Abstract
BACKGROUND
Benign prostatic hyperplasia (BPH) is treated with 5α-reductase inhibitors (5ARI). These drugs inhibit the conversion of testosterone to dihydrotestosterone resulting in apoptosis and prostate shrinkage. Most patients initially respond to 5ARIs; however, failure is common especially in inflamed prostates, and often results in surgery. This communication examines a link between activation of NF-κB and increased expression of SRD5A2 as a potential mechanism by which patients fail 5ARI therapy.
METHODS
Tissue was collected from “Surgical” patients, treated specifically for lower urinary tract symptoms secondary to advanced BPH; and, cancer free transition zone from “Incidental” patients treated for low grade, localized peripheral zone prostate cancer. Clinical, molecular and histopathological profiles were analyzed. Human prostatic stromal and epithelial cell lines were genetically modified to regulate NF-κB activity, androgen receptor (AR) full length (AR-FL), and AR variant 7 (AR-V7) expression.
RESULTS
SRD5A2 is upregulated in advanced BPH. SRD5A2 was significantly associated with prostate volume determined by Transrectal Ultrasound (TRUS), and with more severe lower urinary tract symptoms (LUTS) determined by American Urological Association Symptom Score (AUASS). Synthesis of androgens was seen in cells in which NF-κB was activated. AR-FL and AR-V7 expression increased SRD5A2 expression while forced activation of NF-κB increased all three SRD5A isoforms. Knockdown of SRD5A2 in the epithelial cells resulted in significant reduction in proliferation, AR target gene expression, and response to testosterone (T). In tissue recombinants, canonical NF-κB activation in prostatic epithelium elevated all three SRD5A isoforms and resulted in in vivo growth under castrated conditions.
CONCLUSION
Increased BPH severity in patients correlates with SRD5A2 expression. We demonstrate that NF-κB and AR-V7 upregulate SRD5A expression providing a mechanism to explain failure of 5ARI therapy in BPH patients.
Keywords: p65, inflammation, BPH, androgen receptor, androgen receptor variant 7
INTRODUCTION
Benign Prostatic Hyperplasia (BPH) is the most common urologic disease in men over the age of 50 [1]. BPH is an expansion of the transition zone of the prostate that compresses the urethra, causing increased bladder outlet resistance. This is a cause of lower urinary tract symptoms (LUTS), which include difficult (straining, weak stream, sensation of incomplete emptying) and irritable voiding (frequency, urgency, urge incontinence) [2]. The severity of LUTS is assessed by patient reporting, captured in the American Urological Association Symptom Score (AUASS). There are many potential etiological factors contributing to BPH pathogenesis including metabolic syndrome, growth factors, hormone signaling, and inflammation [3–6]. The strong relationship between LUTS, diabetes, metabolic syndrome, and other risk factors is becoming more appreciated [7,8] suggesting links to the biology and treatment responses of BPH.
There are two major medical approaches for patients presenting with symptoms suggestive of BPH: alpha-adrenergic receptor antagonists (α-blockers) [9] and steroid-5-alpha-reductase (SRD5A) that is blocked by 5α-reductase inhibitors (5ARI) [10]. Alpha-blockers relax the muscle of the prostate and provide symptomatic relief. 5ARIs inhibit the conversion of testosterone (T) to the more potent dihydrotestosterone (DHT) by the SRD5A isoforms, resulting in a reduction of androgen receptor activity [11]. Inhibition leads to epithelial apoptosis, a decreased prostate volume, and thereby reduces LUTS, the risk of acute urinary retention, and the risk of prostate surgery [12,13]. However, a large proportion of patients either fail to respond or become resistant to current medical approaches over time, with some progressing to surgical intervention [13]. Given their age and comorbidities, these patients are often not ideal candidates for surgery [14,15]. Therefore, understanding treatment failure and developing new medical therapies appropriately targeted to these specific patient groups are desirable ways to move forward with precision medicine.
The eukaryotic nuclear factor-kappa B (NF-κB) transcription factor family regulates the expression of numerous genes [16]. NF-κB transcription factors are activated in response to a variety of stress signals, including cytokines and pathogens [17] and signaling occurs through canonical (p50/RelA) and non-canonical (p52/RelB) pathways, resulting in activation of overlapping sets of downstream genes. We have previously shown that elevated NF-κB activation in patients was associated with expression of, as well as its forced activation in cells resulted in an increase in androgen receptor full length (AR-FL) and variant 7 (AR-V7) expression [18].
COOH-terminal truncated variants of the AR (ARVs), including AR-V7, have been described in prostate cancer [19–26] and BPH [18,27]. These AR-Vs lack the ligand-binding domain of AR-FL. AR-Vs are constitutively active, driving AR-regulated transcription and promoting tumor progression under castrate conditions. Expression of AR-V7 is predictive of resistance to Enzalutamide and abiraterone acetate in prostate cancer patients [28]. Gao has reported that AR-Vs can be produced in response to the non-canonical NF-κB pathway, and we have shown that AR-Vs can be induced by the canonical NF-κB pathway [18,29,30]. Currently, however there is not an established mechanistic link between NF-κB and resistance to 5ARI therapy in BPH.
5ARI therapy targets the enzyme 5α-reductase, of which there are three isoforms (SRD5A1, -2, and -3) [31–35]. SRD5A2 is the major 5α-reductase isozyme in the prostate [20–22]. In prostate cancer increased expression of SRD5A1 and SRD5A2 predicts biochemical recurrence, metastatic potential, and reduced efficacy of androgen deprivation treatment [20,21]. SRD5A3 has been linked to glycosylation [34], hormone-refractory prostate cancer [36], and can be inhibited by both Dutasteride and Finasteride [33]. Gene polymorphisms in both SRD5A1 and SRD5A2 are linked to the biochemical recurrence of prostate cancer [31] and these polymorphisms could affect the production rate of DHT and the corresponding local exposure to androgens of androgen-responsive cells, thus undermining the basis of androgen deprivation strategies. Such a scenario is also possible in relation to BPH patient’s response to 5ARI therapy, and we suspected that SRD5A levels could affect 5ARI failure in BPH. The goal of this study was to elucidate mechanisms of 5ARI resistance.
Our previous studies have demonstrated that both NF-κB and AR-V7 are associated with BPH severity, and further we demonstrated that NF-κB induces AR-V7 expression [18]. In this study, we investigated the relationship between activation of NF-κB and/or AR-V7 expression and the levels of SRD5A isoforms in BPH to understand resistance to 5ARI. We utilized human tissue samples, as well as benign human prostate epithelial and stromal cells, to test the consequences of NF-κB, AR-FL, and AR-V7 activation on SRD5A isoforms. Our results provide a mechanism that could contribute to 5ARI therapy failure in some patients. Our data suggest that approaches to inhibit NF-κB activation in combination with a 5ARI could have an impact in reducing treatment failure in BPH.
MATERIALS AND METHODS
Cells, Reagents, and Antibodies
BHPrS1 and NHPrE1 cell lines [37,38] were cultured and transduced with empty vector IKK2 (EV) or constitutively activated KF-κB through IKK2 (EE), over expression of AR-FL and AR-V7 (V7) as previously described [18]. Primary antibodies against 5α-reductase 2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies were purchased from Vector Laboratories (Burlingame, CA).
Transient Knockdown Assays
NHPrE1 and BHPrS1 cells were seeded in 12 well plates and grown in charcoal stripped medium for 3 days until 80% confluent. One day before transfection, growth medium switched to antibiotic-free. siRNA and Lipofectamine were diluted individually in serum-free RPMI1640, combined and then incubated at room temperature for 20 min to produce a siRNA-Lipofectamine 2000 complex. siRNA was then transfected into the NHPrE1 and BHPrS1 cells according to the manufacturer’s protocol (Invitrogen). p65, SRD5A2, and non-targeting siRNA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Viability Assay
Cell viability assay in charcoal stripped medium and was performed using CellTiter-Glo (Promega) according to manufacturer’s instructions. NHPrE1 cells were seeded at 2000 cells/well in a 96-well plate, grown over 3, 5, or 7 days in the presence and absence of testosterone, Finasteride, and/or abiraterone acetate. CellTiter-Glo measurements were taken at several time points to track cell survival.
Determination of Androgen Synthesis by Cells
NHPrE1were grown in CS media for 5 days and cell pellet and media samples were collected in triplicates and analyzed in one analytical run for five androgens (testosterone [T], dihydrotestosterone [DHT], dehydroepiandrosterone [DHEA], androstenedione [ASD], and androsterone [AND]) using a modification of a validated high pressure liquid chromatographic (HPLC) assay with tandem mass spectrometric detection (LC/MS/MS) performed by the Bioanalytics, Metabolomics, and Pharmacokinetics (BMPK) Shared Resource at Roswell Park Cancer Institute (Buffalo, NY) as previously described [39].
Prostate Tissue
Our Benign Prostate Tissue repository has been described previously [14,18,40]. As part of an Institutional Review Board (IRB)-approved protocol, fresh prostate specimens were collected from consented patients undergoing holmium laser enucleation of the transition zone of the prostate (HoLEP) for symptomatic BPH with LUTS, referred to here as “Surgical BPH” and as a control, a pathologist resected transition zone nodules from patients undergoing radical prostatectomy for small volume, low risk, clinically localized peripheral zone prostate cancer, referred to here as “Incidental BPH” as described previously. Twenty-nine Incidental and 36 Surgical patients, a subset of our previous study [18] were analyzed.
Tissue Processing and Pathology
After gross pathological examination, all prostate samples used for this study was processed to maximize utility for multiple assays. This involved dividing samples, flash freezing in liquid nitrogen followed by storage at −80°C until use, as well formalin fixation for paraffin embedding. Samples were reviewed by a pathologist (O.H.) to confirm histologic findings and to exclude those with any foci of cancer as previously described [18].
Quantitative-Real-Time PCR and IHC
For quantitative real-time PCR (qPCR) 50mg flash-frozen tissue from patient of experimental sources was ground using a mortar and pestle in liquid nitrogen and RNA was extracted with Trizol (Ambion). Subsequently, 500 ng RNA was reverse transcribed into cDNA using RT2 First Strand Kit (Qiagen, Valencia, CA). qPCR was performed using IQ SYBR Green Supermix (BioRad, Hercules, CA) and results were analyzed using BioRad CFX manager software. All results were calculated using ΔΔCt analysis and normalized to GAPDH expression. qPCR primers have all been previously described and are all human-specific (no cross reaction to rat or mouse sequences). AR-FL, AR-V7, PSA, and TMPRSS2 primers were described in [18], those for SRD5A1, -2, and -3 were described in [36].
Immunohistochemistry used 4 µm sections which were de-waxed, rehydrated, and endogenous peroxidases were blocked with hydrogen peroxide. Sections were then boiled in citrate and cooled for 1 hr. Primary blocking was 15 min using Vector Elite reagents (Vector Laboratories). Primary antibodies were incubated for 30 min at room temperature at the following concentrations: SRD5A2 (1:25). Slides were washed in PBS. Biotinylated secondary antibodies (Vector Laboratories) were incubated for 30 min at room temperature after. Slides were incubated in ABC-HRP complex (Vector Laboratories Burlingame, CA) for 30 min at room temperature. Bound antibodies were then visualized by incubation with 3,3′ diaminobenzidine tetrahydrochloride (liquid DAB, DAKO). Slides were then rinsed in tap water, counterstained with hematoxylin, and mounted.
Tissue Recombinants and Subrenal Capsule Xenografting
Rat urogenital sinus mesenchyme (rUGM), the inductive mesenchymal cells surrounding the epithelial core of the urogenital sinus, was prepared from E18.5 embryonic fetuses as previously described [41–43]. To prepare tissue recombinants, rat UGM was mixed with human prostate epithelial cells as discussed in the text. The cell mixture was pelleted and resuspended in 50 µl rat-tail collagen (pretitrated to pH 7.4). After polymerization, the collagen was overlaid with growth medium. After incubation overnight at 37°C, the tissue recombinants were grafted under the renal capsule of intact Athymic Nude-Foxn1nu mouse. Mice were castrated, then rUGM recombination with either NHPrE1-EV, NHPrE1-EE, or NHPrE1-AR-V7 cell lines. For each of the three epithelial cell lines we used three mice each carrying two recombinant grafts in Groups 1 and 2 for a total n = 6 grafts per cell line per group. Grafts were inserted under the kidney capsule, and then supplemented with a 25mg testosterone pellet for 2 weeks to allow graft establishment. After 2 weeks mice were separated into two groups. Group 1 received a new testosterone pellet, while Group 2 animals had their testosterone pellet removed and returned to castrate-conditions. Hosts were euthanized at 8 weeks after grafting a portion of these grafts were flash frozen, subjected to mortar and pestle disassociation and Trizol treatment to recover RNA. cDNA was generated using reverse transcription. The other portion was analyzed histologically.
Statistical Analysis
Mann–Whitney tests were used for univariate comparisons of study characteristics between Surgical and Incidental patients. A two-sided P-value of 0.05 or less was considered statistically significant. A two-way ANOVA test was used for comparisons of characteristics between 5α-reductase isoform 1, 5αreductase isoform 2, and 5α-reductase isoform 3 gene expressions in xenografts. Correlation between SRD5A2 1–3 and TRUS and AUASS was evaluated by the determination of the Spearman correlation coefficient (GraphPad Prism).
RESULTS
SRD5A2 Is Upregulated in More Severe BPH and in Patients Exposed to 5ARI Therapy
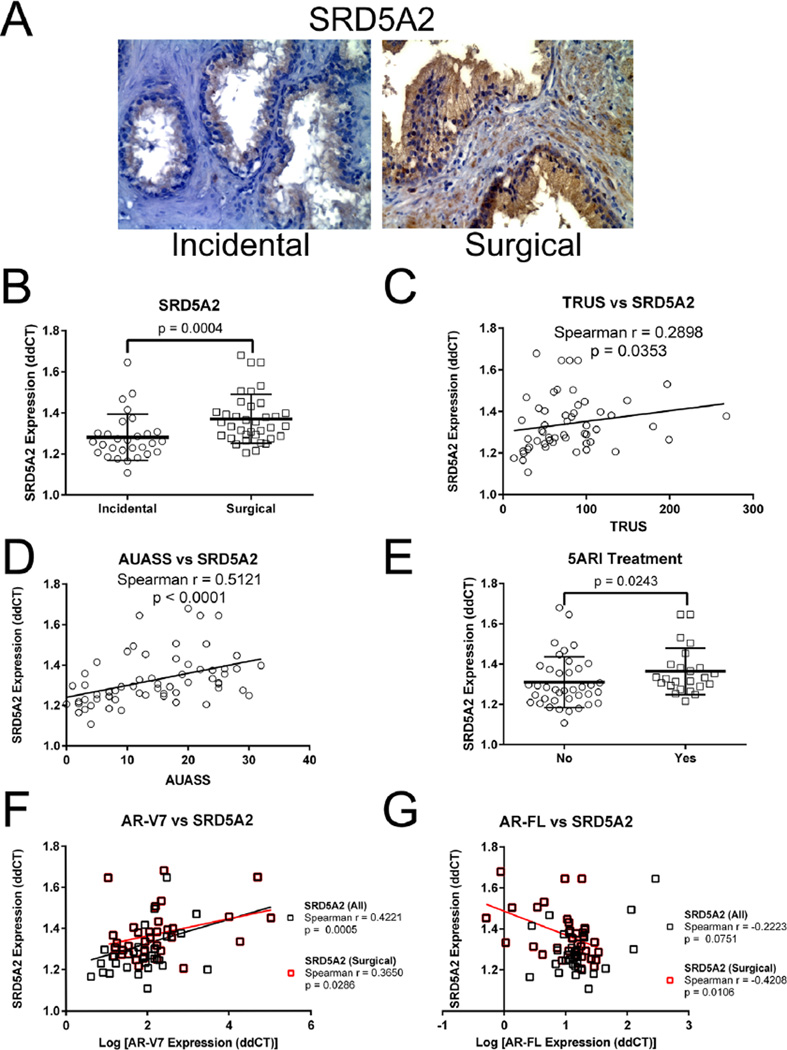
Progressive BPH is commonly associated with inflammation [44]. We recently demonstrated that NFκB expression was significantly higher in Surgical compared to Incidental BPH and, additionally, was correlated with TRUS volume [18]. We wanted to determine whether SRD5A levels in these same patients were associated with symptomatic progression. An initial immunohistochemical analysis was performed to compare expression in clinically advanced Surgical BPH specimens versus Incidental BPH specimens. The data show a complex staining pattern in both groups with a wide variability within the two groups and overlapping patterns between the groups. Two examples are shown in Figure 1A, and it is important to emphasize that examples of these two patterns of expression both occur in Surgical and Incidental patient samples. In some samples staining is essentially localized only to epithelial cells, this pattern has been reported previously [45]. As expected, many samples in both Surgical and Incidental groups have additional staining of variable distribution and intensity in the stroma.
Fig. 1.
SRD5A expression in symptomatic BPH. Immunolocalization of SRD5A2 in incidental and surgical BPH samples. (A) SRD5A2. Consistent with the qPCR data shown in panel B there is a general increase in staining intensity in surgical versus incidental samples; however, there is significant variability and overlap in both intensity and pattern of staining in the groups. The examples shown illustrate an incidental sample where staining is essentially localized only to epithelial cells, this phenotype is common. However many samples, such as the surgical example shown have additional staining of variable distribution and intensity in the stroma. (B) qPCR analysis of SRD5A2 expression in BPH, showing a significant increase in expression of SRD5A2 in the Surgical BPH (n = 36) cohort compared to Incidental BPH (n = 29). Bars are presented as standard deviation, P-value: non-parametric Mann–Whitney test. (C). Spearman’s correlation coefficient analysis of SRD5A2 mRNA expression and TRUS volume showing a significant increase in SRD5A2 expression as TRUS volume increases (n = 53). (D) Spearman’s correlation coefficient analysis of SRD5A2 mRNA expression and AUASS showing a significant increase in SRD5A2 expression as AUASS increases (n = 61). (E) qPCR analysis of SRD5A2 expression in BPH patients. Patients treated with 5α-reductase inhibitors (5ARI) displayed a significant increase in SRD5A2 expression versus patients not exposed to 5ARIs (n = 65). Bars are presented as standard deviation, P-value: non-parametric Mann–Whitney test. (F) Spearman’s correlation coefficient analysis of SRD5A2 and AR-V7 mRNA expression showing a significant increase in SRD5A2 expression as AR-V7 increases (n = 65). (G) Spearman’s correlation coefficient analysis of SRD5A2 shows a trend but no significant decrease of AR-FL mRNA expression n = 65.
To quantitate overall SRD5A expression we utilized qPCR to determine mRNA levels. We showed a significant increase in SRD5A2 expression in the Surgical versus the Incidental BPH (Fig. 1B). SRD5A1 and SRD5A3 were present at low levels in these samples and were not significantly correlated with disease severity (data not shown).
We next investigated whether SRD5A2 expression was associated with increased prostate size and with increased LUTS. We analyzed patients whose AUASS (n = 61) and TRUS volume (n = 53) was included in our data set. There was a significant correlation linking both increased TRUS volume (P = 0.0353) (Fig. 1C) and AUASS (P < 0.0001) (Fig. 1D) with SRD5A2 expression. In addition, SRD5A2 expression was significantly higher (P = 0.0127) in patients who had received 5ARI therapy (Fig. 1E). No significant link was found between either SRD5A1 or SRD5A3 mRNA levels and TRUS volume (data not shown). We examined the relationship between SRD5A2 mRNA levels and those for AR-V7 and AR-FL. We found a significant positive correlation between SRD5A2 and AR-V7 mRNA levels (Fig. 1F) and a weak but not significant negative correlation with AR-FL levels when all patients were considered (Fig. 1G). A subanalysis of this dataset (Fig. 1G, red squares) revealed that when the advanced Surgical patients only were considered this negative correlation became stronger (Spearman r = −0.4208) and significant (P = 0.01).
Chronic Activation of NF-κB Results in Enhanced Expression of SRD5A Isoforms
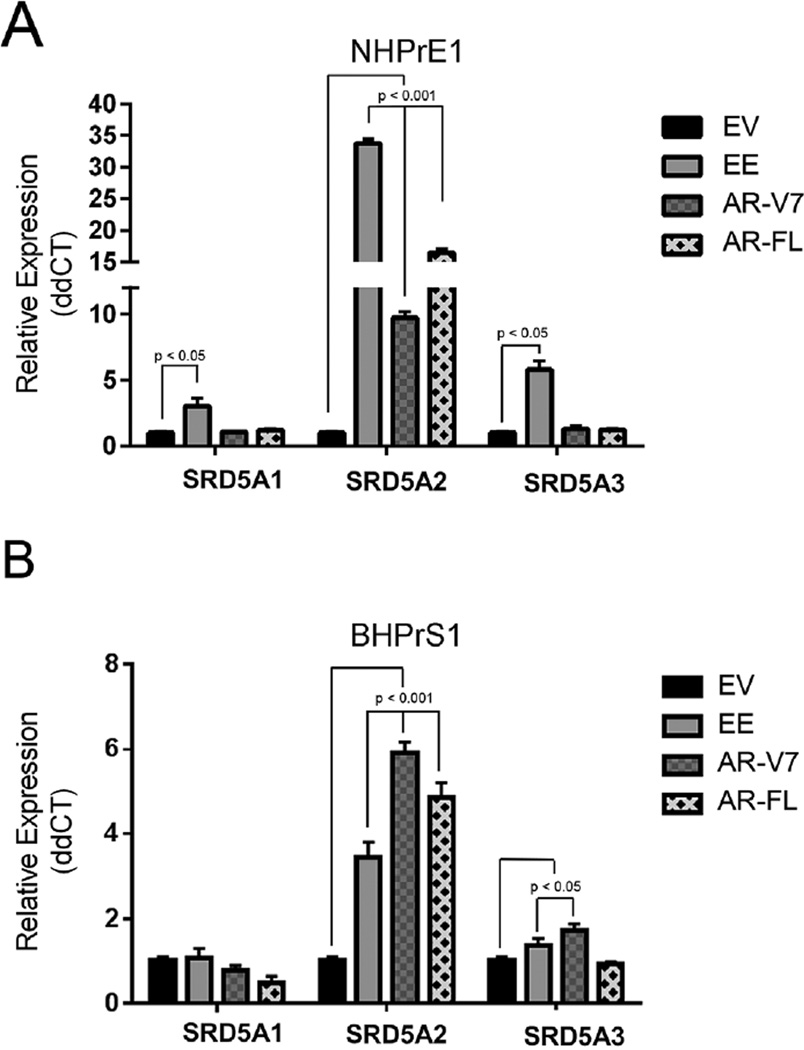
We previously demonstrated that activated NF-κB can induce expression of AR-V7, providing a potential route for resistance to 5ARI [18]. To determine whether chronic activation of NF-κB resulted in increased expression of SRD5A isoforms and influenced cell growth and function, we used previously generated cell lines [18] and show that NHPrE1-EE, a human prostatic epithelial cell line which has activated NF-κB through IKK2, significantly upregulated all three SRD5A isoforms (P < 0.001) when compared to control empty vector (EV) cells (Fig. 2A). Forced expression of AR-V7 or AR-FL showed significant upregulation of only SRD5A2 (P < 0.05) (Fig. 2A). In a stromal cell line BHPrS1, SRD5A2 (P < 0.001) was upregulated in the BHPrS1-EE, BHPrS1-AR-V7, BHPrS1-AR-FL lines, and SRD5A3 (P < 0.05) in BHPrS1-AR-V7 cells (Fig. 2B).
Fig. 2.
NF-κB and AR can regulate SRD5A expression in human cell lines. qPCR analysis of SRD5A1, SRD5A2, and SRD5A3 expression in the benign human prostatic epithelial cell line NHPrE1 (A) and the benign human prostatic stromal cell line BHPrS1 (B) transduced with empty vector (EV), constitutively active NF-κB (EE), overexpressed androgen receptor full length (AR), and androgen receptor variant 7 (V7). (A) Activation of NF-κB in NHPRE1 cells (NHPrE1-EE) resulted in a significant increase expression of all three SRD5A isoforms while expression of AR and AR-V7 (NHPrE1-AR-FL and NHPrE1-AR-V7) resulted in a significant (and much attenuated) increase in SRD5A2 expression only. (B) BHPrS1-EE, BHPrS1-AR-FL, and BHPrS1-AR-V7 demonstrated in a significant increase in SRD5A2 expression. All experiments were performed three times with triplicate repetitions. Bars are presented as standard deviation, P-value: two-way ANOVA.
Inhibition of NF-κB Abrogates SRD5A Expression
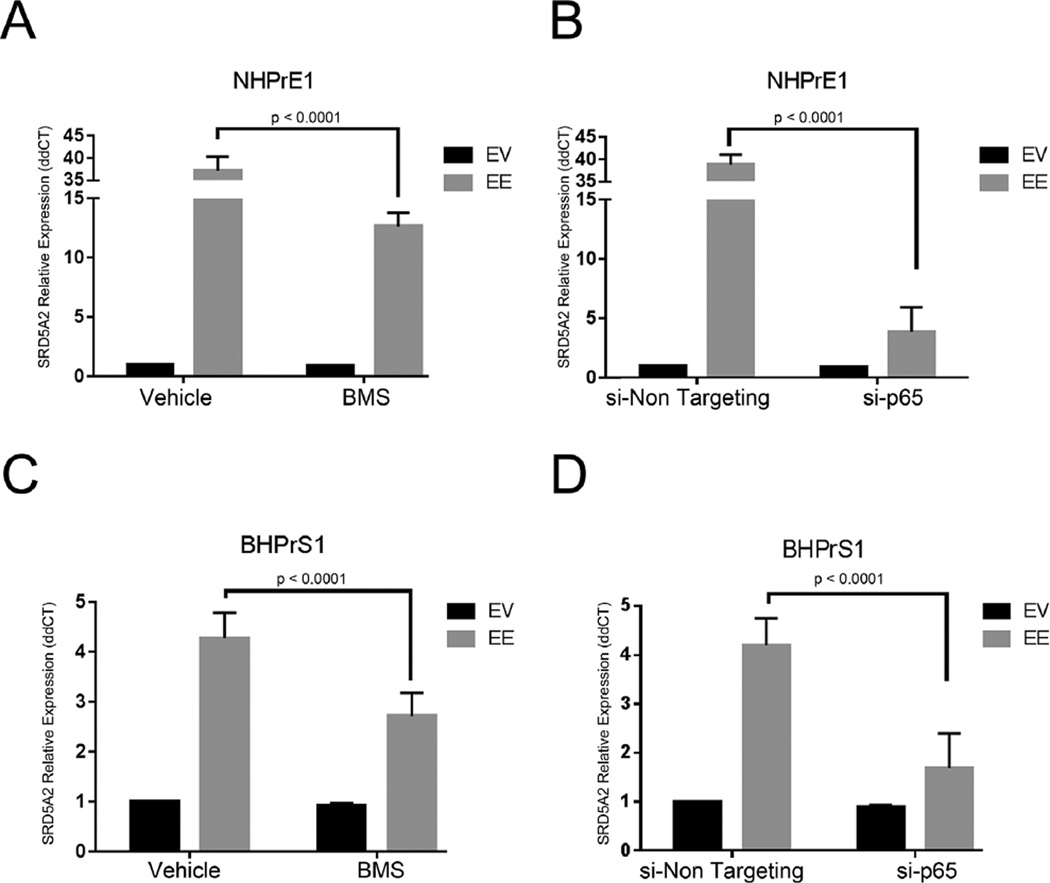
To confirm that the elevation of SRD5A in the NHPrE1-EE or the BHPrS1-EE cells was due to the effects of NF-κB activation, we performed experiments aimed at silencing downstream effectors of this activity. Since the constitutive activation of NF-κB in the EE-transduced cells is regulated by a modified form of IKK2, we used two alternative approaches to suppress NF-κB signaling. We used silencing of NF-κB, in both the epithelial and stromal cell lines through siRNA targeting of p65, and by the allosteric inhibition of IKK2 with the chemical compound BMS-345541. Silencing of NF-κB by allosteric inhibition (Fig. 3A and C) or by siRNA targeting (Fig. 3B and D) significantly (P < 0.0001) reduced SRD5A2 mRNA expression in both benign human prostatic epithelial and stromal cell lines suggesting a general mechanism that NF-κB can exert control over SRD5A2 expression.
Fig. 3.
NF-κB inhibition affects SRD5A2 expression. (A) Inhibition of NF-κB activation by BMS-345541 in NHPrE1-EE and (C) BHPrS1-EE cell lines resulted in a significant decrease in SRD5A2 expression. (B) Silencing of NF-κB using an siRNA towards p65 (si-p65) in NHPrE1-EE and BHPRs-1-EE cells (D) Results in a significant decrease in SRD5A2 expression compared to a non-targeting siRNA (si-Non Targeting). Bar values are standard deviations. P-value determined using non-parametric Mann–Whitney test.
Chronic Activation of NF-κB Induces Testosterone Synthesis
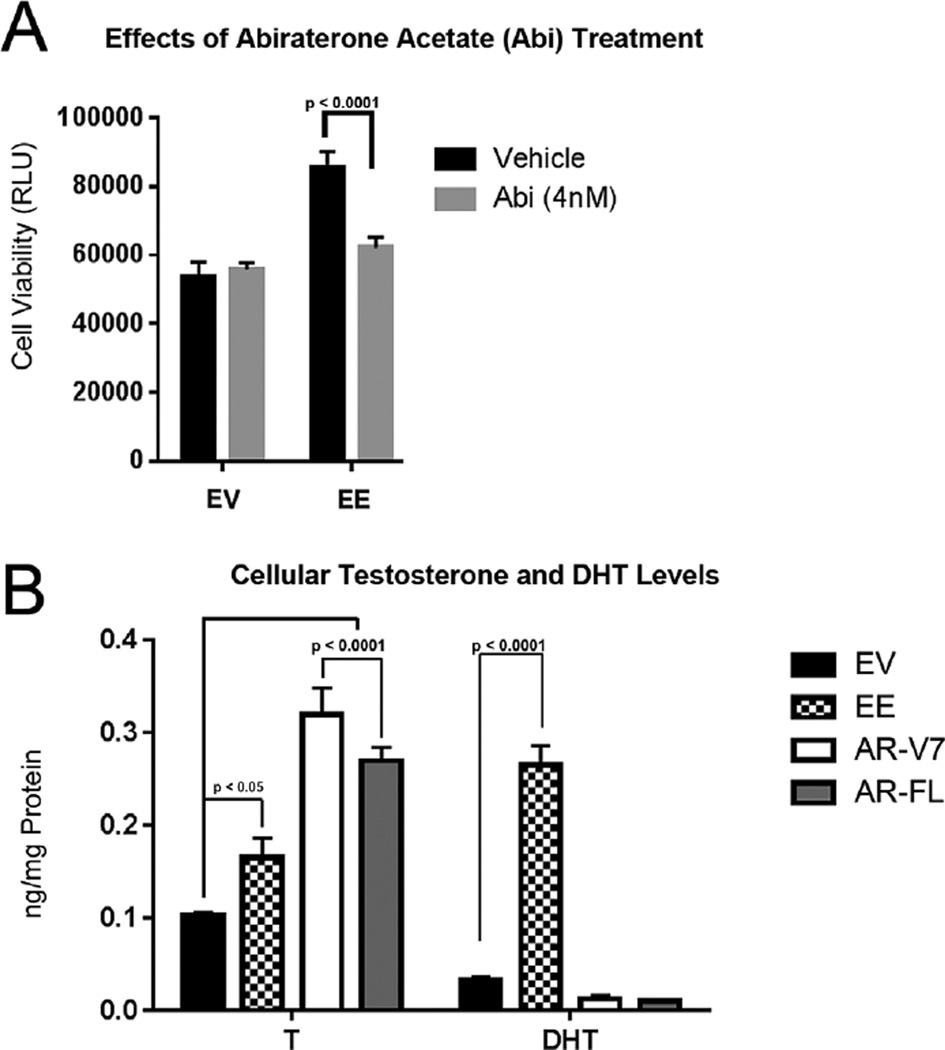
To determine whether expression of activated NF-κB can induce cells to generate increased levels of T and DHT, we used two approaches. First, a pharmacological inhibitor of androgen synthesis, abiraterone acetate (Abi) was used [46,47], and second direct quantitation using high pressure liquid chromatographic assay with tandem mass spectrometric detection (LC/MS/MS) to measure the levels of five endogenous androgens. NHPrE1 cells were grown in charcoal stripped serum (vehicle) or with 4 nM Abi for 5 days. Treatment with Abi in activated NF-κB (NHPrE1-EE) cell line resulted in a significant reduction in cell viability suggesting that suppressing androgen synthesis reduced cellular proliferation (Fig. 4A). LC/MS/MS was used to quantitate testosterone (T), dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), androstenedione (ASD), and androsterone (AND) in NHPrE1cell lines grown in CS serum over a 5- day period. As shown in Figure 4B, the NHPrE1-EE, NHPrE1-AR-V7, and NHPrE1-AR-FL cell lines can produce T. However, the NHPrE1-EE cell line had over an eightfold higher production of DHT when compared to NHPrE1-EV, NHPrE1-AR-FL, and NHPrE1-AR-V7 cells. This is consistent with the increased expression of SRD5A isoforms shown in Figure 2. DHEA, ASD, and AND were below the limit of detection in all cell lines.
Fig. 4.
NF-κB activation increases androgenic effects and DHT synthesis. (A) NHPrE1-EV (empty vector) and NHPrE1-EE (constitutively active NF-κB) cell lines were treated with 4 nM of Abiraterone acetate (Abi) to inhibit androgen synthesis. There was a significant decrease in viability of NHPrE1-EE over a five dayperiod when compared to vehicle. Bars are presented as standard deviation, P-value: two-way ANOVA (B) Measurement of two endogenous androgens (testosterone [T], and dihydrotestosterone [DHT]) in NHPrE1 cell lines grown in charcoal stripped serum over a five dayperiod. NHPrE1-EE cell line showed slightly increased synthesis of T and a large increase in DHT when compared to NHPrE1-EV (n = 3). Bars are presented as standard deviation, P-value: two-way ANOVA.
Knockdown of SRD5A2 Causes a Reduction in Cell Viability and AR Target Gene Expression
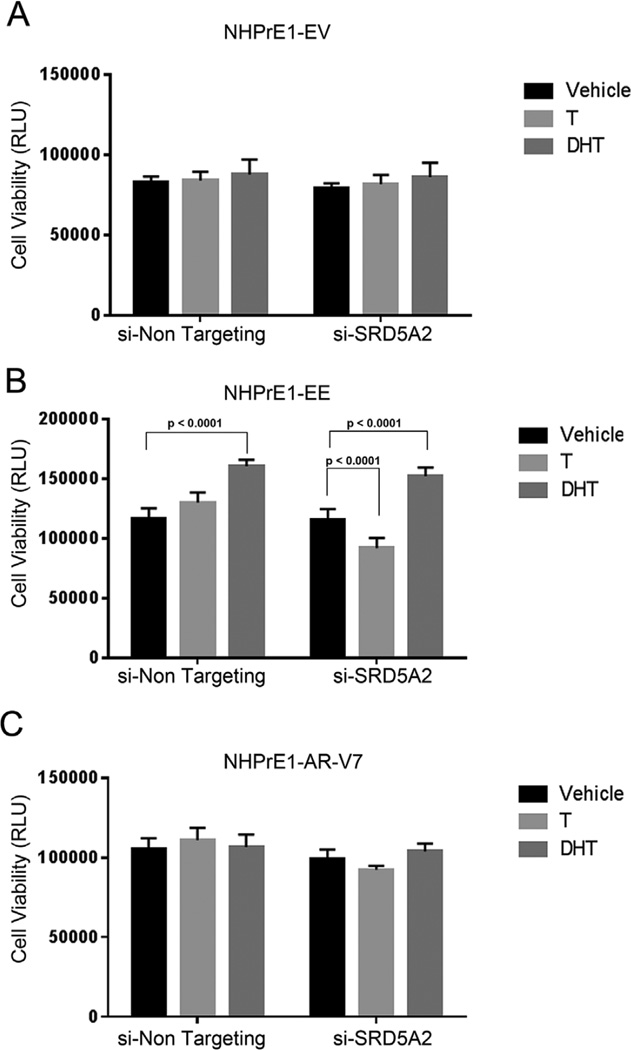
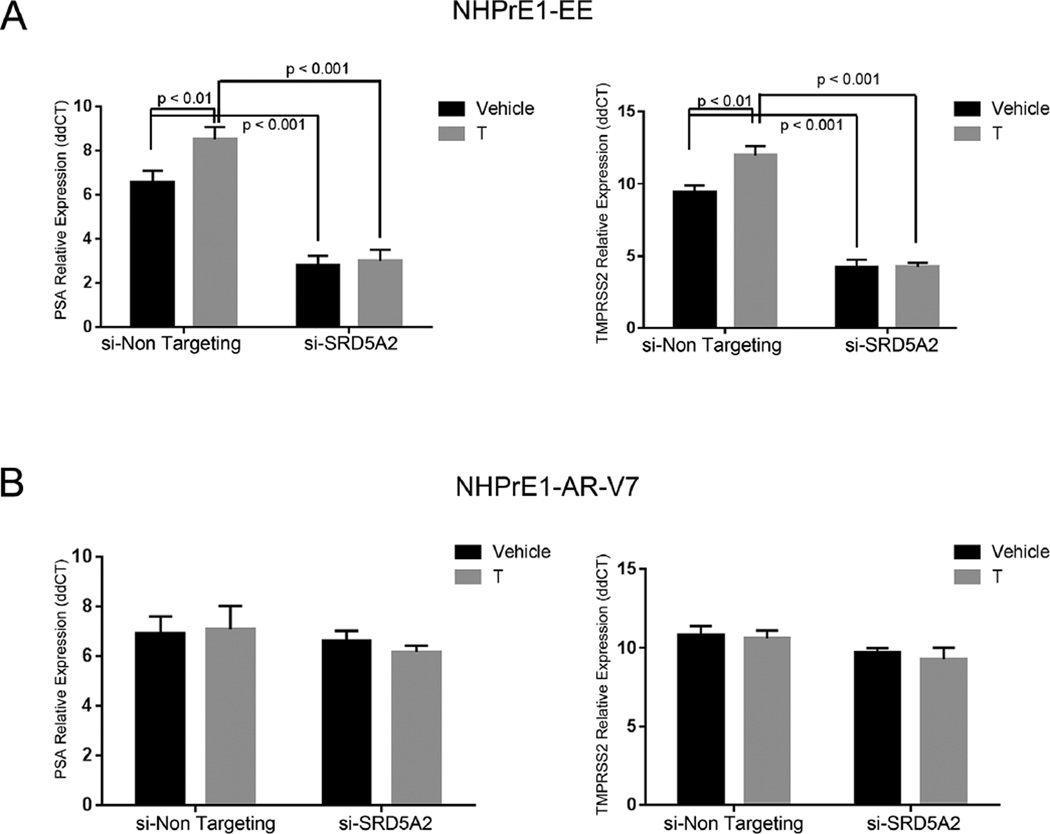
To determine whether there is a contribution of SRD5A2 in maintaining cell viability, we knocked down expression of this isoform using siRNA and assayed for cell viability after 3 days and AR target gene expression after 12 hr. As shown in Figure 5A, neither T or DHT affected the assay in the control (EV) cells. Also, as expected, knockdown of SRD5A2 in NHPrE1-EV cells had no effect on cellular viability in the presence of T or DHT. In the NHPrE1-EE cells androgen synthesis resulted in an increase in cell viability in the non-targeting siRNA cells, while knockdown of SRD5A2 decreased cell viability (P < 0.0001) in the presence of T when compared to a non-targeting siRNA. This effect was rescued by DHT, confirming the necessity for SRD5A activity to convert T to DHT to elicit a response (Fig. 5B). In the NHPrE1-AR-V7 cell line we saw that the effects of T and DHT were minimal in the both the non-targeting siRNA and in the knockdown of SRD5A2 with no significant effects on cell viability (Fig. 5C). When looking at AR target gene expression in the knockdown cell lines using qPCR and normalized against—EV controls, we saw that there was no effect on PSA and TMPRSS2 expression in NHPrE1-EV in the presence or absence of T or DHT or in the face of siRNA knockdown of SRD5A2 (data not shown). In marked contrast NHPrE1-EE cells expressed both PSA and TMPRSS2 and this expression was increased by exogenous testosterone and had a significant decreased (P < 0.01) when SRD5A2 was suppressed with siRNA (Fig. 6A). In the NHPrE1-AR-V7 cell line, there was no change in AR target gene expression with the knockdown of SRD5A2 (Fig. 6B).
Fig. 5.
Knock-down of SRD5A2 decreased viability in cells with activated NF-κB. NHPrE1-EV (empty vector control— A) -EE (constitutively active NF-κB—B), and -AR-V7 (expressing AR-V7—C) cell lines were transfected with siRNA corresponding to SRD5A2 (si-SRD5A2) or a control siRNA (si-Non Targeting) and incubated in the presence of vehicle (Ethanol), testosterone 10−9 M (T) or dihydrotestosterone 10−7 M (DHT). Cell viability was examined over a three day period. (A) Suppression of SRD5A2 had no effect on NHPrE1-EV viability in the presence or absence of androgens. (B) The control (si-Non Targeting) NHPrE1-EE cell line responded to T and DHT and showed a significant (P < 0.0001) increase in viability in the presence of DHT. Knock-down of SRD5A2 resulted in a significant (P < 0.0001) decrease in cell viability even the presence of T but no change in the effects of DHT when compared to control cells. (C) NHPrE1-AR-V7 cells line had no change in viability when SRD5A2 was knocked down even in the presence of androgens. All experiments were performed three times with triplicate repetitions (n = 9). Bars are presented as standard deviation, P-value: two-way ANOVA.
Fig. 6.
Knockdown of SRD5A2 results in a significant decrease in androgen receptor target gene expression in cells with active NF-κB but not in cells overexpressing AR-V7. NHPrE1-EE (constitutively active NF-κB—A) and NHPrE-V7 (expressing AR-V7—B) cell lines transfected with a control siRNA (control) or with siRNA corresponding to SRD5A2 (si-SRD5A2) were incubated in the presence of vehicle or testosterone (T). AR target gene expression was examined 12 hr later. Expression is relative to empty vector control cells that do not express androgen receptor. (A) qPCR of NHPrE1-EE for AR target genes PSA (right) and TMPRSS2 (left) resulted in a significant increase in mRNA expression in the presence of androgens when compared to vehicle (P < 0.001). SRD5A2 knock down cells had a significant decrease in PSA and TMPRSS2 expression even in the presence of androgens when compared to EE (P < 0.01) and lost the ability to respond to androgens when compared SRD5A2 knock down vehicle treated. (B) qPCR inNHPrE1-V7 cell lines for AR target genes PSA and TMPRSS2 resulted in no change in mRNA expression in the presence of testosterone. Bars are presented as standard deviation, P-value: two-way ANOVA. All experiments were performed three times with triplicate repetitions (n = 9).
NF-κB Induces Resistance to Castration
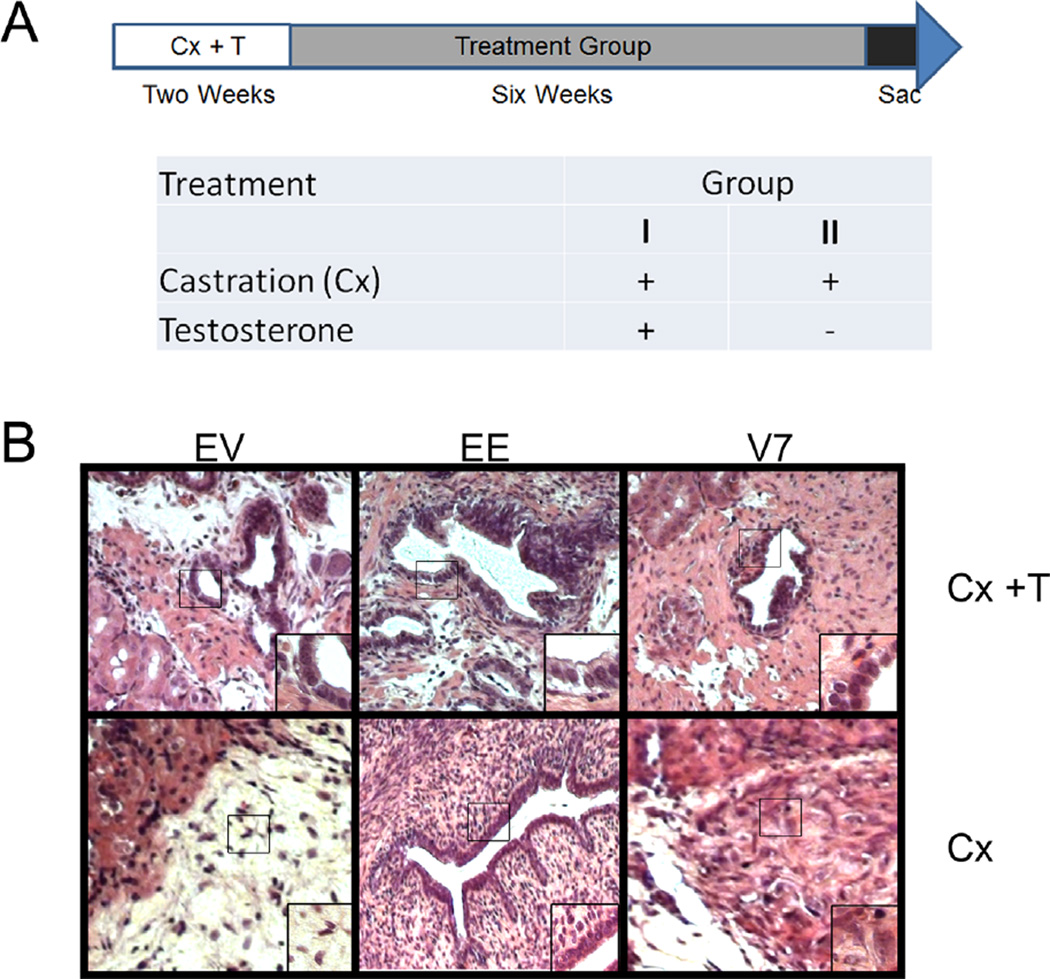
To determine how NHPrE1-EV, NHPrE1-EE, and NHPrE1-AR-V7 cells lines respond to androgen withdrawal in vivo we used a tissue recombination model. The epithelial cells lines were recombined with prostatic inductive rUGM and grafted under the renal capsule of castrated or castrated and testosterone supplemented nude mice. The treatment schema is illustrated in Figure 7A. Each group contained six sub-renal capsule grafts containing cell lines NHPrE1-EV, NHPrE1-EE, or NHPrE1-AR-V7 with rUGM. As proof of principle that the castration and the T pellet in castrated mice regulated androgen target tissues, the host seminal vesicles (SV) and associated prostatic complex were dissected and examined. The marked reduction in the size of the SV and prostate complex confirmed that androgenic stimulation had been effectively removed in these mice (not shown).
Fig. 7.
Constitutively active NF-κB results in xenograft maintenance under castrated conditions. (A) Treatment schema for Athymic Nude-Foxn1nu mice. NHPrE1-EV (empty vector control), NHPrE1-EE (constitutively active NF-κB), and NHPrE1-AR-V7 (overexpressing AR-V7) cells were recombined with rat urogenital sinus mesenchyme and grafted under the kidney capsule of mice. All mice were castrated and supplemented with testosterone (T) pellets for 2 weeks after grafting. After 2 weeks mice were separated into two groups. Group 1 (n = 9 mice) had a replacement T pellet and Group 2 (n = 9 mice) had their T pellet removed. Growth was assessed after 6 weeks and mice were euthanized. (B) Representative H&E images of recombinants containing NHPrE-EV, NHPrE1-EE, and NHPrE1-AR-V7 cells. Under continuous androgenic stimulation these show glandular epithelial structures that had developed. Following castration the NHPrE1-EV and NHPrE1-AR-V7 containing grafts, contain residual epithelium but no obvious retention of glandular structure, consistent with post castration regression in a recombinant. In contrast NHPrE1-EE grafts show a maintenance of glandular architecture, representing protection from regression in the face of androgen ablation.
H&E staining of grafts in mice maintained under continuous androgenic stimulation showed prostatic ductal development, with luminal and basal epithelial cells surrounding a lumen (Fig. 7B). Under castrate conditions NHPrE1-EV and NHPrE1-AR-V7 grafts failed to maintain epithelial glandular architecture. In contrast, grafts in which NF-κB is constitutively activated in the epithelium (the NHPrE1-EE grafts) displayed glandular development (n = 4/6) even under castrate conditions (Fig. 7B).
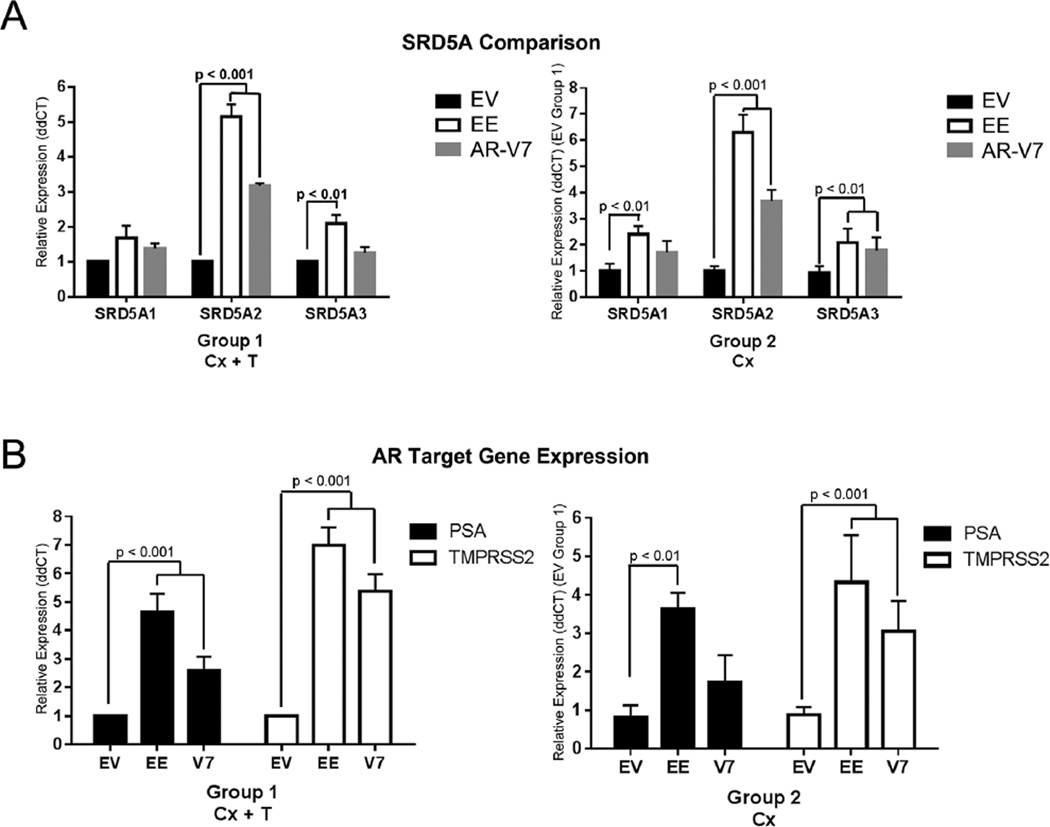
To examined biological activity in the human epithelial cell component of the grafts we used a series of human-specific qPCR primers. As shown in Figure 8A left, in the presence of testosterone, NHPrE1-EE recombinants had a significant increase in SRD5A2 (P < 0.001) and SRD5A3 (P < 0.01) when compared to NHPrE1-EV while NHPrE1-AR-V7 recombinants had a significant increase in only SRD5A2 (P < 0.01). Under castrate conditions, NHPrE1-EE grafts showed a significant increase (P < 0.001) in all three SRD5A isoforms while NHPrE1-AR-V7 grafts only had a significant increase (P < 0.01) in SRD5A2 (Fig. 8A right).
Fig. 8.
Constitutively active NF-κB results in significant increase of SRD5A and androgen receptor target gene expression under castrated conditions. qPCR analysis of mRNA expression of SRD5A isoforms (A) and AR target gene expression (B) were performed on tissue recombinant samples. Expression levels were separately standardized to the control level for each isoform and target gene. In the presence of androgens there was a significant increase in SRD5A2 and SRD5A3 (P < 0.01) in NHPrE1-EE (constitutively active NF-κB) grafts and in SRD5A2 (P < 0.001) in NHPrE1-AR-V7 grafts when compared to NHPrE1-EV (right panel). When compared to NHPrE1-EV grafts in the presence of androgens, NHPrE1-EE grafts under castrated conditions resulted in a significant increase in all three SRD5A (P < 0.01) isoforms, while NHPrE1-AR-V7 grafts showed an increase in SRD5A2 (P < 0.01) (left panel). (B) qPCR of AR target genes PSA and TMPRSS2 in the presence of androgens showed a significant increase in NHPrE1-EE and NHPrE1-V7 (P < 0.001) grafts when compared to NHPrE1-EV (right panel). When compared to AR target gene expression in NHPrE1-EV grafts under castrate conditions, NHPrE1-EE grafts under castrated conditions showed an increase in PSA and TMPRSS2 (P < 0.001) expression while NHPrE-AR-V7 resulted in only a significant increase of TMPRSS2 (P < 0.01) expression (left panel). Bars are presented as standard deviation, P-value: two-way ANOVA. All experiments were performed three times with triplicate repetitions (n = 9).
When looking at the AR target genes PSA and TMPRSS2 in these grafts, both NHPrE1-EE and NHPrE1-AR-V7 showed a significant increase (P < 0.001) in gene expression in the presence of testosterone, as compared to NHPrE1-EV controls (Fig. 8B left). PSA is not present in rat or mouse genomes and the TMPRSS2 primers used for these assays detect only the human gene, so detection is limited to mRNA derived from the human cells in the grafts. Under castrate conditions the NHPrE1-AR-V7 recombination grafts showed a significant decrease in expression of these human AR target genes under castration compared to androgenized conditions while the NHPrE1-EE grafts expressed similar levels of these AR target gene expression in the presence and absence of exogenous androgens (Fig. 8B right).
DISCUSSION
Our goal in this study was to elucidate a mechanism by which BPH patients may become resistant to 5ARI treatment. We investigated the link between activation of NF-κB and SRD5A levels in BPH. In prostate cancer SRD5A1 and SRD5A2 predict biochemical recurrence and reduced efficacy of androgen deprivation therapy [31,32]. In a previous study [18], we demonstrated that both activation of NF-κB and subsequent induction of AR-V7, contributed to resistance to a 5ARI. Here we examine whether the induction of SRD5A expression could also be a player in this phenomenon. We used a previously described repository of human prostate transition zone tissues that represents a range of BPH/LUTS severity. Using these Surgical BPH samples, we saw that SRD5A2 Levels were significantly higher in patients whose disease had progressed and required surgical intervention when compared to TZ from patients with low AUASS (Incidental BPH). We also saw that SRD5A2 significantly correlated with increased TRUS volume and AUASS. TRUS measures the entire volume of the prostate and our group has shown that TRUS volumes of Surgical BPH patients were significantly increased when compared to Incidental BPH (reflecting a general increase in prostate size as the severity of the disease increases). The Surgical BPH patients had failed therapy for severe symptoms while the transition zone tissue from Incidental patients generally represented earlier stages of BPH with no cancer lesions in the TZ and low grade and limited cancer lesions in the peripheral zone. This suggests that any change in prostate volume can be primarily attributed to transitional zone volume. We also saw that SRD5A2 was significantly higher in BPH patients who had been exposed to a 5ARI therapy; although, this result could reflect that more advanced BPH patients are more likely to receive 5ARI therapy. In patient samples, SRD5A2 correlated with expression of AR-V7 (which we have shown can be a downstream consequence of inflammation). Increased levels of SRD5A2 correlate weakly with the down-regulation of AR-FL levels when all patients are examined. It is established that DHT activation of AR-FL feedbacks to down-regulate AR-FL mRNA [48,49]. Thus, the inverse relationship with SRD5A2 and AR-FL suggest higher levels of DHT are present in the prostatic tissue from these BPH patients. It is noteworthy that when only the more advanced Surgical patient samples are considered this negative correlation between SRD5A2 and AR-FL became significant (P = 0.01) and the Spearman correlation was much stronger. This may suggest some changes in dysregulation of androgen action in more advanced disease and that such a contribution becomes more important as BPH progresses. Since SRD5A2 is the primary enzyme in the prostate that converts T to DHT [31–33], its increased expression in more symptomatic patients could help to explain why these patients failed 5ARI therapy and progressed to surgery. Increased NF-κB and associated increased SRD5A2 provide a potential mechanism by which increases in SRD5A enzyme expression might swamp the suppressive action of the 5ARI. While many of these correlations are statistically significant, they are associated with relatively low r-values, suggesting that they represent a partial explanation of what is likely a more complex phenomenon.
To clarify the underlying molecular processes, a series of in vitro experiments was performed to examine the effects of NF-κB on SRD5A levels. We have previously shown that NF-κB is upregulated in the Surgical BPH patient samples and that NF-κB activation can induce AR-FL and AR-V7 expressions [18]. Here, we wanted to determine whether NF-κB activation could affect SRD5A expression in benign human prostatic cell lines. We show that NF-κB activation markedly upregulates SRD5A2 in epithelial and stromal cell lines, with much more modest effects on SRD5A1 and A3. AR-FL and AR-V7 overexpression in epithelial and stromal cell lines upregulated the SRD5A2 isoform. We used LC/MS/MS analysis to demonstrate that the NHPrE1-EV, NHPrE1-EE, NHPrE1-AR-V7, and NHPrE1-AR-FL cell lines can synthesize androgens, although the NHPrE1-EE cells produced the highest levels of DHT, consistent with the large increase in SRD5A2 in these cells. The conversion of T to DHT is likely the most important component of androgen action in BPH patients as suppression of androgen synthesis is not part of the normal care of these patients and as such circulating levels of testicular androgens are normal. Suppression of NF-κB activity using siRNA or BMS-345541 resulted in a significant decrease in SRD5A2 expression by qPCR in the cells in which the pathway is forcibly activated. These results suggest that NF-κB induction of SRD5A isoforms, most significantly of SRD5A2, could help to explain why patients fail 5ARI therapy.
To better understand the relationship between synthesis of T, SRD5A expression, and 5ARI therapy, we used a tissue recombination grafting model, where NHPrE1-EV, NHPrE1-EE, and NHPrE1 AR-V7 cell lines were combined with rUGM and grafted under the kidney capsule in the presence and absence of androgens for 6 weeks. Six weeks after castration the NHPrE1-EE cell line was still able to maintain glandular epithelial development, showing luminal and basal cell arrangement and expressed AR-regulated genes. This is a parallel result to our report that NFκB activation in the mouse prostate results in a hyperplastic, non-malignant castrate resistant prostate in vivo [50]. This shows that under castrated conditions in vivo, activation of NF-κB can circumvent androgen ablation and supports a mechanism by which NF-κB can confer resistance to a 5ARI. Castration results in the absolute removal of testicular androgens whereas 5ARI therapy reduces the conversion of T to DHT. However, these experiments in castrated mice demonstrate the biological effect that chronic action of NF-κB can have on prostate growth. Activation of NF-κB, a consequence of chronic inflammation, can increase T synthesis, SRD5A expression, and DHT production resulting in the expression of androgen regulated genes in vivo. BPH is often thought of as a stromal disease with epithelial growth as a secondary consequence, a concept first described by McNeal and validated by Cunha et al. [43,51]. Paracrine interactions between the stroma and epithelium are important for the normal development of the prostate and also for the development of BPH [52–57]. Immune/inflammatory cells are a component of, and influence the behavior of, the stroma. Activation of NF-κB results in the upregulation of SRD5A enzymes resulting in increased conversion of T to DHT. This provides a mechanism by which inflammation plays a role in BPH progression and 5ARI failure.
In summary, the development of resistance to 5ARI therapy is likely complex and multifactorial. We have previously shown a link between NFκB activation and the expression of constitutively active AR-V7 that suggests on contributing mechanism to this resistance. The data presented suggest an additional link; whereby, aberrant activation of NF-κB drives upregulation SRD5A isoforms which increases the local synthesis of DHT. Increased NF-κB activity and the synthesis of locally produced androgens and elevated levels of SRD5A would drive the activation of AR signaling. Linking together these pathways provides a fundamental mechanism that explains failure of 5ARI therapy for BPH patients. The common theme between these observations is that inflammation that is a common response to the comorbities of BPH, notably obesity [58–61]. This insight can result in new approaches to combat BPH progression and 5ARI failure and devise approaches in precision therapy to treat individual BPH patients.
Acknowledgments
This work was supported through grants from the National Institutes of Health to DWS (DK098277), RJM (R01-CA076142 and R01-DK055748) and SWH (DK103483, DK067049, DK097782, and DK104280). The work was supported in part by the Vanderbilt-Ingram Cancer Center CCSG award No. P30 CA068485 from the National Cancer Institute through the use of the Translational Pathology Shared Resource. LC-MS/MS analysis of androgens was conducted by the Bioanalytics, Metabolomics and Pharmacokinetics (BMPK) Shared Resource of Roswell Park Cancer Institute, and partially supported by National Cancer Institute (NCI) grant P30CA016056. Tissue samples were provided by the Cooperative Human Tissue Network–Western Division, a National Cancer Institute supported resource. The authors gratefully acknowledge the assistance of Ms. Mona Cornwell and Dr. Susan Crawford of the North-Shore Histology and Imaging Core for the optimization of immunohistochemical stains for SRD5A isoforms. The work is solely the responsibility of the authors and does not necessarily represent official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflicts of interest: The authors have nothing to disclose.
REFERENCES
- 1.Platz EA, Joshu CE, Mondul AM, Peskoe SB, Willett WC, Giovannucci E. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. J Urol. 2012;188(2):496–501. doi: 10.1016/j.juro.2012.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. the measurement committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 3.Hammarsten J, Hogstedt B, Holthuis N, Mellstrom D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998;1(3):157–162. doi: 10.1038/sj.pcan.4500221. [DOI] [PubMed] [Google Scholar]
- 4.Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, Landis P, Platz EA. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91(7):2562–2568. doi: 10.1210/jc.2005-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fibbi B, Penna G, Morelli A, Adorini L, Maggi M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;33(3):475–488. doi: 10.1111/j.1365-2605.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 6.Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29–41. doi: 10.1038/nrurol.2010.207. [DOI] [PubMed] [Google Scholar]
- 7.Moul S, McVary KT. Lower urinary tract symptoms, obesity and the metabolic syndrome. Curr Opin Urol. 2010;20(1):7–12. doi: 10.1097/MOU.0b013e3283336f3f. [DOI] [PubMed] [Google Scholar]
- 8.Ozden C, Ozdal OL, Urgancioglu G, Koyuncu H, Gokkaya S, Memis A. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol. 2007;51(1):199–203. doi: 10.1016/j.eururo.2006.05.040. discussion 204–206. [DOI] [PubMed] [Google Scholar]
- 9.Lepor H, Kazzazi A, Djavan B. Alpha-Blockers for benign prostatic hyperplasia: The new era. Curr Opin Urol. 2012;22(1):7–15. doi: 10.1097/MOU.0b013e32834d9bfd. [DOI] [PubMed] [Google Scholar]
- 10.Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G, Aria A, Investigators AS. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60(3):434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- 11.Nacusi LP, Tindall DJ. Targeting 5alpha-reductase for prostate cancer prevention and treatment. Nat Rev Urol. 2011;8(7):378–384. doi: 10.1038/nrurol.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, Albertsen P, Roehrborn CG, Nickel JC, Wang DZ, Taylor AM, Waldstreicher J. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia finasteride long-term efficacy and safety study group. N Engl J Med. 1998;338(9):557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 13.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Dixon CM, Kusek JW, Lepor H, McVary KT, Nyberg LM, Clarke HS, Crawford ED, Diokno A, Foley JP, Foster HE, Jacobs SC, Kaplan SA, Kreder KJ, Lieber MM, Lucia MS, Miller GJ, Menon M, Milam DF, Ramsdell JW, Schenkman NS, Slawin KM, Smith JA. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 14.Lin-Tsai O, Clark PE, Miller NL, Fowke JH, Hameed O, Hayward SW, Strand DW. Surgical intervention for symptomatic benign prostatic hyperplasia is correlated with expression of the AP-1 transcription factor network. Prostate. 2014;74(6):669–679. doi: 10.1002/pros.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu-Yao GL, Barry MJ, Chang CH, Wasson JH, Wennberg JE. Transurethral resection of the prostate among medicare beneficiaries in the United States: Time trends and outcomes. Prostate Patient Outcomes Research Team (PORT) Urology. 1994;44(5):692–698. doi: 10.1016/s0090-4295(94)80208-4. discussion 698–9. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8(11):837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 17.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 18.Austin DC, Strand DW, Love HL, Franco OE, Jang A, Grabowska MM, Miller NL, Hameed O, Clark PE, Fowke JH, Matusik RJ, Jin RJ, Hayward SW. NF-κB and androgen receptor variant expression correlate with human BPH progression. Prostate. 2016;76(5):491–511. doi: 10.1002/pros.23140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71(15):1656–1667. doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun F, Chen HG, Li W, Yang X, Wang X, Jiang R, Guo Z, Chen H, Huang J, Borowsky AD, Qiu Y. Androgen receptor splice variant AR3 promotes prostate cancer via modulating expression of autocrine/paracrine factors. J Biol Chem. 2014;289(3):1529–1539. doi: 10.1074/jbc.M113.492140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 2010;107(39):16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, Vakar-Lopez, Vessella RL, Plymate SR. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS ONE. 2011;6(11):e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Network TCGAR. The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. ARV7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin R, Yamashita H, Yu X, Wang J, Franco OE, Wang Y, Hayward SW, Matusik RJ. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to antiandrogen treatment by decreasing androgen receptor-variant expression. Oncogene. 2015;34(28):3700–3710. doi: 10.1038/onc.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, Gao AC. NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer: Role of androgen receptor and its variants. Mol Cancer Ther. 2013;12(8):1629–1637. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Audet-Walsh E, Bellemare J, Nadeau G, Lacombe L, Fradet Y, Fradet V, Huang SP, Bao BY, Douville P, Girard H, Guillemette C, Levesque E. SRD5A polymorphisms and biochemical failure after radical prostatectomy. Eur Urol. 2011;60(6):1226–1234. doi: 10.1016/j.eururo.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Das K, Lorena PD, Ng LK, Lim D, Shen L, Siow WY, Teh M, Reichardt JK, Salto-Tellez M. Differential expression of steroid 5alpha-reductase isozymes and association with disease severity and angiogenic genes predict their biological role in prostate cancer. Endocr Relat Cancer. 2010;17(3):757–770. doi: 10.1677/ERC-10-0022. [DOI] [PubMed] [Google Scholar]
- 33.Yamana K, Labrie F, Luu-The V. Human type 3 5alphareductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Horm Mol Biol Clin Investig. 2010;2(3):293–299. doi: 10.1515/HMBCI.2010.035. [DOI] [PubMed] [Google Scholar]
- 34.Stiles AR, Russell DW. SRD5 A3: A surprising role in glycosylation. Cell. 2010;142(2):196–198. doi: 10.1016/j.cell.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, Lehle L, Hombauer H, Adamowicz M, Swiezewska E, De Brouwer AP, Blumel P, Sykut-Cegielska J, Houliston S, Swistun D, Ali BR, Dobyns WB, Babovic-Vuksanovic D, van Bokhoven H, Wevers RA, Raetz CR, Freeze HH, Morava E, Al-Gazali L, Gleeson JG. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142(2):203–217. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y, Nakagawa H. Nakagawa Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008;99(1):81–86. doi: 10.1111/j.1349-7006.2007.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, Hayward SW. Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem Cells. 2010;28:344–356. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, 2nd, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71(4):1272–1272. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilton JH, Titus MA, Efstathiou E, Fetterly GJ, Mohler JL. Androgenic biomarker profiling in human matrices and cell culture samples using high throughput, electrospray tandem mass spectrometry. Prostate. 2014;74(7):722–731. doi: 10.1002/pros.22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabowska MM, Kelly SM, Reese AL, Cates JM, Case TC, Zhang J, DeGraff DJ, Strand DW, Miller NL, Clark PE, Hayward SW, Gronostajski RM, Anderson PD, Matusik RJ. Nfib regulates transcriptional networks that control the development of prostatic hyperplasia. Endocrinology. 2016;157(3):1094–1109. doi: 10.1210/en.2015-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: A practical approach. Differentiation. 2003;71(7):402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- 42.Hayward SW, Haughney PC, Lopes ES, Danielpour D, Cunha GR. The rat prostatic epithelial cell line NRP-152 can differentiate in vivo in response to its stromal environment. Prostate. 1999;39(3):205–212. doi: 10.1002/(sici)1097-0045(19990515)39:3<205::aid-pros9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 43.Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;63(3):131–140. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- 44.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: Examination of baseline data from the REDUCE trial. Eur Urol. 2008;54(6):1379–1384. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Titus MA, Gregory CW, Ford OH, 3rd, Schell MJ, Maygarden SJ, Mohler JL. Steroid 5alpha-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res. 2005;11(12):4365–4371. doi: 10.1158/1078-0432.CCR-04-0738. [DOI] [PubMed] [Google Scholar]
- 46.O’Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, Harland S, Robbins A, Halbert G, Nutley B, Jarman M. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90(12):2317–2317. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 48.Wolf DA, Herzinger T, Hermeking H, Blaschke D, Hörz W. Transcriptional and posttranscriptional regulation of human androgen receptor expression by androgen. Mol Endocrinol. 1993;7(7):924–936. doi: 10.1210/mend.7.7.8413317. [DOI] [PubMed] [Google Scholar]
- 49.Yeap BB, Krueger RG, Leedman PJ. Differential posttranscriptional regulation of androgen receptor gene expression by androgen in prostate and Breast cancer cells. Endocrinology. 1999;140(7):3282–3291. doi: 10.1210/endo.140.7.6769. [DOI] [PubMed] [Google Scholar]
- 50.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB, Mehra R, Chinnaiyan AM. The role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10(2):177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNeal JE. Anatomy of the prostate and morphogenesis of BPH. Prog Clin Biol Res. 1984;145:27–53. [PubMed] [Google Scholar]
- 52.Bayne CW, Donnelly F, Chapman K, Bollina P, Buck C, Habib F. A novel coculture model for benign prostatic hyperplasia expressing both isoforms of 5 alpha-reductase. J Clin Endocrinol Metab. 1998;83(1):206–213. doi: 10.1210/jcem.83.1.4486. [DOI] [PubMed] [Google Scholar]
- 53.Evangelou AI, Winter SF, Huss WJ, Bok RA, Greenberg NM. Steroid hormones, polypeptide growth factors, hormone refractory prostate cancer, and the neuroendocrine phenotype. J Cell Biochem. 2004;91(4):671–683. doi: 10.1002/jcb.10771. [DOI] [PubMed] [Google Scholar]
- 54.Hayward SW, Cunha GR. The prostate: Development and physiology. Radiol Clin North Am. 2000;38(1):1–14. doi: 10.1016/s0033-8389(05)70146-9. [DOI] [PubMed] [Google Scholar]
- 55.Hayward SW, Rosen MA, Cunha GR. Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol. 1997;79(Suppl 2):18–26. doi: 10.1111/j.1464-410x.1997.tb16917.x. [DOI] [PubMed] [Google Scholar]
- 56.Nakano K, Fukabori Y, Itoh N, Lu W, Kan M, McKeehan WL, Yamanaka H. Androgen-stimulated human prostate epithelial growth mediated by stromal-derived fibroblast growth factor-10. Endocr J. 1999;46(3):405–413. doi: 10.1507/endocrj.46.405. [DOI] [PubMed] [Google Scholar]
- 57.Peehl DM, Rubin JS. Keratinocyte growth factor: An androgen-regulated mediator of stromal-epithelial interactions in the prostate. World J Urol. 1995;13(5):312–317. doi: 10.1007/BF00185975. [DOI] [PubMed] [Google Scholar]
- 58.Jiang M, Strand DW, Franco OE, Clark PE, Hayward SW. PPARγ: A molecular link between systemic metabolic disease and benign prostate hyperplasia. Differentiation. 2011;82(4–5):220–236. doi: 10.1016/j.diff.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corona G, Vignozzi L, Rastrelli G, Lotti F, Cipriani S, Maggi M. Benign prostatic hyperplasia: A new metabolic disease of the aging male and its correlation with sexual dysfunctions. Int J Endocrinol. 2014;2014:329456. doi: 10.1155/2014/329456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McLaren ID, Jerde TJ, Bushman W. Role of interleukins, IGF and stem cells in BPH. Differentiation. 2011;82(4–5):237–243. doi: 10.1016/j.diff.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parsons JK. Benign prostatic hyperplasia and male lower urinary tract symptoms: Epidemiology and risk factors. Curr Bladder Dysfunct Rep. 2010;5(4):212–218. doi: 10.1007/s11884-010-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]