Abstract
Objectives
Few studies have investigated the complex relationship among asthma control, sleep problems, and health-related quality of life (HRQOL) among children with asthma. This study aimed to test the longitudinal effect of asthma control status on asthma-specific HRQOL through the mechanism of nighttime sleep quality and daytime sleepiness.
Methods
229 dyads of asthmatic children and their parents engaged in the Patient-Reported Outcomes Measurement Information System (PROMIS®) Pediatric Asthma Study with 2 years of follow-up to assess the change of asthma control, sleep problems, and asthma-specific HRQOL. The Asthma Control and Communication Instrument was used to measure asthma control status. Nighttime sleep quality assessment was based on difficulty falling asleep and getting up, and sleep disturbance. The Iowa Pediatric Daytime Sleeping Scale was used to assess daytime sleepiness. The PROMIS Asthma Impact Scale was used to measure asthma-specific HRQOL. Multilevel structural equation modeling was performed to quantify the direct and indirect effects of asthma control status on asthma-specific HRQOL through nighttime sleep quality and daytime sleepiness.
Results
Poorer asthma control status was directly associated with lower asthma-specific HRQOL at within-subject and between-subject levels (p’s<0.05); however, effects of asthma control on asthma-specific HRQOL were indirectly influenced through daytime sleepiness at within-subject level (p<0.05), and through nighttime sleep quality and daytime sleepiness at between-subject level (p<0.05).
Conclusions
Asthma control status is associated with asthma-specific HRQOL, and this association is mediated by nighttime sleep quality and daytime sleepiness. Clinicians should address sleep problems related to asthma control to improve HRQOL for asthmatic children.
Keywords: Asthma control, nighttime sleep quality, daytime sleepiness, health-related quality of life, children, multilevel structural equation modeling
1. INTRODUCTION
Asthma is a common chronic illness which affects approximately 6.9 million (9%) children in the United States [1]. Children with asthma are more likely to experience sleep problems than children without asthma [2, 3]. Depending upon different study designs and population characteristics, the percentage of children with asthma experiencing sleep difficulties ranged from 30% to 40% [4–6]. It is especially evident that children with poorly-controlled asthma are more likely to experience nighttime awakening and sleep difficulties than children with well-controlled asthma [3, 7, 8]. The relationship between sleep problems and health-related quality of life (HRQOL) in asthmatic children has been extensively investigated by previous studies. These studies found that sleep problems such as nighttime sleep disturbance [9, 10] and daytime sleepiness [11] were associated with poorer HRQOL in physical [9, 10], and mental [9, 10] health domains, as well as asthma-specific domains [11].
Studies on the complex associations among asthma control, sleep problems, and HRQOL are still sparse. Our previous study based on the path analytic approach has found that poorly-controlled asthma status was linked to great daytime sleepiness and low asthma-specific HRQOL; more importantly daytime sleepiness significantly mediated the association between asthma control status and asthma-specific HRQOL [11]. Another study investigating the relationships of self-reported sleep quality and sleep duration assessed by actigraphy with psychological well-being found that variability in sleep duration over 7 days rather than sleep onset latency or awakening time was associated with poorer subjective well-being [12]. This study further found that sleep quality mediated the relationship between sleep duration and subjective well-being [12]. However, previous studies have frequently focused on a single aspect of sleep problem such as nighttime sleep quality, sleep duration, or daytime sleepiness; very few studies have included multiple aspects of sleep problems simultaneously. Testing different aspects of sleep problems and their respective roles in the relationship between asthma control and HRQOL can help clarify the mechanisms toward designing interventions to improve HRQOL.
This study aimed to investigate the influence of nighttime sleep quality and daytime sleepiness on the association between asthma control and asthma-specific HRQOL based on the parent study, the Patient-Reported Outcomes Measurement Information System (PROMIS®) Pediatric Asthma Study (PAS), which was funded through the National Institutes of Health (NIH) between 2010 and 2015. PROMIS PAS is a longitudinal study collecting data of asthma control status, nighttime and daytime sleep issues, asthma-specific HRQOL, and psychosocial outcomes from asthmatic children and their parents across multiple time points. Given the nature of the repeated data, a multilevel structure equation modeling (MSEM) approach was specifically used to quantify the effects of asthma control status on asthma-specific HRQOL (defined as direct effects), and the effects of asthma control status on asthma-specific HRQOL through the mechanism of nighttime sleep quality and daytime sleepiness (defined as indirect effects). It was hypothesized that if children experienced poor nighttime sleep quality due to poorly-controlled asthma status, they would subsequently experience excessive daytime sleepiness. Poor nighttime sleep and excessive daytime sleepiness would in turn influence their daily functional status and asthma-specific HRQOL.
2. MATERIALS AND METHODS
2.1 Population and data collection
In the PROMIS PAS, 229 dyads of asthmatic children and their parents were recruited from Florida Medicaid and the State Children’s Health Insurance Program (SCHIP). After University of Florida’s institutional review board approved the research protocol, this study applied the same inclusion and exclusion criteria as the parent study to recruit the study participants, including 1) children’s age between 8 and 17.9 years old, and parents’ age greater than 18 years old; 2) at least 6 months continuous enrollment in Florida Medicaid and the SCHIP; 3) asthma diagnosis (ICD-9-CM: 493.1, 493.2 or other 493.x) listed in the claim and enrollment files of Florida Medicaid and the SCHIP; 4) at least two asthma-related health care visits during the past 12 months; and 5) accessible to both the internet and telephone in the past 6 weeks. Children and parents who were not able to read, speak, and understand English were excluded from the PROMIS PAS.
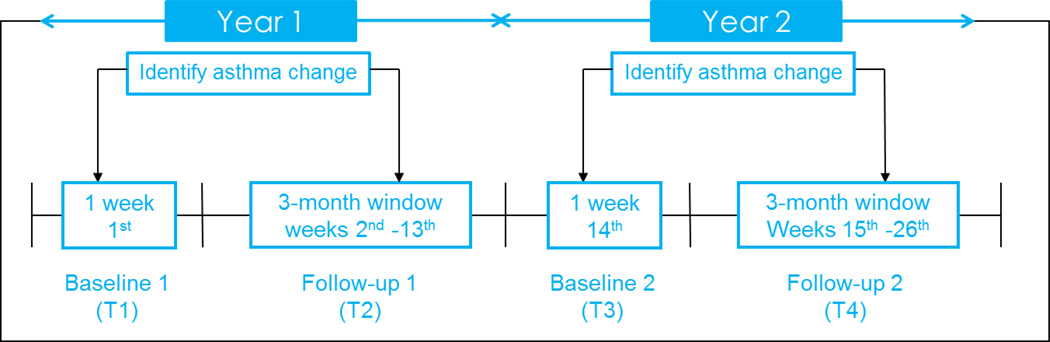
Asthma control status and the corresponding nighttime sleep quality, daytime sleepiness, and asthma-specific HRQOL were identified through a 26-week time window across 2 years (Appendix 1). Briefly, asthma control status and nighttime sleep quality were reported weekly (26 time points in total) by parents through the study website, including the weeks 1 through 13 in the first year and the weeks 14 through 26 in the second year. Changes in asthma control status were identified by comparing the control status in weeks 2 through 13 to the baseline status assessed at week 1 of the first year (T1; baseline of the first year), as well as the control status in weeks 15 through 26 to the baseline status assessed at week 14 of the second year (T3; baseline of the second year). Asthma-specific HRQOL and daytime sleepiness were self-reported by children through telephone interviews at both T1 and T3.
In the PROMIS PAS, the overall asthma control status of individuals was determined by 5 items in the Asthma Control and Communication Instrument (ACCI) measuring asthma control (see below), and then classified as adequately-controlled status if they answered all 5 items as adequately-controlled; otherwise, they were classified as poorly-controlled status [13]. If the first asthma control status changed (worse or better) anytime between weeks 2 and 13 in the first year, HRQoL data were collected from an affected child via a telephone interview during the same week (T2); likewise in the second year between weeks 15 and week 26 (T4). Change in asthma control status could be either from adequately-controlled to poorly-controlled or from poorly-controlled to adequately-controlled. However, if a child’s asthma control status did not change during the follow-up period, we collected HRQOL at the end of follow-up period of each year (T2 and T4). The uniqueness of our study design accounts for the individual difference in the first change of asthma control status and the corresponding change of HRQOL. Additionally, since asthma control status and nighttime sleep quality were collected prior to pediatric daytime sleepiness and asthma-specific HRQOL, a temporal mechanism can be established.
2.2 Measurement and instrument
The ACCI was used to assess children’s asthma control status [13]. The ACCI was developed based on the 2007 National Asthma Education Prevention Program Expert Panel Report-3 (NAEPP EPR-3) [14]. The ACCI has been validated by previous studies and has shown satisfactory psychometric properties including concurrent validity, discriminant validity, and known-group validity [13]. This instrument comprises 11 items; 5 out of the 11 items specifically capture the concept of asthma control status (symptoms, use of rescue medicine, occurrence of asthma attack, activity limitation due to asthma, and nighttime awakenings). One open-ended item is used to collect patient-physician communication. The overall asthma control score of an individual is calculated by summing the scores of the five individual items with a range from 0 to 19. Higher scores indicate worse asthma control status.
Children’s nighttime sleep quality was assessed by 3 items developed by this study: 1) “Last week, how difficult was it for your child to settle and fall asleep after bedtime rituals?”; 2) “Last week, how difficult was it for your child to get up in the morning?”; and 3) “Last week, how many times did your child wake up during the night?”. For each item, a 5-point Likert-type response category ranging from “not at all difficult” to “extremely difficult” or from “never” to “7 times” is used. The overall sleep quality score of an individual is calculated by summing the scores of the 3 individual items with a range from 3 to 15. Higher scores represent worse nighttime sleep quality.
Children’s daytime sleepiness was measured using the Iowa Pediatric Daytime Sleeping Scale (PDSS) that comprises 8 items for a single dimension of daytime sleepiness. PDSS has shown acceptable measurement properties such as internal consistency [15]. Of the 8 items, 7 items are related to sleepiness, and a 5-point Likert-type response category ranging from “always getting sleepy” to “never getting sleepy” is used. One item is related to alertness, and a 5-point Likert-type response category ranging from “never being alert” to “always being alert” is used. All of the items are used to calculate the domain score. Higher scores represent worse daytime sleepiness.
In the PROMIS PAS, the PROMIS pediatric short-forms were used to collect 7 domains of HRQOL including asthma impact, fatigue, depressive symptoms, anxiety, pain interference, peer relationships, and mobility [16–19]. This study focused on asthma-specific HRQOL measured by the asthma impact domain. This domain comprises 8 items selected from a calibrated PROMIS asthma item bank developed by item response theory and qualitative methodologies [20, 21]. For each item, a 5-point Likert-type response category ranging from “never” to “almost always” is used. Scores of individual items are estimated for each child, with a standardized mean score of 50 and SD of 10. Higher scores represent worse asthma-specific HRQOL.
The PROMIS PAS collected baseline socio-demographic characteristics of children and their parents including the children’s age, gender, race/ethnicity, height, weight, and type of chronic conditions, as well as the parents’ age, race/ethnicity, marital status, and education background. Children’s height and weight were used to calculate body mass index (BMI) based on the Centers for Disease Control and Prevention’s criteria [22]. Overweight was defined as a BMI ≥ the 85th percentile and < the 95th percentile among children and teens of the same age and sex.
2.3 Statistical analysis
Descriptive analyses were performed to analyze the distribution of socio-demographic characteristics among the study participants. The percentage or mean and standard deviation (SD) were calculated for the children’s age, gender, race/ethnicity, overweight status, number of chronic conditions, and parents’ age, race/ethnicity, marital status, education background, and family income. In this study, the raw scores of asthma control status, nighttime sleep quality, and daytime sleepiness domain were linearly transformed to a range between 0 (best outcomes) and 100 (worst outcomes). The transformed scores were used in all statistical analyses. The mean and SD of asthma control, nighttime sleep quality, daytime sleepiness, and asthma-specific HRQOL were reported by 4 individual time points. Bivariate analyses were conducted to examine the associations of asthma control status with nighttime sleep quality, daytime sleepiness, asthma-specific HRQOL, and participants’ socio-demographic characteristics, respectively, through these 4 individual time points. Additionally, bivariate analyses were performed to test the associations of HRQOL with nighttime sleep quality, daytime sleepiness, and participants’ socio-demographic characteristics, respectively, by individual time points.
Linear random-intercept models were performed to examine the effects of asthma control, nighttime sleep quality, and daytime sleepiness on asthma-specific HRQOL by treating individual subjects as random effects to account for the clustering effects of repeated assessments of asthma-specific HRQOL outcomes. Different independent variables were included in 4 separate random-intercept models: asthma control alone (Model 1); nighttime sleep quality alone (Model 2); daytime sleepiness alone (Model 3); and asthma control, nighttime sleep quality, and daytime sleepiness (Model 4). The children’s age, gender, race/ethnicity, number of chronic conditions, and the parents’ age, marital status, and educational background were treated as covariates and included in all random-intercept models.
Multilevel structural equation modeling (MSEM) was applied to investigate the direct effect of asthma control status on asthma-specific HRQOL, and the indirect effect of asthma control on asthma-specific HRQOL through the influence of nighttime sleep quality and daytime sleepiness. MSEM has demonstrated several unique features compared with traditional path analytic methods on managing multilevel data, and has been successfully applied to several social behavioral studies [23–25]. First, MSEM is able to incorporate more than one mediator in the path analytic framework. In this study, MSEM was conducted to model the effect of two mediators related to sleep problems (nighttime sleep quality and daytime sleepiness) on the pathway between asthma control status and asthma-specific HRQOL. Second, MSEM is able to model various relationships among independent variables, mediators, and outcome variables at different levels of the data [26, 27]. In this study, because asthma control status, nighttime sleep quality, daytime sleepiness, and asthma-related HRQOL were assessed at 4 time points for each subject, the data can be stratified into two levels: within-subject (or repeated) variation (level 1) and between-subject variation (level 2). The intraclass correlations (ICC) of selected variables were examined to indicate if there was significant between-subject variation, which was calculated as the ratio of between-group variance to the total variance of that variable. ICC ≥ 0.2 suggests significant clustering effects in the data that should be addressed using a multilevel analytic framework [28–30]. Third, MSEM can accommodate missing data and unequal cluster sizes in multilevel study designs [27]. Finally, the use of MSEM allows for adjustment of participants’ socio-demographic factors assumed to confound the associations among variables.
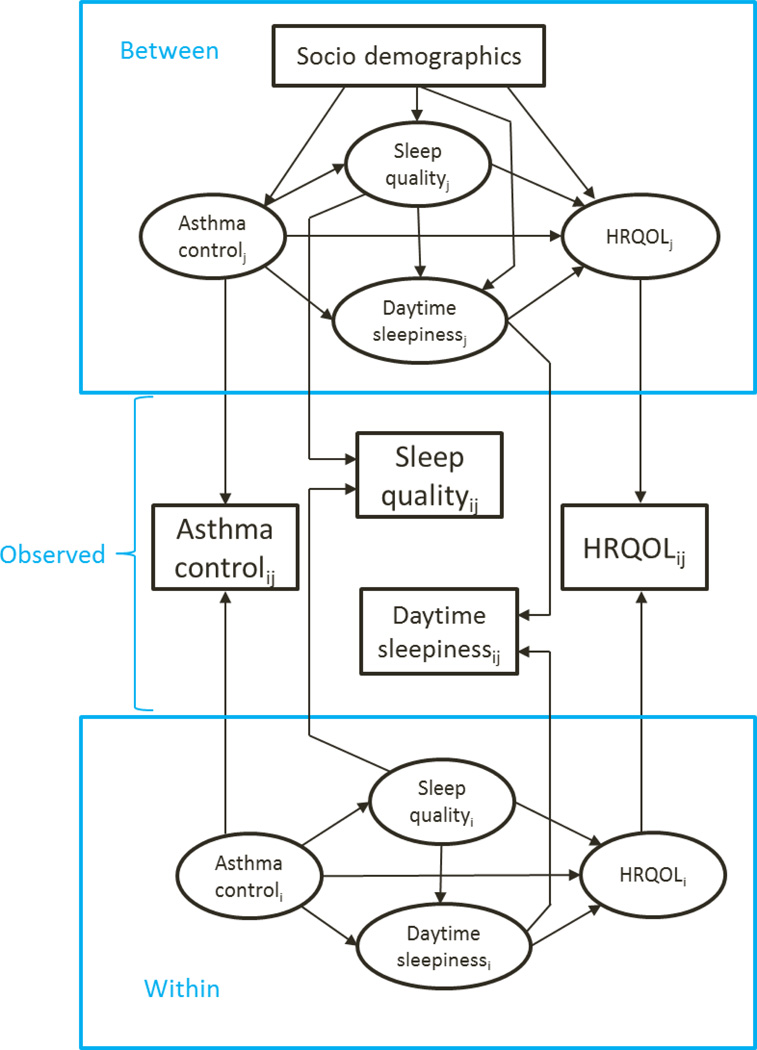
The analytic framework (Figure 1) for MSEM was developed based on Preacher and colleagues’ methodology [27]. The longitudinal design of the PROMIS PAS results in two-level data for statistical analyses: individual values of each variable collected from 4 repeated assessments (i.e., within-subject level) and the mean estimated value of 4 repeated assessments from an individual (i.e., between-subject level). Using information of within-subject and between-subject variance, the relationships among asthma control status, sleep problems, and asthma-specific HRQOL were tested using the scores of latent variables (ovals in Figure 1) that were estimated from the observed variables (rectangles in Figure 1) collected from 4 time points. Monte Carlo simulation was performed to estimate the unbiased confidence intervals (CIs) for indirect effects of asthma control status on asthma-specific HRQOL through nighttime sleep quality and daytime sleepiness. Several studies have reported that Monte Carlo simulation would provide precise CIs when a bootstrap method is not feasible in multilevel context [26, 31, 32]. In this study, Mplus 7.11 (Muthén and Muthén, Los Angeles, CA) was used to perform multilevel path analyses, and SAS 9.3 (SAS Institute, Cary, NC, USA) was used to implement the remaining analyses.
Figure 1.
A framework depicts the associations among asthma control status, sleep problems, and HRQOL at within-subject and between-subject levels
Socio-demographics include the child’s age, gender, race/ethnicity, number of chronic conditions, and the parent’s age, education background and marital status. Index: i indicates the time points (i=1, 2, …, 4), and j indicates the study subjects (j=1, 2, …, 229)
3. RESULTS
3.1. Participant characteristics
Table 1 shows study participants’ characteristics. For children at the baseline of the study in the first year (N=229), the mean age was 12.2 years old (SD: 2.6); 59.0% were boys; 38.0% were non-Hispanic White; and 17.0% had hyperactivity or attention deficit disorder, and 45.0% were overweight. For parents, the mean age was 40.6 years old (SD: 8.7); 51.5% were married; most had an educational background of some college, associate degrees or college degrees (60.2%); and family income was largely between $15,000 and $35,000 (45.5%).
Table 1.
Characteristics of study participants at baseline of the first year (N=229)
| Characteristics | Number of subject (%) or mean (SD) |
|---|---|
| Child’s age in years | 12.24 (2.57) |
| Child’s gender, % | |
| Male | 135 (58.95%) |
| Female | 94 (41.05%) |
| Child’s race/ethnicity, % | |
| White/non-Hispanic | 87 (37.99%) |
| Black/non-Hispanic | 59 (25.76%) |
| Hispanic | 63 (27.51%) |
| Other | 20 (8.73%) |
| Child’s overweight status, % | 95 (45.02%) |
| Most common child’s chronic conditions, % | |
| Attention-deficit/hyperactivity disorder | 39 (17.03%) |
| Premature birth | 26 (11.35%) |
| Mental health conditions such as depression, anxiety, bipolar disorder and other |
7 (3.06%) |
| Epilepsy or other seizure disorders | 6 (2.62%) |
| Inflammatory bowel syndrome, Chron’s disease or other intestinal disorder |
5 (2.18%) |
| Deaf or hard of hearing | 5 (2.18%) |
| Parent’s age in years | 40.60 (8.69) |
| Parent’s race/ethnicity, % | |
| White/non-Hispanic | 97 (42.36%) |
| Black/non-Hispanic | 60 (26.20%) |
| Hispanic | 59 (25.76%) |
| Other | 13 (5.68%) |
| Parent’s education background, % | |
| High school or below | 74 (32.74%) |
| Some college/ technical/associated degree and college degree |
136 (60.18%) |
| Advanced degree | 16 (7.08%) |
| Family income,% | |
| < $14,999 | 47 (20.52%) |
| $15,000– $34,999 | 102 (44.54%) |
| $35,000 –$54,999 | 57 (24.89%) |
| >55,000 | 23 (10.04%) |
| Parent’s marital status,% | |
| Married | 118 (51.53%) |
| Divorced | 45 (19.65%) |
| Living with partner in committed relations | 10 (4.37%) |
| Separated | 9 (3.93%) |
| Other | 40 (14.47%) |
Abbreviation: SD, standard deviation
3.2. Score distribution for the variables of interest
Table 2 shows the results of descriptive analyses for asthma control, nighttime sleep quality, daytime sleepiness, and asthma-specific HRQOL across 4 time points. The scores of these variables were highest at T1, and declined over time. The tests for this trend on the score change over time were indicated by F values, which were significant for nighttime sleep quality (p<0.001), daytime sleepiness (p<0.01), and asthma-specific HRQOL (p<0.01).
Table 2.
Mean and standard deviation of the asthma control status, nighttime sleep quality, daytime sleepiness and asthma-specific HRQOL scores at 4 time points
| T1 (N=218) Mean (SD) |
T2 (N=189) Mean (SD) |
T3 (N=166) Mean (SD) |
T4 (N=158) Mean (SD) |
F-value | |
|---|---|---|---|---|---|
| Asthma control statusa | 16.88 (18.66) | 15.58 (17.78) | 15.30 (19.02) | 13.83 (16.47) | 1.44 |
| Nighttime sleep qualitya | 25.13 (19.35) | 19.46 (16.97) | 19.63 (18.70) | 19.39 (16.78) | 6.55*** |
| Daytime sleepinessa | 45.27 (20.87) | 42.11 (21.44) | 40.40 (21.02) | 39.64 (19.52) | 5.17** |
| Asthma-specific HRQOLa | 48.07 (10.23) | 46.67 (10.04) | 45.47 (10.04) | 44.96 (10.17) | 5.03** |
Abbreviations: SD, standard deviation; HRQOL, health-related quality of life.
Higher scores indicate worse asthma control status, worse nighttime sleep quality, greater daytime sleepiness, and worse asthma-specific HRQOL.
p<0.05;
p<0.01;
p<0.001.
3.3. Bivariate associations
Table 3 shows the bivariate associations of HRQOL with asthma control, nighttime sleep quality, daytime sleepiness and participants’ socio-demographic characteristics, respectively. Data collected from 4 time points were treated as 4 waves of cross-sectional data. Specifically, HRQOL was positively associated with asthma control, nighttime sleep quality, and daytime sleepiness at individual time points (p’s<0.01). Poorer HRQOL was associated with being male children at T4 (p<0.05). Children of parents with a high school education or below were more likely to have impaired asthma-specific HRQOL than those whose parents had a college degree or above in all four models (p’<0.05) at T1 and T2.
Table 3.
Bivariate association of HRQOL with asthma control status, nighttime sleep quality, daytime sleepiness and socio-demographics, respectively, by individual time points
| T1 | T2 | T3 | T4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β (SE) | 95% CI | β (SE) | 95% CI | β (SE) | 95% CI | β (SE) | 95% CI | ||
| Asthma controla | 0.22*** (0.03) |
[0.15, 0.29] | 0.21*** (0.04) |
[0.13, 0.28] | 0.20*** (0.04) |
[0.13, 0.28] | 0.19*** (0.05) |
[0.09, 0.28] | |
| Nighttime sleep qualitya | 0.14*** (0.04) |
[0.07, 0.21] | 0.14** (0.04) |
[0.06, 0.22] | 0.13** (0.04) |
[0.04, 0.21] | 0.16***( 0.05) |
[0.07, 0.25] | |
| Daytime sleepinessa | 0.24*** (0.03) |
[0.18, 0.30] | 0.23*** (0.03) |
[0.17, 0.29] | 0.20*** (0.03) |
[0.13, 0.27] | 0.27*** (0.04) |
[0.20, 0.34] | |
| Child’s age | −0.03 (0.27) |
[−0.56, 0.51] | 0.37 (0.28) |
[−0.18, 0.92] | 0.32 (0.33) |
[−0.33, 0.96] | 0.26 (0.34) |
[−0.41, 0.92] | |
| Child’s gender (Ref: Female) |
|||||||||
| Male | −1.56 (1.40) |
[−4.31, 1.20] | 0.89 (1.48) |
[−2.03, 3.80] | −2.70 (1.56) |
[−5.78, 0.38] | −3.34* (1.61) |
[−6.52, −0.16] | |
| Child’s race/ethnicity (Ref: White) |
|||||||||
| Black | 2.43 (1.76) |
[−1.03, 5.90] | 1.93 (1.87) |
[−1.76, 5.61] | −0.57 (1.97) |
[−4.46, 3.33] | 0.00 (2.03) |
[−4.00, 4.00] | |
| Hispanics | 2.40 (1.72) |
[−0.99, 5.80] | 1.85 (1.83) |
[−1.76, 5.46] | −2.07 (2.01) |
[−6.04, 1.90] | −1.05 (2.15) |
[−5.29, 3.19] | |
| Others | 2.85 (2.66) |
[−2.39, 8.09] | 3.37 (2.72) |
[−2.00, 8.75] | −0.91 (2.78) |
[−6.40, 4.58] | −0.83 (2.85) |
[−6.46, 4.78] | |
| Parent’s age | −0.02 (0.08) |
[−0.18, 0.13] | −0.01 (0.08) |
[−0.17, 0.16] | −0.02 (0.09) |
[−0.19, 0.16] | −0.08 (0.09) |
[−0.25, 0.10] | |
| Marital status (Ref: Married) |
|||||||||
| Not married | 2.13 (1.38) |
[−0.59, 4.84] | 1.75 (1.46) |
[−1.12, 4.63] | 0.38 (1.56) |
[−2.69, 3.46] | 0.60 (1.62) |
[−2.59, 3.80] | |
| Education (Ref: College or above) |
|||||||||
| High school or below | 4.11** (1.46) |
[1.23, 7.00] | 3.35* (1.57) |
[0.24, 6.45] | 1.82 (1.68) |
[−1.50, 5.15] | 2.75 (1.71) |
[−0.63, 6.14] | |
| Smoking status at home (Ref: Not smoking) |
|||||||||
| Smoking at home | −1.72 (1.81) |
[−5.28, 1.83] | −3.10 (1.94) |
[−6.93, 0.72] | −3.39 (2.04) |
[−7.42, 0.64] | −2.07 (2.18) |
[−6.38, 2.24] | |
| Number of chronic conditions | 1.17 (0.87) |
[−0.55, 2.89] | 0.48 (0.91) |
[−1.31, 2.27] | 0.39 (0.98) |
[−1.54, 2.32] | 0.09 (1.00) |
[−1.89, 2.07] | |
Abbreviations: SE, standard error; CI, confidence interval; HRQOL, health-related quality of life.
Higher scores indicate worse asthma control, worse nighttime sleep quality, greater daytime sleepiness, and worse asthma-specific HRQOL.
p<0.05;
p<0.01;
p<0.001.
Bivariate associations of asthma control with nighttime sleep quality, daytime sleepiness, asthma-specific HRQOL, and participants’ socio-demographics were presented in Appendix 2. Asthma control status was positively associated with nighttime sleep quality in individual time points (p’s<0.001). Poorer asthma control was associated with greater daytime sleepiness at T1 and T4 (p’s<0.01). Poorer asthma control was associated with lower asthma-specific HRQOL in individual time points (p’s<0.001). Children with more chronic conditions had poorer asthma control at T1 and T3 than those with fewer chronic conditions (p’s<0.05).
3.4. Multivariate associations
Table 4 shows the associations of asthma-specific HRQOL with asthma control, nighttime sleep quality, daytime sleepiness, and participants’ characteristics estimated by linear random-intercept models. After adjusting for participants’ characteristics, Model 1 reveals that poorer asthma control was significantly associated with lower asthma-specific HRQOL (β: 0.17; p<0.001); Model 2 shows poorer nighttime sleep quality was significantly associated with lower asthma-specific HRQOL (β: 0.12; p<0.001); and Model 3 shows greater daytime sleepiness was significantly associated with lower asthma-specific HRQOL (β: 0.21; p<0.001). When taking asthma control, nighttime sleep quality and daytime sleepiness into consideration (Model 4), only poorer asthma control status (β: 0.14; p<0.001) and greater daytime sleepiness (β: 0.19; p<0.001) were significantly associated with lower asthma-specific HRQOL. Children of parents with a high school education or below were more likely to have impaired asthma-specific HRQOL than those whose parents had a college degree or above in all four models (all p’s<0.05).
Table 4.
Multivariate association of asthma-specific HRQOL with asthma control, nighttime sleep quality, daytime sleepiness, and subject’s socio-demographic characteristics using the random-intercept models
| Model 1 | Model 2 | Model 3 | Model 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β (SE) | 95% CI | β (SE) | 95% CI | β (SE) | 95% CI | β (SE) | 95% CI | ||
| Asthma controla | 0.17*** (0.02) |
[0.13, 0.20] | 0.14*** (0.02) |
[0.11, 0.18] | |||||
| Nighttime sleep qualitya | 0.12*** (0.02) |
[0.08, 0.16] | 0.02 (0.02) |
[−0.02, 0.06] | |||||
| Daytime sleepinessa | 0.21*** (0.02) |
[0.18, 0.24] | 0.19*** (0.02) |
[0.15, 0.22] | |||||
| Child’s age | 0.30 (0.21) |
[−0.12, 0.72] | 0.34 (0.23) |
[−0.11, 0.80] | 0.08 (0.20) |
[−0.31, 0.47] | 0.20 (0.19) |
[−0.18, 0.58] | |
| Child’s gender (Ref: Female) |
|||||||||
| Male | −1.43 (1.04) |
[−3.47, 0.61] | −1.15 (1.11) |
[−3.34, 1.04] | −0.58 (0.96) |
[−2.47, 1.31] | −0.67 (0.93) |
[−2.49, 1.16] | |
| Child’s race/ethnicity (Ref: White) |
|||||||||
| Black | −0.08 (1.34) |
[−2.70, 3.55] | 0.48 (1.43) |
[−2.33, 3.29] | 0.37 (1.23) |
[−2.05, 2.80] | −0.67 (1.19) |
[−3.01, 1.67] | |
| Hispanics | 0.84 (1.30) |
[−1.71, 3.39] | 1.21 (1.40) |
[−1.54, 3.96] | 0.82 (1.20) |
[−1.55, 3.18] | 0.20 (1.17) |
[−2.10, 2.49] | |
| Others | 1.40 (1.92) |
[−2.37, 5.18] | 1.42 (2.04) |
[−2.59, 5.44] | −0.45 (1.80) |
[−3.98, 3.08] | −0.74 (1.70) |
[−4.08, 2.61] | |
| Parent’s age | −0.06 (0.07) |
[−0.19, 0.06] | −0.10 (0.07) |
[−0.24, 0.04] | −0.03 (0.06) |
[−0.15, 0.09] | −0.03 (0.06) |
[−0.15, 0.08] | |
| Marital status (Ref: Married) |
|||||||||
| Not married | 1.30 (1.07) |
[−0.80, 3.40] | 1.02 (1.15) |
[−1.24, 3.28] | 0.47 (0.99) |
[−1.47, 2.41] | 0.35 (0.96) |
[−1.54, 2.24] | |
| Education (Ref: College or above) |
|||||||||
| High school or below | 2.93** (1.12) |
[0.74, 5.12] | 3.13** (1.20) |
[0.78, 5.48] | 2.99** (1.03) |
[0.95, 5.02] | 2.56* (1.00) |
[0.60, 4.52] | |
| Smoking status at home (Ref: Not smoking) |
|||||||||
| Smoking at home | −1.92 (1.36) |
[−4.59, 0.74] | −1.63 (1.46) |
[−4.50, 1.24] | −0.66 (1.27) |
[−3.16, 1.84] | −0.09 (1.22) |
[−2.49, 2.32] | |
| Number of chronic conditions | 0.28 (0.65) |
[−1.00, 1.56] | 0.22 (0.70) |
[−1.16, 1.59] | 0.64 (0.60) |
[−0.54, 1.82] | 0.06 (0.58) |
[−1.08, 1.21] | |
Abbreviations: SE, standard error; CI, confidence interval; HRQOL, health-related quality of life.
Higher scores indicate worse asthma control, worse nighttime sleep quality, greater daytime sleepiness, and worse asthma-specific HRQOL.
p<0.05;
p<0.01;
p<0.001.
3.5. Direct and indirect effects of asthma control on asthma-specific HRQOL
ICCs for the repeated measurements of asthma control, nighttime sleep quality, daytime sleepiness, and asthma-specific HRQOL were 0.21, 0.48, 0.58, and 0.52, respectively. These values were greater than the threshold 0.20, suggesting the existence of clustering effects of the data and the importance of using MSEM approach.
Table 5 shows the estimated coefficients for the variables at both within-subject and between-subject levels in the path analyses based on the MSEM. At the within-subject level, the individual scores of 4 repeated measurements on asthma control, nighttime sleep quality, daytime sleepiness, and asthma-specific HRQOL were used to interpret the relationships among these variables. Results revealed that poorer asthma control status was significantly associated with poorer nighttime sleep quality (β: 0.35; p<0.05), greater daytime sleepiness (β: 0.09; p<0.05), and lower asthma-specific HRQOL (β: 0.12; p<0.05), respectively. Additionally, greater daytime sleepiness was significantly associated with lower asthma-specific HRQOL (β: 0.15, p<0.05). The coefficient, for example 0.15, can be interpreted as an increase of daytime sleepiness by 1 unit, was associated with an increase of asthma-specific HRQOL by 0.15 units. However, poorer nighttime sleep quality was not significantly associated with greater daytime sleepiness (β: 0.09; p>0.05) and lower asthma-specific HRQOL (β: 0.03; p>0.05). The estimated magnitude of the indirect effect of asthma control status on asthma-specific HRQOL was 0.01 through the influence of daytime sleepiness (p<0.05); 0.01 through the influence of nighttime sleep quality (p>0.05); and 0.01 through both nighttime sleep quality and daytime sleepiness (p>0.05). In other words, at the within-subject level, daytime sleepiness rather than nighttime sleep quality significantly explained or mediated the relationship between asthma control status and asthma-specific HRQOL.
Table 5.
The mediating effects of nighttime sleep qualitya and daytime sleepinessa on the association between asthma controla and asthma-specific HRQOLa using the multilevel path analyses+
| Parameter | 95% CI | ||||
|---|---|---|---|---|---|
| β | SE | Lower | Upper | ||
| Within level (within subject) | |||||
| Direct effect | |||||
| Asthma control -> Nighttime sleep quality | 0.35* | 0.04 | 0.28 | 0.42 | |
| Asthma control -> Daytime sleepiness | 0.09* | 0.03 | 0.04 | 0.14 | |
| Asthma control -> Asthma-specific HRQOL | 0.12* | 0.02 | 0.08 | 0.15 | |
| Nighttime sleep quality -> Daytime sleepiness | 0.09 | 0.05 | −0.00 | 0.19 | |
| Nighttime sleep quality -> Asthma-specific HRQOL | 0.03 | 0.03 | −0.01 | 0.08 | |
| Daytime sleepiness -> Asthma-specific HRQOL | 0.15* | 0.03 | 0.11 | 0.20 | |
| Indirect effect | |||||
| Asthma control -> Nighttime sleep quality -> Asthma-specific HRQOL | 0.01 | 0.01 | −0.01 | 0.03 | |
| Asthma control -> Daytime sleepiness -> Asthma-specific HRQOL | 0.01* | 0.01 | 0.00 | 0.02 | |
| Asthma control -> Nighttime sleep quality -> Daytime sleepiness -> Asthma-specific HRQOL | 0.01 | 0.00 | −0.00 | 0.01 | |
| Total indirect effect | 0.03* | 0.01 | 0.00 | 0.05 | |
| Between level (between subjects) | |||||
| Direct effect | |||||
| Asthma control -> Nighttime sleep quality | 1.04* | 0.21 | 0.69 | 1.39 | |
| Asthma control -> Daytime sleepiness | −0.37 | 0.43 | −1.08 | 0.34 | |
| Asthma control -> Asthma-specific HRQOL | 0.72* | 0.23 | 0.35 | 1.10 | |
| Nighttime sleep quality -> Daytime sleepiness | 0.69* | 0.24 | 0.31 | 1.08 | |
| Nighttime sleep quality -> Asthma-specific HRQOL | −0.26 | 0.14 | −0.53 | 0.02 | |
| Daytime sleepiness -> Asthma-specific HRQOL | 0.30* | 0.06 | 0.21 | 0.39 | |
| Indirect effect | |||||
| Asthma control -> Nighttime sleep quality -> Asthma-specific HRQOL | −0.27 | 0.18 | −0.61 | 0.02 | |
| Asthma control -> Daytime sleepiness -> Asthma-specific HRQOL | −0.11 | 0.14 | −0.38 | 0.14 | |
| Asthma control -> Nighttime sleep quality -> Daytime sleepiness -> Asthma-specific HRQOL | 0.22* | 0.11 | 0.06 | 0.44 | |
| Total indirect effect | −0.17 | 0.18 | −0.59 | 0.26 | |
Abbreviations: SE, standard error; CI, confidence interval; HRQOL, health-related quality of life.
Results were adjusted for the child’s age, gender, race/ethnicity, number of chronic conditions, and the parent’s age, education background, and marriage status.
Higher scores indicate worse asthma control, worse nighttime sleep quality, greater daytime sleepiness, and worse asthma-specific HRQOL.
p<0.05.
At the between-subject level, the estimated mean scores of four repeated measurements on asthma control, nighttime sleep quality, daytime sleepiness, and asthma-specific HRQOL were used to interpret the relationships among these variables. Results revealed that poorer asthma control status was significantly associated with poorer nighttime sleep quality (β: 1.04, p<0.05) and lower asthma-specific HRQOL (β: 0.72, p<0.05); however, asthma control was not significantly associated with daytime sleepiness (β: −0.37, p>0.05). Additionally, poorer nighttime sleep quality was significantly associated with greater daytime sleepiness (β: 0.69, p<0.05), but not with asthma-specific HRQOL (β: −0.26, p>0.05). Greater daytime sleepiness was significantly associated with poorer asthma-specific HRQOL (β: 0.30, p<0.05). The estimated magnitude of the indirect effect of asthma control on asthma-specific HRQOL was −0.27 through nighttime sleep quality (p>0.05); −0.01 through daytime sleepiness (p>0.05); and 0.22 through both nighttime sleep quality and daytime sleepiness (p<0.05). In this regard, at the population level, poorer sleep quality and greater daytime sleepiness significantly explained or mediated the association between asthma control and asthma-specific HRQOL.
4. DISCUSSION
We used an innovative MSEM methodology to investigate the complex pathways from asthma control status to HRQOL through the effect of nighttime sleep quality and daytime sleepiness among children with asthma. We found that poorer asthma control status was significantly associated with lower asthma-specific HRQOL at within-subject (or individual) and between-subject (or population) levels. However, the effect of asthma control status on asthma-specific HRQOL was explained through daytime sleepiness at the within-subject level, and through both nighttime sleep quality and daytime sleepiness at the between-subject level. Because asthma control status and nighttime sleep quality were reported prior to daytime sleepiness and asthma-specific HRQOL assessments, the identified pathways could represent causal relationships.
Our findings contribute to the literature by specifying nighttime sleep quality and daytime sleepiness as two important mediators that jointly contribute to the association of asthma control with asthma-specific HRQOL at the population level. Additionally, results derived from the within-subject level indicated that increasing poor asthma control contributed to greater daytime sleepiness, which in turn contributed to poorer asthma-specific HRQOL. Taken together, our results reveal the effect of asthma control status on asthma-specific HRQOL is not straightforward, meaning that the association of worse asthma control with impaired asthma-specific HRQOL is through a dynamic process and multiple aspects of sleep problems in asthmatic children. Literature suggests that asthma status tends to be worse at night due to circadian rhythms and decreased lung function, which leads to more symptoms such as coughing, nocturnal awakenings, and excessive daytime sleepiness [5, 33].
At between-subject level, the beta coefficient of the indirect effect of asthma control on HRQOL through nighttime sleep quality and daytime sleepiness is 0.22, which suggests that the 1 unit change in asthma control is associated with 0.22 unit change of HRQOL. Our recent study has reported that the minimally important difference of PROMIS pediatric measures ranged from 0.2 to 0.3 points on 1 SD metric [34]. Therefore, a small change of asthma control status on average is associated with a significant change of HRQOL that is perceived as meaningful by children and parents.
In this study, 3 items were used to measure nighttime sleep quality (difficulty falling asleep, difficulty getting up, and sleep disturbance). However, the use of this simple, generic measure may not cover the full spectrum of sleep quality, potentially leading to low sensitivity to detect the changes of nighttime sleep quality for children over time. This may explain why the mean nighttime sleep quality and daytime sleepiness mediated the effect of asthma control status on asthma-specific HRQOL at the population level, but the effect of nighttime sleep quality over time was not linked to either daytime sleepiness or asthma-specific HRQOL at the individual level. Using 3 items to assess sleep quality was prone to misclassification of poor sleep quality, and they may not accurately capture the changes of nighttime sleep quality. Future studies should use standard nighttime sleep quality measures to examine the influence of variations in nighttime sleep quality on the asthma control-HRQOL pathway.
Given that nighttime sleep quality and daytime sleepiness are important factors influencing the association of asthma control status with HRQOL, assessing and treating multiple domains of sleep problems may directly benefit children with asthma regarding HRQOL improvement, especially those who have poorer asthma control status. Specifically, physicians could explore asthmatic children’s sleep history and assess nighttime sleep quality and daytime sleepiness on a regular basis. A brief screening on sleep history would be valuable for determining if interventions addressing sleep quality are needed to improve asthmatic children’s HRQOL. Previous studies have reported several therapeutic strategies that effectively improve sleep for children with asthma or other chronic diseases [35–37]. For example, the use of melatonin has significantly reduced sleep latency and the number of awakenings for general children with chronic insomnia [35]. Allergic rhinitis, a common comorbid condition co-occurring with asthma, is also related to sleep problems [38, 39]; therefore, managing rhinitis symptoms and maintaining nasal patency could be an effective way to improve sleep quality for asthmatics with rhinitis [36, 37]. Additionally, cognitive-behavioral therapy such as relaxation and stress reduction strategies could be prescribed for improving sleep quality and reducing sleep disturbance for children and adolescents with insomnia [40, 41]. Future studies need to test the effect of these therapeutic strategies to address sleep problems associated with poor asthma control status.
Very few instruments are available to measure self-reported sleep problems for children and adolescents. A newly developed instrument, the Children’s Report of Sleep Patterns (CRSP), is able to measure the concepts of sleep pattern, sleep hygiene, and sleep disturbances for children [42]. Although our study has established a conceptual framework for testing the influence of nighttime sleep quality and sleepiness on the relationship of asthma control status with HRQOL, future studies are encouraged to apply the CRSP among other instruments to validate our framework by investigating the impact of different aspects of sleep problems on HRQOL among children with asthma.
There are several limitations that should be noted when interpreting the results of this study. First, the study participants were from the Florida Medicaid and the SCHIP, and most of the participants were recruited from families with low economic status. Therefore, our findings may not generalize to other populations. However, this population is vulnerable because they are likely to have the high rate of health disparities due to asthma. Second, multiple allergic factors (e.g., allergic rhinitis) might confound or mediate the relationship between asthma control status and asthma-specific HRQOL. A detailed history of allergies was not collected in this study but should be included in future research. Third, the present study focused on two dimensions of sleep problems and did not include other important sleep parameters such as sleep duration and sleep disordered breathing (SDB). Previous research has found that sleep duration and SDB may influence the relationship between asthma control and HRQOL [43–45]. Further studies need to included other dimensions of sleep problems and accurately quantify children’s sleep duration at night by using objective measures such as actigraphy. Finally, the number of participants used for analyses decreased (28%) from the baseline (T1) to the end of study (T4) (Table 2). We found that there were no significant differences between those who did or did not remain at T4 for analyses in terms of the child’s race/ethnicity, number of chronic conditions, and parental age, educational background, and marital status (all p’s>0.05). However, children who remained at T4 were slightly younger than those did not remain at T4 (p=0.03). Because a child’s age at different time points was not associated with asthma control status, nighttime sleep quality, daytime sleepiness, and HRQOL, we believe the impact of a child’s age on our current findings would be small.
In conclusion, our study found that asthma control status was associated with asthma-specific HRQOL, and this association was explained partly by the influence of nighttime sleep quality and daytime sleepiness. Interventions to address sleep quality and daytime sleepiness may help to minimize the negative effect of poor asthma control on HRQOL in pediatric populations.
Supplementary Material
Acknowledgments
Funding sources:
National Institutes of Health U01 AR052181 (Thompson, Gross, Shenkman, Reeve, DeWalt, Huang) and American Lebanese Syrian Associated Charities (Huang)
PROMIS® was funded with cooperative agreements from the National Institutes of Health (NIH) Common Fund Initiative (Northwestern University, PI: David Cella, PhD, U54AR057951, U01AR052177; Northwestern University, PI: Richard C. Gershon, PhD, U54AR057943; American Institutes for Research, PI: Susan (San) D. Keller, PhD, U54AR057926; State University of New York, Stony Brook, PIs: Joan E. Broderick, PhD and Arthur A. Stone, PhD, U01AR057948, U01AR052170; University of Washington, Seattle, PIs: Heidi M. Crane, MD, MPH, Paul K. Crane, MD, MPH, and Donald L. Patrick, PhD, U01AR057954; University of Washington, Seattle, PI: Dagmar Amtmann, PhD, U01AR052171; University of North Carolina, Chapel Hill, PI: Harry A. Guess, MD, PhD (deceased), Darren A. DeWalt, MD, MPH, U01AR052181; Children’s Hospital of Philadelphia, PI: Christopher B. Forrest, MD, PhD, U01AR057956; Stanford University, PI: James F. Fries, MD, U01AR052158; Boston University, PIs: Alan Jette, PT, PhD, Stephen M. Haley, PhD (deceased), and David Scott Tulsky, PhD (University of Michigan, Ann Arbor), U01AR057929; University of California, Los Angeles, PIs: Dinesh Khanna, MD (University of Michigan, Ann Arbor) and Brennan Spiegel, MD, MSHS, U01AR057936; University of Pittsburgh, PI: Paul A. Pilkonis, PhD, U01AR052155; Georgetown University, PIs: Carol. M. Moinpour, PhD (Fred Hutchinson Cancer Research Center, Seattle) and Arnold L. Potosky, PhD, U01AR057971; Children’s Hospital Medical Center, Cincinnati, PI: Esi M. Morgan DeWitt, MD, MSCE, U01AR057940; University of Maryland, Baltimore, PI: Lisa M. Shulman, MD, U01AR057967; and Duke University, PI: Kevin P. Weinfurt, PhD, U01AR052186). NIH Science Officers on this project have included Deborah Ader, PhD, Vanessa Ameen, MD (deceased), Susan Czajkowski, PhD, Basil Eldadah, MD, PhD, Lawrence Fine, MD, DrPH, Lawrence Fox, MD, PhD, Lynne Haverkos, MD, MPH, Thomas Hilton, PhD, Laura Lee Johnson, PhD, Michael Kozak, PhD, Peter Lyster, PhD, Donald Mattison, MD, Claudia Moy, PhD, Louis Quatrano, PhD, Bryce Reeve, PhD, William Riley, PhD, Peter Scheidt, MD, Ashley Wilder Smith, PhD, MPH, Susana Serrate-Sztein, MD, William Phillip Tonkins, DrPH, Ellen Werner, PhD, Tisha Wiley, PhD, and James Witter, MD, PhD. The contents of this article uses data developed under PROMIS. These contents do not necessarily represent an endorsement by the US Federal Government or PROMIS. See www.nihpromis.org for additional information on the PROMIS® initiative.
Appendix 1: The study design to identify the changed of asthma control status
Appendix 2: Bivariate association of asthma control status with socio-demographics, nighttime sleep quality, daytime sleepiness, and asthma-specific HRQOL, respectively, by individual time points
| Differences in asthma control scores between levels of categorical variables of interest | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||||
| Categorical variables | Mean (SD) |
T- or F- value |
Mean (SD) | T- or F- value |
Mean (SD) | T- or F- value |
Mean (SD) | T- or F- value |
|
| Child’s gender | |||||||||
| Female | 16.54 (18.58) |
−0.23 | 15.00 (16.92) |
−0.39 | 15.35 (17.55) |
−0.02 | 13.75 (16.24) |
−0.05 | |
| Male | 17.13 (18.78) |
16.02 (18.47) |
15.43 (20.18) |
13.89 (16.74) |
|||||
| Child’s race/ethnicity | |||||||||
| White | 14.05 (15.97) |
12.96 (13.05) |
12.54 (16.69) |
13.91 (14.90) |
|||||
| Black | 21.34 (21.03) |
1.98 | 18.13 (20.52) |
0.91 | 20.11 (20.21) |
1.44 | 13.62 (17.90) |
0.01 | |
| Hispanic | 15.75 (18.66) |
16.42 (20.88) |
14.89 (19.70) |
14.10 (15.64) |
|||||
| Other | 20.00 (20.93) |
16.76 (16.20) |
13.53 (20.67) |
13.53 (20.29) |
|||||
| Education | |||||||||
| High school or below | 19.79 (18.91) |
−1.63 | 12.89 (13.06) |
1.52 | 16.76 (16.27) |
−0.69 | 13.53 (14.74) |
0.03 | |
| College degree or above | 15.38 (18.36) |
16.56 (19.37) |
14.55 (19.96) |
13.62 (16.68) |
|||||
| Parent’s marital status | |||||||||
| Married | 14.87 (17.11) |
−1.66 | 14.65 (16.59) |
−0.76 | 13.62 (18.77) |
−1.27 | 12.96 (15.75) |
−0.68 | |
| Others | 19.05 (20.05) |
16.61 (19.05) |
17.37 (19.27) |
14.74 (17.26) |
|||||
| Smoking status at home | |||||||||
| No | 19.23 (18.23) |
23.79 (23.49) |
15.86 (18.23) |
15.19 (18.25) |
|||||
| Yes | 16.37 (18.76) |
0.87 | 13.85 (15.88) |
2.32* | 15.29 (19.27) |
0.15 | 13.56 (16.16) |
0.46 | |
| Correlation of asthma control scores with other continuous variables of interest | ||||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Continuous variables | Correlation coefficient | Correlation coefficient | Correlation coefficient | Correlation coefficient |
| Child’s age in years | 0.04 | −0.06 | −0.10 | −0.15 |
| Number of chronic conditions | 0.14* | 0.14 | 0.26*** | 0.13 |
| Parent’s age in years | −0.06 | 0.00 | −0.08 | −0.09 |
| Nighttime sleep qualitya | 0.51*** | 0.50*** | 0.45*** | 0.51*** |
| Daytime sleepinessa | 0.29*** | 0.12 | −0.03 | 0.20** |
| Asthma-specific HRQOLa | 0.41*** | 0.37*** | 0.38*** | 0.30*** |
Abbreviations: SD, standard deviation; HRQOL, health-related quality of life.
Higher scores indicate worse asthma control, worse nighttime sleep quality, greater daytime sleepiness and worse asthma-specific HRQOL.
p<0.05;
p<0.01;
p<0.001.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
No conflicts of interest to all co-authors.
REFERENCES
- 1.Bloom B, Jones LI, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2012. Vital Health Stat 10. 2013;258:1–81. [PubMed] [Google Scholar]
- 2.Jensen ME, Gibson PG, Collins CE, Hilton JM, Latham-Smith F, Wood LG. Increased sleep latency and reduced sleep duration in children with asthma. Sleep Breath. 2013;17(1):281–287. doi: 10.1007/s11325-012-0687-1. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer LJ, Ullrich M, Szefler SJ. Sleep duration, sleep hygiene, and insomnia in adolescents with asthma. J Allergy Clin Immunol Pract. 2014;2(5):562–569. doi: 10.1016/j.jaip.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chugh IM, Khanna P, Shah A. Nocturnal symptoms and sleep disturbances in clinically stable asthmatic children. Asian Pac J Allergy Immunol. 2006;24(2–3):135–142. [PubMed] [Google Scholar]
- 5.Krouse HJ, Yarandi H, McIntosh J, Cowen C, Selim V. Assessing sleep quality and daytime wakefulness in asthma using wrist actigraphy. J Asthma. 2008;45(5):389–395. doi: 10.1080/02770900801971800. [DOI] [PubMed] [Google Scholar]
- 6.Stores G, Ellis AJ, Wiggs L, Crawford C, Thomson A. Sleep and psychological disturbance in nocturnal asthma. Arch Dis Child. 1998;78(5):413–419. doi: 10.1136/adc.78.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diette GB, Markson L, Skinner EA, Nguyen TT, Algatt-Bergstrom P, Wu AW. Nocturnal asthma in children affects school attendance, school performance, and parents’ work attendance. Arch Pediatr Adolesc Med. 2000;154(9):923–928. doi: 10.1001/archpedi.154.9.923. [DOI] [PubMed] [Google Scholar]
- 8.Strunk RC, Sternberg AL, Bacharier LB, Szefler SJ. Nocturnal awakening caused by asthma in children with mild-to-moderate asthma in the childhood asthma management program. J Allergy Clin Immunol. 2002;110(3):395–403. doi: 10.1067/mai.2002.127433. [DOI] [PubMed] [Google Scholar]
- 9.King MT, Kenny PM, Marks GB. Measures of asthma control and quality of life: longitudinal data provide practical insights into their relative usefulness in different research contexts. Qual Life Res. 2009;18(3):301–312. doi: 10.1007/s11136-009-9448-4. [DOI] [PubMed] [Google Scholar]
- 10.Daniel LC, Boergers J, Kopel SJ, Koinis-Mitchell D. Missed sleep and asthma morbidity in urban children. Ann Allergy Asthma Immunol. 2012;109(1):41–46. doi: 10.1016/j.anai.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Huang IC, Thompson L, Tuli S, Huang SW, Dewalt D, et al. The relationships between asthma control, daytime sleepiness, and quality of life among children with asthma: a path analysis. Sleep Med. 2013;14(7):641–647. doi: 10.1016/j.sleep.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemola S, Ledermann T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One. 2013;8(8):e71292. doi: 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patino CM, Okelo SO, Rand CS, Riekert KA, Krishnan JA, Thompson K, et al. The asthma control and communication instrument: a clinical tool developed for ethnically diverse populations. J Allergy Clin Immunol. 2008;122(5):936, 943.e6. doi: 10.1016/j.jaci.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 15.Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Pietro B. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26(4):455–458. [PubMed] [Google Scholar]
- 16.Walsh TR, Irwin DE, Meier A, Varni JW, DeWalt DA. The use of focus groups in the development of the PROMIS pediatrics item bank. Qual Life Res. 2008;17(5):725–735. doi: 10.1007/s11136-008-9338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin DE, Varni JW, Yeatts K, DeWalt DA. Cognitive interviewing methodology in the development of a pediatric item bank: a patient reported outcomes measurement information system (PROMIS) study. Health Qual Life Outcomes. 2009;7:3. doi: 10.1186/1477-7525-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, et al. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res. 2010;19(4):595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varni JW, Stucky BD, Thissen D, Dewitt EM, Irwin DE, Lai JS, et al. PROMIS pediatric pain interference scale: an item response theory analysis of the pediatric pain item bank. J Pain. 2010;11(11):1109–1119. doi: 10.1016/j.jpain.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thissen D, Varni JW, Stucky BD, Liu Y, Irwin DE, Dewalt DA. Using the PedsQL 3.0 asthma module to obtain scores comparable with those of the PROMIS pediatric asthma impact scale (PAIS) Qual Life Res. 2011;20(9):1497–1505. doi: 10.1007/s11136-011-9874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Centers for Disease Control and Prevention. [Access 10/20/2015];BMI percentile calculator for child and teen english version. http://Nccd.cdc.gov/dnpabmi/calculator.aspx.
- 23.Cano MA, Lam CY, Chen M, Adams CE, Correa-Fernandez V, Stewart DW, et al. Positive smoking outcome expectancies mediate the association between negative affect and smoking urge among women during a quit attempt. Exp Clin Psychopharmacol. 2014;22(4):332–340. doi: 10.1037/a0036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturgeon JA, Zautra AJ, Arewasikporn A. A multilevel structural equation modeling analysis of vulnerabilities and resilience resources influencing affective adaptation to chronic pain. Pain. 2014;155(2):292–298. doi: 10.1016/j.pain.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunkley DM, Ma D, Lee IA, Preacher KJ, Zuroff DC. Advancing complex explanatory conceptualizations of daily negative and positive affect: trigger and maintenance coping action patterns. J Couns Psychol. 2014;61(1):93–109. doi: 10.1037/a0034673. [DOI] [PubMed] [Google Scholar]
- 26.Preacher KJ, Zhang Z, Zyphur MJ. Alternative methods for assessing mediation in multilevel data: the advantages of multilevel SEM. Struct Equ Modeling. 2011;18(2):161–182. [Google Scholar]
- 27.Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods. 2010;15(3):209–233. doi: 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- 28.Caicedo B, Jones K. Investigating neighbourhood effects on health: using community-survey data for developing neighbourhood-related constructs. Rev Salud Publica (Bogota) 2014;16(1):77–89. doi: 10.15446/rsap.v16n1.38665. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q. The impact of ignoring a level of nesting structure in multilevel mixture model: a monte carlo study. SAGE Open. 2012:2158244012442518. [Google Scholar]
- 30.Fenn B, Morris SS, Frost C. Do childhood growth indicators in developing countries cluster? Implications for intervention strategies. Public Health Nutr. 2004;7(7):829–834. doi: 10.1079/phn2004632. [DOI] [PubMed] [Google Scholar]
- 31.Bauer DJ, Preacher KJ, Gil KM. Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: new procedures and recommendations. Psychol Methods. 2006;11(2):142–163. doi: 10.1037/1082-989X.11.2.142. [DOI] [PubMed] [Google Scholar]
- 32.Preacher KJ, James SP. Advantages of Monte Carlo confidence intervals for indirect effects. Commun Methods Meas. 2012;6(2):77–98. [Google Scholar]
- 33.Khan WH, Mohsenin V, D’Ambrosio CM. Sleep in asthma. Clin Chest Med. 2014;35(3):483–493. doi: 10.1016/j.ccm.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Thissen D, Liu Y, Magnus B, Quinn H, Gipson DS, Dampier C, et al. Estimating minimally important difference (MID) in PROMIS pediatric measures using the scale-judgment method. Qual Life Res. 2015:1–11. doi: 10.1007/s11136-015-1058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanenko A, Crabtree VM, Tauman R, Gozal D. Melatonin in children and adolescents with insomnia: a retrospective study. Clin Pediatr (Phila) 2003;42(1):51–58. doi: 10.1177/000992280304200108. [DOI] [PubMed] [Google Scholar]
- 36.Rimmer J, Greenwood A, Bartlett D, Hellgren J. Nasal steroids improve regulation of nasal patency in asthma and mild rhinitis: a randomised, cross-over trial. Eur Arch Otorhinolaryngol. 2012;269(4):1133–1138. doi: 10.1007/s00405-011-1803-8. [DOI] [PubMed] [Google Scholar]
- 37.Profita M, Riccobono L, Bonanno A, Chanez P, Gagliardo R, Montalbano AM, et al. Effect of nebulized beclomethasone on airway inflammation and clinical status of children with allergic asthma and rhinitis: a randomized, double-blind, placebo-controlled study. Int Arch Allergy Immunol. 2013;161(1):53–64. doi: 10.1159/000343137. [DOI] [PubMed] [Google Scholar]
- 38.de Groot EP, Duiverman EJ, Brand PL. Comorbidities of asthma during childhood: possibly important, yet poorly studied. Eur Respir J. 2010;36(3):671–678. doi: 10.1183/09031936.00185709. [DOI] [PubMed] [Google Scholar]
- 39.Gentile D, Bartholow A, Valovirta E, Scadding G, Skoner D. Current and future directions in pediatric allergic rhinitis. J Allergy Clin Immunol Pract. 2013;1(3):214, 26. doi: 10.1016/j.jaip.2013.03.012. quiz 227. [DOI] [PubMed] [Google Scholar]
- 40.Dautovich ND, McNamara J, Williams JM, Cross NJ, McCrae CS. Tackling sleeplessness: psychological treatment options for insomnia. Nat Sci Sleep. 2010;2:23–37. [PMC free article] [PubMed] [Google Scholar]
- 41.Tikotzky L, Sadeh A. The role of cognitive-behavioral therapy in behavioral childhood insomnia. Sleep Med. 2010;11(7):686–691. doi: 10.1016/j.sleep.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Meltzer LJ, Avis KT, Biggs S, Reynolds AC, Crabtree VM, Bevans KB. The children’s report of sleep patterns (CRSP): a self-report measure of sleep for school-aged children. J Clin Sleep Med. 2013;9(3):235–245. doi: 10.5664/jcsm.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosen CL, Palermo TM, Larkin EK, Redline S. Health-related quality of life and sleep-disordered breathing in children. Sleep. 2002;25(6):657–666. [PubMed] [Google Scholar]
- 44.Fagnano M, van Wijngaarden E, Connolly HV, Carno MA, Forbes-Jones E, Halterman JS. Sleep-disordered breathing and behaviors of inner-city children with asthma. Pediatrics. 2009;124(1):218–225. doi: 10.1542/peds.2008-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fagnano M, Bayer AL, Isensee CA, Hernandez T, Halterman JS. Nocturnal asthma symptoms and poor sleep quality among urban school children with asthma. Acad Pediatr. 2011;11(6):493–499. doi: 10.1016/j.acap.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.