Abstract
Introduction
Dexmedetomidine (Dex) has sedative, analgesic, and anesthetic-sparing effects. This meta-analysis examines demonstrated intraoperative and postoperative effects of intraoperative Dex administration during pediatric surgery.
Methods
A search for randomized placebo-controlled trials was conducted to identify clinical trials examining intraoperative Dex use in children, infants, and neonates. Primary outcome was postoperative opioid consumption; secondary outcomes were: postoperative pain intensity and postoperative nausea and vomiting (PONV).
Results
Fourteen randomized controlled trials performed during painful procedures were analyzed. Intraoperative Dex administration was associated with significantly reduced postoperative opioid consumption in the postanesthesia care unit [PACU; risk ratio (RR) = 0.31 (0.17, 0.59), I 2 = 76%, p < 0.0001 and cumulative z score using trial sequential analysis], decreased pain intensity in PACU [standardized mean difference (SMD) = −1.18 (−1.88, −0.48), I 2 = 91%, p < 0.0001] but had no effect upon PONV incidence [RR = 0.67 (0.41, 1.08), I 2 = 0%, p = 0.48]. Subgroup analyses found administering Dex during adenotonsillectomy and using a bolus <0.5 µg/kg (irrespective to the use of a continuous administration) without effects on studies outcomes. Heterogeneity was high among results and a high suspicion of publication bias was present for all analyzed outcomes.
Conclusions
This meta-analysis shows that intraoperative Dex administration in children reduces postoperative opioids consumption and postoperative pain in PACU. According to our results, optimal bolus dose was found to be ≥0.5 µg/kg. Future studies have to explore this particular point and the postoperative analgesic effects of Dex during longer periods.
Keywords: Analgesia, Children, Dexmedetomidine, Meta-analysis, Postoperative pain, Recovery
Introduction
Dexmedetomidine (Dex) is a short-acting α2-adrenoceptor agonist commonly used in adult anesthesia and intensive care [1–3]. It provides analgesia, preserves the ability to be roused, and avoids respiratory depression [3]. Several studies suggest that Dex can be useful in specific anesthetic situations. One recent meta-analysis performed in adults demonstrated that intraoperative Dex reduced intraoperative opioid consumption, pain intensity during PACU stay, opioid consumption during postanesthesia care unit (PACU) or intensive care unit (ICU) stay, and postoperative nausea and vomiting (PONV) incidence during PACU stay [4]. These properties, especially the postoperative effects on analgesia, opioid consumption, and PONV occurrence, are very interesting while they might help in promoting rapid rehabilitation after surgery.
In pediatric populations, numerous studies of intraoperative Dex use have also been performed [2]. However, most are non-randomized trials, small cohorts, or case reports, which complicate making any confident conclusions regarding the effects of intraoperative Dex. In 2013, a meta-analysis in children found Dex [5] to produce the same effects as those observed in adults [5] and in the two last years, two meta-analyses [6, 7] found this compound to strongly prevent emergence agitation. However, the potential of Dex to decrease postoperative pain and provide an opioid-sparing effect remains debated.
The aim of the present study was to perform a systematic review and meta-analysis, updating the previous one published in 2013, and exploring the postoperative effects of Dex on postoperative analgesia quality, opioid consumption, and PONV occurrence.
Methods
This meta-analysis is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Bibliographic Search and Analysis
We conducted this meta-analysis according to the guidelines of the Cochrane Handbook for Systematic Reviews [8] of intervention and the PRISMA statements [9]. Literature databases included PubMed, EMBASE, Cochrane central register of controlled trials, clinical trials register, and open-access journals not indexed in major databases (Directory of Open Access Journals, Open Journal of Anesthesiology, Anesthesiology Research and Practice, Journal of Anesthesia and Clinical Research, Journal of Anesthesiology and Clinical Science, Journal of Anesthesiology and Critical Care Medicine). The following queries were used to discard irrelevant results related to postoperative Dex use: “Dexmedetomidine” and “children or child or infant or infants”. No language restriction was applied for searches. In addition, a manual search of the references found in all selected articles was performed, including reviews and meta-analyses. Identified articles were independently assessed by four anesthesiologists (Myriam Bellon, Alix Le bot, Daphnée Michelet, Julie Hilly) and only those which fulfilled the following criteria were included: randomized-controlled, double-blind studies, patients with neurological and/or psychiatric diseases excluded, standardized protocols for anesthesia, analgesia and rescue analgesics, presence of a control group (placebo with no active anesthetic or analgesic agent) and of at least one outcome in relation to: postoperative analgesia or opioid consumption. Given the potential impact of neurosurgery on postoperative neurological function and the preoperative alteration of those functions in congenital heart diseases, both cardiac surgery and neurosurgery were excluded from the area of this meta-analysis. Abstracts presented at meetings were not included. The most recent search was performed in December 2015.
Each reader evaluated the potential presence of bias and study quality based on the following criteria: randomization and allocation concealment (clear, sufficiently detailed description of methodology demonstrating whether intervention allocations could have been foreseen before or during enrolment), double blinding, incomplete data report statements (concerning excluded patients and data) and selective reporting (presence of studied outcomes report verified). For the studies meeting these criteria, data were then independently collated by two anesthesiologists and included: patient American Anesthesiologists Association (ASA) physical status and age, type of surgery, sedative anesthetic premedication (dose, timing, and route of administration), Dex administration characteristics (doses and timing, bolus and infusion), other hypnotic agents used, intraoperative analgesia administration (both systemic or regional analgesia), postoperative analgesic administered and endpoints of each study. The primary endpoint of the study was the opioid-sparing effect of intraoperative Dex (either expressed as continuous data or as percentage of patients receiving opioids). Secondary endpoints were: the quality of postoperative [either the intensity of postoperative pain or the presence of a significant pain defined as: FLACC (Face, Legs, Activity, Cry, Consolability) >3, visual or numerical pain scale >3, facial pain scale >3, and Objective Pain Scale (OPS) >3] [10–13] and the occurrence of PONV (either both or the presence of vomiting). Other outcomes such as emergence agitation and hemodynamic effects of Dex were not analyzed because of the restrictive search on studies with postoperative analgesia outcomes. When conflicting results were found, the article was rechecked twice by the two anesthesiologists until a consensus was found.
Statistical Analysis
Statistical analyses were performed using Review Manager 5 software (RevMan 5.3, The Cochrane Collaboration, Oxford, UK) and the Trial Sequential Analysis Software (Copenhagen Trial Unit’s Trial Sequential Analysis Software, hereafter: TSA Software, Copenhagen, Sweden). Where original data were expressed as continuous variables, meta-analyses were performed using the mean difference (MD) or standardized mean difference (SMD). SMD is calculated using the formula: difference in mean outcome between studies/standard deviation (SD) of outcome among participants. This method allows aggregation of outcomes measured using different scales (opioid consumption when combining different opioid agents, times when combining hours and minutes, score rating when using five-point or ten-point scales, etc.). In all other cases, outcome incidence analysis was performed using the risk ratios (RR). In order to include a maximum number of appropriate studies and avoid publication bias, incomplete data were obtained by contacting the corresponding author or estimation of the mean and the SD on the basis of the sample size, median, and range according to the method described by Hozo and collaborators [14]. Where no validated method was identified to convert median and interquartile ranges to means and SD, data were discarded. In articles where outcomes were expressed as continuous variables, a partial standardized mean ratio was initially computed for each study, than transformed into partial odds ratio (OR) using Chinn’s formula [15]: LnOR = 1.814 × SMD (Ln: natural logarithm). The data were then included as Ln(OR) and SD(LnOR) in the software (Review Manager 5 software). Overall SDM or RR (and 95% confidence intervals) were then calculated using the inverse variance method [8]. Regarding common cut-off values for SMD, the Dex effect was considered small when the SMD was greater than −0.4, moderate when it was lying between −0.4 and −0.7, and large when it was smaller than −0.7 [8].
Heterogeneity was assessed using I 2 statistics. This approach describes the percentage of the variability in effect estimates (OR, MD, or SMD) that is due to heterogeneity rather than sampling error. According to the Cochrane Review guidelines [8], the threshold for heterogeneity is an I 2 > 40% and a p < 0.1 and indicated the use of a random effect in OR and SMD computation rather than a fixed-effects model. The random-effects model assumes that the observed effects are estimating different intervention effects while a fixed-effects model estimates the same “true” intervention effect. Based on this principle, studies were weighted. In the random-effects model, all studies are equally weighted while in the fixed-effects model, each study is weighted according to the number of included patients. In addition, because of the potential effect of some confounding factors on results, subgroup analyses for Dex effect were performed (when at least two studies included the considered outcome for the considered subgroup) according to: the type of procedure, the mode of administration (bolus alone, or infusion with or without bolus), and the dose of bolus administered (threshold for defining low and high boluses was considered as the mean for number of included studies >30 or the median if the number of included studies <30). Finally, overall results were also computed in studies displaying low-risk bias for all checked items.
In order to confirm results of our meta-analysis on the primary outcome, a second set of analyses were performed using the trial sequential method [16, 17]. This statistical method allows combining effects of studies and previous meta-analysis performed on the same subject to correct results (the adjustment of alpha-risk related to multiple comparison in previous meta-analyses), predict the possibility of a significant result in case of low power of the actual analysis and estimates the effect-size to be included in a meta-analysis (termed the information size for meta-analysis) to find a significant result. This analysis was performed on the freeware Copenhagen Trial Unit’s Trial Sequential Analysis Software, hereafter: TSA Software, Copenhagen, Sweden.
In studies with more than one intervention arm, in order to take into account all data, each arm was considered as a study and compared to the control group. However, given the weight taken by those studies in overall results, a sensitivity analysis was performed by removing one arm and another in order to assess the effect of these studies on outcome. Finally, to avoid calculation failure related to zero values in RevMan and TSA, a 1 or 0.001 was added to all groups when the number of events was equal to 0 in one group (for RevMan and TSA, respectively).
Statistical methods are available to assess the effects of unpublished studies on meta-analysis results (publication bias). Publication bias is assessed by studying the distribution of results on a funnel plot, which is a scatter plot of the intervention effect (RR, MD, or SMD) estimates from individual studies against some measure of each study’s size or precision (standard error of the intervention effect). Funnel plot asymmetry may indicate that some studies went unpublished [18, 19]. This asymmetry can also indicate result heterogeneity or poor methodology in included studies [18, 19]. According to the Cochrane collaborative guideline [8], it is suitable to assess publication bias when analysis aggregates at least ten studies.
Results are expressed as RR, MD, or SMD (95% confidence interval), I 2, p value for I 2 statistics.
Results
Study Selection and Features
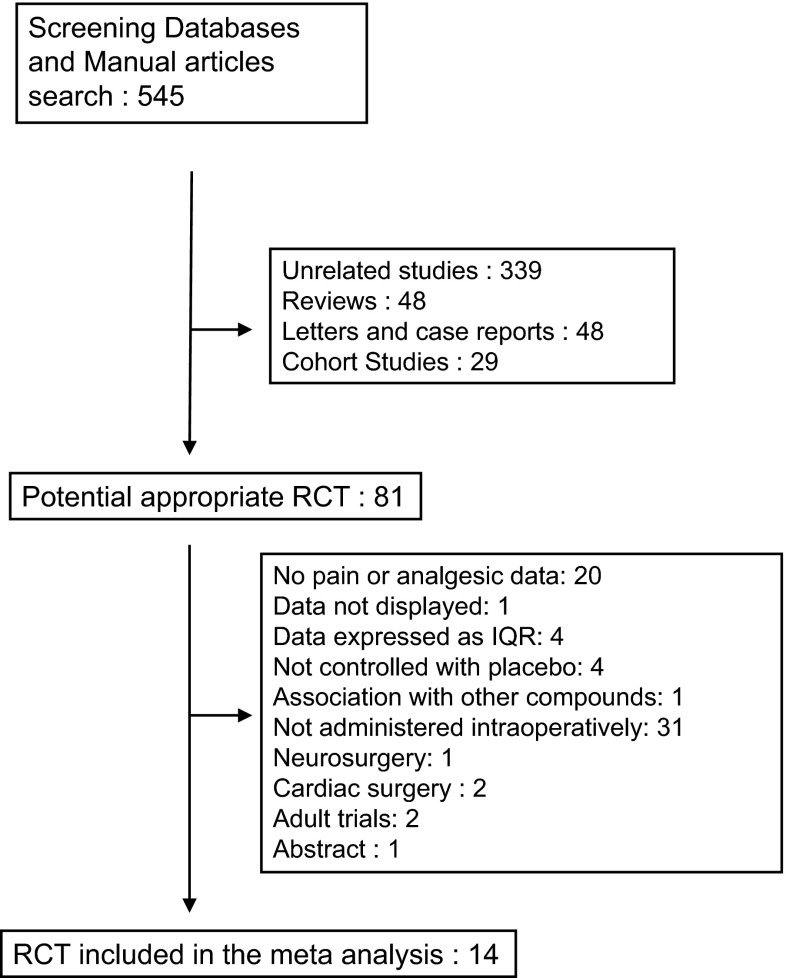
Using the above-described criteria, 545 pediatric studies were found. Analysis allowed the selection of 81 relevant randomized controlled studies. Among these articles, 67 were discarded for at least one of the following reasons: no pain or analgesic data: 20, data not displayed: one, data expressed as interquartile range (IQR): four without a response from authors, not controlled with placebo: four, association with other compounds (ketamine): one, not administered intraoperatively: 31, neurosurgery: one, cardiac surgery: two, adult trials: two and abstract: one.
Characteristics of Included Studies
Analyses were carried out upon 14 articles [20–33] (Table 1). There was no difference in recorded information between assessors and no second analysis was necessary. All studies were performed during surgery (no anesthesia for procedural sedation). Surgery performed consisted of: adenotonsillectomy and outpatient surgeries (Table 1). The selection process is summarized in Fig. 1 and characteristics of included studies are displayed in Table 1. Three studies contained three arms (two groups including patients treated with Dex compared to one control group): the study performed by Ghai and collaborators [23] (both arms using a bolus of Dex), the one performed by Meng and collaborators [28] (both using a bolus mode) and the study performed by Pestieau and collaborator [30] (using either a bolus or a continuous infusion). Postoperative pain intensities were expressed as median (range) in four studies [23, 24, 27, 32] and necessity transformation to mean and SD. Finally, both primary and secondary outcomes interested the PACU period and no data in studies described outcomes after this period.
Table 1.
Relevant methodological features and characteristics of included studies
| Author | ASA | Age | Intervention | Premedication | Intraoperative anesthetics | Intraoperative analgesia | Postoperative analgesia | Timing of Dex | Bolus (µg/kg) | Continuous administration (µg/kg/h) | Primary outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ali 2013 | I–II | 2–6 years | Adenotonsillectomy | Midazolam | Sevoflurane | Dexamethasone + paracetamol | No | End of surgery | 0.3 | 0 | Agitation in PACU |
| Al-Zaben 2010 | I | 1–12 years | Hypospadias | 0 | Sevoflurane | Fentanyl | Morphine + paracetamol | After induction | 1 | 0.7 | Fentanyl, morphine, and paracetamol reduction |
| Erdil 2009 | I | 4–7 years | Adenoidectomy | 0 | Sevoflurane | Dexamethasone + paracetamol | Fentanyl | After induction | 0.5 | 0 | Agitation in PACU |
| Ghai 2015_1 | I–II | 1–6 years | Catact | Midazolam | Sevoflurane | Sub-tenon block + paracetamol | Fentanyl | After induction | 0.15 | 0 | Agitation in PACU |
| Ghai 2015_2 | I–II | 1–6 years | Catact | Midazolam | Sevoflurane | Sub-tenon block + paracetamol | Fentanyl | After induction | 0.3 | 0 | Agitation in PACU |
| Guler 2005 | I | 3–7 years | Adenotonsillectomy | 0 | Sevoflurane | Paracetamol | Fentanyl | End of surgery | 0.5 | 0 | Not defined |
| Hauber 2015 | I–III | 4–10 years | Adenotonsillectomy | 0 | Sevoflurane | Dexamethasone + morphine | Fentanyl | End of surgery | 0.5 | 0 | Agitation in PACU |
| Kim 2014 | I–III | 1–5 years | Strabism | 0 | Propofol + desflurane | Dexamethasone + fentanyl | Fentanyl | After induction | 0 | 0.2 | Agitation in PACU |
| Kim 2014 | I | 1–5 years | Orchidopexy | 0 | Sevoflurane | Caudal | Fentanyl | After induction | 1 | 0.1 | Decrease of sevoflurane ET concentration |
| Meng 2012_1 | I–II | 5–14 years | Adenoidectomy | Midazolam | Propofol + sevoflurane | Sufentanil + remifentanil | Fentanyl | After induction | 0.5 | 0.2 | Agitation in PACU |
| Meng 2012_2 | I–II | 5–14 years | Adenoidectomy | Midazolam | Propofol + sevoflurane | Sufentanil + remifentanil | Fentanyl | After induction | 1 | 0.4 | Not defined |
| Patel 2010 | II–III | 2–10 years | Adenotonsillectomy | 0 | Sevoflurane | Dexamethasone + paracetamol + fentanyl | Morphine | After induction | 2 | 0.7 | Rescue morphine in PACU |
| Pestieau 2011_1 | I–II | 6 months–6 years | Myringotomy | 0 | Sevoflurane | 0 | Paracetamol | After induction intranasal | 1 | 0 | Analgesic rescue |
| Pestieau 2011_2 | I–II | 6 months–6 years | Myringotomy | 0 | Sevoflurane | 0 | Paracetamol | After induction intranasal | 2 | 0 | Analgesic rescue |
| Sato 2010 | I–II | 1–9 years | Outpatient surgery | 0 | Sevoflurane | Paracetamol + NSAIDs | Fentanyl | After induction | 0.3 | 0 | Agitation in PACU |
| Shukry 2005 | I–II | 1–10 years | Outpatient surgery | 0 | Sevoflurane | Fentanyl | Morphine | After induction | 0 | 0.2 | Not defined |
| Soliman 2015 | I–II | 4–14 years | Adenotonsillectomy | 0 | Propofol + sevoflurane | Fentanyl + dexamethasone | Fentanyl + paracetamol | After induction | 0.5 | 0.2 | Agitation in PACU |
All studies compared Dex to inactive placebo. Studies with more than one Dex arm are displayed as author, name, year of publication_1 and author name, year of publication_2
ASA American Anesthesiologists Association, Dex dexmedetomidine, PACU postanethesia care unit, Paracetamol acetaminophen
Fig. 1.
Meta-analysis flowchart. IQR interquartile range, RCT randomized controlled trial
Overall Results
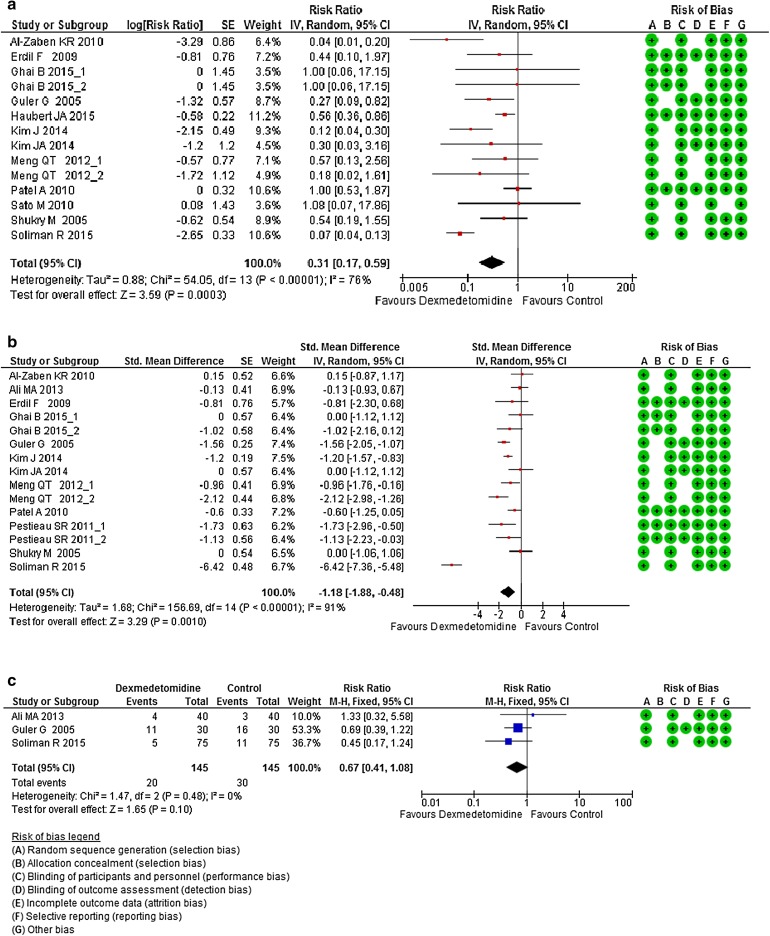
Seven hundred and seventy patients received Dex and 693 received placebo. Overall, the results showed that intraoperative Dex administration was significantly associated with an opioid-sparing effect [RR = 0.31 (0.17, 0.59), I 2 = 76%, p < 0.0001; Fig. 2a]. Sensitivity analyses for this outcome including only one arm of studies with more than on Dex group [23, 28] still found a Dex opioid-sparing effect [RR = 0.31 (0.16, 0.61), I 2 = 79%, p < 0.0001 and RR = 0.29 (0.14, 0.57), I 2 = 79%, p < 0.0001, interaction test: X 2 = 0.03, df = 1, I 2 = 0%, p = 0.87]. Dex also displayed an improvement in postoperative pain management [SMD = −1.18 (−1.88, −0.48), I 2 = 91%, p < 0.0001; Fig. 2b]. Sensitivity analyses including only one arm of studies with more than one Dex group [23, 28, 30] still displayed a significant reduction of pain in Dex-treated patients [RR = 0.33 (0.14, 0.76), I 2 = 93%, p < 0.0001 and RR = 0.29 (0.12, 0.66), I 2 = 93%, p < 0.0001, interaction test: X 2 = 0, df = 1, I 2 = 0%, p = 0.95]. PONV was carried on three studies and found Dex ineffective in decreasing their occurrence [RR = 0.67 (0.41, 1.08), I 2 = 0%, p = 0.48; Fig. 2c].
Fig. 2.
a Forest plot of meta-analysis of the effects of Dex versus placebo on opioid consumption in the PACU. b Forest plot of meta-analysis of the effects of Dex versus placebo on postoperative pain intensity in the PACU. c Forest plot of meta-analysis of the effects of dexmedetomidine versus placebo on postoperative nausea and vomiting in the PACU. The square in front of each study (first author and year of publication) is the RR for individual trials, and the corresponding horizontal line is the 95% CI. The lozenge at the bottom represents pooled OR with 95% CI. Studies with more than one Dex arm are displayed as author, name, year of publication_1, and author name year of publication_2 (see Table 1 for exact description of each arm). CI confidence interval, Dex dexmedetomidine, OR odds ratio, PACU postanesthesia care unit, RR risk ratio, SE standard error, SMD standardized mean difference
Subgroup Analyses
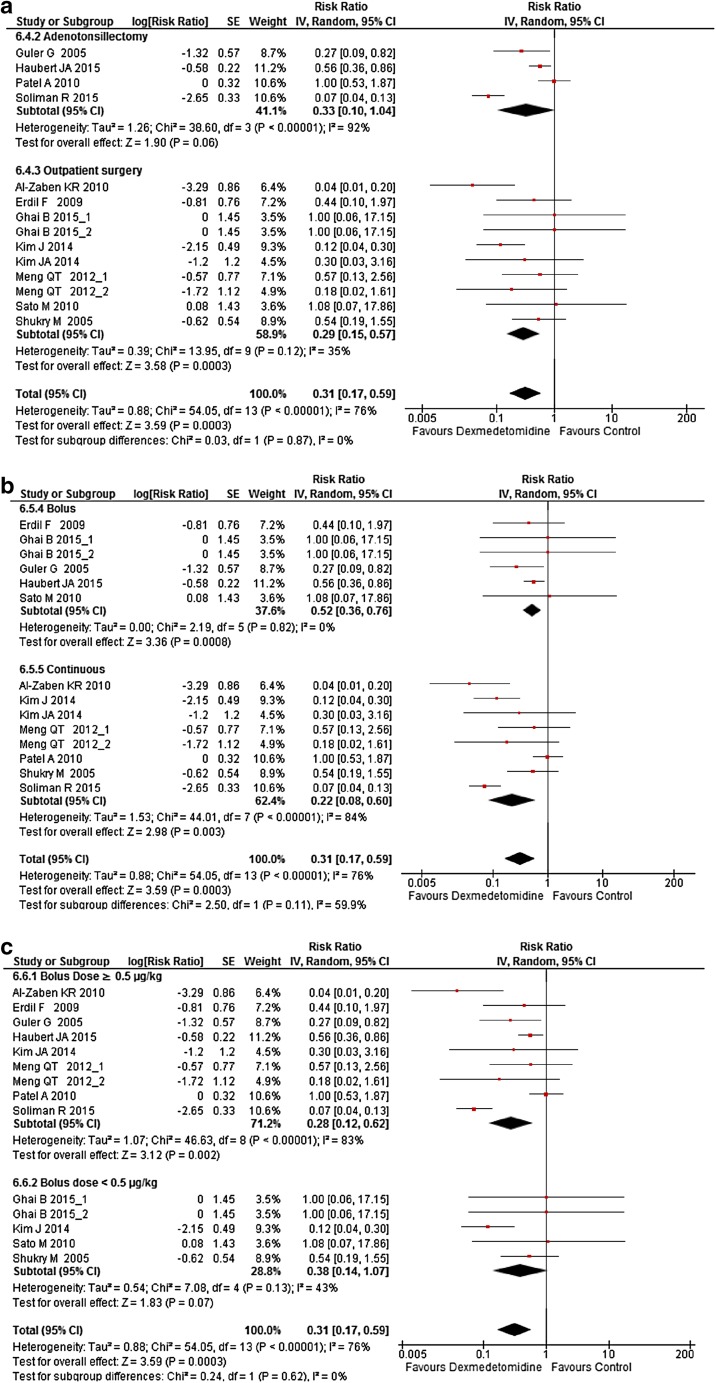
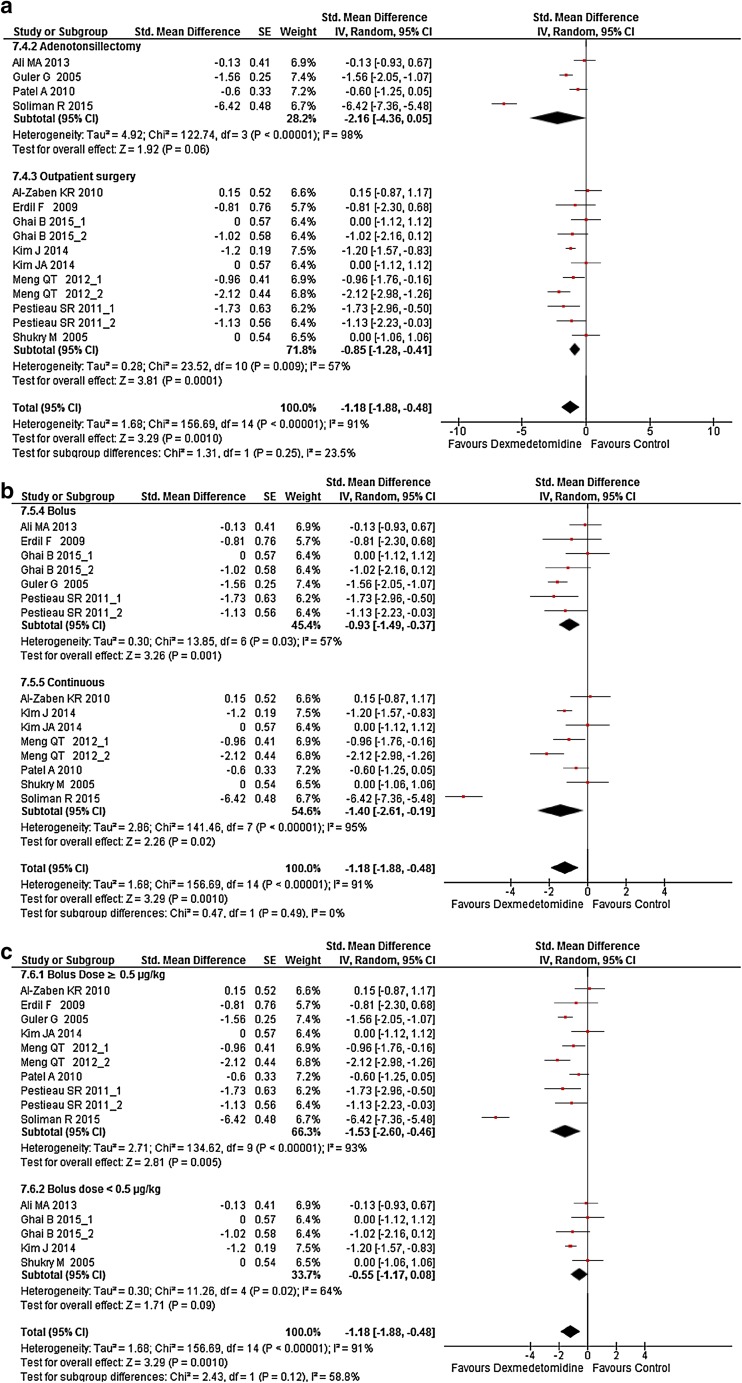
Subgroup analyses were carried on the following criteria: adenotonsillectomy versus outpatient surgery, bolus administration of Dex versus continuous administration and low-dose bolus versus high-dose bolus. High and low median bolus were chosen according to the median value of boluses used in studies (0.5 µg/kg), accordingly, low bolus doses were <0.5 µg/kg and high bolus doses were ≥0.5 µg/kg. Dex was still found to decrease both postoperative opioid consumption (Fig. 3a–c) and postoperative pain intensity (Fig. 4a–c) except after adenotonsillectomy and when boluses were <0.5 µg/kg irrespective to the presence or absence of a continuous administration (Figs. 3a, 4a). However, subgroup analyses interaction did not find a significant difference between paired subgroups for all outcomes (Figs. 3, 4).
Fig. 3.
Forest plot of subgroup analysis of the effect a of the surgery, b of the bolus mode versus the bolus plus continuous mode, and c the effect of a bolus of ≥0.5 µg/kg versus a bolus <0.5 µg/kg, on Dex opioid-sparing effect in the postanesthesia care unit. The square in front of each study (first author and year of publication) is the RR for individual trials, and the corresponding horizontal line is the 95% CI. The lozenge at the bottom represents pooled OR with 95% CI. The test for subgroup difference represents the interaction test between groups. Studies with more than one Dex arm are displayed as author name, year of publication_1, and author name, year of publication_2 (see Table 1 for exact description of each arm). CI confidence interval, Dex dexmedetomidine, RR risk ratio, SE standard error
Fig. 4.
Forest plot of subgroup analysis of a the surgery, b the bolus mode versus the bolus plus continuous mode, and c the effect of a bolus of Dex ≥0.5 µg/kg versus a bolus <0.5 µg/kg, on Dex effect on postoperative pain intensity in the postanesthesia care unit. The square in front of each study (first author and year of publication) is the SMD for individual trials, and the corresponding horizontal line is the 95% CI. The lozenge at the bottom represents pooled OR with 95% CI. The test for subgroup difference represents the interaction test between groups. Studies with more than one Dex arm are displayed as author name, year of publication_1, and author name year of publication_2 (see Table 1 for exact description of each arm). CI confidence interval, Dex dexmedetomidine SE standard error, SMD standardized mean difference
Effect of Study’s Bias on Results
Including in the analysis studies with low-bias risks, found Dex effective in decreasing opioid consumption in PACU [three studies, RR = 0.44 (0.36, 0.54), I 2 = 20%, p = 0.28] and postoperative pain intensity [three studies, SMD = −0.89 (−1.38, −0.41), I 2 = 0%, p = 0.43].
However, given the low number of studies of low-bias included in this meta-analysis for primary outcome, we performed a trial sequential analysis (TSA) including studies expressed as discrete data (nine studies on overall 12 available [21–28, 31] for the primary outcome because no inverse variance method is available in the TSA software) and data of low-bias risk studies to compute the relative risk reduction in order to determine the number of patients needed to found a significant result [16, 17].
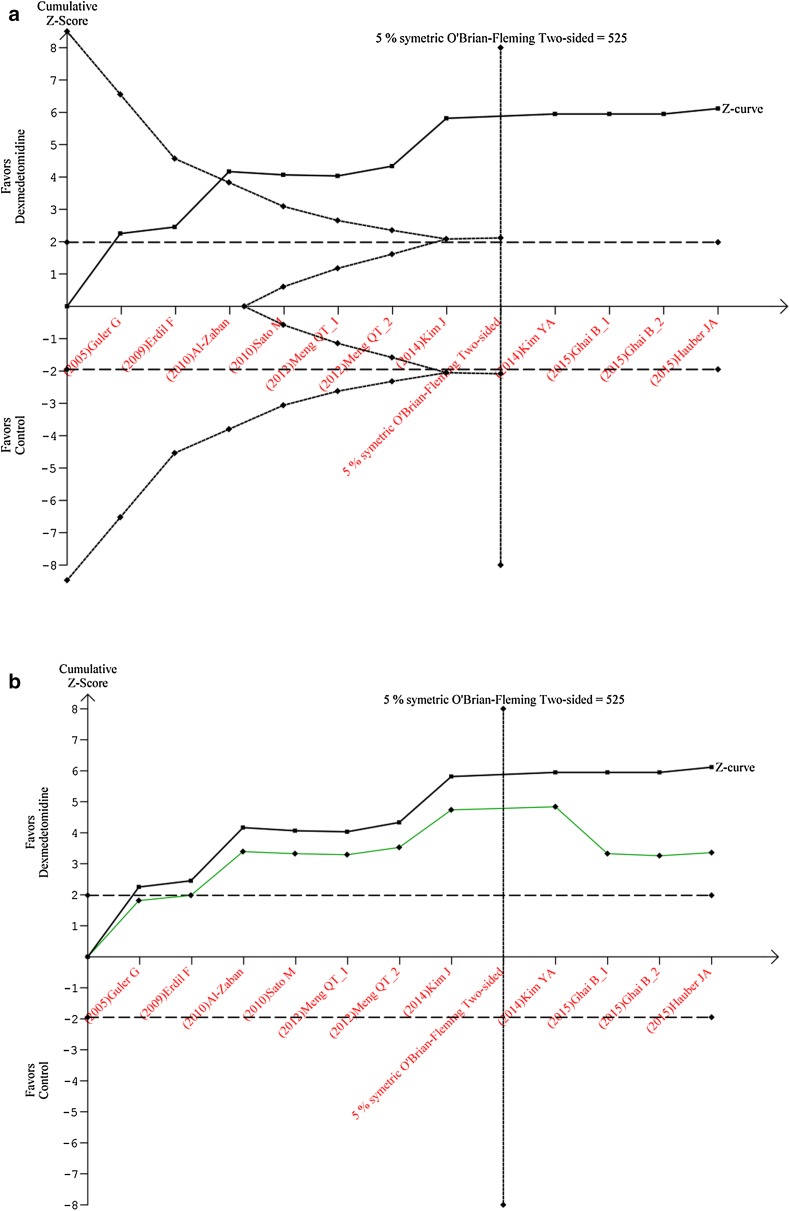
Results of TSA, confirmed the opioid-sparing effect of Dex [RR = 0.49 (0.39, 0.62), I 2 = 0%, p = 0.44] and a cumulative z scores above the significant threshold (Fig. 5). This analysis also found the number of patients to be included in this meta-analysis with an alpha risk of 5 % and a power of 80 % to detect a relative risk reduction of 34 % of 525 patients (Fig. 5a). Finally, introducing a correction for previous analysis [5], found Dex to continue exhibiting a significant opioid-sparing effect (Fig. 5b). The cumulative results of RevMan and TSA analyses clearly indicate that the opioid-sparing effect of Dex is valid assumption during pediatric surgery.
Fig. 5.
a Trial sequential analysis graph (x-axis studies effect, y-axis cumulative z scores). The displaying in the full line displays the cumulative z score, the horizontal dotted line the boundaries of significance (results in the region within these boundaries are non-significant), the vertical line the meta-analysis information size (size of patients to be included in order to show a significant outcome: 525). The etched lines the upper inward-sloping represents the trial sequential monitoring boundary and the lower outward-sloping the futility region. Given the evolution of the z score outside the futility region and crossing the monitoring boundary curve (constructed with low-risk bias studies), the opioid-sparing effect of dexmedetomidine is confirmed. b Correction for previous meta-analyses of trial sequential analysis graph: (x-axis studies effect, y-axis cumulative z scores). The upper curve represents the actual z scores analysis without correction and the lower one the corrected z scores taking in account previous analyses
Publication Bias Analyses
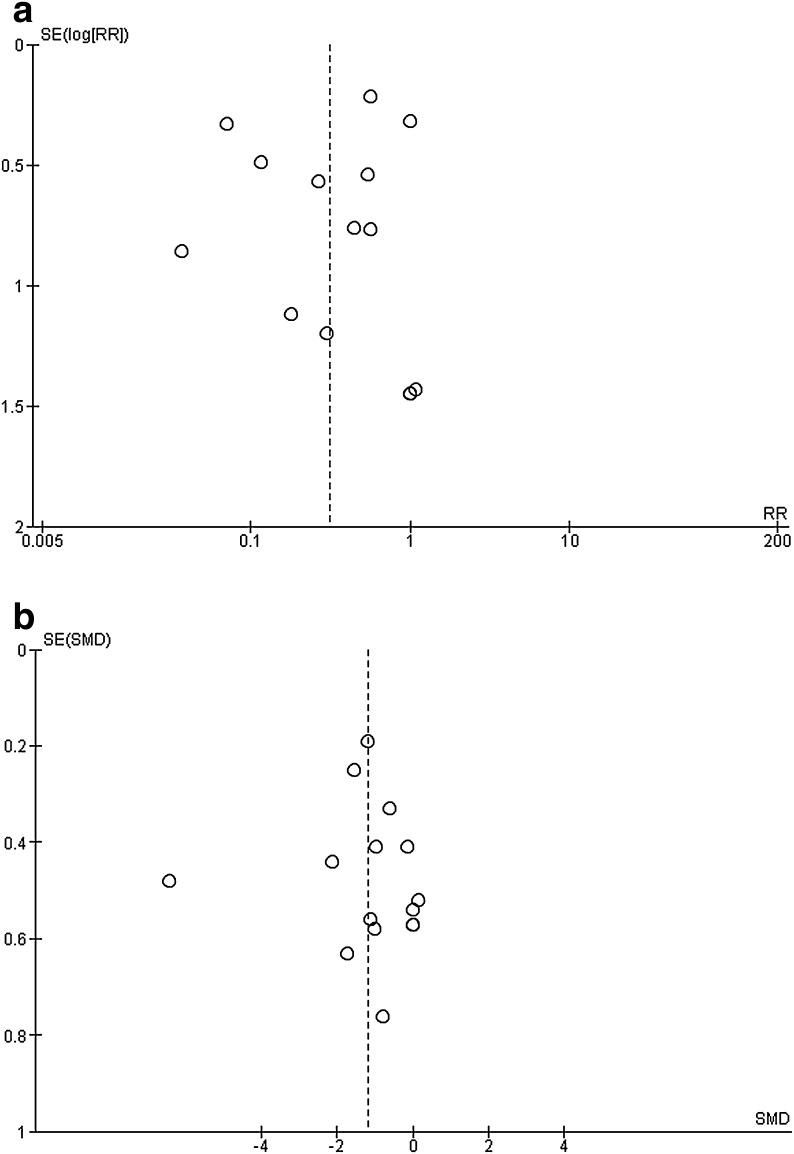
Concerning publication bias, according to Cochrane recommendations (see Methods section for publication bias), two outcomes were examined, namely, opioid consumption and postoperative pain and. Both funnel plots (Fig. 6a and b, respectively) displayed an asymmetry that might indicate either a great heterogeneity in results or a publication bias related to unpublished negative results.
Fig. 6.
a Funnel plot of Dex effect upon opioid consumption in PACU. b Funnel plot of Dex effect upon postoperative pain intensity in PACU. Graphs display the intervention effect (RR or SMD) estimates from individual studies in the x-axis against some measure of each study’s size or precision (standard error of the intervention effect) in the y-axis. Dex dexmedetomidine, PACU postanesthesia care unit, RR risk ratio, SE standard error, SMD standardized mean difference
Discussion
The two main findings of the present study are the following: Intraoperative administration of Dex either as a bolus or a continuous administration has a postoperative opioid-sparing effect and improves the quality of postoperative pain. The result of the primary outcome was confirmed using the TSA with and without correction for previous meta-analysis on the same outcome. Despite the absence of a significant difference between subgroups, analyses according to the surgery performed, the mode of Dex administration, and the dose of the Dex bolus found that both adenotonsillectomy and low bolus doses (<0.5 µg/kg) impact Dex opioid-sparing effect and postoperative pain quality. PONV occurrence was not decreased by Dex, but this outcome was supported by results of three studies.
Results of the current meta-analysis were similar to those found in the previous one published in 2013 [5] concerning postoperative pain management. However, the current meta-analysis included more studies (overall 14 studies in comparison to the 11 included in 2013) and compared Dex to placebo. Moreover, the primary outcome of our study was computed on 12 studies in comparison to the four used for the same outcome in the previous meta-analysis [5]. This clearly confirms the postoperative analgesic and opioid-sparing effect of Dex. The observed postoperative effect of Dex might involve its pharmacokinetics properties: Dex has an elimination half-life of approximately 2 h, and as such may reduce pain intensity (and opioid consumption) for some time after surgery, especially in non-major surgeries such those performed in the included studies (both in the current meta-analysis and the previous one). Another interesting finding of this study is the ineffective effect of Dex during adenotonsillectomy. Dex did not exhibit the opioid-sparing effect nor does it improve the quality of postoperative pain management during adenotonsillectomy. This might result from the intense postoperative pain after this procedure. This hypothesis is strongly suggested by the absence of Dex effect on both opioid consumption and postoperative pain during this procedure. The second hypothesis is derived from the subgroup analyses concerning the bolus dose. Boluses <0.5 µg/kg, either followed by a continuous administration or not, were found ineffective in decreasing both postoperative pain intensity and opioid consumption. However, this hypothesis is unlikely given that all studies performed during adenotonsillectomy used boluses of Dex ≥0.5 µg/kg. An alternative explanation for this result is the amount of intraoperative opioid administered intraoperatively with the development of a subsequent hyperalgesia [34–37]. This hypothesis is strongly supported by the association of intraoperative and postoperative opioid-sparing effect of Dex observed in some studies included in this meta-analysis: Patel’s studies [29] found no intraoperative and postoperative opioid-sparing effect of Dex. In contrast, using the same anesthesia protocol, Soliman and collaborators [33] found both an intraoperative and a postoperative opioid-sparing effect of Dex. Preventing opioid-induced hyperalgesia might therefore represent an alternative hypothesis explaining the postoperative opioid-sparing effect of Dex found in our meta-analysis and might represent an interesting hypothesis to explore in future studies. Finally, the absence of a postoperative opioid-sparing effect of Dex during adenotonsillectomy might also result from the limited number of studies included to compute this subgroup (four studies).
Interestingly, doses used in included studies ranged from 0.3 to 2 µg/kg (median of 0.5 µg/kg) and continuous administration during the intraoperative period ranged from 0.2 to 0.7 µg/kg/h (median of 0.2 µg/kg/h). These doses were lower than those commonly used during procedural sedation (especially during pediatric imaging: bolus of 1 µg/kg and continuous infusion of 0.5–2 µg/kg/h) [38]. This difference is logical given that during painful procedures, Dex is used in combination with other opioid and hypnotic agents while imaging requires sedation that can be achieved using Dex as the sole anesthetic agent. Our results indicate that optimal bolus dose of Dex to produce its analgesic and opioid-sparing effects must be ≥0.5 µg/kg. Although, this interesting finding had to be further explored, this result gives an interesting indication on the optimal dose of Dex to be used to improve postoperative pain management.
Despite the reduction of opioids consumption, Dex did not affect the incidence PONV in our meta-analysis. However, this probably reflects the heterogeneity of results and the small number of studies focusing on this outcome (three studies) [4, 39]. This explanation is highly supported by the finding in the recent meta-analysis on the same topic in adults that included more studies.
The results presented in this meta-analysis are of a great interest for management of postoperative rehabilitation in pediatric patients. Every effort if made today in order to decrease postoperative opioid administration [40]. This allows a rapid switch from the intravenous administration of those compounds (often administered via a patient- or nurse-controlled analgesia) to an oral administration of non-opioid analgesics [40, 41]. This accelerates the discharge from the hospital while most surgical care can be performed at home. In addition, decreasing the amount of morphine has been shown to decrease opioid-related side effects such nausea, vomiting, and constipation; and decrease the time of first oral intake, even after abdominal surgery. Altogether, results of this meta-analysis strongly encourage studies on the effects of Dex on rapid postoperative rehabilitation.
Limitations of the Study
This meta-analysis suffers many limitations. The primary outcome of the current meta-analysis (postoperative opioid consumption) was the primary outcome of only two individual trials (Table 1). As a consequence, most data were computed with secondary outcomes of individual studies. However, using the trial sequence analysis allow to confirm our results and the adequate patients included in this meta-analysis. Data from studies designed with more than one active group were analyzed with each arm considered as a separate study. Although this would increase the weight of the considered study in the analysis, this allowed avoiding publication bias.
Subgroup analyses were performed with the aim of reducing heterogeneity and to identify factors influencing results. However, this goal was not achieved for most outcomes. This probably explains the absence of statistical difference between subgroups (interaction test) even when showing different results on outcomes. Consequently, our results must be interpreted cautiously, especially for outcomes involving lesser numbers of analyzed studies. Using funnel plots, we demonstrated suspected publication bias for two outcomes—pain intensity in PACU and postoperative opioid consumption in PACU—indicating that some studies of these outcomes with negative results were not published. Alternatively, this funnel plot asymmetry might also result from the great heterogeneity between studies.
The current meta-analysis was designed to examine the postoperative effects of Dex versus placebo during pediatric surgery. As such, no conclusions can be made about the efficacy of Dex in comparison to other sedative or analgesic agents such as morphine. Our study is also limited regarding the effects of Dex on intraoperative hemodynamics. This outcome was excluded from our meta-analysis for the following reasons: heterogeneity in numerical expression of this outcome, heterogeneity in types of surgery and Dex infusion regimes, which could all result in hemodynamic disturbances, and the absence of an exhaustive search for articles displaying this outcome. Finally, due to the design of the included studies, no data for postoperative analgesia are available after discharge from the PACU. This represents the most challenging point for future studies.
Conclusions
Our meta-analysis shows that intraoperative Dex, when compared to placebo, is associated with reduced in postoperative opioid consumption and an improvement of pain management during PACU stay. More studies are necessary to assess the dose-effect of Dex on postoperative pain management and its benefice during longer postoperative period in order to precise its advantages during rapid postoperative rehabilitation programs.
Acknowledgments
No funding or sponsorship was received for this study or in the publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Myriam Bellon, Alix Le bot, Daphnée Michelet, Julie Hilly, Mathieu Maesani, Christopher Brasher, and Souhayl Dahmani have nothing to disclose.
Compliance with Ethics Guidelines
This meta-analysis is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. [DOI] [PubMed]
- 2.Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115(2):171–182. doi: 10.1093/bja/aev226. [DOI] [PubMed] [Google Scholar]
- 3.Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28(1):3–6. doi: 10.1097/EJA.0b013e32833e266d. [DOI] [PubMed] [Google Scholar]
- 4.Le Bot A, Michelet D, Hilly J, Maesani M, Dilly MP, Brasher C, et al. Efficacy of intraoperative dexmedetomidine compared with placebo for surgery in adults: a meta-analysis of published studies. Minerva Anestesiol. 2015;81(10):1105–1117. [PubMed] [Google Scholar]
- 5.Schnabel A, Reichl SU, Poepping DM, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of intraoperative dexmedetomidine for acute postoperative pain in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2013;23(2):170–179. doi: 10.1111/pan.12030. [DOI] [PubMed] [Google Scholar]
- 6.Zhu M, Wang H, Zhu A, Niu K, Wang G. Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: different administration and different dosage. PLoS ONE. 2015;10(4):e0123728. doi: 10.1371/journal.pone.0123728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Hu J, Liu X, Yan J. Effects of intravenous dexmedetomidine on emergence agitation in children under sevoflurane anesthesia: a meta-analysis of randomized controlled trials. PLoS ONE. 2014;9(6):e99718. doi: 10.1371/journal.pone.0099718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cochrane collaborative. Cochrane handbook. 2015. http://www.cochrane-handbook.org. Accessed 31 Dec 2015.
- 9.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Crellin D, Sullivan TP, Babl FE, O’Sullivan R, Hutchinson A. Analysis of the validation of existing behavioral pain and distress scales for use in the procedural setting. Paediatr Anaesth. 2007;17(8):720–33. [DOI] [PubMed]
- 11.Voepel-Lewis T, Zanotti J, Dammeyer JA, Merkel S. Reliability and validity of the face, legs, activity, cry, consolability behavioral tool in assessing acute pain in critically ill patients. Am J Crit Care. 2010;19(1):55–61 (quiz 2). [DOI] [PubMed]
- 12.Solodiuk JC, Scott-Sutherland J, Meyers M, Myette B, Shusterman C, Karian VE, et al. Validation of the Individualized Numeric Rating Scale (INRS): a pain assessment tool for nonverbal children with intellectual disability. Pain. 2010;150(2):231–6. [DOI] [PubMed]
- 13.von Baeyer CL, Spagrud LJ. Systematic review of observational (behavioral) measures of pain for children and adolescents aged 3 to 18 years. Pain. 2007;127(1–2):140–50. [DOI] [PubMed]
- 14.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::AID-SIM784>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Afshari A, Wetterslev J. When may systematic reviews and meta-analyses be considered reliable? Eur J Anaesthesiol. 2015;32(2):85–87. doi: 10.1097/EJA.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Whitehead A, Simmonds M. Sequential methods for random-effects meta-analysis. Stat Med. 2015;30(9):903–921. doi: 10.1002/sim.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton AJ, Higgins JP. Recent developments in meta-analysis. Stat Med. 2008;27(5):625–650. doi: 10.1002/sim.2934. [DOI] [PubMed] [Google Scholar]
- 20.Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi J Anaesth. 2013;7(3):296–300. doi: 10.4103/1658-354X.115363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Zaben KR, Qudaisat IY, Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Oweidi AS, et al. Intraoperative administration of dexmedetomidine reduces the analgesic requirements for children undergoing hypospadius surgery. Eur J Anaesthesiol. 2010;27(3):247–252. doi: 10.1097/EJA.0b013e32833522bf. [DOI] [PubMed] [Google Scholar]
- 22.Erdil F, Demirbilek S, Begec Z, Ozturk E, Ulger MH, Ersoy MO. The effects of dexmedetomidine and fentanyl on emergence characteristics after adenoidectomy in children. Anaesth Intensive Care. 2009;37(4):571–576. doi: 10.1177/0310057X0903700405. [DOI] [PubMed] [Google Scholar]
- 23.Ghai B, Jain D, Coutinho P, Wig J. Effect of low dose dexmedetomidine on emergence delirium and recovery profile following sevoflurane induction in pediatric cataract surgeries, effect of low dose dexmedetomidine on emergence delirium and recovery profile following sevoflurane induction in pediatric cataract surgeries. J Anesthesiol. 2015;2015/11/02/2015/11/02;2015, 2015:e617074.
- 24.Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. 2005;15(9):762–766. doi: 10.1111/j.1460-9592.2004.01541.x. [DOI] [PubMed] [Google Scholar]
- 25.Hauber JA, Davis PJ, Bendel LP, Martyn SV, McCarthy DL, Evans MC, et al. Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth Analg. 2015;121(5):1308–1315. doi: 10.1213/ANE.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Kim SY, Lee JH, Kang YR, Koo BN. Low-dose dexmedetomidine reduces emergence agitation after desflurane anaesthesia in children undergoing strabismus surgery. Yonsei Med J. 2014;55(2):508–516. doi: 10.3349/ymj.2014.55.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim NY, Kim SY, Yoon HJ, Kil HK. Effect of dexmedetomidine on sevoflurane requirements and emergence agitation in children undergoing ambulatory surgery. Yonsei Med J. 2014;55(1):209–215. doi: 10.3349/ymj.2014.55.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng QT, Xia ZY, Luo T, Wu Y, Tang LH, Zhao B, et al. Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: a case-control study. Int J Pediatr Otorhinolaryngol. 2012;76(7):1036–1041. doi: 10.1016/j.ijporl.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Patel A, Davidson M, Tran MC, Quraishi H, Schoenberg C, Sant M, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg. 2010;111(4):1004–1010. doi: 10.1213/ANE.0b013e3181ee82fa. [DOI] [PubMed] [Google Scholar]
- 30.Pestieau SR, Quezado ZM, Johnson YJ, Anderson JL, Cheng YI, McCarter RJ, et al. High-dose dexmedetomidine increases the opioid-free interval and decreases opioid requirement after tonsillectomy in children. Can J Anaesth. 2011;58(6):540–550. doi: 10.1007/s12630-011-9493-7. [DOI] [PubMed] [Google Scholar]
- 31.Sato M, Shirakami G, Tazuke-Nishimura M, Matsuura S, Tanimoto K, Fukuda K. Effect of single-dose dexmedetomidine on emergence agitation and recovery profiles after sevoflurane anesthesia in pediatric ambulatory surgery. J Anesth. 2010;24(5):675–682. doi: 10.1007/s00540-010-0976-4. [DOI] [PubMed] [Google Scholar]
- 32.Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth. 2005;15(12):1098–1104. doi: 10.1111/j.1460-9592.2005.01660.x. [DOI] [PubMed] [Google Scholar]
- 33.Soliman R, Alshehri A. Effect of dexmedetomidine on emergence agitation in children undergoing adenotonsillectomy under sevoflurane anesthesia: a randomized controlled study. Egypt J Anaesth. 2015;31:283–9.
- 34.Belgrade M, Hall S. Dexmedetomidine infusion for the management of opioid-induced hyperalgesia. Pain Med. 2010;11(12):1819–1826. doi: 10.1111/j.1526-4637.2010.00973.x. [DOI] [PubMed] [Google Scholar]
- 35.Hallett BR, Chalkiadis GA. Suspected opioid-induced hyperalgesia in an infant. Br J Anaesth. 2012;108(1):116–118. doi: 10.1093/bja/aer332. [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, Stoicea N, Soghomonyan S, Bergese SD. Intraoperative use of remifentanil and opioid induced hyperalgesia/acute opioid tolerance: systematic review. Front Pharmacol. 2014;5:108. doi: 10.3389/fphar.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145–61. [PubMed]
- 38.Fang H, Yang L, Wang X, Zhu H. Clinical efficacy of dexmedetomidine versus propofol in children undergoing magnetic resonance imaging: a meta-analysis. Int J Clin Exp Med. 2015;8(8):11881–11889. [PMC free article] [PubMed] [Google Scholar]
- 39.Liang X, Zhou M, Feng JJ, Wu L, Fang SP, Ge XY, et al. Efficacy of dexmedetomidine on postoperative nausea and vomiting: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015;8(6):8450–8471. [PMC free article] [PubMed] [Google Scholar]
- 40.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 41.Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 Suppl 3):12–15. doi: 10.1016/j.arth.2007.05.040. [DOI] [PubMed] [Google Scholar]