Abstract
Weight loss and food intake disturbances that often precede cognitive decline and diagnosis have been extensively reported in Alzheimer’s disease patients. Previously, we observed that transgenic mice overexpressing tau seemed to eat more food, yet weigh less than non-transgenic littermates. Thus the present longitudinal study measured the time course of changes in metabolic state over the lifespan of the tau depositing Tg4510 mouse model of tau deposition. Although body weight was comparable to non-transgenic littermates at 2 months of age, Tg4510 mice weighed less at older ages. This was accompanied by the accumulation of tau pathology and by dramatically increased activity in all phases of the 24-hour cycle. Resting metabolic rate was also increased at 7 months of age. At 12 months near the end of the Tg4510 lifespan, there was a wasting phase, with a considerable decrease of resting metabolic rate, although hyperactivity was maintained. These diverse changes in metabolism in a mouse model of tau deposition are discussed in the context of known changes in energy metabolism in Alzheimer’s disease.
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by the accumulation of beta amyloid (Aβ), which forms extracellular plaques, neurofibrillary tangles composed of tau protein, and cognitive declines, such as episodic memory disorder, visuospatial impairment, and deteriorating executive function (Baudic, et al., 2006). Additionally, AD is characterized by non-cognitive symptomatology. Weight loss is a non-cognitive symptom that is commonly reported in AD patients and was described by Alois Alzheimer in his original report from 1906 (Gillette, et al., 2007,Tamura, et al., 2007). Many epidemiological studies have since confirmed this initial observation and it is now recognized as a clinical feature of AD (McKhann, et al., 1984), impacting 20% to 45% of patients (Gillette-Guyonnet, et al., 2005,Gillette-Guyonnet, et al., 2000,Guerin, et al., 2005a,Guerin, et al., 2005b,Vellas, et al., 2005). Weight loss in demented patients is associated with adverse outcomes, such as an accelerated progression of AD, a higher rate of institutionalization (Andrieu, et al., 2001) and increased mortality (Faxen-Irving, et al., 2005,Gambassi, et al., 1999,White, et al., 1998). Although dementia-associated weight loss often begins before the onset of the clinical syndrome and accelerates by the time of diagnosis, it is unclear if it is a cause or a consequence of AD pathology (Inelmen, et al., 2010).
Overall, to decrease body mass, energy intake must be lower than energy expenditure (Morton, et al., 2006), although food intake in people with AD is usually normal or sometimes even increased (Burns, et al., 1989,Keene and Hope, 1997,Niskanen, et al., 1993,Spindler, et al., 1996). Indeed, paradoxical overeating concomitant with weight loss has been observed in patients with AD (Wolf-Klein, et al., 1992) as well as in frontotemporal dementia (FTD), another tauopathy (Bozeat, et al., 2000,Piguet, 2011,Snowden, et al., 2001). The observation of significant weight loss in the absence of decreased food intake suggests the possibility of a hypermetabolic state in AD (Vloeberghs, et al., 2008,Wolf-Klein, et al., 1992). Hypermetabolism is the physiological state of increased metabolic activity, which is typically characterized by an elevated basal metabolic rate. It is uncertain whether abnormalities in metabolism exist in AD patients because no consistent changes (either increase or reduction) in basal metabolism have been reported (Donaldson, et al., 1996,Niskanen, et al., 1993). Nevertheless, many factors contribute to daily metabolism. High energy expenditure may be caused, for example, by increased physical activity levels such as wandering or excessive pacing, which are recognized symptoms in AD (Devanand, et al., 1997,Devanand, et al., 1992,Lopez, et al., 1999). Circadian behaviors are also altered in AD patients with increased activity during evening hours,“ sundowning” (Scarmeas, et al., 2007) sleep fragmentation at night, and a tendency to sleep during daytime (Ancoli-Israel, et al., 1994).
These characteristics are consistent with observations made previously in different mouse models of AD pathology. Indeed, lower body weight and increased feeding behavior have been described in different models of amyloid deposition (Morgan and Gordon, 2008,Pugh, et al., 2007,Vloeberghs, et al., 2008). Specifically, Tg2576 APP mice exhibit lower weight without alterations in feeding behavior (Brownlow, et al., 2013,Ishii, et al., 2014), but associated with increased energy expenditure (Ishii, et al., 2014) or increased activity in open field tests (Brownlow, et al., 2013). In addition, 3xtgAD mice display increased body weight at 3 and 6 months of age (Adebakin, et al., 2012,Knight, et al., 2012) with increased appetite starting at 2 months (Knight, et al., 2012) that continues up to 12 months, although body weight is decreased at 12 months compared to non-transgenic mice due to an increase in metabolism (Knight, et al., 2012). A slight increase in activity during the day was also reported in these mice at 10 months of age (Knight, et al., 2013), although a different cohort showed no difference in body weight and hypoactivity in open field tests at 14–20 months of age (Filali, et al., 2012). Abnormalities in circadian rhythmicity were also observed in these mice, which may explain these discrepancies in the subsequent cohorts (Sterniczuk, et al., 2010).
Hyperactivity and increased agitation have been reported in mouse models of tau deposition such as THY-22 and Tg4510 (Jul, et al., 2015,Van der Jeugd, et al., 2013) together with a decrease in body weight (Brownlow, et al., 2013,Brownlow, et al., 2014,Leboucher, et al., 2013). Despite all of these observations of altered body weight in mouse models of AD, there is no current report of energy balance variations in a mouse model of tauopathy to our knowledge.
Therefore, the aim of this study was to examine longitudinal changes in body weight, food intake, and energy expenditure in addition to locomotor activity in a mouse model of tau deposition, Tg4510 mice. These mice develop progressive pathology with histologically discernible tau deposits similar to that found in AD patients at 3 months of age, leading to neuronal loss and atrophy by 6 months of age (Dickey, et al., 2009,Santacruz, et al., 2005).
2. Methods
2.1. Animals and experimental design
All animal testing procedures were approved by the Institutional Animal Care and Use Committee of the University of South Florida and were performed in accordance with the eighth edition “Guide for the Care and Use of Laboratory Animals,” published by the National Academy of Science, the National Academies Press, Washington, DC (2011).
Parental mutant tau and tetracycline-controlled transactivator protein mouse lines were maintained separately and bred to produce Tg4510 mice as described previously (Santacruz, et al., 2005). Because the two transgenes segregate independently, offspring can carry neither transgene (nontransgenic), a single transgene alone (tau only or tTA only), or both transgenes (Tg4510). The Tg4510 mouse expresses tau with a P301L mutation, which is associated with an autosomal dominantly inherited dementia referred to as frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) (Hutton, et al., 1998). This model differs from most other tau transgenic mice in that the majority of tau pathology and neurodegeneration is found in the forebrain rather than the spinal cord. This neuroanatomical specificity is caused by tet-transactivator being regulated by the CaM kinase II promoter, resulting in expression predominantly in forebrain neurons. These mice develop progressive pathology with histologically discernible tau deposits consistently observed at 3 months, progressing through a series of conformational and hyperphosphorylated forms analogous to that found in AD patients, leading to neuronal loss and atrophy by 6 months of age (Dickey, et al., 2009,Santacruz, et al., 2005). Mice were housed individually and maintained on a twelve-hour light/dark cycle. Food (NIH-31, Harlan) and water were given ad libitum. Mice in the USF Tg4510 colony were weighed routinely. Weight data collected from 2594 mice were binned into monthly averages for each genotype. These data are presented in Figure 2B.
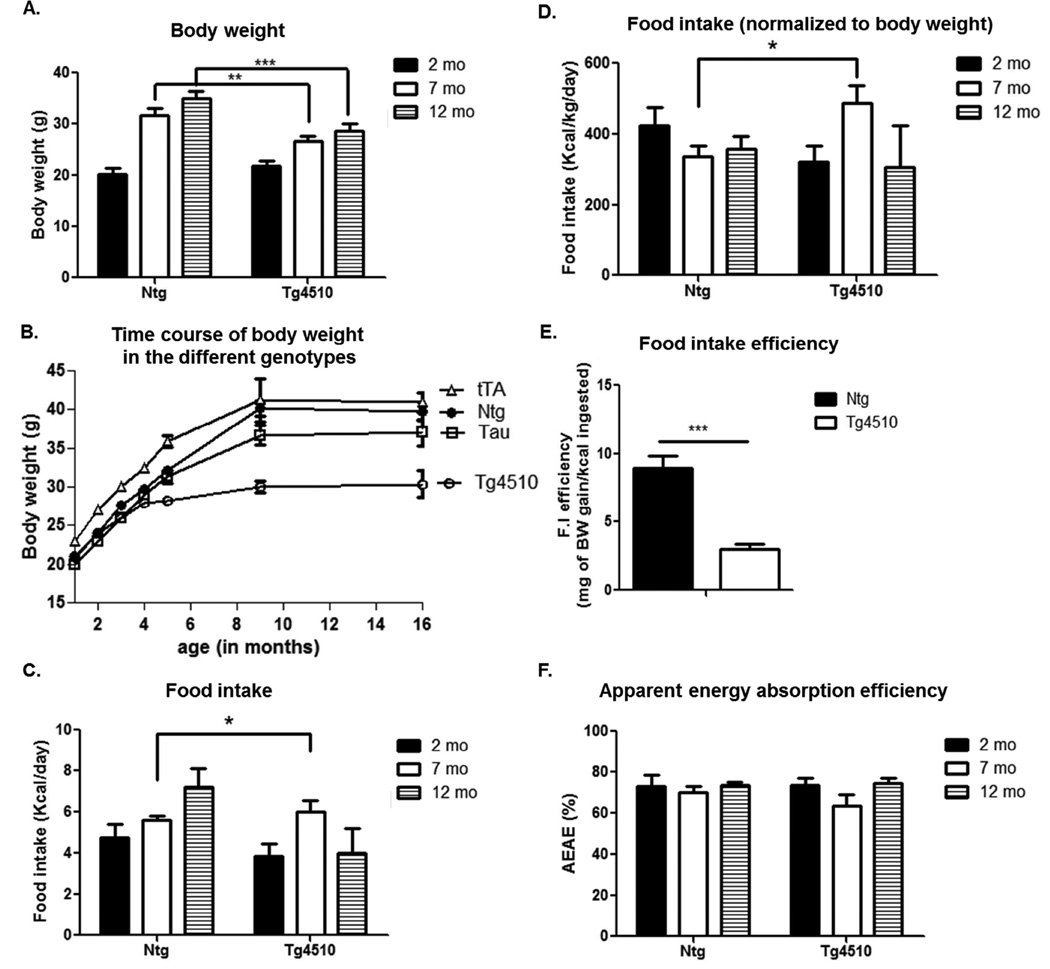
Figure 2. Body weight and energy intake.
Time course of body weight in Tg4510 mice versus non-transgenic littermates (Ntg) at 2 (black bars), 7 (white bars) and 12 months of age (shaded bars) (A). Evolution of body weight in all littermates: non-transgenic, tau only (Tau), tet only (tTA) and tau + tet (Tg4510) over the course of aging (B; data extracted from the mouse colony database). 24 h food intake raw (C) or normalized to body weight (D), food intake efficiency (E) and apparent energy absorption efficiency (F), in Tg4510 mice versus non-transgenic littermates at 2 (black bars), 7 (white bars) and 12 months of age (shaded bars). Food intake efficiency was calculated as mg of body weight gain/kcal consumed over a period of 6 months (between 4 and 10 months of age). Statistical analysis was performed using two-way ANOVA. Data are presented as mean +/− SEM *** p<0.001, ** p<0.01, *p<0.05.
For the longitudinal study in metabolism cages, a group of 2 month-old Tg4510 transgenic mice (n=6) and control non-transgenic littermates (FVB/129S F1 hybrid background, n=6) with an equal number of males and females were used. The same group of animals was tested again at both 7 and 12 months of age. Tet (tTA) only control mice were tested in metabolic caging at the 7 month-old time point (n=4). Additional groups of mice were euthanized at the same time points (2 month-old n=5, 7 month-old n=10, and 12 months old n=4) for histology, biochemistry and tissue dissection.
2.2. Energy expenditure and locomotor activity analysis with indirect calorimetry
Mice were analyzed for whole body energy expenditure (kJ/h), oxygen consumption and carbon dioxide production, respiratory exchange rate (VCO2/VO2), food intake (Kcal) and locomotor activity (Counts/hour) using calorimetric cages (Phenomaster, TSE Systems, Chesterfield, MO, USA) with bedding, food and water measurements, as previously described (Joly-Amado, et al., 2012). Composition of gases was compared to a reference empty cage and whole animal energy expenditure was calculated according to the Weir equation for respiratory gas exchange measurements (Weir, 1949). Each cage was equipped with an array of highly sensitive feeding and drinking sensors for automated online measurement and locomotion monitored with an infrared light beam-based system. Night over day activity ratio was calculated by dividing the total activity at night by the total activity during the day for each mouse.
Mice were individually housed with free access to food and water ad libitum with lights on from 6am to 6pm and an ambient temperature of 22 ± 1°C. All animals were acclimated to the metabolism cages for 24 hours before experimental measurements were recorded. Data analysis was performed using O2 consumption, CO2 production (expressed in ml/h), and energy expenditure (kJ/h). Data were analyzed using ANCOVA as recommended by (Tschop, et al., 2012). Resting metabolic rate (RMR) was estimated by using general linear mixed effects modelling (GLM-MM, SPSS software version 22) with intercepts of oxygen consumption and activity plots when the activity value = 0.
2.3. Apparent energy absorption efficiency (AEAE) and food efficiency
Measurements of energy intake were made at 2, 7 and 12 months. In the morning, bedding was changed and a weighed portion of food was added to the hopper. Twenty-four hours later, the food remaining in the hopper was reweighed and feces were removed from the cage, dried to a constant mass at 60°C for 48h and weighed again. The gross energy content of feces was measured by bomb calorimetry. We calculated the daily energy intake of each mouse by calculating food consumption of each mouse (g/day) from food removed from hopper (g/day) – uneaten food from the cage floor (g/day). The energy lost through defecation (kcal/day) was calculated from dry fecal production (g/day) × fecal energy content (kcal/g of dry mass). The apparent energy absorption efficiency (AEAE) was calculated as the percentage of gross energy intake that was digested of the daily energy intake (Krol, et al., 2007). Food efficiency was calculated as described in (Joly-Amado, et al., 2012). Briefly, the body weight gain over a period of 6 months was normalized to the amount of calories ingested over the same period (between 4 and 10 months of age).
2.4. Feces analysis
To measure the energy content of each biological sample, a sample was combusted with an Isoperibol Calorimeter 6200 instrument with a model 1108 oxygen bomb (Parr Instrument Co, Moline, IL) following manufacturer instructions. Briefly, after preparation, the pellet was weighed and placed in a model 1108 oxygen bomb with contact to a 10-cm fuse wire connected to a 2901EB ignition unit (Parr Instrument Co). The bomb was placed in a bomb cylinder surrounded by 2000 mL distilled water. The heat produced at the combustion of the pellet was sensed as a rise of water temperature. Bombs were calibrated using benzoic acid before each use. To give the energy equivalent (W) per change in water temperature (ΔT), amount of benzoic acid standards with a temperature rise equivalent to the samples tested were conducted every 10 burns. The energy content of the pellet (ES) was calculated as follows: Es = W × ΔT / the weight of the pellet. The total number of calories in the sample was calculated on the basis of weight of the sample.
2.5. Tissue Collection
Three different groups of transgenic mice and age matched non-transgenic littermates were euthanized at 2, 7 and 12 months of age with a solution containing pentobarbital and phenytoin, then transcardially perfused with 25 ml of 0.9% normal saline solution. Mice were placed on an isothermal pad during perfusion to minimize generation of artefactual tau phosphorylation, which has been reported to result from lowered body temperature caused by the euthanizing solution (Maurin, et al., 2014,Planel, et al., 2007). Brains were collected immediately following perfusion. One hemisphere was dissected and frozen for western analysis and the second hemisphere was immersion fixed in 4% phosphate-buffered paraformaldehyde for 24 h. The fixed hemispheres were cryoprotected in successive incubations of 10%, 20% and 30% sucrose solutions for 24 h each. Subsequently, brains were frozen on a cold stage and sectioned in the horizontal or coronal plane (25 µm thickness) on a sliding microtome and stored in Dulbecco’s phosphate buffered saline with 10 mM sodium azide solution at 4°C. Brown adipose tissue, white adipose tissue (specifically perirenal and subcutaneous depots) and muscle (as extensor digitorum longus muscle [EDL], tibialis and soleus) were dissected and weighed. For white adipose tissue, data are presented as the sum of both perirenal and subcutaneous depots. For muscle tissue, data are presented as the sum of EDL, tibialis and soleus. Blood was collected from intracardiac puncture using EDTA and then centrifuged at 1000 g for 15 min at 4C for plasma collection.
2.6. Histopathology
Stereological principles were employed to select the sections stained for each marker. Immunohistochemical procedural methods were described (Gordon, et al., 2002). Sections from all animals were placed in a multi-sample staining tray and endogenous peroxidase was blocked (10% methanol, 3% H2O2 in phosphate buffered saline, 10 mM NaPO4, 154 NaCl, pH 7.4, PBS;30 min). Tissue samples were permeabilized with 0.2% lysine, 1%Triton X-100 in PBS solution and incubated overnight in anti-tau phosphorylated at Ser396 (rabbit polyclonal, Anaspec). Sections were washed in PBS, and then incubated in corresponding biotinylated secondary antibody (VectorLaboratories, Burlingame, CA). The tissue was again washed after 2 hours and incubated with Vectastain® Elite® ABC kit (Vector Laboratories, Burlingame, CA) for enzyme conjugation. Finally, sections were stained using 0.05% diaminobenzidine, 0.5% nickel ammonium sulfate and 0.03% H2O2. Tissue sections were mounted onto slides, dehydrated, cover slipped and scanned for analysis using slide scanner Axio Scan Z.1 (Zeiss Inc). Hippocampal and cortex volume (expressed in mm3) was calculated by measuring hippocampal area of 8 sections (25 µm thickness) utilizing Zeiss software (Zeiss Inc.) and applying the formula: V = ΣA × T, where ΣA = sum of the hippocampal areas on the sections analyzed, and T = section interval × t (section thickness after tissue processing). Stained sections were imaged using Zeiss digital scanning microscope and Image Analysis software.
2.7. Western blotting and ELISA
Tissues for Western blot analysis were prepared as previously described (Brownlow, et al., 2014). Briefly, brown adipose tissue was quickly minced in homogenization RIPA buffer containing protease inhibitor cocktail (Sigma Aldrich) and phosphatase inhibitor cocktails I and II (Sigma Aldrich) and centrifuged at 14000 rpm for 30min. The supernatant was collected and protein concentrations were determined by the BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of proteins (40µg/well) were loaded in each well of a 4–12% SDS-PAGE gel and transferred to a 0.2 µm pore size nitrocellulose membrane and immunoblotted with anti-mouse Uncoupling protein 1 (UCP1) (alpha diagnostics, Texas), anti-mouse Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α) (Abcam, Cambridge, Ma, USA) and anti-mouse β-actin (sigma) at 1:1000-fold dilution. Fluorescently tagged secondary antibodies (IRDye 800CW, LI-COR Biosciences) were used at a dilution of 1:10,000. Band intensities were quantified by densitometric analysis and normalized to the band intensity of β-actin using the Odyssey imaging system (LI-COR Biosciences). Measurement of plasma leptin concentration was performed using a mouse leptin ELISA kit (Life Technologies, CA, USA) according to the manufacturer’s instructions.
2.8. Statistical methods
Data were analyzed by two-way ANOVA or repeated measures ANOVA followed by Fisher’s protected least significant difference post hoc tests (statview) or ANCOVA (SPSS version 22). In all tests, a P-value <0.05 was considered significant. All data are presented as mean values ± standard error of the mean unless specified otherwise.
3. Results
3.1. Age- related pathology in Tg4510 mice
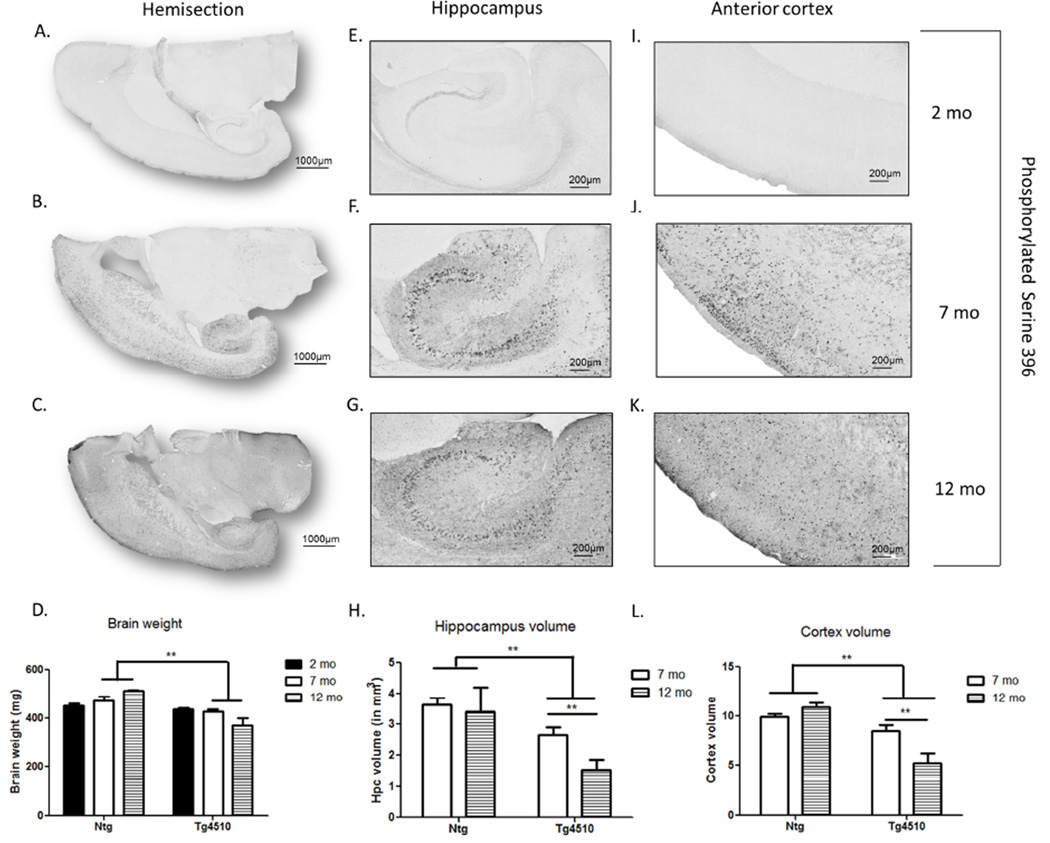
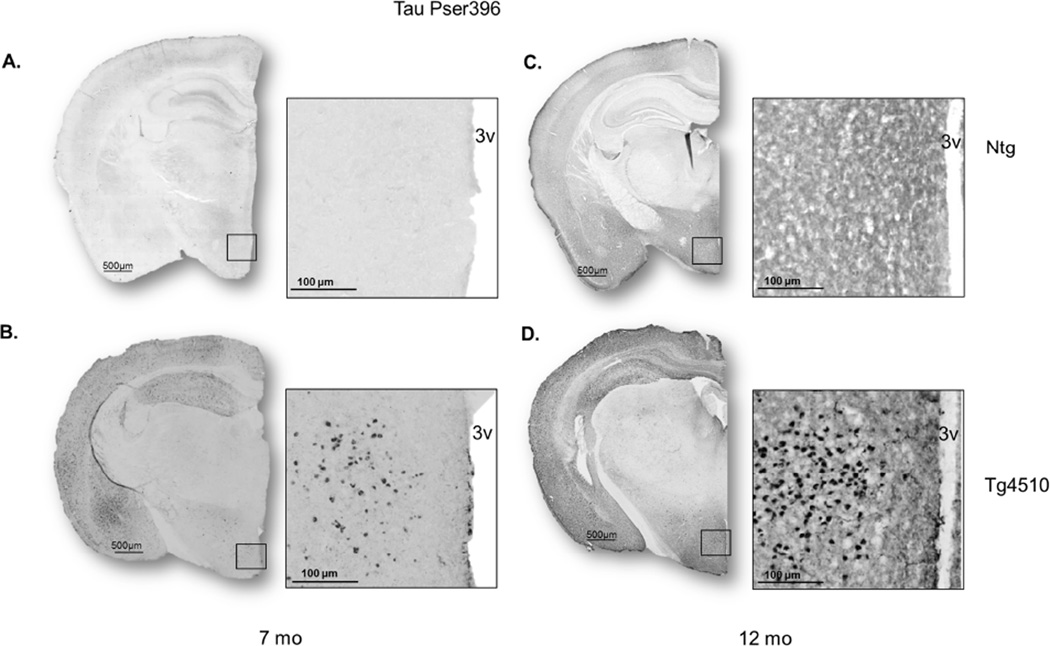
Consistent with previous studies (Santacruz, et al., 2005), no hyperphosphorylated tau (pser396) was detected in 2 month-old Tg4510 mice (Fig. 1A,E,I) or in non-transgenic littermates (data not shown). At 7 months of age, pser396 was detected in the forebrain (Fig. 1B), more specifically in neurons in both hippocampus (Fig. 1F) and cortex (Fig. 1J). Pser396 staining spread to the parenchyma at 12 months of age (Fig. 1C,G,K). The increased expression of phosphorylated tau was accompanied by decreased brain weight in Tg4510 mice at both 7 and 12 mo (Fig. 1D). There was no genotype difference in brain weight at 2 months. In addition, decreased hippocampal volume (Fig. 1H) and decreased cortex volume (Fig. 1L) were observed in Tg4510 mice at 7 and 12 months of age compared to non-transgenic littermates, but not at 2 months (data not shown). In addition, in Tg4510 mice hippocampal and cortical volume were significantly reduced between 7 month and 12 months (Fig. 1H,L), reflecting the worsening of pathology with aging.
Figure 1. Evolution of pathology over the course of aging in Tg4510 mice.
Micrograph representation of staining with tau phosphorylated at Ser396 in Tg4510 at 2 (A,E,I), 7 (B,F,J) and 12 months of age (C,G,K). Brain weight post euthanization in 2-, 7-, and 12-month-old non-transgenic littermates (Ntg) and Tg4510 (D). Hippocampal volume (H) and cortex volume (L) expressed in mm3 in 7 and 12 month-old Tg4510 mice was calculated by measuring hippocampal area of 8 sections (25 µm thickness) utilizing Mirax software (Zeiss Inc.) and applying the formula: V = ΣA × T. Two-way ANOVA showed a significant decrease in hippocampal and cortex volume in Tg4510 mice compared to non-transgenic littermates at both 7 and 12 months of age. Hippocampal and cortex volume in Tg4510 decreased significantly between 7 and 12 months of age. Data are presented as mean ± S.E.M. **p < 0.01.
3.2. Age related body weight loss in Tg4510 mice was independent of food intake
No differences were observed in body weight between 2 month Tg4510 mice and non-transgenic littermates. However, as mice in the longitudinal study aged, body weight was significantly lower at 7 and 12 months in Tg4510 mice compared to non-transgenic littermates (Fig. 2A). These age related differences in body weight were confirmed in our collection of body weight information from over 2594 mice derived from our colony over the past 8 years. ANOVA revealed main effects of age and genotype as expected (Fig 2B). Post-hoc analysis at 1 month of age revealed a significant difference between all genotypes, with tau only littermates displaying a slight but significant decrease in body weight compared to non-transgenic littermates (20.5g +/− 0.13 versus 20.9 +/− 0.14, n=608 and 547, respectively). Tg4510 (tau + tet) as well as tet only mice displayed a significant increase in body weight compared to non-transgenic littermates (21.8 +/− 0.17 and 23.4 +/− 0.16 versus 20.9 +/− 0.14, n=383, 474 and 547, respectively). However, body weight was not different between tau or Tg4510 mice compared to non-transgenic littermates at 2 months of age, although tet only mice (tTA) were still significantly heavier. At 3 months of age Tg4510 mice started to have lower body weight compared to all other genotypes and that difference grew larger over the ensuing year. Tau only mice body weights remained similar to non-transgenic littermates over the course of aging after 2 months of age. Tet only mice body weights remained significantly higher than all other genotypes until 9 months of age. Together, these data show that the reduced body weight in the Tg4510 mice is not due to weight differences caused by one of the background transgenes (tTA in tet only or tau in tau only) but most likely due to the increased expression of tau and accumulation of tau deposits in the Tg4510 mice.
No difference was observed in daily food intake (Fig. 2C) or food intake normalized to body weight (Fig. 2D) in Tg4510 mice compared to non-transgenic littermates at 2 months and 12 months. Remarkably, 7 months old Tg4510 mice displayed higher food intake than non-transgenic littermates, indicating that reductions in food intake could not account for the lower body weight (Fig. 2C,D). However, food intake efficiency, i.e body weight gain normalized to the amount of calories ingested over a period of 6 months (from 4 to 10 months of age) was dramatically decreased in Tg4510 mice compared to non-transgenic littermates (Fig. 2E). The apparent energy absorption efficiency (AEAE; derived from calories remaining in feces) was not different among the different ages between Tg4510 and non-transgenic littermates. Thus body weight loss or reduction in food intake efficiency was not due to defective energy absorption (Fig. 2F).
3.3. Decrease in white but not brown fat depots in Tg4510 mice
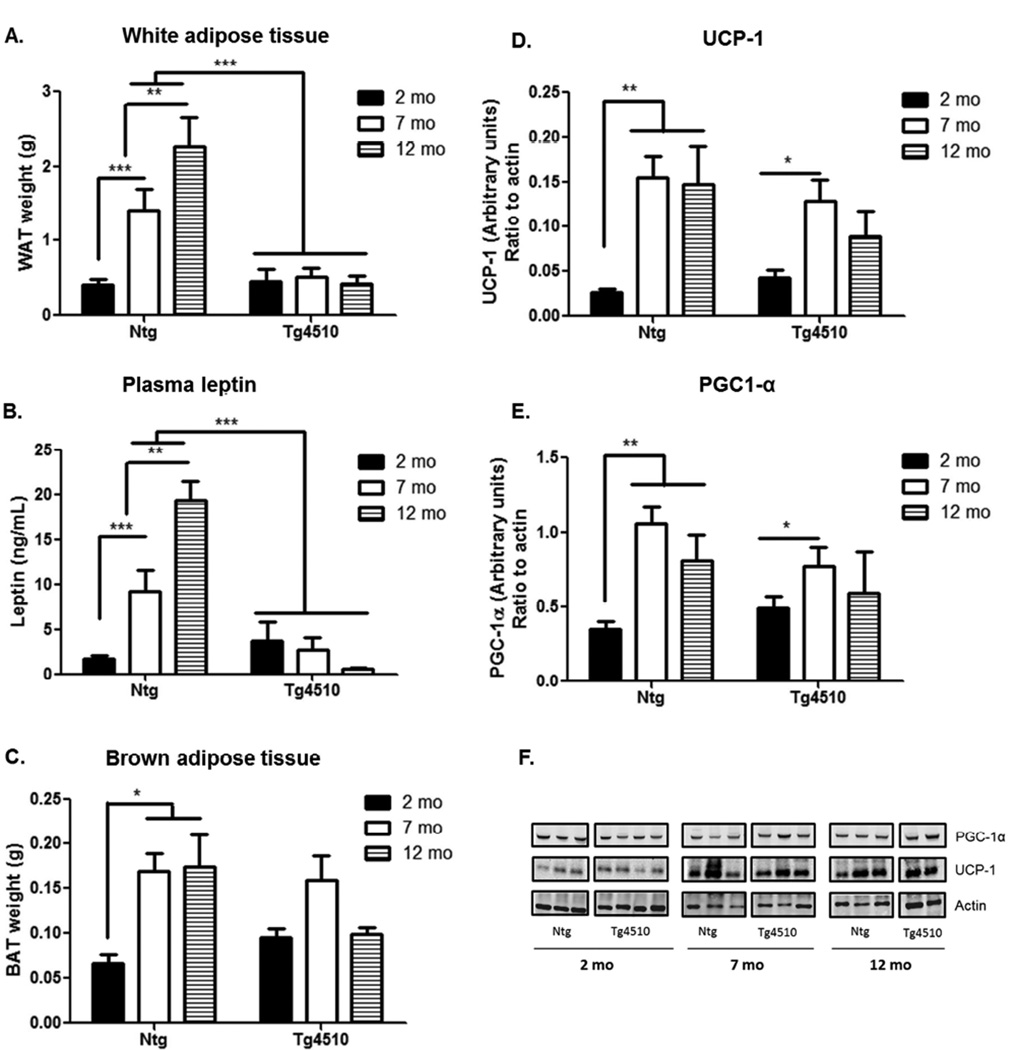
Consistent with the decrease in body weight, 7 and 12 months old Tg4510 mice showed a dramatic decrease in the size of the white fat pads compared to age-matched, non-transgenic mice (Fig. 3A). Indeed, while white adipose tissue content increased significantly over the course of aging in non-transgenic littermates, it remained low in Tg4510 mice, with no significant increase with aging. Measurement of leptin concentration in plasma matched the variations observed in white adipose tissue content in both non-transgenic and Tg4510 mice (Fig. 3B). At two months of age there were no differences in the amount of white fat pads or plasma leptin level between Tg4510 and non-transgenic littermates (Fig. 3A,B).
Figure 3. Adipose tissue analysis.
The time course of changes in white adipose tissue weight (A), plasma levels of leptin (B), brown adipose tissue weight (C) and levels of UCP-1 (D, F) and PGC1-α (E,F) are presented. UCP-1 (Uncoupling protein 1) and PGC-1 (Proliferator-activated receptor γ coactivator 1) in brown adipose tissue was assessed by western blot analysis (F), and band intensity was normalized to actin in transgenic Tg4510 (Tau + tet) mice and non-transgenic littermates (Ntg) at 2 (black bars), 7 (white bars) and 12 months of age (shaded bars). Statistical analysis was performed using two-way ANOVA. Data are presented as mean +/− SEM *** p<0.001, ** p<0.01, *p<0.05.
There were no differences between age-matched Tg4510 and non-transgenic littermates in muscle mass (data not shown) or in brown adipose tissue weight (Fig. 3C), although there were main effects of aging. Similarly, basal levels of UCP-1 (Uncoupling protein 1, Fig. 3D,F) and PGC1-α (Proliferator-activated receptor γ coactivator 1, Fig. 3E,F) were similar in age-matched Tg4510 and non-transgenic littermates in brown adipose tissue when assessed by western blot.
3.4. A progressive hyperactivity phenotype developed in Tg4510 mice
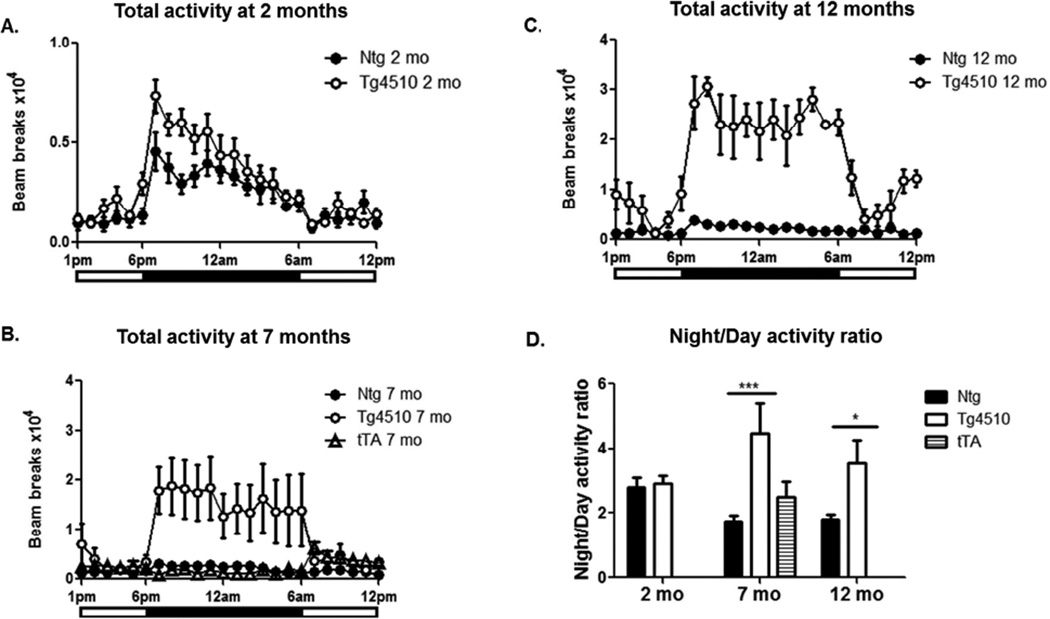
At 2 months of age, there were no significant differences in average activity levels or in the timing or amplitude of daily activity rhythms between transgenic and non-transgenic littermates (Fig. 4A). The peak in activity observed at the beginning of the dark phase was observed at the same time in Tg4510 mice and non-transgenic littermates. Even though the amplitude of the peak appeared consistently higher in transgenic mice, the difference was not statistically significant (Fig. 4A). There was no genotype difference of activity partitioning during the day and at night at 2 months (Fig. 4D). Prominently, at 7 months, a significant increase was observed in the amplitude of daily activity profiles for Tg4510 mice compared to non-transgenic littermates (Fig. 4B; note change in Y axis scaling). The genotype difference was greater at night than during the day, with Tg4510 mice reaching a 2-fold increase during the day and nearly 7-fold at night. At this age we also included tTA littermate mice in the metabolic cages. As expected, no difference in locomotor activity was observed in tTA mice compared to non-transgenic animals (Fig. 4B). At 12 months, the genotype differences in activity were even greater than at 7 months during both day and night reaching a 4.5- fold increase in Tg4510 mice during the day and an 8-fold increase at night (Fig. 4C). The genotype difference in the ratio of night activity over day activity is illustrated in Fig. 4D. Interestingly, night over day ratio activity decreased with aging in non-transgenic mice, but Tg4510 mice displayed an increase in this ratio, largely due to increased activity in the dark phase (rather than decreased activity during the light phase) (Fig. 4D). This difference suggests transgenic mice are still sensitive to circadian cues since they are more active at night than during the day.
Figure 4. Progressive hyperactive phenotype in Tg4510 mice.
Total activity is presented for 2-month-old (A), 7-month-old (B) and 12-month-old (C) Tg4510 mice (open circles) compared to non-transgenic littermates (Ntg; black circles) and tet only mice (tTA, open triangles). Both 7 and 12-month-old, but not 2- month- old Tg4510 mice display a significant increase in activity when using ANOVA for repeated measures to compare day and night. Data are presented as mean of activity counts per hour over a period of 4 nights and 3 days. Note change in Y axis scale in B and C vs A. Night over day activity ratio (D) in Tg4510 tau mice (white bars) compared to non-transgenic littermates (Ntg, black bars) at 2, 7 and 12 months; an additional group of tet only mice was introduced at 7 months (tTA, shaded bars). Statistical analysis was performed using ANOVA for repeated measures and two-way ANOVA. ***p<0.001, *p<0.05
3.5. Changes in metabolism and fuel utilization
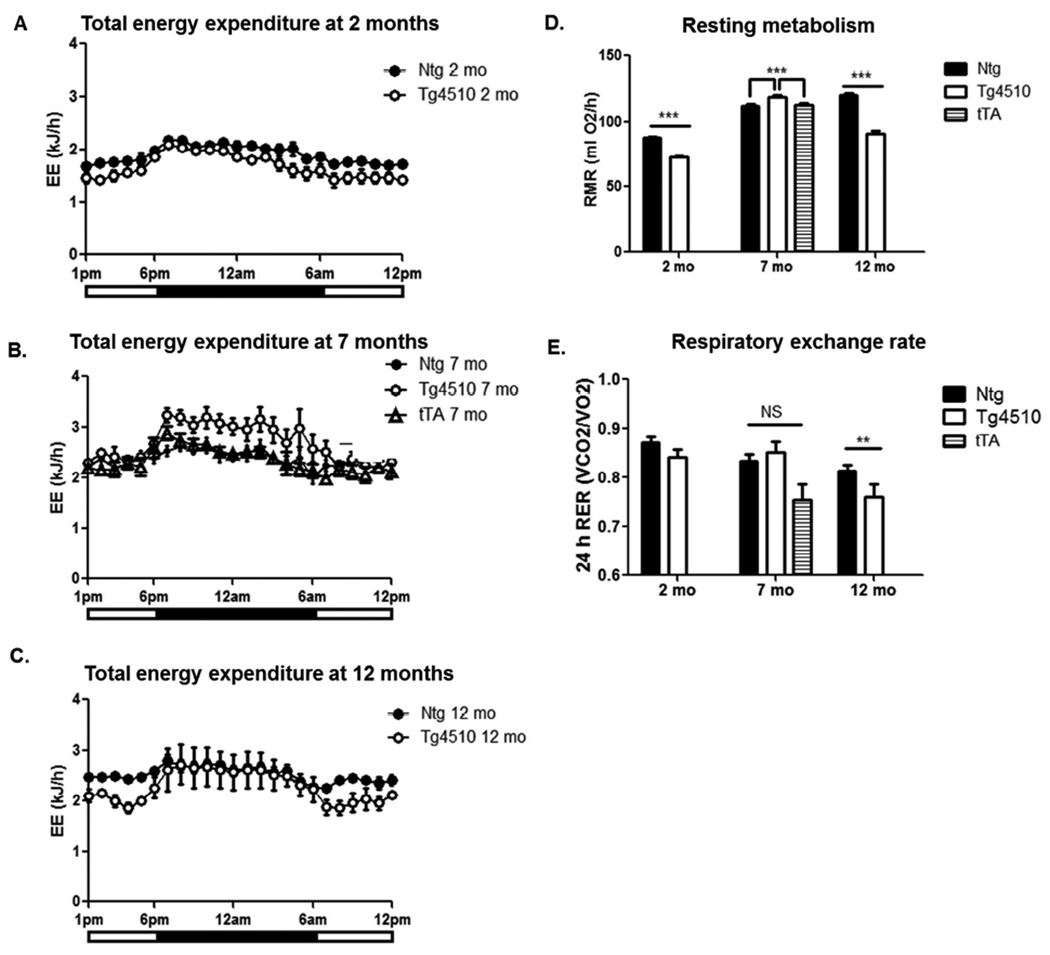
Total daily energy expenditure (DEE) was significantly lower in 2 month old Tg4510 mice compared to non-transgenic mice (Fig. 5A). Although 24 hour DEE was not significantly different in 7 month old Tg4510 mice compared to non-transgenic and tTA littermates, Tg4510 mice exhibited increased energy expenditure at night (Fig. 5B). Total 24 hour DEE was not significantly different in 12 months old Tg4510 mice compared to non-transgenic littermates, although we observed significantly lower energy expenditure during the day (Fig. 5C). Resting metabolic rate (RMR) was calculated using values of oxygen consumption when activity was null. RMR was lower in 2 month old Tg4510 mice compared to controls, whereas it was higher than both non-transgenic and tTA littermates at 7 months old, suggesting a switch in metabolic efficiency (Fig. 5D). Later in life, resting metabolism was lower in Tg4510 mice again (Fig. 5D).
Figure 5. Age-dependent changes in metabolism in Tg4510 mice and non-transgenic littermates.
The graphs present total energy expenditure in 2-month-old (A), 7-month-old (B) and 12-month-old (C) in Tg4510 mice (open circles) compared to non-transgenic littermates (Ntg; black circles) and tet only (tTA; open triangles) when relevant. The resting metabolic rate (RMR) averaged over a period of 3 nights and 4 days (D), and the value for respiratory exchange rate RER (VCO2/VO2) averaged over a period of 24h (E) in Tg4510 mice (white bars), non-transgenic littermates (Ntg, black bars) at 2, 7 and 12 months of age are shown. An additional group of tet only mice was introduced at 7 months (tTA, shaded bars). Statistical analysis was performed using repeated measure ANOVA or two-way ANOVA when appropriate. Data are presented as mean +/− SEM ***p<0.001, **p<0.01.
The 24h respiratory exchange rate (RER) was not significantly different at 2 and 7 months between Tg4510 mice and non-transgenic or tTA littermates. However, 12 month-old Tg4510 mice displayed a significantly lower RER compared to non-transgenic mice, 0.75 vs. 0.81 respectively (Fig. 5E), suggesting a preference towards fat as a metabolic fuel (Longo, et al., 2010). Circadian analysis of RER shows that 12-month-old Tg4510 mice stayed around 0.75 in both night and day, whereas non-transgenic mice displayed circadian changes in RER with a higher RER at night than during the day, reflecting the consumption and use of dietary carbohydrates as the main source of metabolic fuel during the night (data not shown). Overall, lower RER suggests Tg4510 mice are biased towards fat as the primary metabolic fuel, whereas non-transgenic littermates would be on a mixed substrate oxidation, which parallels our observations in fat pad size.
3.6. Tau pathology in hypothalamus over the course of aging in Tg4510 mice
Immunostaining against hyperphosphorylated tau (pser396) in coronal sections revealed the presence of pathology in the hypothalamus of Tg4510 mice at 7 (Fig. 6B) and 12 months of age (Fig. 6D), which was not present in non-transgenic littermates (Fig. 6A,C). No staining was detected in 2- month-old mice (see Fig. 1A).
Figure 6. Tau pathology in the hypothalamus over the course of aging.
Micrographs representing staining of tau phosphorylated at Ser396 in non-transgenic littermates (A, C) and Tg4510 mice (B, D) at 7 (A, B) and 12 months of age (C, D) in coronal sections (25 µm thickness) utilizing Mirax software (Zeiss Inc.). The boxed area on the left panels of each pair are enlarged on the right. Cellular staining for phosphorylated tau is present in the hypothalamus.
All data are summarized in table 1.
Table 1.
Values for each dependent measure reported here.
| 2 months | 7 months | 12 months | ||||
|---|---|---|---|---|---|---|
| Ntg | Tg4510 | Ntg | Tg4510 | Ntg | Tg4510 | |
|
Diurnal locomotor activity (24h counts *10^4) |
1.43 +/−0.18 | 1.65 +/− 0.26 | 1.73 +/− 0.24 | 4.12 +/− 0.12a | 1.68 +/− 0.28 | 7.45 +/− 1.91a |
|
Nocturnal locomotor activity (24h counts *10^4) |
3.76 +/− 036 | 6.84 +/− 0.68 | 2.75 +/− 0.26 | 18.86 +/− 6.9a | 2.95 +/− 0.47 | 22.95 +/− 6.93a |
|
Energy expenditure (kJ/day) |
38.62 +/− 2.14 | 33.66 +/−1.29 | 36.52 +/−1.07 | 43.75 +/− 1.11 | 35.55 +/− 1.37 | 38.77 +/− 5.96 |
| RER (ml O2/h) | 0.87 +/− 0.01 | 0.84 +/− 0.01 | 0.83 +/− 0.01 | 0.85 +/− 0.02 | 0.81 +/− 0.01 | 0.76 +/− 0.02a |
| Resting metabolism | 87.8 +/− 0.8 | 72.7 +/− 1.0a | 112.2 +/− 1.4 | 118.5 +/− 1.4a | 120.4 +/− 1.1 | 91 +/− 2.4a |
| Body weight (g) | 20.3 +/− 1.1 | 21.9 +/− 0.9 | 31.6 +/− 1.4 | 26.6 +/− 0.9a | 35.1 +/− 1.3 | 28.6 +/− 1.3a, |
| Brain weight (mg) | 449.4 +/− 11.3 | 436.3 +/− 5.1 | 472.9 +/− 17.1 | 425.4 +/−11.4a | 512.3 +/− 3.1 | 368.2 +/− 30.1a,b |
|
Food intake (kcal/day) |
424.7 +/− 49.9 | 322.3 +/− 44.3 | 336.4 +/− 29.7 | 486.7 +/− 50.3a | 357.7 +/− 37.4 | 305.6 +/− 118.2 |
| AEAE (%) | 73.0 +/− 5.6 | 73.8 +/− 3.4 | 69.9 +/− 3.3 | 63.5 +/− 5.4 | 73.8 +/− 1.3 | 74.7 +/− 2.1 |
| WAT (mg) | 406 +/− 78 | 451 +/− 161 | 1412 +/− 278c | 515 +/− 119a | 2261 +/− 400c | 429 +/− 99a |
|
Plasma leptin (ng/mL) |
1.72 +/− 0.38 | 3.77 +/− 2.11 | 9.31 +/− 2.31c | 2.76 +/− 1.43a | 19.39 +/− 2.140c |
0.58 +/− 0.20a |
| BAT (mg) | 66.9 +/− 9.1 | 95.2 +/− 10.4 | 169 +/− 20.3 | 158.4 +/− 28.2 | 174.5 +/− 36.1 | 99.3 +/− 6.8 |
|
UCP1 (Arbitrary Units) Ratio to actin |
0.02 +/− 0.01 | 0.04 +/− 0.01 | 0.15 +/− 0.02c | 0.13 +/− 0.02 | 0.15 +/− 0.04c | 0.09 +/− 0.02 |
|
PGC-1 α(Arbitrary Units) Ratio to actin |
0.36 +/− 0.05 | 0.49 +/− 0.08 | 1.06 +/− 0.11c | 0.77 +/− 0.13 | 0.81 +/− 0.17c | 0.60 +/− 0.27 |
: significantly different from age-matched non-transgenic littermates.
: significantly different from 7 months old Tg4510.
: significantly different from 2 months old non-transgenic.
Abbreviations: AEAE (Apparent energy absorption efficiency), BAT (Brown adipose tissue), NTg (Non-transgenic), PGC-1-α (proliferator-activated receptor γ coactivator 1), RER (Respiratory exchange rate), UCP-1 (uncoupling protein 1), WAT (White adipose tissue).
4. Discussion
In this study, we showed metabolic disturbances in a mouse model of tau deposition that resembled some non-cognitive symptomatology reported in both AD patients and mice with amyloid deposition. Our results indicated that increased tau pathology in Tg4510 mice was accompanied by lower body weight, and associated with reduced fat depot size. This body weight loss was not caused by a decrease in food intake or energy absorption but was accompanied by a significant decrease in food efficiency (i.e body weight gain normalized to calorie intake) in Tg4510 mice compared to non-transgenic littermates. These observations were correlated with a progressive increase in locomotor activity. Indeed, no body weight difference was observed at 2 months when locomotor activity was the same for both genotypes, while a lower body weight at 7 months and 12 months of age was correlated with a hyperactive phenotype. Hyperactivity seemed to be related to the presence of hyperphosphorylated tau since no significant difference in locomotor activity was observed at 2 months, when no hyperphosphorylated tau was detectable, but was present at 7 and 12 months when there was significant tau pathology. This is consistent with results from a previous study of locomotor activity in Tg4510 mice where hyperactivity was attenuated by transgene suppression with doxycycline (Jul, et al., 2015). However, the authors mentioned that activity was not normalized to non-transgenic levels, suggesting that factors other than tau accumulation could be involved. Although hyperactivity in Tg4510 was observed during both night and day, the ratio of night over day activity was maintained or exaggerated, indicating a sustained responsiveness to circadian cues unlike other mouse models of AD (Sterniczuk, et al., 2010).
Classical literature using brain lesions identified the prefrontal cortical region as one that results in hyperactivity when damaged (Zubek and DeLorenzo, 1952), confirming earlier work in monkeys (Richter and Hines, 1938). Subsequent studies using lesions in rats identified the dorsal hippocampus as a specific region underlying persistent increases in exploratory behavior in rats (Kimble, 1963). Confirming a role of the dorsal hippocampus in locomotor activity, MacLean (MacLean, 1957) found that stimulation of this structure arrested movements in freely behaving rats. Lesions in amygdala performed in the rat also showed a role for this structure in regulation of spontaneous locomotor activity (Korczynski and Fonberg, 1979). In the Tg4510 mouse model, there is accumulation of Gallyas positive tau inclusions and loss of neurons in all of these structures (Cook, et al., 2014,Dickey, et al., 2009,Santacruz, et al., 2005). We have found 25% reductions in hippocampal volume and 30% losses of neurons in the hippocampus of Tg4510 animals at 7 months (Brownlow, et al., 2014,Nash, et al., 2013). Thus, one interpretation of the hyperactivity in our study suggests damage or injury to areas of the limbic system known to control locomotor activity.
Our results also show variations of daily energy expenditure (DEE) over the course of aging. DEE is the sum of multiple physiological processes including resting metabolism (i.e. minimum energy requirements for cell activity), the thermogenic effect of food (energy used for digestion) and locomotor activity; the last being considered as the largest contributor to individual differences (Lowell and Spiegelman, 2000). Notably in our study, group differences in DEE did not always associate with locomotor activity. Instead, energy expenditure correlated stronger to changes in resting metabolic rate. We observed a switch to increased food intake and resting metabolic rate in Tg4510 mice as the mice transitioned from the asymptomatic phase (2 months) when overexpression of tau has not yet led to pathological lesions, to the symptomatic phase where tau pathology is associated with tangles, brain atrophy and cognitive impairments (7 months). The end stage of the tau phenotype (12 months) resulted in a reduction in resting metabolic rate that was not observed in non-transgenic mice between 7 and 12 months of age. There was further a decline in DEE in 12 month old Tg4510 mice relative to non-transgenic mice, in spite of retention of the elevated locomotor activity during the dark phase of the cycle. This was associated with a reduced respiratory exchange rate, implying greater metabolism of fat relative to carbohydrate. Indeed, there was a specific loss of white adipose tissue mass in the transgenic mice, whereas muscle and brown fat mass remained similar to non-transgenic mice.
To maintain weight, increased energy utilization must be compensated for by an increase in energy intake. Therefore, the decrease in body weight in both tau mice models and AD patients should be compensated by an increased energy intake or by diminished energy expenditure. At a cellular level, the main mechanism involved in differences in metabolism is uncoupling and proton leak across the inner mitochondrial membrane which is under the control of carrier proteins (Dulloo and Samec, 2001) like uncoupling protein-1 (UCP-1) in brown adipose tissue. In the conditions of our experiment, no differences were observed in the levels of PGC1-α and its target UCP-1 in brown adipose tissue, suggesting that this mechanism was not engaged. Nevertheless, 12 month old mice seemed to trigger compensatory mechanisms like decreasing RMR and using more energy efficient fuels, such as lipids, as suggested by the observed decrease in respiratory exchange ratio and fat pad loss. However, these mechanisms were not sufficient to overcome the reduced weight gain occurring at younger ages. Indeed, even though we observed an increase in food intake at 7 months, it was not sufficient to compensate for the hyperactivity and maintain body weight. We verified that this was not due to a defect in energy absorption as fecal energy content did not differ between transgenic and non-transgenic littermates at any of the ages tested. This argues that loss of brain integration of peripheral signals or a deregulation of orexigenic pathways was involved. Information regarding nutrient status and energy stores is communicated to the brain through diverse endocrine and afferent neural signals where it is subsequently integrated in the hypothalamus to modulate food behavior and energy expenditure in order to maintain body weight (Morton, et al., 2006). Experiments performed 60 years ago showed that lesions of the ventromedial hypothalamus in the rat led to hyperphagia, decreased energy expenditure and increased body weight gain whereas lesions of the lateral hypothalamic area would result in decreased food intake (Anand and Brobeck, 1951). Hypothalamic lesions and volume loss is also reported in patients with AD and FTD and have been linked to disturbances in feeding behavior observed in these diseases (Piguet, et al., 2011). In this study we showed that Tg4510 mice are characterized by hyperphosphorylated tau in the hypothalamus at both 7 months and 12 months of age that may be linked to the variations observed in energy expenditure, food efficiency and body weight. In addition, white adipose tissue and plasma leptin concentration were dramatically decreased in aging Tg4510 mice compared to non-transgenic littermates, which indicate either a deregulation of fat sensing (the also called “adipostat”) located in the hypothalamus (Kennedy, 1953), or a default in efferent response to the peripheral organs involved in energy storage such as liver and adipose tissue. Further analysis will be interesting to determine if specific areas or neuronal populations of the hypothalamus are differentially affected by tau pathology.
Recent literature (Ishii, et al., 2014) showed that APP Tg2576 mice had hypothalamic leptin signaling dysfunction leading to early body weight deficits and increased energy expenditure without alterations in feeding behavior. These observations appeared correlated to poor function of the strong orexigenic effect of neuropeptide Y (NPY) in the hypothalamus. The phenotype described in this study resembled our data and similar mechanisms might be involved in Tg4510 mice. In a different experiment, (Adebakin, et al., 2012) reported that administration of an anorectic factor involved in meal termination, cholecystokinin (CCK), in 3xTgAD mice had no effect on food intake compared to non-transgenic controls. Detection of CCK by the brainstem appeared intact in these mice but efferent mechanisms might have been lost. In patients, some studies have also shown a reduction in content of the anorexic neurotransmitter serotonin (5HT) in the brain of both AD and FTD patients (Huey, et al., 2006) and a decrease in the density of some of its receptors (Cross, et al., 1984,Tsang, et al., 2010). In tau mice, a reduction in serotonergic neurons in THY-Tau22 mice was reported (Van der Jeugd, et al., 2013). Serotonin is involved in mood, sleep, feeding and energy balance (Lam and Heisler, 2007) and could be involved in the deregulation of homeostasis observed in both patients and mice models.
One advantage of longitudinal studies of this type is one can observe changes within the same mice circumventing cohort effects contributing to different outcomes at different ages. The differences seen in asymptomatic, symptomatic and end stages of the tau phenotype may be the reason different studies sometimes report contradictory results in patients as well as mouse models. It is conceivable that changes in energy metabolism over the course of Alzheimer’s disease have a biphasic response of initially elevated metabolism followed by reduced metabolism as pathology becomes more severe, as seen in our Tg4510 mouse model of tauopathy. Changes in peripheral metabolism may be used as a noninvasive, potential clinical marker for the different stages of the disease, reflecting changes in the central nervous system.
Finally, part of the phenotype observed in this study, especially at 12 months old, resembled some symptoms observed in cachexia. Cachexia, also known as wasting syndrome, is often observed in patients with cancer and has been reported in patients with AD (Koopmans, et al., 2007), being listed as the cause of death more often than dementia itself. The metabolic deregulation of cachexia leads to progressive weight loss and predicts both morbidity and mortality. It is feasible that cachexia-related metabolic disturbances could contribute to cognitive impairments in patients with AD, as in patients with cancer. Therefore, it remains important to determine how metabolism becomes dysregulated over time in AD. Understanding the mechanisms involved in such dysregulation over the course of dementia could lead to the development of effective interventions for ensuring metabolic homeostasis and may slow down the progression of the disease.
5. Conclusion
This is the first longitudinal study showing differences in metabolic rate, body weight and body composition in a mouse model of tau pathology. These findings raise new questions about the role of tau in body weight loss and metabolic deregulation related to AD pathology especially regarding the hypothesis in which hypermetabolism is responsible for weight loss in AD patients. Indeed, we report that resting metabolism was differently affected with aging in a manner which correlates with the severity of pathology. However, the reduced weight gain observed in our study appears due to a progressive hyperactive phenotype plus increased metabolic rate that is not adequately compensated for by increased food intake in 7 months old mice. Interestingly, body weight loss was associated with a dramatic reduction in white adipose tissue content, and a consequent decrease in plasma leptin concentration. As mice reach the end stage of the phenotype (average longevity is 14–15 months), hyperactivity is retained but is compensated by a reduced basal metabolic rate, despite a lower energy intake at 12 months of age.
Highlights.
Age related body weight loss in Tg4510 tau mice was associated with a decrease in fat mass but was independent of food intake.
Reduced weight gain in Tg4510 tau mice appears due to a progressive hyperactive phenotype
Basal metabolism was differently affected with aging in a manner which correlates with the severity of pathology.
Acknowledgments
This work was supported by Alzheimer Association grant IIRG-10-174448 (D.G.M.), NIH grant NS076308 (D.G.M.) and Byrd Alzheimer’s Institute BRD 712. We thank the Comparative Medicine staff for their assistance in caring for the mice. We also thank John Kuhn, PhD, and Yolanda Daza, Department of Chemical & Biomedical Engineering, University of South Florida for use of the calorimeter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There is no actual or potential conflict of interest.
Bibliography
- Adebakin A, Bradley J, Gumusgoz S, Waters EJ, Lawrence CB. Impaired satiation and increased feeding behaviour in the triple-transgenic Alzheimer's disease mouse model. PloS one. 2012;7(10):e45179. doi: 10.1371/journal.pone.0045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand BK, Brobeck JR. Localization of a "feeding center" in the hypothalamus of the rat. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 1951;77(2):323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Klauber MR, Gillin JC, Campbell SS, Hofstetter CR. Sleep in non-institutionalized Alzheimer's disease patients. Aging (Milan, Italy) 1994;6(6):451–458. doi: 10.1007/BF03324277. [DOI] [PubMed] [Google Scholar]
- Andrieu S, Reynish W, Nourhashemi F, Ousset PJ, Grandjean H, Grand A, Albarede JL, Vellas B. Nutritional risk factors for institutional placement in Alzheimer's disease after one year follow-up. The journal of nutrition, health & aging. 2001;5(2):113–117. [PubMed] [Google Scholar]
- Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, Traykov L. Executive function deficits in early Alzheimer's disease and their relations with episodic memory. Arch Clin Neuropsychol. 2006;21(1):15–21. doi: 10.1016/j.acn.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? Journal of neurology, neurosurgery, and psychiatry. 2000;69(2):178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow ML, Benner L, D'Agostino D, Gordon MN, Morgan D. Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer's pathology. PloS one. 2013;8(9):e75713. doi: 10.1371/journal.pone.0075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow ML, Joly-Amado A, Azam S, Elza M, Selenica ML, Pappas C, Small B, Engelman R, Gordon MN, Morgan D. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behavioural brain research. 2014;271:79–88. doi: 10.1016/j.bbr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Burns A, Marsh A, Bender DA. Dietary intake and clinical, anthropometric and biochemical indices of malnutrition in elderly demented patients and non-demented subjects. Psychological medicine. 1989;19(2):383–391. doi: 10.1017/s0033291700012423. [DOI] [PubMed] [Google Scholar]
- Cook C, Dunmore JH, Murray ME, Scheffel K, Shukoor N, Tong J, Castanedes-Casey M, Phillips V, Rousseau L, Penuliar MS, Kurti A, Dickson DW, Petrucelli L, Fryer JD. Severe amygdala dysfunction in a MAPT transgenic mouse model of frontotemporal dementia. Neurobiol Aging. 2014;35(7):1769–1777. doi: 10.1016/j.neurobiolaging.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AJ, Crow TJ, Ferrier IN, Johnson JA, Bloom SR, Corsellis JA. Serotonin receptor changes in dementia of the Alzheimer type. Journal of neurochemistry. 1984;43(6):1574–1581. doi: 10.1111/j.1471-4159.1984.tb06081.x. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Jacobs DM, Tang MX, Del Castillo-Castaneda C, Sano M, Marder K, Bell K, Bylsma FW, Brandt J, Albert M, Stern Y. The course of psychopathologic features in mild to moderate Alzheimer disease. Archives of general psychiatry. 1997;54(3):257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Miller L, Richards M, Marder K, Bell K, Mayeux R, Stern Y. The Columbia University Scale for Psychopathology in Alzheimer's disease. Archives of neurology. 1992;49(4):371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- Dickey C, Kraft C, Jinwal U, Koren J, Johnson A, Anderson L, Lebson L, Lee D, Dickson D, de Silva R, Binder LI, Morgan D, Lewis J. Aging analysis reveals slowed tau turnover and enhanced stress response in a mouse model of tauopathy. The American journal of pathology. 2009;174(1):228–238. doi: 10.2353/ajpath.2009.080764. [pii] 10.2353/ajpath.2009.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson KE, Carpenter WH, Toth MJ, Goran MI, Newhouse P, Poehlman ET. No evidence for a higher resting metabolic rate in noninstitutionalized Alzheimer's disease patients. Journal of the American Geriatrics Society. 1996;44(10):1232–1234. doi: 10.1111/j.1532-5415.1996.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Samec S. Uncoupling proteins: their roles in adaptive thermogenesis and substrate metabolism reconsidered. The British journal of nutrition. 2001;86(2):123–139. doi: 10.1079/bjn2001412. [DOI] [PubMed] [Google Scholar]
- Faxen-Irving G, Basun H, Cederholm T. Nutritional and cognitive relationships and long-term mortality in patients with various dementia disorders. Age and ageing. 2005;34(2):136–141. doi: 10.1093/ageing/afi023. [DOI] [PubMed] [Google Scholar]
- Filali M, Lalonde R, Theriault P, Julien C, Calon F, Planel E. Cognitive and non-cognitive behaviors in the triple transgenic mouse model of Alzheimer's disease expressing mutated APP, PS1, and Mapt (3xTg-AD) Behavioural brain research. 2012;234(2):334–342. doi: 10.1016/j.bbr.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Gambassi G, Landi F, Lapane KL, Sgadari A, Mor V, Bernabei R. Predictors of mortality in patients with Alzheimer's disease living in nursing homes. Journal of neurology, neurosurgery, and psychiatry. 1999;67(1):59–65. doi: 10.1136/jnnp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette-Guyonnet S, Cortes F, Cantet C, Vellas B. Long-term cholinergic treatment is not associated with greater risk of weight loss during Alzheimer's disease: data from the French REAL.FR cohort. The journal of nutrition, health & aging. 2005;9(2):69–73. [PubMed] [Google Scholar]
- Gillette-Guyonnet S, Nourhashemi F, Andrieu S, de Glisezinski I, Ousset PJ, Riviere D, Albarede JL, Vellas B. Weight loss in Alzheimer disease. The American journal of clinical nutrition. 2000;71(2):637s–642s. doi: 10.1093/ajcn/71.2.637s. [DOI] [PubMed] [Google Scholar]
- Gillette GS, Abellan VK, Alix E, Andrieu S, Belmin J, Berrut G, Bonnefoy M, Brocker P, Constans T, Ferry M, Ghisolfi-Marque A, Girard L, Gonthier R, Guerin O, Hervy MP, Jouanny P, Laurain MC, Lechowski L, Nourhashemi F, Raynaud-Simon A, Ritz P, Roche J, Rolland Y, Salva T, Vellas B. IANA (International Academy on Nutrition and Aging) Expert Group: weight loss and Alzheimer's disease. JNutrHealth Aging. 2007;11(1):38–48. [PubMed] [Google Scholar]
- Gordon MN, Holcomb LA, Jantzen PT, DiCarlo G, Wilcock D, Boyett KL, Connor K, Melachrino JO, O'Callaghan JP, Morgan D. Time course of the development of Alzheimer-like pathology in the doubly transgenic PS1+APP mouse. Exp Neurol. 2002;173:183–195. doi: 10.1006/exnr.2001.7754. [DOI] [PubMed] [Google Scholar]
- Guerin O, Andrieu S, Schneider SM, Milano M, Boulahssass R, Brocker P, Vellas B. Different modes of weight loss in Alzheimer disease: a prospective study of 395 patients. The American journal of clinical nutrition. 2005a;82(2):435–441. doi: 10.1093/ajcn.82.2.435. [DOI] [PubMed] [Google Scholar]
- Guerin O, Soto ME, Brocker P, Robert PH, Benoit M, Vellas B. Nutritional status assessment during Alzheimer's disease: results after one year (the REAL French Study Group) The journal of nutrition, health & aging. 2005b;9(2):81–84. [PubMed] [Google Scholar]
- Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66(1):17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Inelmen EM, Sergi G, Coin A, Girardi A, Manzato E. An open-ended question: Alzheimer's disease and involuntary weight loss: which comes first? Aging clinical and experimental research. 2010;22(3):192–197. doi: 10.1007/BF03324796. [DOI] [PubMed] [Google Scholar]
- Ishii M, Wang G, Racchumi G, Dyke JP. Transgenic mice overexpressing amyloid precursor protein exhibit early metabolic deficits and a pathologically low leptin state associated with hypothalamic dysfunction in arcuate neuropeptide Y neurons. 2014;34(27):9096–9106. doi: 10.1523/JNEUROSCI.0872-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, Rouch C, Kassis N, Dairou J, Cani PD, Ventura-Clapier R, Prola A, Flamment M, Foufelle F, Magnan C, Luquet S. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. The EMBO journal. 2012;31(22):4276–4288. doi: 10.1038/emboj.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jul P, Volbracht C, de Jong IE, Helboe L, Elvang AB, Pedersen JT. Hyperactivity with Agitative-Like Behavior in a Mouse Tauopathy Model. Journal of Alzheimer's disease : JAD. 2015;49(3):783–795. doi: 10.3233/JAD-150292. [DOI] [PubMed] [Google Scholar]
- Keene JM, Hope T. Hyperphagia in dementia, 2. Food choices and their macronutrient contents in hyperphagia, dementia and ageing. Appetite. 1997;28(2):167–175. doi: 10.1006/appe.1996.0068. [DOI] [PubMed] [Google Scholar]
- Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proceedings of the Royal Society of London Series B, Biological sciences. 1953;140(901):578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Kimble DP. The effects of bilateral hippocampal lesions in rats. Journal of Comparative and Physiological Psychology. 1963;56:273–283. doi: 10.1037/h0048903. [DOI] [PubMed] [Google Scholar]
- Knight EM, Brown TM, Gumusgoz S, Smith JC, Waters EJ, Allan SM, Lawrence CB. Agerelated changes in core body temperature and activity in triple-transgenic Alzheimer's disease (3xTgAD) mice. Dis Model Mech. 2013;6(1):160–170. doi: 10.1242/dmm.010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight EM, Verkhratsky A, Luckman SM, Allan SM, Lawrence CB. Hypermetabolism in a triple-transgenic mouse model of Alzheimer's disease. Neurobiol Aging. 2012;33(1):187–193. doi: 10.1016/j.neurobiolaging.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Koopmans RT, van der Sterren KJ, van der Steen JT. The 'natural' endpoint of dementia: death from cachexia or dehydration following palliative care? International journal of geriatric psychiatry. 2007;22(4):350–355. doi: 10.1002/gps.1680. [DOI] [PubMed] [Google Scholar]
- Korczynski R, Fonberg E. Spontaneous locomotor activity and food and water intake in rats with medial amygdala lesions. Acta neurobiologiae experimentalis. 1979;39(4):227–240. [PubMed] [Google Scholar]
- Krol E, Murphy M, Speakman JR. Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. The Journal of experimental biology. 2007;210(Pt 23):4233–4243. doi: 10.1242/jeb.009779. [DOI] [PubMed] [Google Scholar]
- Lam DD, Heisler LK. Serotonin and energy balance: molecular mechanisms and implications for type 2 diabetes. Expert reviews in molecular medicine. 2007;9(5):1–24. doi: 10.1017/S1462399407000245. [DOI] [PubMed] [Google Scholar]
- Leboucher A, Laurent C, Fernandez-Gomez FJ, Burnouf S, Troquier L, Eddarkaoui S, Demeyer D, Caillierez R, Zommer N, Vallez E, Bantubungi K, Breton C, Pigny P, Buee-Scherrer V, Staels B, Hamdane M, Tailleux A, Buee L, Blum D. Detrimental effects of diet-induced obesity on tau pathology are independent of insulin resistance in tau transgenic mice. Diabetes. 2013;62(5):1681–1688. doi: 10.2337/db12-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo KA, Charoenthongtrakul S, Giuliana DJ, Govek EK, McDonagh T, Distefano PS, Geddes BJ. The 24-hour respiratory quotient predicts energy intake and changes in body mass. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298(3):R747–R754. doi: 10.1152/ajpregu.00476.2009. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Wisniewski SR, Becker JT, Boller F, DeKosky ST. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer disease. Archives of neurology. 1999;56(10):1266–1272. doi: 10.1001/archneur.56.10.1266. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404(6778):652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- MacLean PD. Chemical and electrical stimulation of hippocampus in unrestrained animals. Archives of Neurology and Psychiatry. 1957;78:113–142. doi: 10.1001/archneurpsyc.1957.02330380003001. [DOI] [PubMed] [Google Scholar]
- Maurin H, Lechat B, Borghgraef P, Devijver H, Jaworski T, Van Leuven F. Terminal hypothermic Tau.P301L mice have increased Tau phosphorylation independently of glycogen synthase kinase 3alpha/beta. The European journal of neuroscience. 2014;40(2):2442–2453. doi: 10.1111/ejn.12595. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morgan D, Gordon MN. Amyloid, hyperactivity, and metabolism: theoretical comment on Vloeberghs et al. (2008) Behavioral neuroscience. 2008;122(3):730–732. doi: 10.1037/0735-7044.122.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Nash KR, Lee DC, Hunt JB, Jr, Morganti JM, Selenica ML, Moran P, Reid P, Brownlow M, Guang-Yu Yang C, Savalia M, Gemma C, Bickford PC, Gordon MN, Morgan D. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol Aging. 2013;34(6):1540–1548. doi: 10.1016/j.neurobiolaging.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskanen L, Piirainen M, Koljonen M, Uusitupa M. Resting energy expenditure in relation to energy intake in patients with Alzheimer's disease, multi-infarct dementia and in control women. Age and ageing. 1993;22(2):132–137. doi: 10.1093/ageing/22.2.132. [DOI] [PubMed] [Google Scholar]
- Piguet O. Eating disturbance in behavioural-variant frontotemporal dementia. Journal of molecular neuroscience : MN. 2011;45(3):589–593. doi: 10.1007/s12031-011-9547-x. [DOI] [PubMed] [Google Scholar]
- Piguet O, Petersen A, Yin Ka Lam B, Gabery S, Murphy K, Hodges JR, Halliday GM. Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Annals of neurology. 2011;69(2):312–319. doi: 10.1002/ana.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, Krishnamurthy P, Herman M, Wang L, Schachter JB, Nelson RB, Lau LF, Duff KE. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(12):3090–3097. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh PL, Richardson JC, Bate ST, Upton N, Sunter D. Non-cognitive behaviours in an APP/PS1 transgenic model of Alzheimer's disease. BehavBrain Res. 2007;178(1):18–28. doi: 10.1016/j.bbr.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Richter CP, Hines M. Increased spontaneous activity produced in monkeys by brain lesions. Brain. 1938;61:1–16. [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Blacker D, Albert M, Hadjigeorgiou G, Dubois B, Devanand D, Honig L, Stern Y. Disruptive behavior as a predictor in Alzheimer disease. Archives of neurology. 2007;64(12):1755–1761. doi: 10.1001/archneur.64.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. Journal of neurology, neurosurgery, and psychiatry. 2001;70(3):323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler AA, Renvall MJ, Nichols JF, Ramsdell JW. Nutritional status of patients with Alzheimer's disease: a 1-year study. Journal of the American Dietetic Association. 1996;96(10):1013–1018. doi: 10.1016/S0002-8223(96)00270-2. [DOI] [PubMed] [Google Scholar]
- Sterniczuk R, Dyck RH, Laferla FM, Antle MC. Characterization of the 3xTg-AD mouse model of Alzheimer's disease: part 1. Circadian changes. Brain research. 2010;1348:139–148. doi: 10.1016/j.brainres.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Tamura BK, Masaki KH, Blanchette P. Weight loss in patients with Alzheimer's disease. Journal of nutrition for the elderly. 2007;26(3–4):21–38. doi: 10.1300/j052v26n03_02. [DOI] [PubMed] [Google Scholar]
- Tsang SW, Keene J, Hope T, Spence I, Francis PT, Wong PT, Chen CP, Lai MK. A serotoninergic basis for hyperphagic eating changes in Alzheimer's disease. Journal of the neurological sciences. 2010;288(1–2):151–155. doi: 10.1016/j.jns.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Muller TD, Munzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nature methods. 2012;9(1):57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Jeugd A, Blum D, Raison S, Eddarkaoui S, Buee L, D'Hooge R. Observations in THYTau22 mice that resemble behavioral and psychological signs and symptoms of dementia. Behavioural brain research. 2013;242:34–39. doi: 10.1016/j.bbr.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Vellas B, Lauque S, Gillette-Guyonnet S, Andrieu S, Cortes F, Nourhashemi F, Cantet C, Ousset PJ, Grandjean H. Impact of nutritional status on the evolution of Alzheimer's disease and on response to acetylcholinesterase inhibitor treatment. The journal of nutrition, health & aging. 2005;9(2):75–80. [PubMed] [Google Scholar]
- Vloeberghs E, Van Dam D, Franck F, Serroyen J, Geert M, Staufenbiel M, De Deyn PP. Altered ingestive behavior, weight changes, and intact olfactory sense in an APP overexpression model. Behavioral neuroscience. 2008;122(3):491–497. doi: 10.1037/0735-7044.122.3.491. [DOI] [PubMed] [Google Scholar]
- Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1–2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H, Pieper C, Schmader K. The association of weight change in Alzheimer's disease with severity of disease and mortality: a longitudinal analysis. Journal of the American Geriatrics Society. 1998;46(10):1223–1227. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- Wolf-Klein GP, Silverstone FA, Levy AP. Nutritional patterns and weight change in Alzheimer patients. International psychogeriatrics / IPA. 1992;4(1):103–118. doi: 10.1017/s1041610292000930. [DOI] [PubMed] [Google Scholar]
- Zubek JP, DeLorenzo AJ. The cerebral cortex and locomotor activity in rats. Canadian Journal of psychology. 1952;6:55–70. doi: 10.1037/h0083554. [DOI] [PubMed] [Google Scholar]