Abstract
Objective/Background
Circadian rhythm sleep-wake disorders often manifest during the adolescent years. Measurement of circadian phase such as the Dim Light Melatonin Onset (DLMO) improves diagnosis and treatment of these disorders, but financial and time costs limit the use of DLMO phase assessments in clinic. The current analysis aims to inform a cost-effective and efficient protocol to measure the DLMO in older adolescents by reducing the number of samples and total sampling duration.
Patients/Methods
A total of 66 healthy adolescents (26 males) aged 14.8 to 17.8 years participated in a study in which sleep was fixed for one week before they came to the laboratory for saliva collection in dim light (<20 lux). Two partial 6-h salivary melatonin profiles were derived for each participant. Both profiles began 5 h before bedtime and ended 1 h after bedtime, but one profile was derived from samples taken every 30 mins (13 samples) and the other from samples taken every 60 mins (7 samples). Three standard thresholds (first 3 melatonin values mean + 2 SDs, 3 pg/mL, and 4 pg/mL) were used to compute the DLMO. Agreement between DLMOs derived from 30-min and 60-min sampling rates was determined using a Bland-Altman analysis; agreement between sampling rate DLMOs was defined as ± 1 h.
Results and Conclusions
Within a 6-h sampling window, 60-min sampling provided DLMO estimates that were within ± 1 h of DLMO from 30-min sampling, but only when an absolute threshold (3 pg/mL or 4 pg/mL) was used to compute the DLMO. Future analyses should be extended to include adolescents with circadian rhythm sleep-wake disorders.
Keywords: dim light melatonin onset, DLMO, circadian, adolescent, sampling rate, circadian rhythm sleep-wake disorder
1. Introduction
Melatonin, a neurohormone that is secreted by the pineal gland, is commonly used to estimate the timing (phase) of the central circadian clock when measured in dim light [1]. Melatonin has a distinct circadian pattern; in a healthy nocturnal-sleeping individual, circulating melatonin concentration is low during the waking day, shows a distinct rise about 1 to 3 hours before bedtime, remains high throughout sleep, and decreases close to wake-up time. The onset (dim light melatonin onset, DLMO), offset (dim light melatonin offset, DLMOff), and midpoint are often used to mark the phase of the endogenous melatonin rhythm. Several factors make melatonin phase markers more appealing than other outputs of the central clock. First, phase estimates from the melatonin rhythm are less variable and more reliable than phase measures from other circadian outputs, like core body temperature and cortisol [2, 3]. Furthermore, the melatonin rhythm is less prone to masking by behavior (e.g., sleep), and if measured from saliva samples, is also less invasive than the procedure necessary to measure core body temperature (wearing a rectal probe or swallowing a capsule). Finally, it is not necessary to measure melatonin over 24 h or even overnight to determine a reliable phase marker; collecting salivary melatonin around habitual bedtime is usually adequate to estimate the DLMO. Therefore, many researchers measure the DLMO to collect a reliable, non-invasive circadian phase marker.
Measuring the DLMO significantly improves diagnosis and treatment of circadian rhythm sleep-wake disorders [4–6] and the ICSD-3 encourages DLMO measurement in diagnosing these disorders [7]. Part of the challenge facing sleep clinics attempting to measure DLMO phase is the financial and time expense. Melatonin assays can be costly (~$12 per sample in the US) and while sampling can be limited to the evening, it still must be done over the course of several hours. Reducing the number of samples collected can reduce cost (but not time). One previous study in healthy adults showed that 60-min sampling (17 samples) provides adequate estimates of the DLMO when compared to 30-min sampling (33 samples) [8]. Another study of adults with and without sleep complaints showed that DLMO estimates derived from fitting a curve to 11 well-placed saliva samples over 20 h (“sparse-sampling schedule”) were within 20 minutes of DLMOs computed using 24 saliva samples collected hourly [9]. Both of these studies, however, examined melatonin profiles derived from 16 to 20 h of sampling which is impractical for a clinical setting. Another study of patients with suspected circadian rhythm sleep-wake disorders (CRSWDs) collected hourly samples within a narrow 5-h sampling window, but failed to capture almost a quarter of the DLMOs [10]. These failures may have resulted from the investigators setting the sampling window relative to age and not based on habitual sleep/wake times; the latter has been shown to predict circadian phase well in adults [11, 12] and adolescents [13]. These concerns raise the need for a comprehensive analysis of DLMO estimates using fewer samples over a well-timed, narrow sampling window.
Adolescents may be particularly vulnerable to CRSWDs as this is the age when symptoms of Delayed Sleep-Wake Phase Disorder (DSPD) often begin to emerge [14, 15]. Sleep timing shifts later [16–19] in healthy adolescents, driven in part by homeostatic and circadian rhythm changes that have been linked to puberty [20–22]. Whether DSPD is an exaggeration of this normal developmental sleep regulatory processes or whether it has another etiological pathway is unknown. In either case, efficient and cost-effective estimates of phase in adolescents are needed to diagnose a possible underlying circadian problem and to provide an accurate temporal context for administering timed treatment, such as bright light [23–26] to shift the system in the desired direction.
The current analysis aims to inform the development of a cost-effective, efficient, and standardized protocol to measure the dim light melatonin onset (DLMO) in older adolescents. We sampled saliva within a 6-h sampling window timed relative to bedtime and examined whether 60-min sampling provided a similar estimate of the DLMO as 30-min sampling in this age group. Three standard threshold methods were used to compute the DLMO for both sampling rates. We also describe cases of missed or potentially spurious DLMO estimates when melatonin is sampled within a 6-h sampling window.
2. Method
2.1 Participants
Participants and a parent responded to study advertisements in the community (e.g., information sessions, flyers, post cards, etc.). After a preliminary phone interview, the child and at least one parent (usually the mother) visited the laboratory for a tour of the facility and to complete questionnaires to determine eligibility.
A total of 66 adolescents (26 males) aged 14.8 to 17.8 years (mean ± SD = 16.1 ± 0.7 years) participated in one of two studies from which baseline data were analyzed. Thirty-four participants completed a study during the school year (October to May; “school-year study”); some of these data have been previously reported [27]. Thirty-two participants were enrolled in a study during summer months (June, July, and August; “summer study”). For both studies, a parent reported on a questionnaire that their adolescent was healthy and without a personal history of a sleep disorder, psychotic disorder, bipolar disorder, or neurological disorder, and that their child had no diagnosis of any chronic medical conditions or developmental disorder. Participants and their parent reported that participants were medication-free, except for one female participant in the summer study who was taking an oral contraceptive. Participants did not endorse depressive symptoms as indicated by a score of 16 or lower on the Center for Epidemiologic Studies – Depression (CES-D) scale [28]. Participants did not travel more than 2 time zones within the month before starting the study. Participants reported their usual sleep duration was between 6 and 10 hours. Circadian phase preference was measured with the Morningness Questionnaire of Smith and colleagues [29]; morning types (≥ 44) and evening types (≤ 20) were defined by 2 SDs above and below the mean of a larger separate sample of older adolescents (n=148). The majority of participants (n=62) were neither types, three were morning types, and one was an evening type. Participants completed the study based on their availability in groups of one to six.
These studies were approved by their respective Institutional Review Boards (Brown University and Lifespan for the school-year study and Rush University Medical Center for the summer study) and were performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki. A parent of the participant was present during the consenting process and provided written consent for their child to participate in the study, and study participants co-signed the consent forms to acknowledge their assent to participate. Participants were paid for participating in the studies.
2.2 Study Protocols
Study protocols were 2 or 4 weeks, and both studies required that participants sleep at home on a fixed baseline sleep schedule during the first week of the study before collecting salivary melatonin in the laboratory. Only this first baseline melatonin profile was included in the current analysis. Sleep schedules for both studies were assigned based on habitual sleep times just prior to participation in the study. Participants in the summer study were given a 9-hour sleep opportunity, while those in the school-year study were given a 7.5-hour sleep opportunity during this baseline week. Sleep duration differed between the studies to reflect an adolescent’s typical sleep duration during the school year and during summer vacation. Compliance to the baseline sleep schedule was monitored by wrist actigraphs (Actiwatch Spectrum, Mini-Mitter, Bend, OR , USA and Octagonal Basic; Ambulatory Monitoring, Ardsley, NY, USA) worn on the non-dominant wrist, sleep diaries, and daily timestamped telephone messages to the laboratory at bedtime and wake time. Actigraph records and sleep diaries were reviewed with participants 2 to 3 times during the baseline week to clarify inconsistencies and ensure compliance with the protocol. If participants were not compliant with the sleep schedule, then they were dropped from the study.
Following a week of baseline sleep at home, participants came to the laboratory for the saliva collection procedure. Saliva sampling in the school-year study occurred across 6 hours, starting 5 h before scheduled bedtime and ending 1 h after scheduled bedtime. Bedtimes ranged from 22:00 to 23:30; therefore, the earliest sampling window was 17:00 to 23:00 and the latest sampling window was 18:30 to 00:30. In the summer study, sampling occurred from 15:00 to 03:30 for everyone; however, the present analysis used samples taken in the same 6-h window as the school-year study, i.e., 5 h before to 1 h after scheduled bedtime. Bedtimes for the summer study ranged from 21:00 to 02:00; therefore, the earliest sampling window was 16:00 to 22:00 and the latest was 21:00 to 03:00. Non-steroidal anti-inflammatory drugs (NSAIDs), alcohol, nicotine, and recreational drugs were prohibited throughout both studies. Chocolate and caffeine were prohibited in the 72 h before and during saliva collection. Participants self-reported abstaining from these substances when they arrived to the laboratory for the saliva collection procedure. Bananas were not consumed during saliva collection to avoid cross-reactivity with the melatonin assay. Approximately 2 mL of saliva was collected every 30 minutes in dim light (< 20 lux) using Salivettes (Sarstedt, Newton, NC, USA) over the 6-h sampling window. Participants were seated for at least 5 minutes before and during each saliva sample. Saliva samples were immediately centrifuged after collection and frozen. These samples were later radioimmunoassayed (RIA) for melatonin concentration using commercially available kits (Alpco, Salem NH, USA). An individual’s samples were analyzed in the same batch. The intra-assay coefficients of variation for low (evening) and high (nighttime) levels of salivary melatonin were 4.1% and 4.8%, respectively. The inter-assay coefficients of variation for low and high levels of salivary melatonin were 6.6% and 8.4%, respectively. The functional least detectable dose of the assay (minimum salivary melatonin concentration measured with an intra-assay coefficient of variation of less than 10%) was 0.9 pg/mL.
2.3 Data Analysis
We examined 2 melatonin profiles for each participant in this analysis; both profiles began 5 h before scheduled bedtime and ended 1 h after scheduled bedtime. The difference between these profiles was the sampling interval: one profile was derived from samples taken every 30 minutes (“30-min sampling profile”; 13 samples total) and the other from samples taken every 60 minutes (“60-min sampling profile”; 7 samples total). The 60-min sampling-rate profile was obtained from the 30-min melatonin profile by removing every other sample beginning 4.5 hours before habitual bedtime.
Two of the most frequently-used and objective methods to compute the DLMO from a partial melatonin profile are (1) an absolute threshold and (2) a threshold calculated at 2 SDs above low baseline (daytime) values [30]. The absolute threshold most often used for plasma melatonin is 10 pg/mL based on the original work of Lewy and colleagues [1]. Because salivary melatonin levels are approximately 30% [31] to 40% [32] of plasma levels, many use the absolute thresholds of 3 or 4 pg/mL to determine salivary DLMO. Carskadon and colleagues were the first to propose a 4 pg/mL threshold for determining salivary DLMO in adolescents [22]. For each sampling rate profile, the DLMO was calculated using 3 threshold methods: a fixed 3 pg/mL threshold, a fixed 4 pg/mL threshold, and a relative threshold (“2 SD”). The 2SD threshold method uses the average of the first three melatonin data points plus two standard deviations of the same three points; this is similar to the threshold proposed by Voultsios and colleagues [31]. For all threshold methods, the DLMO was operationally defined as the time point that melatonin values exceeded the threshold value and remained above the threshold. Linear interpolation of the times between the melatonin points immediately below and above the threshold value was used to calculate the precise DLMO clock time.
2.4 Statistical Analysis
A Bland-Altman analysis [33] was used to determine agreement between the DLMOs derived from the two sampling rates (30-min and 60-min) for each threshold method. This method is more appropriate than computing a correlation coefficient because a significant correlation indicates that the two estimates are associated, but does not necessarily indicate that the two methods of measuring the DLMO agree. Thus, a high correlation may obscure lack of agreement between DLMO estimates [33]. Data were first examined using a Bland-Altman plot, in which the mean of the 30-min and 60-min DLMOs (estimate of the true value) was plotted on the x-axis and the difference between the two DLMOs derived from each sampling frequency (measurement error) was plotted on the y-axis. Bias estimates (the average difference between sampling rates) were compared to 0 using a one-sample t-test. Multiple linear regressions were computed to determine whether bias estimates were systematically varying by mean clock time of the DLMO, the study (school-year vs. summer), sex, or race (African American or other race)1. We defined a priori our agreement measure for DLMO estimates from each sampling profile as 95% Limits of Agreement (average difference ± 1.96*SD of the difference) within ± 1 h because 1 h is the lowest sampling rate in the current analysis.
3. Results
3.1 Agreement between DLMOs derived from 30-min and 60-min sampling melatonin profiles
Of the 66 participants included in the analysis, DLMO was estimated for both the 30-min and 60-min sampling-rate profiles for 64 participants (97%) when using the 2 SD threshold and for 60 participants (91%) when using the 4 pg/mL threshold or the 3 pg/mL threshold. We were unable to compute the 60-min sampling rate DLMO for 2 participants using the 2 SD threshold and for 6 participants using the 4 pg/mL and 3 pg/mL thresholds (Table 1; see section 3.2 below). Average (SD) DLMOs for each sampling rate profile and threshold method are listed in Table 1.
Table 1.
Mean ± SD DLMO and number of DLMOs missed for each sampling rate and threshold method.
| 30-min sampling profile | DLMO threshold method
|
||
|---|---|---|---|
| 2 SD | 4 pg/mL | 3 pg/mL | |
| DLMO (hh:mm ± mins) | 20:47 ± 75 | 21:30 ± 77 | 21:17 ± 75 |
| melatonin levels < threshold (N) | -- | 4 | 3 |
| melatonin levels > threshold (N) | -- | 1 | 2 |
| Total Missed (N; % total sample1) | 0 (0%) | 5 (8%) | 5 (8%) |
|
| |||
| 60-min sampling profile | |||
|
| |||
| DLMO (hh:mm ± mins) | 20:53 ± 63 | 21:28 ± 79 | 21:14 ± 77 |
| melatonin levels < threshold (N) | 2 | 5 | 4 |
| melatonin levels > threshold (N) | -- | 1 | 2 |
| Total Missed (N;% of total sample1) | 2 (3%) | 6 (9%) | 6 (9%) |
N=66
DLMO estimates using 30-min and 60-min sampling rates were significantly correlated for all 3 threshold methods examined; however, the correlation for the 2SD method (r = 0.87, p < .001) was weaker than the correlations for both absolute threshold methods (r’s = 0.99, p’s < .001; z = 7.13, p < .05).
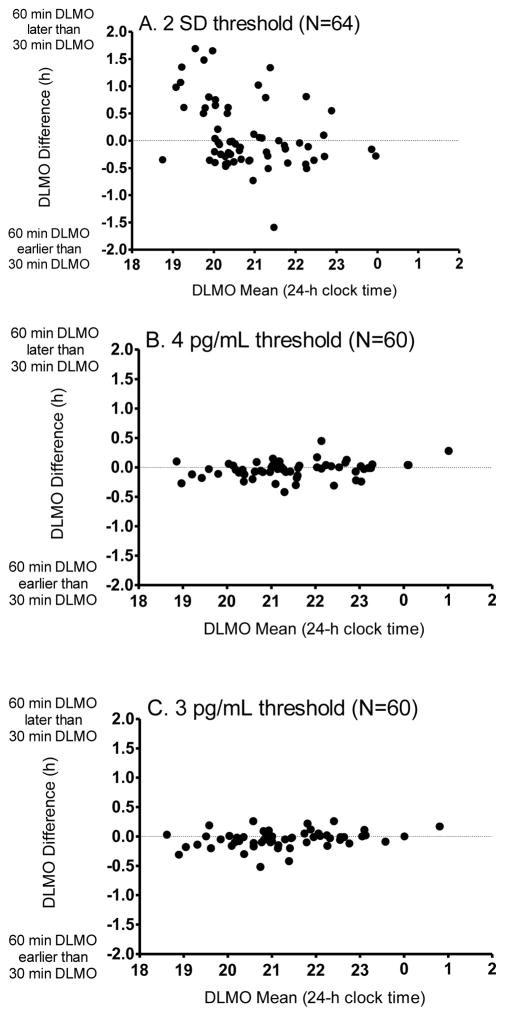
Bland-Altman plots for each threshold method are illustrated in Figure 1. DLMO estimates computed using the 2 SD method for both sampling profiles differed most from one another, as indicated by frequent and large deviations from the “true” estimate (Figure 1A). The overall difference between sampling rates using the 2SD threshold did not show a statistically significant bias (bias estimate = 6 ± 38 min; t(63)=1.3, p=.20); however, a negative linear association (R = −.37, p = .01) indicated that 60-min DLMOs were later than 30-min DLMOs for individuals with early DLMOs. This finding was accounted for by the group of adolescents who were studied during the school year (B=0.34, p =.03) and not by sex or race. The difference between DLMO measurements using the two sampling rates and the 2SD threshold method was estimated between −1.13 and 1.34 h (95% Limits of Agreement), which extends beyond our a priori criterion of 1 h. A total of 8 out of 64 (13%) DLMO estimates differed by more than 1 h. Therefore, with a narrow 6-h sampling window in the evening and a 2 SD threshold method, DLMOs derived using a 60-min sampling rate can show substantial disagreement with a DLMO derived from a 30-min sampling rate.
Figure 1.
Bland-Altman plots for the 2 SD threshold (A), 4 pg/mL absolute threshold (B), and the 3 pg/mL absolute threshold (C). For each plot, the mean of the 30-min and 60-min DLMOs (estimate of the true DLMO value) is identified on the x-axis. The difference between the two DLMOs derived from each sampling rate is shown on the y-axis. Sample sizes included in the analyses are indicated for each threshold method.
DLMO differences between the two sampling rates were less variable using an absolute threshold (4 pg/mL or 3 pg/mL; see Figure 1 B and C). Although the bias estimate was small (−2 ± 8 min) for the 4 pg/mL method, it was different from 0 min [t(59)=1.98, p=.05], indicating that the 60-min DLMO was systematically earlier than the 30-min DLMO. A positive association (R=0.26, p=.04) indicated that this bias is more likely to occur when DLMOs were early during the 6-h window. DLMO deviations did not differ by sex, race, or school status. The difference between measurements using these two sampling rates and the 4 pg/mL threshold method lay between −0.32 and 0.25 h (95% Limits of Agreement) which is within our a priori criterion of 1 h. Similar results were seen for the 3 pg/mL absolute threshold. Therefore, either of these absolute thresholds applied to samples collected in a 6-h sampling window in the evening achieve reliably similar DLMOs from a 60-min sampling rate compared to a 30-min sampling rate.
3.2 Missed and Potentially Spurious DLMOs
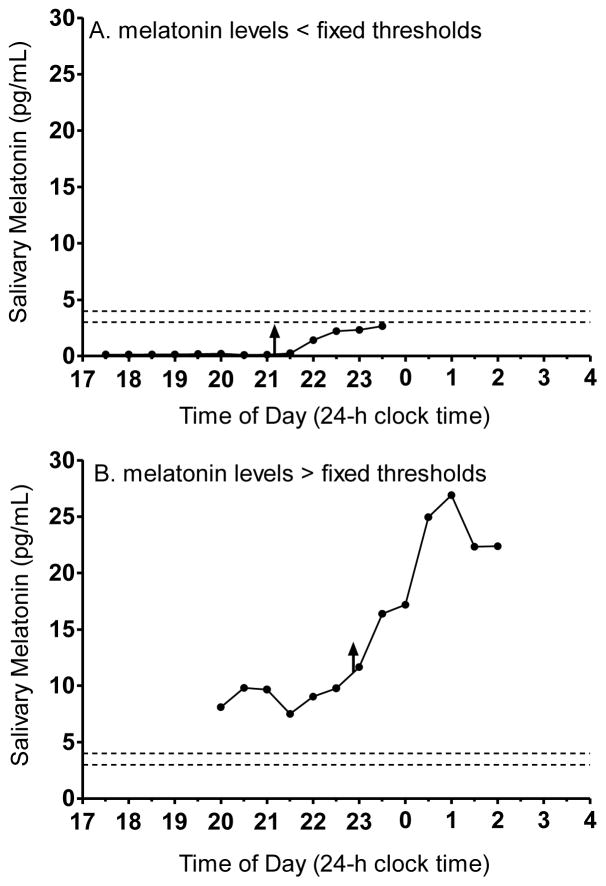
We were unable to compute the DLMOs in 0 to 9% of the 6-h melatonin profiles, depending on the sampling rate and the threshold method (see Table 1). DLMOs missed in the 30-min sampling profile were the same participants for whom DLMO was missed in the 60-min sampling profile. Also, DLMOs missed using the 3 pg/mL threshold method also were missed using the 4 pg/mL threshold method. The number of missed DLMOs was roughly equal for males and females. DLMOs were missed when melatonin values did not reach the threshold or if melatonin values started higher than the threshold. Figure 2 illustrates an example of each scenario where we were unable to compute the DLMO using the 3 pg/mL and 4 pg/mL thresholds from the 30-min sampling profile. The first scenario, in which all melatonin levels were less than the threshold (Figure 2A), occurred most often for participants in the school-year study. Sampling ended 1 h after schedule bedtime; thus, melatonin onset as defined by an absolute threshold was likely more than 1 h after scheduled school-year bedtime in these few adolescents. Instances in which all melatonin values were above the threshold (Figure 2B) only occurred for participants in the summer study. It is possible that these youngsters were either low melatonin secretors (Figure 2A) or high melatonin secretors (Figure 2B); however, without more data it is difficult to determine whether these absolute thresholds are inappropriate for these individuals or if the melatonin onset was not captured in the selected sampling window.
Figure 2.
Examples of 30-minute sampling melatonin profiles in which the DLMO was missed when using the fixed threshold methods, 3 pg/mL and 4 pg/mL. Each threshold is indicated by the dashed horizontal lines. A: DLMO was not computed because melatonin values were lower than both fixed thresholds. B: DLMO was not computed because melatonin values were higher than both fixed thresholds. The upward facing arrows indicate the DLMOs computed using the 2 SD threshold approach (21:09 for the profile in A and 22:48 for the profile in B).
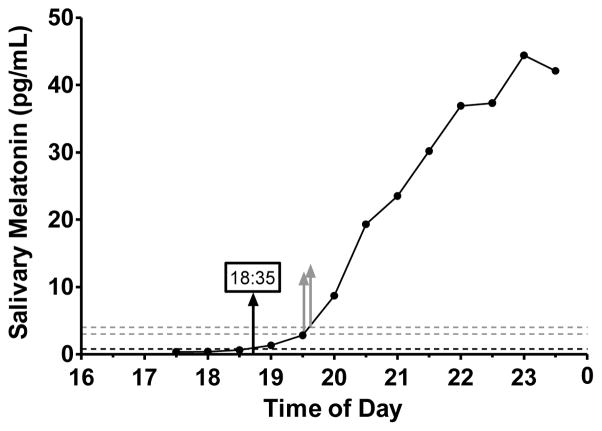
The 2 SD threshold method yielded a DLMO for all 30-min sampling profiles and for all except two 60-min sampling profiles. The 2 SD threshold values ranged from 0.16 to 17.18 pg/mL (median = 1.11, IQR = 1.50 pg/mL) for the 30-min sampling profiles and 0.17 to 37.10 pg/mL (median = 1.28, IQR = 2.30 pg/mL) for the 60-min sampling profiles. As seen by these ranges, a number of 2 SD thresholds were quite low. Of the 66 participants included in the analysis, almost half (n=30) had 2 SD thresholds lower than the functional sensitivity (<0.9 pg/mL) of the assay in the 30-min sampling condition. A low threshold may result in an early and perhaps spurious DLMO estimate that does not indicate the distinct rise of the melatonin rhythm. In Figure 3, for example, the rise occurred after 19:00 when we visually inspect the profile, yet the 2 SD DLMO was computed at 18:35. To determine the frequency in which DLMOs may be too early using the 2 SD method, we computed the slope between the interpolating points (e.g., 18:30 and 19:00 in Figure 3) and the slope of the entire profile. DLMOs were defined as too early if the slope of the line connecting the points bracketing the threshold was less than the slope of the entire profile. Of the 30 participants with low (<0.9 pg/mL) thresholds using 30-min sampling, 20 DLMOs were categorized as too early. Two authors (SJC and TAM) visually inspected these 20 profiles and agreed that 18 computed DLMOs were earlier than the distinct rise of the melatonin rhythm. Twenty-three 60-min sampling profiles had 2 SD thresholds less than 0.9 pg/mL and 10 DLMOs were defined as too early using a descriptive comparison of slopes as described above. Visual inspection confirmed that 9 out of 10 DLMOs were earlier than the distinct rise of melatonin rhythm.
Figure 3.
Example of a melatonin profile in which the 2 SD threshold (0.8 pg/mL) is lower than the functional sensitivity (<0.9 pg/mL) of the melatonin assay. The DLMO estimate (black upward facing arrow) appears earlier than the rise of the melatonin. Gray dashed lines mark the 3 pg/mL and 4 pg/mL thresholds and gray upward-facing arrows indicate the DLMO derived from the 3 pg/mL (DLMO=19:31) and 4 pg/mL (DLMO=19:36) thresholds.
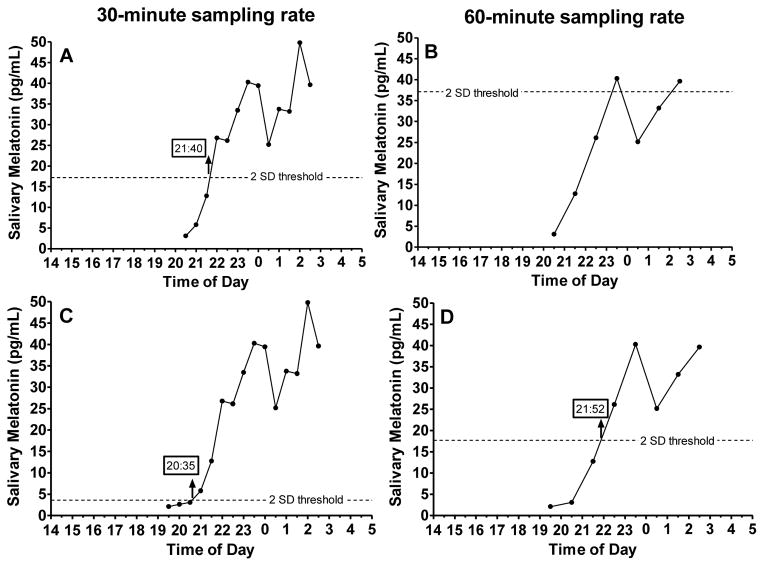
In addition to low thresholds, several 2 SD thresholds were considered outliers (> 75th percentile + 1.5 x IQR). These outliers occurred when the first 3 melatonin values were high, variable, or both. We identified three 30-min sampling profiles with 2 SD thresholds that were outliers (thresholds > 5.9 pg/mL) and nine 60-min sampling profiles with 2 SD thresholds that were outliers (thresholds > 6.2 pg/mL). Figure 4A illustrates an example of one participant’s 30-min sampling profile, in which the calculated threshold was 17.2 pg/mL. In this case, the threshold was not derived from low daytime levels, which was the original intention of this threshold method [31]. When only hourly samples were available (Figure 4B), the first 3 points of this profile were higher and more variable than in the 30-min sampling profile, producing such a high threshold that the DLMO could not be accurately determined from the 60-min samples. High thresholds run the risk of computing a DLMO that is later than actual melatonin onset.
Figure 4.
Example of a 30-minute (A) and a 60-minute (B) 6-h sampling profile in which the 2SD thresholds were outliers. All profiles in this figure are from the same individual who participated in the summer study with a scheduled bedtime of 01:30. The 2 SD threshold was 17.2 pg/mL in the 30-minute sampling profile and 37.1 pg/mL in the 60-minute sampling profile. By adding to the 30-min (C) and 60-min (D) profiles samples acquired 1 h earlier, we illustrate that low daytime levels were missed by our 6-h sampling window and adding one hour to the sampling window can change the 2SD threshold and therefore the DLMO estimate. DLMOs computed from the available samples are indicated by upward-facing arrows with times provided in the box.
Participants in the summer study provided more samples than the analyzed 6-h window, which provided the opportunity to determine whether a longer sampling window would change the DLMO estimate compared to the 6-h window. Sampling began at 15:00 and the earliest scheduled bedtime was 21:00 in the summer study. Therefore, the sampling window could be extended 1 h earlier to begin 6 h before bedtime for all summer study participants. The last sample remained at 1 h after bedtime, making the longer sampling window 7 h. In a post-hoc analysis, we compared DLMOs computed from the original 6-h sampling window to DLMOs computed from the extended 7-h sampling window in the summer group only (n=32). We computed DLMO’s using the 2SD threshold only because adding more samples would not change the 3 and 4 pg/mL absolute thresholds and therefore would not change the DLMO estimates. Agreement between the DLMOs computed from the 6-h and 7-h sampling windows were within our 1-h criterion for the 30-min sampling rate (95% Limits of Agreement: −0.88 to 0.89 h), but were not within our 1-h criterion for the 60-min sampling rate (95% Limits of Agreement: −1.45 to 1.02 h). Of the 32 participants included in this sub-analysis, the DLMO changed by more than 1 h for three 30-minute profiles (9%) when we used a 7-h sampling window and the 2SD threshold. The DLMO changed by one or more hours in seven 60-min sampling profiles (22%) when we used the 7-h sampling window and the 2SD threshold. Figure 4C and 4D illustrate an example of how the threshold and therefore DLMO changed when the sampling window was lengthened by one hour. When we reintroduced the early samples in the 30-min (Figure 4C) sampling profiles, the 2 SD threshold decreased to 3.6 pg/mL, resulting in an earlier DLMO than when computed from the 6-h sampling window (Figure 4A). The 2SD threshold also decreased in the 60-min sampling profile (Figure 4D); however, the difference in DLMO estimates between the two sampling rates remained large (77 minutes). Therefore, adding one hour to the sampling window can change the DLMO estimate by more than 1 h when using the 2SD threshold method.
4. Discussion
The aim of the current analysis was to inform development of a cost-effective and efficient method to measure accurate circadian phase using salivary melatonin in older adolescents. We tested agreement between 30-min and 60-min sampling rates when samples were collected within a narrow 6-h window timed relative to habitual sleep. The number of samples was substantially lower (7 vs. 13) in the 60-min sampling protocol, and therefore could reduce assay costs. Our analysis indicates that within a 6-h sampling window beginning 5 h before habitual bedtime, 60-min sampling will provide a similar estimate of the melatonin onset phase as 30-min sampling, but only when an absolute threshold (3 pg/mL or 4 pg/mL) is used to compute the DLMO. Our descriptive analysis also indicates that it may not be possible to compute the DLMO in a small number of youngsters using this approach and these absolute thresholds. Nevertheless, we feel confident using the fixed threshold method because, although the 2 SD threshold computed a DLMO for all but two profiles, this method produced spurious results with a 6-h partial melatonin profile.
A similar analysis completed in adults [8] also showed that 60-min sampling produced results similar to 30-min sampling DLMOs when samples were collected over 16 h beginning 6 h before bedtime. Similar to the current analysis, DLMO estimates were more variable using the 2 SD threshold compared to a fixed 3 pg/mL threshold. In that study, the 60-min sampling profile DLMO differed from the 30-min sampling profile by an average of only 8 ± 25 min using the 2 SD threshold; however, the maximum difference between the two sampling rate DLMOs was 2 h, and 7 participants (~6% of the sample) showed differences of more than 1 h. Of note, 13 participants (~11% of the sample) were omitted from their analysis because they did not have six low daytime melatonin levels necessary to compute the 2SD DLMO in both the 30-min and the 60-min sampling profiles (similar to the participant depicted in Figure 4 A and B). Removing these participants from the set likely reduced the variability and differences that may otherwise have been seen between sampling rates using the 2 SD method. When a fixed 3 pg/mL threshold was used to compute the DLMO in that study, differences between the DLMOs using a 60-min and a 30-min sampling rate averaged 6 ± 11 min, and the maximum difference between the DLMOs computed from the two sampling rates was 1.1 h. One participant showed a difference of more than 1 h when the fixed 3 pg/mL threshold was used, and 3 participants (2.5% of the sample) were omitted because values did not reach threshold. This previous study in adults and our current analysis suggest that 60-min sampling provides as accurate an estimate of the DLMO as 30-min sampling. Our analysis, however, indicated that applying the 2 SD threshold method can substantially impact agreement between DLMO estimates derived from a 30-min and 60-min sampling rate. Therefore, unlike the study completed in adults [8], we would recommend caution when using the 2 SD threshold to compute the DLMO from a partial melatonin profile.
Several methods to compute the DLMO have been reported, providing a challenge to know which method is most appropriate. In a consensus report, Benloucif and colleagues [30] suggested that when feasible, a low threshold (2 SD or < 3 pg/mL) should be reported to facilitate DLMO comparisons between partial and overnight melatonin profiles across studies, but also indicated that there was limited evidence to support any one low-threshold method. It could be argued from the current analysis that that the 2 SD method may compute spurious phase estimates when only 6 h of melatonin data are available. Therefore, it is critical to plot and visually inspect melatonin profiles for spurious results (e.g., due to a threshold that is too low like in Figure 3 or a threshold that is too high like in Figure 4 A and B) and not rely on automated computations of the DLMO. Given the large variability in DLMO estimates and potential for spurious results using the 2 SD method found in the current analysis, we recommend including a fixed (3 pg/mL or 4 pg/mL) threshold when computing DLMO from a partial melatonin profile to facilitate comparison between studies. A 4 pg/mL threshold may be preferred for studies of children and adolescents as most previous studies that collected salivary melatonin in this age group use this threshold (e.g., [13, 22, 37–40]).
Our data came from a 6-h sampling window that began 5 h before scheduled bedtime. In our previous study of adolescents, we found that self-reported sleep times predict DLMO to within ± 2 hours of the measured DLMO [13]. We used the equations derived from these regression models to time the melatonin sampling window for these participants. This 6-h sampling window ending 1 h after bedtime is feasible for a young person and their family to complete successfully. Of course, missing the rise of melatonin is a risk with such a narrow time frame, particularly among individuals in whom a circadian rhythm sleep-wake disorder is suspected. Keijzer and colleagues, for example, collected hourly salivary melatonin samples in patients with suspected CRSWDs (~42% were children) using a 5-h sampling window and missed almost 25% [10], perhaps because sampling was timed based on age (e.g., 19:00-00:00 for children aged 6 to 12 years, 20:00-01:00 for adolescents aged 13–15 years, and 21:00-02:00 for patients 16 and older) and not relative to habitual sleep times. Alternatively, the 5-h window may be too narrow and needed to be extended slightly. Using the 4 pg/mL threshold, Keijzer and colleagues missed approximately 11.5% of DLMOs because values were too low (< 3 pg/mL) and 7.4 % because values started higher than the threshold. (Another 5% were missed due to unexplained curve fluctuation or insufficient samples.) In our analysis with a sampling window of only 1 h longer and timed relative to bedtime, our rate of misses using the 4 pg/mL threshold was descriptively smaller (7.8% and 1.5%, respectively). Most of our misses were due to values not reaching threshold, which suggests that extending the window to 2 h after bedtime may help capture more DLMOs, though this comes at the expenses of time, sampling cost, and patient burden. It is also possible that some of these youngsters were low melatonin secretors and extending the sampling window would not help. Future analyses may consider a systematic examination of sampling window duration and timing relative to sleep to determine the most efficient and high-yielding protocol to determine DLMO phase in adolescents presenting with sleep problems.
The current analysis is not without limitations. Melatonin profiles examined in this study were collected from healthy normally-sleeping adolescents who kept a strict sleep-wake schedule for a week before DLMO was measured and not a clinical sample. Patients with sleep/wake complaints may show greater variability in DLMO timing and therefore may require a wider sampling window to capture the onset of melatonin. In this instance, a cost-saving option may be to collect saliva samples over a longer time period, but assay the samples taken 5 h before to 1 h after habitual bedtime to determine whether the DLMO can be captured. The extra samples could be kept on reserve and assayed only if needed. Also, the 60-min sampling melatonin profile was derived from the 30-min sampling profile because this was a secondary analysis of previous datasets. It remains unknown whether collecting saliva samples at different sampling rates on separate evenings would provide similar results. Females were slightly over-represented in this secondary data analysis (~60% female); thus, repeating this analysis with a more equal distribution of males and females may be warranted. Despite these limitations, these data start to inform a cost-effective and efficient method to measure circadian phase for clinical purposes, which can help in diagnosis and treatment of sleep/wake scheduling disorders common during the adolescent years.
HIGHLIGHTS.
Compared 30-min and 60-min melatonin sampling rates to compute DLMO in adolescents
Sampled over a 6-h interval to reduce time cost and increase feasibility
Sampling rate did not impact DLMO estimates when we applied a fixed threshold
Informs a cost-effective and efficient method to measure DLMO for clinical use
Acknowledgments
We are grateful to Isaac Gross, Jacqueline Muñoz, Marissa Dziepak, Brock Peiffer, Amy Feehan, Julia Kleinhenz, Asantewaa Ture, Michael Steinert, Devon Langston, Haein Sung, Victoria Tomaka, Anna Ishikawa, Ieva Misiunaite, Neha Singla, Andrew Kalweit, Athina Bouroukas, Chelsea Fournier, James Farrell, and Jazmin Garcia for their assistance with data collection for these studies. We would also like to acknowledge the participants and their families for their dedication and cooperation while participating in these research projects. This work was supported by grants from Apollo Health, Inc., the National Institute of Mental Health (F31 MH078662), and the National Heart Lung and Blood Institute (R01 HL105395) awarded to S.J.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Mental Health (NIMH) or the National Heart Lung and Blood Institute (NHLBI). Apollo Health, Inc., NIMH and NHLBI had no involvement in designing the study, data collection, data analysis, and interpretation, writing of the manuscript, or in the decision to submit the manuscript for publication. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Footnotes
We examine race because recent studies indicate that African Americans have a shorter intrinsic circadian period compared to other races [34–36], and this difference that may impact the phase relationship between DLMO and sleep timing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christina Suh, Email: suhzee11@gmail.com.
Thomas A. Molina, Email: Thomas_A_Molina@rush.edu.
Louis F. Fogg, Email: Louis_Fogg@rush.edu.
Katherine M. Sharkey, Email: katherine_sharkey@brown.edu.
Mary A. Carskadon, Email: Mary_A_Carskadon@Brown.edu.
References
- 1.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker of circadian phase position. J Biol Rhythms. 1999;14:227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 2.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–93. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 3.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–88. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 4.Lockley SW. Timed melatonin treatment for delayed sleep phase syndrome: the importance of knowing circadian phase. Sleep. 2005;28:1214–6. doi: 10.1093/sleep/28.10.1214. [DOI] [PubMed] [Google Scholar]
- 5.Rahman SA, Kayumov L, Tchmoutina EA, Shapiro CM. Clinical efficacy of dim light melatonin onset testing in diagnosing delayed sleep phase syndrome. Sleep Med. 2009;10:549–55. doi: 10.1016/j.sleep.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Keijzer H, Smits MG, Duffy JF, Curfs LM. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev. 2014;18:333–9. doi: 10.1016/j.smrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 7.The International Classification of Sleep Disorders-3: Diagnostic and Coding Manual. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 8.Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int. 2011;28:714–8. doi: 10.3109/07420528.2011.597531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Someren EJ, Nagtegaal E. Improving melatonin circadian phase estimates. Sleep Med. 2007;8:590–601. doi: 10.1016/j.sleep.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Keijzer H, Smits MG, Peeters T, Looman CW, Endenburg SC, Gunnewiek JM. Evaluation of salivary melatonin measurements for Dim Light Melatonin Onset calculations in patients with possible sleep-wake rhythm disorders. Clin Chim Acta. 2011;412:1616–20. doi: 10.1016/j.cca.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- 12.Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14:229–37. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley SJ, Acebo C, Fallone G, Carskadon MA. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. 2006;29:1632–41. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- 14.Weitzman ED, Czeisler CA, Coleman RM, Spielman AJ, Zimmerman JC, Dement WC. Delayed sleep phase syndrome: a chronobiological disorder with sleep-onset insomnia. Archives of General Psychiatry. 1981;38:737–46. doi: 10.1001/archpsyc.1981.01780320017001. [DOI] [PubMed] [Google Scholar]
- 15.Thorpy MJ, Korman E, Spielman AJ, Glovinsky PB. Delayed sleep phase syndrome in adolescents. Journal of Adolescent Health Care. 1988;9:22–7. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 16.Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–6. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- 17.Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the Transition to Adolescence: A Longitudinal Study. Sleep. 2009;32:1602–9. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–12. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Crowley SJ, Van Reen E, LeBourgeois MK, Acebo C, Tarokh L, Seifer R, et al. A Longitudinal Assessment of Sleep Timing, Circadian Phase, and Phase Angle of Entrainment across Human Adolescence. PLoS One. 2014;9:e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 21.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Ann NY Acad Sci. 2004;1021:276–91. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- 22.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–89. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- 23.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 24.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(3):945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1h pulse of bright white light. J Physiol. 2012;590:3035–45. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Revell VL, Molina TA, Eastman CI. Human phase response curve to intermittent blue light using a commercially available device. J Physiol. 2012;590:4859–68. doi: 10.1113/jphysiol.2012.235416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27:1469–92. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 29.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 30.Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 32.Deacon SJ, Arendt J. Phase-shifts in melatonin, 6-sulphatoxymelatonin and alertness rhythms after treatment with moderately bright light at night. Clinical Endocrinology. 1994;40:413–20. doi: 10.1111/j.1365-2265.1994.tb03940.x. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb;8:307–10. [PubMed] [Google Scholar]
- 34.Smith MR, Burgess HJ, Fogg LF, Eastman CI. Racial differences in the human endogenous circadian period. PLoS One. 2009;4:e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eastman CI, Molina TA, Dziepak ME, Smith MR. Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans) Chronobiol Int. 2012;29:1072–7. doi: 10.3109/07420528.2012.700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eastman CI, Suh C, Tomaka VA, Crowley SJ. Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci Rep. 2015;5:8381. doi: 10.1038/srep08381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–81. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 38.Lebourgeois MK, Carskadon MA, Akacem LD, Simpkin CT, Wright KP, Jr, Achermann P, et al. Circadian phase and its relationship to nighttime sleep in toddlers. J Biol Rhythms. 2013;28:322–31. doi: 10.1177/0748730413506543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased sensitivity of the circadian system to light in early/mid puberty. J Clin Endocrinol Metab. 2015:jc20152775. doi: 10.1210/jc.2015-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Figueiro MG, Rea MS. Evening daylight may cause adolescents to sleep less in spring than in winter. Chronobiol Int. 2010;27:1242–58. doi: 10.3109/07420528.2010.487965. [DOI] [PMC free article] [PubMed] [Google Scholar]