Abstract
Thymic epithelial cells (TECs) play important roles in T cell generation. Mechanisms that control TEC development and function are still not well defined. The mammalian or mechanistic target of rapamycin complex 2 (mTORC2) signals to regulate cell survival, nutrient uptake, and metabolism. We report here that mice with TEC-specific ablation of Rictor, a critical and unique adaptor molecule in mTORC2, display thymic atrophy, which accompanies decreased TEC numbers in the medulla. Moreover, generation of multiple T cell lineages, including conventional TCRαβ T cells, regulatory T cells, iNKT cells, and TCRγδ T cells, was reduced in TEC-specific Rictor-deficient mice. Our data demonstrate that mTORC2 in TECs is important for normal thymopoiesis and efficient T cell generation.
Introduction
Multiple T cell lineages such as conventional TCRαβ T (cαβT) cells, NKT cells, regulatory T cells (Tregs), and TCRγδ T (γδT) cells are generated in the thymus; some acquire effector function during intrathymic development (1, 2). A normal thymic environment is crucial to ensure that these T cell lineages develop properly and establish a repertoire of T cells that are functional but also self-tolerant (3). The thymus comprises many cell lineages of both hematopoietic and non-hematopoietic origin. Thymic epithelial cells (TECs) are essential for thymopoiesis. Defects in TECs can block thymus development, as athymus nude mice exemplify, because of a loss-of-function mutation in Foxn1 that results in the absence of T cells (4–6). TECs are defined into cortical (c) and medullary (m) TECs that reside in the cortex and medullar regions of the thymus, respectively. After early T cell progenitors seed in the thymus, they develop sequentially from the CD4−CD8− double negative (DN) to the CD4+CD8+ double positive (DP) and the CD4+CD8− and CD4−CD8+ single positive (SP) stages. SP thymocytes eventually migrate from the thymus to populate peripheral lymphoid organs (2). cTECs present self-peptide MHC complexes to the TCR expressed on DP thymocytes to ensure that these cells survive, a process also called positive selection (7–10). mTECs promiscuously express tissue-restricted antigens (TRAs) to trigger the death of highly self-reactive CD4+ or CD8+ SP thymocytes that migrate from the cortex, a process called negative selection, and to induce Treg generation (7–9). Promiscuous expression of TRAs in mTECs, maturation of mTECs, and establishment of central tolerance depends on Aire (11), a deficiency of which impairs mTEC maturation and function, resulting in multi-organ autoimmune diseases (4–6).
The mammalian or mechanistic target of rapamycin (mTOR) is a serine/threonine kinase that integrates multiple signals to control cell growth, proliferation, survival, and metabolism. It signals through two complexes: mTORC1 and mTORC2. mTORC1 contains a crucial and unique adaptor molecule called Raptor and is sensitive to acute rapamycin inhibition, while mTORC2 contains Rictor and is resistant to acute rapamycin inhibition (12, 13). Many studies have demonstrated that mTOR is activated in both thymocytes and peripheral T cells following TCR engagement and intrinsically controls the development and/or function of cαβT-cells, iNKT-cells, and Tregs (14–21). Recently, we have found that the mTORC1 signal in TECs plays crucial roles in thymopoiesis and thymic function. Ablation of Raptor in TECs causes impaired TEC maturation, decreased mTEC to cTEC ratios, and altered thymic architecture, leading to severe thymic atrophy, reduced recruitment of early thymic progenitors and impaired development of virtually all T cell lineages (22). We report here that mTORC2/Rictor in TECs signals for proper thymopoiesis and T cell generation. TEC-specific deletion of mTORC2/Rictor causes moderate thymic atrophy, decreased mTEC numbers, and moderately reduced production of virtually all T-cell lineages in the thymus.
Methods
Mice
Rictorf/f mice (23) were obtained from the Jackson Laboratory and further backcrossed to C57Bl/6J background for at least four generations. Foxn1Cre mice (24) were gifts from Dr. Nancy Manley (University of Georgia). Mice were all housed under specific pathogen-free conditions and experiments described were carried out under the approval of the Institutional Animal Care and Use Committee of Duke University.
TEC Preparation
Thymic single-cell suspension as previously described with modifications (22, 25, 26). In brief, thymi were cut into small pieces (about 2mm), which were directly digested in FBS-free RPMI-1640 containing 10mg/ml collagenase type IV (Worthington) and 50mg/ml DNase I (Worthington) at 37 °C with constant orbital shaking at 100–150 rpm for 10 minutes. After vortex, fragments were allowed to settle down; the supernatants were collected, filtered through a 70μm nylon mesh, and kept on ice; settled remains were digested similarly twice and repeated a third time if necessary. After the last digestion, cells were combined and filtered. After centrifuging the pellets at 472g for 5 minutes, pellets were washed with 10ml RPMI-containing 10% FBS (RPMI-10) and resuspended in either cold FACS buffer (5Mm EDTA, 2%FBS in PBS) or RPMI-10.
Antibodies and flow cytometry
The FITC-conjugated TCR-Vβ usage kit, including anti-TCRβ2 (clone B20.6), β3 (clone KJ25), β4 (clone KT4), β5.1/5.2 (clone MR9-4), β6 (clone RR4-7), β7 (clone TR310), β8.1/8.2 (clone MR5-2), β8.3 (clone IB3.3), β9 (clone MR10-2), β10b (clone B21.5), β11 (clone RR3-15), β12 (clone MR11-1), β13 (clone MR12-3), β14 (clone 14-2), and β17a (clone KJ23), was purchased from BD Pharmingen. Fluorochrome-conjugated anti- B220 (clone RA3-6B2), CD3e (clone 145-2C11), CD4 (clone GK1.5), CD8 (clone 53-6.7), CD19 (clone 6D5), CD24 (clone M1/69), CD25 (clone PC61.5), CD27 (clone LG.3A10), CD40 (clone 3/23 ), CD44 (clone IM7), CD45 (clone 30-F11), CD45.2 (clone 104), CD62L (clone MEL-14), CD80 (clone 16-10A1), c-kit/CD117 (clone 2B8), CD11b (clone M170), CD11c (clone N418), EpCAM/CD326 (clone G8.8), F4/80 (clone BM8), Gr1 (clone RB6-8C5), Ly51 (clone 6C3), IFN-γ (clone XMG1.2), IL-17A (clone TC11-18 H10.1), MHC II-I-A/I-E (clone M5/114.15.2 ), NK1.1 (clone PK136), TCR-β (clone H57-597), TCRγδ (clone GL3), 9/Erythroid Cells (clone TER-119), Annexin-V, Streptavidin, and Qa-2 (clone 695H1-9-9) were purchased from Biolegend. CD1d tetramer was acquired from the NIH tetramer facility. Ulex Europaeus Agglutinin I (UEA-1, clone B-1065) was from Vector Laboratories. Foxp3 (clone FJK-61s), RORγt (clone AFKJS-9), Tbet (clone 4B10) and PLZF (clone 21F7) were obtained from eBioscience. Phospho-S6 (Ser235/236, d57.2.2E) and phospho-AKT (Ser473, 193H12) antibodies were purchased from Cell Signal Technology. Cells were stained for surface molecules using 2% FBS–PBS. Cell death was identified by using the Violet Live/Dead cell kit (Invitrogen) or annexin-V and 7-AAD. Intracellular staining for Foxp3 was performed using the eBioscience Foxp3 Staining Buffer Set. Phospho-S6 and -Akt were stained using the BD Biosciences Cytofix/Cytoperm and Perm/Wash solutions. Stained samples were acquired on a FACS Canto-II (BD Biosciences) flow cytometer. Data was analyzed with FlowJo software (Tree Star).
Intracellular cytokine detection
Cytokine expression in thymic γδT cells were detected as previously described (27). Briefly, ten million thymocytes were stimulated with phorbol 12-myrustate 13-acetatae (PMA, 50ng/ml) plus ionomycin (500 ng/ml) in the presence of GolgiPlug (1ng/ml) for 4 hours. After cell surface staining, intracellular staining for IL-17A and IFNγ were performed using the BD Biosciences Cytofix/Cytoperm and Perm/Wash solutions.
BrdU incorporation
Three-week-old mice were intraperitoneally (i.p.) with 5-bromo-2-deoxyuridine (BrdU, Sigma; 1mg/mouse or 50mg/kg in 100μl PBS). Four hours after injection, thymocytes were cell surface stained with indicated antibodies, then intracellularly stained with a FITC-labeled anti-BrdU antibody using a BrdU Flow Kit (BD Biosciences) according to the manufacturer’s protocol. For BrdU incorporation in TECs, mice were injected with BrdU daily for three days and euthanized on day 4 to detect BrdU incorporation.
Histology and immunofluorescence microscopy
For hematoxylin and eosin (H&E) staining, thymi were fixed in 10% formalin solution for 1 day and then transferred into 70% ethanol. Paraffin-thin sections of thymus were stained with H&E according to standard protocols. For immunofluorescence, thymi were embedded in OCT (Leica Biosystems Richmond, Inc., Richmond) and frozen immediately at −80°C. Frozen thin sections (5μm) were fixed in a 1:1 mixture of acetone and methanol at −20 °C for 8 minutes, washed in PBS, and blocked in PBS containing 3% BSA with 0.1% Tritonx-100 for 30–45 minutes at room temperature (RT). Subsequently, samples were incubated with primary rat-anti-mouse-keratin 8 (Troma-1, DSHB, University of Iowa, 1:50 dilution) and rabbit-anti-mouse-keratin 5 (PRB-160P, Covance; 1:200 dilution) antibodies, followed by secondary Rhodamine-conjugated-donkey anti-rabbit IgG (1:400 dilution) and FITC-conjugated-goat anti-rat IgG (1:400 dilution, Santa Cruz Biotechnology). Sections were mounted with Vector mounting solution containing DAPI (Vector) and allowed to dry overnight at RT or 4 degrees in the dark before imaging. Images were captured using a Zeiss ApoTome Microscope and organized using Photoshop software.
Statistical analysis
All statistical analysis was performed using Prism 5 (GraphPad) software. Comparisons were made using a two-tailed Student t-test. Error bars indicate SEM. P-values less than 0.05 were considered significant.
Results
TEC-specific deletion of Rictor/mTORC2 caused moderate thymic atrophy
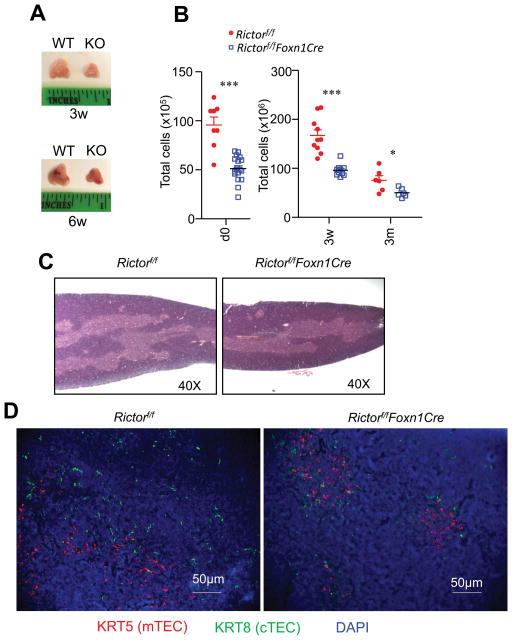
To investigate the role of mTORC2 in TECs for thymopoiesis, we bred Rictorf/f mice with Foxn1Cre mice, which selectively ablated floxed alleles in TECs starting on embryonic day 11.5 (23). We examined Rictorf/f-Foxn1Cre (KO) mice and Rictorf/f control (WT) mice at the newborn stage (day 0), on day 21 after birth (3 w), and at 3 months of age (3 m). As shown in Figure 1A, thymi from RictorKO mice were smaller than WT controls at 3 and 6 weeks. Correlated with thymic atrophy, total thymic cellularity in RictorKO mice decreased about 46.6% (day 0), 42.5% (3 weeks), and 33.9% (3 months) compared with WT controls (Figure 1B). Either H&E staining (Figure 1C) or immunofluorescence microscopic imaging with anti-keratin (KRT) 5 and KRT8 to stain mTECs and cTECs respectively (Figure 1D) of thymic thin sections showed no obvious disruption of medulla and cortex structure in RictorKO thymic. Together, our data demonstrated that mTORC2 deficiency in TECs caused moderate thymic atrophy, indicating an important role for mTORC2 in these cells for proper thymopoiesis.
Figure 1. TEC-specific deletion of mTORC2/Rictor cause moderate thymic atrophy.
A. Thymus size from 3-week- and 6-week-old Rictorf/fFoxn1Cre (KO) and Rictorf/f (WT) mice. B. Total thymic cellularity in mice with indicated ages. Each circle or square represents one WT or KO mouse, respectively. Bars represent mean ± SEM. C. H&E staining of thymus thin-sections from a pair of 6-week-old Rictorf/fFoxn1Cre and Rictorf/f mice. D. Immunofluorescence analysis of thymus cryo-sections from a pair of –week-old Rictorf/fFoxn1Cre and Rictorf/f mice. Cryo-sections were stained with primary rabbit anti-mouse-KRT5 and rat anti-mouse-KRT8 antibodies followed by secondary Rhodamine-labeled donkey anti-rabbit and FITC-labeled goat anti-rat IgG antibodies. Data shown represent or have been calculated from at least three experiments. *p<0.05, ***p<0.001 determined by unpaired two-tailed Student’s t-test.
Reduction of TEC numbers in the absence of mTORC2
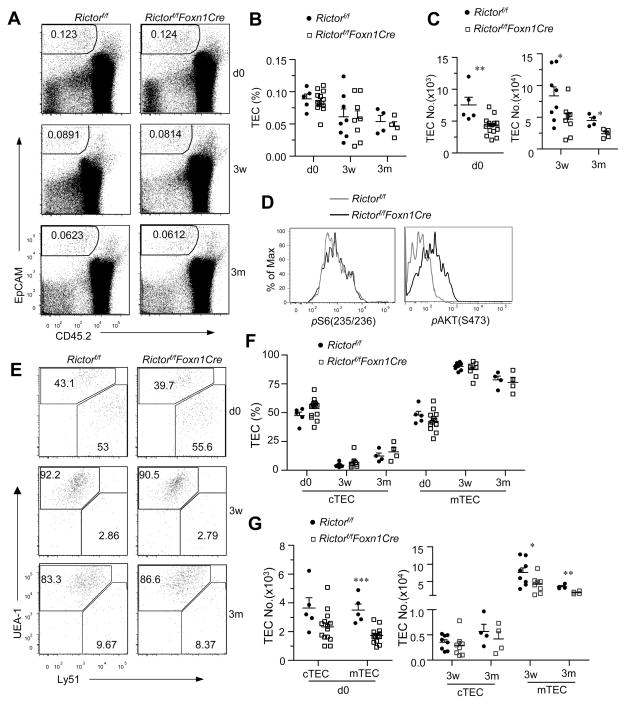
Thymic atrophy in RictorKO mice suggests that TEC development and/or function depends on mTORC2 activity. The percentages of CD45−EpCAM+ TECs did not obviously differ between WT and RictorKO thymi in newborn, 3-week-old and 3-month-old mice (Figure 2A and 2B). However, total TEC numbers in RictorKO thymi decreased by about 45% compared to WT controls (Figure 2C). Such decreases of CD45−EpCAM+ TECs in RictorKO mice correlated with decreased Akt phosphorylation at serine 473 residue, an mTORC2 dependent event, but not S6 phosphorylation, an mTORC1 dependent event (Figure 2D). Within TECs, the percentages of Ly51−UEA-1+ mTECs and Ly51+UEA-1− cTECs were similar between WT and RictorKO mice (Figure 2E, 2F). Although the averages of total cTEC numbers in RictorKO thymi were slightly lower than WT controls, such differences were not statistically significant (p>0.05). Total mTECs in RictorKO mice were decreased at varied degrees compared to WT controls (Figure 2G). Thus, mTECs appear to rely more on Rictor/mTORC2 for their generation/maintenance. Further studies are necessary to determine why mTECs were more sensitive than cTECs to mTORC2 deficiency.
Figure 2. Diminished mTECs in the absence of mTORC2.
Single-cell suspension from Rictorf/f-Foxn1Cre and Rictorf/f mice of indicated ages were stained with indicated antibodies and analyzed by flow cytometry. A. Representative dot-plots of CD45.2 and EpCAM expression. B. Percentages of EpCAM+CD45− TECs in thymus. C. Absolute numbers of TECs. D. Overlaid histogram shows phosphor-S6 (235/236) and -AKT (S473) intensity in gated CD45−EpCAM+TECs. E. Representative dot-plots of Ly51 and UEA-1 expression in gated EpCAM+CD45− TECs. F. Percentages of mTECs (UEA-1+Ly51−) and cTECs (Ly51+UEA-1−) gated from TECs. G. Absolute numbers of mTECs and cTECs. Data shown are representative or calculated from at least three experiments. Each circle or square represents one Rictorf/f or Rictorf/f-Foxn1Cre mouse, respectively. *, p<0.05; **, p<0.01; ***, p<0.001 determined by unpaired two-tail Student t-test.
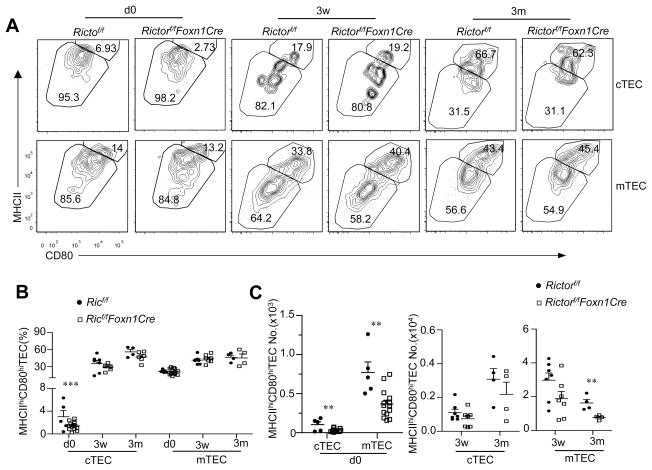
Both cTECs and mTECs developed from MHC-IIlowCD80low immature stage to MHC-IIhiCD80hi mature stage (28, 29). We observed no obvious decrease of MHC-IIhiCD80hi percentages within mTECs or cTECs in RictorKO mice at newborn, 3 weeks, or 3 months except for a slight decrease of these cells in newborn cTECs (Figure 3A, 3B). Total MHCIIhi cTEC numbers in RictorKO thymi were similar to those of WT controls with the exception of an approximate 70% decrease in newborn thymi (Figure 3C). MHCIIhi mTEC numbers were decreased in newborn and 3 months old RictorKO mice. At 3 weeks, MHCIIhi mTEC numbers showed a tendency to decrease compared with WT controls, although such a decrease was not statistically significant. TECs from RictorKO thymi did not display obvious differences in expansion (Supplemental Figure S1A, S1B) or survival (Supplemental Figure S1C, S1D) compared with WT controls. Thus, with the exception of cTECs in newborn mice, no obviously impaired differentiation occurred from MHC-IIlowCD80low stage to MHC-IIhiCD80hi stage for either mTECs or cTECs.
Figure 3. TEC maturation in TEC-specific deletion of mTORC2/Rictor.
Single cell suspension from Rictorf/f-Foxn1Cre and Rictorf/f mice of indicated ages were stained with indicated antibodies and analyzed by flow cytometry. A. Contour plots show CD80 and MHCII expression in gated mTECs and cTECs. B. Percentages of MHCIIhiCD80hi mature TECs at the indicated ages. C. Total numbers of MHCIIhiCD80hi mature TECs. Data shown are representative or calculated from at least four experiments except Day 0, which was calculated from three experiments. Each circle or square represents one Rictorf/f or Rictorf/f-Foxn1Cre mouse, respectively. **, p<0.01; ***, p<0.001 determined by unpaired two-tail Student t-test.
Rictor/mTORC2 deficiency in TECs decreased T cell generation
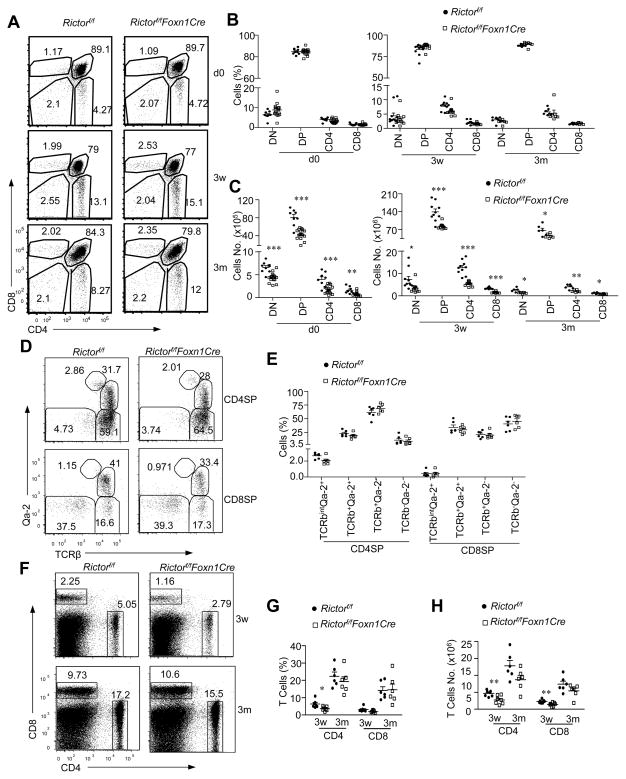
To examine whether TEC-specific mTORC2 deficiency affected T cell development, we stained thymocytes for CD4 and CD8. In newborn, 3-week-old, and 3-month-old mice, DN, DP, CD4SP, and CD8SP percentages did not obviously differ between WT and RictorKO mice (Figure 4A,4B). Qa-2 staining of SP thymocytes showed similar ratios of TCRβ+Qa-2+ mature SP thymocytes in both WT and RictorKO mice (Figure 4D, 4E), further supporting that no obvious developmental blockade occurred in RictorKO mice. Decreased total thymic cellularity in the absolute numbers of DN, DP, and SP thymocytes caused a decrease of about 30–60% in RictorKO thymus (Figure 4C). RictorKO thymocyte subsets demonstrated similar BrdU incorporation and survival compared with WT controls (Supplemental Figure S2A, S2B), suggesting that reduced T cell generation was not caused by impaired expansion or survival.
Figure 4. Impaired cαβT-cell development in Rictorf/fFoxn1Cre mice.
A. CD4 and CD8 staining of thymocytes from newborn (day 0), 3-week- (3 w) and 3-month- (3 m) old Rictorf/f and Rictor/fFoxn1Cre mice. B., C. Percentages (B) and absolute numbers (C) of indicated thymocyte populations. D. Representative dot plots showing Qa-2 and TCRβ staining of CD4+ SP and CD8+ SP thymocytes from 3 months old mice. E. Percentages of TCRβ+Qa-2+, TCRβ+Qa-2−, and TCRβ−Qa-2− populations within CD4SP and CD8 SP thymosytes (n=6). F. Representative dot plots showing CD4 and CD8 staining of splenocytes. G., H. Percentages (G) and absolute numbers (H) of splenic CD4 and CD8 T cells (3w, n=7; 3m, n=6). Data shown are representative or calculated from at least four experiments except Day 0, which we calculated from three experiments. Each circle or square represents one Rictorf/f or Rictorf/fFoxn1Cre mouse, respectively. *, p<0.05; **, p<0.01; ***, p<0.001 determined by unpaired two-tail Student t-test.
We further examined how Rictor deficiency might affect T cells in the periphery. At 3 weeks, CD4 T cell percentages and numbers in the spleen decreased by about 40% compared with WT controls (Figure 4F–4H). Although CD8 T cell percentages did not significantly differ from WT control, CD8 T cell numbers decreased 39%. At 3 months, both CD4 and CD8 T cells from RictorKO mice were similar to WT controls in both percentages and total numbers. Moreover, the ratios of CD44−CD62L+ naïve, CD44+CD62L+ central memory, and CD44+CD62L− effector memory T cells did not obviously differ between RictorKO and WT mice (supplement Figure S3). Together, these observations suggested that inefficient T cell generation resulting from Rictor deficiency in TECs delayed T cell populating in the periphery in young mice.
Effects of Rictor deficiency in TECs on early T cell development
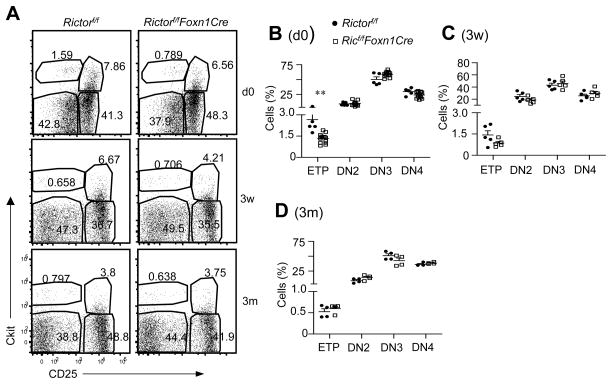
To examine whether mTORC2 in TECs regulated early T cell development, we assessed DN subsets in Rictorf/f-Foxn1Cre mice and control mice based on cKit and CD25 expression. In newborn mice, the relative ratios of early T cell progenitors (ETPs, Lin−cKit+CD25−CD24+) decreased 50%. However, the relative ratios of DN2 (cKit+CD25+), DN3 (cKit−CD25+), and DN4 (cKit−CD25−) subsets within Rictorf/f-Foxn1Cre Lin− thymocytes did not obviously differ from WT controls (Figure 5A and 5B). At 3 weeks and 3 months, the relative ratios of all these DN subsets were similar between Rictorf/f-Foxn1Cre and control mice (Figure 5A, 5C, and 5D). Together, these observations suggested that mTORC2/Rictor deficiency in TECs did not cause obvious T cell developmental blockade at DN stages with the exception of an initial decrease of ETP ratios in newborn thymus.
Figure 5. Effects of Rictor deficiency on DN thymocyte development.
A. Representative dot-plots showing cKit and CD25 expression on live gated Lin−(CD4−CD8−CD3−TCRβ−B220−CD19−TCRγδ−NK1.1−CD11b−CD11c−Gr1−Ter119−) CD24+ thymocytes from newborn, 3-week-old and 3-month-old Rictorf/f and Rictorf/fFoxn1Cre mice. B – D. Scatter plots represent mean ± SEM of ETP and DN subset percentages in newborn (B), 3-week-old (C), and 3-month-old (D) mice. Data shown are representative or calculated from at least four experiments. **, p<0.01 determined by unpaired two-tailed Student t-test.
Effects of mTORC2 deficiency in TECs on Treg and γδT cell development
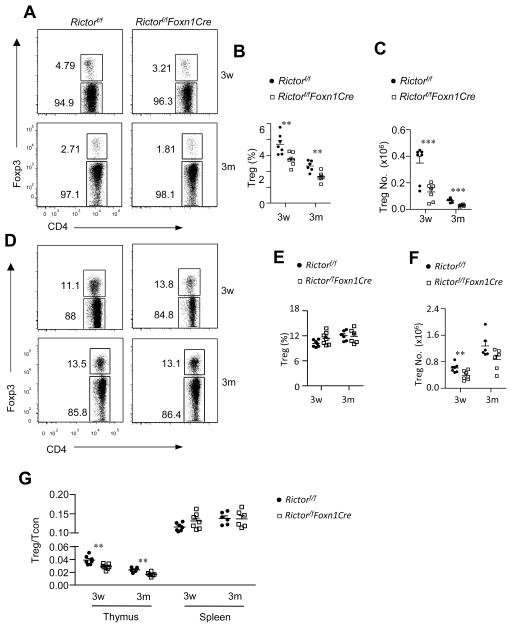
Recent evidence demonstrates an important role for mTECs in nTreg development (8, 9). In RictorKO thymus, the percentages of Foxp3+ Tregs within CD4 SP cells decreased about 26% and 29% in the thymus at 3 weeks and 3 months, respectively (Figure 6A, 6B), accompanying obvious decreases of total Treg numbers in the thymus (Figure 6C). These observations suggest that mTORC2 in TECs promotes Treg development. In the spleen, Treg percentages within CD4 T cells were similar between WT and RictorKO mice (Figure 6D, 6E). Treg numbers in RictorKO mice decreased at 3 weeks. Although Treg numbers in RictorKO mice displayed a trend of decrease at 3 months, their differences from WT controls were not statistically significant (Figure 6F). While Treg to CD4+Foxp3− conventional T cell (Tcon) ratios decreased in RictorKO thymus, we did not observe such decreases in the spleen (Figure 6G), likely because of increased Treg expansion or survival in RictorKO mice.
Figure 6. Impaired nTreg development in RictorKO thymus.
Thymocytes and splenocytes from Rictorf/f and Rictorf/f-Foxn1Cre mice were cell surface stained for CD4 and CD8 and intracellularly stained for Foxp3. A. Representative dot plots showing CD4 and Foxp3 staining in gated CD4SP thymocytes. B. Percentages of Foxp3+ Tregs in CD4SP thymocytes (3w, n=7; 3m, n=6). C. Total Treg numbers in the thymus (3w, n=7; 3m, n=6). D. Representative dot plots showing CD4 and Foxp3 staining in gated CD4+ splenocytes. B. Percentages of Foxp3+ Tregs in CD4+ splenocytes (3w, n=7; 3m, n=6). F. Total Treg numbers in the spleen (3w, n=7; 3m, n=6). G. Treg to Tcon ratios in the thymus and spleen (3w, n=7; 3m, n=6). Data shown are representative or calculated from four experiments. Each circle or square represents one Rictorf/f or Rictorf/fFoxn1Cre mouse, respectively. **, p<0.01; ***, p<0.001 determined by unpaired two-tail Student t-test.
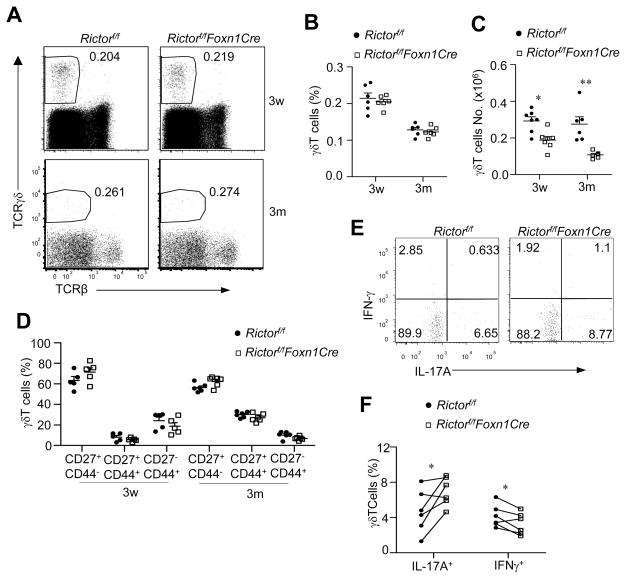
γδT-cells are another lineage of T cells that develops in the thymus (30, 31). Percentages of γδT-cells in RictorKO thymi were similar to controls at 3 weeks and 3 months (Figure 7A, 7B). However, total γδT-cell numbers decreased about 36% at 3 weeks and 61% at 3 months in RictorKO thymi (Figure 7C), indicating that efficient γδT-cell generation was dependent on mTORC2 in TECs. γδT-cells can differentiate into distinct effector lineages within the thymus (30, 32). IL-17-producing γδT (γδT17) cells are predominantly generated in fetal and newborn thymus (33, 34). Two recent studies have revealed that thymic epithelial cells may foster a thymic environment for temporal control of γδT17 differentiation (22, 35) and that such temporal control of γδT17 differentiation requires mTORC1 in TECs (22). γδT17 cells mainly reside in the CD44+CD27− population, while IFNγ-producing γδT (γδT1) cells are enriched in the CD27+ population (34). Although the related ratios of these γδT populations in WT and RictorKO thymi were similar (Figure 7D), within RictorKO thymic γδT cells, γδT17 cell percentages slightly increased, but γδT1 cell percentages slightly decreased (Figure 7E, 7F), suggesting that mTORC2 in TECs may weakly control thymic environment to shape γδ effector differentiation.
Figure 7. Effects of mTORC2/Rictor deficiency on γδT cell development.
A. Representative dot plots showing TCRγδ and TCRβ expression of 3-week- and 3-month-old Rictorf/f and Rictorf/f-Foxn1Cre thymocytes. B. γδT percentages (B) and numbers (C) in indicated ages in thymus (3w, n=6; 3m, n=6). D. Scatter graph represents mean ± SEM of percentages of the indicated γδT populations based on CD27 and CD44 expression (3w, n=5; 3m, n=6). E. Representative dot plots showing IL-17A and IFNγ producing cells in gated γδT cells in the thymus. F. Percentage s of IL-17A+ γδT Cells and IFNγ+ γδT in the thymus. Data shown represent or are calculated from at least five experiments. *, p<0.05; **, p<0.01 determined by unpaired (B–D) and paired (F) two-tailed Student t-test.
mTORC2 signaling in TECs promotes iNKT-cell development
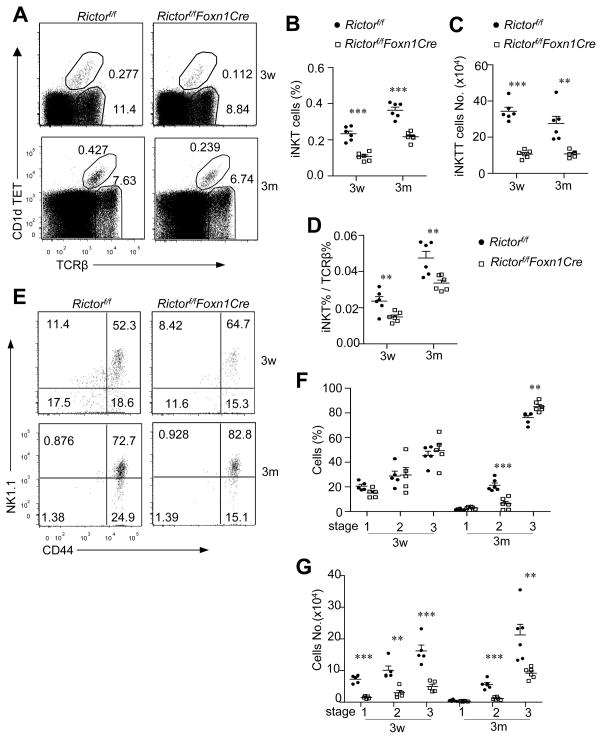
The invariant NKT (iNKT) cells express the invariant Vα14-Jα18 TCRα chain in mice and are positively selected by self-lipid ligand-CD1d complex expressed on DP thymocytes (36). mTOR and its tight regulation by TSC1 in developing thymocytes intrinsically control iNKT cell development and function (15, 16, 21, 37–39). We have recently revealed that mTORC1 in TECs promotes iNKT cell development (22). Using PBS57-loaded CD1d-Tetramer (CD1dTET) and TCRβ staining, we observed decreased thymic CD1dTET+TCRβ+ iNKT-cells in 3-week- and 3 month-old RictorKO mice (Figure 8A, 8B), accompanying more severe decreases of iNKT-cell total numbers (Figure 8C). Moreover, the ratios of iNKT to cαβT cells decreased about 30–40% in RictorKO thymus (Figure 8D), suggesting more defective iNKT generation than cαβT-cells in these mice. Development of iNKT cells are defined into multiple stages based on CD44 and NK1.1 expression. At 3 weeks, the relative percentages of stages 1 (CD24−CD44−NK1.1−), 2 (CD24−CD44+NK1.1−), and 3 (CD24−CD44+NK1.1+) iNKT-cells in these mice were similar to WT controls (Figure 8E, 8F), suggesting no obvious developmental blockade during iNKT cell development. At 3 months, the relative ratio of stage 1 iNKT cells was similar for WT and RictorKO mice; however, the ratio of stage 2 decreased and the ratio of stage 3 iNKT cells relatively increased in RictorKO mice, suggesting a potential accelerated iNKT cell terminal maturation in adult RictorKO mice. Of note, the absolute numbers of iNKT cells at all developmental stages decreased markedly in both 3-week- and 3-month-old RictorKO mice (Figure 8G). Together, these observations indicate that efficient iNKT-cell generation is dependent on mTORC2 signaling in TECs.
Figure 8. Severe iNKT-cell developmental defect in Rictorf/fFoxn1Cre mice.
Thymocytes from 3 weeks and 3 months old Rictorf/f and Rictorf/fFoxn1Cre mice were stained with PBS57 loaded CD1d-Tetramer (CD1dTET), TCRβ, and other indicated molecules. A. CD1dTET and TCRβ staining of live-gated thymocytes. B, C. Percentages (B) and absolute numbers (C) of iNKT cells (3w, n=6; 3m, n=6). Horizontal bars represent means and SEM. D. iNKT cells to cαβT cell ratios in individual mice (3w, n=6; 3m, n=6). E. Representative dot plots show CD44 and NK1.1 expression in gated iNKT cells. F., G. Percentages (F) and absolute numbers (G) of indicated iNKT cell populations (n=6). *, p<0.05; **, p<0.01; ***, p<0.001 determined by two-tailed tailed-Student t-test.
Discussion
Both cell-intrinsic and -extrinsic factors regulate intrathymic T cell development. Previous studies have found that Rictor/mTORC2 is involved in early T cell development at the DN stage but plays minimal roles for T cell development at DP and SP stages (19, 40–42). In Rictorf/f mice carrying the Mx1-Cre transgene, systemic deletion of Rictor/mTORC2 after polyinosinic-polycytidylic acid injection for two weeks causes thymic atrophy. Such an effect has been mainly attributed to an intrinsic role of mTORC2 in early T cell development; a role of mTORC2 in TECs was not assessed (42). Using mice deficient in Rictor in TECs, we have shown that Rictor/mTOR2 plays important roles in thymopoiesis and T cell generation. Rictor/mTORC2 deficiency causes moderate thymic atrophy correlated with decreases of total thymic TEC numbers. Abnormal development/function of TECs in the absence of Rictor/mTORC2 leads to moderately impaired development of cαβT cells, Tregs, and γδT cells, but more severe impairment of iNKT cells. Data from this study and our recent demonstration of a critical role for mTORC1 in TECs for thymopoiesis and T cell generation (22) establish mTOR as a central regulator in TECs to extrinsically control T cell development.
We have shown that TEC numbers decrease when Rictor/mTORC2 is ablated in these cells. mTORC2 phosphorylates multiple substrates to increase their activities, including Akt, PKCα, and the serum- and glucocorticoid-induced protein kinase 1 (SGK1) (12). Phosphorylation of Akt promotes cell survival and nutrients such as glucose uptake. We have found that Akt phosphorylation at serine 473 residue decreases in RictorKO TECs. However, RictorKO TECs appear to survive in a manner similar to that of WT TECs, suggesting that decreased TECs in RictorKO mice may not be caused by increased death of these cells. Although Akt promotes glucose uptake in T cells and several other cell types (43), glucose uptake by Rictor/mTORC2-deficient TECs does not obviously decrease, suggesting that Rictor/mTORC2 might not be crucial to control TEC metabolism via glucose uptake regulation. Interestingly, Wnt signaling has been reported to control thymus development and function (44–46). Akt phosphorylates and inactivates GSK3β (48), which phosphorylates β-catenin to induce its degradation and thus inhibit Wnt signaling (47). Future studies should explore whether an mTORC2-Ake-GSK3β-β-catenin axis in TECs controls thymopoiesis and T cell development. Similarly, PKCα controls cytoskeleton arrangement and cell polarity. SGK1 regulates cell survival and lipid metabolism (12). Whether PKCα and SGK1 phosphorylation is affected by mTORC2 deficient in TECs and how such alternation may potentially contribute to the phenotypes we observed in RictorKO mice remains to be investigated.
In Rictor/mTORC2-deficient mice, mTEC numbers diminish, while cTEC numbers are not significantly affected. Interestingly, Raptor/mTORC1 deficiency in TECs results in severe mTEC reduction but only slightly affects cTEC numbers (22). Thus, mTECs appear to rely more on both mTORC1 and mTORC2 for their expansion/maintenance. At present, we do not know why mTECs are hypersensitive to mTOR deficiency, which could relate to their difference in metabolism. That deficiency of either mTORC1 or mTORC2 decreases mTECs raises the possibility that deficiency of one of these complexes in TECs could affect the other complex. Cross-regulation of mTORC1 and mTORC2 has been documented in other cells (12, 21). Because mTORC1 activity is not obviously altered in mTORC2 deficient TECs, the phenotype we observed in mTORC2-deficient mice is unlikely to have resulted from dysregulation of mTORC1 in these cells.
Our data have also revealed important roles for Rictor/mTORC2 in extrinsically promoting intrathymic development of virtually all T cell lineages, including convention αβT cells, Tregs, γδT cells, and iNKT cells. Because the developmental stages for each of these lineages are proportionally affected and there is no obvious developmental blockade at particular developmental stages, Rictor/mTORC2 appears to regulate overall T cell production. Interestingly, mTECs play important roles in self-tolerance via inducing negative selection of highly self-reactive T cells and Treg generation. Although Rictor/mTORC2 deficiency affects mTECs more severely than it affects cTECs, we have not observed obvious autoimmune diseases in Rictor/mTORC2-deficient mice. Thus, mTECs, even in limited numbers, can manage negative selection sufficiently to maintain central tolerance in RictorKO mice. Additionally, Tregs are generated in Rictor/mTORC2 deficient mice, and their expansion in the peripheral lymphoid organs is not hindered, which may compensate for potential inefficiency of negative selection in these mice. Future studies with more definitive approaches are needed to address whether mTORC2 deficiency affects negative selection in TECs.
Although Rictor/mTORC2 deficiency affects development of all T cell lineages, iNKT cells appear to be more severely reduced than cαβT cells in Rictor/mTORC2-deficient mice. Although positive selection of iNKT cells depends on engaging the iVα14TCR with self-lipid ligands presented by CD1d expressed on DP thymocytes in the cortex rather than TECs (36), mTECs have been found to promote late stage iNKT-cell development by producing IL-15 (49). It would be interesting to determine if Rictor/mTORC2 regulates iNKT cell development via producing IL-15 and other cytokines.
Age-associated and stress-, irradiation-, or chemical-induced thymic involution/atrophy can lead to reduced T cell production, T cell repertoire contraction, weakened adaptive immunity, and inclination for autoimmunity (50–53). Rapamycin and its derivatives are widely used for immune suppression and for anti-tumor therapies. Although rapamycin displays more selective effects on mTORC1, it also inhibits mTORC2 at higher concentrations. Moreover, new generation of pan-mTOR inhibitors have also been recently developed for use in clinical settings (54). Given our findings that deficiency of either mTORC2 or mTORC1 causes thymic atrophy and decreases T cell production, it would be important to consider potential side effects of these mTOR inhibitors on thymic function.
Supplementary Material
Acknowledgments
We thank Dr. Nancy Manley for kindly providing us with the Foxn1Cre mice and the NIH tetramer core facility for providing the CD1d tetramer.
This study was supported by the National Institutes of Health (R01AI079088 and R01AI101206).
Abbreviations used in this article
- mTORC1
mammalian or mechanistic target of rapamycin complex 1
- nTORC2
mammalian or mechanistic target of rapamycin complex 2
- TEC
thymic epithelial cell
- mTEC
medullar thymic epithelial cell
- cTEC
cortical thymic epithelial cell
- DP
double positive
- DN
double negative
- SP
single positive
- TRA
tissue-restricted antigen
Footnotes
Author contributions: HXW designed and performed experiments, analyzed data, and wrote the paper. JSC and SC performed experiments and analyzed data. YRQ participated in data analysis. XPZ conceived the project, designed experiments, participated in data analysis, and wrote the paper.
Conflict of interests: The authors declare no conflict of interests.
References
- 1.Fahl SP, Coffey F, Wiest DL. Origins of gammadelta T cell effector subsets: a riddle wrapped in an enigma. J Immunol. 2014;193:4289–4294. doi: 10.4049/jimmunol.1401813. [DOI] [PubMed] [Google Scholar]
- 2.Yang Q, Jeremiah Bell J, Bhandoola A. T-cell lineage determination. Immunol Rev. 2010;238:12–22. doi: 10.1111/j.1600-065X.2010.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson G, Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 2012;33:256–263. doi: 10.1016/j.it.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Nehls M, Kyewski B, Messerle M, Waldschutz R, Schuddekopf K, Smith AJ, Boehm T. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996;272:886–889. doi: 10.1126/science.272.5263.886. [DOI] [PubMed] [Google Scholar]
- 5.Su DM, Navarre S, Oh WJ, Condie BG, Manley NR. A domain of Foxn1 required for crosstalk-dependent thymic epithelial cell differentiation. Nat Immunol. 2003;4:1128–1135. doi: 10.1038/ni983. [DOI] [PubMed] [Google Scholar]
- 6.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 7.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med. 2013;210:675–681. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coquet JM, Ribot JC, Babala N, Middendorp S, van der Horst G, Xiao Y, Neves JF, Fonseca-Pereira D, Jacobs H, Pennington DJ, Silva-Santos B, Borst J. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27-CD70 pathway. J Exp Med. 2013;210:715–728. doi: 10.1084/jem.20112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 12.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien TF, Zhong XP. The role and regulation of mTOR in T-lymphocyte function. Arch ImmunolTherap Exp. 2012;60:173–181. doi: 10.1007/s00005-012-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Yang J, Yang K, Wang H, Gorentla B, Shin J, Qiu Y, Que LG, Foster WM, Xia Z, Chi H, Zhong XP. iNKT cells require TSC1 for terminal maturation and effector lineage fate decisions. J Clin Invest. 2014;124:1685–1698. doi: 10.1172/JCI69780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin J, Wang S, Deng W, Wu J, Gao J, Zhong XP. Mechanistic target of rapamycin complex 1 is critical for invariant natural killer T-cell development and effector function. Proc Natl Acad Sci USA. 2014;111:E776–783. doi: 10.1073/pnas.1315435111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie DL, Wu J, Lou YL, Zhong XP. Tumor suppressor TSC1 is critical for T-cell anergy. Proc Natl Acad Sci USA. 2012;109:14152–14157. doi: 10.1073/pnas.1119744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K, Nam KT, Cho SH, Gudapati P, Hwang Y, Park DS, Potter R, Chen J, Volanakis E, Boothby M. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J Exp Med. 2012;209:713–728. doi: 10.1084/jem.20111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshii T, Kasada A, Hatakeyama T, Ohtani M, Tadokoro Y, Naka K, Ikenoue T, Ikawa T, Kawamoto H, Fehling HJ, Araki K, Yamamura K, Matsuda S, Hirao A. Loss of mTOR complex 1 induces developmental blockage in early T-lymphopoiesis and eradicates T-cell acute lymphoblastic leukemia cells. Proc Natl Acad Sci USA. 2014;111:3805–3810. doi: 10.1073/pnas.1320265111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Gorentla B, Zhong XP, Shin J. mTOR and its tight regulation for iNKT cell development and effector function. Mol Immunol. 2015;68:536–545. doi: 10.1016/j.molimm.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HX, Shin J, Wang S, Gorentla B, Lin X, Gao J, Qiu YR, Zhong XP. mTORC1 in Thymic Epithelial Cells Is Critical for Thymopoiesis, T-Cell Generation, and Temporal Control of gammadeltaT17 Development and TCRgamma/delta Recombination. PLoS Biol. 2016;14:e1002370. doi: 10.1371/journal.pbio.1002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magee JA, Ikenoue T, Nakada D, Lee JY, Guan KL, Morrison SJ. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell. 2012;11:415–428. doi: 10.1016/j.stem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon J, Xiao S, Hughes B, 3rd, Su DM, Navarre SP, Condie BG, Manley NR. Specific expression of lacZ and cre recombinase in fetal thymic epithelial cells by multiplex gene targeting at the Foxn1 locus. BMC Dev Biol. 2007;7:69. doi: 10.1186/1471-213X-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray DH, Fletcher AL, Hammett M, Seach N, Ueno T, Young LF, Barbuto J, Boyd RL, Chidgey AP. Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Methods. 2008;329:56–66. doi: 10.1016/j.jim.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Wang HX, Qiu YR, Zhong XP. Intercellular Protein Transfer from Thymocytes to Thymic Epithelial Cells. PloS one. 2016;11:e0152641. doi: 10.1371/journal.pone.0152641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Ci X, Gorentla B, Sullivan SA, Stone JC, Zhang W, Pereira P, Lu J, Zhong XP. Differential requirement of RasGRP1 for gammadelta T cell development and activation. J Immunol. 2012;189:61–71. doi: 10.4049/jimmunol.1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakib S, Desanti GE, Jenkinson WE, Parnell SM, Jenkinson EJ, Anderson G. Checkpoints in the development of thymic cortical epithelial cells. J Immunol. 2009;182:130–137. doi: 10.4049/jimmunol.182.1.130. [DOI] [PubMed] [Google Scholar]
- 29.Nowell CS, Bredenkamp N, Tetelin S, Jin X, Tischner C, Vaidya H, Sheridan JM, Stenhouse FH, Heussen R, Smith AJ, Blackburn CC. Foxn1 regulates lineage progression in cortical and medullary thymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genet. 2011;7:e1002348. doi: 10.1371/journal.pgen.1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Ann Rev Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- 31.Born WK, Kemal Aydintug M, O’Brien RL. Diversity of γδ T-cell antigens. Cell Mol Immunol. 2013;10:13–20. doi: 10.1038/cmi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 33.Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, Chennupati V, Fohse L, Naumann R, Weiss S, Krueger A, Forster R, Prinz I. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nature Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nitta T, Muro R, Shimizu Y, Nitta S, Oda H, Ohte Y, Goto M, Yanobu-Takanashi R, Narita T, Takayanagi H, Yasuda H, Okamura T, Murata S, Suzuki H. The thymic cortical epithelium determines the TCR repertoire of IL-17-producing γδ T cells. EMBO Rep. 2015;16:638–653. doi: 10.15252/embr.201540096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, Bendelac A, Littman DR. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Shin J, Xie D, Wang H, Gao J, Zhong XP. Tuberous sclerosis 1 promotes invariant NKT cell anergy and inhibits invariant NKT cell-mediated antitumor immunity. J Immunol. 2014;192:2643–2650. doi: 10.4049/jimmunol.1302076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prevot N, Pyaram K, Bischoff E, Sen JM, Powell JD, Chang CH. Mammalian target of rapamycin complex 2 regulates invariant NKT cell development and function independent of promyelocytic leukemia zinc-finger. J Immunol. 2015;194:223–230. doi: 10.4049/jimmunol.1401985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Tschumi BO, Corgnac S, Ruegg MA, Hall MN, Mach JP, Romero P, Donda A. Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function. J Immunol. 2014;193:1759–1765. doi: 10.4049/jimmunol.1400769. [DOI] [PubMed] [Google Scholar]
- 40.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang F, Wu Q, Ikenoue T, Guan KL, Liu Y, Zheng P. A critical role for Rictor in T lymphopoiesis. J Immunol. 2012;189:1850–1857. doi: 10.4049/jimmunol.1201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, Hollander GA. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol. 2002;3:1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- 45.Zuklys S, Gill J, Keller MP, Hauri-Hohl M, Zhanybekova S, Balciunaite G, Na KJ, Jeker LT, Hafen K, Tsukamoto N, Amagai T, Taketo MM, Krenger W, Hollander GA. Stabilized beta-catenin in thymic epithelial cells blocks thymus development and function. J Immunol. 2009;182:2997–3007. doi: 10.4049/jimmunol.0713723. [DOI] [PubMed] [Google Scholar]
- 46.Heinonen KM, Vanegas JR, Brochu S, Shan J, Vainio SJ, Perreault C. Wnt4 regulates thymic cellularity through the expansion of thymic epithelial cells and early thymic progenitors. Blood. 2011;118:5163–5173. doi: 10.1182/blood-2011-04-350553. [DOI] [PubMed] [Google Scholar]
- 47.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 48.Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White AJ, Jenkinson WE, Cowan JE, Parnell SM, Bacon A, Jones ND, Jenkinson EJ, Anderson G. An essential role for medullary thymic epithelial cells during the intrathymic development of invariant NKT cells. J Immunol. 2014;192:2659–2666. doi: 10.4049/jimmunol.1303057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su DM, Aw D, Palmer DB. Immunosenescence: a product of the environment? Curr Opin Immunol. 2013 doi: 10.1016/j.coi.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 51.Boehm T, Swann JB. Thymus involution and regeneration: two sides of the same coin? Nat Rev Immunol. 2013;13:831–838. doi: 10.1038/nri3534. [DOI] [PubMed] [Google Scholar]
- 52.Holland AM, van den Brink MR. Rejuvenation of the aging T cell compartment. Curr Opin Immunol. 2009;21:454–459. doi: 10.1016/j.coi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coder BD, Wang H, Ruan L, Su DM. Thymic involution perturbs negative selection leading to autoreactive T cells that induce chronic inflammation. J Immunol. 2015;194:5825–5837. doi: 10.4049/jimmunol.1500082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Li XR, Zhang J. Current status and future perspectives of PI3K and mTOR inhibitor as anticancer drugs in breast cancer. Curr Cancer Drug Targets. 2013;13:175–187. doi: 10.2174/1568009611313020007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.