Abstract
Majority of gram negative pathogenic bacteria are responsible for extended spectrum β-lactamases (ESBLs) production, which show resistance to some newer generation of antibiotics. The study was aimed at evaluating the prevalence of ESBL and antibiotic susceptibility pattern of Pseudomonas isolates collected during 2010 to 2014 from tertiary care hospitals of Peshawar, Pakistan. Out of 3450 samples, 334 Pseudomonas spp. isolates comprised of 232 indoor and 102 outdoor patients were obtained from different specimens and their susceptibility pattern was determined against 20 antibiotics. Antimicrobial susceptibility testing was carried out using the Kirby-Bauer agar diffusion method and ESBL production was detected by Synergy Disc Diffusion technique. The mean age group of the patients was 29.9 + 9.15 years. Meronem showed best activity (91.02%) from class carbapenem, β-lactam and β-lactamase inhibitors exhibited 69.16% activity, and doxycycline had a diminished activity (10.18%) to Pseudomonas spp. Outdoor isolates were more resistant than the indoor and during the course of the study the sensitivity rate of antibiotics was gradually reducing. ESBL production was observed in 44.32% while the remaining was non-ESBL. The moderate active antibiotics were amikacin (50.7%), SCF (51.4%), TZP (52.7%), and MXF (54.1%) among ESBL producing isolates. Lack of antibiotic policy, irrational uses (3GCs particularly), and the emergence of antibiotic resistant organisms in hospitals may be causes of high antibiotic resistance.
1. Introduction
Genus Pseudomonas is an important member of the family Pseudomonadaceae. They are in a straight or sometimes in marginally bent form in shape, characteristically aerobic in nature, and flagellated [1]. According to Obritsch et al. [2], Pseudomonas is in the third rank to cause UTIs and dermatitis, otitis, conjunctivitis, GIT, soft tissue, bone, and joint infections are also often caused by these species [3]. Studies conducted on HIV-infected patients reported a progressive increase of gram-negative bacilli, including Pseudomonas spp. In fact, Pseudomonas spp. induced invasive infection is observed with growing frequency among immunocompromised hosts and patients with some predisposing conditions, such as malignancies, extremes of age, neutropenia, prolonged hospitalization, surgery, trauma, and instrumentation [4].
Pseudomonas strains were isolated from wound of burn patients (22 to 73%) [5]. It is the main causative agent of morbidity and mortality in patients of grown age with cystic fibrosis of the respiratory tract infection [6]. The antimicrobial resistance is one of the most important complications which are associated with Pseudomonas. A limited number of antibiotics are effective, because of their tendency to be resistant naturally to a number of pathogens [7], and resistance rises at an elevated frequency to those therapeutic drugs that were previously sensitive to Pseudomonas [8]. Antimicrobial resistance in nosocomial settings is linked with adverse clinical consequences and higher costs [9]. Extended spectrum beta lactamases (ESBLs) are the enzymes that hydrolyze and induce resistance to cephalosporin monobactams [10]. Various studies from subcontinent suggested the prevalence rate of ESBL production of Pseudomonas spp. is 22 to 36% [11, 12]. The two drugs' synergetic activity is considered to be most effective in the treatment of pseudomonal infections, by using penicillin in combination with aminoglycosides and carbapenems (β-lactamase inhibitors) or antipseudomonal penicillin alone [13].
The purpose of this study is to determine the rate of occurrence, pattern of antimicrobial susceptibility, and prevalence of ESBL production of these bacteria from three main tertiary care hospitals in Peshawar region of KPK, Pakistan, as limited work has been previously conducted on this subject.
2. Methodology
2.1. Collection of Bacterial Isolates (Specimens)
This study was conducted in Microbiology Section at Pathology Department, Khyber Teaching Hospital, Peshawar, from 2010 to 2014. During the study period, 3450 samples were collected from three main tertiary care hospitals of Peshawar KPK, Pakistan. The specimens collected were comprised of pus (wounds, burns, ear, TS, and HVS), urine, and blood from indoor patients of gynaecology, surgery, medicine, and burns units and outdoor patients.
The samples from the suspected patient were introduced to inoculation on blood agar, nutrient agar, MacConkey agar, and CLED agar and, after overnight aerobic incubation at 37°C, they were examined for bacterial growth. Grown colonies were isolated and identified on the basis of their colony morphology, staining characters, pigment production, motility, and relevant biochemical tests as per standard laboratory methods of identification [14, 15].
2.2. Antimicrobial Susceptibility Protocol
For inoculums preparation, tryptic soy broth (CM129-OXOID) was made by pouring 4-5 mL of broth medium in screw capped tubes and sterilized by autoclaving at 121°C for 15 minutes at 15 psi. The media were cooled and kept in an incubator for 24 hours at 35°C prior to inoculation. The inoculum density was standardized to a final concentration of 1.5 × 108 CFU/mL according to CLSI and placed in an incubator for 2–6 hours at 35°C to check sterility.
Routinely used different groups of antibiotics (purchased from Oxoid, England) were subjected to determine the antimicrobial susceptibility pattern by using disc diffusion method [16, 17]. Minimum inhibitory concentrations (MICs) were found by agar dilution method for representative antibiotics of different groups. The standard break points were standardized with CLSI reported by Cockerill [18].
2.3. Detection of ESBL
The isolates were screened according to CLSI as prescribed by Hawser et al. [19], to assess the prevalence of ESBL in Pseudomonas spp. Isolates stored at −20°C were refreshed on tryptic soy agar medium for ESBL production by using disc diffusion method.
2.4. Synergy Disc Diffusion Method
In the initial screening of ESBL production, disc diffusion method was used. Discs of cefotaxime (CTX = 30 μg), ceftazidime (CAZ = 30 μg), ceftriaxone (CRO = 30 μg), and aztreonam (AZM = 30 μg) were placed at a distance of 25–30 mm from amoxicillin + clavulanic acid. Amoxicillin + clavulanic acid (AMC = 20 + 10 μg) was placed in the center of the inoculated plates containing Müller Hinton agar according to the CLSI recommendations [19]. Zones of inhibition around the 3G cephalosporin discs and aztreonam were observed after overnight incubation at 37°C. When the zones of inhibition around the third-generation cephalosporin discs and aztreonam were extended on the sides nearest to AMC, the isolate is said to be ESBL producer.
2.5. Phenotypic Detection of ESBL
In the phenotypic confirmatory test, the test organisms were inoculated on Müller Hinton agar (MHA) and discs of ceftazidime (30 μg) and cefotaxime (30 μg) alone and a disc in combination with clavulanic acid (30/10 μg) were placed on the inoculated agar for each isolate. Both the discs were placed 25 mm apart (center to center) on a lawn culture of the test plate and incubated for 24 hours at 37°C. An increase in zone of (≥5 mm) for either antimicrobial agent tested in combination with clavulanic acid versus its zone when tested alone was designated as ESBL positive. Klebsiella pneumonia ATCC (700603) and E. coli ATCC (25922) were used as positive and negative control strains, respectively.
2.6. Statistical Analysis
The data were analyzed by using X 2 test through SPSS version 16.0.
3. Results
A total of 3450 samples were collected from the three main tertiary care hospitals of Peshawar, namely, Khyber Teaching Hospital, Lady Reading Hospital, and Hayatabad Medical Complex. Pseudomonas spp. obtained from different sources are listed in Figure 1. These samples were further screened for antimicrobial susceptibility and ESBLs prevalence. Specimens were taken from different sources: pus 162 (48.50%), urine 67 (20.05), blood 16 (4.79%), and HVS, along with throat and ear swabs 32 (9.58%).
Figure 1.
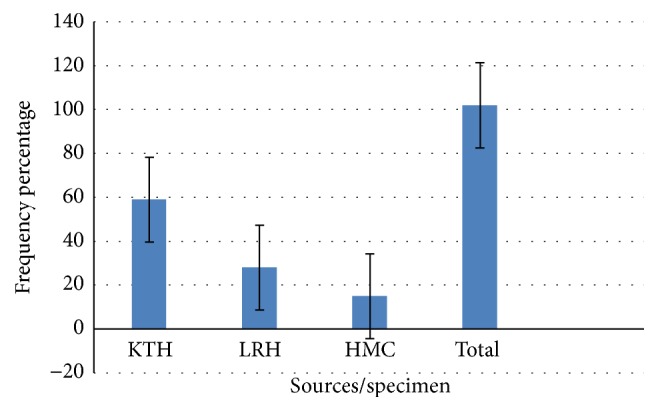
Prevalence rate of Pseudomonas spp. isolates from different specimen.
A total of 334 isolates comprising 232 (69.46%) indoor and 102 (30.54%) outdoor patients including males and females as mentioned in Figure 2 (male to female ratio 1 : 1.4) were recovered as Pseudomonas positive, out of which most of the isolates were taken from Khyber Teaching Hospital 191 (57.18%), followed by Lady Reading Hospital 79 (23.65%) and Hayatabad Medical Complex, Peshawar 64 (19.16%) with mean age 25.9 ± 9.15 years. Details are given in Figure 3.
Figure 2.
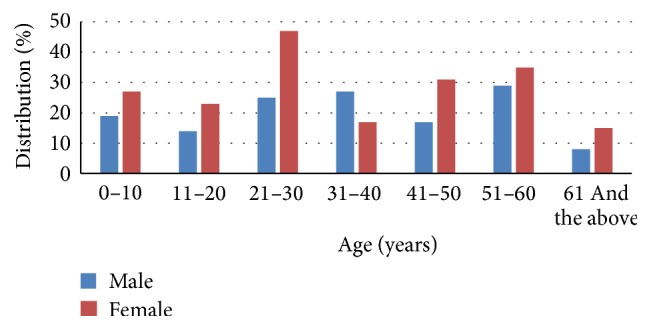
Genderwise distribution of male and female among different age groups.
Figure 3.
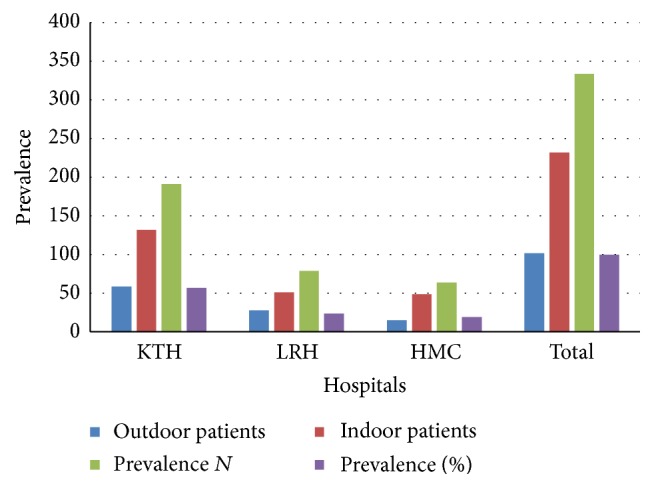
Prevalence of Pseudomonas spp. in different hospitals.
3.1. Susceptibility Pattern of Pseudomonas spp. to Various Antimicrobial Agents
In β-lactam agents, the frequency of susceptibility to cephalosporins' 2nd generation (cefaclor) 21.26%, 3rd generation (ceftazidime and ceftriaxone) 33.23% and 36.23%, respectively, while the 4th generation (cefepime) was 48.5%, showed a higher activity among the cephalosporin's group. While, in β-lactam agents, the most potent antimicrobial agent was imipenem (84.43%) and Meronem (91.02%) from class carbapenems against Pseudomonas spp., least activity was observed by amoxicillin 14.97%.
Among the β-lactams and β-lactamase inhibitors, maximum activity was observed by cefoperazone + sulbactam (69.16%) and then by piperacillin + tazobactam (60.78%) and amoxicillin + clavulanic acid (24.5%). Susceptibility pattern against amikacin was 66.0% and gentamycin was 19.6% in aminoglycosides.
In class fluoroquinolones, 61.7% isolates were found susceptible to moxifloxacin, 44.6% to gatifloxacin and 55.7% to sparfloxacin, 50.3% ciprofloxacin, and 35% to enoxacin. In the macrolides group of antibiotics, clarithromycin had a good activity (42.51%), while to erythromycin it was 19.16%. In class tetracycline, 10.18% strains were found sensitive to doxycycline. Nevertheless, none of the antibiotics was found completely resistant to the Pseudomonas spp. The resistance rate was highest for tetracycline followed by penicillin and the isolates were coresistant to macrolides and fluoroquinolones (Table 1).
Table 1.
Cumulative susceptibility pattern of Pseudomonas spp. to various antimicrobial agents.
| Antimicrobial agent | Codes | Sensitive N (%) |
Intermediate N (%) |
Resistant N (%) |
|---|---|---|---|---|
| Amoxicillin | AML | 50 (14.97) | 39 (11.68) | 245 (73.35) |
| Amoxicillin + clavulanic acid | AMC | 82 (24.55) | 26 (7.78) | 226 (67.66) |
| Piperacillin + tazobactam | TZP | 203 (60.78) | 55 (16.47) | 76 (22.75) |
| Cefoperazone + sulbactam | SCF | 231 (69.16) | 44 (13.17) | 59 (17.66) |
| Cefaclor | CEC | 71 (21.26) | 22 (6.59) | 241 (72.16) |
| Ceftazidime | CAZ | 111 (33.23) | 27 (8.08) | 196 (58.68) |
| Ceftriaxone | CRO | 121 (36.23) | 37 (11.08) | 176 (52.69) |
| Cefepime | FEP | 162 (48.50) | 17 (5.09) | 155 (46.41) |
| Sparfloxacin | SPX | 186 (55.69) | 14 (4.19) | 134 (40.12) |
| Ciprofloxacin | CIP | 168 (50.30) | 19 (5.69) | 147 (44.01) |
| Gatifloxacin | GTX | 149 (44.61) | 84 (25.15) | 101 (30.24) |
| Enoxacin | ENX | 117 (35.03) | 37 (11.08) | 180 (53.89) |
| Moxifloxacin | MXF | 206 (61.68) | 35 (10.48) | 93 (27.84) |
| Erythromycin | E | 64 (19.16) | 22 (6.59) | 248 (74.25) |
| Clarithromycin | CLR | 142 (42.51) | 37 (11.08) | 155 (46.41) |
| Meropenem | MEM | 304 (91.02) | 4 (1.20) | 26 (7.78) |
| Imipenem | IPM | 282 (84.43) | 15 (4.49) | 37 (11.08) |
| Gentamycin | CN | 62 (18.56) | 24 (7.19) | 248 (74.25) |
| Amikacin | AK | 216 (64.67) | 17 (5.09) | 101 (30.24) |
| Doxycycline | DO | 34 (10.18) | 19 (5.69) | 281 (84.13) |
3.2. Susceptibility Pattern of Strain in Indoor and Outdoor Patients
Among the 334 Pseudomonas positive isolates, 102 were from outdoor patients while 232 were recovered from indoor patients. Outdoor isolates showed a higher frequency of sensitivity to almost all the antibiotics (Table 2). Imipenem (88.79%) and Meronem (96.08%) were highly active antibiotics from the class of carbapenems in indoor isolates, while outdoor isolates were 81.47% and 91.18% susceptible towards these two antibiotics. 30.39% isolates were susceptible to cefaclor, 41.18% to ceftazidime, 44% to ceftriaxone, and 59% to cefepime (4th generation cephalosporin) of β-lactam agents from outdoor isolates, while 17.2% were susceptible to cefaclor, 29.24% to ceftazidime, 32.76% to ceftriaxone, and 44% to cefepime for indoor patients. These results showed that the frequency of susceptibility is higher for outdoor isolates.
Table 2.
Comparative susceptibility pattern between indoor and outdoor patients.
| Antimicrobial agents | Hospitalized patients n = 232 | Outdoor patients n = 102 | P value | ||
|---|---|---|---|---|---|
| Sensitive N (%) |
Resistant N (%) |
Sensitive N (%) |
Resistant N (%) |
||
| AML | 22 (9.48) | 210 (90.52) | 28 (27.45) | 74 (72.55) | <0.001 |
| AMC | 45 (19.40) | 187 (80.60) | 37 (36.27) | 65 (63.73) | <0.0001 |
| TZP | 132 (56.90) | 100 (43.10) | 71 (69.61) | 31 (30.39) | <0.03 |
| SCF | 151 (65.09) | 81 (34.91) | 80 (78.43) | 22 (21.57) | <0.02 |
| CEC | 40 (17.24) | 192 (82.76) | 31 (30.39) | 71 (69.61) | <0.006 |
| CAZ | 69 (29.74) | 163 (70.26) | 42 (41.18) | 60 (58.82) | <0.041 |
| CRO | 76 (32.76) | 156 (67.24) | 45 (44.12) | 57 (55.88) | <0.467 |
| FEP | 102 (43.97) | 130 (56.03) | 60 (58.82) | 42 (41.18) | <0.0123 |
| ENX | 75 (32.33) | 157 (67.67) | 42 (41.18) | 60 (58.82) | <0.022 |
| GTX | 95 (40.95) | 137 (59.05) | 54 (52.94) | 48 (47.06) | <0.05 |
| CIP | 108 (46.55) | 124 (53.45) | 60 (58.82) | 42 (41.18) | <0.0388 |
| SPX | 128 (55.17) | 104 (44.83) | 58 (56.86) | 44 (43.14) | <0.0427 |
| MXF | 134 (57.76) | 98 (42.24) | 72 (70.59) | 30 (29.41) | <0.03 |
| E | 35 (15.09) | 197 (84.91) | 29 (28.43) | 73 (71.57) | <0.0137 |
| CLR | 88 (37.93) | 144 (62.07) | 54 (52.94) | 48 (47.06) | <0.0106 |
| CN | 38 (16.38) | 194 (83.62) | 24 (23.53) | 78 (76.47) | <0.0193 |
| AK | 140 (60.34) | 92 (39.66) | 76 (74.51) | 26 (25.49) | <0.01 |
| DO | 12 (5.17) | 220 (94.83) | 22 (21.57) | 80 (78.43) | <0.0001 |
| MEM | 206 (88.79) | 26 (11.21) | 98 (96.08) | 4 (3.92) | <0.032 |
| IPM | 189 (81.47) | 43 (18.53) | 93 (91.18) | 9 (8.82) | <0.0242 |
Amongst the β-lactams and β-lactamase (combined) inhibitors, cefoperazone-sulbactam was 65% active, piperacillin-tazobactam was 56.9%, and Augmentin was 19.4% active against indoor isolates of Pseudomonas spp., while the percent activities of these combined antibiotics against the outdoor isolates of the pathogen were 78.43%, 69.6%, and 36.27% by cefoperazone-sulbactam, piperacillin-tazobactam, and amoxicillin + clavulanic, respectively. Amoxicillin showed 9.48% (indoor) and 27.45 (outdoor) susceptibility rates to the positive isolates.
Moxifloxacin had a maximum activity among the fluoroquinolones against outdoor isolated followed by ciprofloxacin, sparfloxacin, gatifloxacin, and then enoxacin with 70.59%, 58.82%, 56.86%, 52.94%, and 41.18% sensitivity, respectively. In contrast, the rate of susceptibility of moxifloxacin was 57.76%, sparfloxacin 55.17%, ciprofloxacin 46.55%, gatifloxacin 40.95%, and enoxacin 32.33% in hospitalized patients.
In amino glycosides, amikacin (indoor patients = 60.34%, outdoor patients = 74.51%) had a better activity than gentamycin (16.38 and 23.53% indoor and outdoor patients, resp.). In doxycycline, the only tetracycline that had a diminished rate of activeness for both indoor and outdoor patients, 5.17% and 21.57% (Table 3). Overall susceptibility rate in the hospital was affected which might be due to increase of use of antibiotics and nosocomial environment.
Table 3.
Yearwise susceptibility pattern (sensitivity) of Pseudomonas spp. to different antibiotics.
| Agents | 2010 N (%) |
2011 N (%) |
2012 N (%) |
2013 N (%) |
2014 N (%) |
Total N (%) |
|---|---|---|---|---|---|---|
| AML | 10 (18.5) | 14 (17.9) | 13 (13.8) | 8 (12.1) | 5 (11.9) | 50 (15.0) |
| AMC | 17 (31.5) | 22 (28.2) | 18 (19.1) | 17 (25.8) | 8 (19.0) | 82 (24.6) |
| TZP | 35 (64.8) | 47 (60.3) | 57 (60.6) | 40 (60.6) | 24 (57.1) | 203 (60.8) |
| SCF | 42 (77.8) | 55 (70.5) | 63 (67.0) | 45 (68.2) | 26 (61.9) | 231 (69.2) |
| CEC | 13 (24.1) | 17 (21.8) | 19 (20.2) | 14 (21.2) | 8 (19.0) | 71 (21.3) |
| CAZ | 22 (40.7) | 27 (34.6) | 29 (30.9) | 23 (34.8) | 10 (23.8) | 111 (33.2) |
| CRO | 21 (38.9) | 30 (38.5) | 33 (35.1) | 20 (30.3) | 17 (40.5) | 121 (36.2) |
| FEP | 29 (53.7) | 44 (56.4) | 41 (43.6) | 29 (43.9) | 19 (45.2) | 162 (48.5) |
| SPX | 34 (63.0) | 44 (56.4) | 51 (54.3) | 35 (53.0) | 22 (52.4) | 186 (55.7) |
| CIP | 31 (57.4) | 37 (47.4) | 47 (50.0) | 34 (51.5) | 19 (45.2) | 168 (50.3) |
| ENX | 21 (38.9) | 25 (32.1) | 34 (36.2) | 24 (36.4) | 13 (31.0) | 117 (35.0) |
| GTX | 28 (51.9) | 39 (50.0) | 40 (42.6) | 27 (40.9) | 15 (35.7) | 149 (44.6) |
| MXF | 37 (68.5) | 52 (66.7) | 55 (58.5) | 38 (57.6) | 24 (57.1) | 206 (61.7) |
| E | 11 (20.4) | 14 (17.9) | 18 (19.1) | 13 (19.7) | 8 (19.0) | 64 (19.2) |
| CLR | 27 (50.0) | 37 (47.4) | 38 (40.4) | 24 (36.4) | 16 (38.1) | 142 (42.5) |
| MEM | 49 (90.7) | 72 (92.3) | 85 (90.4) | 60 (90.9) | 38 (90.5) | 304 (91.0) |
| IPM | 46 (85.2) | 67 (85.9) | 79 (84.0) | 54 (81.8) | 36 (85.7) | 282 (84.4) |
| CN | 11 (20.4) | 15 (19.2) | 18 (19.1) | 11 (16.7) | 7 (16.7) | 62 (18.6) |
| AK | 36 (66.7) | 49 (62.8) | 63 (67.0) | 42 (63.6) | 26 (61.9) | 216 (64.7) |
| DO | 7 (13.0) | 9 (11.5) | 9 (9.6) | 6 (9.1) | 3 (7.1) | 34 (10.2) |
3.3. Yearwise Susceptibility Pattern of Isolates to Different Antibiotics
The changes in the susceptibility pattern during the study period from 2010 to 2014 against all types of clinical specimens were checked for various classes of antibiotics. The sensitivity pattern of combined β-lactams and β-lactamase inhibitors was 77.8% in 2010 and 70.5% in 2011, while a slight drift has been seen in the last 3 consecutive years which was 66%, 68%, and 61% for 2012-2013 and 2014 against SCF. AMC showed an average 15% sensitivity rate throughout the study period. The range was acceptable that is 11.9%–18.5%.
Carbapenem exhibited greater activity over other antimicrobial agents against Pseudomonas spp. during the course of the study. Within the same class, Meronem had a better activity than the imipenem over the entire duration; however, both carbapenems had a range of 92.8% to 89.4% and 85.3% to 92%, respectively.
Among the beta lactams, all generation of cephalosporin used in the study had consistently diminished rate of sensitivity: cefaclor was 24.1% sensitive in 2010, while it has fluctuated to 19% in 2014; ceftazidime had an average of 34.14% for the entire period. However, the 4th generation cephalosporin had shown an increase in effectiveness rate from 53.7% in 2010 to 56.4% in 2011 and then a sudden decline (43%) in the next three years.
Relatively a steady decrease has been examined with the following percent of susceptibility rates to SPX over the study periods 63.8%, 56.4% 54.3%, 53%, and then 52.4%. Among the fluoroquinolones, sensitivity rate for GTX was found 52% and 50% in 2010 and 2011, respectively. However, a gradual decrease was noted in the next three years (42.6%, 40.9%, and 35.7%). CIP frequency range was 45.2% to 57.4%. Also for MXF, isolates showed a reduction in susceptibility ranges from 68.5% in 2010 to 57.1% in 2014.
Erythromycin and clarithromycin were the least reliably active reagents against the tested Pseudomonas spp. Overall, there was a moderate decrease in susceptibility rate to the antibiotic analyzed over the last five years of the study. Table 3 reflects yearwise susceptibility pattern of isolates against individual antibiotic.
3.4. MIC (Minimum Inhibitory Concentrations)
Percent susceptible strains and MICs 50% and 90% inhibition range were calculated for each of the antibiotics. The values show concordance between disc zone diameter (mm) and MICs values for antibiotics (Table 4).
Table 4.
In vitro susceptibility of ESBL and non-ESBL producing Pseudomonas spp. shows the MICs against the tested strain (MIC 50 μg/mL and MIC 90 μg/mL).
| Antibiotics | % susceptibility | MIC 50 μg/mL | MIC 90 μg/mL |
|---|---|---|---|
| AMC | 21.56 | 16 | 32 |
| AML | 14.97 | 32 | 64 |
| SCF | 66.17 | 4 | 16 |
| CEC | 21.26 | 128 | 256 |
| CAZ | 34.13 | 64 | 64 |
| CRO | 36 | 32 | 128 |
| FEP | 48.5 | 8 | 32 |
| CIP | 46.71 | 2 | 16 |
| GTX | 52.40 | 16 | 128 |
| MXF | 58.08 | 0.5 | 2 |
| MEM | 91.02 | 1 | 4 |
| IPM | 87.43 | 1 | 8 |
| CN | 19.16 | 16 | 32 |
| AK | 67.07 | 4 | 4 |
| DO | 10.18 | 256 | 512 |
Production of ESBLs was observed in 148 (44.32%) of the isolates and the remaining 186 (55.68%) were non-ESBL producers (Figure 4). A high resistance rate was observed in the ESBL positive isolates as compared to non-ESBL strains (Table 5).
Figure 4.
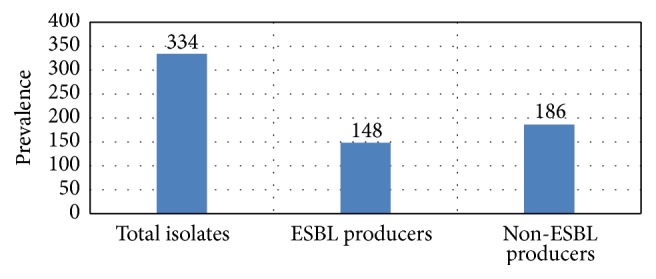
Prevalence of ESBL in clinically pathogenic Pseudomonas spp.
Table 5.
In vitro (%) susceptibility of ESBL and non-ESBL produced by Pseudomonas spp.
| Antimicrobials | ESBL producers | Non-ESBL producers | P value | ||
|---|---|---|---|---|---|
| Susceptible N (%) |
Resistant N (%) |
Susceptible N (%) |
Resistant N (%) |
||
| AML | 14 (9.5) | 134 (90.5) | 36 (19.4) | 150 (80.6) | <0.018 |
| AMC | 24 (16.2) | 124 (83.8) | 58 (31.2) | 128 (68.8) | 0.0025 |
| TZP | 78 (52.7) | 70 (47.3) | 125 (67.2) | 61 (32.8) | <0.01 |
| SCF | 76 (51.4) | 72 (48.6) | 155 (83.3) | 31 (16.7) | <0.0001 |
| CEC | 21 (14.2) | 127 (85.8) | 50 (26.9) | 136 (73.1) | <0.007 |
| CAZ | 30 (20.3) | 118 (79.7) | 81 (43.5) | 105 (56.5) | <0.0001 |
| CRO | 22 (14.3) | 126 (85.1) | 99 (53.2) | 87 (46.8) | <0.0001 |
| FEP | 33 (22.3) | 115 (77.7) | 129 (69.4) | 57 (30.6) | <0.0001 |
| SPX | 62 (41.9) | 86 (58.1) | 124 (66.7) | 62 (33.3) | <0.0001 |
| CIP | 55 (37.2) | 93 (62.8) | 113 (60.8) | 73 (39.2) | <0.0001 |
| ENX | 43 (29.1) | 105 (70.9) | 74 (39.8) | 112 (60.2) | <0.05 |
| GTX | 55 (37.2) | 93 (62.8) | 94 (50.5) | 92 (49.5) | <0.01 |
| MXF | 80 (54.1) | 68 (45.9) | 116 (62.4) | 70 (37.6) | <0.13 |
| E | 36 (24.3) | 112 (75.7) | 28 (15.1) | 158 (84.9) | <0.325 |
| CLR | 66 (44.6) | 80 (54.1) | 76 (40.9) | 110 (59.1) | <0.43 |
| MEM | 128 (86.5) | 20 (13.5) | 176 (94.6) | 10 (5.4) | <0.0098 |
| IPM | 120 (81.1) | 28 (18.9) | 172 (92.5) | 14 (7.5) | <0.0018 |
| CN | 20 (13.5) | 128 (86.5) | 42 (22.6) | 144 (77.4) | <0.034 |
| AK | 75 (50.7) | 73 (49.3) | 141 (75.8) | 45 (24.2) | <0.0001 |
| DO | 9 (6.1) | 139 (93.9) | 25 (13.4) | 161 (86.6) | <0.0426 |
A significant difference was found in susceptibility to the carbapenems, quinolones, and β-lactam/β-lactamase inhibitors, statistically. Resistance of ESBLs to the other class of antibiotics like penicillin and macrolides was a little a bit higher than the non-ESBLs but statistically it was not significant.
The resistance conferred by ESBLs producing Pseudomonas spp. to cephalosporin (CEC, CAZ, CRO, and CFP) was 14.2%, 20.3%, 14.3%, and 22.3%, respectively, contrary to the non-ESBLs. Both ESBL and non-ESBL producers isolates were almost resistant to tetracycline. Good susceptibility was observed with amikacin in both ESBL (50.7%) and non-ESBL producers (75.8%). On the other hand, pseudomonal susceptibility against antibiotics from the β-lactams and β-lactamase inhibitors was observed. Augmentin was 16.2% and 31.2% for ESBL and non-ESBL producers, respectively. It was 37.2% and 50.5% for ESBLs and non-ESBLs producing isolate against GTX. Noble activity has been shown for both ESBL and non-ESBL by the class carbapenems and the considerable activities by SCF, MXF, and AK, which has a higher activity than cephalosporin. A higher resistance for AMC, AML, and DO was evaluated in comparison to SPR and SPX of β-lactam inhibitors and CLR and CIP member of quinolones given in Table 5.
3.5. ESBL and Non-ESBL Producing Pseudomonas spp. (Hospitalwise)
The overall frequency of isolation of ESBL producing organism at different hospitals is depicted in Figure 5. The distribution of ESBL isolates varied, considerably in each hospital. The highest incidence (46.88%) was observed at Hayatabad Medical Complex (HMC) followed (45.03%) by Khyber Teaching Hospital (KTH). The prevalence was comparatively small (40.51%) from Lady Reading Hospital (LRH).
Figure 5.
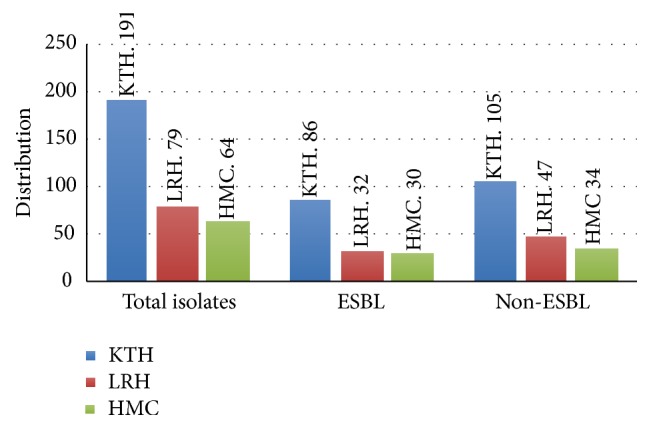
Hospitalwise distribution of ESBL and Non-ESBL producing Pseudomonas spp.
The statistical analysis indicates that there is no association between ESBL and hospitals; means hospitals are not involved to produce ESBL. While in outdoor patients females are 1.158 times more likely than male patients who produce positive ESBL, male indoor patients are 0.802 times less likely than female outdoor patients who produce positive ESBL
4. Discussion
Surveillance is a key to the control of antimicrobial resistance. Facts and figures found by surveillance events can be used to direct empirical prescribing of antimicrobial agents, to identify newly developing resistances, to determine importance for research, and to evaluate involvement strategies and potential control trials aimed at dropping the prevalence of resistant pathogens [20].
The rate of susceptibility was most productive for antimicrobial agent of class carbapenem against Pseudomonas spp. [21]. Supported current results as 90% of strains were susceptible to Meronem and 84% to imipenem of class carbapenems.
The results are in agreement with Zhanel et al.'s [22] findings as reported for both sparfloxacin and moxifloxacin 58%, followed by ciprofloxacin 46.7%. In this study, amoxicillin and amoxiclav have established 85% and 78.44% resistance. The study conducted in Pakistan reported by Khan et al. [23] had a high resistance rate of penicillin that is 98%; our findings are also in agreement with other studies as reported by Sasirekha et al. [24] and Ullah et al. [12] with respect to penicillin's. In the present study cefaclor, ceftriaxone, ceftazidime, and cefepime were found to be 17.24%, 33.19%, 33.62%, and 43.97% acquired from hospital and 30.39%, 35.29%, 44.12%, and 58.82% susceptible from the outdoor (OPD) patients infected by Pseudomonas spp., respectively. Susceptibility to fourth-generation cefepime reported in India was 32% [25] and in Bulgaria 42% [26] against Pseudomonas spp. isolates and cephalosporin especially third generation has been used for gram-negative bacteria treatment [27]. The results of the third generation are in close agreement with other studies [26, 28].
Aminoglycosides have good activity against clinically important gram-negative bacilli [29]. In the present study among the non-beta lactams, 68.53% isolates were susceptible to amikacin, followed by 16.38% to gentamicin. It was similar to what is reported in the literature [24]; in France a higher susceptibility rate of 86% of amikacin was reported by Cavallo et al. [30]. Several studies showed that amikacin was more sensitive than gentamicin and our results also support the above arguments. In 2010 gentamicin was 59% resistant in India [24] and 55.5% in Bangladesh [31], while in Bulgaria it was 36.11% recorded in Pseudomonas spp. by Strateva et al. [26].
Pseudomonads have more adoptability than Enterobacteriaceae in developing drug resistance by diverse means. The production of ESBLs found more resistance at different stages to expanded spectrum cephalosporins [32].
Production of β-lactamases by bacteria which hydrolyzes the penicillin, cephalosporin, and other β-lactams leads to inactivation of the drug. Extended spectrum beta lactamases (ESBLs) are enzymes that hydrolyze and induce resistance to cephalosporins (ceftriaxone, ceftazidime, cefepime, and cefotaxime) and monobactams (aztreonam) [10, 33]. Cross resistance in Pseudomonas spp. has been identified to be the emerging risk factor to imipenem, formerly used fluoroquinolone [34], and a study reported that imipenem-resistant to Pseudomonas spp. isolates showed resistance to ciprofloxacin or levofloxacin, signifying that cross resistance is established for imipenem [35]. Multidrug resistant Pseudomonas isolates, sometimes, develop Pan-drug resistance, resistant to all the antibiotics except colistin [35]. β-lactams are hydrophilic antibiotics, utilizing the pore forming proteins (OprD in Pseudomonas and OmpF in E. coli) to enter inside the cell, whereas larger hydrophobic antibiotics and macrolides cross the lipid bilayer through diffusion. Alteration in lipid or proteins conformation of OM barrier accounts for survival of the antibiotic-resistant strains and pinpoints its position in antibiotic susceptibility [36].
ESBLs prevalence in this particular study was recorded as 44.32%, which was very similar to the studies conducted by (Ali et al. [37], Jabeen et al. [38], and Ullah et al. [12]) from Pakistan, 40%, 43%, and 58.7% ESBLs producers. Lower rates were recorded by Anjum and Mir [39] in Pakistan which observed 33% and this incidence is superior to continental surveys performed in South America (18.1%), Europe (11%), North America (7.5%), and Asia-Pacific (14.2%) parts [19, 40].
The findings of this study indicate that indoor ESBLs producing Pseudomonas isolates were more resistant towards third-generation antibiotics, for example, cephalosporins (78% to 86%) as compared with outdoor isolates, supported by the studies of Babypadmini and Appalaraju [41], who found 84%, and Sasirekha et al. [24], who reported 75 to 85% resistance rate to cephalosporins. Most of the ESBLs producing organisms were found coresistant to fluoroquinolones and aminoglycosides which correlate with the study done by Denholm et al. [42] and Jabeen et al. [38]. It is because of the genes, encoding that these β-lactamases are often situated at large plasmids which also encode resistant genes for other antibiotics, together with sulfonamides, tetracycline, chloramphenicol, trimethoprim, and aminoglycosides [43].
Use of cephalosporin is not only associated with ESBL infection, but also seen to be a risk factor for colonization with ESBL producing organisms [44]. As a result, higher percentage of ESBL producing Pseudomonas spp. because of selected stress is forced by excessive use of the 3rd-generation cephalosporins in this research. This association has been best displayed by interventional study which demonstrated decline in the frequency of ESBL pooling from 8% to 6% due to control use of third-generation cephalosporins [45]. ESBLs occurrence was significantly higher among isolates from inpatients than outpatients (P = 0.002).
In this study, we used two combinations with clavulanic acid (CAZ/CAZC and CTX/CTXC) and found that Pseudomonas spp. revealed higher production of ESBLs in CAZ/CAZC and are closely proximate to the findings of other researches [46, 47]. As a result, laboratories should perform the ESBLs confirmatory test to both resistant and sensitive strains. The marker of ESBLs that is cefotaxime, ceftazidime, and ceftriaxone is no longer recommended.
Consequently a quick response is needed to identify the ESBLs producing organisms that in future proper antibiotic practice and infection managing procedures can be employed.
5. Conclusion
High level of resistance of antibiotics to Pseudomonas spp. may be due to lack of antibiotic policy, irrational use of antibiotics, particularly 3GCs, and the emergence of antibiotic-resistant organisms in hospitals, resulting in multidrug resistance in Pseudomonas spp.
Competing Interests
The authors declare no competing interests.
References
- 1.Haas D., Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Reviews Microbiology. 2005;3(4):307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 2.Obritsch M. D., Fish D. N., MacLaren R., Jung R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25(10):1353–1364. doi: 10.1592/phco.2005.25.10.1353. [DOI] [PubMed] [Google Scholar]
- 3.Pier G., Ramphal R. Pseudomonas aeruginosa. Principles and Practice of Infectious Diseases. 2005;6(2):2587–2615. [Google Scholar]
- 4.Manfredi R., Nanetti A., Ferri M., Chiodo F. Pseudomonas spp. complications in patients with HIV disease: an eight-year clinical and microbiological survey. European Journal of Epidemiology. 2000;16(2):111–118. doi: 10.1023/a:1007626410724. [DOI] [PubMed] [Google Scholar]
- 5.Japoni A., Farshad S., Alborzi A. Pseudomonas aeruginosa: burn infection, treatment and antibacterial resistance. Iranian Red Crescent Medical Journal. 2009;11(3):244–253. [Google Scholar]
- 6.Ciofu O., Hansen C. R., Høiby N. Respiratory bacterial infections in cystic fibrosis. Current Opinion in Pulmonary Medicine. 2013;19(3):251–258. doi: 10.1097/MCP.0b013e32835f1afc. [DOI] [PubMed] [Google Scholar]
- 7.Ruimy R., Genauzeau E., Barnabe C., Beaulieu A., Tibayrenc M., Andremont A. Genetic diversity of Pseudomonas aeruginosa strains isolated from ventilated patients with nosocomial pneumonia, cancer patients with bacteremia, and environmental water. Infection and Immunity. 2001;69(1):584–588. doi: 10.1128/iai.69.1.584-588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akanji B. O., Ajele J. O., Onasanya A., Oyelakm O. Genetic fingerprinting of Pseudomonas aeruginosa involved in nosocomial infection as revealed by RAPD-PCR markers. Biotechnology. 2011;10(1):70–77. doi: 10.3923/biotech.2011.70.77. [DOI] [Google Scholar]
- 9.Afunwa R. A., Odimegwu D. C., Iroha R. I., Esimone C. O. Antimicrobial resistance status and prevalence rates of extended spectrum beta-lactamase (ESBL) producers isolated from a mixed human population. Bosnian Journal of Basic Medical Sciences. 2011;11(2):91–96. doi: 10.17305/bjbms.2011.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peirano G., Pitout J. D. D. Molecular epidemiology of Escherichia coli producing CTX-M β-lactamases: the worldwide emergence of clone ST131 O25:H4. International Journal of Antimicrobial Agents. 2010;35(4):316–321. doi: 10.1016/j.ijantimicag.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Bell J. M., Chitsaz M., Turnidge J. D., Barton M., Walters L. J., Jones R. N. Prevalence and significance of a negative extended-spectrum β-lactamase (ESBL) confirmation test result after a positive ESBL screening test result for isolates of Escherichia coli and Klebsiella pneumoniae: results from the SENTRY Asia-Pacific surveillance program. Journal of Clinical Microbiology. 2007;45(5):1478–1482. doi: 10.1128/jcm.02470-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullah F., Malik S. A., Ahmed J. Antimicrobial susceptibility and ESBL prevalence in Pseudomonas aeruginosa isolated from burn patients in the North West of Pakistan. Burns. 2009;35(7):1020–1025. doi: 10.1016/j.burns.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Walkty A., DeCorby M., Nichol K., Mulvey M. R., Hoban D., Zhanel G. Antimicrobial susceptibility of Pseudomonas aeruginosa isolates obtained from patients in Canadian intensive care units as part of the Canadian National Intensive Care Unit study. Diagnostic Microbiology and Infectious Disease. 2008;61(2):217–221. doi: 10.1016/j.diagmicrobio.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Cowan S. T., Barrow G. I., Steel K. J., Feltham R. K. A. Cowan and Steel's Manual for Identification of Medical Bacteria. 3rd. Cambridge University Press; 2004. [Google Scholar]
- 15.Chessbrough M. District Laboratory Practice in Tropical Countries. New York, NY, USA: Cambridge University Press; 2006. [Google Scholar]
- 16.Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 17.Deshpande L. M., Fix A. M., Pfaller M. A., Jones R. N. Emerging elevated mupirocin resistance rates among staphylococcal isolates in the SENTRY Antimicrobial Surveillance Program (2000): correlations of results from disk diffusion, Etest and reference dilution methods. Diagnostic Microbiology and Infectious Disease. 2002;42(4):283–290. doi: 10.1016/s0732-8893(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 18.Cockerill F. R. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-First Informational Supplement. Clinical and Laboratory Standards Institute (CLSI); 2011. [Google Scholar]
- 19.Hawser S. P., Bouchillon S. K., Lascols C., et al. Susceptibility of European Escherichia coli clinical isolates from intra-abdominal infections, extended-spectrum β-lactamase occurrence, resistance distribution, and molecular characterization of ertapenem-resistant isolates (SMART 2008-2009) Clinical Microbiology and Infection. 2012;18(3):253–259. doi: 10.1111/j.1469-0691.2011.03550.x. [DOI] [PubMed] [Google Scholar]
- 20.Gao Z. S., Shen H. H., Zheng M., Frewer L. J., Gilissen L. J., editors. Multidisciplinary Approaches to Allergies. New York, NY, USA: Springer Science & Business Media; 2013. [Google Scholar]
- 21.Turner P. J. Meropenem and imipenem activity against Pseudomonas aeruginosa isolates from the MYSTIC Program. Diagnostic Microbiology and Infectious Disease. 2006;56(3):341–344. doi: 10.1016/j.diagmicrobio.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Zhanel G. G., Laing N. M., Nichol K. A., et al. Antibiotic activity against urinary tract infection (UTI) isolates of vancomycin-resistant enterococci (VRE): results from the 2002 North American vancomycin resistant enterococci susceptibility study (NAVRESS) Journal of Antimicrobial Chemotherapy. 2003;52(3):382–388. doi: 10.1093/jac/dkg352. [DOI] [PubMed] [Google Scholar]
- 23.Khan J. A., Iqbal Z., Ur Rahman S., Farzana K., Khan A. Prevalence and resistance pattern of Pseudomonas aeruginosa against various antibiotics. Pakistan Journal of Pharmaceutical Sciences. 2008;21(3):311–315. [PubMed] [Google Scholar]
- 24.Sasirekha B., Manasa R., Ramya P., Sneha R. Frequency and antimicrobial sensitivity pattern of extended spectrum β-lactamases producing E. coli and Klebsiella pneumoniae isolated in a tertiary care hospital. Al Ameen Journal of Medical Sciences. 2010;3(4):265–271. [Google Scholar]
- 25.Chaudhury A. In vitro activity of Cefpirome: a new fourth generation cephalosporin. Indian Journal of Medical Microbiology. 2003;21(1):52–55. [PubMed] [Google Scholar]
- 26.Strateva T., Ouzounova-Raykova V., Markova B., Todorova A., Marteva-Proevska Y., Mitov I. Widespread detection of VEB-1-type extended-spectrum beta-lactamases among nosocomial ceftazidime-resistant Pseudomonas aeruginosa isolates in Sofia, Bulgaria. Journal of Chemotherapy. 2007;19(2):140–145. doi: 10.1179/joc.2007.19.2.140. [DOI] [PubMed] [Google Scholar]
- 27.Samaha-Kfoury J. N., Araj G. F. Recent developments in β lactamases and extended spectrum β lactamases. The British Medical Journal. 2003;327(7425):1209–1213. doi: 10.1136/bmj.327.7425.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Revathi G., Puri J., Jain B. K. Bacteriology of burns. Burns. 1998;24(4):347–349. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez L. S., III, Spencer J. P. Aminoglycosides: a practical review. American Family Physician. 1998;58(8):1811–1820. [PubMed] [Google Scholar]
- 30.Cavallo J. D., Hocquet D., Plesiat P., Fabre R., Roussel-Delvallez M. Susceptibility of Pseudomonas aeruginosa to antimicrobials: a 2004 French multicentre hospital study. Journal of Antimicrobial Chemotherapy. 2007;59(5):1021–1024. doi: 10.1093/jac/dkm076. [DOI] [PubMed] [Google Scholar]
- 31.Haque S. F., Ali S.-Z., Mohammed T., Khan A. U. Prevalence of plasmid mediated bla TEM-1 and bla CTX-M-15 type extended spectrum beta-lactamases in patients with sepsis. Asian Pacific Journal of Tropical Medicine. 2012;5(2):98–102. doi: 10.1016/s1995-7645(12)60003-0. [DOI] [PubMed] [Google Scholar]
- 32.Castanheira M., Toleman M. A., Jones R. N., Schmidt F. J., Walsh T. R. Molecular characterization of a β-lactamase gene, blaGIM.1, encoding a new subclass of metallo-β-lactamase. Antimicrobial Agents and Chemotherapy. 2004;48(12):4654–4661. doi: 10.1128/aac.48.12.4654-4661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livermore D. M. β-lactamases in laboratory and clinical resistance. Clinical Microbiology Reviews. 1995;8(4):557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furtado G. H. C., Bergamasco M. D., Menezes F. G., et al. Imipenem-resistant Pseudomonas aeruginosa infection at a medical-surgical intensive care unit: risk factors and mortality. Journal of Critical Care. 2009;24(4):625.e9–625.e14. doi: 10.1016/j.jcrc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Rajkumari N., John N. V., Mathur P., Misra M. C. Antimicrobial resistance in Pseudomonas sp. causing infections in trauma patients: a 6 year experience from a south asian country. Journal of Global Infectious Diseases. 2014;6(4):182–185. doi: 10.4103/0974-777x.145250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Den Engelsen C., van der Werf C., Matute A. J., Delgado E., Schurink C. A. M., Hoepelman A. I. M. Infectious diseases and the use of antibiotics in outpatients at the emergency department of the University Hospital of León, Nicaragua. International Journal of Infectious Diseases. 2009;13(3):349–354. doi: 10.1016/j.ijid.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Ali A. M., Rafi S., Qureshi A. H. Frequency of extended spectrum beta lactamase producing gram negative bacilli among clinical isolates at clinical laboratories of Army Medical College, Rawalpindi. Journal of Ayub Medical College, Abbottabad. 2004;16(1):35–37. [PubMed] [Google Scholar]
- 38.Jabeen K., Zafar A., Hasan R. Frequency and sensitivity pattern of Extended Spectrum beta Lactamase producing isolates in a tertiary care hospital laboratory of Pakistan. Journal of the Pakistan Medical Association. 2005;55(10):436–439. [PubMed] [Google Scholar]
- 39.Anjum F., Mir A. Susceptibility pattern of Pseudomonas aeruginosa against various antibiotics. African Journal of Microbiology Research. 2010;4(10):1005–1012. [Google Scholar]
- 40.Turner P. J. Extended-spectrum β-lactamases. Clinical Infectious Diseases. 2005;41(4):S273–S275. doi: 10.1086/430789. [DOI] [PubMed] [Google Scholar]
- 41.Babypadmini S., Appalaraju B. Extended spectrum β-lactamases in urinary isolates of Escherichia coli and Klebsiella pneumoniae—prevalence and susceptibility pattern in a tertiary care hospital. Indian Journal of Medical Microbiology. 2004;22(3):172–174. [PubMed] [Google Scholar]
- 42.Denholm J. T., Huysmans M., Spelman D. Community acquisition of ESBL-producing Escherichia coil: a growing concern. The Medical Journal of Australia. 2009;190(1):45–46. doi: 10.5694/j.1326-5377.2009.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 43.Perez F., Endimiani A., Hujer K. M., Bonomo R. A. The continuing challenge of ESBLs. Current Opinion in Pharmacology. 2007;7(5):459–469. doi: 10.1016/j.coph.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy S. S. S., Mello M. J. G., Gusmão-filho F. A. R., Correia J. B. Colonisation by extended-spectrum β-lactamase-producing Klebsiella spp. in a paediatric intensive care unit. The Journal of Hospital Infection. 2010;76(1):66–69. doi: 10.1016/j.jhin.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Bisson G., Fishman N. O., Patel J. B., Edelstein P. H., Lautenbach E. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species: risk factors for colonization and impact of antimicrobial formulary interventions on colonization prevalence. Infection Control and Hospital Epidemiology. 2002;23(5):254–260. doi: 10.1086/502045. [DOI] [PubMed] [Google Scholar]
- 46.Rahman M. M., Haq J. A., Hossain M. A., Sultana R., Islam F., Islam A. H. M. S. Prevalence of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in an urban hospital in Dhaka, Bangladesh. International Journal of Antimicrobial Agents. 2004;24(5):508–510. doi: 10.1016/j.ijantimicag.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Thomson K. S., Sanders C. C., Washington J. A., II High-level resistance to cefotaxime and ceftazidime in Klebsiella pneumoniae isolates from Cleveland, Ohio. Antimicrobial Agents and Chemotherapy. 1991;35(5):1001–1003. doi: 10.1128/AAC.35.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]


