Abstract
Objective
Venous thromboembolism frequently results in thrombi formation near or within the pocket of a venous valve as the result of recirculating hemodynamics, which has been largely attributed to hypoxia-induced tissue factor (TF) expression. Numerical models are now capable of assessing the spatiotemporal behavior of the TF initiated coagulation cascade under non-uniform hemodynamics. The aim of this study was to use such a numerical simulation to analyze the degree and location of thrombin formation with respect to tissue factor position in the presence of disturbed flow induced by an open venous valve.
Method
Thrombin formation was simulated using a computational model that captures the hemodynamics, kinetics and chemical transport of 22 biochemical species. Disturbed flow is described by the presence of a valve in the equilibrium phase of the valve cycle with leaflets in a fully open position. Three different positions of TF downstream of the valve opening were investigated.
Results
The critical amount of TF required to initiate a thrombotic response is reduced by up to 80% when positioned underneath the recirculating regions near the valve opening. Additionally, due to the increased surface area of the open valve cusp, in conjunction with recirculating hemodynamics, it was observed that thrombin is generated inside the valve pocket even when the exposed region of TF is downstream of the valve.
Conclusions
The presence of pro-thrombotic surface reactions, in conjunction with recirculating hemodynamics, provides an additional mechanism for thrombus formation in venous valves, which does not require direct damage or dysfunction to the valve itself.
Introduction
In accordance with Virchow’s Triad, three factors contribute to venous thromboembolism (VTE), including hemodynamics, blood composition, and vessel wall injury. Injury to the vessel wall occurs when the endothelial layer is damaged or activated leading to expression of tissue factor (TF), which can initiate the coagulation cascade.1 The endothelium is particularly susceptible to activation at valvular sinuses where vortices can promote recirculating areas of relative stasis2, aggregation of red blood cells3, and regions of hypoxia that can lead to P-selectin expression with a concomitant increase in local TF expression through a number of mechanisms.4–11 This increase in TF expression may partly explain why a majority of venous thrombi occur within the pockets of venous valves. However, there may be additional mechanisms that relate to the unique mass transport conditions that result from recirculating flow.
Thrombin generation occurs on the surface of blood vessels where hemodynamics can significantly affect chemical transport and, as a result, reaction rates. Significantly, in the venous system, valves produce vortices near and inside the valvular pocket, altering the chemical transport within this region. Although it has been speculated that secondary vortices lead to the creation of potential stagnation zones with the accumulation of prothrombotic substances, such as thrombin, this supposition has been difficult to study experimentally. Due to the complex nature of the clotting cascade, numerical models have been developed in an effort to understand the relationship between clotting factors and clot formation.12–18 While some of these numerical models include blood flow, none have considered disturbed flow, which is likely a critical component in the development of VTE, as venous valves induce eddies that both lead to the induction of TF and increase the residence time of locally produced clotting factors.19–21 In this report, we expand upon a recently described numerical model that highlights the significance of surface reactions18, 22 through the incorporation of disturbed flow induced by an open venous valve in the equilibrium phase of the valve cycle. We analyzed the degree and location of thrombin formation with respect to TF position and compare these results to the case of non-disturbed flow, when a valve is not present.
Methods
Numerical Model
Thrombin generation initiated by surface-bound TF under specified flow conditions is modeled using the finite element method, implemented in COMSOL Multiphysics 4.3a. Chemical and flow parameters are governed by partial differential equations with initial conditions specified by initial factor/inhibitor concentrations and desired shear rates. Chemical transport is captured in standard convection-diffusion equations with boundary conditions determined by the chemical kinetics at each surface. The numerical model considers up to 60 minutes of reaction time.
Chemical reaction scheme
The numerical model employed considers 22 biochemical reactions and 44 distinct chemical species and has been described in detail elsewhere.22 In short, the presence of surface-bound TF initiates the cascade by reversibly binding to activated factor VIIa, forming extrinsic tenase. Extrinsic tenase activates factor X and IX, where activated factor Xa activates factors VII and V. Surface species prothrombinase is formed by the binding of activated factors Xa and Va. Prothrombinase, in turn, activates factors VII, as well as factor II (prothrombin) producing thrombin. Production of thrombin initiates a positive feedback loop with activation of factors VIII and V, which then allows for the formation of surface-bound intrinsic tenase. Intrinsic tenase amplifies the cascade by activating factor X, even if TF is no longer present. Thrombin formation is inhibited by the presence of activated protein C, antithrombin III (ATIII), tissue factor pathway inhibitor, and surface bound thrombomodulin (TM). This numerical model includes surface binding events, as key drivers of the cascade. Specifically, extrinsic tenase, intrinsic tenase, prothrombinase and TM are all considered as surface bound species.
Initial conditions
Initially, the concentration of all activated compounds are set to zero with the exception of factor VIIa, which is set to 1% of the non-activated concentration.23 The remaining plasma species were set to mean physiological levels.24 It has been shown that TM surface density is enhanced in the valvular sinus endothelium over that expressed in the vein luminal endothelium10, however quantifying this surface density is challenging. As an approximation, the surface density of surface bound TM was set at 1000 fmol/cm2, as prior studies have demonstrated that at this level the generation of activated protein C is transport limited.18, 22 This estimation ensures that the inhibition of thrombin formation will not be limited by TM expression. A range of surface bound TF densities were considered from ~0.1 to 2 fmol/cm2.
Flow profile
Flow profiles are represented by incompressible Navier-Stokes equations with inlet profiles based on a venous shear rate of 50 s−1. At a venous shear of 50 s−1, blood flow is in a transition region between Newtonian and non-Newtonian flow.25 While non-Newtonian effects may be present, particularly in the region of the valvular pocket, using the Newtonian equations simplifies the model while providing a close approximation of the chemical behavior of the coagulation cascade in the presence of vortices.
Venous structure
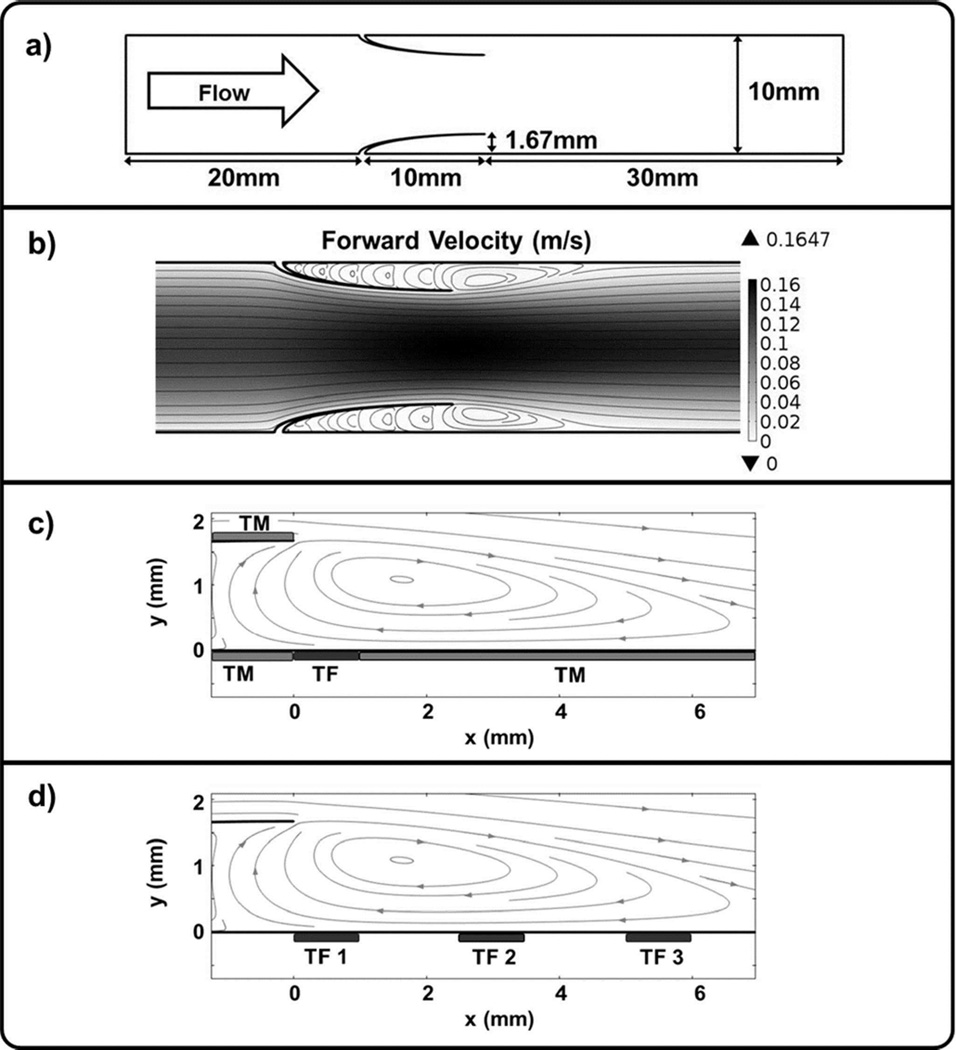
In this model, blood flows from left to right through a 10 mm diameter vein past an open valve (Figure 1A, B). The valve is comprised of two cusps creating pockets that are 10 mm deep with an opening extending 1/6 of the total vein diameter.26 The simulation considers 20 mm upstream and 30 mm downstream of the venous valve, for a total of 60 mm of simulated vessel length. The movement of venous valve leaflets is complex and can be delineated into four distinct phases of the valve cycle including opening, equilibrium, closing, and closed.20 Dynamic models of valve behavior have been developed that use fluid-structure interaction modeling.27–29 However, adding the non-linear behavior of chemical kinetics and mass transport necessary to accurately describe the coagulation cascade to models of this type would be computationally prohibitive. Thus, due to the complexity of capturing the structural dynamics of the valve cycle along with changes in hemodynamic flow patterns and thrombin formation, a simplified approach was elected for this initial investigation. Specifically, the selected model system only considers the valve in the equilibrium phase of the valve cycle with leaflets in a fully open position. Of note, a dynamic model that includes the case of a closing valve would have greater regions of low shear stress and stagnation in the valve pocket, leading to enhanced rates of thrombin formation.18, 30, 31 Therefore, the selected model provides a conservative estimate of thrombin formation in valvular regions. These limitations aside, we believe the approach described herein provides a useful starting point for coupling venous valve hemodynamics and the chemical mechanism of the coagulation cascade.
Figure 1. Valve Flow Model.
a) Model geometry. b) Forward velocity (m/s) near valve pockets with grey streamlines indicating flow direction. A large vortex forms at each valve opening with smaller counter-vortices developing inside the valve pockets. c) The main vortex passes over a region of TF, as well as adjacent regions of TM. d) Three different TF locations, 1 mm in length, were considered at 0, 2.5, and 5 mm downstream from the valve opening.
Surface sites of tissue factor and thrombomodulin expression
A 1 mm region of TF is placed downstream of the valve opening along the bottom wall (Figure 1C). TM, a cofactor that is expressed on endothelial cells, is placed to the left and right of the TF region extending to the end of the simulated region downstream, as well as inside the valve cusp. In order to examine the impact of TF location, three different TF positions were investigated: 0, 2.5 and 5 mm downstream from the valve opening (Figure 1D). In each case, the region of TM begins immediately adjacent to the site of TF.
Results
Flow profile
The simulated flow profile is axially symmetric with parabolic flow upstream and downstream from the valve opening (Figure 1B). Fluid velocity increases as the vessel diameter decreases with the open valve. As fluid exits the valve, counter-rotating vortices develop inside the valve pockets and extend approximately 5 mm downstream, which is consistent with experimental studies.20, 32, 33 As noted below, the presence of counter-rotating vortices provides a chemical transport mechanism that allows for prothrombotic species to enter the valve pocket.
Impact of TF surface density
TF surface density determines whether or not the coagulation cascade will propagate. Once a threshold concentration of thrombin is produced, a positive feedback loop facilitates the propagation of the cascade. We have previously demonstrated that shear rate influences the TF surface density required to generate a threshold concentration of thrombin that is sufficient to propagate the cascade.18, 22 This observation supports the notion that mass transport has an important impact on the sensitivity of a given hemodynamic environment to generate thrombin induced by surface-bound TF. Each of the TF positions considered in these studies experiences different mass transport conditions, as a consequence of the large vortex near the valve opening (Figure 1D).
The relationship between the location and density of surface-bound TF and maximum thrombin concentration, measured immediately distal or upstream of the TF/TM interface just within the valve pocket, was evaluated in the presence and absence of a model valve (Figure 1D, Figure 2). Positions 1 and 3, which represent the distal and proximal regions of the vortex, are the most sensitive, requiring the least amount of TF to promote thrombin generation. However, all three cases with disturbed flow were more sensitive than the case where a valve is not present. A thrombin concentration of 10 nM, which is approximately 1% of the maximum thrombin concentration that can be generated, leads to significant platelet activation and has often been cited as a threshold level for physiologically significant thrombin production.34 Given this threshold, 4- to 6-fold less surface-bound TF is required to exceed this threshold than that required in the absence of disturbed flow.
Figure 2. Thrombin Generation.
a) Maximum thrombin (nM) at the leftmost TF/TM interface for varying TF surface density levels. Three different positions of the 1 mm TF region were investigated (Figure 1) and compared with the results from the non-disturbed model. TM density is 1000 fmol/cm2 and coagulation factors/inhibitors were set to mean physiological levels. The initial shear rate at the inlet is 50 s−1. Position 1, at the valve opening, results in the most sensitive thrombin response, requiring the least amount of TF. In the case of Position 1, TF = 0.24 fmol/cm2 is the TF level just below the thrombin threshold point of 10 nM. b) Thrombin generation at the TF/TM interface (x = 0, y = 0) for a 0.3 fmol/cm2 TF surface density at Position 1 over 60 min. Thrombin levels peak around 30 min and then begin to decrease due to inhibition. c) Thrombin generation over the entire area for a 0.3 fmol/cm2 TF surface density at Position 1. A burst of thrombin generation occurs at approximately 30 min and then slowly tapers off.
Thrombin generation over time
We evaluated thrombin production in response to surface bound TF located in the immediate vicinity of the valve opening over a 60 minute period in order to account for coagulation cascade initiation, propagation, and the onset of inhibition (Figure 2B). Thrombin concentration slowly increases within the first 25 minutes of the initiation of the simulation and once a thrombin concentration of ~ 100 nM is reached, thrombin production increases rapidly reaching a maximum at 30 minutes, at which time inhibitory reactions limit further thrombin generation and thrombin concentration decreases.
Location of thrombin generation
The spatial distribution of thrombin concentration in response to surface bound TF located in the immediate vicinity of the valve opening was evaluated over a 60 minute period (Figure 2C). During the first 20 minutes of the simulation little thrombin is present (< 14 nM), but after 30 minutes there is burst of thrombin generation (700 nM), which extends towards and inside the valve pocket. Thrombin is distributed not only along the vein wall, but also within the inside of the valve cusp. The counter-rotating vortices draw activated coagulation compounds inside the pocket and up towards the cusp. Thrombin generation along both the valve wall and cusp is possible. Even though the simulation did not explicitly incorporate surface-bound TF in these locations, thrombin is present due to the local generation of intrinsic tenase at these sites, a surface-bound species.
Thrombin concentrations are highest near surfaces due to the presence of surface-bound extrinsic and intrinsic tenase and prothrombinase. The steady state thrombin concentrations at the vein wall and valve cusp surfaces was evaluated after a 60 minute simulation period (Figure 3). When TF surface density was selected at a level below a surface density threshold required for maximal thrombin generation, only small amounts of thrombin are generated along the vein wall near the valve opening (x = 0 mm), with negligible amounts along the valve cusp (Figure 3A). Regardless of which TF position is considered, above a threshold of TF surface density that leads to maximal thrombin generation, high thrombin concentrations are observed within the valve pocket, both along the vein wall and the valve cusp (Figure 3 B–D). Despite the position of sites of surface bound TF near the distal apex of the main vortex, 5 mm downstream of the valve opening, retrograde flow is sufficient to transport key coagulation elements to within the valve pocket, further propagating the cascade.
Figure 3. Thrombin vs TF Position.
Thrombin generation occurs predominantly along surfaces both along the vein wall (y = 0) and along the inside of the valve cusp (see Figure 2). TM density was set to 1000 fmol/cm2 and coagulation factors/inhibitors were set to mean physiological levels. The initial shear rate at the inlet is 50 s−1. Thrombin levels are reported along the wall at t = 60 min. a) Position 1 with TF below cutoff, b) Position 1 with TF above cutoff, c) Position 2 with TF above cutoff, and d) Position 3 with TF above cutoff. Despite the TF region occurring away from the valve opening for Position 2 and 3, thrombin is still formed in significant quantities inside the valve pocket, on both the vein wall and valve cusp.
Discussion
Most venous thrombi that are unrelated to trauma arise in the valves of the calf veins, typically in the deepest recess of the valve sinus, as documented by both autopsy35–38 and venography39–42 studies. Moreover, there is a direct correlation between the frequency of venous thromboemolism and the number of lower extremity venous valves.43 The presence of valves inherently alters the local venous hemodynamics, where open valves lead to disturbed flow with eddies forming at the valve opening. Karino and Motomiya33 visualized eddy formations using model particles flowing through a two-leaflet valve in a dog saphenous vein. It was revealed that large vortices form near the opening with a second set of smaller counter-rotating vortices deep within the valve pockets. The flow profile simulated in this report demonstrates similar behavior, with alternating vortices throughout the valve pocket.
Simulated results suggest that the degree of thrombin formation is impacted by the location of surface bound TF relative to these vortices and, significantly, TF need not be located within the deepest recess of the vein valve itself. Indeed, valve hemodynamics may enhance the sensitivity of surface induced thrombin formation, even when the site of expressed TF is located outside the anatomic region of the valve. In particular, the coagulation cascade was most sensitive to TF expression when the site of expressed TF was nearest the apices of the largest vortex, at regions of very low shear. Shear rate has been shown to impact coagulation in both computational18, 30, 31 and experimental in vitro studies44, 45, where low shear rate enhances thrombin production. Lower shear rates near positions of surface bound TF retain key procoagulants near the reactive surface, increasing the local residence time for critical chemical interactions, leading to enhanced thrombin formation.
In addition to valve induced eddies, the open valve provides supplemental surface area for key reactions to occur. After sufficient thrombin is formed along the vein wall, thrombin generation is initiated inside the valve along the valve cusp. As products of the coagulation cascade move and recirculate with the flow vortex, they are able to react with the surface of the valve cusp and form intrinsic tenase and prothrombinase, which can then propagate the cascade along this new surface. Nonetheless, during the first 30 minutes of this simulation relatively low levels of thrombin were generated in response to a TF induced trigger. This suggests that a sufficient lag time may exist for effective inhibition of an impending deep venous thrombosis by circulating site-targeted anti-thrombotic agents. This could provide a surveillance strategy for use of anti-thrombotic agents that circulate at a low systemic concentration, minimizing bleeding risk, but which display the capacity to locally concentrate at sites of thrombin generation. The application of a numerical model that accurately emphasizes the formation and reaction of surface species was essential to these findings.
Conclusions
Several known factors enhance the susceptibility of venous valves to thrombus formation, including lower oxygen tension, low blood flow, and aggregation of red blood cells. Simulated coagulation results confirms the notion that local hemodynamics also lead to a system that is significantly more sensitive to the initiation of thrombin formation by surface bound TF. Expression of surface bound TF at or near the apex of a valvular vortex significantly reduces the amount of TF required to initiate thrombin production, as compared TF located within a region of non-disturbed blood flow. Furthermore, in all cases where expression exceeds a threshold TF surface density and is located distal or upstream from the main vortex, significant amounts of thrombin are formed within all exposed surfaces of the valve pocket. In each position considered, the site of TF was located outside the valve pocket, with the furthest position 5 mm away from the opening. These investigations suggest that thrombus formation within the venous valve pocket does not require direct damage or dysfunction to the valve itself. While these conclusions have not been independently supported by experimental data, we hope that these studies will motivate further biological investigations that aid in the design of implantable blood-contacting devices.
Clinical Relevance.
The mechanisms by which venous valve flow dynamics promotes thrombin generation, suggested by these numerical results, may aid ongoing efforts directed at the development of venous implants and artificial venous valves. Moreover, the lag time associated with the generation of a thrombin burst in the venous circulation supports the use of site-targeted anti-thrombotic agents that circulate at a low systemic concentration, minimizing bleeding risk, but which display the capacity to locally concentrate at sites of thrombin generation.
Acknowledgements
This work was supported by grants from the National Institutes of Health (DK06927, HL106018 and HL56819).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
E.V.D. and E.L.C. jointly conceived the project. Computational modeling was carried out by E.V.D. All authors analyzed the data and contributed to the preparation of the manuscript. E.V.D. current position is in the Mathematical Modeling and Analysis Group at MIT Lincoln Labs, Lexington, MA.
References
- 1.Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Ann Rev Physiol. 2011;73:515–525. doi: 10.1146/annurev-physiol-042210-121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122(7):2331. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musallam K, Porter J, Sfeir P, Tamim H, Richards T, Lotta L, et al. Raised haematocrit concentration and the risk of death and vascular complications after major surgery. British J Surg. 2013;100(8):1030–1036. doi: 10.1002/bjs.9176. [DOI] [PubMed] [Google Scholar]
- 4.Lawson C, Yan S, Yan SF, Liao H, Zhou YS, Sobel J, et al. Monocytes and tissue factor promote thrombosis in a murine model of oxygen deprivation. J Clin Invest. 1997;99(7):1729. doi: 10.1172/JCI119337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101(3):439–451. [PubMed] [Google Scholar]
- 6.Closse C, Seigneur M, Renard M, Pruvost A, Dumain P, Belloc F, et al. Influence of hypoxia and hypoxia-reoxygenation on endothelial P-selectin expression. Thrombosis Res. 1997;85(2):159–164. doi: 10.1016/s0049-3848(96)00233-2. [DOI] [PubMed] [Google Scholar]
- 7.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197(11):1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell–derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206(9):1913–1927. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bovill EG, van der Vliet A. Venous valvular stasis-associated hypoxia and thrombosis: What is the link? Ann Rev Physiol. 2011;73:527–545. doi: 10.1146/annurev-physiol-012110-142305. [DOI] [PubMed] [Google Scholar]
- 10.Brooks EG, Trotman W, Wadsworth MP, Taatjes DJ, Evans MF, Ittleman FP, et al. Valves of the deep venous system: An overlooked risk factor. Blood. 2009;114(6):1276–1279. doi: 10.1182/blood-2009-03-209981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamer J, Malone P, Silver I. The PO2 in venous valve pockets: its possible bearing on thrombogenesis. British J Surg. 1981;68(3):166–170. doi: 10.1002/bjs.1800680308. [DOI] [PubMed] [Google Scholar]
- 12.Kastrup CJ, Runyon MK, Shen F, Ismagilov RF. Modular chemical mechanism predicts spatiotemporal dynamics of initiation in the complex network of hemostasis. Proc Natl Acad Sci U S A. 2006;103(43):15747–15752. doi: 10.1073/pnas.0605560103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277(21):18322–18333. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 14.Jones KC, Mann KG. A model for the tissue factor pathway to thrombin. II. A mathematical simulation. J Biol Chem. 1994;269(37):23367–23373. [PubMed] [Google Scholar]
- 15.Zarnitsina V, Pokhilko A, Ataullakhanov F. A mathematical model for the spatio-temporal dynamics of intrinsic pathway of blood coagulation. I. The model description. Thrombosis Res. 1996;84(4):225–236. doi: 10.1016/s0049-3848(96)00182-x. [DOI] [PubMed] [Google Scholar]
- 16.Zarnitsina V, Pokhilko A, Ataullakhanov F. A mathematical model for the spatio-temporal dynamics of intrinsic pathway of blood coagulation. II. Results. Thrombosis Res. 1996;84(5):333–344. doi: 10.1016/s0049-3848(96)00197-1. [DOI] [PubMed] [Google Scholar]
- 17.Fogelson AL, Tania N. Coagulation under flow: The influence of flow-mediated transport on the initiation and inhibition of coagulation. Pathophysiol Haemostasis Thrombosis. 2005;34(2–3):91–108. doi: 10.1159/000089930. [DOI] [PubMed] [Google Scholar]
- 18.Jordan S, Chaikof E. Simulated surface-induced thrombin generation in a flow field. Biophysical J. 2011;101(2):276. doi: 10.1016/j.bpj.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajd F, Vidmar J, Fabjan A, Blinc A, Kralj E, Bizjak N, et al. Impact of altered venous hemodynamic conditions on the formation of platelet layers in thromboemboli. Thrombosis Res. 2011 doi: 10.1016/j.thromres.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Lurie F, Kistner RL, Eklof B, Kessler D. Mechanism of venous valve closure and role of the valve in circulation: A new concept. J Vasc Surg. 2003;38(5):955–961. doi: 10.1016/s0741-5214(03)00711-0. [DOI] [PubMed] [Google Scholar]
- 21.Buxton GA, Clarke N. Computational phlebology: The simulation of a vein valve. J Biol Physics. 2006;32(6):507–521. doi: 10.1007/s10867-007-9033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dydek EV, Chaikof EL. Simulated thrombin generation in the presence of surface-bound heparin and circulating tissue factor. Ann Biomed Eng. 2015 doi: 10.1007/s10439-015-1377-5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrissey JH. Tissue factor modulation of factor VIIa activity: Use in measuring trace levels of factor VIIa in plasma. Thromb Haemost. 1995;74(1):185–188. [PubMed] [Google Scholar]
- 24.Butenas S, van't Veer C, Mann KG. "Normal" thrombin generation. Blood. 1999;94(7):2169–2178. [PubMed] [Google Scholar]
- 25.Merrill EW. Rheology of blood. Physiol Rev. 1969;49(4):863–888. doi: 10.1152/physrev.1969.49.4.863. [DOI] [PubMed] [Google Scholar]
- 26.Lurie F. The functioning of venous valves in normal and pathological conditions. Medicographia. 2008;30(2):95–99. [Google Scholar]
- 27.van Loon R, Anderson PD, van de Vosse FN. A fluid–structure interaction method with solid-rigid contact for heart valve dynamics. J Comput Physics. 2006;217(2):806–823. [Google Scholar]
- 28.De Hart J, Peters G, Schreurs P, Baaijens F. A three-dimensional computational analysis of fluid–structure interaction in the aortic valve. J Biomechanics. 2003;36(1):103–112. doi: 10.1016/s0021-9290(02)00244-0. [DOI] [PubMed] [Google Scholar]
- 29.Tien W-H, Chen H, Berwick Z, Krieger J, Chambers S, Dabiri D, et al. Characterization of a bioprosthetic bicuspid venous valve hemodynamics: Implications for mechanism of valve dynamics. Europ J Vasc Endovasc Surg. 2014;48(4):459–464. doi: 10.1016/j.ejvs.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 30.Kuharsky AL, Fogelson AL. Surface-mediated control of blood coagulation: The role of binding site densities and platelet deposition. Biophysical J. 2001;80(3):1050–1074. doi: 10.1016/S0006-3495(01)76085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Runyon MK, Kastrup CJ, Johnson-Kerner BL, Van Ha TG, Ismagilov RF. Effects of shear rate on propagation of blood clotting determined using microfluidics and numerical simulations. J Am Chem Soc. 2008;130(11):3458–3464. doi: 10.1021/ja076301r. [DOI] [PubMed] [Google Scholar]
- 32.Karino T, Goldsmith HL, Motomiya M, Mabuchi S, Sohara Y. Flow patterns in vessels of simple and complex geometriesa. Ann NY Acad Sci. 1987;516(1):422–441. doi: 10.1111/j.1749-6632.1987.tb33063.x. [DOI] [PubMed] [Google Scholar]
- 33.Karino T, Motomiya M. Flow through a venous valve and its implication for thrombus formation. Thrombosis Res. 1984;36(3):245–257. doi: 10.1016/0049-3848(84)90224-x. [DOI] [PubMed] [Google Scholar]
- 34.Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor–induced blood coagulation. Blood. 2002;100(1):148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 35.Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism. A clinicopathological study in injured and burned patients. British J Surg. 1961;48(211):475–489. doi: 10.1002/bjs.18004821103. [DOI] [PubMed] [Google Scholar]
- 36.Teviotdale B, Gwynne J. Deep calf vein thrombosis and pulmonary embolism: A necropsy study. New Zealand Med J. 1967;66(420):530. [PubMed] [Google Scholar]
- 37.Lund F, Diener L, Ericsson JL. Postmortem intraosseous phlebography as an aid in studies of venous thromboembolism with application on a geriatric clientele. Angiology. 1969;20(3):155–176. doi: 10.1177/000331976902000306. [DOI] [PubMed] [Google Scholar]
- 38.Sevitt S. The structure and growth of valve-pocket thrombi in femoral veins. J Clin Pathol. 1974;27(7):517–528. doi: 10.1136/jcp.27.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLachlin AD, McLachlin JA, Jory TA, Rawling EG. Venous stasis in the lower extremities. Ann Surg. 1960;152(4):678. doi: 10.1097/00000658-196010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cotton L, Clark C. Symposium on thrombosis: Anatomical localization of venous thrombosis. Ann Royal Coll Surg Engl. 1965;36(4):214. [PMC free article] [PubMed] [Google Scholar]
- 41.Nicolaides A, Kakkar V, Field E, Renney J. The origin of deep vein thrombosis: A venographic study. British J Radiol. 1971;44(525):653–663. doi: 10.1259/0007-1285-44-525-653. [DOI] [PubMed] [Google Scholar]
- 42.Browse N, Thomas ML. Source of non-lethal pulmonary emboli. Lancet. 1974;303(7851):258–259. doi: 10.1016/s0140-6736(74)92559-8. [DOI] [PubMed] [Google Scholar]
- 43.Liu G, Ferris E, Reifsteck J, Baker M. Effect of anatomic variations on deep venous thrombosis of the lower extremity. Am J Roentgenol. 1986;146(4):845–848. doi: 10.2214/ajr.146.4.845. [DOI] [PubMed] [Google Scholar]
- 44.Okorie UM, Denney WS, Chatterjee MS, Neeves KB, Diamond SL. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: Amplification of 100 fM circulating tissue factor requires flow. Blood. 2008;111(7):3507–3513. doi: 10.1182/blood-2007-08-106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neeves K, Illing D, Diamond S. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophysical J. 2010;98(7):1344–1352. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]