altmann et al., 2014). Interestingly, in transgenic mouse models of AD, female mice are also more affected by AD in that they develop more severe AD-like neuropathology (Carroll et al., 2010; Hirata-Fukae et al., 2008; Schäfer et al., 2006), and have greater cognitive impairments (Blázquez et al., 2014; Carroll et al., 2010).
Several lines of evidence indicate that some of these sex differences in AD risk can be attributed to sex steroid hormones. That is, sex steroid hormones are protective against AD in both sexes, and age-related loss of both estrogens in women (Manly et al., 2000; Pike et al., 2009) and of androgens (Hogervorst et al., 2001; Moffat et al., 2004; Paoletti et al., 2004) in men, have been shown to increase AD risk. Moreover, low brain and circulating estrogen in women (Rosario et al., 2011; Yue et al., 2005) and testosterone in men (Rosario et al., 2011; 2004) have been associated with increased AD risk (Pike et al., 2009). Likewise, our lab and others have demonstrated similar relationships in animal models, such that in a mouse model of AD, androgen depletion in males (McAllister et al., 2010; Rosario et al., 2006) and estrogen depletion in females (Carroll et al., 2007) increases AD-like pathology, changes that are reversed by androgen and estrogen treatment. Collectively, human and rodent data suggest that the age-related depletion of sex steroid hormones, which diminishes beneficial activational effects of hormones in adult brain, places the brain at increased vulnerability for development of AD. Interestingly, emerging data also suggest that organizational effects of sex steroids hormones in the developmental sexual differentiation of the brain may confer greater risk in the female brain (Carroll et al., 2010).
3. Sex differences in obesity
A. Epidemiological studies
Rates of obesity are fairly similar between the sexes, at roughly 33% of men and 36% of women in the US being obese (Ogden et al., 2014). However, sex differences exist in the way that obesity manifests itself and in obesity-related complications. For example, some studies have found that rates of metabolic syndrome are somewhat lower in women (Hadaegh et al., 2013; Pradhan, 2013), and that women are protected against some but not all obesity-related complications (Syme et al., 2008). Yet, over a timespan of about 20 years, the prevalence of metabolic syndrome has increased most significantly among young women aged 20–39 years (Pradhan, 2013). Moreover, increases in BMI are positively associated with inflammation in women, but not men, suggesting that relative increases in BMI may be more harmful in women (Ahonen et al., 2012). In addition, one study found that obese adolescent girls have higher free fatty acid flux than do obese boys, which predicts development of insulin resistance (Adler-Wailes et al., 2013).
A number of studies have demonstrated that premenopausal women are protected against obesity, but that this protective effect is lost at menopause (Bloor and Symonds, 2014; Meyer et al., 2011; Sugiyama and Agellon, 2012). Some of these sex differences may be attributed to the way in which men and women store fat; that is, women tend to deposit excess fat in the lower body, whereas men develop mainly visceral fat deposits (Bloor and Symonds, 2014). Subcutaneous fat in the lower body has a greater capacity to store lipids and undergo tissue remodeling, which may contribute to better metabolic outcomes in premenopausal women (Bloor and Symonds, 2014). However, these sex differences diminish after menopause, suggesting a role for sex steroid hormones (discussed below).
Sex differences have also been found in brain responses to obesity. For example, men and women differ in the way they respond to food cues, suggesting that women have lower cognitive control of brain responses to food stimulation (Wang et al., 2009). In concordance with this finding, obese women have greater activation of brain regions regulating cognition and emotion in response to high calorie foods (Geliebter et al., 2013). Moreover, men have higher hypothalamic-pituitary-adrenal axis activity to suppress food intake after consuming a meal (Martens et al., 2012), demonstrating that responses to food intake also exhibit sex differences. Finally, the adverse effects of obesity on the brain may be exaggerated in women, as one study found that although both sexes had axonal degeneration with obesity, only obese women showed a positive correlation between BMI, serum leptin levels, and myelin degeneration (Mueller et al., 2011). In summary, though there are no sex differences in rates of obesity, there appear to be significant differences in obesity-related outcomes and in brain responses between the sexes.
B. Experimental Studies
Experimental work in animal models has confirmed the existence of sex differences in obesity. Most studies have demonstrated that female rodents are protected against metabolic impairments associated with DIO. For example, despite similar inflammatory cytokine expression in adipose tissue, female mice had less infiltrating macrophages, later onset of glucose homeostasis impairments, better insulin sensitivity, and less fat deposition in the liver than did male mice (Medrikova et al., 2012). In another study, obese male mice had increased levels of inflammatory cytokines and macrophage infiltration in adipose tissue, whereas obese female mice had an increase in anti-inflammatory T cells in adipose tissue (Pettersson et al., 2012). Female rodents have greater expandability of adipocytes which may allow them to maintain insulin sensitivity in response to HFD (Amengual-Cladera et al., 2012; Medrikova et al., 2012). Additionally, female adipocytes have greater insulin sensitivity and lipid production, and higher levels of proteins involved in glucose and lipid metabolism (Macotela et al., 2009). Adipocytes of castrated male mice have increased insulin sensitivity and lipogenesis, whereas adipocytes from ovariectomized female mice have decreased lipogenesis (Macotela et al., 2009), pointing to the role of sex steroid hormones in sex differences in adipose tissue properties.
Females also appear to be protected against the adverse effects of HFD on the brain; male mice developed more metabolic and cognitive impairments and had alterations in hippocampal synaptic plasticity that female mice did not (Hwang et al., 2010). Moreover, in one mouse model of diabetes, the NSY mouse, 98% of males become diabetic by 48 weeks of age, versus only 31% of females (Ueda et al., 1995), and another study found that though both sexes became obese, only male rats lacking the leptin receptor developed T2D (Moralejo et al., 2010).
However, the opposite has also been demonstrated, in that female rats had a greater increase in body weight, despite having lower energy intake than males (Nadal-Casellas et al., 2012). Furthermore, obese female rats had reduced insulin signaling in brown adipose tissue, while males had increased mitochondrial function, which may have served to suppress oxidative damage and reduce insulin signaling impairments (Nadal-Casellas et al., 2012). Though a few studies have shown greater detrimental effects in females, the majority of research suggests that female rodents are protected against some aspects of obesity and related complications. As discussed below, evidence suggests that sex steroid hormones may be particularly important in regulating these sex differences.
C. Estrogens and Obesity
Sex differences in obesity may be partly accounted for by sex steroid hormones, and estrogens have a multitude of roles in obesity and metabolic syndrome. As mentioned previously, sex differences in fat storage are diminished after menopause, with postmenopausal women showing a shift from subcutaneous to visceral fat deposition (Brown and Clegg, 2010; Meyer et al., 2011; Shi and Clegg, 2009; White and Tchoukalova, 2014). A number of findings point to the role of a loss of estrogen in this shift after menopause. For example, aromatase is required for the conversion of androgens to estrogens, and both male and female mice lacking aromatase have increases in adiposity and insulin resistance (Jones et al., 2000; Takeda et al., 2003), which can be reversed by estrogen treatment (Takeda et al., 2003). Moreover, the main source of estrogens in postmenopausal women is the adipose tissue, where androgens are converted into estrogens. Interestingly, aromatase activity in adipose tissue increases with age as well as with obesity (Meyer et al., 2011). Estrogens also have a number of important roles in insulin homeostasis, including increasing the release of insulin from the islet of Langerhans, preventing β-cell apoptosis and improving insulin’s action (Meyer et al., 2011).
The effects of ovariectomy (OVX) and estrogen replacement in rodent models highlight the importance of estrogen in regulating adiposity, body weight, and metabolism. For instance, OVX mice were shown to have pancreatic beta cell dysfunction that could be reversed by hormone replacement (Bailey and Ahmed-Sorour, 1980). Both male and female aromatase knockout mice have a number of metabolic disturbances that can be restored with estrogen replacement (Simpson et al., 2005). Furthermore, one study found that estrogen replacement and exercise had additive beneficial effects in reversing the metabolic disturbances associated with OVX and HFD (Zoth et al., 2010). Thus, loss of ovarian hormones causes metabolic disturbances in both the presence and absence of HFD, which can be reversed by estrogen replacement.
In addition to its effects peripherally, estrogen signaling in brain is required for energy homeostasis. Specifically, estrogen receptor α (ERα) signaling is important in this regard, as both male and female mice knocked out for ERα have increased adiposity and metabolic disturbances even on a regular chow diet (Heine et al., 2000). Moreover, ERα signaling in hypothalamus appears to be especially crucial, as blocking ERα only in the ventromedial hypothalamus causes obesity, hyperphagia, and hyperglycemia in rodents (Musatov et al., 2007). Thus, estrogen has roles in adiposity, insulin homeostasis, body weight, and energy homeostasis.
D. Testosterone and obesity
Just as depletion of estrogens at menopause in women is associated with increased risk of obesity and metabolic syndrome, so too is the age-related decline of androgens in men. Low testosterone levels are associated with insulin resistance, metabolic syndrome, and development of T2D (Kapoor et al., 2007; 2005; Stellato et al., 2000; Zitzmann, 2009). Obesity, T2D, and metabolic syndrome are also associated with low testosterone levels in pre-pubertal (Mogri et al., 2013) and young men (Chandel et al., 2008; Goncharov et al., 2008).
There appears to be a bi-directional relationship between testosterone loss and obesity, such that low testosterone levels predispose to obesity, and obesity causes decreases in testosterone levels (De Maddalena et al., 2012; Grossmann et al., 2014). In accord with this idea, weight loss has been found to increase testosterone levels. For example, free testosterone levels increase in men after bariatric surgery and are associated with improved insulin and glucose sensitivity (Botella-Carretero et al., 2013), but men with low testosterone levels are at an increased risk of regaining weight (Wang et al., 2013). Conversely, testosterone supplementation can also lower risks of obesity and metabolic syndrome as long-term testosterone replacement in older men is often associated with reduced body weight, waist circumference, and BMI, and with a reduction in symptoms of metabolic syndrome (Yassin et al., 2014). Additionally, our lab has shown that loss of testosterone in male rodents exacerbates effects of DIO, whereas testosterone replacement protects against DIO-induced hyperglycemia and hyperinsulinemia (Jayaraman et al., 2014).
One mechanism by which obesity may contribute to testosterone loss is via excess adipose tissue. Levels of aromatase, which converts testosterone to estradiol, are high in adipose tissue (Meyer et al., 2011). Thus, increased adiposity would result in increased activity of aromatase and thereby increased metabolism of testosterone to estrogens (Blouin et al., 2006). Additionally, male mice maintained on HFD have decreased testosterone levels associated with apoptosis of Leydig cells in the testes and reduced testicular weight and function (Zhao et al., 2014). Thus, it is likely that both, increased metabolism of testosterone in adipose tissue and decreased testosterone production in the testes, are mechanisms by which obesity drives testosterone loss. Consistent with this possibility, obese men placed on caloric restriction have increased serum testosterone levels, due to both an improvement in testicular function and a decrease in the conversion of testosterone to estrogens in adipose tissue (Schulte et al., 2014). Moreover, testosterone inhibits adipogenesis, as testosterone supplementation has been found to inhibit pluripotent stem cells and pre-adipocytes from becoming adipocytes (White and Tchoukalova, 2014). These studies point to the existence of a vicious cycle in which low testosterone and obesity interact to promote development of both conditions.
4. Interactions between sex differences, obesity, and Alzheimer’s disease
A. Epidemiological studies
Although few studies have explored sex differences in the effects of obesity on AD risk, available evidence suggests that the adverse effects of obesity on cognition and AD risk are stronger in women than in men. One study found that only in women did obesity increase risk of AD, and diabetes increase risk of vascular dementia (Hayden et al., 2006). In contrast, Whitmer and colleagues found no sex differences in the association between BMI and AD risk (Whitmer et al., 2007). Many studies controlled for sex rather than examining sex differences (Debette et al., 2010; Exalto et al., 2014; Ho et al., 2010; Hughes et al., 2009; Isaac et al., 2011), suggesting that sex differences may be more prevalent than the literature currently reflects. Sex differences have also been demonstrated in the effects of obesity on blood brain barrier disruption (a risk factor for AD), with overweight and obese women, but not men, showing increased disruption (Gustafson et al., 2007). Interestingly, greater blood brain barrier disruption at old age correlates with availability of sex steroid hormones at middle age (Gustafson et al., 2007).
Independent of AD, sex differences are observed in the effects of obesity on age-related cognitive decline. Specifically, in contrast to the finding that obesity increases AD risk only in women; obesity, hypertension, and high adiposity were associated with cognitive decline in men but not in women (Elias et al., 2003; Kanaya et al., 2009). In addition, sex differences have been found in effects of obesity on brain structure, with obese men having gray matter loss and alterations not found in obese women (Taki et al., 2008). In contrast, increases in BMI have been associated with temporal lobe atrophy in women (Gustafson et al., 2004), and obesity is linked with myelin degeneration in women but not men (Mueller et al., 2011). Though the factors underlying the observed sex differences are unclear, the greater prevalence of vascular risk factors in men may indicate that men are more susceptible to the effects of obesity on vascular outcomes. In summary, findings on sex differences in the effects of obesity on AD, cognition, and brain structure are mixed, and future studies should address the role of sex in these relationships.
B. Experimental studies
The literature on sex differences in obesity and AD risk in experimental models is extremely limited, but studies have generally shown that male mice may be more sensitive to the effects of dietary manipulations. Our own lab has demonstrated that although both male and female AD transgenic mice become obese on HFD, only males exhibit significant ectopic fat accumulation, hyperglycemia, and hyperinsulinemia (Barron et al., 2013). Despite differential effects of HFD on metabolic outcomes, both males and females have increased Aβ deposition (Barron et al., 2013), suggesting that AD-related pathology is accelerated by other obesity-induced effects, perhaps including inflammation.
Though not directly related to AD risk, other studies in animal models have demonstrated that neural effects of obesity are generally more pronounced in males. For example, obese male mice have learning and memory impairments and reductions in synaptic plasticity that are not present in female mice (Hwang et al., 2010). Additionally, male mice have been shown to be more sensitive to the beneficial effects of diet. That is, male mice fed a rodent diet supplemented with phyto-nutrients and fish oils had improved working memory and hippocampal mitochondrial function, whereas female mice did not show improvements with diet supplementation (Wolf et al., 2012). A major limitation in our understanding of this issue is that most studies use only male rodents to examine dietary effects on brain changes and AD outcomes; thus, sex differences in obesity and cognitive decline/AD risk may be far more prevalent than currently assumed.
5. Mechanisms underlying sex differences in obesity and Alzheimer’s disease
A. Apolipoprotein ε 4
A.1. Apolipoprotein ε 4 and Alzheimer’s disease
Apolipoprotein E is a cholesterol transporter that has three isoforms that vary by a single amino acid: apoE ε2, ε3, and ε4, with the ε4 allele (apoE4) being the strongest genetic risk factor for AD (Corder et al., 1993; Saunders et al., 1993; Strittmatter et al., 1993). Only ~12% of the general population are apoE4 carriers (de-Andrade et al., 2000), yet its frequency increases to ~50% in AD patients (Ward et al., 2012). Moreover, the onset of AD occurs earlier in apoE4 carriers (Corder et al., 1993), and ~40% of healthy middle-aged versus ~8% of non-carriers have Aβ accumulation (Lathe et al., 2014; Liu et al., 2013). Apart from increasing AD risk, apoE4 is associated with greater cognitive decline over a 6 year period in healthy middle-aged adults (Blair et al., 2005). ApoE4 is also linked with greater AD-like pathology in mouse models, where it has been shown to potentiate oligomerization of Aβ (Belinson and Michaelson, 2009), and accelerate and worsen Aβ plaque formation (Youmans et al., 2012).
ApoE4 is also associated with changes in vascular pathology and in blood brain barrier function. Specifically, AD patients who are apoE4 carriers have greater arteriosclerosis and cerebral amyloid angiopathy (Premkumar et al., 1996; Yip et al., 2005), and though cerebral amyloid angiopathy is rare in the absence of AD, it is found in otherwise healthy homozygous ApoE4 carriers (Walker et al., 2000). Mice expressing human apoE4 have reduced cerebral vascularization at a young age and increased vascular atrophy at old age (Alata et al., 2015). Moreover, apoE4 is associated with increased blood brain barrier permeability and breakdown in both humans (Halliday et al., 2013) and in mouse models (Bell et al., 2012; Nishitsuji et al., 2011). Thus, apoE4 may be acting through several different mechanisms, including regulation of Aβ oligomerization and deposition, and disruption of the vasculature and the blood brain barrier.
Notably, carriers of one apoE4 allele have a ~30% lifetime risk of AD, meaning that a significant proportion of apoE4 carriers never develop the disease (Genin et al., 2011). Thus, apoE4 is neither necessary nor sufficient for AD. Consequently, apoE4 must interact with other risk factors, perhaps including obesity and diabetes, to influence AD risk.
A.2. Apolipoprotein ε4 and obesity
Abundant evidence indicates that apoE4 is a risk factor for several metabolic disturbances and adverse cardiovascular outcomes, including hypertension (Niu et al., 2009), increased systolic blood pressure and carotid artery thickness (Atabek et al., 2012), increased triglyceride and low density lipoprotein cholesterol levels (de-Andrade et al., 2000; Kypreos et al., 2009), decreased high density lipoprotein cholesterol levels (Zarkesh et al., 2012), and higher pancreatic islet amyloidosis in diabetic patients (Guan et al., 2013). Female apoE4 carriers have been found to have increased central adiposity (Oh et al., 2001) and frequency of apoE4 is increased in metabolic syndrome patients of either sex (Sima et al., 2007). Additionally, among obese men, those with apoE4 have higher insulin and glucose levels (Elosua et al., 2003; Marques-Vidal et al., 2003). In a DIO mouse model, apoE4 was associated with greater metabolic impairments and adipocyte hypertrophy (Arbones-Mainar et al., 2008). However, other studies fail to find a significant relationship between apoE4 and metabolic disturbances including insulin resistance (Meigs et al., 2000; Ragogna et al., 2011). The reason for these discordant findings is unclear, but may reflect the growing appreciation of gene-environment interactions in mediating the effects of apoE4 in aging and age-related diseases (Corella and Ordovas, 2014). For example, deleterious effects of apoE4 on heart disease risk and outcomes are preferentially observed in the context of high saturated fat diets (Corella et al., 2011; Yang et al., 2007).
A number of mechanisms that may underlie the association between obesity and apoE4 have been identified. For example, apoE isoforms have been shown to differentially interact with hormones important in nutrient sensing and homeostasis, including adiponectin (Arbones-Mainar et al., 2008) and leptin (Fewlass et al., 2004). Moreover, apoE acts in the hypothalamus to suppress food intake (Shen et al., 2008), but how this might differ by apoE genotype in not known. Importantly, apoE4 carriers have lower levels of hippocampal insulin degrading enzyme, which is also involved in Aβ clearance (Cook et al., 2003; Du et al., 2009; Edland, 2004). MCI patients with apoE4 have higher levels of the more toxic, lipid depleted Aβ, while all apoE4 carriers have an increase in lipid depleted apoE, which is less effective at clearing Aβ (Hanson et al., 2013). Interestingly, levels of lipid depleted Aβ are increased in the presence of a diet high in saturated fats and glycemic index, and decreased with a low saturated fat and glycemic index diet (Hanson et al., 2013).
As with cardiovascular disease, the combination of obesity and apoE4 may also affect AD outcomes. For example, mid-life obesity (Ghebranious et al., 2011) and high fat and calorie intake (Luchsinger et al., 2002), are associated with greater risk for AD only in apoE4 subjects. Men with both type 2 diabetes and the apoE4 allele have a 5.5 times greater risk of AD, as well as greater AD neuropathology than men with neither risk factor (Peila et al., 2002). Further, the relationship between obesity and diminished cognitive functions appears to be strongest in apoE4 carriers (Zade et al., 2013). Thus, gene-environment interactions between apoE4 and obesity appear to be important regulators of cognitive decline and AD risk.
A.3. Sex differences in apolipoprotein ε4, obesity, and Alzheimer’s disease
ApoE4 is the primary genetic risk factor for late-onset AD. Interestingly, the increased AD risk associated with apoE4 disproportionately affects women: AD risk is increased approximately 4-fold and 10-fold in women with one and two apoE4 alleles, respectively, whereas men show essentially no increased risk with one apoE4 allele and a 4-fold increase in risk with two apoE4 alleles (Payami et al., 1994). Additionally, female apoE4 carriers exhibit a significantly greater risk than male apoE4 carriers of converting to MCI, and from MCI to dementia (Altmann et al., 2014). ApoE4 is also more strongly linked to cognitive dysfunction (Beydoun et al., 2012) and brain atrophy (Holland et al., 2013; Liu et al., 2010) in women. Healthy older female, but not male, apoE4 carriers have decreased default mode network activity that is correlated with cerebrospinal fluid levels of tau protein (Damoiseaux et al., 2012). Similarly, female but not male apoE4 mice exhibit cognitive impairments (Raber et al., 2002).
The loss of estrogen at menopause appears to increase risk of AD, whereas estrogen treatments may be protective against AD (Pike et al., 2009). However, these relationships may vary depending on apoE genotype. For example, estrogen receptor polymorphisms have been associated with increased risk of AD but only in apoE4-carrying women (Fernandez-Martinez et al., 2013). Further, estrogen-based hormone therapy is associated with memory improvement and slower cognitive decline in non-apoE4 carriers, but not in apoE4-carrying women (Burkhardt et al., 2004; Yaffe et al., 2000). In fact, estradiol may have deleterious effects in the context of apoE4, as female AD patients with high estradiol and the apoE4 allele have an increase in neuropsychiatric symptoms (Xing et al., 2012). Adverse effects of estradiol and apoE4 have also been demonstrated in mice, where estrogen treatment in apoE2 and apoE3 mice reduced Aβ pathology, but increased pathology in apoE4 mice (Kunzler et al., 2014). How estradiol and apoE4 interact is unclear. Estradiol has been shown to increase apoE expression in astrocytes and microglia (Stone et al., 1997; Struble et al., 2007). The particular receptor it acts on may be important, as activation of estrogen receptor α up-regulates apoE expression, while activation of estrogen receptor β down-regulates apoE (Wang et al., 2006). One protective action of estradiol that is compromised by apoE4 is its ability to inhibit pro-inflammatory actions of microglia (Brown et al., 2008). In contrast to evidence that apoE4 may negate or even reverse the neural benefits of estradiol, other reports show that estrogen-based hormone therapy exerts cognitive benefits (Ryan et al., 2009), may reduce AD risk (Rippon et al., 2006), and slows cellular aging (Jacobs et al., 2013) in women with apoE4. Thus, although apoE4 is clearly associated with greater neural risk in women, the role of estradiol in this relationship remains unclear.
Like estrogen loss in women, age-related testosterone depletion in men is a risk factor for development of AD (Pike et al., 2009; Vest and Pike, 2013), and apoE4 interacts with the relationship between testosterone and AD. First, cognitively normal aged men that are apoE4 carriers exhibit significantly lower levels of testosterone than non-carriers (Hogervorst et al., 2002). Second, testosterone levels are inversely associated with hippocampal volume in middle-aged men with apoE4 (Panizzon et al., 2010), but positively correlated with verbal episodic memory (Panizzon et al., 2014). Conversely, one study reported testosterone levels were positively associated with cognitive performance in middle-aged men lacking apoE4, but negatively associated with performance in apoE4 carriers (Burkhardt et al., 2006); however, this study may have been limited by a small sample size. Interactions of androgens and apoE have also been demonstrated in mouse models. Androgens have reduced binding to the androgen receptor in the presence of apoE4 (Raber, 2004). Additionally, apoE4 but not apoE3 mice show cognitive impairments following testosterone depletion (Pfankuch et al., 2005) and pharmacological antagonism of the androgen receptor (Raber et al., 2002). Interestingly, androgen treatment also improves cognitive performance in female apoE4 mice (Raber et al., 2002). Collectively, the limited available data suggest that apoE4 and low testosterone are interactive risk factors for AD. As with estrogen-based hormone therapy in women, it remains unclear whether testosterone treatment in men could mitigate deleterious effects of apoE4. Also uncertain is whether obesity modulates interactions between apoE and sex steroid hormones in men and women.
B. Inflammation
B.1. Inflammation and Alzheimer’s disease
Multiple lines of evidence have established inflammation as a key component in the initiation and/or progression of AD pathogenesis (Wyss-Coray and Rogers, 2012). Aging, the most significant risk factor for late-onset AD, is associated with an increase in chronic inflammation (Singh and Newman, 2011). Age-associated increases in pro-inflammatory cytokines can increase Aβ levels, which in turn can activate microglia and astrocytes to perpetuate a cycle of inflammation and Aβ production (Blasko et al., 2004). Indeed, higher levels of circulating inflammatory cytokines are associated with increased risk of developing AD (Tan et al., 2007). In the central nervous system, pro-inflammatory cytokines including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are upregulated in CSF even before detectable Aβ pathology (Avila-Muñoz and Arias, 2014; Eikelenboom et al., 2011). Moreover, higher levels of inflammatory cytokines are associated with greater cognitive decline (Rafnsson et al., 2007) and greater loss of entorhinal cortex volume (Avila-Muñoz and Arias, 2014), as well as greater loss of total brain volume than would be expected for a given age (Jefferson et al., 2007). Cerebral inflammation, independent of other AD pathology markers, predicts early death caused by dementia (Nägga et al., 2014).
The roles of heritability and environmental factors in the association between inflammation and AD have also been demonstrated. Recent genetic evidence links polymorphisms in several components of the immune system as risk factors for AD, including CD 33 (Bertram et al., 2008; Hollingworth et al., 2011; Naj et al., 2011), TREM2 (Kleinberger et al., 2014), clusterin, and CR1 (Harold et al., 2009; Lambert et al., 2009). Further, the production of IL-1β, TNF-α, and interferon γ in response to the pro-inflammatory stimulant lipopolysaccharide (LPS) is greater in children with a parental history of AD (van Exel et al., 2009). Environmental factors that increase inflammation are also implicated in promoting AD. Traumatic brain injury, for example, is associated with increased risk of AD, and chronic neuroinflammation has been suggested as a mediator of this relationship (Breunig et al., 2013). Increased inflammation in response to air pollution is well established, and even children living in highly polluted areas show increased pro-inflammatory cytokines in brain that correlate with Aβ and tau pathology (Calderón-Garcidueñas et al., 2012).
Activated microglia and astrocytes are found surrounding Aβ plaques in AD, and are associated with increased production of pro-inflammatory factors (Glass et al., 2010). Interestingly, response sites for NFκB, a major upstream regulator of inflammatory cytokine production, have been found in the promoters of genes involved in production of Aβ, and pro-inflammatory cytokines increase the expression of these Aβ-related genes in neurons (Glass et al., 2010; Sastre et al., 2008). LPS increases production of pro-inflammatory cytokines and Aβ in both wildtype mice (Brugg et al., 1995), and in AD-transgenic mice (Sheng et al., 2003). Conversely, anti-inflammatory treatments reduce Aβ production and Aβ plaque deposition (Yan et al., 2003) in rodent models and may have some efficacy in AD prevention (Zandi et al., 2002), although the literature in this area is mixed. The specific role of different components of neuroinflammation in AD pathogenesis is unclear, as some studies have found reduced Aβ pathology in response to increasing microglia (Boissonneault et al., 2009), whereas others have found that attenuating pro-inflammatory cascades decrease Aβ pathology (Heneka et al., 2013). Thus, some aspects of neuroinflammation appear to be beneficial while others are harmful, indicating a need for more research.
B.2. Obesity and inflammation
Strong links between obesity and chronic inflammation have been established over the last several years (Weisberg et al., 2003; Zeyda and Stulnig, 2009). Not only does obesity appear to drive inflammation, but chronic inflammation can also disrupt metabolic processes to further drive obesity (Thaler and M. W. Schwartz, 2010). For example, healthy men with high levels of pro-inflammatory cytokines in serum had an increased risk of weight gain over a 6 year period (Engström et al., 2003). Conversely, obese subjects placed on a very low calorie diet for 28 days had a decrease in pro- and an increase in anti-inflammatory cytokines (Clément et al., 2004), pointing to the role of diet in the association between obesity and inflammation. In fact, saturated fatty acids have been shown to induce secretion of pro-inflammatory factors in culture (Gupta et al., 2012), whereas polyunsaturated fatty acids improve obesity-associated inflammation (Liu et al., 2013). Inflammation also contributes to insulin resistance (McNelis and Olefsky, 2014), a central feature of the metabolic syndrome. For example, signaling through the inflammatory NFκB pathway can result in insulin resistance peripherally (Arkan et al., 2005), as well as leptin and insulin resistance in the hypothalamus (García-Cáceres et al., 2013). Thus, inflammatory cascades associated with obesity are widely believed to contribute to the relationship between obesity and development of metabolic syndrome and T2D.
Obesity is also associated with central nervous system inflammation. For example, a high fat diet has been linked with a 30% increase in immune cell infiltration into the brain (Buckman et al., 2014). Inflammation in the hypothalamus is a central feature of obesity and high fat diets (De Souza et al., 2005; Milanski et al., 2009; Thaler et al., 2012). In fact, hypothalamic inflammation precedes diet-induced obesity since it is present after even a few days of high fat diet consumption (Thaler and Schwartz, 2010), and neonatally fed mice show preservation of an increased inflammatory response in hypothalamus even in adulthood (Ziko et al., 2014). Furthermore, there are also significant interactions between the hypothalamus and periphery in diet-induced obesity and inflammation. Administering anti-inflammatory antibodies into brains of obese rats led to decreased hypothalamic inflammation, increased leptin sensitivity in hypothalamus, and increased insulin sensitivity in liver, along with restoring liver glucose production (Milanski et al., 2012), demonstrating that manipulating central inflammation has direct effects on the periphery.
A relationship between obesity, inflammation, and cognition has also been established (Freeman et al., 2014). An increase in inflammatory markers in subjects with metabolic syndrome predicted subsequent cognitive decline (Yaffe et al., 2003). Subjects with both metabolic syndrome and high levels inflammation had the worst cognitive performance (Dik et al., 2007). Interestingly, one study found that cognitive performance improved and inflammation decreased after bariatric surgery, though the two did not correlate (Hawkins et al., 2014). Similar findings have been reported in rodent models. For example, levels of the pro-inflammatory cytokine IL-1β correlated with adiposity and cognitive impairment in the db/db mouse model of obesity (Erion et al., 2014). Moreover, treatment with an IL-1β antagonist prevented obesity-associated cognitive impairments and synaptic dysfunction (Erion et al., 2014). Finally, associations between obesity and inflammation have been shown in the context of neuropsychiatric disorders such as depression, in both humans (Castanon et al., 2014; Soczynska et al., 2010; Viscogliosi et al., 2012), and in mice (André et al., 2014).
Moreover, the hypothalamus has been proposed to play a central role in the relationship between obesity, inflammation, and cognition. That is, inflammatory cytokines and infiltrating immune cells are thought to act in hypothalamus to activate local inflammation. This inflammation then causes synaptic remodeling and degeneration in hypothalamus, thereby affecting any regions to which the hypothalamus projects, including brain regions important in cognition (Miller and Spencer, 2014). Additionally, greater hypothalamic damage was associated with higher levels of inflammatory cytokines and worse cognitive performance in obese subjects (Puig et al., 2015).
Thus, there is strong evidence linking obesity with both peripheral and central inflammation, and with downstream effects including insulin resistance and development of the metabolic syndrome, and cognitive impairments. The short-term inflammatory effects of dietary components are likely to have different effects from the long-term chronic inflammation associated with obesity, though both aspects of the inflammatory cascade are likely important.
B.3. Sex differences in inflammation, obesity, and Alzheimer’s disease
The role of inflammation in obesity is observed in both males and females, but there are significant sex differences in obesity-induced inflammatory responses. For example, several studies point to greater inflammation in response to obesity in women than in men (Ahonen et al., 2012; Khera et al., 2009; Mascarenhas-Melo et al., 2013; Petty et al., 2010; Rudnicka et al., 2011). Higher levels of inflammation also correlate more strongly with risk of T2D in women than in men, independent of BMI or adiposity (Thorand et al., 2007). Interestingly, non-diabetic premenopausal women are protected against metabolic disease and inflammation, but this protective effect is lost in the presence of either, T2D or menopause (Mascarenhas-Melo et al., 2013). Thus, it appears that obesity not only induces a greater inflammatory response in women, but higher levels of inflammation are also associated with an increased risk of metabolic disturbances, including T2D, in women.
Though no studies have directly examined sex differences in inflammation in the context of AD, a few studies have evaluated this relationship in terms of cognitive performance. Some research suggests that this relationship is stronger in women. For example, high inflammation was associated with poor cognitive performance in women but not men (Canon and Crimmins, 2011; Trollor et al., 2011). Similarly, another study found a correlation between high inflammation and mild cognitive impairment only in women (Trollor et al., 2010). In contrast, men may be vulnerable to other neural consequences of neuroinflammation. In support of this idea, the association between inflammatory cytokines and decreased brain volume is reportedly stronger in men (Jefferson et al., 2007). In an AD transgenic mouse model, the immune system is more impaired in male versus female mice (Giménez-Llort et al., 2008). Overall, findings point to increased inflammation in response to obesity in females, as well as a greater association between inflammation and cognitive decline in women. These sex differences may be mediated in part by sex steroid hormones, as estrogens and androgens are known to modulate inflammation (Spence and Voskuhl, 2012; Tsilidis et al., 2013).
The anti-inflammatory role of estrogens has been well established in the literature (Arevalo et al., 2010; Ritzel et al., 2013), but this effect may be especially important in the context of obesity. For example, estradiol has been demonstrated to have anti-inflammatory effects in adipose tissue, neurons, and in the cardiovascular system, that may protect these tissues from the pro-inflammatory effects of high fat diet and obesity (Brown and Clegg, 2010). In support of this idea, ovariectomized female mice and males show higher weight gain on high fat diet, whereas mice treated with estrogen do not develop adipocyte hypertrophy nor adipose tissue inflammation, and are protected against liver steatosis and insulin resistance (Stubbins et al., 2011). Moreover, estrogen exerts its anti-inflammatory actions via ERα, as demonstrated by the fact that mice lacking ERα have increased adipose tissue inflammation even before obesity (Davis et al., 2013). Interestingly, mice lacking ERα specifically in adipocytes also have increased inflammation, and this is especially true in male mice (Davis et al., 2013). Pregnant mice on high fat diet show decreases in visceral adipose hypertrophy, inflammation, and less glucose intolerance during late gestation, a time that correlates with increases in visceral adipose tissue ERα signaling (Pedroni et al., 2014). The role of estrogens in inflammation is further supported by the fact that both ERα and ERβ are found on monocytes and macrophages, and estrogens act on these receptors to block pro-inflammatory responses (Vegeto et al., 2003). Additionally, estrogens modulate inflammation in the context of AD, such that ovariectomized mice have increased microglial reactivity around Aβ plaques, but this is reversed by estradiol treatment (Vegeto et al., 2008).
Like estrogens, androgens also have anti-inflammatory effects that may be important in the context of obesity and AD. Numerous studies have found that testosterone levels in men are inversely associated with C-reactive protein (CRP) (Kupelian et al., 2010; Tsilidis et al., 2013; Zhang et al., 2012). Even in young men, clinically low testosterone levels are associated with increased expression of TNFα and other inflammatory factors (Bobjer et al., 2013). Older men with androgen deficiency have higher levels of inflammatory cytokines (Maggio et al., 2006), which are reduced by testosterone treatment (Malkin et al., 2004). Moreover, low testosterone levels were shown to correlate with increased mortality, independent of metabolic syndrome, diabetes, or cardiovascular disease, but dependent on levels of the inflammatory enzymes, IL-6 and CRP (Laughlin et al., 2008), pointing to the interplay between androgens and inflammation. Experimental evidence suggests that androgens can reduce indices of inflammation (Norata et al., 2006; Schwinge et al., 2015). There is some evidence that testosterone treatment in aging men can lower inflammatory markers such as CRP (Giltay et al., 2008; Kalinchenko et al., 2010), though efficacy remains to be firmly established.
Testosterone and inflammation have also been demonstrated to interact in the context of obesity. Men with low testosterone are more likely to have metabolic syndrome and high levels of CRP (Laaksonen et al., 2003). In men with metabolic syndrome, lower levels of testosterone were associated with higher levels of IL-6 (Gautier et al., 2013). Similarly, men with T2D also have lower testosterone and higher CRP levels (Bhatia et al., 2006). In animal models, inflammatory effects of obesity can be attenuated by testosterone treatment (Vignozzi et al., 2011). Research on the association of testosterone and inflammation in AD are limited, however, one study demonstrated that men with AD have lower testosterone and luteinizing hormone levels, the latter of which inversely correlate with levels of TNFα (Butchart et al., 2013). Our lab recently demonstrated that testosterone depletion exacerbates metabolic, pro-inflammatory, and peripheral nerve injury outcomes of DIO in male mice, effects that were reversed by testosterone treatment (Jayaraman et al., 2014). Moreover, we have found that DIO-induced increases in Aβ levels are associated with neuroinflammation, exacerbated by testosterone loss, and prevented by testosterone treatment (unpublished observations). Thus, the anti-inflammatory effects of testosterone are likely to be important in the relationship between obesity and AD.
C. Interactions between inflammation and apoE in obesity and AD
In addition to being independent factors in AD pathogenesis, apoE4 and inflammation also have important interactions with each other and with obesity (Figure 1). One established function of apoE is regulation of inflammation. In support of this role, glia from apoE knock-out mice exhibit increased pro-inflammatory responses after exposures to Aβ (LaDu et al., 2001) and LPS (Lynch et al., 2001). Importantly, apoE isoforms differ in their inflammatory effects. That is, apoE4 is associated with greater levels of pro-inflammatory cytokines, both in humans (Colton et al., 2004; Gale et al., 2014) and in mouse models (Colton et al., 2004; Lynch et al., 2003; Ophir et al., 2005; Vitek et al., 2009). Interestingly, apoE4 carriers have lower expression of apoE and, as young adults, increased levels of pro-inflammatory cytokines that decrease with age, though this study may be limited by a small sample size (Ringman et al., 2012). However, apoE can also take on a pro-inflammatory role when overproduced by activated microglia, and this pro-inflammatory response is stronger in the presence of apoE4 than apoE3 (Guo et al., 2004). Additionally, LPS stimulation in the presence of apoE4 is associated with increased endoplasmic reticulum stress and macrophage cell death (Cash et al., 2012), greater neuron damage (Maezawa et al., 2006a), and failure to regenerate dendrites (Maezawa et al., 2006b). Effects of apoE on inflammation may vary by cell type, as one study found that apoE3 astrocytes displayed increased astrogliosis after LPS, while apoE4 astrocytes had no response (Ophir et al., 2003). Thus, apoE appears to be an important regulator of inflammatory processes, with apoE4 generally having a more pro-inflammatory effect than apoE3.
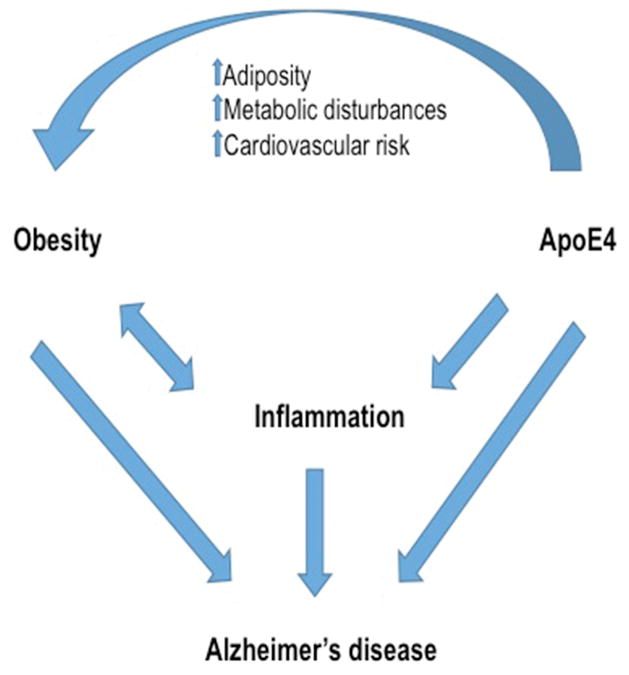
Figure 1. Interactions between obesity, apoE4 and inflammation in Alzheimer’s disease.
Alzheimer’s disease (AD) risk and pathology are increased by a number of factors and pathways, many of which interact with each other. As diagrammed here and described in the text, apoE4 and obesity independently increase AD risk as well as inflammation, which also contributes to AD risk. Moreover, apoE4 carriers have been shown to be more at risk for a number of obesity-related complications, including increased adiposity, metabolic disturbances, and cardiovascular risk. Thus, apoE4 and obesity appear to both independently and cooperatively increase inflammation and AD risk.
The relationship between apoE and inflammation is also important in the context of AD (Keene et al., 2011). Among AD patients, those with the apoE4 allele had greater baseline and stimulated levels of IL-1β (Olgiati et al., 2010). ApoE co-localizes with microglia around Aβ plaques (Liu et al., 2013), and apoE4 mice have greater microgliosis and astrogliosis in response to Aβ than do apoE3 mice (Belinson and Michaelson, 2009). Additionally, mice with both human apoE4 and familial AD mutations have higher levels of pro-inflammatory cytokines and increased microglial reactivity surrounding Aβ plaques than do their apoE3 counterparts (Rodriguez et al., 2014). Further, apoE4 macrophages are less effective at clearing Aβ (Zhao et al., 2009). ApoE4, but not apoE2 or apoE3, activates a pro-inflammatory factor in pericytes that causes blood brain barrier breakdown (Bell et al., 2012). Thus, apoE4’s pro-inflammatory actions may contribute to the finding that apoE4 carriers have increased blood brain barrier breakdown (Halliday et al., 2013). Interestingly, non-steroidal anti-inflammatory drugs have been found to reduce risk for AD only in apoE4 carriers (Barger and Harmon, 1997; Schram et al., 2007), reinforcing the possibility of significant interactions between apoE4 and inflammation in AD.
The interplay between apoE and inflammation in the context of obesity has not been well studied. Unfortunately, available evidence is inconsistent. Several studies indicate that apoE knockout mice show reduced gains in fat mass and body weight in response to HFD (Arbones-Mainar et al., 2008; Bartelt et al., 2010; Pereira et al., 2012; Wang et al., 2012). Nonetheless, how apoE status affects obesity-associated inflammation is unclear, as one study reported that apoE knockout mice show a stronger pro-inflammatory response in adipose tissue than wildtype mice (Pereira et al., 2012), whereas another study found that apoE knockout mice on HFD have lower levels of inflammatory cytokines in adipose tissue and skeletal muscle (Wang et al., 2012). Interestingly, apoE4 mice fed a HFD are more susceptible to motor deficits after stroke, and this is accompanied by increased inflammation (Dhungana et al., 2013). Overall, the literature demonstrates an interaction between apoE and obesity, but more research is needed to clarify how this relationship affects inflammation.
Interestingly, there appear to be sex differences in the interactions between apoE and inflammation. For example, among nonagenarians, those who carry the apoE4 allele have lower levels of the inflammatory marker CRP, an outcome that is more robust in women (Rontu et al., 2006). Experimental work in animal models has also found sex differences in this regard, with apoE4 being associated with a greater pro-inflammatory response in males but not in females (Colton et al., 2005), suggesting that apoE4 carrying males may have an exaggerated inflammatory response. In addition, female apoE4 mice are less responsive to the anti-inflammatory effects of 17-β-estradiol (Brown et al., 2008), and male apoE4 mice are less responsive to the anti-inflammatory effects of DHT (Brown et al., 2007). Thus, both male and female apoE4 carriers may be less responsive to the anti-inflammatory effects of sex steroid hormones (Brown et al., 2008; 2007).
6. Conclusions
As reviewed in this article, there are significant sex differences in obesity and AD, as well as in mechanisms that may be underlying these disease processes (see Table 1 for a summary of sex differences in the AD risk factors discussed in this article). For one, obesity, and specifically central adiposity at midlife are significant risk factors for AD later in life (Emmerzaal et al., 2015; Fitzpatrick et al., 2009; Meng et al., 2014; Profenno et al., 2010; Xu et al., 2011). This relationship may be especially important for women, as women are both more at risk for AD (Li and Singh, 2014), and experience an increase in central adiposity at menopause (Meyer et al., 2011; Sugiyama and Agellon, 2012). While there exists some evidence of sex differences in obesity-associated AD risk (Barron et al., 2013; Hwang et al., 2010; Wolf et al., 2012), results are inconclusive and more work is needed to determine the nature of this relationship. Two factors that may be mechanistically important in the relationship between obesity and AD are apoE4 and inflammation. First, apoE4 is a risk factor for AD (Saunders et al., 1993; Strittmatter et al., 1993) and may contribute to obesity-related complications and metabolic syndrome (de-Andrade et al., 2000; Niu et al., 2009; Sima et al., 2007). Moreover, sex differences exist in the effects of apoE, with the apoE4-associated risk of AD being significantly higher in women (Altmann et al., 2014; Payami et al., 1994). Chronic inflammation is associated with both AD (Glass et al., 2010) and obesity (Engström et al., 2003; Yaffe et al., 2003) and several studies have demonstrated that the pro-inflammatory effects of obesity are stronger in women (Ahonen et al., 2012; Khera et al., 2009; Mascarenhas-Melo et al., 2013; Rudnicka et al., 2011). Finally, apoE and inflammation also interact with each other, such that apoE4 is associated with greater pro-inflammatory responses (Cash et al., 2012; Colton et al., 2004; Gale et al., 2014; Lynch et al., 2003). In summary, apoE4 and inflammation are important factors in the association between obesity and AD, and their differing actions in males and females may be significant in explaining sex differences in these conditions.
Table 1.
Sex differences in the Alzheimer’s disease risk factors obesity, apolipoprotein E (apoE4), and inflammation.
| AD risk factor | Sex differences | |
|---|---|---|
| Males | Females | |
| Obesity | Reduced by testosterone Causes greater visceral fat deposits Causes stronger metabolic and neurological effects in male rodents |
Reduced by estrogens Causes greater subcutaneous fat deposits Food cues cause greater neural activation Increased risk of obesity after menopause |
| ApoE4 | Alzheimer risk increased ~4X by two apoE4 alleles Lower testosterone in apoE4 carriers Testosterone has beneficial cognitive effects in apoE4 humans and rodents |
Alzheimer risk increased ~4X by one apoE4 alleles and ~10X by two apoE4 alleles Greater increase in cognitive decline Estrogen may not be beneficial in apoE4-carrying women |
| Inflammation | Reduced by testosterone Inflammatory effects of obesity attenuated by testosterone |
Reduced by estrogens Increased obesity associated with greater inflammation in women |
Though this article has focused mainly on apoE4 and inflammation as mechanisms underlying sex differences in obesity and AD, a number of other factors may also play important roles in this relationship. Vascular factors including blood brain barrier breakdown, are associated with both, obesity and AD. As previously discussed, a link between the blood brain barrier, inflammation and apoE4 was recently discovered. That is, apoE4 is associated with increased inflammation around pericytes that then leads to blood brain barrier breakdown (Halliday et al., 2013; Zlokovic, 2013). Though sex differences in blood brain barrier function are unknown, it has been established that men are at increased risk of a number of cardiovascular risk factors (Crea et al., 2015). Thus, it may be the case that, though obesity is a risk factor for AD in both men and women, there may be different pathways between the sexes through which obesity acts to influence AD risk. For example, several studies point to women having greater inflammation in response to obesity, whereas obese men have more cardiovascular risk factors. Yet, both inflammation and vascular risk factors are associated with AD, such that obesity may use different signaling pathways in men and women to drive AD pathogenesis. Moreover, apoE4 will also interact with these pathways and its interactions are likely to vary between the sexes.
Though a number of genetic and environmental risk factors for sporadic AD have been identified, none of these is conclusive in predicting who will develop the disease. Even amongst those who carry the apoE4 allele, there is only a 30% lifetime risk of AD (Genin 2011), so that a number of other factors must also influence risk. Research has generally focused on examining risk factors in isolation, however, AD is a multifactorial disease. Continuing AD research must consider not only the interactions between genetic and environmental factors, but also how these are differentially affected by sex.
Highlights.
Sex and obesity independently and interactively affect Alzheimer’s disease.
Alzheimer’s disease risk is increased by both apolipoprotein E and inflammation.
Sex and obesity interactions may involve apolipoprotein E and inflammation.
Acknowledgments
This work was supported by NIA grants AG034103 (CJP) and AG026572 (RD Brinton and CJP/Project 3).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
V. Alexandra Moser, Email: vmoser@usc.edu.
Christian J. Pike, Email: cjpike@usc.edu.
References
- Adler-Wailes DC, Periwal V, Ali AH, Brady SM, McDuffie JR, Uwaifo GI, Tanofsky-Kraff M, Salaita CG, Hubbard VS, Reynolds JC, Chow CC, Sumner AE, Yanovski JA. Sex-Associated Differences in Free Fatty Acid Flux of Obese Adolescents. J Clin Endocr Metab. 2013;98:1676–1684. doi: 10.1210/jc.2012-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen T, Vanhala M, Kautiainen H, Kumpusalo E, Saltevo J. Sex Differences in the Association of Adiponectin and Low-Grade Inflammation With Changes in the Body Mass Index From Youth to Middle Age. GENM. 2012;9:1–8. doi: 10.1016/j.genm.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E _4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cerebr Blood F Met. 2015;35:86–94. doi: 10.1038/jcbfm.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann A, Tian L, Henderson VW, Greicius MD Alzheimer’s Disease Neuroimaging Initiative Investigators. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual-Cladera E, Lladó I, Gianotti M, Proenza AM. Sex differences in the effect of high-fat diet feeding on rat white adipose tissue mitochondrial function and insulin sensitivity. Metabolis. 2012;61:1108–1117. doi: 10.1016/j.metabol.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Amtul Z, Westaway D, Cechetto DF, Rozmahel RF. Oleic Acid Ameliorates Amyloidosis in Cellular and Mouse Models of Alzheimer’s Disease. Brain Pathol. 2010;21:321–329. doi: 10.1111/j.1750-3639.2010.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André C, Dinel AL, Ferreira G, Layé S, Castanon N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun. 2014;41:10–21. doi: 10.1016/j.bbi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Arbones-Mainar JM, Johnson LA, Altenburg MK, Maeda N. Differential modulation of diet-induced obesity and adipocyte functionality by human apolipoprotein E3 and E4 in mice. Int J Obes Relat Metab Disord. 2008;32:1595–1605. doi: 10.1038/ijo.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochem Biophys Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Atabek ME, Özkul Y, Eklioğlu BS, Kurtoğlu S, Baykara M. Association between apolipoprotein E polymorphism and subclinic atherosclerosis in patients with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2012;4:8–13. doi: 10.4274/jcrpe.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Muñoz E, Arias C. When astrocytes become harmful: functional and inflammatory responses that contribute to Alzheimer’s disease. Ageing Res Rev. 2014;18:29–40. doi: 10.1016/j.arr.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Ahmed-Sorour H. Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion. Diabetologia. 1980;19:475–481. doi: 10.1007/BF00281829. [DOI] [PubMed] [Google Scholar]
- Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- Barnard ND, Bunner AE, Agarwal U. Saturated and trans fats and dementia: a systematic review. Neurobiol Aging. 2014;35:S65–S73. doi: 10.1016/j.neurobiolaging.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Barron AM, Rosario ER, Elteriefi R, Pike CJ. Sex-Specific Effects of High Fat Diet on Indices of Metabolic Syndrome in 3xTg-AD Mice: Implications for Alzheimer’s Disease. PLoS ONE. 2013;8:e78554. doi: 10.1371/journal.pone.0078554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A, Beil FT, Schinke T, Roeser K, Ruether W, Heeren J, Niemeier A. Apolipoprotein E-dependent inverse regulation of vertebral bone and adipose tissue mass in C57Bl/6 mice: Modulation by diet-induced obesity. Bone. 2010;47:736–745. doi: 10.1016/j.bone.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Belinson H, Michaelson DM. ApoE4-dependent Aβ-mediated neurodegeneration is associated with inflammatory activation in the hippocampus but not the septum. J Neural Transm. 2009;116:1427–1434. doi: 10.1007/s00702-009-0218-9. [DOI] [PubMed] [Google Scholar]
- Bell RD. The imbalance of vascular molecules in Alzheimer’s disease. J Alzheimers Dis. 2012;32:699–709. doi: 10.3233/JAD-2012-121060. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-León J, Mitchell AJ, Hernández-Gallego J, Bermejo-Pareja F. Obesity and impaired cognitive functioning in the elderly: a population-based cross-sectional study (NEDICES) Eur J Neurol. 2013;20:899–e77. doi: 10.1111/ene.12083. [DOI] [PubMed] [Google Scholar]
- Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M, Hogan MF, Schjeide BMM, Hooli B, DiVito J, Ionita I, Jiang H, Laird N, Moscarillo T, Ohlsen KL, Elliott K, Wang X, Hu-Lince D, Ryder M, Murphy A, Wagner SL, Blacker D, Becker KD, Tanzi RE. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser LM, Gill DP, Monsell SE, Brenowitz W, Meranus DH, Kukull W, Gustafson DR. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:36–43. doi: 10.1097/WAD.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, O’Brien R, Zonderman AB. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33:720–731. doi: 10.1016/j.neurobiolaging.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P. Low Testosterone and High C-Reactive Protein Concentrations Predict Low Hematocrit in Type 2 Diabetes. Diabetes Care. 2006;29:2289–2294. doi: 10.2337/dc06-0637. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Blair CK, Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E, Boerwinkle E. APOE genotype and cognitive decline in a middle-aged cohort. Neurology. 2005;64:268–276. doi: 10.1212/01.WNL.0000149643.91367.8A. [DOI] [PubMed] [Google Scholar]
- Blasko I, Beer R, Bigl M, Apelt J, Franz G, Rudzki D, Ransmayr G, Kampfl A, Schliebs R. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease beta-secretase (BACE-1) J Neural Transm. 2004;111:523–536. doi: 10.1007/s00702-003-0095-6. [DOI] [PubMed] [Google Scholar]
- Blázquez G, Cañete T, Tobeña A, Giménez-Llort L, Fernández-Teruel A. Cognitive and emotional profiles of aged Alzheimer’s disease (3xTgAD) mice: Effects of environmental enrichment and sexual dimorphism. Behav Brain Res. 2014;268:185–201. doi: 10.1016/j.bbr.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Bloor ID, Symonds ME. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm Behav. 2014;66:95–103. doi: 10.1016/j.yhbeh.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Blouin K, Richard C, Brochu G, Hould FS, Lebel S, Marceau S, Biron S, Luu-The V, Tchernof A. Androgen inactivation and steroid-converting enzyme expression in abdominal adipose tissue in men. J Endocrinol. 2006;191:637–649. doi: 10.1677/joe.1.06365. [DOI] [PubMed] [Google Scholar]
- Bobjer J, Katrinaki M, Tsatsanis C, Lundberg Giwercman Y, Giwercman A. Negative Association between Testosterone Concentration and Inflammatory Markers in Young Men: A Nested Cross-Sectional Study. PLoS ONE. 2013;8:e61466. doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- Botella-Carretero JI, Balsa JA, Gómez-Martin JM, Peromingo R, Huerta L, Carrasco M, Arrieta F, Zamarron I, Martin-Hidalgo A, Vazquez C. Circulating free testosterone in obese men after bariatric surgery increases in parallel with insulin sensitivity. J Endocrinol Invest. 2013;36:227–232. doi: 10.3275/8469. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Guillot-Sestier MV, Town T. Brain injury, neuroinflammation and Alzheimer’s disease. Front Aging Neurosci. 2013;5:26. doi: 10.3389/fnagi.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol Psychiatry. 2013;18:864–874. doi: 10.1038/mp.2012.162. [DOI] [PubMed] [Google Scholar]
- Brown CM, Choi E, Xu Q, Vitek MP, Colton CA. The APOE4 genotype alters the response of microglia and macrophages to 17β-estradiol. Neurobiol Aging. 2008;29:1783–1794. doi: 10.1016/j.neurobiolaging.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Xu Q, Okhubo N, Vitek MP, Colton CA. Androgen-Mediated Immune Function Is Altered by the Apolipoprotein E Gene. Endocrinology. 2007;148:3383–3390. doi: 10.1210/en.2006-1200. [DOI] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugg B, Dubreuil YL, Huber G, Wollman EE, Delhaye-Bouchaud N, Mariani J. Inflammatory processes induce beta-amyloid precursor protein changes in mouse brain. Proc Natl Acad Sci USA. 1995;92:3032–3035. doi: 10.1073/pnas.92.7.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, Weller K, Ellacott KLJ. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt MS, Foster JK, Clarnette RM, Chubb SAP, Bruce DG, Drummond PD, Martins RN, Yeap BB. Interaction between Testosterone and Apolipoprotein E ε4 Status on Cognition in Healthy Older Men. J Clin Endocr Metab. 2006;91:1168–1172. doi: 10.1210/jc.2005-1072. [DOI] [PubMed] [Google Scholar]
- Burkhardt MS, Foster JK, Laws SM, Baker LD, Craft S, Gandy SE, Stuckey BGA, Clarnette R, Nolan D, Hewson-Bower B, Martins RN. Oestrogen replacement therapy may improve memory functioning in the absence of APOE epsilon4. J Alzheimers Dis. 2004;6:221–228. doi: 10.3233/jad-2004-6302. [DOI] [PubMed] [Google Scholar]
- Butchart J, Birch B, Bassily R, Wolfe L, Holmes C. Male sex hormones and systemic inflammation in Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27:153–156. doi: 10.1097/WAD.0b013e318258cd63. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Kavanaugh M, Block M, D’Angiulli A, Delgado-Chávez R, Torres-Jardón R, González-Maciel A, Reynoso-Robles R, Osnaya N, Villarreal-Calderon R, Guo R, Hua Z, Zhu H, Perry G, Diaz P. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis. 2012;28:93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- Canon ME, Crimmins EM. Sex differences in the association between muscle quality, inflammatory markers, and cognitive decline. J Nutr Health Aging. 2011;15:695–698. doi: 10.1007/s12603-011-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Lu H, Lewis TL, Li L. Intake of Sucrose-sweetened Water Induces Insulin Resistance and Exacerbates Memory Deficits and Amyloidosis in a Transgenic Mouse Model of Alzheimer Disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and Estrogen Regulate Alzheimer-Like Neuropathology in Female 3xTg-AD Mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Kreimer S, Villamagna A, Gentzschein E, Stanczyk FZ, Pike CJ. Sex differences in β-amyloid accumulation in 3xTg-AD mice: Role of neonatal sex steroid hormone exposure. Brain Res. 2010;1366:233–245. doi: 10.1016/j.brainres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C, Katz PS, Dutta S, Katakam PV, Moreira PI, Busija DW. Increased susceptibility to amyloid-β toxicity in rat brain microvascular endothelial cells under hyperglycemic conditions. J Alzheimers Dis. 2014;38:75–83. doi: 10.3233/JAD-130464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JG, Kuhel DG, Basford JE, Jaeschke A, Chatterjee TK, Weintraub NL, Hui DY. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J Biol Chem. 2012;287:27876–27884. doi: 10.1074/jbc.M112.377549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N, Lasselin J, Capuron L. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol. 2014;5:74–74. doi: 10.3389/fendo.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel A, Dhindsa S, Topiwala S, Chaudhuri A, Dandona P. Testosterone Concentration in Young Patients With Diabetes. Diabetes Care. 2008;31:2013–2017. doi: 10.2337/dc08-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflamm. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- Colton CA, Brown CM, Vitek MP. Sex steroids, APOE genotype and the innate immune system. Neurobiol Aging. 2005;26:363–372. doi: 10.1016/j.neurobiolaging.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Colton CA, Needham LK, Brown C, Cook D, Rasheed K, Burke JR, Strittmatter WJ, Schmechel DE, Vitek MP. APOE genotype-specific differences in human and mouse macrophage nitric oxide production. J Neuroimmunol. 2004;147:62–67. doi: 10.1016/j.jneuroim.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, Schellenberg GD, Jin LW, Kovacina KS, Craft S. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-ε4 allele. Am J Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corella D, Ordovas JM. Aging and cardiovascular diseases: the role of gene-diet interactions. Ageing Res Rev. 2014;18:53–73. doi: 10.1016/j.arr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Corella D, Portolés O, Arriola L, Chirlaque MD, Barrricarte A, Francés F, Huerta JM, Larrañaga N, Martínez C, Martinez-Camblor P, Molina E, Navarro C, Quirós JR, Rodríguez L, Sánchez MJ, Ros E, Sala N, González CA, Moreno-Iribas C. Saturated fat intake and alcohol consumption modulate the association between the APOE polymorphism and risk of future coronary heart disease: a nested case-control study in the Spanish EPIC cohort. The J Nutr Biochem. 2011;22:487–494. doi: 10.1016/j.jnutbio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Crea F, Battipaglia I, Andreotti F. Sex differences in mechanisms, presentation and management of ischaemic heart disease. Atherosclerosis. 2015;241:157–168. doi: 10.1016/j.atherosclerosis.2015.04.802. [DOI] [PubMed] [Google Scholar]
- Currais A, Prior M, Lo D, Jolivalt C, Schubert D, Maher P. Diabetes exacerbates amyloid and neurovascular pathology in aging-accelerated mice. Aging Cell. 2012;11:1017–1026. doi: 10.1111/acel.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD Alzheimer’s Disease Neuroimaging Initiative. Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, Zheng W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–122. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KE, Neinast MD, Sun K, Skiles WM, Bills JD, Zehr JA, Zeve D, Hahner LD, Cox DW, Gent LM, Xu Y, Wang ZV, Khan SA, Clegg DJ. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2:227–242. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maddalena C, Vodo S, Petroni A, Aloisi AM. Impact of testosterone on body fat composition. J Cell Physiol. 2012;227:3744–3748. doi: 10.1002/jcp.24096. [DOI] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJA, Velloso LA. Consumption of a Fat-Rich Diet Activates a Proinflammatory Response and Induces Insulin Resistance in the Hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- de-Andrade FM, Larrandaburu M, Callegari-Jacques SM, Gastaldo G, Hutz MH. Association of apolipoprotein E polymorphism with plasma lipids and Alzheimer’s disease in a Southern Brazilian population. Braz J Med Biol Res. 2000;33 doi: 10.1590/s0100-879x2000000500007. [DOI] [PubMed] [Google Scholar]
- Debette S, Beiser A, Hoffmann U, DeCarli C, O’Donnell CJ, Massaro JM, Au R, Himali JJ, Wolf PA, Fox CS, Seshadri S. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68:136–144. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhungana H, Rolova T, Savchenko E, Wojciechowski S, Savolainen K, Ruotsalainen AK, Sullivan PM, Koistinaho J, Malm T. Western-type diet modulates inflammatory responses and impairs functional outcome following permanent middle cerebral artery occlusion in aged mice expressing the human apolipoprotein E4 allele. J Neuroinflamm. 2013;10:102. doi: 10.1186/1742-2094-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Comijs HC, Deeg DJH, Kok A, Yaffe K, Penninx BW. Contribution of Metabolic Syndrome Components to Cognition in Older Individuals. Diabetes Care. 2007;30:2655–2660. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- Doruk H, Naharci MI, Bozoglu E, Isik AT, Kilic S. The relationship between body mass index and incidental mild cognitive impairment, Alzheimer’s disease and vascular dementia in elderly. J Nutr Health Aging. 2010;14:834–838. doi: 10.1007/s12603-010-0113-y. [DOI] [PubMed] [Google Scholar]
- Du J, Chang J, Guo S, Zhang Q, Wang Z. ApoE 4 reduces the expression of Aβ degrading enzyme IDE by activating the NMDA receptor in hippocampal neurons. Neurosci Lett. 2009;464:140–145. doi: 10.1016/j.neulet.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Edland SD. Insulin-degrading enzyme, apolipoprotein E, and Alzheimer’s disease. J Mol Neurosci. 2004;23:213–217. doi: 10.1385/JMN:23:3:213. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Veerhuis R, van Exel E, Hoozemans JJM, Rozemuller AJM, van Gool WA. The early involvement of the innate immunity in the pathogenesis of late-onset Alzheimer’s disease: neuropathological, epidemiological and genetic evidence. Curr Alzheimer Res. 2011;8:142–150. doi: 10.2174/156720511795256080. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Elosua R, Demissie S, Cupples LA, Meigs JB, Wilson PWF, Schaefer EJ, Corella D, Ordovas JM. Obesity modulates the association among APOE genotype, insulin, and glucose in men. Obes Res. 2003;11:1502–1508. doi: 10.1038/oby.2003.201. [DOI] [PubMed] [Google Scholar]
- Emmerzaal TL, Kiliaan AJ, Gustafson DR. 2003–2013: a decade of body mass index, Alzheimer’s disease, and dementia. J Alzheimers Dis. 2015;43:739–755. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- Engström G, Hedblad B, Stavenow L, Lind P, Janzon L, Lindgärde F. Inflammation-sensitive plasma proteins are associated with future weight gain. Diabetes. 2003;52:2097–2101. doi: 10.2337/diabetes.52.8.2097. [DOI] [PubMed] [Google Scholar]
- Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM. Obesity Elicits Interleukin 1-Mediated Deficits in Hippocampal Synaptic Plasticity. J Neurosci. 2014;34:2618–2631. doi: 10.1523/JNEUROSCI.4200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10:562–570. doi: 10.1016/j.jalz.2013.05.1772. [DOI] [PubMed] [Google Scholar]
- Feng L, Chong MS, Lim WS, Lee TS, Collinson SL, Yap P, Ng TP. Metabolic syndrome and amnestic mild cognitive impairment: singapore longitudinal ageing study-2 findings. J Alzheimer Dis. 2013;34:649–657. doi: 10.3233/JAD-121885. [DOI] [PubMed] [Google Scholar]
- Fernandez-Martinez M, Elcoroaristizabal Martin X, Blanco Martin E, Galdos Alcelay L, Ugarriza Serrano I, Gomez Busto F, Alvarez-Alvarez M, Molano Salazar A, Bereincua Gandarias R, Ingles Borda S, Uterga Valiente JM, Indakoetxea Juanbeltz B, Gomez Beldarrain MA, Moraza Lopez J, Barandiaran Amillano M, de Pancorbo MM. Oestrogen receptor polymorphisms are an associated risk factor for mild cognitive impairment and Alzheimer disease in women APOE 4 carriers: a case-control study. BMJ Open. 2013;3:e003200–e003200. doi: 10.1136/bmjopen-2013-003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C, Nacmias B, Bagnoli S, Piaceri I, Lombardi G, Pradella S, Tedde A, Sorbi S. Imaging and Cognitive Reserve Studies Predict Dementia in Presymptomatic Alzheimer’s Disease Subjects. Neurodegener Dis. 2014;13:157–159. doi: 10.1159/000353690. [DOI] [PubMed] [Google Scholar]
- Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. FASEB J. 2004;18:1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci. 2011;125:943–955. doi: 10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: A review of proposed mechanisms. Nutr Neurosci. 2014;17:241–251. doi: 10.1179/1476830513Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SC, Gao L, Mikacenic C, Coyle SM, Rafaels N, Murray Dudenkov T, Madenspacher JH, Draper DW, Ge W, Aloor JJ, Azzam KM, Lai L, Blackshear PJ, Calvano SE, Barnes KC, Lowry SF, Corbett S, Wurfel MM, Fessler MB. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J Allergy Clin Immunol. 2014;134:127–134. doi: 10.1016/j.jaci.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cáceres C, Yi CX, Tschöp MH. Hypothalamic Astrocytes in Obesity. Endocrinol Metab Clin North Am. 2013;42:57–66. doi: 10.1016/j.ecl.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Gautier A, Bonnet F, Dubois S, Massart C, Grosheny C, Bachelot A, Aubé C, Balkau B, Ducluzeau PH. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin Endocrinol. 2013;78:373–378. doi: 10.1111/j.1365-2265.2012.04401.x. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res. 2013;243:91–96. doi: 10.1016/j.bbr.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O, Bullido MJ, Engelborghs S, De Deyn P, Berr C, Pasquier F, Dubois B, Tognoni G, Fiévet N, Brouwers N, Bettens K, Arosio B, Coto E, Del Zompo M, Mateo I, Epelbaum J, Frank-Garcia A, Helisalmi S, Porcellini E, Pilotto A, Forti P, Ferri R, Scarpini E, Siciliano G, Solfrizzi V, Sorbi S, Spalletta G, Valdivieso F, Vepsäläinen S, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Hanon O, Piccardi P, Annoni G, Seripa D, Galimberti D, Licastro F, Soininen H, Dartigues JF, Kamboh MI, Van Broeckhoven C, Lambert JC, Amouyel P, Campion D. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry. 2011;16:903–907. doi: 10.1038/mp.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebranious N, Mukesh B, Giampietro PF, Glurich I, Mickel SF, Waring SC, McCarty CA. A pilot study of gene/gene and gene/environment interactions in Alzheimer disease. Clin Med Res. 2011;9:17–25. doi: 10.3121/cmr.2010.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay EJ, Haider A, Saad F, Gooren LJ. C-reactive protein levels and ageing male symptoms in hypogonadal men treated with testosterone supplementation. Andrologia. 2008;40:398–400. doi: 10.1111/j.1439-0272.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- Giménez-Llort L, Arranz L, Maté I, De la Fuente M. Gender-specific neuroimmunoendocrine aging in a triple-transgenic 3xTg-AD mouse model for Alzheimer’s disease and its relation with longevity. Neuroimmunomodulat. 2008;15:331–343. doi: 10.1159/000156475. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms Underlying Inflammation in Neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov NP, Katsya GV, Chagina NA, Gooren LJ. Three definitions of metabolic syndrome applied to a sample of young obese men and their relation with plasma testosterone. Aging Male. 2008;11:118–122. doi: 10.1080/13685530802204629. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MOW, Rothhaar TL, Grösgen S, Burg VK, Hundsdörfer B, Haupenthal VJ, Friess P, Kins S, Grimm HS, Hartmann T. Trans fatty acids enhance amyloidogenic processing of the Alzheimer amyloid precursor protein (APP) J Nutr Biochem. 2012;23:1214–1223. doi: 10.1016/j.jnutbio.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Tang Fui M, Dupuis P. Lowered testosterone in male obesity: Mechanisms, morbidity and management. Asian J Androl. 2014;16:223. doi: 10.4103/1008-682X.122365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J, Zhao HL, Sui Y, He L, Lee HM, Lai FMM, Tong PCY, Chan JCN. Histopathological correlations of islet amyloidosis with apolipoprotein E polymorphisms in type 2 diabetic Chinese patients. Pancreas. 2013;42:1129–1137. doi: 10.1097/MPA.0b013e3182965e6e. [DOI] [PubMed] [Google Scholar]
- Guo L, LaDu MJ, Van Eldik LJ. A dual role for apolipoprotein e in neuroinflammation: anti- and pro-inflammatory activity. J Mol Neurosci. 2004;23:205–212. doi: 10.1385/JMN:23:3:205. [DOI] [PubMed] [Google Scholar]
- Gupta S, Knight AG, Gupta S, Keller JN, Bruce-Keller AJ. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem. 2012;120:1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson D, Lissner L, Bengtsson C, Björkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Bäckman K, Waern M, Ostling S, Guo X, Zandi P, Mielke MM, Bengtsson C, Skoog I. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73:1559–1566. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med. 2007;262:643–650. doi: 10.1111/j.1365-2796.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- Hadaegh F, Hasheminia M, Lotfaliany M, Mohebi R, Azizi F, Tohidi M. Incidence of Metabolic Syndrome over 9 Years Follow-Up; the Importance of Sex Differences in the Role of Insulin Resistance and Other Risk Factors. PLoS ONE. 2013;8:e76304. doi: 10.1371/journal.pone.0076304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR, Pomara N, Sagare AP, Mack WJ, Frangione B, Zlokovic BV. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013;70:1198–1200. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AJ, Bayer-Carter JL, Green PS, Montine TJ, Wilkinson CW, Baker LD, Watson GS, Bonner LM, Callaghan M, Leverenz JB, Tsai E, Postupna N, Zhang J, Lampe J, Craft S. Effect of Apolipoprotein E Genotype and Diet on Apolipoprotein E Lipidation and Amyloid Peptides. JAMA Neurol. 2013;70:972–980. doi: 10.1001/jamaneurol.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins MAW, Alosco ML, Spitznagel MB, Strain G, Devlin M, Cohen R, Crosby RD, Mitchell JE, Gunstad J. The Association Between Reduced Inflammation and Cognitive Gains After Bariatric Surgery. Psychosom Med. 2015;77:688–696. doi: 10.1097/PSY.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, Tschanz JT, Norton MC, Pieper CF, Munger RG, Breitner JCS, Welsh-Bohmer KA, Welsh-Bohmer KA. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease andcontributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata-Fukae C, Li HF, Hoe HS, Gray AJ, Minami SS, Hamada K, Niikura T, Hua F, Tsukagoshi-Nagai H, Horikoshi-Sakuraba Y, Mughal M, Rebeck GW, LaFerla FM, Mattson MP, Iwata N, Saido TC, Klein WL, Duff KE, Aisen PS, Matsuoka Y. Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 2008;1216:92–103. doi: 10.1016/j.brainres.2008.03.079. [DOI] [PubMed] [Google Scholar]
- Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund-Levi Y, Faxen-Irving G, Wahlund LO, Basun H, Eriksdotter M, Schultzberg M. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J Alzheimers Dis. 2013;35:697–713. doi: 10.3233/JAD-130131. [DOI] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Lee S, Hibar D, Dinov ID, Stein JL, Jack CR, Jr, Weiner MW, Toga AW, Thompson PM. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging. 2010;31:1326–1339. doi: 10.1016/j.neurobiolaging.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Lehmann DJ, Warden DR, McBroom J, Smith AD. Apolipoprotein E epsilon4 and testosterone interact in the risk of Alzheimer’s disease in men. Int J Geriatr Psychiatry. 2002;17:938–940. doi: 10.1002/gps.714. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer’s disease. Neuroendocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- Holland D, Desikan RS, Dale AM, McEvoy LK Alzheimer’s Disease Neuroimaging Initiative. Higher rates of decline for women and apolipoprotein E epsilon4 carriers. Am J Neuroradiol. 2013;34:2287–2293. doi: 10.3174/ajnr.A3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TM, Konanur VR, Taing L, Usui R, Kayser BD, Goran MI, Kanoski SE. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus. 2014;25:227–239. doi: 10.1002/hipo.22368. [DOI] [PubMed] [Google Scholar]
- Hughe TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72:1741–1746. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman K, Strickland S, Norris EH. The APOE _4/_4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J Cereb Blood Flow Metab. 2013;33:1251–1258. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC, Ho IK, Yang WS, Chiou LC. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity. 2010;18:463–469. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac V, Sim S, Zheng H, Zagorodnov V, Tai ES, Chee M. Adverse Associations between Visceral Adiposity, Brain Structure, and Cognitive Performance in Healthy Elderly. Front Aging Neurosci. 2011;3:12. doi: 10.3389/fnagi.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Kroenke C, Lin J, Epel ES, Kenna HA, Blackburn EH, Rasgon NL. Accelerated Cell Aging in Female APOE-ε4 Carriers: Implications for Hormone Therapy Use. PLoS ONE. 2013;8:e54713. doi: 10.1371/journal.pone.0054713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Lent-Schochet D, Pike CJ. Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J Neuroinflamm. 2014;11:162. doi: 10.1186/s12974-014-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Pike CJ. Alzheimer’s Disease and Type 2 Diabetes: Multiple Mechanisms Contribute to Interactions. Curr Diab Rep. 2014;14:476. doi: 10.1007/s11892-014-0476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, Larson MG, Meigs JB, Keaney JF, Lipinska I, Kathiresan S, Benjamin EJ, DeCarli C. Inflammatory biomarkers are associated with total brain volume: The Framingham Heart Study. Neurology. 2007;68:1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivalt CG, Lee CA, Beiswenger KK, Smith JL, Orlov M, Torrance MA, Masliah E. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: Parallels with Alzheimer’s disease and correction by insulin. J Neurosci Res. 2008;86:3265–3274. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, Calon F. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging. 2010;31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJG, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol. 2010;73:602–612. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Lindquist K, Harris TB, Launer L, Rosano C, Satterfield S, Yaffe K Health ABC Study. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch Neurol. 2009;66:329–335. doi: 10.1001/archneurol.2008.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21:207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and Biochemical Assessment of Hypogonadism in Men With Type 2 Diabetes: Correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30:911–917. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Malkin CJ, Channer KS, Jones TH. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol. 2005;63:239–250. doi: 10.1111/j.1365-2265.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- Keene CD, Cudaback E, Li X, Montine KS, Montine TJ. Apolipoprotein E isoforms and regulation of the innate immune response in brain of patients with Alzheimer’s disease. Curr Opin Neurobiol. 2011;21:920–928. doi: 10.1016/j.conb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Raine LB, Donovan SM, Hillman CH. IV The cognitive implications of obesity and nutrition in childhood. Monogr Soc Res Child Dev. 2014;79:51–71. doi: 10.1111/mono.12130. [DOI] [PubMed] [Google Scholar]
- Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, de Lemos JA. Sex Differences in the Relationship between C-Reactive Protein and Body Fat. J Clin Endocr Metab. 2009;94:3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased Tau Phosphorylation and Cleavage in Mouse Models of Type 1 and Type 2 Diabetes. Endocrinology. 2009;150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Backus C, Oh S, Feldman EL. Hyperglycemia-induced tau cleavage in vitro and in vivo: A possible link between diabetes and Alzheimer’s disease. J Alzheimers Dis. 2013;34:727–739. doi: 10.3233/JAD-121669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger G, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6:243ra86–243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- Knight EM, Martins IVA, Gümüsgöz S, Allan SM, Lawrence CB. High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol Aging. 2014;35:1821–1832. doi: 10.1016/j.neurobiolaging.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohjima M, Sun Y, Chan L. Increased Food Intake Leads to Obesity and Insulin Resistance in the Tg2576 Alzheimer’s Disease Mouse Model. Endocrinology. 2010;151:1532–1540. doi: 10.1210/en.2009-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzler J, Youmans KL, Yu C, LaDu MJ, Tai LM. APOE modulates the effect of estrogen therapy on Aβ accumulation EFAD-Tg mice. Neurosci Lett. 2014;560:131–136. doi: 10.1016/j.neulet.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C-reactive protein levels in men. Clin Endocrinol. 2010;72:527–533. doi: 10.1111/j.1365-2265.2009.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypreos KE, Karagiannides I, Fotiadou EH, Karavia EA, Brinkmeier MS, Giakoumi SM, Tsompanidi EM. Mechanisms of obesity and related pathologies: Role of apolipoprotein E in the development of obesity. FEBS J. 2009;276:5720–5728. doi: 10.1111/j.1742-4658.2009.07301.x. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Salonen R, Rauramaa R, Salonen JT. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol. 2003;149:601–608. doi: 10.1530/eje.0.1490601. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, Van Eldik LJ. Apolipoprotein E and apolipoprotein E receptors modulate A beta-induced glial neuroinflammatory responses. Neurochem Int. 2001;39:427–434. doi: 10.1016/s0197-0186(01)00050-x. [DOI] [PubMed] [Google Scholar]
- LaFerla FM. Pathways linking Aβ and tau pathologies. Biochem Soc Trans. 2010;38:993. doi: 10.1042/BST0380993. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A. The emerging role of dietary fructose in obesity and cognitive decline. Nutr J. 2013;12:1–1. doi: 10.1186/1475-2891-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lathe R, Sapronova A, Kotelevtsev Y. Atherosclerosis and Alzheimer - diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr. 2014;14:1–30. doi: 10.1186/1471-2318-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low Serum Testosterone and Mortality in Older Men. J Clin Endocr Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebbadi M, Julien C, Phivilay A, Tremblay C, Emond V, Kang JX, Calon F. Endogenous conversion of omega-6 into omega-3 fatty acids improves neuropathology in an animal model of Alzheimer’s disease. J Alzheimers Dis. 2011;27:853–869. doi: 10.3233/JAD-2011-111010. [DOI] [PubMed] [Google Scholar]
- Leboucher A, Laurent C, Fernandez-Gomez FJ, Burnouf S, Troquier L, Eddarkaoui S, Demeyer D, Caillierez R, Zommer N, Vallez E, Bantubungi K, Breton C, Pigny P, Buée-Scherrer V, Staels B, Hamdane M, Tailleux A, Buée L, Blum D. Detrimental effects of diet-induced obesity on τ pathology are independent of insulin resistance in τ transgenic mice. Diabetes. 2013;62:1681–1688. doi: 10.2337/db12-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Deng J, Sheng W, Zuo Z. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Be. 2012;101:564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Singh M. Sex differences in cognitive impairment and Alzheimer’s disease. Front Neuroendocrin. 2014;35:385–403. doi: 10.1016/j.yfrne.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Sima AAF. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HQ, Qiu Y, Mu Y, Zhang XJ, Liu L, Hou XH, Zhang L, Xu XN, Ji AL, Cao R, Yang RH, Wang F. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr Res. 2013;33:849–858. doi: 10.1016/j.nutres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Paajanen T, Westman E, Wahlund LO, Simmons A, Tunnard C, Sobow T, Proitsi P, Powell J, Mecocci P, Tsolaki M, Vellas B, Muehlboeck S, Evans A, Spenger C, Lovestone S, Soininen H, Soininen H. Effect of APOE ε4 allele on cortical thicknesses and volumes: the AddNeuroMed study. J Alzheimers Dis. 2010;21:947–966. doi: 10.3233/JAD-2010-100201. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Cheng D, Tang MX, Schupf N, Mayeux R. Central obesity in the elderly is related to late-onset Alzheimer disease. Alzheimer Dis Assoc Disord. 2012;26:101–105. doi: 10.1097/WAD.0b013e318222f0d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–113. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE Genotype and an ApoE-mimetic Peptide Modify the Systemic and Central Nervous System Inflammatory Response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and Depot Differences in Adipocyte Insulin Sensitivity and Glucose Metabolism. Diabetes. 2009;58:803–812. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Nivison M, Montine KS, Maeda N, Montine TJ. Neurotoxicity from innate immune response is greatest with targeted replacement of E4 allele of apolipoprotein E gene and is mediated by microglial p38MAPK. FASEB J. 2006a;20:797–799. doi: 10.1096/fj.05-5423fje. [DOI] [PubMed] [Google Scholar]
- Maezawa I, Zaja-Milatovic S, Milatovic D, Stephen C, Sokal I, Maeda N, Montine TJ, Montine KS. Apolipoprotein E isoform-dependent dendritic recovery of hippocampal neurons following activation of innate immunity. J Neuroinflamm. 2006b;3:21. doi: 10.1186/1742-2094-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L. Correlation between Testosterone and the Inflammatory Marker Soluble Interleukin-6 Receptor in Older Men. J Clin Endocr Metab. 2006;91:345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The Effect of Testosterone Replacement on Endogenous Inflammatory Cytokines and Lipid Profiles in Hypogonadal Men. J Clin Endocr Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Merchant CA, Jacobs DM, Small SA, Bell K, Ferin M, Mayeux R. Endogenous estrogen levels and Alzheimer’s disease among postmenopausal women. Neurology. 2000;54:833–837. doi: 10.1212/wnl.54.4.833. [DOI] [PubMed] [Google Scholar]
- Marques-Vidal P, Bongard V, Ruidavets JB, Fauvel J, Hanaire-Broutin H, Perret B, Ferrières J. Obesity and alcohol modulate the effect of apolipoprotein E polymorphism on lipids and insulin. Obes Res. 2003;11:1200–1206. doi: 10.1038/oby.2003.165. [DOI] [PubMed] [Google Scholar]
- Martens EAP, Lemmens SGT, Adam TCM, Westerterp-Plantenga MS. Sex differences in HPA axis activity in response to a meal. Physiol Behav. 2012;106:272–277. doi: 10.1016/j.physbeh.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Mascarenhas-Melo F, Marado D, Palavra F, Sereno J, Coelho Á, Pinto R, Teixeira-Lemos E, Teixeira F, Reis F. Diabetes abrogates sex differences and aggravates cardiometabolic risk in postmenopausal women. Cardiovasc Diabetol. 2013;12:61. doi: 10.1186/1475-2840-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoccoli G, Dagostino MP, Vinciguerra M, Ciccone F, Paroni G, Seripa D, Addante F, Montella RC, De Cosmo S, Sera F, Greco A. An association study between epicardial fat thickness and cognitive impairment in the elderly. Am J Physiol Heart Circ. 2014;307:H1269–H1276. doi: 10.1152/ajpheart.00175.2014. [DOI] [PubMed] [Google Scholar]
- McAllister C, Long J, Bowers A, Walker A, Cao P, Honda SI, Harada N, Staufenbiel M, Shen Y, Li R. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010;30:7326–7334. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNelis JC, Olefsky JM. Macrophages, Immunity, and Metabolic Disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes Relat Metab Disord. 2012;36:262–272. doi: 10.1038/ijo.2011.87. [DOI] [PubMed] [Google Scholar]
- Mehla J, Chauhan BC, Chauhan NB. Experimental induction of type 2 diabetes in aging-accelerated mice triggered Alzheimer-like pathology and memory deficits. J Alzheimers Dis. 2014;39:145–162. doi: 10.3233/JAD-131238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs JB, Ordovas JM, Cupples LA, Singer DE, Nathan DM, Schaefer EJ, Wilson PW. Apolipoprotein E isoform polymorphisms are not associated with insulin resistance: the Framingham Offspring Study. Diabetes Care. 2000;23:669–674. doi: 10.2337/diacare.23.5.669. [DOI] [PubMed] [Google Scholar]
- Meng XF, Yu JT, Wang HF, Tan MS, Wang C, Tan CC, Tan L. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42:1295–1310. doi: 10.3233/JAD-140954. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol. 2011;203:259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA, Romanatto T, Pascoal LB, Caricilli AM, Torsoni MA. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012;61:1455–1462. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DML, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JBC, Bordin S, Saad MJ, Velloso LA. Saturated Fatty Acids Produce an Inflammatory Response Predominantly through the Activation of TLR4 Signaling in Hypothalamus: Implications for the Pathogenesis of Obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Mogri M, Dhindsa S, Quattrin T, Ghanim H, Dandona P. Testosterone concentrations in young pubertal and post-pubertal obese males. Clin Endocrinol. 2013;78:593–599. doi: 10.1111/cen.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moralejo DH, Hansen CT, Treuting P, Hessner MJ, Fuller JM, Van Yserloo B, Jensen R, Osborne W, Kwitek AE, Lernmark A. Differential effects of leptin receptor mutation on male and female BBDR. Gimap5-/Gimap5- spontaneously diabetic rats. Physiol Genomics. 2010;41:9–20. doi: 10.1152/physiolgenomics.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI. High-sugar diets, type 2 diabetes and Alzheimer_s disease. Curr Opin Clin Nutr Metab Care. 2013;16:440–445. doi: 10.1097/MCO.0b013e328361c7d1. [DOI] [PubMed] [Google Scholar]
- Morris GP, Clark IA, Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol Commun. 2014;2:135. doi: 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Tangney CC. Dietary fat composition and dementia risk. Neurobiol Aging. 2014;35:S59–S64. doi: 10.1016/j.neurobiolaging.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Anwander A, Möller HE, Horstmann A, Lepsien J, Busse F, Mohammadi S, Schroeter ML, Stumvoll M, Villringer A, Pleger B. Sex-Dependent Influences of Obesity on Cerebral White Matter Investigated by Diffusion-Tensor Imaging. PLoS ONE. 2011;6:e18544. doi: 10.1371/journal.pone.0018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Knight NS, Cochlin LE, McAleese S, Deacon RMJ, Rawlins JNP, Clarke K. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J. 2009;23:4353–4360. doi: 10.1096/fj.09-139691. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Casellas A, Bauzá-Thorbrügge M, Proenza AM, Gianotti M, Lladó I. Sex-dependent differences in rat brown adipose tissue mitochondrial biogenesis and insulin signaling parameters in response to an obesogenic diet. Mol Cell Biochem. 2012;373:125–135. doi: 10.1007/s11010-012-1481-x. [DOI] [PubMed] [Google Scholar]
- Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägga K, Wattmo C, Zhang Y, Wahlund LO, Palmqvist S. Cerebral inflammation is an underlying mechanism of early death in Alzheimer’s disease: a 13-year cause-specific multivariate mortality study. Alzheimers Res Ther. 2014;6:1–8. doi: 10.1186/alzrt271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedowicz DM, Reeves VL, Platt TL, Kohler K, Beckett TL, Powell DK, Lee TL, Sexton TR, Song ES, Brewer LD, Latimer CS, Kraner SD, Larson KL, Ozcan S, Norris CM, Hersh LB, Porter NM, Wilcock DM, Murphy MP. Obesity and diabetes cause cognitive dysfunction in the absence of accelerated β-amyloid deposition in a novel murine model of mixed or vascular dementia. Acta Neuropathol Comm. 2014;2:64. doi: 10.1186/2051-5960-2-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E Regulates the Integrity of Tight Junctions in an Isoform-dependent Manner in an in Vitro Blood-Brain Barrier Model. J Biol Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Qi Y, Qian Y, Gao P, Zhu D. The relationship between apolipoprotein E e2/e3/e4 polymorphisms and hypertension: a meta-analysis of six studies comprising 1812 cases and 1762 controls. Hypertens Res. 2009;32:1060–1066. doi: 10.1038/hr.2009.164. [DOI] [PubMed] [Google Scholar]
- Norata GD, Tibolla G, Seccomandi PM, Poletti A, Catapano AL. Dihydrotestosterone Decreases Tumor Necrosis Factor-α and Lipopolysaccharide-Induced Inflammatory Response in Human Endothelial Cells. J Clin Endocr Metab. 2006;91:546–554. doi: 10.1210/jc.2005-1664. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA. 2014;311:806. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Barrett-Connor E, Barrett-Connor E. Apolipoprotein E polymorphism and lipid levels differ by gender and family history of diabetes: the Rancho Bernardo Study. Clin Genet. 2001;60:132–137. doi: 10.1034/j.1399-0004.2001.600207.x. [DOI] [PubMed] [Google Scholar]
- Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, Leenders I, Broersen L, Lütjohann D, Hartmann T, Tanila H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis. 2006;23:563–572. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Olgiati P, Politis A, Malitas P, Albani D, Dusi S, Polito L, De Mauro S, Zisaki A, Piperi C, Stamouli E, Mailis A, Batelli S, Forloni G, De Ronchi D, Kalofoutis A, Liappas I, Serretti A. APOE epsilon-4 allele and cytokine production in Alzheimer’s disease. Int J Geriatr Psychiatry. 2010;25:338–344. doi: 10.1002/gps.2344. [DOI] [PubMed] [Google Scholar]
- Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-κB signaling cascade. Neurobiol Dis. 2005;20:709–718. doi: 10.1016/j.nbd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Ophir G, Meilin S, Efrati M, Chapman J, Karussis D, Roses A, Michaelson DM. Human apoE3 but not apoE4 rescues impaired astrocyte activation in apoE null mice. Neurobiol Dis. 2003;12:56–64. doi: 10.1016/s0969-9961(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Orr ME, Salinas A, Buffenstein R, Oddo S. Mammalian target of rapamycin hyperactivity mediates the detrimental effects of a high sucrose diet on Alzheimer’s disease pathology. Neurobiol Aging. 2014;35:1233–1242. doi: 10.1016/j.neurobiolaging.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger R, Dale AM, Eaves LJ, Eyler LT, Fischl B, Fennema-Notestine C, Franz CE, Grant MD, Jak AJ, Jacobson KC, Lyons MJ, Mendoza SP, Neale MC, Prom-Wormley EC, Seidman LJ, Tsuang MT, Xian H, Kremen WS. Testosterone modifies the effect of APOE genotype on hippocampal volume in middle-aged men. Neurology. 2010;75:874–880. doi: 10.1212/WNL.0b013e3181f11deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Hauger R, Xian H, Vuoksimaa E, Spoon KM, Mendoza SP, Jacobson KC, Vasilopoulos T, Rana BK, McKenzie R, McCaffery JM, Lyons MJ, Kremen WS, Franz CE. Interaction of APOE genotype and testosterone on episodic memory in middle-aged men. Neurobiol Aging. 2014;35:1778.e1–8. doi: 10.1016/j.neurobiolaging.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti AM, Congia S, Lello S, Tedde D, Orrù M, Pistis M, Pilloni M, Zedda P, Loddo A, Melis GB. Low androgenization index in elderly women and elderly men with Alzheimer’s disease. Neurology. 2004;62:301–303. doi: 10.1212/01.wnl.0000094199.60829.f5. [DOI] [PubMed] [Google Scholar]
- Payami H, Montee KR, Kaye JA, Bird TD, Yu CE, Wijsman EM, Schellenberg GD. Alzheimer’s Disease, Apolipoprotein E4, and Gender. JAMA. 1994;271:1316–1317. [PubMed] [Google Scholar]
- Pedroni SMA, Turban S, Kipari T, Dunbar DR, McInnes K, Saunders PTK, Morton NM, Norman JE. Pregnancy in Obese Mice Protects Selectively against Visceral Adiposity and Is Associated with Increased Adipocyte Estrogen Signalling. PLoS ONE. 2014;9:e94680. doi: 10.1371/journal.pone.0094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- Pereira TM, Teixeira F, Aguilar E, Matoso RO, Soares FLP, Ferreira AFB, Alvarez-Leite JI. Differences in adipose tissue inflammation and oxidative status in C57BL/6 and ApoE−/− mice fed high fat diet. Anim Sci J. 2012;83:549–555. doi: 10.1111/j.1740-0929.2011.00982.x. [DOI] [PubMed] [Google Scholar]
- Pettersson US, Waldén TB, Carlsson PO, Jansson L, Phillipson M. Female Mice are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue. PLoS ONE. 2012;7:e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty KH, Li K, Dong Y, Fortenberry J, Stallmann-Jorgensen I, Guo D, Zhu H. Sex dimorphisms in inflammatory markers and adiposity in African-American youth. Int J Pediatr Obes. 2010;5:327–333. doi: 10.3109/17477160903497019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfankuch T, Rizk A, Olsen R, Poage C, Raber J. Role of circulating androgen levels in effects of apoE4 on cognitive function. Brain Res. 2005;1053:88–96. doi: 10.1016/j.brainres.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrin. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, Yu WH, Luchsinger JA, Wadzinski B, Duff KE, Takashima A. Insulin Dysfunction Induces In Vivo Tau Hyperphosphorylation through Distinct Mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD. Sex Differences in the Metabolic Syndrome: Implications for Cardiovascular Health in Women. Clin Chem. 2013;60:44–52. doi: 10.1373/clinchem.2013.202549. [DOI] [PubMed] [Google Scholar]
- Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am J Pathol. 1996;148:2083–2095. [PMC free article] [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Puig J, Blasco G, Daunis-i-Estadella J, Molina X, Xifra G, Ricart W, Pedraza S, Fernández-Aranda F, Fernández-Real JM. Hypothalamic Damage Is Associated With Inflammatory Markers and Worse Cognitive Performance in Obese Subjects. J Clin Endocr Metab. 2015;100:E276–E281. doi: 10.1210/jc.2014-2682. [DOI] [PubMed] [Google Scholar]
- Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, Evans SJW, Pocock SJ. BMI and risk of dementia in two million people over two decades: A retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:431–436. doi: 10.1016/S2213-8587(15)00033-9. [DOI] [PubMed] [Google Scholar]
- Raber J. Androgens, apoE, and Alzheimer’s disease. Sci Aging Knowledge Environ. 2004;11:re2. doi: 10.1126/sageke.2004.11.re2. [DOI] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens Protect against Apolipoprotein E4-Induced Cognitive Deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, Lowe GDO, Fowkes FGR. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc. 2007;55:700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Ragogna F, Lattuada G, Ruotolo G, Luzi L, Perseghin G. Lack of association of apoE ε4 allele with insulin resistance. Acta Diabetol. 2011;49:25–32. doi: 10.1007/s00592-011-0255-3. [DOI] [PubMed] [Google Scholar]
- Ramos-Rodriguez JJ, Ortiz O, Jimenez-Palomares M, Kay KR, Berrocoso E, Murillo-Carretero MI, Perdomo G, Spires-Jones T, Cozar-Castellano I, Lechuga-Sancho AM, Garcia-Alloza M. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrino. 2013;38:2462–2475. doi: 10.1016/j.psyneuen.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Reinert KRS, Po’e EK, Barkin SL. The Relationship between Executive Function and Obesity in Children and Adolescents: A Systematic Literature Review. J Obes. 2013;2013:1–10. doi: 10.1155/2013/820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Elashoff D, Geschwind DH, Welsh BT, Gylys KH, Lee C, Cummings JL, Cole GM. Plasma signaling proteins in persons at genetic risk for Alzheimer disease: influence of APOE genotype. Arch Neurol. 2012;69:757–764. doi: 10.1001/archneurol.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon GA, Tang MX, Lee JH, Lantigua R, Medrano M, Mayeux R. Familial Alzheimer disease in Latinos: Interaction between APOE, stroke, and estrogen replacement. Neurology. 2006;66:35–40. doi: 10.1212/01.wnl.0000191300.38571.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Horm Behav. 2013;63:238–253. doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez GA, Tai LM, LaDu MJ, Rebeck GW. Human APOE4 increases microglia reactivity at Aβ plaques in a mouse model of Aβ deposition. J Neuroinflamm. 2014;11:111. doi: 10.1186/1742-2094-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rontu R, Ojala P, Hervonen A, Goebeler S, Karhunen PJ, Nikkila M, Kunnas T, Jylha M, Eklund C, Hurme M, Lehtimaki T. Apolipoprotein E genotype is related to plasma levels of C-reactive protein and lipids and to longevity in nonagenarians. Clin Endocrinol. 2006;64:265–270. doi: 10.1111/j.1365-2265.2006.02455.x. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens Regulate the Development of Neuropathology in a Triple Transgenic Mouse Model of Alzheimer’s Disease. J Neurosci. 2006;26:13384–13389. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011;32:604–613. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292:1431–1432. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- Ross AP, Bartness TJ, Mielke JG, Parent MB. A high fructose diet impairs spatial memory in male rats. Neurobiol Learn Mem. 2009;92:410–416. doi: 10.1016/j.nlm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicka AR, Rumley A, Whincup PH, Lowe GD, Strachan DP. Sex differences in the relationship between inflammatory and hemostatic biomarkers and metabolic syndrome: British 1958 Birth Cohort. J Thromb Haemost. 2011;9:2337–2344. doi: 10.1111/j.1538-7836.2011.04517.x. [DOI] [PubMed] [Google Scholar]
- Ryan J, Carrière I, Scali J, Dartigues JF, Tzourio C, Poncet M, Ritchie K, Ancelin ML. Characteristics of hormone therapy, cognitive function, and dementia: the prospective 3C Study. Neurology. 2009;73:1729–1737. doi: 10.1212/WNL.0b013e3181c34b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras K, Sachdev PS. Diabetes and the elderly brain: sweet memories? Ther Adv Endocrinol Metab. 2012;3:189–196. doi: 10.1177/2042018812469645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Walter J, Gentleman SM. Interactions between APP secretases and inflammatory mediators. J Neuroinflamm. 2008;5:25. doi: 10.1186/1742-2094-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schäfer S, Wirths O, Multhaup G, Bayer TA. Gender dependent APP processing in a transgenic mouse model of Alzheimer’s disease. J Neural Transm. 2006;114:387–394. doi: 10.1007/s00702-006-0580-9. [DOI] [PubMed] [Google Scholar]
- Schram MT, Euser SM, De Craen AJM, Witteman JC, Frölich M, Hofman A, Jolles J, Breteler MMB, Westendorp RGJ. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55:708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- Schulte D, Hahn M, Oberhäuser F, Malchau G, Schubert M, Heppner C, Müller N, Güdelhöfer H, Faust M, Krone W, Laudes M. Caloric Restriction Increases Serum Testosterone Concentrations in Obese Male Subjects by Two Distinct Mechanisms. Horm Metab Res. 2014;46:283–286. doi: 10.1055/s-0033-1358678. [DOI] [PubMed] [Google Scholar]
- Schwartz DH, Leonard G, Perron M, Richer L, Syme C, Veillette S, Pausova Z, Paus T. Visceral fat is associated with lower executive functioning in adolescents. Int J Obes Relat Metab Disord. 2013;37:1336–1343. doi: 10.1038/ijo.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinge D, Carambia A, Quaas A, Krech T, Wegscheid C, Tiegs G, Prinz I, Lohse AW, Herkel J, Schramm C. Testosterone Suppresses Hepatic Inflammation by the Downregulation of IL-17, CXCL-9, and CXCL-10 in a Mouse Model of Experimental Acute Cholangitis. J Immunol. 2015;194:2522–2530. doi: 10.4049/jimmunol.1400076. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s Disease. Cold Spring Harb Perspect Biol. 2011;3:a004457–a004457. doi: 10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp ES, Gatz M. Relationship Between Education and Dementia. Alzheimer Dis Assoc Disord. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Tso P, Woods SC, Clegg DJ, Barber KL, Carey K, Liu M. Brain Apolipoprotein E: an Important Regulator of Food Intake in Rats. Diabetes. 2008;57:2092–2098. doi: 10.2337/db08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid β peptide in APPswe transgenic mice. Neurobiol Dis. 2003;14:133–145. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97:199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima A, Iordan A, Stancu C. Apolipoprotein E polymorphism--a risk factor for metabolic syndrome. Clin Chem Lab Med. 2007;45:1149–1153. doi: 10.1515/CCLM.2007.258. [DOI] [PubMed] [Google Scholar]
- Simpson E, Jones M, Misso M, Hewitt K, Hill R, maffei L, Carani C, Boon WC. Estrogen, a fundamental player in energy homeostasis. J Steroid Biochem Mol Biol. 2005;95:3–8. doi: 10.1016/j.jsbmb.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soczynska JK, Kennedy SH, Woldeyohannes HO, Liauw SS, Alsuwaidan M, Yim CY, McIntyre RS. Mood Disorders and Obesity: Understanding Inflammation as a Pathophysiological Nexus. Neuromol Med. 2010;13:93–116. doi: 10.1007/s12017-010-8140-8. [DOI] [PubMed] [Google Scholar]
- Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33:105–115. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Hajian H, Finch CE. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp Neurol. 1997;143:313–318. doi: 10.1006/exnr.1996.6360. [DOI] [PubMed] [Google Scholar]
- Strachan MWJ, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struble RG, Nathan BP, Cady C, Cheng X, McAsey M. Estradiol regulation of astroglia and apolipoprotein E: An important role in neuronal regeneration. Exp Gerontol. 2007;42:54–63. doi: 10.1016/j.exger.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Stubbins RE, Najjar K, Holcomb VB, Hong J, Núñez NP. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes, Obes Metab. 2011;14:58–66. doi: 10.1111/j.1463-1326.2011.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama MG, Agellon LB. Sex differences in lipid metabolism and metabolic disease risk. Biochem Cell Biol. 2012;90:124–141. doi: 10.1139/o11-067. [DOI] [PubMed] [Google Scholar]
- Syme C, Abrahamowicz M, Leonard GT, Perron M, Pitiot A, Qiu X, Richer L, Totman J, Veillette S, Xiao Y, Gaudet D, Paus T, Pausova Z. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence: sex differences and underlying mechanisms. Arch Pediatr Adolesc Med. 2008;162:453–461. doi: 10.1001/archpedi.162.5.453. [DOI] [PubMed] [Google Scholar]
- Takalo M, Haapasalo A, Martiskainen H, Kurkinen KMA, Koivisto H, Miettinen P, Khandelwal VKM, Kemppainen S, Kaminska D, Mäkinen P, Leinonen V, Pihlajamäki J, Soininen H, Laakso M, Tanila H, Hiltunen M. High-fat diet increases tau expression in the brain of T2DM and AD mice independently of peripheral metabolic status. J Nutr Biochem. 2014;25:634–641. doi: 10.1016/j.jnutbio.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Takechi R, Pallebage-Gamarallage MM, Lam V, Giles C, Mamo JC. Aging-Related Changes in Blood-Brain Barrier Integrity and the Effect of Dietary Fat. Neurodegener Dis. 2013;12:125–135. doi: 10.1159/000343211. [DOI] [PubMed] [Google Scholar]
- Takeda K, Toda K, Saibara T, Nakagawa M, Saika K, Onishi T, Sugiura T, Shizuta Y. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol. 2003;176:237–246. doi: 10.1677/joe.0.1760237. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H. Relationship Between Body Mass Index and Gray Matter Volume in 1,428 Healthy Individuals. Obesity. 2008;16:119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006296. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Schwartz MW. Minireview: Inflammation and Obesity Pathogenesis: The Hypothalamus Heats Up. Endocrinology. 2010;151:4109–4115. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorand B, Baumert J, Kolb H, Meisinger C, Chambless L, Koenig W, Herder C. Sex Differences in the Prediction of Type 2 Diabetes by Inflammatory Markers: Results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Diabetes Care. 2007;30:854–860. doi: 10.2337/dc06-1693. [DOI] [PubMed] [Google Scholar]
- Tolppanen AM, Solomon A, Kulmala J, Kåreholt I, Ngandu T, Rusanen M, Laatikainen T, Soininen H, Kivipelto M. Leisure-time physical activity from mid- to late life, body mass index, and risk of dementia. Alzheimers Dement. 2015;11:434–443.e6. doi: 10.1016/j.jalz.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Trollor JN, Smith E, Agars E, Kuan SA, Baune BT, Campbell L, Samaras K, Crawford J, Lux O, Kochan NA, Brodaty H, Sachdev P. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age. 2011;34:1295–1308. doi: 10.1007/s11357-011-9301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, Samaras K, Crawford J, Brodaty H, Sachdev P. Systemic Inflammation Is Associated with MCI and Its Subtypes: The Sydney Memory and Aging Study. Dement Geriatr Cogn Disord. 2010;30:569–578. doi: 10.1159/000322092. [DOI] [PubMed] [Google Scholar]
- Tsilidis KK, Rohrmann S, McGlynn KA, Nyante SJ, Lopez DS, Bradwin G, Feinleib M, Joshu CE, Kanarek N, Nelson WG, Selvin E, Platz EA. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology. 2013;1:919–928. doi: 10.1111/j.2047-2927.2013.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Ikegami H, Yamato E, Fu J, Fukuda M, Shen G, Kawaguchi Y, Takekawa K, Fujioka Y, Fujisawa T. The NSY mouse: a new animal model of spontaneous NIDDM with moderate obesity. Diabetologia. 1995;38:503–508. doi: 10.1007/BF00400717. [DOI] [PubMed] [Google Scholar]
- van Exel E, Eikelenboom P, Comijs H, Frölich M, Smit JH, Stek ML, Scheltens P, Eefsting JE, Westendorp RGJ. Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Arch Gen Psychiatry. 2009;66:1263–1270. doi: 10.1001/archgenpsychiatry.2009.146. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29:507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest RS, Pike CJ. Gender, sex steroid hormones, and Alzheimer’s disease. Horm Behav. 2013;63:301–307. doi: 10.1016/j.yhbeh.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignozzi L, Morelli A, Sarchielli E, Comeglio P, Filippi S, Cellai I, Maneschi E, Serni S, Gacci M, Carini M, Piccinni MP, Saad F, Adorini L, Vannelli GB, Maggi M. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J Endocrinol. 2011;212:71–84. doi: 10.1530/JOE-11-0289. [DOI] [PubMed] [Google Scholar]
- Viscogliosi G, Andreozzi P, Chiriac IM, Cipriani E, Servello A, Marigliano B, Ettorre E, Marigliano V. Depressive symptoms in older people with metabolic syndrome: is there a relationship with inflammation? Int J Geriatr Psychiatry. 2012;28:242–247. doi: 10.1002/gps.3817. [DOI] [PubMed] [Google Scholar]
- Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2009;30:1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Pahnke J, Madauss M, Vogelgesang S, Pahnke A, Herbst EW, Stausske D, Walther R, Kessler C, Warzok RW. Apolipoprotein E4 promotes the early deposition of Aβ42 and then Aβ40 in the elderly. Acta Neuropathol. 2000;100:36–42. doi: 10.1007/s004010051190. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, Thanos PK, Geliebter A, Biegon A, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Perrard XD, Perrard JL, Mukherjee A, Rosales C, Chen Y, Smith CW, Pownall HJ, Ballantyne CM, Wu H. ApoE and the role of very low density lipoproteins in adipose tissue inflammation. Atherosclerosis. 2012;223:342–349. doi: 10.1016/j.atherosclerosis.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Irwin RW, Brinton RD. Activation of estrogen receptor alpha increases and estrogen receptor beta decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Natl Acad Sci USA. 2006;103:16983–16988. doi: 10.1073/pnas.0608128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Menheere PPCA, Astrup A, Andersen MR, van Baak MA, Larsen TM, Jebb S, Kafatos A, Pfeiffer AFH, Martinez JA, Handjieva-Darlenska T, Hlavaty P, Viguerie N, Langin D, Saris WHM, Mariman ECM Diogenes consortium. Metabolic syndrome, circulating RBP4, testosterone, and SHBG predict weight regain at 6 months after weight loss in men. Obesity. 2013;21:1997–2006. doi: 10.1002/oby.20311. [DOI] [PubMed] [Google Scholar]
- Wang X, Zheng W, Xie JW, Wang T, Wang SL, Teng WP, Wang ZY. Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol Neurodegen. 2010;5:46–46. doi: 10.1186/1750-1326-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Arrighi HM. Prevalence of Apolipoprotein E4 Genotype and Homozygotes (APOE e4/4) among Patients Diagnosed with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2012;38:1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White UA, Tchoukalova YD. Sex dimorphism and depot differences in adipose tissue function. Biochem Biophys Acta. 2014;1842:377–392. doi: 10.1016/j.bbadis.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Quesenberry CP, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- Wolf AB, Braden BB, Bimonte-Nelson H, Kusne Y, Young N, Engler-Chiurazzi E, Garcia AN, Walker DG, Moses GSD, Tran H, LaFerla F, Lue L, Lombardo NE, Valla J. Broad-based nutritional supplementation in 3xTg mice corrects mitochondrial function and indicates sex-specificity in response to Alzheimer’s disease intervention. J Alzheimers Dis. 2012;32:217–232. doi: 10.3233/JAD-2012-120478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer Disease--A Brief Review of the Basic Science and Clinical Literature. Cold Spring Harb Perspect Med. 2012;2:a006346–a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Qin W, Li F, Jia XF, Jia J. Apolipoprotein E ε4 Status Modifies the Effects of Sex Hormones on Neuropsychiatric Symptoms of Alzheimer’s Disease. Dement Geriatr Cogn Disord. 2012;33:35–42. doi: 10.1159/000336600. [DOI] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk A population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Haan MN, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: Evidence of gene-environment interaction. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HT, Sheen YJ, Kao CD, Chang CA, Hu YC, Lin JL. Association between the characteristics of metabolic syndrome and Alzheimer’s disease. Metab Brain Dis. 2013;28:597–604. doi: 10.1007/s11011-013-9406-2. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ruiz-Narvaez E, Kraft P, Campos H. Effect of apolipoprotein E genotype and saturated fat intake on plasma lipids and myocardial infarction in the Central Valley of Costa Rica. Hum Biol. 2007;79:637–647. doi: 10.1353/hub.2008.0010. [DOI] [PubMed] [Google Scholar]
- Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-Term Testosterone Treatment in Elderly Men with Hypogonadism and Erectile Dysfunction Reduces Obesity Parameters and Improves Metabolic Syndrome and Health-Related Quality of Life. J Sex Med. 2014;11:1567–1576. doi: 10.1111/jsm.12523. [DOI] [PubMed] [Google Scholar]
- Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130:e856–e864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- Yoon DH, Choi SH, Yu JH, Ha JH, Ryu SH, Park DH. The relationship between visceral adiposity and cognitive performance in older adults. Age Ageing. 2012;41:456–461. doi: 10.1093/ageing/afs018. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Nomiyama K, Kawano H, Ueda K. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L, Kanekiyo T, Gan M, Kim J, Eimer WA, Estus S, Rebeck GW, Weeber EJ, Bu G, Yu C, LaDu MJ. APOE4-specific Changes in Amyloid-Beta Accumulation in a New Transgenic Mouse Model of Alzheimer Disease. J Biol Chem. 2012;287:41774–41786. doi: 10.1074/jbc.M112.407957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Lu M, Lancaster T, Cao P, Honda SI, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci USA. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zade D, Beiser A, McGlinchey R, Au R, Seshadri S, Palumbo C, Wolf PA, DeCarli C, Milberg W. Apolipoprotein Epsilon 4 Allele Modifies Waist-to-Hip Ratio Effects on Cognition and Brain Structure. J Stroke Cerebrovasc Dis. 2013;22:119–125. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JCS, Breitner JCS. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- Zarkesh M, Daneshpour MS, Faam B, Hedayati M, Azizi F. Is there any association of apolipoprotein E gene polymorphism with obesity status and lipid profiles? Tehran Lipid and Glucose Study (TLGS) Gene. 2012;509:282–285. doi: 10.1016/j.gene.2012.07.048. [DOI] [PubMed] [Google Scholar]
- Zempel H, Mandelkow E. Lost after translation: missorting of Tau protein and consequences for Alzheimer disease. Trends Neurosci. 2014;37:721–732. doi: 10.1016/j.tins.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Zerbi V, Jansen D, Wiesmann M, Fang X, Broersen LM, Veltien A, Heerschap A, Kiliaan AJ. Multinutrient diets improve cerebral perfusion and neuroprotection in a murine model of Alzheimer’s disease. Neurobiol Aging. 2014;35:600–613. doi: 10.1016/j.neurobiolaging.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance–a mini-review. Gerontology. 2009;55:379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao Y, Tan A, Yang X, Zhang H, Zhang S, Wu C, Lu Z, Wang M, Liao M, Qin X, Li L, Hu Y, Mo Z. Endogenous sex hormones and C-reactive protein in healthy chinese men. Clin Endocrinol. 2012;78:60–66. doi: 10.1111/j.1365-2265.2012.04359.x. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhai L, Liu Z, Wu S, Xu L. Leptin Level and Oxidative Stress Contribute to Obesity-Induced Low Testosterone in Murine Testicular Tissue. Oxid Med Cell Longev. 2014;2014:1–14. doi: 10.1155/2014/190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Lin S, Bales KR, Gelfanova V, Koger D, Delong C, Hale J, Liu F, Hunter JM, Paul SM. Macrophage-mediated degradation of beta-amyloid via an apolipoprotein E isoform-dependent mechanism. J Neurosci. 2009;29:3603–3612. doi: 10.1523/JNEUROSCI.5302-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziko I, De Luca S, Dinan T, Barwood JM, Sominsky L, Cai G, Kenny R, Stokes L, Jenkins TA, Spencer SJ. Neonatal overfeeding alters hypothalamic microglial profiles and central responses to immune challenge long-term. Brain Behav Immun. 2014;41:32–43. doi: 10.1016/j.bbi.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673–681. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular Effects of Apolipoprotein E. JAMA Neurol. 2013;70:440. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoth N, Weigt C, Laudenbach-Leschowski U, Diel P. Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. J Steroid Biochem. 2010;122:100–105. doi: 10.1016/j.jsbmb.2010.03.029. [DOI] [PubMed] [Google Scholar]